Abstract
Cannabis products in which cannabidiol (CBD) is the primary chemical constituent (CBD-dominant) are increasingly popular and widely available. The impact of CBD exposure on urine drug testing has not been well studied. This study characterized the urinary pharmacokinetic profile of 100-mg oral and vaporized CBD, vaporized CBD-dominant cannabis (100-mg CBD; 3.7-mg ∆9-THC) and placebo in healthy adults (n = 6) using a within-subjects crossover design. Urine specimens were collected before and for 5 days after drug administration. Immunoassay (IA) screening (cutoffs of 20, 50 and 100 ng/mL) and LC–MS-MS confirmatory tests (cutoff of 15 ng/mL) for 11-nor-9-carboxy-∆9-tetrahydrocannabinol (∆9-THCCOOH) were performed; urine was also analyzed for CBD and other cannabinoids. Urinary concentrations of CBD were higher after oral (mean Cmax: 776 ng/mL) versus vaporized CBD (mean Cmax: 261 ng/mL). CBD concentrations peaked 5 h after oral CBD ingestion and within 1 h after inhalation of vaporized CBD. After pure CBD administration, only 1 out of 218 urine specimens screened positive for ∆9-THCCOOH (20-ng/mL IA cutoff) and no specimens exceeded the 15-ng/mL confirmatory cutoff. After inhalation of CBD-dominant cannabis vapor, nine samples screened positive at the 20-ng/mL IA cutoff, and two of those samples screened positive at the 50-ng/mL IA cutoff. Four samples that screened positive (two at 20 ng/mL and two at 50 ng/mL) confirmed positive with concentrations of ∆9-THCCOOH exceeding 15 ng/mL. These data indicate that acute dosing of pure CBD will not result in a positive urine drug test using current federal workplace drug testing guidelines (50-ng/mL IA cutoff with 15-ng/mL confirmatory cutoff). However, CBD products that also contain ∆9-THC may produce positive urine results for ∆9-THCCOOH. Accurate labeling and regulation of ∆9-THC content in CBD/hemp products are needed to prevent unexpected positive drug tests and unintended drug effects.
Introduction
Recent national and international policy reforms have made cannabis legal in an unprecedented number of jurisdictions. Medicinal cannabis use is permitted in 34 US states, the District of Columbia and various international locations (e.g., Australia, much of the European Union), while non-medicinal, or “recreational,” cannabis use is permitted in 11 US states, Canada and Uruguay. Moreover, the legalization of hemp (defined in the USA as cannabis plants with ≤0.3% ∆-9-tetrahydrocannabinol (1), ∆9-THC, the primary psychoactive constituent of cannabis) is also expanding. For instance, the Agriculture Improvement Act of 2018 (aka the “Farm Bill”), recently removed hemp and its derivative products from the list of controlled substances in the USA (https://www.congress.gov/115/bills/hr2/BILLS-115hr2enr.pdf). These and other policy reforms have led to the development of a litany of products that contain cannabis, or individual cannabinoids, which are widely available for retail purchase.
Cannabidiol (CBD) is a key chemical constituent of many cannabis and hemp products (2). CBD has garnered widespread attention for its purported therapeutic benefits, and a cannabis-derived CBD product (Epidiolex) has been approved by the US Food and Drug Administration (FDA) for treatment of pediatric seizure disorders. In addition, many individuals use non-FDA-approved CBD-dominant cannabis or hemp products as therapeutics for various health conditions, as well as for general wellness (3–8). Products that contain high concentrations of CBD are widely available in cannabis dispensaries, and, in the case of hemp-derived CBD products, a variety of other retail locations (including in jurisdictions where cannabis remains illegal) (2). Beyond CBD-dominant cannabis plant material, which can be inhaled using conventional methods (e.g., joints, bowls and vaporizers), there are many products that contain concentrated hemp or cannabis-derived CBD extracts, which are intended for oral (e.g., tinctures), pulmonary (e.g., vaporizers or vape pens), topical and other methods of administration (2, 8, 9).
Due to the proliferation of CBD-dominant products, there is an urgent need to understand the impact CBD use has on urine drug-testing programs commonly used in workplace, criminal justice, drug treatment and other settings. Urine remains the primary biological matrix for drug testing and the most commonly targeted analyte to evaluate cannabis exposure is 11-nor-9-carboxy-∆9-tetrahydrocannabinol (∆9-THCCOOH), a metabolite of ∆9-THC (10, 11). Though CBD is not an analyte of interest in extant drug-testing procedures, there are several ways that the use of CBD products could theoretically produce a positive result for cannabis on a urine drug test. First, as a result of the unregulated nature of the cannabis industry, products advertised as containing only CBD often contain ∆9-THC in concentrations that range from trace levels to levels capable of producing intoxication/impairment (9, 12). Further, hemp-derived CBD products can legally contain up to 0.3% ∆9-THC (1). Even the FDA-approved CBD medication Epidiolex may contain trace levels (<0.1%) of ∆9-THC (13). Thus, individuals who use CBD products may inadvertently expose themselves to ∆9-THC and potentially increase their risk of testing positive for cannabis. Second, some evidence suggests that acidic gastric fluid can convert CBD to THC, though whether this conversion happens in the human gut remains a hotly debated topic (14–16). When CBD is introduced, in vitro, to acidic conditions analogous to the human gut, it can be converted to ∆8 and ∆9-THC (17, 18). The limited supportive in vivo evidence for the metabolic conversion of CBD to ∆9-THC includes a case study in which ∆8 and ∆9-THC were detected in the urine of woman taking daily oral doses of CBD (600 mg) (19) and another study that detected ∆9-THC in serum and brain tissue of rodents given oral and subcutaneous (but not vaporized) doses of pure CBD (10 or 60 mg/kg) (20). Detractors of these findings note that the in vitro conditions under which CBD was converted to ∆8 and ∆9-THC were too artificial (i.e., not reflective of the human gut), and that clinical studies that have administered extremely high oral doses of CBD have generally not observed THC-like subjective effects or impairment in study participants, which presumably would occur if CBD converted to ∆9-THC or its active metabolite 11-hydroxy-∆-9THC (11-OH-∆9-THC) (15, 16).
The present study was conducted to evaluate, under controlled conditions, the urine drug-testing outcomes of acute administration of CBD via both oral ingestion and vaporization, which represent two common routes of CBD product administration. In addition, we evaluated vaporized whole-plant cannabis that had a CBD-dominant chemotype, but that also contained a low concentration of ∆9-THC. This human laboratory study investigated the likelihood that an acute dose of CBD (orally ingested or and inhaled with a vaporizer), can, by itself, impact urine drug testing for cannabis. It also allowed for a comparative evaluation to an acute inhaled dose of a high CBD/low ∆9-THC concentration botanical cannabis product.
Method
Participants
Study volunteers were recruited using media advertisements and word-of-mouth. Individuals who appeared eligible after a brief telephone interview were invited for a screening visit at the Johns Hopkins Behavioral Pharmacology Research Unit (BPRU). At this screening visit, participants provided written informed consent, and completed procedures to ascertain study eligibility.
In order to be eligible, participants were required to: (i) be in good health (as determined using medical history, a 12-lead electrocardiogram or EKG, blood chemistry, hematology, and serology analysis and a physical examination); (ii) self-report no cannabis use for at least one month prior to screening; (iii) have prior experience inhaling cannabis; (iv) test negative for recent use of cannabis and other illicit drugs (via urinalysis) and alcohol (via breathalyzer) at screening and at the beginning of each study visit; (v) be between the ages of 18 and 45 and have a body mass index (BMI) between 19 and 36 kg/m2 and (vi) for females, test negative for pregnancy (tested via serum at screening and via urine before each session). Additional factors that were exclusionary included: current use of prescription/OTC medications or other drug products (e.g., herbal supplements), which would interfere with the participants’ safety (namely those metabolized via CYP2D6, CYP2C9 and CYP2B10 enzymes, or which induce/inhibit CYP3A4 enzymes; (21, 22); and use of dronabinol in the past 6 months or hemp seeds/hemp oil in the past 3 months.
A total of six participants provided informed consent and completed all study procedures (three men; three women). Table I details demographic and select substance use characteristics for each individual. Participants were predominantly Caucasian, all non-tobacco users, and on average, had not used cannabis for 127 days (range 32–365) at study entry. Mean BMI’s were 25.9 kg/m2 for men and 29.2 kg/m2 for women.
Table I.
Participant Characteristics
| ID# | Gender | Age | Race | Ethnicity (hispanic: Y/N) | Height (ft’ in) |
Weight (lbs) |
BMI (kg/m2) | Last cannabis use (days) | Cigarette smoker (Y/N) |
Session order |
|---|---|---|---|---|---|---|---|---|---|---|
| 038 | M | 27 | White | N | 5’73/4 | 182 | 27.9 | 365 | N | c,b,d,a |
| 053 | F | 31 | White | Y | 5’11/2 | 194 | 36.1 | 32 | N | c,b,d,a |
| 054 | F | 29 | Black | N | 5’101/2 | 204 | 28.9 | 150 | N | a,c,b,d |
| 063 | F | 38 | White | N | 5’31/4 | 128 | 22.5 | 36 | N | b,d,a,c |
| 066 | M | 23 | White | N | 5’6 | 159 | 25.7 | 60 | N | d,a,c,b |
| 068 | M | 37 | White | N | 6’41/2 | 202 | 24.3 | 120 | N | d,a,c,b |
Note: a = placebo; b = 100-mg oral CBD; c = 100-mg vaporized CBD; d = vaporized cannabis (100-mg CBD: 3.7-mg ∆9-THC).
The Institutional Review Board of Johns Hopkins University School of Medicine approved this study, which was conducted in accordance with ethical standards established in the Helsinki Declaration. Participants were compensated for their time following each study visit.
Study design and procedure
All participants completed four experimental conditions, each spanning five consecutive days. For each condition, participants were housed in a closed residential research unit on Days 1–3 (58 h total) and completed brief outpatient visits on Days 4 and 5. This study used a double-dummy dosing procedure to control for expectancy effects, meaning participants received both an oral and vaporized study dose in each study condition. The four study conditions were: (i) oral ingestion of placebo CBD followed by inhalation of 100-mg vaporized CBD; (ii) oral ingestion of 100-mg CBD followed by inhalation of vaporized placebo cannabis; (iii) oral ingestion of placebo CBD followed by inhalation of CBD-dominant cannabis (100-mg CBD; 3.7-mg ∆9-THC) and (iv) oral ingestion of placebo CBD followed by inhalation of placebo cannabis (placebo condition). Experimental sessions were completed in a randomized order and dose administration across sessions was separated by at least 1 week to facilitate drug washout between visits. Participants and research staff were both blinded to the study doses in each session.
Study drug
Two separate batches of cannabis (CBD-dominant and placebo) were obtained for this study from the National Institute on Drug Abuse (NIDA) Drug Supply Program. The CBD-dominant batch of cannabis contained (based on dry weight percent): 10.5% CBD, 0.39% ∆-9-THC, 0.02% ∆-8-THC and 0.05% Cannabinol (CBN). The placebo cannabis batch contained 0.001% ∆-9-THC, 0.003% CBD, 0.005% CBN and had no detectable ∆-8-THC. The same quantity of plant material (953 mg) was used for both active (total CBD dose = 100 mg) and placebo cannabis vapor administration sessions. Cannabis was vaporized using the Volcano Medic® (Storz and Bickel, Tuttelingen, Germany) desktop vaporizer with the temperature set at 204°C (400°F).
Pure CBD in crystalline powder form (purity by HPLC = 100%) was obtained from Albany Molecular Research Inc. for this study. Independent testing confirmed ∆9-THC was not present in this product. For oral dosing, the Johns Hopkins BPRU Pharmacy placed 100-mg CBD into a size 00 gelcap and filled the remaining space with microcrystalline cellulose. Placebo capsules were identical gelcaps, but filled only with cellulose. For vaporization, the Volcano Medic® was used to heat and aerosolize the CBD powder (placed on a stainless-steel dosing pad). All study drugs were prepared and dispensed by the Johns Hopkins BPRU Pharmacy.
With respect to dose selection, a 100-mg CBD dose was selected for two primary reasons. First, 1 mL (single unit dose) of the FDA-approved CBD medication Epidiolex contains 100-mg CBD. Second, 100 mg is approximately the amount of CBD a person would inhale from a 1g cannabis cigarette containing 10% CBD, which is a typical amount of cannabis consumed by frequent cannabis users; 10% CBD potency is also common for CBD-dominant cannabis flowers sold in cannabis dispensaries. We maintained the 100-mg CBD dose for the botanical cannabis product to enable comparison with the pure CBD dose conditions. The inclusion of 3.7-mg ∆9-THC equates to a 25:1 CBD:THC ratio, which is a common ratio for CBD-dominant cannabis products currently in the retail market. Moreover, the ∆9-THC concentration of 0.39% in the botanical product is a close proxy to what is currently defined as hemp in the USA.
Experimental session procedures
On Day 1 of each experimental dosing session, participants arrived at ~07:30 h. Upon arrival, participants completed a urine drug test, a urine pregnancy test (females only) and an alcohol breathalyzer; they were required to test negative on all tests to participate in each session. Participants also self-reported their use of cannabis, alcohol and tobacco since the last laboratory visit using the Timeline Follow-Back questionnaire (23) and were asked about concomitant medication. Baseline pharmacodynamic measures (i.e., cognitive performance, subjective effects and vital signs) and baseline biological specimens (i.e., urine, blood, oral fluid and hair) were also collected at this time. After completing baseline assessments and consuming a standard low-fat breakfast (toast and jam), participants swallowed an oral gelcap containing either placebo or 100-mg CBD, and exactly 1 h later, inhaled the vaporized study dose (either pure CBD, placebo or CBD-dominant cannabis) using the Volcano Medic®. Each vaporized study dose was heated at 204°C (400°F) and captured in a balloon; participants inhaled three full balloons ad libitum within 10 min to ensure complete dose delivery. New balloons were used for each session to avoid contamination from previous study doses. Each balloon was covered with an opaque bag to minimize aerosol visibility to participants and study staff. For ~8 h after drug administration, participants stayed at the BPRU, where they provided all urine voids and completed pharmacodynamic assessments. Participants were then taken to a nearby closed residential research unit, where they resided and were monitored for the next 2 days and continued to provide all urine voids (they were discharged 58 h after oral dosing). On the second day after discharge from the residential unit (Days 4 and 5 relative to dose administration), participants returned to the BPRU for brief outpatient visits (one on each day), where they provided single urine specimens. These outpatient visits were completed at the participant’s convenience, and thus the times for biological specimen collection on Days 4 and 5 varied across participants/conditions.
On Day 1 of each experimental dosing session, urine samples were collected at baseline and 1, 2, 3 and 4 h after oral drug administration. Following the 4 h collection time point, urine voids were pooled between 4–6, 6–8, 8–10, 10–12, 12–22, 22–26, 26–30, 30–34, 34–46, 46–50, 50–54 and 54–58 h post-oral drug administration. The exact timing of urine collection varied occasionally across participants/sessions (by ~±5 min) due to a variety of reasons (e.g., participants were unable to void immediately). Thus, these time points should be viewed as nominal values. At the end of each of these pooled time periods, participants were asked to void. Single urine specimens were also collected on Days 4 and 5 after dosing for each drug condition (specific post-dosing times for these specimens are provided in Table II). Following collection of each specimen, urine was aliquoted into two 30 mL polypropylene bottles. All specimens were then wrapped with parafilm and stored at –20°C until they were shipped overnight (on dry ice) to the Clinical Reference Laboratory (CRL; Lenexa, KS) for testing.
Table II.
Analyses of Urine Specimens Following Administration of Oral and Vaporized CBD and CBD-dominant Cannabis
| Subject# | Time (h) | Dose (mg) | Volume (mL) | Creatinine (mg/dL) |
20 IA ∆9-THC-COOH (ng/mL) |
50 IA ∆9-THC-COOH (ng/mL) |
100 IA ∆9-THC-COOH (ng/mL) |
CBD (ng/mL) | ∆9-THC-COOH (ng/mL) | ∆8-THC-COOH (ng/mL) | THC-VA (ng/mL) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 038_M | BL | 100 CBD_O | 100 | 88.4 | –1 | 0 | 2 | 1.3 | 0.0 | 0.0 | 0.0 |
| 038 | 1.5 | 100 CBD_O | 175 | 26.2 | 3 | 4 | 4 | 3.3 | 0.0 | 0.0 | 0.0 |
| 038 | 2 | 100 CBD_O | 75 | 78.2 | 1 | 0 | 2 | 13.7 | 0.0 | 0.0 | 0.0 |
| 038 | 3 | 100 CBD_O | 175 | 38.0 | 2 | 2 | 4 | 12.4 | 0.0 | 0.0 | 0.0 |
| 038 | 4 | 100 CBD_O | 150 | 40.0 | 9 | 9 | 10 | 682.5 | 0.0 | 0.0 | 0.0 |
| 038 | 4–6 | 100 CBD_O | 75 | 34.5 | 14 | 14 | 10 | 924.8 | 0.0 | 0.0 | 0.0 |
| 038 | 6–8 | 100 CBD_O | 175 | 28.5 | 9 | 8 | 8 | 812.3 | 0.0 | 0.0 | 0.0 |
| 038 | 8–10 | 100 CBD_O | 100 | 62.1 | 31 | 26 | 20 | 2,941.0 | 0.0 | 0.0 | 0.0 |
| 038 | 10–12 | 100 CBD_O | 400 | 5.8 | 5 | 4 | 5 | 122.0 | 0.0 | 0.0 | 0.0 |
| 038 | 12–22 | 100 CBD_O | 1,670 | 49.0 | 13 | 11 | 12 | 89.8 | 0.0 | 0.0 | 0.0 |
| 038 | 22–26 | 100 CBD_O | 800 | 40.9 | 11 | 10 | 11 | 60.4 | 0.0 | 0.0 | 0.0 |
| 038 | 26–30 | 100 CBD_O | 1,500 | 19.3 | 9 | 9 | 8 | 20.2 | 0.0 | 0.0 | 0.0 |
| 038 | 30–34 | 100 CBD_O | 1,300 | 27.1 | 10 | 8 | 8 | 22.6 | 0.0 | 0.0 | 0.0 |
| 038 | 34–46 | 100 CBD_O | 1,950 | 48.2 | 9 | 6 | 7 | 23.0 | 0.0 | 0.0 | 0.0 |
| 038 | 46–50 | 100 CBD_O | 1,100 | 38.7 | 6 | 7 | 5 | 14.7 | 0.0 | 0.0 | 0.0 |
| 038 | 50–54 | 100 CBD_O | 1,000 | 25.1 | 6 | 6 | 6 | 15.2 | 0.0 | 0.0 | 0.0 |
| 038 | 54–58 | 100 CBD_O | 1,300 | 32.1 | 3 | 4 | 4 | 6.7 | 0.0 | 0.0 | 0.0 |
| 038 | 77 | 100 CBD_O | 75 | 154.9 | 2 | 2 | 3 | 16.6 | 0.0 | 0.0 | 0.0 |
| 038 | 98 | 100 CBD_O | 125 | 120.8 | 2 | 2 | 3 | 14.3 | 0.0 | 0.0 | 0.0 |
| 038 | BL | 100 CBD_V | 125 | 81.0 | –3 | –5 | –1 | 0 | 0.0 | 0.0 | 0.0 |
| 038 | 1.5 | 100 CBD_V | 200 | 29.5 | 0 | –1 | 1 | 631.1 | 0.0 | 0.0 | 0.0 |
| 038 | 2 | 100 CBD_V | 75 | 31.8 | 2 | –2 | 2 | 416.4 | 0.0 | 0.0 | 0.0 |
| 038 | 3 | 100 CBD_V | 125 | 54.6 | –3 | –4 | 1 | 366.9 | 0.0 | 0.0 | 0.0 |
| 038 | 4 | 100 CBD_V | 50 | 132.9 | 0 | –1 | 0 | 546.6 | 1.2 | 0.0 | 0.0 |
| 038 | 4–6 | 100 CBD_V | 150 | 51.4 | –2 | –2 | 1 | 159.1 | 0.0 | 0.0 | 0.0 |
| 038 | 6–8 | 100 CBD_V | 175 | 31.1 | –2 | –4 | 2 | 47.9 | 0.0 | 0.0 | 0.0 |
| 038 | 8–10 | 100 CBD_V | 400 | 47.0 | –2 | –3 | 2 | 38.6 | 0.0 | 0.0 | 0.0 |
| 038 | 10–12 | 100 CBD_V | 1,000 | 14.2 | –2 | –4 | 1 | 5.2 | 0.0 | 0.0 | 0.0 |
| 038 | 12–22 | 100 CBD_V | 855 | 99.8 | –6 | –4 | –1 | 14.2 | 0.0 | 0.0 | 0.0 |
| 038 | 22–26 | 100 CBD_V | 600 | 25.7 | –2 | –4 | 0 | 1.9 | 0.0 | 0.0 | 0.0 |
| 038 | 26–30 | 100 CBD_V | 1,600 | 48.2 | –4 | –4 | 0 | 9.3 | 0.0 | 0.0 | 0.0 |
| 038 | 30–34 | 100 CBD_V | 1,150 | 26.8 | –3 | –4 | –2 | 2.4 | 0.0 | 0.0 | 0.0 |
| 038 | 34–46 | 100 CBD_V | 1,650 | 61.3 | –4 | –3 | 0 | 2.2 | 0.0 | 0.0 | 0.0 |
| 038 | 46–50 | 100 CBD_V | 1,950 | 21.7 | –3 | –3 | 0 | 1.1 | 0.0 | 0.0 | 0.0 |
| 038 | 50–54 | 100 CBD_V | 1,250 | 22.4 | –5 | –3 | 1 | 0.7 | 0.0 | 0.0 | 0.0 |
| 038 | 54–58 | 100 CBD_V | 1,475 | 92.4 | –8 | –8 | –4 | 0.0 | 0.0 | 0.0 | 0.0 |
| 038 | 77 | 100 CBD_V | 50 | 105.0 | –2 | –4 | 0 | 2.2 | 0.0 | 0.0 | 0.0 |
| 038 | 96 | 100 CBD_V | 100 | 143.4 | –4 | –3 | –2 | 4.2 | 0.0 | 0.0 | 0.0 |
| 038 | BL | 100 CBD/4 THC | 100 | 89.5 | –6 | –8 | –4 | 2.9 | 0.0 | 0.0 | 0.0 |
| 038 | 1.5 | 100 CBD/4 THC | 151 | 18.1 | 2 | 0 | 3 | 506.0 | 0.0 | 0.0 | 0.0 |
| 038 | 2 | 100 CBD/4 THC | 149 | 12.6 | 6 | 4 | 4 | 233.5 | 1.3 | 0.0 | 0.0 |
| 038 | 3 | 100 CBD/4 THC | 110 | 55.9 | 50 | 36 | 24 | 539.1 | 11.2 | 0.4 | 1.2 |
| 038 | 4 | 100 CBD/4 THC | 62 | 64.6 | 59 | 48 | 30 | 424.4 | 19.1 | 0.8 | 1.8 |
| 038 | 4–6 | 100 CBD/4 THC | 125 | 134.2 | 73 | 59 | 37 | 363.6 | 29.9 | 1.2 | 2.9 |
| 038 | 6–8 | 100 CBD/4 THC | ms | ms | ms | ms | ms | ms | ms | ms | ms |
| 038 | 8–10 | 100 CBD/4 THC | 600 | 38.3 | 16 | 13 | 10 | 49.6 | 10.4 | 0.3 | 0.0 |
| 038 | 10–12 | 100 CBD/4 THC | 300 | 52.2 | 7 | 6 | 5 | 26.4 | 5.4 | 0.0 | 0.0 |
| 038 | 12–22 | 100 CBD/4 THC | 1,525 | 51.4 | 6 | 4 | 3 | 15.8 | 5.4 | 0.0 | 0.0 |
| 038 | 22–26 | 100 CBD/4 THC | 500 | 52.4 | 7 | 6 | 5 | 22.9 | 5.7 | 0.0 | 0.0 |
| 038 | 26–30 | 100 CBD/4 THC | 1,300 | 7.3 | –2 | –1 | 1 | 3.9 | 1.6 | 0.0 | 0.0 |
| 038 | 30–34 | 100 CBD/4 THC | 700 | 34.7 | 0 | –1 | 1 | 9.0 | 3.1 | 0.0 | 0.0 |
| 038 | 34–46 | 100 CBD/4 THC | 1,700 | 57.8 | 0 | 1 | 1 | 6.2 | 2.6 | 0.0 | 0.0 |
| 038 | 46–50 | 100 CBD/4 THC | 300 | 73.5 | –1 | 0 | 0 | 10.5 | 2.5 | 0.0 | 0.0 |
| 038 | 50–54 | 100 CBD/4 THC | 500 | 40.1 | –1 | –3 | 0 | 5.8 | 1.3 | 0.0 | 0.0 |
| 038 | 54–58 | 100 CBD/4 THC | 900 | 48.6 | –3 | –3 | –1 | 3.1 | 1.1 | 0.0 | 0.0 |
| 038 | 77 | 100 CBD/4 THC | 25 | 236.1 | –5 | –6 | –4 | 8.8 | 2.3 | 0.0 | 0.0 |
| 038 | 95 | 100 CBD/4 THC | 50 | 214.0 | –7 | –5 | –4 | 16.1 | 2.6 | 0.0 | 0.0 |
| 053_F | BL | 100 CBD_O | 20 | 79.4 | 1 | 0 | 0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 053 | 1.5 | 100 CBD_O | 30 | 94.8 | 1 | 1 | 2 | 138.4 | 0.0 | 0.0 | 0.0 |
| 053 | 2 | 100 CBD_O | 20 | 90.3 | 1 | 2 | 3 | 210.2 | 0.0 | 0.0 | 0.0 |
| 053 | 3 | 100 CBD_O | ms | ms | ms | ms | ms | ms | ms | ms | ms |
| 053 | 4 | 100 CBD_O | 100 | 83.7 | 2 | 1 | 4 | 121.1 | 0.0 | 0.0 | 0.0 |
| 053 | 4–6 | 100 CBD_O | 75 | 37.7 | 3 | 0 | 1 | 26.0 | 0.0 | 0.0 | 0.0 |
| 053 | 6–8 | 100 CBD_O | 80 | 83.6 | –2 | –2 | –1 | 22.7 | 0.0 | 0.0 | 0.0 |
| 053 | 8–10 | 100 CBD_O | 75 | 26.5 | 2 | 1 | 1 | 3.3 | 0.0 | 0.0 | 0.0 |
| 053 | 10–12 | 100 CBD_O | 495 | 23.6 | 2 | 0 | 3 | 2.7 | 0.0 | 0.0 | 0.0 |
| 053 | 12–22 | 100 CBD_O | 2,500 | 16.0 | 4 | 2 | 3 | 0.6 | 0.0 | 0.0 | 0.0 |
| 053 | 22–26 | 100 CBD_O | 300 | 12.6 | 2 | 3 | 4 | 0.4 | 0.0 | 0.0 | 0.0 |
| 053 | 26–30 | 100 CBD_O | ms | ms | ms | ms | ms | ms | ms | ms | ms |
| 053 | 30–34 | 100 CBD_O | 550 | 23.8 | 1 | 1 | 3 | 0.2 | 0.0 | 0.0 | 0.0 |
| 053 | 34–46 | 100 CBD_O | 1,960 | 31.5 | 2 | 3 | 4 | 0.1 | 0.0 | 0.0 | 0.0 |
| 053 | 46–50 | 100 CBD_O | 500 | 74.7 | 0 | –1 | 1 | 0.3 | 0.0 | 0.0 | 0.0 |
| 053 | 50–54 | 100 CBD_O | 1,050 | 23.1 | 0 | 2 | 3 | 0.0 | 0.0 | 0.0 | 0.0 |
| 053 | 54–58 | 100 CBD_O | 300 | 60.6 | 0 | 1 | 1 | 0.0 | 0.0 | 0.0 | 0.0 |
| 053 | 76 | 100 CBD_O | 125 | 41.6 | 0 | 1 | 0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 053 | 96 | 100 CBD_O | 75 | 164.2 | –4 | –2 | –2 | 0.0 | 0.0 | 0.0 | 0.0 |
| 053 | BL | 100 CBD_V | 40 | 153.0 | –9 | –9 | –4 | 0.0 | 0.0 | 0.0 | 0.0 |
| 053 | 1.5 | 100 CBD_V | ms | ms | ms | ms | ms | ms | ms | ms | ms |
| 053 | 2 | 100 CBD_V | 75 | 35.6 | –4 | –4 | 0 | 15.3 | 0.0 | 0.0 | 0.0 |
| 053 | 3 | 100 CBD_V | 75 | 15.0 | –3 | –4 | 1 | 3.1 | 0.0 | 0.0 | 0.0 |
| 053 | 4 | 100 CBD_V | 100 | 72.1 | –6 | –6 | 0 | 6.5 | 0.0 | 0.0 | 0.0 |
| 053 | 4–6 | 100 CBD_V | 150 | 53.9 | –7 | –7 | –3 | 6.0 | 0.0 | 0.0 | 0.0 |
| 053 | 6–8 | 100 CBD_V | 75 | 23.5 | –4 | –5 | –2 | 1.4 | 0.0 | 0.0 | 0.0 |
| 053 | 8–10 | 100 CBD_V | 50 | 113.0 | –10 | –10 | –3 | 3.1 | 0.0 | 0.0 | 0.0 |
| 053 | 10–12 | 100 CBD_V | 1,100 | 11.3 | –3 | –4 | 2 | 0.0 | 0.0 | 0.0 | 0.0 |
| 053 | 12–22 | 100 CBD_V | 1,100 | 47.3 | –3 | –4 | 0 | 0.3 | 0.0 | 0.0 | 0.0 |
| 053 | 22–26 | 100 CBD_V | 550 | 51.8 | –6 | –4 | 0 | 0.3 | 0.0 | 0.0 | 0.0 |
| 053 | 26–30 | 100 CBD_V | 2,000 | 12.4 | –4 | –4 | 1 | 0.0 | 0.0 | 0.0 | 0.0 |
| 053 | 30–34 | 100 CBD_V | 1,080 | 23.2 | –4 | –5 | 0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 053 | 34–46 | 100 CBD_V | 1,500 | 42.9 | –5 | –5 | 0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 053 | 46–50 | 100 CBD_V | 600 | 52.2 | –7 | –6 | –1 | 0.0 | 0.0 | 0.0 | 0.0 |
| 053 | 50–54 | 100 CBD_V | 550 | 37.2 | –7 | –6 | –2 | 0.0 | 0.0 | 0.0 | 0.0 |
| 053 | 54–58 | 100 CBD_V | 1,100 | 22.2 | –6 | –3 | 1 | 0.0 | 0.0 | 0.0 | 0.0 |
| 053 | 71 | 100 CBD_V | 175 | 64.9 | –9 | –8 | –1 | 0.0 | 0.0 | 0.0 | 0.0 |
| 053 | 102 | 100 CBD_V | 50 | 178.2 | –11 | –10 | –6 | 0.0 | 0.0 | 0.0 | 0.0 |
| 053 | BL | 100 CBD/4 THC | 30 | 113.9 | –9 | –7 | –6 | 0.0 | 0.0 | 0.0 | 0.0 |
| 053 | 1.5 | 100 CBD/4 THC | ms | ms | ms | ms | ms | ms | ms | ms | ms |
| 053 | 2 | 100 CBD/4 THC | 55 | 31.1 | 0 | –1 | 2 | 126.1 | 0.0 | 0.0 | 0.0 |
| 053 | 3 | 100 CBD/4 THC | 100 | 23.0 | 0 | –1 | 2 | 38.7 | 0.0 | 0.0 | 0.0 |
| 053 | 4 | 100 CBD/4 THC | ms | ms | ms | ms | ms | ms | ms | ms | ms |
| 053 | 4–6 | 100 CBD/4 THC | 125 | ms | ms | ms | ms | ms | ms | ms | ms |
| 053 | 6–8 | 100 CBD/4 THC | 175 | 22.6 | –4 | –3 | 0 | 10.0 | 0.0 | 0.0 | 0.0 |
| 053 | 8–10 | 100 CBD/4 THC | 180 | 36.8 | –5 | –4 | –1 | 10.1 | 0.0 | 0.0 | 0.0 |
| 053 | 10–12 | 100 CBD/4 THC | 450 | 18.4 | –2 | –4 | 1 | 2.3 | 0.0 | 0.0 | 0.0 |
| 053 | 12–22 | 100 CBD/4 THC | 1,500 | 20.4 | –3 | –2 | –1 | 0.8 | 0.0 | 0.0 | 0.0 |
| 053 | 22–26 | 100 CBD/4 THC | 180 | 125.0 | –5 | –4 | –2 | 3.6 | 1.2 | 0.0 | 0.0 |
| 053 | 26–30 | 100 CBD/4 THC | 950 | 12.1 | –4 | –2 | –1 | 0.3 | 0.0 | 0.0 | 0.0 |
| 053 | 30–34 | 100 CBD/4 THC | 800 | 15.9 | –4 | –3 | –2 | 0.2 | 0.0 | 0.0 | 0.0 |
| 053 | 34–46 | 100 CBD/4 THC | 1,500 | 18.9 | –2 | –3 | –1 | 0.0 | 0.0 | 0.0 | 0.0 |
| 053 | 46–50 | 100 CBD/4 THC | 450 | 33.9 | –6 | –4 | –2 | 0.4 | 0.0 | 0.0 | 0.0 |
| 053 | 50–54 | 100 CBD/4 THC | 380 | 36.1 | –6 | –5 | –2 | 0.3 | 0.0 | 0.0 | 0.0 |
| 053 | 54–58 | 100 CBD/4 THC | 1,000 | 26.4 | –5 | –4 | –2 | 0.2 | 0.0 | 0.0 | 0.0 |
| 053 | Day 4 | 100 CBD/4 THC | ms | ms | ms | ms | ms | ms | ms | 0.0 | 0.0 |
| 053 | 98 | 100 CBD/4 THC | 15 | 314.3 | –21 | –18 | –13 | 1.1 | 0.0 | 0.0 | 0.0 |
| 054_F | BL | 100 CBD_O | 125 | 122.4 | –1 | 2 | 3 | 0.3 | 2.3 | 0.0 | 0.0 |
| 054 | 1.5 | 100 CBD_O | 175 | 107.7 | 1 | 1 | 1 | 40.1 | 2.9 | 0.0 | 0.0 |
| 054 | 2 | 100 CBD_O | 100 | 48.9 | 3 | 4 | 4 | 127.2 | 1.2 | 0.0 | 0.0 |
| 054 | 3 | 100 CBD_O | 175 | 21.5 | 4 | 3 | 4 | 61.7 | 0.0 | 0.0 | 0.0 |
| 054 | 4 | 100 CBD_O | 100 | 71.4 | 5 | 5 | 5 | 496.1 | 1.5 | 0.0 | 0.0 |
| 054 | 4–6 | 100 CBD_O | 200 | 124.9 | 6 | 6 | 5 | 383.5 | 2.2 | 0.0 | 0.0 |
| 054 | 6–8 | 100 CBD_O | 275 | 90.2 | 7 | 7 | 6 | 123.2 | 2.0 | 0.0 | 0.0 |
| 054 | 8–10 | 100 CBD_O | 30 | 125.3 | 8 | 6 | 7 | 75.2 | 2.0 | 0.0 | 0.0 |
| 054 | 10–12 | 100 CBD_O | 490 | 38.3 | 4 | 5 | 5 | 11.7 | 0.0 | 0.0 | 0.0 |
| 054 | 12–22 | 100 CBD_O | 800 | 87.7 | 6 | 6 | 5 | 12.4 | 1.8 | 0.0 | 0.0 |
| 054 | 22–26 | 100 CBD_O | 1,120 | 41.3 | 4 | 4 | 4 | 6.1 | 1.0 | 0.0 | 0.0 |
| 054 | 26–30 | 100 CBD_O | 760 | 49.3 | 2 | 2 | 1 | 6.3 | 1.0 | 0.0 | 0.0 |
| 054 | 30–34 | 100 CBD_O | 430 | 68.1 | 3 | 3 | 2 | 8.8 | 1.3 | 0.0 | 0.0 |
| 054 | 34–46 | 100 CBD_O | 1,300 | 68.0 | 4 | 2 | 5 | 4.0 | 1.0 | 0.0 | 0.0 |
| 054 | 46–50 | 100 CBD_O | 750 | 43.7 | 3 | 4 | 2 | 2.5 | 0.0 | 0.0 | 0.0 |
| 054 | 50–54 | 100 CBD_O | 500 | 57.3 | 0 | –1 | 2 | 3.7 | 0.0 | 0.0 | 0.0 |
| 054 | 54–58 | 100 CBD_O | 350 | 91.1 | 1 | –1 | 2 | 3.7 | 0.0 | 0.0 | 0.0 |
| 054 | 79 | 100 CBD_O | 70 | 184.1 | –5 | –5 | –3 | 4.3 | 1.8 | 0.0 | 0.0 |
| 054 | 103 | 100 CBD_O | 100 | 144.8 | –2 | –3 | 0 | 1.8 | 1.3 | 0.0 | 0.0 |
| 054 | BL | 100 CBD_V | 125 | 88.2 | –10 | –11 | –3 | 0.0 | 0.0 | 0.0 | 0.0 |
| 054 | 1.5 | 100 CBD_V | 250 | 24.7 | 0 | –1 | 0 | 60.1 | 0.0 | 0.0 | 0.0 |
| 054 | 2 | 100 CBD_V | 175 | 29.0 | –5 | –6 | –1 | 79.3 | 0.0 | 0.0 | 0.0 |
| 054 | 3 | 100 CBD_V | 100 | 45.5 | –6 | –8 | –2 | 63.9 | 0.0 | 0.0 | 0.0 |
| 054 | 4 | 100 CBD_V | 130 | ms | ms | ms | ms | ms | ms | ms | ms |
| 054 | 4–6 | 100 CBD_V | 645 | 24.7 | –5 | –5 | –1 | 13.1 | 0.0 | 0.0 | 0.0 |
| 054 | 6–8 | 100 CBD_V | 300 | 52.1 | –7 | –8 | 1 | 7.1 | 0.0 | 0.0 | 0.0 |
| 054 | 8–10 | 100 CBD_V | 325 | 63.2 | –6 | –6 | –2 | 4.6 | 0.0 | 0.0 | 0.0 |
| 054 | 10–12 | 100 CBD_V | 350 | 47.6 | –6 | –6 | –1 | 2.3 | 0.0 | 0.0 | 0.0 |
| 054 | 12–22 | 100 CBD_V | 1,200 | 73.9 | –7 | –7 | –1 | 2.0 | 0.0 | 0.0 | 0.0 |
| 054 | 22–26 | 100 CBD_V | 1,250 | 40.9 | –5 | –7 | 1 | 1.0 | 0.0 | 0.0 | 0.0 |
| 054 | 26–30 | 100 CBD_V | 240 | 80.5 | –8 | –9 | –1 | 0.7 | 0.0 | 0.0 | 0.0 |
| 054 | 30–34 | 100 CBD_V | 1,300 | 32.8 | –5 | –6 | 0 | 0.3 | 0.0 | 0.0 | 0.0 |
| 054 | 34–46 | 100 CBD_V | 910 | 99.3 | –9 | –10 | –3 | 0.9 | 0.0 | 0.0 | 0.0 |
| 054 | 46–50 | 100 CBD_V | 400 | 115.7 | –9 | –8 | –2 | 0.9 | 0.0 | 0.0 | 0.0 |
| 054 | 50–54 | 100 CBD_V | 1,100 | ms | ms | ms | ms | ms | ms | ms | ms |
| 054 | 54–58 | 100 CBD_V | 250 | 175.3 | –11 | –11 | –3 | 1.2 | 0.0 | 0.0 | 0.0 |
| 054 | 76 | 100 CBD_V | 100 | 103.9 | –12 | –10 | –4 | 0.5 | 0.0 | 0.0 | 0.0 |
| 054 | 103 | 100 CBD_V | 150 | 165.0 | –15 | –14 | –6 | 0.7 | 0.0 | 0.0 | 0.0 |
| 054 | BL | 100 CBD/4 THC | 175 | 79.0 | –9 | –7 | –4 | 0.2 | 0.0 | 0.0 | 0.0 |
| 054 | 1.5 | 100 CBD/4 THC | 200 | 21.4 | –3 | –4 | –1 | 157.9 | 0.0 | 0.0 | 0.0 |
| 054 | 2 | 100 CBD/4 THC | 200 | 26.2 | –1 | 0 | 1 | 160.1 | 0.0 | 0.0 | 0.0 |
| 054 | 3 | 100 CBD/4 THC | 75 | 116.7 | 22 | 18 | 13 | 232.8 | 4.5 | 0.2 | 1.7 |
| 054 | 4 | 100 CBD/4 THC | 25 | 128.7 | 26 | 19 | 15 | 155.3 | 5.5 | 0.3 | 2.0 |
| 054 | 4–6 | 100 CBD/4 THC | 200 | 70.5 | 10 | 8 | 7 | 56.9 | 4.7 | 0.3 | 1.1 |
| 054 | 6–8 | 100 CBD/4 THC | 200 | 73.1 | 7 | 6 | 4 | 28.6 | 4.3 | 0.3 | 0.0 |
| 054 | 8–10 | 100 CBD/4 THC | 250 | 158.9 | 10 | 8 | 5 | 44.6 | 7.3 | 0.5 | 1.6 |
| 054 | 10–12 | 100 CBD/4 THC | 125 | 158.8 | 1 | 1 | 1 | 23.9 | 6.8 | 0.5 | 1.0 |
| 054 | 12–22 | 100 CBD/4 THC | 700 | 122.6 | 1 | 2 | 0 | 11.3 | 4.1 | 0.3 | 0.0 |
| 054 | 22–26 | 100 CBD/4 THC | ms | 61.3 | –2 | 0 | 1 | 4.1 | 2.0 | 0.0 | 0.0 |
| 054 | 26–30 | 100 CBD/4 THC | ms | 101.0 | –1 | 1 | –3 | 7.2 | 4.5 | 0.2 | 0.0 |
| 054 | 30–34 | 100 CBD/4 THC | 200 | 136.0 | –4 | –2 | –1 | 5.8 | 3.7 | 0.2 | 0.0 |
| 054 | 34–46 | 100 CBD/4 THC | 850 | 121.2 | –4 | –2 | –2 | 3.4 | 2.5 | 0.0 | 0.0 |
| 054 | 46–50 | 100 CBD/4 THC | 450 | 97.3 | –5 | –4 | –2 | 2.7 | 1.8 | 0.0 | 0.0 |
| 054 | 50–54 | 100 CBD/4 THC | 800 | 36.0 | –4 | –4 | –1 | 1.2 | 0.0 | 0.0 | 0.0 |
| 054 | 54–58 | 100 CBD/4 THC | ms | 67.8 | –5 | –6 | –4 | 1.6 | 1.3 | 0.0 | 0.0 |
| 054 | 71 | 100 CBD/4 THC | 150 | 137.2 | –6 | –5 | –2 | 2.4 | 2.0 | 0.0 | 0.0 |
| 054 | 95 | 100 CBD/4 THC | 100 | 245.3 | –11 | –9 | –6 | 3.1 | 2.2 | 0.0 | 0.0 |
| 063_F | BL | 100 CBD_O | 75 | 59.8 | –1 | 0 | 2 | 0.0 | 0.0 | 0.0 | 0.0 |
| 063 | 1.5 | 100 CBD_O | 80 | 25.9 | 1 | 2 | 2 | 87.4 | 0.0 | 0.0 | 0.0 |
| 063 | 2 | 100 CBD_O | 77 | 21.7 | 2 | 3 | 3 | 7.8 | 0.0 | 0.0 | 0.0 |
| 063 | 3 | 100 CBD_O | 150 | 23.3 | 3 | 2 | 4 | 214.6 | 0.0 | 0.0 | 0.0 |
| 063 | 4 | 100 CBD_O | 60 | 20.2 | 2 | 3 | 4 | 117.3 | 0.0 | 0.0 | 0.0 |
| 063 | 4–6 | 100 CBD_O | 235 | 24.6 | 1 | 3 | 3 | 93.9 | 0.0 | 0.0 | 0.0 |
| 063 | 6–8 | 100 CBD_O | ms | ms | ms | ms | ms | ms | ms | ms | ms |
| 063 | 8–10 | 100 CBD_O | 620 | 22.7 | 1 | 2 | 2 | 41.9 | 0.0 | 0.0 | 0.0 |
| 063 | 10–12 | 100 CBD_O | 500 | 15.0 | 4 | 4 | 4 | 6.2 | 0.0 | 0.0 | 0.0 |
| 063 | 12–22 | 100 CBD_O | 700 | 60.5 | 3 | 2 | 4 | 17.0 | 0.0 | 0.0 | 0.0 |
| 063 | 22–26 | 100 CBD_O | 250 | 44.0 | 2 | 1 | 4 | 18.2 | 0.0 | 0.0 | 0.0 |
| 063 | 26–30 | 100 CBD_O | 1,100 | 23.4 | 3 | 2 | 3 | 8.1 | 0.0 | 0.0 | 0.0 |
| 063 | 30–34 | 100 CBD_O | 1,200 | 14.8 | 4 | 1 | 3 | 6.3 | 0.0 | 0.0 | 0.0 |
| 063 | 34–46 | 100 CBD_O | 1,700 | 33.7 | 1 | 2 | 2 | 10.2 | 0.0 | 0.0 | 0.0 |
| 063 | 46–50 | 100 CBD_O | 600 | 25.5 | 1 | 2 | 4 | 6.9 | 0.0 | 0.0 | 0.0 |
| 063 | 50–54 | 100 CBD_O | 1,250 | 14.3 | 3 | 1 | 4 | 3.3 | 0.0 | 0.0 | 0.0 |
| 063 | 54–58 | 100 CBD_O | 1,200 | 22.3 | 2 | 3 | 4 | 3.6 | 0.0 | 0.0 | 0.0 |
| 063 | 74 | 100 CBD_O | 125 | 63.9 | –1 | 0 | 1 | 6.4 | 0.0 | 0.0 | 0.0 |
| 063 | 102 | 100 CBD_O | 150 | 13.1 | 3 | 2 | 2 | 0.0 | 0.0 | 0.0 | 0.0 |
| 063 | BL | 100 CBD_V | 30 | 51.0 | –7 | –7 | –1 | 0.2 | 0.0 | 0.0 | 0.0 |
| 063 | 1.5 | 100 CBD_V | 175 | 11.9 | –4 | –4 | 0 | 179.6 | 0.0 | 0.0 | 0.0 |
| 063 | 2 | 100 CBD_V | 75 | 28.1 | –5 | –5 | 1 | 248.7 | 0.0 | 0.0 | 0.0 |
| 063 | 3 | 100 CBD_V | 125 | 42.7 | –5 | –6 | 0 | 189.7 | 0.0 | 0.0 | 0.0 |
| 063 | 4 | 100 CBD_V | 175 | 11.1 | –6 | –4 | 1 | 39.9 | 0.0 | 0.0 | 0.0 |
| 063 | 4–6 | 100 CBD_V | 400 | 15.2 | –4 | –4 | –1 | 18.5 | 0.0 | 0.0 | 0.0 |
| 063 | 6–8 | 100 CBD_V | 200 | 17.4 | –5 | –4 | 1 | 8.7 | 0.0 | 0.0 | 0.0 |
| 063 | 8–10 | 100 CBD_V | 600 | 22.5 | –6 | –6 | –3 | 5.6 | 0.0 | 0.0 | 0.0 |
| 063 | 10–12 | 100 CBD_V | 500 | 13.5 | –4 | –5 | 0 | 3.0 | 0.0 | 0.0 | 0.0 |
| 063 | 12–22 | 100 CBD_V | 1,000 | 43.4 | –6 | –4 | –1 | 5.9 | 0.0 | 0.0 | 0.0 |
| 063 | 22–26 | 100 CBD_V | 500 | 59.7 | –8 | –11 | –2 | 8.5 | 0.0 | 0.0 | 0.0 |
| 063 | 26–30 | 100 CBD_V | 1,350 | 21.6 | –4 | –3 | 0 | 2.1 | 0.0 | 0.0 | 0.0 |
| 063 | 30–34 | 100 CBD_V | 750 | 29.3 | –4 | –7 | 0 | 1.9 | 0.0 | 0.0 | 0.0 |
| 063 | 34–46 | 100 CBD_V | 500 | 36.1 | –6 | –7 | 0 | 1.8 | 0.0 | 0.0 | 0.0 |
| 063 | 46–50 | 100 CBD_V | 400 | 65.4 | –7 | –7 | –2 | 3.6 | 0.0 | 0.0 | 0.0 |
| 063 | 50–54 | 100 CBD_V | 1,300 | 16.3 | –5 | –5 | 0 | 0.7 | 0.0 | 0.0 | 0.0 |
| 063 | 54–58 | 100 CBD_V | ms | 27.7 | –6 | –5 | –1 | 1.0 | 0.0 | 0.0 | 0.0 |
| 063 | 78 | 100 CBD_V | 200 | ms | ms | ms | ms | ms | ms | ms | ms |
| 063 | 98 | 100 CBD_V | 125 | 27.3 | –5 | –5 | –3 | 0.4 | 0.0 | 0.0 | 0.0 |
| 063 | BL | 100 CBD/4 THC | 50 | 149.5 | –10 | –8 | –6 | 0.0 | 0.0 | 0.0 | 0.0 |
| 063 | 1.5 | 100 CBD/4 THC | 175 | 45.8 | –2 | –1 | 0 | 195.9 | 0.0 | 0.0 | 0.0 |
| 063 | 2 | 100 CBD/4 THC | 75 | 27.8 | 6 | 6 | 5 | 181.2 | 1.0 | 0.0 | 0.0 |
| 063 | 3 | 100 CBD/4 THC | 150 | 11.9 | 0 | –1 | 0 | 39.2 | 0.0 | 0.0 | 0.0 |
| 063 | 4 | 100 CBD/4 THC | 225 | 14.4 | 0 | 2 | 1 | 28.3 | 1.3 | 0.0 | 0.0 |
| 063 | 4–6 | 100 CBD/4 THC | 200 | 13.2 | –2 | –1 | 0 | 13.0 | 1.1 | 0.0 | 0.0 |
| 063 | 6–8 | 100 CBD/4 THC | 175 | 17.4 | –1 | –2 | –1 | 6.7 | 1.0 | 0.0 | 0.0 |
| 063 | 8–10 | 100 CBD/4 THC | 300 | 28.9 | –1 | –1 | 0 | 4.5 | 1.0 | 0.0 | 0.0 |
| 063 | 10–12 | 100 CBD/4 THC | 200 | 26.8 | –2 | –3 | 0 | 2.4 | 0.0 | 0.0 | 0.0 |
| 063 | 12–22 | 100 CBD/4 THC | 1,100 | 36.0 | –2 | –2 | –1 | 3.4 | 1.0 | 0.0 | 0.0 |
| 063 | 22–26 | 100 CBD/4 THC | 400 | 66.4 | –3 | –3 | 0 | 9.0 | 1.6 | 0.0 | 0.0 |
| 063 | 26–30 | 100 CBD/4 THC | 1,300 | 18.0 | –3 | –4 | –2 | 2.7 | 0.0 | 0.0 | 0.0 |
| 063 | 30–34 | 100 CBD/4 THC | 450 | 43.1 | –2 | –3 | –1 | 5.3 | 0.0 | 0.0 | 0.0 |
| 063 | 34–46 | 100 CBD/4 THC | 1,800 | 26.9 | –3 | –2 | –2 | 2.4 | 0.0 | 0.0 | 0.0 |
| 063 | 46–50 | 100 CBD/4 THC | 650 | 21.0 | –4 | –4 | –1 | 1.3 | 0.0 | 0.0 | 0.0 |
| 063 | 50–54 | 100 CBD/4 THC | 1,100 | 24.8 | –4 | –4 | –2 | 0.9 | 0.0 | 0.0 | 0.0 |
| 063 | 54–58 | 100 CBD/4 THC | 400 | 45.0 | –4 | –4 | –2 | 1.0 | 0.0 | 0.0 | 0.0 |
| 063 | 74 | 100 CBD/4 THC | 150 | 25.6 | –4 | –4 | –3 | 0.5 | 0.0 | 0.0 | 0.0 |
| 063 | 96 | 100 CBD/4 THC | 60 | 81.8 | –5 | –6 | –4 | 6.1 | 0.0 | 0.0 | 0.0 |
| 066_M | BL | 100 CBD_O | 45 | 134.6 | –2 | –3 | –1 | 0.0 | 0.0 | 0.0 | 0.0 |
| 066 | 1.5 | 100 CBD_O | 50 | 173.9 | –4 | –4 | –1 | 13.9 | 0.0 | 0.0 | 0.0 |
| 066 | 2 | 100 CBD_O | ms | ms | ms | ms | ms | ms | ms | ms | ms |
| 066 | 3 | 100 CBD_O | 110 | 94.7 | –2 | –2 | 0 | 110.7 | 0.0 | 0.0 | 0.0 |
| 066 | 4 | 100 CBD_O | 225 | 28.6 | 3 | 1 | 3 | 156.5 | 0.0 | 0.0 | 0.0 |
| 066 | 4–6 | 100 CBD_O | 150 | 106.2 | –1 | 0 | 2 | 526.0 | 0.0 | 0.0 | 0.0 |
| 066 | 6–8 | 100 CBD_O | 175 | 77.5 | 0 | 0 | 3 | 260.6 | 0.0 | 0.0 | 0.0 |
| 066 | 8–10 | 100 CBD_O | 300 | 35.0 | 3 | 3 | 2 | 33.7 | 0.0 | 0.0 | 0.0 |
| 066 | 10–12 | 100 CBD_O | 300 | 75.9 | 0 | 0 | 1 | 54.2 | 0.0 | 0.0 | 0.0 |
| 066 | 12–22 | 100 CBD_O | 275 | 175.7 | 6 | 7 | 5 | 65.3 | 0.0 | 0.0 | 0.0 |
| 066 | 22–26 | 100 CBD_O | 150 | 133.6 | 8 | 7 | 5 | 57.4 | 0.0 | 0.0 | 0.0 |
| 066 | 26–30 | 100 CBD_O | 250 | 144.8 | 1 | 2 | 4 | 16.8 | 0.0 | 0.0 | 0.0 |
| 066 | 30–34 | 100 CBD_O | 300 | 116.6 | 1 | 1 | 0 | 5.4 | 0.0 | 0.0 | 0.0 |
| 066 | 34–46 | 100 CBD_O | 1,500 | 73.8 | 3 | 4 | 2 | 1.8 | 0.0 | 0.0 | 0.0 |
| 066 | 46–50 | 100 CBD_O | 200 | 203.2 | 0 | 1 | 1 | 5.1 | 0.0 | 0.0 | 0.0 |
| 066 | 50–54 | 100 CBD_O | 250 | 99.2 | –1 | –1 | 0 | 1.4 | 0.0 | 0.0 | 0.0 |
| 066 | 54–58 | 100 CBD_O | 600 | 59.0 | 0 | 0 | 0 | 0.6 | 0.0 | 0.0 | 0.0 |
| 066 | 79 | 100 CBD_O | 125 | 309.6 | –7 | –4 | –4 | 1.7 | 0.0 | 0.0 | 0.0 |
| 066 | 96 | 100 CBD_O | 100 | 343.0 | –5 | –5 | –3 | 1.2 | 0.0 | 0.0 | 0.0 |
| 066 | BL | 100 CBD_V | 75 | 353.9 | –17 | –15 | –7 | 0.0 | 0.0 | 0.0 | 0.0 |
| 066 | 1.5 | 100 CBD_V | 75 | 323.7 | –13 | –10 | –6 | 394.2 | 0.0 | 0.0 | 0.0 |
| 066 | 2 | 100 CBD_V | ms | ms | ms | ms | ms | ms | ms | ms | ms |
| 066 | 3 | 100 CBD_V | 125 | 134.2 | –8 | –8 | –4 | 201.3 | 0.0 | 0.0 | 0.0 |
| 066 | 4 | 100 CBD_V | 125 | 97.0 | –8 | –7 | –4 | 47.6 | 0.0 | 0.0 | 0.0 |
| 066 | 4–6 | 100 CBD_V | 220 | 77.5 | –6 | –8 | –2 | 27.2 | 0.0 | 0.0 | 0.0 |
| 066 | 6–8 | 100 CBD_V | 100 | 129.6 | –10 | –13 | –6 | 17.5 | 0.0 | 0.0 | 0.0 |
| 066 | 8–10 | 100 CBD_V | 100 | 71.5 | –8 | –8 | –2 | 3.4 | 0.0 | 0.0 | 0.0 |
| 066 | 10–12 | 100 CBD_V | 220 | 61.8 | –7 | –8 | –3 | 2.5 | 0.0 | 0.0 | 0.0 |
| 066 | 12–22 | 100 CBD_V | 275 | 174.3 | –8 | –8 | –4 | 2.0 | 0.0 | 0.0 | 0.0 |
| 066 | 22–26 | 100 CBD_V | 230 | 134.5 | –9 | –12 | –5 | 3.5 | 0.0 | 0.0 | 0.0 |
| 066 | 26–30 | 100 CBD_V | 540 | 53.7 | –6 | –7 | –2 | 0.7 | 0.0 | 0.0 | 0.0 |
| 066 | 30–34 | 100 CBD_V | 400 | 51.9 | –7 | –6 | –3 | 0.5 | 0.0 | 0.0 | 0.0 |
| 066 | 34–46 | 100 CBD_V | 450 | 175.2 | –9 | –9 | –2 | 0.6 | 0.0 | 0.0 | 0.0 |
| 066 | 46–50 | 100 CBD_V | 155 | ms | ms | ms | ms | ms | ms | ms | ms |
| 066 | 50–54 | 100 CBD_V | 300 | 86.8 | –9 | –8 | –3 | 0.4 | 0.0 | 0.0 | 0.0 |
| 066 | 54–58 | 100 CBD_V | 420 | 96.0 | –3 | –3 | –2 | 0.4 | 0.0 | 0.0 | 0.0 |
| 066 | 73 | 100 CBD_V | 50 | 284.4 | –12 | –14 | –5 | 0.5 | 0.0 | 0.0 | 0.0 |
| 066 | 102 | 100 CBD_V | 125 | 318.8 | –14 | –12 | –7 | 0.0 | 0.0 | 0.0 | 0.0 |
| 066 | BL | 100 CBD/4 THC | 60 | 292.8 | –13 | –10 | –8 | 0.0 | 1.0 | 0.0 | 0.0 |
| 066 | 1.5 | 100 CBD/4 THC | 75 | 249.3 | –7 | –6 | –5 | 216.0 | 1.2 | 0.0 | 0.0 |
| 066 | 2 | 100 CBD/4 THC | 50 | 227.2 | 4 | 4 | 2 | 351.0 | 3.0 | 0.0 | 1.3 |
| 066 | 3 | 100 CBD/4 THC | 25 | 223.2 | 4 | 3 | 2 | 198.3 | 3.9 | 0.0 | 1.2 |
| 066 | 4 | 100 CBD/4 THC | 50 | 217.0 | –2 | –2 | –2 | 111.4 | 4.7 | 0.0 | 1.0 |
| 066 | 4–6 | 100 CBD/4 THC | 125 | 180.9 | –5 | –5 | –4 | 36.2 | 3.3 | 0.0 | 0.0 |
| 066 | 6–8 | 100 CBD/4 THC | 100 | 192.0 | –3 | –4 | –4 | 38.5 | 4.0 | 0.0 | 0.0 |
| 066 | 8–10 | 100 CBD/4 THC | 80 | 216.7 | –4 | –4 | –4 | 18.9 | 2.6 | 0.0 | 0.0 |
| 066 | 10–12 | 100 CBD/4 THC | ms | ms | ms | ms | ms | ms | ms | ms | ms |
| 066 | 12–22 | 100 CBD/4 THC | 520 | 172.1 | –5 | –5 | –2 | 6.7 | 2.2 | 0.0 | 0.0 |
| 066 | 22–26 | 100 CBD/4 THC | 450 | 72.6 | –5 | –3 | –3 | 2.6 | 0.0 | 0.0 | 0.0 |
| 066 | 26–30 | 100 CBD/4 THC | 600 | 57.9 | –6 | –5 | –3 | 1.2 | 0.0 | 0.0 | 0.0 |
| 066 | 30–34 | 100 CBD/4 THC | 680 | 59.0 | –4 | –6 | –4 | 0.6 | 0.0 | 0.0 | 0.0 |
| 066 | 34–46 | 100 CBD/4 THC | 750 | 138.4 | –8 | –7 | –5 | 0.9 | 0.0 | 0.0 | 0.0 |
| 066 | 46–50 | 100 CBD/4 THC | 200 | ms | ms | ms | ms | ms | ms | ms | ms |
| 066 | 50–54 | 100 CBD/4 THC | 390 | 63.3 | –6 | –5 | –3 | 0.6 | 0.0 | 0.0 | 0.0 |
| 066 | 54–58 | 100 CBD/4 THC | ms | 101.4 | –7 | –6 | –5 | 0.6 | 0.0 | 0.0 | 0.0 |
| 066 | 72 | 100 CBD/4 THC | 425 | 280.8 | –10 | –7 | –6 | 0.6 | 0.0 | 0.0 | 0.0 |
| 066 | 96 | 100 CBD/4 THC | 350 | 202.6 | –11 | –9 | –7 | 0.6 | 0.0 | 0.0 | 0.0 |
| 068_M | BL | 100 CBD_O | 125 | 138.3 | –2 | –2 | –1 | 1.3 | 1.3 | 0.0 | 0.0 |
| 068 | 1.5 | 100 CBD_O | 425 | 54.5 | 1 | 1 | 1 | 6.4 | 0.0 | 0.0 | 0.0 |
| 068 | 2 | 100 CBD_O | 60 | 50.9 | 1 | 1 | 1 | 32.1 | 0.0 | 0.0 | 0.0 |
| 068 | 3 | 100 CBD_O | 275 | 28.2 | 2 | 1 | 2 | 28.4 | 0.0 | 0.0 | 0.0 |
| 068 | 4 | 100 CBD_O | 125 | 67.6 | 3 | 2 | 1 | 200.5 | 0.0 | 0.0 | 0.0 |
| 068 | 4–6 | 100 CBD_O | 225 | 91.8 | 1 | 0 | –1 | 135.8 | 1.2 | 0.0 | 0.0 |
| 068 | 6–8 | 100 CBD_O | 250 | 61.2 | 2 | 2 | 1 | 236.3 | 0.0 | 0.0 | 0.0 |
| 068 | 8–10 | 100 CBD_O | 125 | 103.2 | 0 | –1 | –1 | 269.6 | 1.0 | 0.0 | 0.0 |
| 068 | 10–12 | 100 CBD_O | 300 | 85.7 | 0 | 0 | –1 | 85.4 | 1.0 | 0.0 | 0.0 |
| 068 | 12–22 | 100 CBD_O | 1,500 | 43.0 | 1 | 1 | 2 | 28.7 | 0.0 | 0.0 | 0.0 |
| 068 | 22–26 | 100 CBD_O | 1,050 | 17.8 | 3 | 4 | 1 | 4.1 | 0.0 | 0.0 | 0.0 |
| 068 | 26–30 | 100 CBD_O | 450 | 72.0 | 0 | 0 | –1 | 7.2 | 0.0 | 0.0 | 0.0 |
| 068 | 30–34 | 100 CBD_O | 1,000 | 35.2 | 2 | 1 | –1 | 1.5 | 0.0 | 0.0 | 0.0 |
| 068 | 34–46 | 100 CBD_O | 1,600 | 44.8 | 1 | –1 | 1 | 1.0 | 0.0 | 0.0 | 0.0 |
| 068 | 46–50 | 100 CBD_O | ms | 75.3 | 0 | –1 | –1 | 1.1 | 0.0 | 0.0 | 0.0 |
| 068 | 50–54 | 100 CBD_O | ms | 45.1 | –2 | –1 | –1 | 0.7 | 0.0 | 0.0 | 0.0 |
| 068 | 54–58 | 100 CBD_O | 600 | 35.5 | 1 | 0 | 0 | 0.4 | 0.0 | 0.0 | 0.0 |
| 068 | 72 | 100 CBD_O | 550 | 101.9 | –1 | –3 | 0 | 1.0 | 0.0 | 0.0 | 0.0 |
| 068 | 96 | 100 CBD_O | 350 | 42.6 | 1 | 1 | 2 | 0.3 | 0.0 | 0.0 | 0.0 |
| 068 | BL | 100 CBD_V | 75 | 104.7 | –5 | –6 | 0 | 0.7 | 1.5 | 0.0 | 0.0 |
| 068 | 1.5 | 100 CBD_V | 550 | 13.3 | 2 | 1 | 3 | 194.5 | 0.0 | 0.0 | 0.0 |
| 068 | 2 | 100 CBD_V | 200 | 20.9 | –3 | –6 | 1 | 83.3 | 0.0 | 0.0 | 0.0 |
| 068 | 3 | 100 CBD_V | 750 | 11.5 | –3 | –4 | 1 | 24.9 | 0.0 | 0.0 | 0.0 |
| 068 | 4 | 100 CBD_V | 200 | 52.7 | –3 | –3 | 3 | 63.6 | 1.0 | 0.0 | 0.0 |
| 068 | 4–6 | 100 CBD_V | ms | 45.6 | –3 | –4 | 2 | 40.1 | 0.0 | 0.0 | 0.0 |
| 068 | 6–8 | 100 CBD_V | 550 | 34.6 | –3 | –4 | 0 | 10.8 | 0.0 | 0.0 | 0.0 |
| 068 | 8–10 | 100 CBD_V | 200 | 71.8 | –4 | –5 | –2 | 14.3 | 1.3 | 0.0 | 0.0 |
| 068 | 10–12 | 100 CBD_V | 300 | 58.4 | –4 | –5 | 1 | 8.5 | 1.1 | 0.0 | 0.0 |
| 068 | 12–22 | 100 CBD_V | 3,700 | 12.7 | –5 | –5 | –1 | 1.3 | 0.0 | 0.0 | 0.0 |
| 068 | 22–26 | 100 CBD_V | 1,600 | 23.6 | –3 | –5 | 0 | 1.3 | 0.0 | 0.0 | 0.0 |
| 068 | 26–30 | 100 CBD_V | 3,000 | 13.0 | –4 | –7 | 0 | 0.7 | 0.0 | 0.0 | 0.0 |
| 068 | 30–34 | 100 CBD_V | 2,200 | 18.4 | –5 | –6 | 0 | 0.6 | 0.0 | 0.0 | 0.0 |
| 068 | 34–46 | 100 CBD_V | 4,900 | 15.3 | –4 | –4 | –2 | 0.6 | 0.0 | 0.0 | 0.0 |
| 068 | 46–50 | 100 CBD_V | 2,150 | 12.4 | –5 | –5 | –1 | 0.6 | 0.0 | 0.0 | 0.0 |
| 068 | 50–54 | 100 CBD_V | 2,600 | 13.7 | –4 | –5 | –1 | 0.5 | 0.0 | 0.0 | 0.0 |
| 068 | 54–58 | 100 CBD_V | 2,600 | 27.7 | –5 | –4 | 0 | 1.3 | 0.0 | 0.0 | 0.0 |
| 068 | 72 | 100 CBD_V | 1,150 | 41.1 | –4 | –7 | –1 | 0.8 | 0.0 | 0.0 | 0.0 |
| 068 | 96 | 100 CBD_V | 100 | 161.0 | –8 | –9 | –4 | 3.1 | 2.0 | 0.0 | 0.0 |
| 068 | BL | 100 CBD/4 THC | ms | 36.3 | –3 | –4 | –2 | 0.0 | 0.0 | 0.0 | 0.0 |
| 068 | 1.5 | 100 CBD/4 THC | 250 | 22.4 | 3 | 3 | 4 | 399.0 | 1.1 | 0.0 | 0.0 |
| 068 | 2 | 100 CBD/4 THC | 75 | 10.6 | 6 | 6 | 4 | 20.5 | 2.8 | 0.0 | 0.0 |
| 068 | 3 | 100 CBD/4 THC | 250 | 11.8 | 5 | 4 | 5 | 40.2 | 2.1 | 0.0 | 0.0 |
| 068 | 4 | 100 CBD/4 THC | 225 | 15.1 | 6 | 6 | 5 | 16.4 | 4.4 | 0.0 | 0.0 |
| 068 | 4–6 | 100 CBD/4 THC | 350 | 80.8 | 72 | 61 | 38 | 61.6 | 23.2 | 0.5 | 2.5 |
| 068 | 6–8 | 100 CBD/4 THC | 250 | 61.5 | 43 | 34 | 21 | 27.3 | 15.5 | 0.4 | 1.8 |
| 068 | 8–10 | 100 CBD/4 THC | 180 | 74.7 | 30 | 25 | 18 | 20.4 | 11.1 | 0.3 | 1.6 |
| 068 | 10–12 | 100 CBD/4 THC | 600 | 29.5 | 11 | 10 | 7 | 5.8 | 6.0 | 0.0 | 0.0 |
| 068 | 12–22 | 100 CBD/4 THC | 1,500 | 43.5 | 21 | 16 | 11 | 5.1 | 9.3 | 0.0 | 0.0 |
| 068 | 22–26 | 100 CBD/4 THC | 1,550 | 18.2 | 4 | 3 | 2 | 1.2 | 2.0 | 0.0 | 0.0 |
| 068 | 26–30 | 100 CBD/4 THC | 1,500 | 25.2 | 2 | 2 | 2 | 1.6 | 2.2 | 0.0 | 0.0 |
| 068 | 30–34 | 100 CBD/4 THC | 1,100 | 33.3 | 2 | 2 | 1 | 7.1 | 3.4 | 0.0 | 0.0 |
| 068 | 34–46 | 100 CBD/4 THC | 2,500 | 32.9 | 0 | 2 | 2 | 2.3 | 2.5 | 0.0 | 0.0 |
| 068 | 46–50 | 100 CBD/4 THC | 800 | 36.5 | –2 | –2 | 0 | 1.0 | 1.2 | 0.0 | 0.0 |
| 068 | 50–54 | 100 CBD/4 THC | 1,200 | 27.0 | –4 | –3 | –1 | 0.6 | 0.0 | 0.0 | 0.0 |
| 068 | 54–58 | 100 CBD/4 THC | 1,100 | 39.1 | –3 | –3 | –1 | 0.6 | 0.0 | 0.0 | 0.0 |
| 068 | 72 | 100 CBD/4 THC | 750 | 68.9 | –2 | –2 | –1 | 1.2 | 1.4 | 0.0 | 0.0 |
| 068 | 96 | 100 CBD/4 THC | 350 | 67.6 | –1 | 0 | –1 | 4.5 | 1.8 | 0.0 | 0.0 |
Note: ms = missing sample; O = oral dose; V = vaporized dose. Time = h relative to oral dosing (dose inhalation occurred 1 h after oral dosing). BL = baseline; M = male and F = female.
Immunoassay
Initial analyses of specimens by IAs were conducted according to manufacturer’s procedure with the DRI Cannabinoid Assay (Thermo Fisher Scientific, Fremont, CA) on a Beckman AU5800 analyzer for cannabinoids in urine and calibrated with DRI 20-, 50- and 100-ng/mL calibrators. The manufacturer’s package insert indicated the following cannabinoids produced a positive result [calibrated at 50 ng/mL with 11-nor-∆9-tetrahydrocannabinol-9-carboxlic acid (∆9-THCCOOH)] at the indicated concentrations: 11-hydroxy-∆9-tetrahydrocannabinol (11-OH-∆9-THC), 100 ng/mL; ∆8-THCCOOH, 100 ng/mL; 8-β-hydroxy-∆9-THC (8-β-OH-∆9-THC), 100 ng/mL; 8-β,11-dihydroxy-∆9-THC (8,11-diOH-∆9-THC), 50 ng/mL; ∆9-THC, 50 ng/mL; cannabinol (CBN), 100 ng/mL and CBD, 10,000 ng/mL. Creatinine was determined with Siemens modified Jaffe reagent. Specific gravity was determined with a Rudolph J57 refractometer. Determinations of pH were made with Axiom pH reagents (Axiom Diagnostics, Tampa, FL).
Hydrolysis methods for confirmation
It was anticipated that two types of conjugated metabolites would be present in urine specimens from this study (i.e., ether-linked CBD and acid-linked THCCOOH). Because ether-linked cannabinoid conjugates are less susceptible to base-hydrolysis, a separate enzyme hydrolysis method was developed for potential ether-linked conjugates. Base hydrolysis was conducted with 0.1 mL of 5N KOH solution added to 0.3 mL of urine specimens, calibrators and controls and 0.1 mL of internal standard solution. Samples were incubated at 50°C for 15 min. Following incubation, 0.1 mL of 5N formic acid and 0.4 mL of potassium phosphate buffer, pH 6.8 was added prior to extraction. Enzyme hydrolysis was conducted with 0.1 mL of BGTurbo® solution (Kura Biotec, Rancho Dominguez, CA) added to 0.3 mL of urine specimens, calibrators and controls and 0.1 mL of internal standard solution. Samples were incubated at 50°C for 30 min. Following incubation, 0.5 mL of potassium phosphate buffer, pH 6.8 was added prior to extraction.
Extraction
Base and enzyme hydrolyzed samples were extracted with Clean Screen XCEL II 3 mL/130-mg SPE cartridges (UCT, Bristol, PA). After sample passage through the cartridge, the extraction column was washed with 3 mL of hexane and eluted with 2 mL of solvent (49/49/2 hexane/ethyl acetate/acetic acid). Extracts were evaporated and reconstituted with 0.4M of equal parts of 0.1% formic acid in water and methanol and analyzed in separate runs (base hydrolyzed and enzyme hydrolyzed samples by LC–MS-MS).
LC–MS-MS analyses
Extracts from base hydrolyzed samples were analyzed by LC–MS-MS for the following cannabinoids: ∆9-THCCOOH, ∆8-THCCOOH, 11-nor-∆9-tetrahydrocannabivarin-9-carboxlic acid (THCVA) and 8-β-OH-∆9-THC. 8-β-OH-∆9-THC was included in the base hydrolysis because in preliminary studies it was found to be stable in the base hydrolysis procedure but not in the enzyme hydrolysis procedure. Extracts from enzyme hydrolyzed samples were analyzed by LC/MS/MS for the following cannabinoids: ∆9-THC, ∆8-THC, 8,11-diOH-∆9-THC, 11-OH-∆9-THC, THCV, CBD and CBN (see Table III). Analyses were conducted with an API6500 QTrap by electrospray ionization (in positive or negative mode) with a source temperature of 450°C.
Table III.
LC–MS-MS Method Development Metrics
| Analyte | Internal standard | Ionization mode | Transitions (± 0.3 amu) |
Retention time (± 0.3 min) |
|---|---|---|---|---|
| Enzyme hydrolysis assay | ||||
| 8,11-diOH-∆9-THC | Positive | 347.3 > 311.4 347.3 > 293.3 |
2.3 | |
| 8,11-diOH-∆9-THC-D6 | Positive | 353.3 > 317.4 353.3 > 299.3 |
2.3 | |
| 11-OH-THC | Positive | 331.2 > 193.1 331.2 > 201.1 |
4.6 | |
| 11-OH-THC-D3 | Positive | 334.2 > 196.1 334.2 > 201.1 |
4.6 | |
| THCV | Positive | 287.3 > 165.1 287.3 > 231.1 |
5.5 | |
| CBD-D3 | Positive | 318.2 > 196.2 318.2 > 123.2 |
7.3 | |
| CBD | Positive | 315.2 > 193.2 315.2 > 123.2 |
5.8 | |
| CBD-D3 | Positive | 318.2 > 196.2 318.2 > 123.2 |
5.8 | |
| CBN | Positive | 311.1 > 223.1 311.1 > 241.0 |
7.3 | |
| CBN-D3 | Positive | 314.1 > 223.1 314.1 > 241.0 |
7.3 | |
| ∆9-THC | Positive | 315.1 > 193.2 315.1 > 259.2 |
8.0 | |
| ∆9-THC-D3 | Positive | 318.1 > 196.2 318.1 > 262.2 |
8.0 | |
| ∆8-THC | Positive | 315.1 > 193.2 315.1 > 259.2 |
8.2 | |
| ∆8-THC-D9 | Positive | 324.1 > 202.2 324.1 > 268.2 |
8.2 | |
| Base hydrolysis assay | ||||
| 8-β-OH-∆9-THC | Positive | 331.2 > 201.1 331.2 > 271.1 |
3.5 | |
| 11-OH-THC-D3 | Positive | 334.2 > 196.1 334.2 > 201.1 |
5.1 | |
| THCVA | Negative | 315.2 > 217.1 315.2 > 163.1 |
3.05 | |
| ∆8-THCCOOH-D6 | 349.1 > 251.1 349.1 > 191.1 |
5.25 | ||
| ∆8-THCCOOH | Negative | 343.1 > 245.1 343.1 > 191.1 |
5.25 | |
| ∆8-THCCOOH-D6 | Negative | 349.1 > 251.1 349.1 > 191.1 |
5.25 | |
| ∆9-THCCOOH | Negative | 343.1 > 245.1 343.1 > 191.1 |
5.45 | |
| ∆9-THCCOOH-D9 | Negative | 352.1 > 254.1 352.1 > 194.1 |
5.45 |
The linearity was determined by five replicate analyses of the analytes with a single point calibrator at 10 ng/mL for all analytes. The analytical range was verified with four levels below the calibrator and five levels above the calibrator. The limit of detection (LOD) for ∆9-THCCOOH, 8-OH-THC, THCVA and 8,11-diOH-THC was 1.0 ng/mL; the LOD for other analytes was 0.25 ng/mL. The upper limit of linearity and carry over limit for all analytes was 1,000 ng/mL. The criterion for acceptance of results was based on the ion ratio of ±20% for the analyte and internal standard, relative retention time of ±2%, internal standard response of 20–200%, asymmetry of peak from ≥0.5 to ≤3.0, and resolution of a co-eluting peak at ≥90%. The analytical range for the low control (40% of calibrator) and positive control (125% of calibrator) was ±20% of target. Analytes were reported as negative if the value was less than the LOD.
The recovery of the solid phase extraction was performed by the addition of internal standard to post-extraction and analyzed samples. The percent recovery for the analytes ranged from a low of 52% for ∆8-THC to 86% for 8-OH-THC. At 4 (low control for the batch, n = 5) and 12.5 ng/mL (positive control for the batch, n = 5), the within-run precision was 1.5–3.6% CV, respectively, and the between-run precision was 2.9–16.4% CV, respectively. The percent bias was for these analyses ranged from –5.5 to 3.8%.
An interference study was performed to test 127 compounds, including over-the-counter drugs, prescription drugs and drugs of abuse, for specific interference. About 12 interference standard solutions were prepared and diluted in negative and low-control samples. Of which, 10 negative samples were randomly selected and used to perform a quantitative matrix effect study to evaluate for unidentified method interferences. An aliquot of each sample was fortified with analyte to the low-control concentration and analyzed with a corresponding aliquot of the negative sample. The interference study results were evaluated for acceptance using the criterion established for participant samples.
Data presentation and analysis
Participant demographics and LC–MS-MS urine results are presented for each individual participant and summarized using descriptive statistics. Sensitivity, specificity and agreement between immunoassay (IA) and LC–MS-MS results were calculated for ∆9-THCCOOH for all non-placebo conditions. There were three IA screening cutoffs used in these analyses: 20, 50 and 100 ng/mL. The confirmatory LC–MS-MS cutoff was 15 ng/mL for all analyses, which corresponds with the mandatory guidelines for federal workplace drug testing (24).
Urinary ∆9-THCCOOH test results were categorized into the following four categories: true positive (TP; IA response ≥ cutoff concentration and LC–MS-MS positive, i.e., ≥ 15 ng/mL), true negative (TN; IA response < cutoff concentration and LC–MS-MS negative, i.e., < 15 ng/mL), false positive (FP; IA response ≥ cutoff concentration and LC–MS-MS negative, i.e., < 15 ng/mL) or false negative (FN; IA response < cutoff concentration and LC–MS-MS positive, i.e., ≥ 15 ng/mL). Sensitivity, specificity and agreement were calculated using the following formulas: sensitivity (100 × [TP/(TP + FN)]), specificity (100 × [TN/(TN + FP)]) and agreement (100 × [(TP + TN)/(TP + TN + FP + FN)]).
Results
Table II displays full IA and LC–MS-MS urinary results for select cannabinoids for each participant and time point (note that 8,11-diOH-THC, THCV, ∆-9-THC and 8-OH-THC were not detected in urine during any session, and 11-OH-THC, CBN and ∆-8-THC were only detected at trace concentrations (<1 ng/mL) and are thus not listed in Table II). Figure 1 displays mean urinary CBD and ∆9-THCCOOH concentrations before and after drug administration, and Table IV displays Cmax and Tmax values and time to first and last detection for ∆9-THCCOOH and CBD for each individual participant. Figure 2 shows the urinary ∆9-THCCOOH concentrations across time in the CBD-dominant cannabis condition for each participant.
Figure 1.
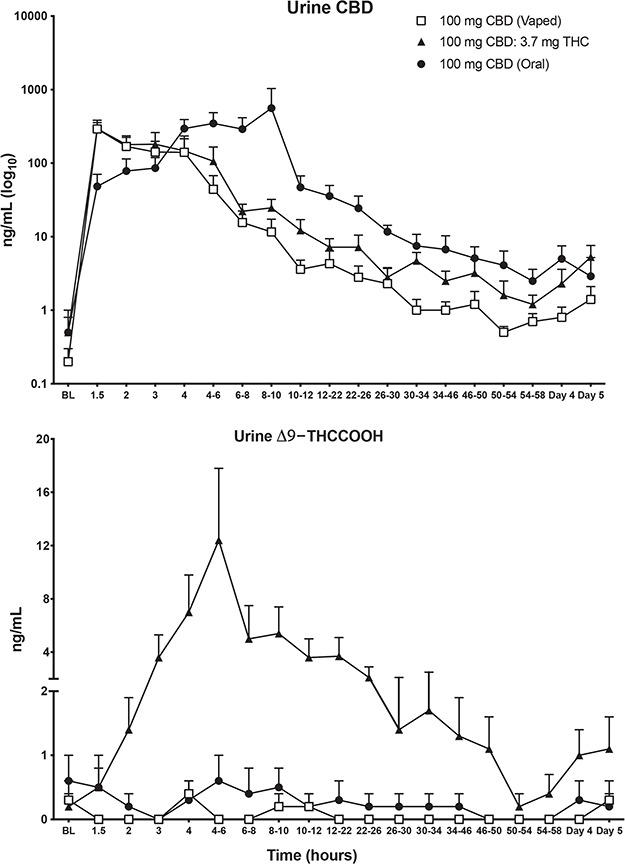
Quantitative urinary concentrations (mean ± SEM) of CBD and ∆9-THCCOOH. ∆9-THCCOOH is on log10 axis; CBD is on linear axis. BL = baseline.
Table IV.
Maximum Concentrations (Cmax), Time to Maximum Concentrations (Tmax) and Detection Windows for Cannabinoids in Urine Following Administration of Oral and Vaporized CBD and CBD-dominant Cannabis
| Subject ID# | CBD Cmax (ng/mL) |
CBD Tmax (h) |
CBD dose excreted (%) | CBD first detected (h) |
CBD last detected (h) |
∆9-THC-COOH Cmax (ng/mL) |
∆9-THC-COOH Tmax (h) |
∆9-THC-COOH first detected (h) |
∆9-THC-COOH last detected (h) |
|---|---|---|---|---|---|---|---|---|---|
| Placebo | |||||||||
| 038 | 14.1 | 5 | N/A | BL | 103 | 2 | 5 | BL | 5 |
| 053 | 3.1 | 1.5 | N/A | BL | 97 | ND | ND | ND | ND |
| 054 | 0.4 | 2 | N/A | 1.5 | 3 | ND | ND | ND | ND |
| 063 | 2.3 | 48 | N/A | BL | 73 | ND | ND | ND | ND |
| 066 | 2 | 2 | N/A | 1.5 | 52 | ND | ND | ND | ND |
| 068 | 4.7 | 32 | N/A | BL | 96 | 2.2 | BL | BL | BL |
| Mean (total) | 4.4 | 15.1 | 0.7 | 2.5 | |||||
| 100-mg oral CBD | |||||||||
| 038 | 2,941 | 9 | 1.0 | BL | 98 | ND | ND | ND | ND |
| 053 | 210.2 | 2 | 0.03 | 1.5 | 48 | ND | ND | ND | ND |
| 054 | 496.1 | 4 | 0.22 | BL | 103 | 2.9 | 1.5 | BL | 103 |
| 063 | 214.6 | 3 | 0.12 | 1.5 | 74 | ND | ND | ND | ND |
| 066 | 526 | 5 | 0.22 | 1.5 | 96 | ND | ND | ND | ND |
| 068 | 269.6 | 9 | 0.23 | BL | 96 | 1.3 | BL | BL | 11 |
| Mean (total) | 776.3 | 5.3 | 0.30 | 0.7 | 0.8 | ||||
| 100-mg vaporized CBD | |||||||||
| 038 | 631.1 | 0.5 | 0.26 | 0.5 | 95 | 1.2 | 3 | 3 | 3 |
| 053 | 15.3 | 1 | 0.003 | 1 | 23 | ND | ND | ND | ND |
| 054 | 79.3 | 1 | 0.05 | 0.5 | 102 | ND | ND | ND | ND |
| 063 | 248.7 | 1 | 0.09 | BL | 97 | ND | ND | ND | ND |
| 066 | 394.2 | 0.5 | 0.05 | 0.5 | 72 | ND | ND | ND | ND |
| 068 | 194.5 | 0.5 | 0.17 | BL | 95 | 2 | 96 | BL | 95 |
| Mean (total) | 260.5 | 0.75 | 0.10 | 0.5 | 54 | ||||
| Vaporized cannabis (100-mg CBD/3.7-mg THC) | |||||||||
| 038 | 539.1 | 2 | 0.28 | BL | 94 | 29.9 | 4 | 1 | 94 |
| 053 | 126.1 | 1 | 0.01 | 1 | 97 | 1.2 | 23 | 23 | 23 |
| 054 | 232.8 | 2 | 0.11 | BL | 94 | 7.3 | 8 | 2 | 94 |
| 063 | 195.9 | 0.5 | 0.08 | 0.5 | 95 | 1.6 | 23 | 1 | 23 |
| 066 | 351 | 1 | 0.06 | 0.5 | 95 | 4.7 | 3 | BL | 16 |
| 068 | 399 | 0.5 | 0.17 | 0.5 | 95 | 23.2 | 4 | 0.5 | 95 |
| Mean (total) | 307.3 | 1.2 | 0.12 | 11.3 | 10.8 | ||||
Note: N/A = not applicable for that analyte; ND = analyte not detected for that participant/condition; BL = baseline. Midpoint time value used for pooled specimens. Tmax and time to first and last detection are relative to oral dosing for 100-mg oral CBD and relative to dose inhalation for 100-mg vaporized CBD and CBD-dominant cannabis. Note: 038, 066 and 068 were males; 053, 054 and 063 were females.
Figure 2.
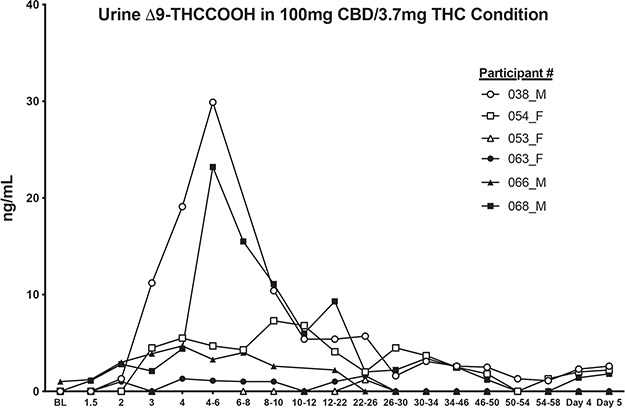
Quantitative urinary concentrations of ∆9-THCCOOH for all six participants following administration of CBD-dominant cannabis (100-mg CBD; 3.7-mg ∆9-THC). Numbers in legend refer to individual participants (see Tables I, II and IV). BL = baseline; M = male and F = female.
LC–MS-MS results
100-mg oral CBD
Following oral administration of 100-mg CBD, urinary Cmax concentrations for CBD ranged from 214 to 2,941 ng/mL (mean Cmax: 776.3 ng/mL), while Tmax values for CBD ranged from 2 to 9 h after oral dosing (mean Tmax: 5.3 h). On average, urinary CBD concentrations were higher for men (mean Cmax for men: 1,245.5 ng/mL) compared to women (mean Cmax for women: 307.0 ng/mL) following oral dosing. The overall percentage of the 100-mg oral CBD dose that was excreted as total drug (free and hydrolyzed) in urine ranged from 0.03 to 1.0 % (mean: 0.3 %). For three out of six participants, CBD was still detected in urine 5 days after acute oral CBD dosing (Day 5 collection times ranged from 96 to 103 h post-dosing). Notably, despite the 1-week washout between doses, three participants, each of whom had vaporized CBD the previous week, had detectable urinary CBD at the baseline timepoint for the oral-dosing session. Thus, urinary levels of CBD were elevated at baseline for these individuals due to residual CBD from the prior dose. For the three participants with no CBD present in urine at baseline, CBD was first detected in urine 1.5 h after oral ingestion and last detected between 48 and 96 h.
Trace amounts of ∆9-THCCOOH were detected during oral CBD dosing sessions for two study participants (#054 and #068). In both cases, ∆9-THCCOOH was detected at baseline (prior to drug administration) and only sporadically at subsequent time points, typically in specimens that also had higher creatinine concentrations compared with samples in which ∆9-THCCOOH was not detected. Participant #054 received the oral CBD dose during the third week of study participation, but was not exposed to the ∆9-THC-containing cannabis dose in the study prior to this dosing session. Participant #068 received the oral CBD dose during the fourth week of study participation and was administered the ∆9-THC-containing cannabis vapor dose during Week 1. Note, low concentrations of ∆9-THCCOOH were also measured at multiple time points for Participant #68 during the placebo (Week 2) and CBD vapor (Week 3) dosing sessions as well. Because ∆9-THCCOOH was present at baseline, was not consistently observed, and was detected only at very low concentrations (1.0–2.9 ng/mL), these results most likely reflect residual excretion of prior ∆9-THC exposure. ∆8-THC was detected for two participants, both at a concentration of 0.3 ng/mL (#038 at the 8–10 h time point and #066 at the 4–6 h time point). The remaining cannabinoids were not detected following oral CBD ingestion.
100-mg vaporized CBD
For the 100-mg vaporized CBD condition, urinary Cmax concentrations for CBD ranged from 15 to 631 ng/mL (mean Cmax: 261 ng/mL) while Tmax values for CBD ranged from 0.5 to 1 h after inhalation (mean Tmax: 0.8 h). On average, urinary CBD concentrations were higher for men (mean Cmax for men: 406.6 ng/mL) compared to women (mean Cmax for women: 114.4 ng/mL) following CBD inhalation. The overall percentage of the 100-mg vaporized CBD dose that was excreted in urine ranged from 0.003 to 0.3% (mean: 0.10 %). For participants #063 and #068 in the 100-mg vaporized CBD condition, CBD was first detected in urine at baseline and last detected on Day 5 (95–97 h post-dose inhalation). For the remaining four participants (participants #038, #053, #054 and #066), CBD was detected 0.5–1 h after dose inhalation, and lasted detected between 23 and 102 h post-administration.
Trace amounts of ∆9-THCCOOH were detected for two participants in the 100-mg vaporized CBD condition (participants #038 and #068). For participant #038, ∆9-THCCOOH was detected at only one time point (3 h post-inhalation; concentration = 1.2 ng/mL). For participant #068, ∆9-THCCOOH concentration was 1.5 ng/mL at baseline, fell below the limit of quantification for several hours, and then was detected again 3, 7–9 and 9–11 h after dose inhalation. ∆9-THCCOOH was subsequently detected again for this participant on Day 5 (95 h after dose inhalation) at 2 ng/mL. 11-OH-THC (not included in Table II) was detected for participant #054 (concentration = 0.5 ng/mL) at the 34–46 h time point and for participant #066 (concentration = 0.2 ng/mL) at the 30–34 h time point; remaining cannabinoids were not detected in the 100-mg vaporized CBD condition.
CBD-dominant cannabis
Following inhalation of CBD-dominant cannabis (containing ~100-mg CBD and 3.7-mg ∆9-THC; Figure 2), urinary Cmax concentrations for CBD ranged from 126 to 539 ng/mL (mean Cmax: 307 ng/mL) while Tmax values for CBD ranged from 0.5 to 2 h after inhalation (mean Tmax: 1.2 h; Table IV). On average, urinary CBD concentrations were higher for men (mean Cmax for men: 429.7 ng/mL) compared to women (mean Cmax for women: 184.9 ng/mL) following cannabis inhalation. The overall percentage of the 100-mg vaporized CBD dose that was excreted in urine ranged from 0.01 to 0.3% (mean: 0.12%). CBD was first detected at baseline for two participants (#38 and #54) and between 0.5 and 1 h after dose inhalation for the other four participants. CBD was detected in urine until Day 5 (94–97 h post-cannabis inhalation) for all study participants. ∆9-THCCOOH was also detected for all participants in the CBD-dominant cannabis condition; Cmax values ranged from 1.2 to 29.9 ng/mL. Tmax values for ∆9-THCCOOH ranged from 3 to 23 h post-cannabis inhalation. Men excreted higher concentrations of ∆9-THCCOOH (mean Cmax for men: 19.3 ng/mL) compared to women (mean Cmax for women: 3.4 ng/mL), on average. There was large inter-individual variability with respect to first and last detection for ∆9-THCCOOH (Table IV). For 3/6 participants (i.e., #038, #054 and #068), ∆9-THCCOOH concentrations were first detected shortly after cannabis inhalation (0.5–2 h) and last detected on Day 5 (94–95 h). For participant #053, ∆9-THCCOOH was only detected once, at the 22–26 h pooled urine collection time point, while for participant #063 the window for detection for ∆9-THCCOOH was 1–23 h post-cannabis inhalation. For participant #066, ∆9-THCCOOH was first detected at baseline and last detected 16 h post-cannabis inhalation. Inhalation of CBD-dominant cannabis resulted in detection of several other cannabinoids for participants #038, #054, #066 and #068. Specifically, THCVA was detected in all four of these individuals, CBN was detected in #038, #054 and #068; ∆8-THC was detected in #066 and #068 and ∆8-THCCOOH was detected in #038, #054 and #068 (see Table II).
Of note, following inhalation of CBD-dominant cannabis, two participants (#038 and #068; both males) excreted ∆9-THCCOOH concentrations above 15 ng/mL (the confirmatory cutoff concentration listed in the Mandatory Guidelines for federal workplace drug testing). Specifically, participant #038 provided two specimens (at the 4 and 4–6 h collection points) and Participant #068 provided two specimens (at the 4–6 and 6–8 h collection points) that exceeded 15 ng/mL (see Figure 2). ∆9-THCCOOH concentrations were well below 15 ng/mL in both oral and vaporized CBD conditions.
Sensitivity, specificity and agreement
Sensitivity, specificity and agreement results between IA and LC–MS-MS for urinary ∆9-THCCOOH concentrations are presented in Table V. Specifically, three different IA screening cutoffs (20, 50 and 100 ng/mL) were compared to the confirmatory LC–MS-MS results (confirmation of positive test was always: ≥15 ng/mL). For the 100-mg oral CBD condition, using the 20 ng/mL IA screening cutoff, one specimen was deemed a false positive while the remaining 109 specimens were deemed true negatives. The lone specimen that was a false positive at the 20 ng/mL IA cutoff occurred for participant #038 at the 8–10 h pooled urine collection time point (this specimen also contained trace amounts of ∆8-THC; Table II). When tested at the 50 and 100 ng/mL IA cutoffs, all specimens from the 100-mg oral CBD condition were considered true negatives. For the 100-mg vaporized CBD condition, all specimens (108/108) were characterized as true negatives at each of the three IA screening cutoffs. Specificity (ability to detect true negatives for ∆9-THCCOOH), was 99.1% at the 20 ng/mL IA cutoff and 100% at the 50 and 100 ng/mL IA cutoffs. Sensitivity (ability to detect true positives for ∆9-THCCOOH) could not be measured in the oral or vaporized CBD conditions because ∆9-THC was not administered.
Table V.
Comparisons of IA Responses to Confirmation Analyses (LC–MS-MS) in Urine Specimens Following Administration of Oral and Vaporized CBD and CBD-dominant Cannabis
| Urine ∆9-THCCOOH IA (cutoff = 20 ng/mL) vs ∆9-THCCOOH LC–MS-MS (confirmation = 15 ng/mL) |
Urine ∆9-THCCOOH IA (cutoff = 50 ng/mL) vs ∆9-THCCOOH LC–MS-MS (confirmation = 15 ng/mL) |
Urine ∆9-THCCOOH IA (cutoff = 100 ng/mL) vs ∆9-THCCOOH LC–MS-MS (confirmation = 15 ng/mL) |
|
|---|---|---|---|
| 100-mg oral CBD | |||
| #True positive (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| #True negative (%) | 109 (99.1) | 110 (100.0) | 110 (100.0) |
| #False positive (%) | 1 (0.9) | 0 (0.0) | 0 (0.0) |
| #False negative (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sensitivity (%) | 0 | 0 | 0 |
| Specificity (%) | 99.1 | 100.0 | 100.0 |
| Agreement (%) | 99.1 | 100.0 | 100.0 |
| 100-mg Vaporized CBD | |||
| #True positive (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| #True negative (%) | 108 (100.0) | 108 (100.0) | 108 (100.0) |
| #False positive (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| #False negative (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sensitivity (%) | 0 | 0 | 0 |
| Specificity (%) | 100.0 | 100.0 | 100.0 |
| Agreement (%) | 100.0 | 100.0 | 100.0 |
| Vaporized cannabis (100-mg CBD/3.7-mg THC) | |||
| #True positive (%) | 4 (3.7) | 2 (1.9) | 0 (0.0) |
| #True negative (%) | 99 (91.7) | 104 (96.3) | 104 (96.3) |
| #False positive (%) | 5 (4.6) | 0 (0.0) | 0 (0.0) |
| #False negative (%) | 0 (0.0) | 2 (1.9) | 4 (3.7) |
| Sensitivity (%) | 100.0 | 50.0 | 0 |
| Specificity (%) | 95.2 | 100.0 | 100.0 |
| Agreement (%) | 95.4 | 98.1 | 96.3 |
As noted above, there were four specimens (two from #038 and two from #068) with ∆9-THCCOOH concentrations that exceed 15 ng/mL in the CBD-dominant cannabis condition. At the 20-ng/mL IA screening cutoff, each of these four samples were confirmed as true positives. At the 50-ng/mL cutoff (screening cutoff suggested by the Mandatory Guidelines for federal workplace drug testing), two of the samples were considered true positives (one from #038 and one from #068) and the remaining two were categorized as false negatives. All four specimens over 15 ng/mL were categorized as false negatives at the 100-ng/mL IA screening cutoff. Sensitivity, specificity and agreement in the CBD-dominant cannabis condition were: 100, 95 and 95% at the 20-ng/mL IA cutoff, 50, 100 and 98% at the 50-ng/mL IA cutoff, and 0, 100 and 96% at the 100-ng/mL IA cutoff.
Adverse events
No adverse events occurred in this study.
Discussion
Oral and inhalable CBD products have become ubiquitous in both legal and illicit cannabis markets. Commercial CBD products often contain low levels of ∆9-THC (1, 9, 12, 13) and there is limited pre-clinical evidence suggesting that CBD may be converted to ∆8 and ∆9-THC in the human gut (17, 18). Given these issues, there is an urgent need to understand whether CBD products can influence results of urine drug tests, which remains the primary method to evaluate recent cannabis use in the workplace and many other settings.
In the present study, acute administration of neither 100-mg oral CBD nor 100-mg vaporized CBD produced a positive urine toxicology result based on current US drug testing guidelines (screening via IA at a cutoff of 50-ng/mL ∆9-THCCOOH and confirmation via LC/MS/MS at a cut-off of 15 ng/mL). Only 1 of 218 specimens screened positive (at 20-ng/mL ∆9-THCCOOH cutoff) after administration of pure CBD (oral dose of 100-mg encapsulated CBD), and no samples screened positive at 50 or 100ng/mL after pure CBD administration. The specimen that screened positive at 20 ng/mL occurred at the 8–10 h timepoint for participant #038, which also coincided with peak urine CBD concentration (2,941 ng/mL) for that participant. LC–MS-MS testing of that sample showed no ∆9-THC or ∆9-THCCOOH, suggesting that the positive screen may have been due to cross-reactivity with the IA assay at the very high CBD concentration or other CBD metabolites. Additional research is needed to identify factors that contribute to the observed increased IA activity in the presence of high urine CBD concentrations.
Another important finding of the present study was that there was no indication that orally administered CBD converted to ∆8-THC or ∆9-THC, as has been observed in pre-clinical studies (17, 18). Though trace amounts of ∆9-THC metabolites were observed in some specimens obtained during experimental sessions in which pure CBD was administered, the detection was sporadic with respect to time (i.e., very few consecutive samples had ∆9-THCCOOH above LOQ when pure CBD was administered), also occurred in baseline and placebo session samples, and was typically in samples with high creatinine concentration relative to surrounding time points with no detection. Together, this suggests the low concentration of ∆9-THCCOOH and other cannabinoids detected in these samples was likely due to prior exposure to ∆9-THC.
This study also demonstrated that inhaling vaporized CBD-dominant cannabis (approximate concentrations of CBD and ∆9-THC were 100 and 3.7 mg, respectively) can produce positive results for IA screening assays up to 50 ng/mL and LC–MS-MS confirmatory testing at a cutoff of 15-ng/mL ∆9-THCCOOH. Specifically, following inhalation of CBD-dominant cannabis, two participants (#038 and #068) each produced two urine specimens, between 4 and 8 h post-cannabis administration, with ∆9-THCCOOH concentrations above the widely used confirmatory cutoff of 15 ng/mL; all specimens tested positive at the 20-ng/mL IA cutoff and 50% of specimens tested positive at the 50-ng/mL IA cutoff. The implications of this outcome will vary, depending on diverse regulatory and national drug control regulations. For example, the CBD-dominant cannabis product used in this study contained 0.39% ∆9-THC by dry weight, which exceeds the 0.3% THC concentration limit for “hemp” products that have been legalized in the USA under the 2018 “Farm Bill” (1), but this product would be legal in Canada. Thus, additional studies are needed to determine the impact of acute and chronic exposure to CBD-dominant products that conform to various legal definitions (e.g., <0.3% ∆9-THC for hemp in the USA). Nevertheless, the current data suggest that individuals subject to drug testing should be aware that even modest amounts of ∆9-THC in a CBD/hemp product may contribute to a positive urine drug test. Such consumer awareness is critical given that retail “CBD” products labeled as being free of ∆9-THC often contain ∆9-THC at concentrations comparable to, or above, that of the cannabis used in this study (9, 12).
To our knowledge, this study is the first to directly compare the urinary excretion profile of CBD and other cannabinoids across multiple routes of CBD administration (oral and vaporized). Overall, peak urinary concentrations of CBD were later relative to dose administration, but generally much higher, following oral administration of CBD compared with inhalation. CBD remained present in urine until the final collection point (i.e., 5 days after administration) for 4/6 oral administration sessions and 4/6 vaporized dosing sessions. In both oral and vaporized pure CBD sessions, most cannabinoids were not detected at all (CBN, 8,11-diOH-THC, THCV, THCVA, ∆9-THC, 8-OH-THC and ∆8-THCCOOH) and the remaining cannabinoids examined (∆8-THC, ∆9-THCCOOH and 11-OH-∆9-THC) were only detected at trace levels in a minority of samples collected. Importantly, this study also enabled a direct pharmacokinetic comparison between the same dose of vaporized CBD (100 mg), with and without a low dose of ∆9-THC. Interestingly, though peak urinary CBD concentrations were similar for vaporized CBD and vaporized CBD-dominant cannabis (with 3.7-mg ∆9-THC), CBD concentrations tended to be higher after administration of CBD-dominant cannabis at later urine collection time points (see Figure 1), suggesting that the simultaneous co-administration of CBD and ∆9-THC altered the elimination of CBD. The seemingly slower elimination rate of CBD in the presence ∆9-THC lends support to the notion that CBD and ∆9-THC can inhibit each other’s metabolism because they are hydrolyzed by similar cytochrome P450 enzymes (20, 22, 25, 26). Last, inhalation of CBD-dominant cannabis resulted in detection of additional cannabinoids for some participants including ∆9-THCCOOH, THCVA, CBN, ∆8-THC and ∆8-THCCOOH.
Several limitations of this study warrant discussion. First, in some cases, the time between experimental sessions was seemingly not long enough for sufficient drug washout. For example, there were several occasions where CBD and/or ∆9-THCCOOH were detected at baseline (prior to drug administration), suggesting the study dose from the week prior had not been completely eliminated. Future controlled studies with multiple CBD/∆9-THC dosing conditions may consider extending the window of observation after drug exposure to longer than 5 days, as this could help to characterize the full urinary excretion profile of CBD and other cannabinoids and also elucidate unequivocally, whether CBD is converted to ∆9-THC in the human gut. Second, this study was limited by the use of only one type of vaporizer, one batch of CBD-dominant cannabis, and one dose of CBD and ∆9-THC. Additional studies are needed to evaluate a greater range of acute doses of CBD, and to evaluate chronic CBD dosing. Last, the small, homogeneous sample in this study limits the overall generality of these findings.
Conclusion
Acute oral ingestion or inhalation of a 100-mg dose of CBD did not result in positive urine drug test when using screening and confirmatory cutoffs in the Mandatory Guidelines for federal workplace drug testing. In contrast, inhalation of cannabis containing 100-mg CBD and 3.7-mg ∆9-THC resulted in positive test results for two out of six participants. Urinary concentrations of CBD were higher, and peaked later, when CBD was orally ingested compared with when it was inhaled. CBD appeared to be eliminated at a slower rate when ∆9-THC was simultaneously administered, compared with administration of CBD alone. Study results did not indicate that CBD converts to ∆-8 and/or ∆-9-THC in the human gut under the conditions tested, but future research should examine whether this occurs in conditions in which the gut is more acidic (e.g., during a fasted state). Several areas of need for additional research have been identified through this study, which is of ever-greater importance with the increased availability and legality of CBD/hemp products.
Funding
This research was supported by the Substance Abuse and Mental Health Services Administration (SAMHSA) and the NIDA (T32DA07209).
Acknowledgments
We thank the support staff of the Johns Hopkins University BPRU for outstanding contributions to the implementation of this study. We also thank the many individuals involved with the NIDA Drug Supply Program for providing their services and cannabis for the conduct of this study.
References
- 1. Bridges M., Hanson K. (2017) Regulating hemp and cannabis-based products. NCSL Legisbrief, 25, 1–2. [PubMed] [Google Scholar]
- 2. Corroon J., Kight R. (2018) Regulatory status of cannabidiol in the united states: a perspective. Cannabis and Cannabinoid Research, 3, 190–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walsh Z., Gonzalez R., Crosby K., S. Thiessen M., Carroll C., Bonn-Miller M.O. (2017) Medical cannabis and mental health: a guided systematic review. Clinical Psychology Review, 51, 15–29. [DOI] [PubMed] [Google Scholar]
- 4. Maroon J., Bost J. (2018) Review of the neurological benefits of phytocannabinoids. Surgical Neurology International, 9, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blessing E.M., Steenkamp M.M., Manzanares J., Marmar C.R. (2015) Cannabidiol as a potential treatment for anxiety disorders. Neurotherapeutics, 12, 825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Poleg S., Golubchik P., Offen D., Weizman A. (2019) Cannabidiol as a suggested candidate for treatment of autism spectrum disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 89, 90–96. [DOI] [PubMed] [Google Scholar]
- 7. Devinsky O., Cross J.H., Wright S. (2017) Trial of cannabidiol for drug-resistant seizures in the dravet syndrome. The New England Journal of Medicine, 377, 699–700. [DOI] [PubMed] [Google Scholar]
- 8. Corroon J., Phillips J.A. (2018) A cross-sectional study of cannabidiol users. Cannabis and Cannabinoid Research, 3, 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonn-Miller M.O., Loflin M.J.E., Thomas B.F., Marcu J.P., Hyke T., Vandrey R. (2017) Labeling accuracy of cannabidiol extracts sold online. JAMA, 318, 1708–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Macdonald S., Hall W., Roman P., Stockwell T., Coghlan M., Nesvaag S. (2010) Testing for cannabis in the work-place: a review of the evidence. Addiction, 105, 408–416. [DOI] [PubMed] [Google Scholar]
- 11. Kulig K. (2017) Interpretation of workplace tests for cannabinoids. Journal of Medical Toxicology, 13, 106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Poklis J.L., Mulder H.A., Peace M.R. (2019) The unexpected identification of the cannabimimetic, 5f-adb, and dextromethorphan in commercially available cannabidiol e-liquids. Forensic Science International, 294, 25–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drug Enforcement Administration DoJ (2018) Schedules of controlled substances: placement in schedule v of certain fda-approved drugs containing cannabidiol; corresponding change to permit requirements. Final order. Federal Register, 83, 48950–48953. [PubMed] [Google Scholar]
- 14. Bonn-Miller M.O., Banks S.L., Sebree T. (2017) Conversion of cannabidiol following oral administration: authors' response to grotenhermen et al. Doi: 10.1089/can.2016.0036. Cannabis and Cannabinoid Research, 2, 5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nahler G., Grotenhermen F., Zuardi A.W., Crippa J.A.S. (2017) A conversion of oral cannabidiol to delta9-tetrahydrocannabinol seems not to occur in humans. Cannabis and Cannabinoid Research, 2, 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grotenhermen F., Russo E., Zuardi A.W. (2017) Even high doses of oral cannabidol do not cause thc-like effects in humans: comment on merrick et al. Cannabis and Cannabinoid Research 2016;1(1):102–112; Doi: 10.1089/can.2015.0004. Cannabis and Cannabinoid Research, 2, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Merrick J., Lane B., Sebree T., Yaksh T., O'Neill C., Banks S.L. (2016) Identification of psychoactive degradants of cannabidiol in simulated gastric and physiological fluid. Cannabis and Cannabinoid Research, 1, 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Watanabe K., Itokawa Y., Yamaori S., Funahashi T., Kimura T., Kaji T. et al. (2007) Conversion of cannabidiol to ∆9-tetrahydrocannabinol and related cannabinoids in artificial gastric juice, and their pharmacological effects in mice. Forensic Toxicology, 25, 16–21. [Google Scholar]
- 19. Harvey D.J., Mechoulam R. (1990) Metabolites of cannabidiol identified in human urine. Xenobiotica, 20, 303–320. [DOI] [PubMed] [Google Scholar]
- 20. Hlozek T., Uttl L., Kaderabek L., Balikova M., Lhotkova E., Horsley R.R. et al. (2017) Pharmacokinetic and behavioural profile of THC, CBD, and THC+CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. Eur Neuropsychopharmacol, 27, 1223–1237. [DOI] [PubMed] [Google Scholar]
- 21. Cox E.J., Maharao N., Patilea-Vrana G., Unadkat J.D., Rettie A.E., McCune J.S. et al. (2019) A marijuana-drug interaction primer: precipitants, pharmacology, and pharmacokinetics. Pharmacology & Therapeutics.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zendulka O., Dovrtelova G., Noskova K., Turjap M., Sulcova A., Hanus L. et al. (2016) Cannabinoids and cytochrome p450 interactions. Current Drug Metabolism, 17, 206–226. [DOI] [PubMed] [Google Scholar]
- 23. Sobell L.C., Sobell M.B.. Timeline follow-back: a technique for assessing self-reported alcohol consumption In: Allen J.P., Litten R.Z. (eds). Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Human Press: Totowa, NJ, 1992; pp41–72. [Google Scholar]
- 24. SAMHSA (2017) Mandatory guidelines for federal workplace drug testing programs. Federal Register, 82, 7920–7970. [Google Scholar]
- 25. Huestis M.A. (2007) Human cannabinoid pharmacokinetics. Chemistry and Biodiversity, 4, 1770–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ujvary I., Hanus L. (2016) Human metabolites of cannabidiol: a review on their formation, biological activity, and relevance in therapy. Cannabis and Cannabinoid Research, 1, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]


