Summary
Solar hydrogen and electricity are promising high energy-density renewable sources. Although photochemistry or photovoltaics are attractive routes, special challenge arises in sunlight conversion efficiency. To improve efficiency, various semiconductor materials have been proposed with selective sunlight absorption. Here, we reported a hybrid system synergizing photo-thermochemical hydrogen and photovoltaics, harvesting full-spectrum sunlight in a cascade manner. A simple suspension of Au-TiO2 in water/methanol serves as a spectrum selector, absorbing ultraviolet-visible and infrared energy for rapid photo-thermochemical hydrogen production. The transmitted visible and near-infrared energy fits the photovoltaic bandgap and retains the high efficiency of a commercial photovoltaic cell under different solar concentration values. The experimental design achieved an overall efficiency of 4.2% under 12 suns solar concentration. Furthermore, the results demonstrated a reduced energy loss in full-spectrum energy conversion into hydrogen and electricity. Such simple integration of photo-thermochemical hydrogen and photovoltaics would create a pathway toward cascading use of sunlight energy.
Subject Areas: Electrochemical Energy Conversion, Energy Resources, Energy Materials
Graphical Abstract
Highlights
-
•
An integration of both photothermal H2 and PV was proposed at full solar spectrum
-
•
Absorbed UV-vis and IR generate H2 faster than reported full-spectrum catalysis
-
•
Transmitted Vis and near-IR bands retain the high efficiency of commercial PV cells
-
•
A novel device was designed with experimental overall efficiency of 4.2% at 12 suns
Electrochemical Energy Conversion; Energy Resources; Energy Materials
Introduction
Generating clean and storable hydrogen from sunlight has been pursued as a fundamental technology for the future of humanity (IEA, 2019). The past several decades have seen a wide array of research on fundamental and applied sciences and engineering toward an efficient and economic sunlight-hydrogen energy conversion process. The concept of “one-pot” solar-hydrogen conversion process is highlighted by using a single solar reactor, ensuring outstanding feasibility and scalability (Ozin, 2017). By its nature, the sunlight energy source includes the broad UV, visible, and infrared bands, generating both electrical and thermal effects for hydrogen generation. By directly using the driving forces, solar photochemical and solar thermochemical conversions emerge for producing “one-pot” solar hydrogen (Tavasoli and Ozin, 2018).
In the photochemical process, the photo-excited charge carriers as a driving force is naturally high-grade electrical energy. Typically, the photocatalytic water splitting is a process from charge carriers to chemical energy in hydrogen, with a leading solar-to-hydrogen efficiency of 1.1% (Wang et al., 2016). Although it is a high-quality electrical-chemical process, the photocatalysis usually has a low efficiency. Thermodynamically, one reason may be the photochemically used high-grade UV band only covers 5% in full spectrum energy, losing most of the Vis-IR band. On the other hand, the high quality of photon energy is severely damaged associating with the irreversibility, because the sunlight has variable spectral energy quantity and quality and is hard to fit with a single bandgap. At the full-spectrum scale, efforts have been made to derive more charge carriers with lower-energy grade from broadband sunlight photons at longer wavelength (Cao et al., 2019, Yang et al., 2019). To reinforce the energy grade, the broadband water splitting requires the assistance of the Z-scheme or sacrificial reagents (Liang et al., 2018, Qi et al., 2018, Liu et al., 2018, Fang et al., 2019). However, a previous study still revealed a dilemma between the energy quantity and quality: the most popular BiVO4 has a recombination of 50%–70% in the UV band and >80% in the Vis band (Zhao et al., 2014). The recombined charge carrier then dissipated as low-temperature heat, causing irreversibility. Toward the 5%–10% solar-to-hydrogen efficiency target for industrial scalable solar photochemistry (Pinaud et al., 2013), the breakthrough is needed on improving both the quality and quantity of photocatalytic utilization. One is to match the semiconductor bandgap to full spectrum photon energy. Then, the gap in energy grade can be minimized between the energy donor and acceptor, reducing the irreversible energy loss. Another is to properly utilize the long-wavelength sunlight with photon energy lower than bandgap, assisting the H2 production.
Originating from the needs of photochemical conversion, the photo-thermocatalytic conversion is proposed by synergizing the photo-thermal driving forces (Ghoussoub et al., 2019). Ozin et al. demonstrated that a nano-heater under sunlight irradiation can produce localized heat to improve the surface reaction rate (Jia et al., 2016). The localized heat was also found to assist the low-grade photons in bandgap excitation (Li et al., 2019b). The Vis and IR sunlight can generate localized heat in processes like recombination, surface plasmonic resonance (SPR), or even the reactant molecular absorption of sunlight. Through controlling such processes, the low-grade Vis and IR photons can join the production of photo-thermal H2 instead of being dissipated as low-temperature heat. In the photochemical component of photo-thermocatalysis, the UV-vis-generated charge carriers may provide a bypass circumventing the thermal activation barrier, reducing the activation energy (Ghoussoub et al., 2019, Li et al., 2019c). In addition, the consumption of photo-generated holes also benefits from an elevated temperature, often induced by localized heat from the IR band (Fang et al., 2019). Then the photochemical process provides more photo-excited electrons, accelerating the surface H2 evolution. The photo-thermocatalysis is no longer an individual photochemical or thermochemical process (Tang et al., 2017) but a photo-thermal integrated systematic utilization at a broadband solar spectrum scale.
Besides the photo-thermocatalytic conversion, there is another method for photo-thermal integrated systems that use spectral splitting. Typically, a band-pass spectrum splitter transmits the near infrared (NIR) band for photovoltaic (PV) electricity generation, while absorbing the UV-vis-IR band for solar thermochemistry or solar heat generation (Tang et al., 2018, Weinstein et al., 2018). In recent reports, even the PV itself can become a spectrum selector transmitting below-bandgap photons for solar heat (Xu et al., 2020). Such spectral splitting methods matched the photovoltaic (PV) conversion with the Vis-IR band, where the PV is more efficient than with full-spectrum, thus improving the overall efficiency. This inspires us to a potential synergy of PV and solar photo-thermochemistry. A not only spectral selective but photo-thermal catalytic solution can enable a synergy system of solar H2 and PV electricity, both at their most desirable solar spectrum band.
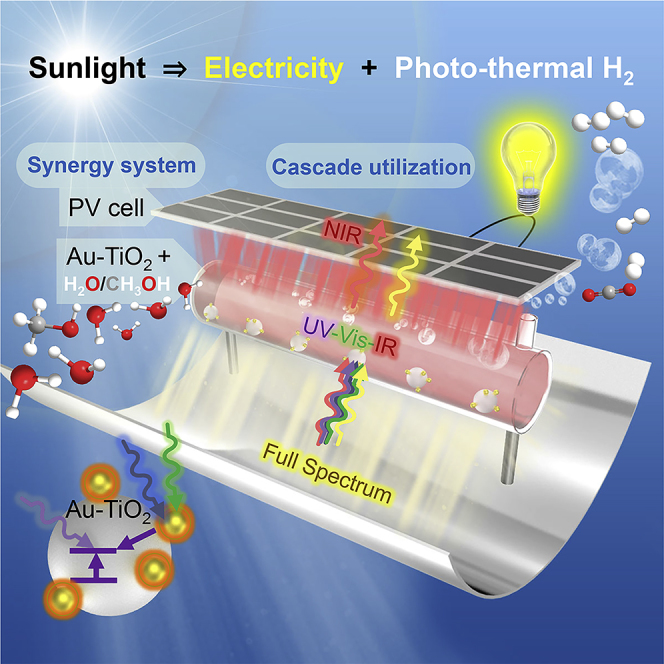
Herein, we provide a solar energy system co-generating H2 and electricity in a cascade-like conversion of the concentrated sunlight. Matching the energy grade between the solar spectrum and the conversions, the system uses not only the UV-vis/IR band for photo-thermal H2 but also the Vis and near-infrared (NIR) band for PV electricity. We designed an experimental device to identify the key problems in the cascade-like conversion of the concentrated sunlight. A simple Au-TiO2 suspended reaction solution is designed like a spectral selector, absorbing the UV-IR while transmitting the Vis-NIR bands. Under an input sunlight intensity as high as 15 suns, we found that the H2 productivity using the UV-vis and IR narrow band gained a 30%–40% improvement to full-spectrum absorptive sophisticated photo-thermocatalysis. The transmitted visible and near-infrared energy coincided with the photovoltaic bandgap and maintained the high efficiency of a commercial photovoltaic cell under different solar concentration values. The experimental design achieved an overall efficiency of 4.2% for a solar concentration of 12 suns. Further analysis on energy flow enlightened that through selectively absorbing the useful photons, it is easier to separate charge carriers.
Results and Discussion
Synergizing Concentrated Photo-Thermal Hydrogen and Photovoltaics
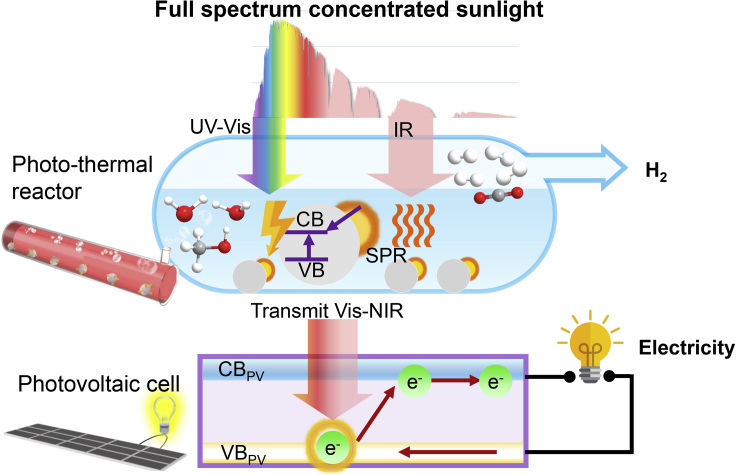
The research in photocatalysis has provided various means to expand the spectrum absorption, such as using compound bandgap semiconductors with a core-shell, anchored, or a multilayer structure (Liu et al., 2017a). In the present work, we selected a simple Au-TiO2 catalyst and obtained spectral selectivity along with absorptivity. The hybrid system, schematically shown in Figure 1, proposes a cascade pathway converting the concentrated sunlight into photo-thermal H2 and PV electricity. A parabolic trough solar collector concentrates the sunlight to 15 suns. In the upstream photo-thermal H2 process, the concentrated sunlight enters the Au-TiO2 mixed methanol/water reaction solution. The reaction solution functions as a spectrum “selector,” absorbing the UV-vis/IR band while transmitting the Vis-NIR band. Through such a selector, the energy of UV-vis/IR band drives the photo-thermocatalysis and is converted into the chemical energy in H2. In the downstream photovoltaic process, the integrated PV cell receives the Vis-NIR band transmitted out of the reaction solution. In contrast, an individual PV is prone to relaxation or carrier recombination in the UV-vis/IR band, generating waste heat. Based on the second law of thermodynamics, the waste heat generation is the destruction of the potential for conversion into electricity or chemical energy. By selecting the UV-vis/IR for upstream and transmitting Vis-NIR for downstream, this work maintains the energy grade of the sunlight converted into chemical energy and electricity.
Figure 1.
Synergizing Concentrated Photo-Thermal Hydrogen and Photovoltaics
Schematic of the concentrated solar system synergizing photo-thermal H2 and PV electricity in a cascade pathway.
Selective Absorption for Photo-Thermal Hydrogen
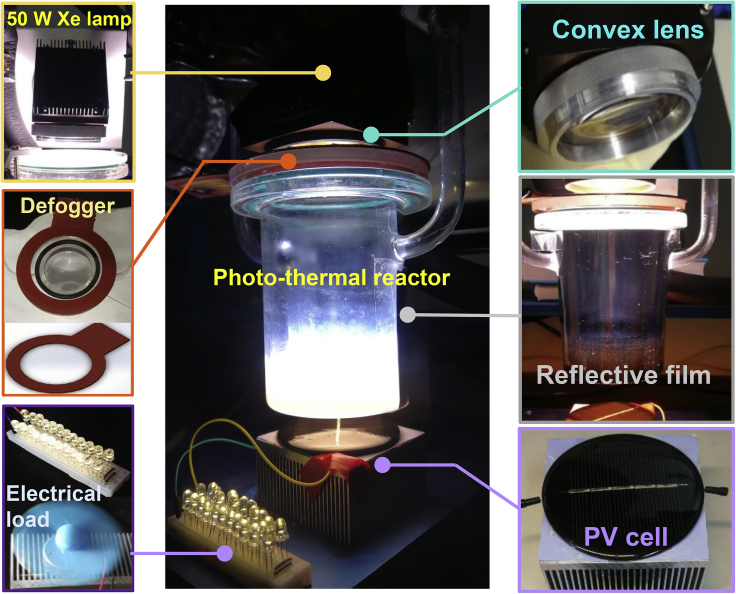
Figure 2 shows the experimental apparatus for the cascading production of photo-thermal H2 and electricity from full-spectrum sunlight (more details in Figures S10 and S11). We assembled a convex lens on a 300 W xenon lamp and achieved a concentrated sunlight input of 15 suns. The intensity was measured at the same location and orientation of the surface of the reaction solution (Li et al., 2019a). For photo-thermocatalysis, loaded Au was controlled at an average d = 15 nm (Figures S1 and S12) to acquire an absorbing peak at 400–700 nm Vis band. The morphology remained stable after 40 h of experiments (Figures S1 and S2). By dispersing Au-TiO2 in 10% vol methanol, a near-homogeneous volumetric liquid absorber was obtained with UV-vis/IR absorption and Vis-NIR transmittance. Then, an over 95% transmittivity quartz reactor was adopted to contain the liquid absorber to minimize the optical losses. The reactor cap was blurred by the reactant steam; thus, we pasted a defogger heating the cap at 110°C to prevent blurring. There also existed outward scattering around the side of the reactor; therefore, an over 95% reflective film was used to recollect the scattering. Consequently, most of the scattered and unabsorbed sunlight was transmitted out of the reactor bottom. The total transmitted sunlight was measured using a spectrometer as 700–1,100 nm wavelength in the Vis-NIR band (Figure S3). Furthermore, the transmitted NIR coincided with the 1.2- to 1.3-eV bandgap of mono-crystalline silicon, driving the commercial PV cell to obtain electricity.
Figure 2.
Selective Absorption for Photo-Thermal Hydrogen
Experimental apparatus for the cascading production of photo-thermal H2 and electricity from full-spectrum sunlight.
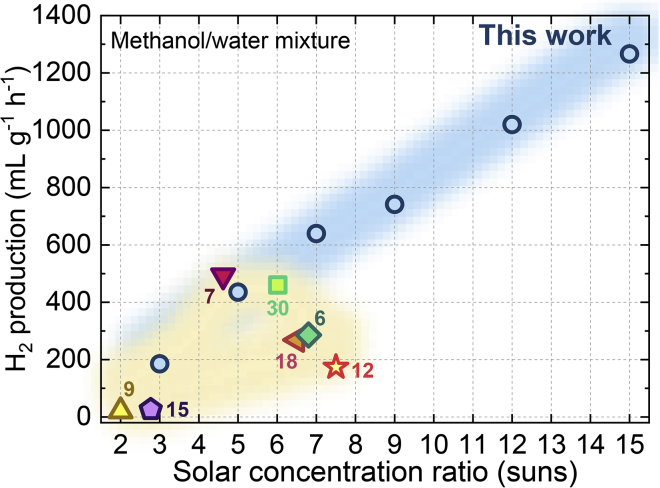
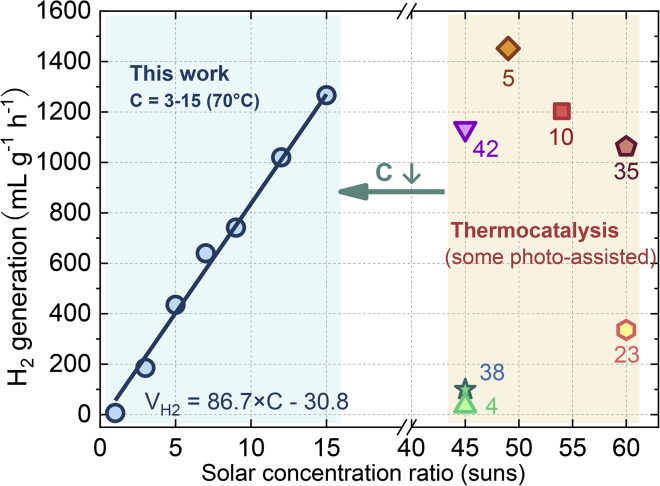
Figure 3 shows the photo-thermocatalysis performance using UV-vis/IR spectrum under concentrated sunlight of C = 1–15. The H2 generation rate increased in proportion to the increasing solar concentration ratio, and the highest rate was 750–1,300 mL gcat−1 h−1 at C = 9–15 (see Video S1). Even at C = 7, we used the UV-vis/IR band to generate H2 at 435 mL h−1 g−1. The results indicate that the UV-vis/IR narrow band is sufficient for H2 generation owing to the high UV-vis energy grade (>2 eV) for charge carrier excitation and an elevated IR phonon energy grade by photo-thermal synergy. Using broadband absorptive catalysts such as SiO2/Ag@TiO2 or Pt-TiO2, previous studies also reported photo-thermal H2 generation rates of <460 mL h−1 g−1 at C = 2–7 with the same sacrificial reagent (for detailed data see Table S1) (Chiarello et al., 2010, Chiarello et al., 2014, Elbanna et al., 2017, Gao et al., 2016, Highfield et al., 2009, Huaxu et al., 2017, Liu et al., 2017b). Figure 3 shows that, although the present work adopted the simplest and less active Au-TiO2 catalyst, a 30%–50% increase in H2 productivity was observed compared with previously reported values. Considering the energy donor as the absorbed sunlight and the acceptor as the photo-thermal reaction, the acceptor side responds better to the charge carriers from the UV-vis band and phonons from the IR band. If the catalyst absorption is monotonously expanded, the additional Vis-NIR band may provide more photons with lower energy. Such Vis-NIR charge carriers have insufficient energy for H2 generation but may produce phonon heat to inhibit the transfer of charge carriers. Owing to spectral selectivity, photo-thermocatalysis avoided handling the Vis-NIR band, increasing the H2 productivity.
Figure 3.
Photo-Thermal H2 Generation Rate from UV-Vis/IR Spectrum in This Work and from Full-Spectrum in Previous Studies
The solution is not stirred to clarify the movement of bubbles
Figure 3 suggests that solar concentration plays an important role in photo-thermal H2 generation. Solar concentration pressurizes the photon gas before it enters the turbine (Bejan, 2016), improving the capacity of sunlight to do work. In concentrated sunlight-H2 conversion, the capacity to do work appears as H2 productivity. In the present work, the H2 productivity increases linearly as the sunlight is concentrated to 3–15 suns. The primary cause for such linear promotion is the densified charge carrier flow and localized surface heating under the solar concentration. The charge carrier flow may be explained by electromagnetic simulation, where an SPR Au localized electrical field is observed. The localized electricity field can selectively absorb the UV-vis band and channel the charge carrier generation process near the TiO2 surface (Figures S4A and S4B). This can avoid the bulk phase recombination loss, especially under the concentrated sunlight (Sigle et al., 2015). Furthermore, the effect of concentrated solar heating at 35°C–85°C and C = 5–15 (Figures S5A and S5B) increased the H2 production rate by two to three times. The reason is that the thermal effect activated more adsorbed molecules to react with the surface charge carriers, suppressing the competent process of surface recombination (Panayotov and Morris, 2016). In comparison, the pure semiconductor photocatalytic H2 was seldom explored at a concentration ratio higher than C = 6 (Wei et al., 2018). On the one hand, the SPR effect in the present work may avoid heating from the excessive IR band or bulk recombination, which can reduce the charge carrier mobility in purely semiconductor photocatalysis (Neumann et al., 2013). On the other hand, the IR heat-assisted surface reaction avoided the loss of IR sunlight quality to waste heat in pure semiconductor photocatalysis. Therefore, the photo-thermocatalysis in this work presents an opportunity to increase the solar concentration.
We expected an increase in the ability to generate H2 under concentrated sunlight. A breakthrough made by Turner et al. (Khaselev and Turner, 1998). promoted the sunlight-photocurrent efficiency from 7% to 12.4% for a photo-electrochemical (PEC) cell by elevating the solar concentration to C = 11.9. The results of Turner et al. encouraged us to measure the photocurrent for Au-TiO2 nanoparticles, which absorbs a narrow UV-vis band from the full spectrum. Figure S6 shows the continuously rising shape of the transient photocurrent character. If the transient photocurrent has a decay spike under the concentrated sunlight, there may be a possible aggregation of the energy loss in surface recombination (Sigle et al., 2015, Xue et al., 2017). The likely reason is a steady surface reaction coupled with an ever-increasing surface charge carrier productivity; thus, excessive charge carriers from the broad band may be dissipated in the recombination. Here, the selective absorption only generates charge carriers from the UV/Vis-IR narrow band (see absorptivity curve measured in Figure S3 and the absorption of Au nanoparticles simulated in Figure S4C). Moreover, no spike is observed in the transient photocurrent, indicating no charge accumulation during the test. We collected the steady photocurrent under different solar concentration values and depicted the results in Figure 4. In the present work, the steady photocurrent increases linearly from 0.20 to 1.30 μA with the concentration ratio of C. Individual photocatalysis typically shows a logarithmic photocurrent increase with solar concentration, slower than the linear increase (Bell et al., 2013). Using the UV/Vis-IR narrow band absorption, this work avoids the ever-increasing recombination loss in individual photocatalysis (Pihosh et al., 2015), reducing the irreversibility from the Vis-NIR band.
Figure 4.
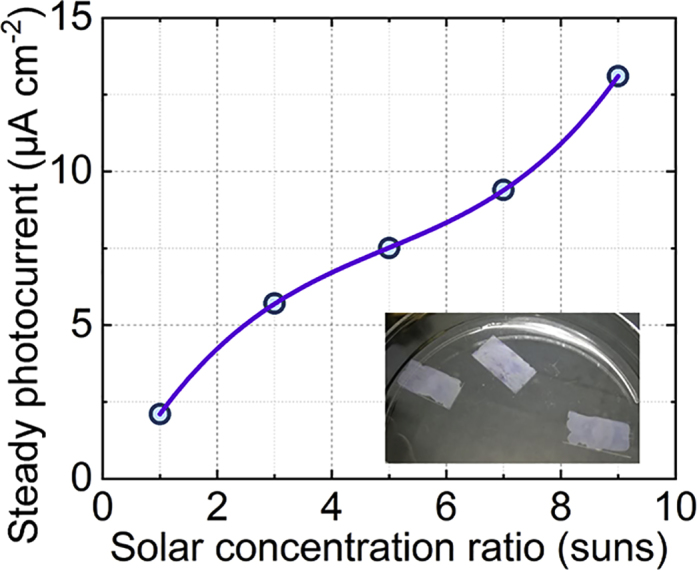
The Steady Photocurrent under Solar Concentration of C = 1–9
For experimental details see Figure S13.
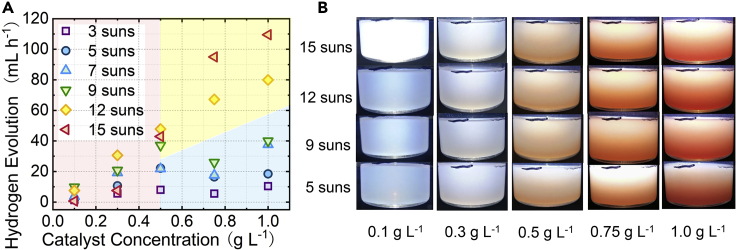
Figure 5A presents the influence of the catalyst mass concentration on photo-thermal H2 generation. The effects differ in three conditions. The first is a condition of 0.1–0.5 g L−1 and high solar concentration (the red region), with a H2 generation rate <30 mL h−1 even at C = 15. Although the solar concentration is high, the low catalyst concentration has weak absorption and loses the UV-vis band in transmission. The second condition is a high catalyst concentration and low solar concentration (the blue region), where the H2 generation rate is only slightly increased to 40 mL h−1 at 1 g L−1 and C = 7. C = 7 is too weak to penetrate the nano catalyst layer and can only illuminate the catalyst nanoparticles near the skin of the reaction solution (Figure 5B). With surface reaction as the rate-determining step, most surface charge carriers recombine, limiting the H2 production on each catalyst particle. As the solution skin only contains a part of all suspended catalysts, the H2 production rate of the solution is limited as a result. The third condition (the yellow region) reveals a strong interaction between the high solar concentration and high catalyst concentration. Compared with C < 10, the UV-vis sunlight at C = 15 may be intensive enough to easily arrive at each nanocatalyst particle of 1.0 g L−1 (Figure 5B), elevating the rate of H2 conversion to the highest level of 110 mL h−1.
Figure 5.
The Photo-Thermal Reacion under Various Solar Concentration and Catalyst Concentration
(A) Influence of catalyst mass concentration (0.1–1.0 g L−1) on H2 production rate.
(B) The appearance of the reaction solution of different volume concentrations under 5–15 suns.
We compared the photo-thermocatalysis method with the state-of-the-art solar thermocatalysis method where the concentrated full-spectrum sunlight as a heat source drives the water-splitting reaction for H2 (Hong et al., 2005). Such kind of solar thermochemical water splitting thermodynamically requires a reaction temperature over 2,500°C (Fletcher and Moen, 1977). A recent benchmark is the 100 kWth pilot by Steinfeld et al. (Villasmil et al., 2017). By multistep processes instead of direct process (Steinfeld, 2002, Gokon et al., 2013), the solar-to-hydrogen efficiency was elevated to 3% with high-grade input of 1,827°C heat at >3,000 suns. Jin et al. proposed solar-driven methanol decomposition for hydrogen at temperature of 200°C and solar-to-hydrogen efficiency achieved by 45% (Hong et al., 2005, Hong et al., 2012). In Figure 6, pure thermocatalysis provides a 300–1,400 mL gcat−1 h−1 H2 generation rate (Caravaca et al., 2016, Caudillo-Flores et al., 2019, Fang et al., 2019, Kuo et al., 2019, Papavasillou et al., 2004, Pu et al., 2019, Sun et al., 2019), requiring a reaction temperature of 200°C–300°C (for a parabolic solar concentrator Hong et al., 2012, see Table S2). With the same reactant and a similar H2 generation rate, the present work reduces the solar concentration to C = 15. The main reason is that solar thermocatalysis begins with the conversion of full-spectrum sunlight into reaction heat. Reaction heat is transferred as a phonon or the thermal motion of molecules, both of which have a disordered state of movement. To drive the ordered exchange of electrons in a reaction, the disordered phonon or the thermal motion requires high temperatures to overcome the high activation energy barrier. Furthermore, the high-concentration sunlight energy is like that of a compressed photon gas with high work availability, suffering energy degradation and arising the irreversibility in the solar thermocatalysis process. Therefore, it is important to consider lowering the solar concentration ratio. By contrast, there is a possibility for the proposed photo-thermal catalysis process to reduce the irreversibility. This is because the photochemical component provides a direct photon-H2 conversion with lower activation energy barrier, rather than only relying on the thermal activation of molecules (Wang et al., 2018a). In addition, the thermal component enables more activated reactant molecules to pass the activation energy barrier and promotes the H2 generation rate. In this case, a low solar concentration would be enough for the need of the photo-thermal catalysis process.
Figure 6.
Solar Concentration Ratio Required by Photo-Thermally Driven or Thermally Driven Reaction
Transmitted Sunlight for Photovoltaics
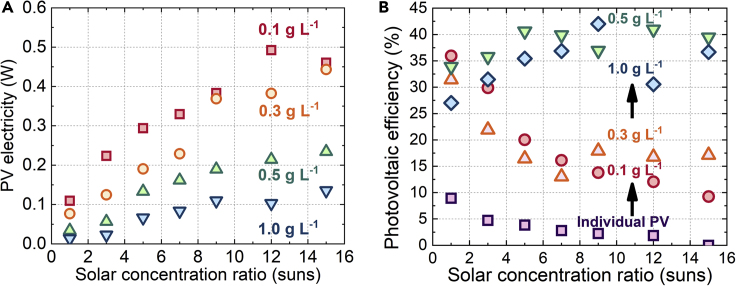
The measured transmitted sunlight is distributed in the 600- to 1,200-nm Vis-NIR narrow band, accounting for 5%–20% of the full-spectrum energy (Figures S3, S7A, and S7B). Therefore, we integrated the commercial 2.5-V P-type mono-crystalline silicon PV cell with the 1.2- to 1.3-eV bandgap, which is expected to accommodate the transmitted energy. Then, we assessed the power generation capability by a voltammetry measuring method (Kiermasch et al., 2019), which ran a voltage sweep on a high-sensitivity programmable electrical load (details in Figure S14). For each case, the voltage-current curve of the PV cell was monitored to find the maximum power output (see Figures S8A–S8F). As a comparison experiment, the individual PV was also studied using full-spectrum input. Figure 7A shows the electricity output of the integrated PV. Using the 600- to 1,200-nm narrow band energy, the PV produces 0.3–0.6 W electricity. Since the PV electricity increases with the transmitted Vis-NIR band, the PV maximum power output increases linearly with the solar concentration ranging from 1 to 15 and decreases with the catalyst concentration varying from 0.1 to 1.0 g L−1. The PV output at any condition can drive the continuous operation of a motor fan or 25 LEDs (Videos S2 and S3).
Figure 7.
Transmitted Sunlight for Photovoltaics
Measured PV (A) electricity output and (B) efficiency with or without integration with the photo-thermal reaction.
Figure 7B depicts the efficiency of the same PV cell with or without the integration with photo-thermal reactions. For the same solar concentration C, the PV efficiency continuously increases from <10% (individual PV) to 40% (1.0 g L−1) when integrated with the photo-thermal reaction at higher catalyst concentrations because of the decreasing PV heat relaxation loss in the UV/IR band, which is intercepted by the photo-thermal reaction solution for H2 generation. For the same catalyst concentration, the PV efficiency behaves differently with the increasing solar concentration. For the individual PV or integrated PV with <0.5 g L−1, the PV efficiency continuously decreases from 35% to <10% with increasing C. The reason is the aggregated heat recombination caused by an increasing sunlight intensity in UV and IR bands transmitted from the reaction solution. The highest PV efficiency of ∼40% was found when the PV cell was integrated with the catalyst concentration of 0.5–1.0 g L−1. Under this condition, the UV/IR band from the 15-sun concentrated sunlight is fully intercepted by the photo-thermal reaction. A sufficient amount and the exact band of Vis-NIR sunlight is transmitted, coinciding with the photovoltaic bandgap and improving the PV efficiency.
Efficiency of the Co-generation System
In this section, we assessed the performance of the synergic system of both H2 and electricity. Equation 1 was used to describe the overall efficiency of sunlight conversion into H2 and electricity.
| (Equation 1) |
In Equation 1, the denominator consists of two terms. One is the production of the Gibbs free energy in H2 (ΔGH2) multiplied by the molar amount of H2 (VH2/22.4), representing the chemical energy output from the UV-vis/IR band. Another is the PV power UPV IPV, representing the electricity output from the Vis-NIR band. The nominator is the full-spectrum spectral energy multiplied by the solar concentration ratio C, representing the input full-spectrum concentrated sunlight. The input Gibbs free energy of methanol consumed by the photo-thermal reaction is also considered as ΔGCH3OH⋅nCH3OH, calculated according to the consumption ratio provided in the isotope tracer experiment (Fang et al., 2019). The reason for using is to equalize the energy grade of the chemical energy in H2 to that of the electricity (Coridan et al., 2015).
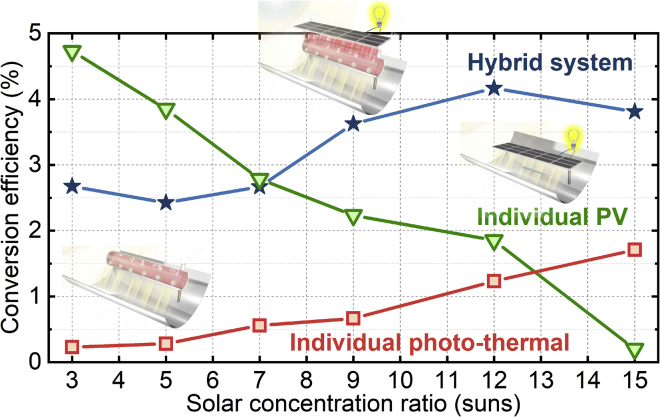
Using Equation 1, we can calculate the overall efficiency according to C, , and PPV from the experimental results (the calculation process is presented in Supplemental Information). As depicted in Figure 8, the overall system efficiency increases with the solar concentration ratio C, finally reaching 3.8%–4.2% at C = 12–15. The comparisons were also performed for the experimental results from the individual cases of PV and photo-thermal H2 generation (the experimental setup is presented in Supplemental Information). With the increase in C, the individual PV efficiency continuously decreases to 0.2% and the individual photo-thermal H2 efficiency slightly increases to 2%. In comparison, the hybrid system efficiency is 3 percentage points higher than both individual cases at a high solar concentration (C > 7).
Figure 8.
Efficiency of the Co-generation System
Overall efficiency of the hybrid system in comparison with the individual PV and individual photo-thermocatalysis.
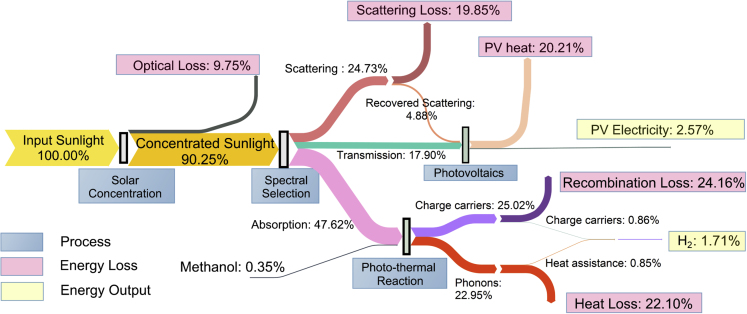
To further reveal the energy loss reduction mechanism at each spectral band, we performed an energy flow analysis for the hybrid system (the calculation process is presented in Supplemental Information). The results are shown as the Sankey diagram in Figure 9 (data listed in Table S3). For comparison, the individual cases were also assessed (Figures S9A and S9B and Table S3). From the flow direction of the solar energy, the system can be assessed in the upstream (solar concentration and spectral selection) and downstream processes (photo-thermocatalysis and PV generation).
Figure 9.
Energy Flow Analysis of the Hybrid System
In upstream processes, the input full-spectrum sunlight is first concentrated to intensify the photon flux and improve the capacity to do work, accounting for 9.75% of the system input for optical loss. Then, in the spectral selection process, the concentrated sunlight is selected into UV-vis/IR and Vis-NIR bands. Since part of the Vis-NIR band can be easily scattered in spectral selection, we used the reflection film to partly recover the scattered sunlight (accounting for 4.88% in system input) for PV conversion. In comparison, the individual photo-thermocatalysis (Figure S9A) did not utilize but dissipated the Vis-NIR band as transmission and scattering losses, comprising 38.63% of the system input.
After the spectral selection, the downstream processes included the photovoltaic and photo-thermocatalytic processes. For the photovoltaic process, the case of the integrated PV cell received the Vis-NIR band (22.78% in system input). The photon energy of 1.1–1.3 eV at the Vis-NIR band coincided with the bandgap of the P-type mono-crystalline silicon and had low recombination loss in the PV cell. Thus, the PV generated electricity was 2.57% in the system input, with 20.21% of waste heat. In the case of individual PV (Figure S9B), the mono-silicon PV cell received the full-spectrum concentrated sunlight (90.25%). A considerable amount of the UV-vis band (2–4 eV) and IR band (<1.1 eV) was dissipated through heat relaxation when received by the mono-Si bandgap of 1.1–1.3 eV. In addition, the UV and IR heat relaxation reduced the charge mobility and further aggregated the recombination loss in the Vis-NIR band. Therefore, the individual PV produced 88.75% waste heat and only 1.90% electricity output.
For photo-thermocatalysis, the UV-vis band (24.67%) and IR band (22.95%) were absorbed to generate charge carriers and phonons. Phonons from the IR band joined the UV-vis charge carriers for H2 production and collaborated for the 1.71% photo-thermal H2 production. In the first step, the concentrated UV-vis band generated charge carriers gathered on the catalyst surface. Then, the IR-induced localized heating activated more adsorbed molecules to react with the surface charge carriers to generate H2, suppressing the competing process of surface recombination (Panayotov and Morris, 2016). In individual photocatalysis, the IR band is expected to generate charge carriers through up-conversion, which combines two NIR or IR photons into one UV or Vis photon. Although the up-conversion is innovative and worth studying, the highest ratio ever achieved for up-conversion is 0.1%–4.0%; thus, it is not ready for potential industrial applications (Zhang et al., 2017). In contrast, photo-thermocatalysis uses IR heat as the assistive diving force and directly elevates the energy of the IR band to chemical energy stored in the H-H bond. Such elevation in the IR energy grade competes with the heat relaxation process, reducing the loss of the IR band to waste heat.
The results of the hybrid system reveal the potential of the full-spectrum cascade conversion for photothermal H2 and PV. One advantage of the hybrid system is its cost-effectiveness in scalable applications. Reducing the dependency on specific and sophisticated materials, this system adopts commercial P25 TiO2 loaded with 5 mol% Au and the commercial mono-silicon PV cell. Such simplicity in materials can help save the synthesis steps for an economical scale-up (Tang et al., 2019). The input solar concentration ratio of 15 suns in the experiments can be found on a parabolic or Fresnel solar collector. Such solar collectors at 40–50 suns have been commonly used for 30–40 years in solar thermal power applications, and the results of this work are encouraging in saving the area of the collector mirror. Although such solar concentrators have 60%–70% optical efficiency, this value may exceed 80% with double-axis tracking (Wang et al., 2018b). The hybrid system can also accommodate the innovations in materials technology, such as structure engineered catalyst (narrow-bandgap semiconductor or non-precious SPR metal loading) and third-generation PV cells (GaAs) (NREL, 2019), with an expected 5–10 percentage points of increase in the overall efficiency.
The sunlight intensity intrinsically varies throughout the year. In general, the production of solar fuel and solar electricity should consider the average annual utilization, which is an important indicator of the system availability to solar energy. For the proposed hybrid system, a satisfying annual utilization is expected because the system integrates the PV power output and H2 energy storage. In summer days, the system uses abundant solar irradiation to generate more H2 for long-term storage. When solar irradiation decreases in winter, the stored H2 can generate electricity in a fuel cell or a gas turbine, compensating the gap between the PV output and the electricity demand. Therefore, the proposed hybrid system provides a potential pathway for highly cost-effective sunlight utilization throughout the year.
In summary, the present work demonstrated a concentrated solar energy system co-producing H2 and electricity from a cascade pathway of solar spectrum. In the experimental design, a simple Au-TiO2 water-splitting reaction was accommodated as a spectral selector, which absorbed the UV-vis/IR band for photo-thermal H2 and transmitted the Vis-NIR band. By selecting the high-grade UV-vis band for charge carriers and collaborating with the IR heat, the H2 generation rate increased super-linearly at 3–15 suns to the highest rate of 1,260 mL g h−1. The transmitted Vis-NIR band coincided with the PV bandgap, reducing the heat relaxation and recombination thus retaining high efficiency under concentrated sunlight. An overall efficiency of 4.2% was obtained at 12 suns. The energy flow analysis demonstrated a reduced energy loss in full spectrum compared with the individual photo-thermocatalysis or the PV cell. Reduction in solar concentration and the simplicity of materials enables the control of the construction cost of industrial solar collectors. Furthermore, the adjustable H2/electricity output can resolve the fundamental concern on the intrinsic and seasonal nature of sunlight. Therefore, the hybrid system can be the basis for concurrent H2 and electricity harnessing by cascading the full-spectrum concentrated sunlight with integrated conversion processes and can be an alternative for individual PV, PEC, or photocatalysis systems in the future.
Limitations of the Study
An optimized design of nanostructure (like core-shell or 2D) and material (like TiOx) could potentially improve the spectral selectivity and reaction activity of the current catalyst. A simulation on the reactor scale can help further understand the influence of solar concentration ratio and mass concentration on system overall efficiency, facilitating the potential application of the proposed system.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work is supported by the Basic Science Center Program for Ordered Energy Conversion of the National Natural Science Foundation of China (No. 51888103), the National Natural Science Foundation of China (No. 51590904), and the Key Research Program of the Chinese Academy of Sciences (No. KFZD-SW-418).
Author Contributions
Conceptualization, S.T. and H.H.; Methodology, S.T. and J.S.; Investigation, S.T., X.X., and W.Y.; Resources, S.T., X.X., and W.Y.; Writing – Original Draft, S.T. and H.H.; Writing – Review & Editing, H.H., S.T., L.W., and Y.X.; Visualization – S.T.; Funding Acquisition, H.H. and H.J.; Supervision, H.H., H.J., and Y.X.
Declaration of Interests
The authors declare no competing interests.
Published: April 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101012.
Supplemental Information
References
- Bejan A. John Wiley & Sons, Inc.; 2016. Advanced Engineering Thermodynamics. [Google Scholar]
- Bell S., Will G., Bell J. Light intensity effects on photocatalytic water splitting with a titania catalyst. Int. J. Hydr. Energy. 2013;38:6938–6947. [Google Scholar]
- Cao Y., Zhou P., Tu Y., Liu Z., Dong B.-W., Azad A., Ma D., Wang D., Zhang X., Yang Y. Modification of TiO2 nanoparticles with organodiboron molecules inducing stable surface Ti3+ complex. iScience. 2019;20:195–204. doi: 10.1016/j.isci.2019.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caravaca A., Daly H., Smith M., Mills A., Chansai S., Hardacre C. Continuous flow gas phase photoreforming of methanol at elevated reaction temperatures sensitised by Pt/TiO2. React. Chem. Eng. 2016;1:649–657. [Google Scholar]
- Caudillo-Flores U., Agostini G., Marini C., Kubacka A., Fernandez-Garcia M. Hydrogen thermo-photo production using Ru/TiO2: heat and light synergistic effects. Appl. Catal. B Environ. 2019;256:117790. [Google Scholar]
- Chiarello G.L., Aguirre M.H., Selli E. Hydrogen production by photocatalytic steam reforming of methanol on noble metal-modified TiO2. J. Catal. 2010;273:182–190. [Google Scholar]
- Chiarello G.L., Dozzi M.V., Scavini M., Grunwaldt J.D., Selli E. One step flame-made fluorinated Pt/TiO2 photocatalysts for hydrogen production. Appl. Catal. B Environ. 2014;160:144–151. [Google Scholar]
- Coridan R.H., Nielander A.C., Francis S.A., Mcdowell M.T., Dix V., Chatman S.M., Lewis N.S. Methods for comparing the performance of energy-conversion systems for use in solar fuels and solar electricity generation. Energy Environ. Sci. 2015;8:2886–2901. [Google Scholar]
- Elbanna O., Kim S., Fujitsuka M., Majima T. TiO2 mesocrystals composited with gold nanorods for highly efficient visible-NIR-photocatalytic hydrogen production. Nano Energy. 2017;35:1–8. [Google Scholar]
- Fang S., Sun Z., Hu Y.H. Insights into the thermo-photo catalytic production of hydrogen from water on a low-cost NiOx-loaded TiO2 catalyst. ACS Catal. 2019;9:5047–5056. [Google Scholar]
- Fletcher E.A., Moen R.L. Hydrogen and oxygen from water. Science. 1977;197:1050–1056. doi: 10.1126/science.197.4308.1050. [DOI] [PubMed] [Google Scholar]
- Gao M., Connor P.K.N., Ho G.W. Plasmonic photothermic directed broadband sunlight harnessing for seawater catalysis and desalination. Energy Environ. Sci. 2016;9:3151–3160. [Google Scholar]
- Ghoussoub M., Xia M., Duchesne P.N., Segal D., Ozin G. Principles of photothermal gas-phase heterogeneous CO2 catalysis. Energy Environ. Sci. 2019;12:1122–1142. [Google Scholar]
- Gokon N., Sagawa S., Kodama T. Comparative study of activity of cerium oxide at thermal reduction temperatures of 1300–1550°C for solar thermochemical two-step water-splitting cycle. Int. J. Hydr. Energy. 2013;38:14402–14414. [Google Scholar]
- Highfield J.G., Chen M.H., Nguyen P.T., Chen Z. Mechanistic investigations of photo-driven processes over TiO2 by in-situ DRIFTS-MS: Part 1. Platinization and methanol reforming. Energy Environ. Sci. 2009;2:991–1002. [Google Scholar]
- Hong H., Jin H., Ji J., Wang Z., Cai R. Solar thermal power cycle with integration of methanol decomposition and middle-temperature solar thermal energy. Sol. Energy. 2005;78:49–58. [Google Scholar]
- Hong H., Liu Q., Jin H. Operational performance of the development of a 15 kW parabolic trough mid-temperature solar receiver/reactor for hydrogen production. Appl. Energy. 2012;90:137–141. [Google Scholar]
- Huaxu L., Fuqiang W., Ziming C., Shengpeng H., Bing X., Xiangtao G., Bo L., Jianyu T., Xiangzheng L., Ruiyang C. Analyzing the effects of reaction temperature on photo-thermo chemical synergetic catalytic water splitting under full-spectrum solar irradiation: an experimental and thermodynamic investigation. Int. J. Hydr. Energy. 2017;42:12133–12142. [Google Scholar]
- IEA . 2019. The Future of Hydrogen.www.iea.org/publications/reports/thefutureofhydrogen/ [Google Scholar]
- Jia J., O'brien P.G., He L., Qiao Q., Fei T., Reyes L.M., Burrow T.E., Dong Y.C., Liao K., Varela M. Visible and near-infrared photothermal catalyzed hydrogenation of gaseous CO2 over nanostructured Pd@Nb2O5. Adv. Sci. 2016;3:13. doi: 10.1002/advs.201600189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaselev O., Turner J.A. A monolithic photovoltaic-photoelectrochemical device for hydrogen production via water splitting. Science. 1998;280:425–427. doi: 10.1126/science.280.5362.425. [DOI] [PubMed] [Google Scholar]
- Kiermasch D., Gil-Escrig L., Bolink H.J., Tvingstedt K. Effects of masking on open-circuit voltage and fill factor in solar cells. Joule. 2019;3:16–26. [Google Scholar]
- Li X., Ni G., Cooper T., Xu N., Li J., Zhou L., Hu X., Zhu B., Yao P., Zhu J. Measuring conversion efficiency of solar vapor generation. Joule. 2019;3:1798–1803. [Google Scholar]
- Li Y., Wang C., Song M., Li D., Zhang X., Liu Y. TiO2-x/CoOx photocatalyst sparkles in photothermocatalytic reduction of CO2 with H2O steam. Appl. Catal. B Environ. 2019;243:760–770. [Google Scholar]
- Kuo M.-T., Chen Y.-Y., Hung W.-Y., Lin S.-F., Lin H.-P., Hsu C.-H., Shih H.-Y., Xie W.-A., Li S.-N. Synthesis of mesoporous CuFe/silicates catalyst for methanol steam reforming. Int. J. Hydr. Energy. 2019;44:14416–14423. [Google Scholar]
- Li Y.F., Lu W., Chen K., Duchesne P., Jelle A., Xia M.K., Wood T.E., Ulmer U., Ozin G.A. Cu atoms on nanowire Pd/HyWO3-x, bronzes enhance the solar reverse water gas shift reaction. J. Am. Chem. Soc. 2019;141:14991–14996. doi: 10.1021/jacs.9b08030. [DOI] [PubMed] [Google Scholar]
- Liang L., Li X., Sun Y., Tan Y., Jiao X., Ju H., Qi Z., Zhu J., Xie Y. Infrared light-driven CO2 overall splitting at room temperature. Joule. 2018;2:1004–1016. [Google Scholar]
- Liu G., Ma L., Yin L.-C., Wan G., Zhu H., Zhen C., Yang Y., Liang Y., Tan J., Cheng H.-M. Selective chemical epitaxial growth of TiO2 Islands on ferroelectric PbTiO3 crystals to boost photocatalytic activity. Joule. 2018;2:1095–1107. [Google Scholar]
- Liu X., Iocozzia J., Wang Y., Cui X., Chen Y., Zhao S., Li Z., Lin Z. Noble metal-metal oxide nanohybrids with tailored nanostructures for efficient solar energy conversion, photocatalysis and environmental remediation. Energy Environ. Sci. 2017;10:402–434. [Google Scholar]
- Liu X., Ye L., Ma Z., Han C., Wang L., Jia Z., Su F., Xie H. Photothermal effect of infrared light to enhance solar catalytic hydrogen generation. Catal. Commun. 2017;102:13–16. [Google Scholar]
- Neumann O., Urban A.S., Day J., Lal S., Nordlander P., Halas N.J. Solar vapor generation enabled by nanoparticles. ACS Nano. 2013;7:42–49. doi: 10.1021/nn304948h. [DOI] [PubMed] [Google Scholar]
- NREL . 2019. Best Research-Cell Efficiency Chart.http://www.nrel.gov/pv/assets/images/efficiency-chart.png [Google Scholar]
- Ozin G.A. “One-Pot” solar fuels. Joule. 2017;1:19–23. [Google Scholar]
- Panayotov D.A., Morris J.R. Surface chemistry of Au/TiO2: Thermally and photolytically activated reactions. Surf. Sci. Rep. 2016;71:77–271. [Google Scholar]
- Papavasillou J., Avgouropoulos G., Ioannides T. Production of hydrogen via combined steam reforming of methanol over CuO-CeO2 catalysts. Catal. Commun. 2004;5:231–235. [Google Scholar]
- Pihosh Y., Turkevych I., Mawatari K., Uemura J., Kazoe Y., Kosar S., Makita K., Sugaya T., Matsui T., Fujita D. Photocatalytic generation of hydrogen by core-shell WO(3)/BiVO(4) nanorods with ultimate water splitting efficiency. Sci. Rep. 2015;5:11141. doi: 10.1038/srep11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinaud B.A., Benck J.D., Seitz L.C., Forman A.J., Chen Z., Deutsch T.G., James B.D., Baum K.N., Baum G.N., Ardo S. Technical and economic feasibility of centralized facilities for solar hydrogen production via photocatalysis and photoelectrochemistry. Energy Environ. Sci. 2013;6:1983–2002. [Google Scholar]
- Pu Y.-C., Li S.-R., Yan S., Huang X., Wang D., Ye Y.-Y., Liu Y.-Q. An improved Cu/ZnO catalyst promoted by Sc2O3 for hydrogen production from methanol reforming. Fuel. 2019;241:607–615. [Google Scholar]
- Qi Y., Zhao Y., Gao Y., Li D., Li Z., Zhang F., Li C. Redox-Based visible-light-driven Z-scheme overall water splitting with apparent quantum efficiency exceeding 10% Joule. 2018;2:2393–2402. [Google Scholar]
- Sigle D.O., Zhang L., Ithurria S., Dubertret B., Baumberg J.J. Ultrathin CdSe in plasmonic nanogaps for enhanced photocatalytic water splitting. J. Phys. Chem. Lett. 2015;6:1099–1103. doi: 10.1021/acs.jpclett.5b00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinfeld A. Solar hydrogen production via a two-step water-splitting thermochemical cycle based on Zn/ZnO redox reactions. Int. J. Hydr. Energy. 2002;27:611–619. [Google Scholar]
- Sun Z.X., Fang S.Y., Lin Y., Hu Y.H. Photo-assisted methanol steam reforming on solid solution of Cu-Zn-Ti oxide. Chem. Eng. J. 2019;375:121909. [Google Scholar]
- Tang S., Hong H., Jin H., Xuan Y. A cascading solar hybrid system for co-producing electricity and solar syngas with nanofluid spectrum selector. Appl. Energy. 2019;248:231–240. [Google Scholar]
- Tang S., Hong H., Sun J., Qu W. Efficient path of distributed solar energy system synergetically combining photovoltaics with solar-syngas fuel cell. Energ. Convers. Manage. 2018;173:704–714. [Google Scholar]
- Tang S.L., Sun J., Hong H., Liu Q.B. Solar fuel from photo-thermal catalytic reactions with spectrum-selectivity: a review. Front. Energy. 2017;11:437–451. [Google Scholar]
- Tavasoli A., Ozin G. Green syngas by solar dry reforming. Joule. 2018;2:571–575. [Google Scholar]
- Villasmil W., Cooper T., Koepf E., Meier A., Steinfeld A. Coupled concentrating optics, heat transfer, and thermochemical modeling of a 100-kW th high-temperature solar reactor for the thermal dissociation of ZnO. J. Sol. Energy Eng. 2017;139:021015. [Google Scholar]
- Wang Q., Hisatomi T., Jia Q., Tokudome H., Zhong M., Wang C., Pan Z., Takata T., Nakabayashi M., Shibata N. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1% Nat. Mater. 2016;15:611–615. doi: 10.1038/nmat4589. [DOI] [PubMed] [Google Scholar]
- Wang L., Ghoussoub M., Wang H., Shao Y., Sun W., Tountas A.A., Wood T.E., Li H., Loh J.Y.Y., Dong Y. Photocatalytic hydrogenation of carbon dioxide with high selectivity to methanol at atmospheric pressure. Joule. 2018;2:1369–1381. [Google Scholar]
- Wang R., Qu W., Hong H., Sun J., Jin H. Experimental performance of 300 kWth prototype of parabolic trough collector with rotatable axis and irreversibility analysis. Energy. 2018;161:595–609. [Google Scholar]
- Wei Q.Y., Yang Y., Liu H.J., Hou J.Y., Liu M.C., Cao F., Zhao L. Experimental study on direct solar photocatalytic water splitting for hydrogen production using surface uniform concentrators. Int. J. Hydr. Energy. 2018;43:13745–13753. [Google Scholar]
- Weinstein L.A., Mcenaney K., Strobach E., Yang S., Bhatia B., Zhao L., Huang Y., Loomis J., Cao F., Boriskina S.V. A hybrid electric and thermal solar receiver. Joule. 2018;2:962–975. [Google Scholar]
- Xu N., Zhu P., Sheng Y., Zhou L., Li X., Tan H., Zhu S., Zhu J. Synergistic tandem solar electricity-water generators. Joule. 2020;4:347–358. [Google Scholar]
- Xue C., An H., Yan X., Li J., Yang B., Wei J., Yang G. Spatial charge separation and transfer in ultrathin CdIn2S4/rGO nanosheet arrays decorated by ZnS quantum dots for efficient visible-light-driven hydrogen evolution. Nano Energy. 2017;39:513–523. [Google Scholar]
- Yang Z., Jiang Y., Zhang W., Ding Y., Jiang Y., Yin J., Zhang P., Luo H. Solid-state, low-cost, and green synthesis and robust photochemical hydrogen evolution performance of ternary TiO2/MgTiO3/C photocatalysts. iScience. 2019;14:15–26. doi: 10.1016/j.isci.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Yang S., Li J., Gao W., Deng Y., Dong W., Zhao C., Lu G. Visible-to-ultraviolet Upconvertion: energy transfer, material matrix, and synthesis strategies. Appl. Catal. B Environ. 2017;206:89–103. [Google Scholar]
- Zhao X., Luo W., Feng J., Li M., Li Z., Yu T., Zou Z. Quantitative analysis and visualized evidence for high charge separation efficiency in a solid-liquid bulk heterojunction. Adv. Energy Mater. 2014;4:1301785. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The solution is not stirred to clarify the movement of bubbles