Abstract
A 38-year-old African American male presented with progressive pain, swelling, numbness, and warmth of the left upper extremity ten days before admission. A chest computerized tomography scan showed a large 8.3 cm × 6.1 cm x 9.9 cm anterior mediastinal mass with compression of the left brachiocephalic vein and superior vena cava. A venous doppler showed multiple occlusive venous thrombi in bilateral upper extremities, including the bilateral internal jugular and subclavian veins, as well as the left subclavian, axillary, cephalic, brachial and median cubital veins.
Further laboratory workup came positive for acetylcholine receptor binding antibody suggesting myasthenia gravis, but the patient was asymptomatic for myasthenia gravis. A percutaneous core CT guided biopsy pathology resulted in a predominant T-cell population CD5 positive with few B cells; the immunophenotypic features suggested Type B2 thymoma. To the best of our knowledge, this case is the only reported thymoma presenting with bilateral deep vein thrombosis of the upper extremities. The deep vein thrombosis therapy was enoxaparin 1mg/kg subcutaneously every 12 hours and dexamethasone 4mg intravenously every 4 hours as an anti-inflammatory drug for thymoma related compression of the mediastinum. The patient was referred to a tertiary oncological medical center for a total thymectomy, chemotherapy, and adjuvant radiotherapy.
Keywords: Thymoma, Deep vein thrombosis, Paraneoplastic syndrome
Abbreviations: CT, Computed Tomography; DVT, Deep vein thrombosis
1. Introduction
Thymic tumors originate from the thymus gland located in the anterior mediastinum in 90% of cases and the remainder in other areas of the mediastinum, including the neck and the heart [1]. Thymic tumors account for 30% of all mediastinal neoplasms in adults with an incidence of 1.5 per million person-years. Thymic carcinoma incidence is 0.2–0.5 per million [2]. Up to 30% percent of thymomas are found as an incidental finding on radiologic imaging without symptoms [3].
In symptomatic patients, the symptoms can relate to direct mediastinal involvement or a paraneoplastic syndrome. Thirty percent of thymomas have symptoms related to direct mediastinal involvement include thoracic outlet syndrome, cough, dyspnea, neck mass, chest pain, arm or facial swelling [3]. Paraneoplastic syndromes may cause muscle weakness, ptosis, diplopia, dysphagia, dizziness, frequent infections, fatigue, and anemia. Superior vena cava syndrome, phrenic nerve involvement causing hemidiaphragm paralysis, and recurrent laryngeal nerve infiltration causing hoarseness to appear if the tumor invades the surrounding tissue [4].
Though not all thymic tumors are malignant, they are notorious for their association with paraneoplastic syndromes [5]. The most well-studied paraneoplastic syndrome is myasthenia gravis and occurs in approximately 30% of patients with thymomas [3].
There are paraneoplastic syndromes related to connective tissue diseases like polymyositis, rheumatoid arthritis, scleroderma, systemic lupus erythematosus. Nervous system paraneoplastic syndromes include limbic encephalopathy, myasthenia gravis, myotonic dystrophy, Eaton-Lambert syndrome, and sensorimotor radiculopathy. Hematologic paraneoplastic syndromes are agranulocytosis, hemolytic anemia, pernicious anemia, pure red cell aplasia, and hypogammaglobulinemia. Specific organs are involved with autoimmune diseases like acute pericarditis, Addison's disease, alopecia areata, Cushing's syndrome, myocarditis, nephrotic syndrome, pernicious anemia, thyroiditis, hyperparathyroidism, ulcerative colitis, pemphigus, chronic mucocutaneous candidiasis, and sarcoidosis [6].
2. Case presentation
The patient is a 38-year-old African American male with a past medical history of essential hypertension, asthma, and 25 pack-years of tobacco use. He initially presented to the Emergency Department with three days of left upper extremity pain, which was exacerbated by movement. He denied trauma. The patient reported that he had a similar episode in the right upper extremity in the recent past that resolved spontaneously.
The Emergency Department evaluation revealed a blood pressure of 126/93 mmHg, pulse of 77 beats per minute, respiratory rate of 18 breaths per minute, peripheral oxygen saturation of 100% on room air. The physical exam showed left arm tenderness exacerbated by movement. There were no abrasions, bruising, decreased range of motion, deformity, erythema, swelling, and tenderness. The remaining physical exam was unremarkable. There was no additional diagnostic workup during this visit. The patient was diagnosed with cervical radiculopathy and received ketorolac 60 mg intramuscularly and orphenadrine citrate 60 mg intramuscularly. He was discharged with a prescription of naproxen 500 mg by mouth one tablet every 6 hours and methocarbamol 500 mg two tablets by mouth every 6 hours. The patient was advised to follow up with a primary care physician if the symptoms persisted.
One week later, the patient returned to the Emergency Department due to persistent and progressively worsening symptoms of increased pain in the left upper extremity, particularly in the left biceps and dorsal aspect of the forearm, exacerbated with movement with edema, erythema, and numbness in the same distribution. There was a new onset of moderate pleuritic chest pain. Vital signs revealed a blood pressure 137/86 mmHg, a pulse of 76 beats per minute, a respiratory rate of 18 breaths per minute, temperature 36.8 °C (98.2 °F), and peripheral oxygen saturation of 100% on room air. At the time of presentation, the physical exam findings were remarkable for tenderness, increased warmth, and diffuse swelling of the left upper extremity.
Laboratories included a complete blood count which showed white blood cell count of 10,400 cells/cmm, lymphocytes 27.8%, and neutrophils 65.2%, red blood cell 4.75 mil/cmm, Hgb 14.6 g/dL, hematocrit 43.3 g/dL, mean corpuscular volume 91.2 fl, platelet 258 k/cmm. The complete metabolic panel demonstrated sodium 136 mmol/L, potassium 4.0 mmol/L, bicarbonate 27 mmol/L, blood urea nitrogen 17 mg/dL, creatinine 1.1 mg/dL, aspartate aminotransferase 18 U/L, alanine aminotransferase 19 U/L, alkaline phosphatase 64 and U/L, bilirubin total 0.9 mg/dL.
A left upper extremity Doppler was performed in the Emergency Department and confirmed deep vein thrombosis extending from the subclavian to the axillary vein and into the left brachial vein. The patient treatment consisted of enoxaparin 1mg/kg subcutaneously every 12 h with admission to the general medical floor for upper extremity deep vein thrombosis.
Upon further questioning, the patient reported dyspnea on exertion, facial flushing, night sweats, and intermittent blurry vision for a month. The physical exam demonstrated 3+ pulses at the neck, lower and upper extremities bilaterally. There were no palpable masses or lymph nodes, facial swelling, increased jugular venous distention, distention of chest wall veins, or focal motor deficits.
Due to the extensive nature of the upper extremity thrombosis and the patient's young age, a comprehensive workup was performed, which included a computerized tomography of the chest, abdomen, pelvis, bilateral upper extremity venous doppler, and hypercoagulation profile. Hypercoagulable profile yielded negative results for factor V Leiden, protein C and S studies, and lupus anticoagulant. The antinuclear antibodies were negative with complement C4 serum of 20 mg/mL; the anticardiolipin antibodies were negative (IgG <9 U/mL, IGM <9 U/mL, IGA <9 U/mL).
The CT of the chest with and without contrast showed a large 8.3 cm × 6.1 cm x 9.9 cm mediastinal mass with compression of the left brachiocephalic vein and superior vena cava. Additionally, the CT of the chest displayed an extension of thrombus into the left internal jugular vein. A bilateral upper extremity venous doppler showed multiple occlusive venous thrombi in both upper extremities, including the jugular and subclavian veins. On the left side, there was occlusion of other veins, including the axillary, cephalic, brachial, and median cubital veins. Computerized tomography of the abdomen and pelvis with oral and intravenous contrast was unremarkable.
After the above findings, an additional workup while in the hospital revealed a thyroid-stimulating hormone 1.07 mU/mL, C-reactive protein 0.84 mg/dL, erythrocyte sedimentation rate 9 mm/hr, homocysteine 11.1 μmol/L, complement C3 serum 137 mg/dL, Anti-DNA 2 IU/mL, Antichromatin antibody <0.2 AI, Factor VII assay 91%activity, Hemoglobin's solubility test negative for sickle cell anemia, Alpha-fetoprotein tumor marker 3.5 ng/mL, Human chorionic gonadotropin 0.23mUnit/ml and a positive acetylcholine receptor antibody 1.31 nmol/L. A urine drug screen was positive for marijuana.
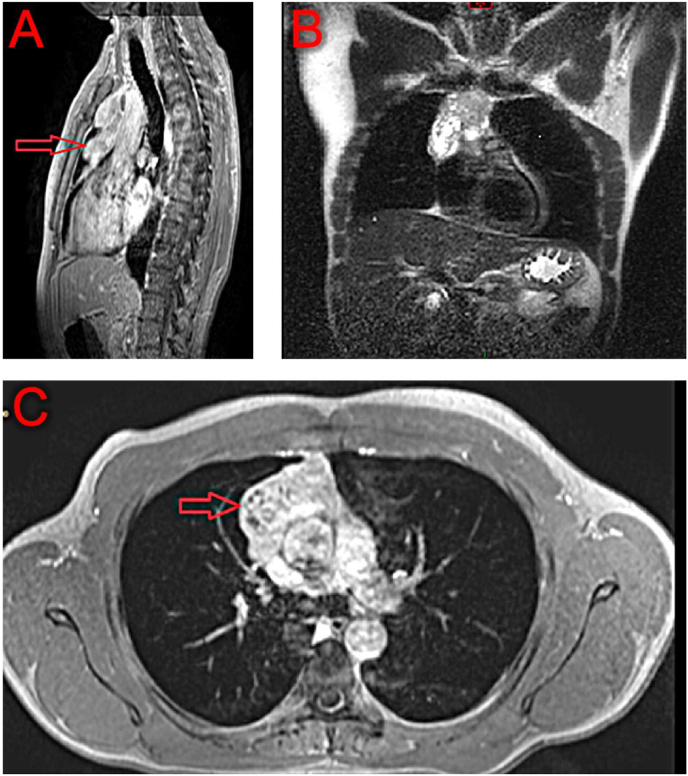
We performed magnetic resonance imaging of the thorax to define the anterior mediastinal mass and its involvement of the mediastinal blood vessels. The magnetic resonance imaging (Fig. 5, Fig. 6) confirmed an anterior mediastinal mass, with mass effect on the superior vena cava, and marked mass effect on the distal left brachiocephalic vein.
Fig. 5.
MRI of the chest with red arrows pointing to the thymoma. (A) Sagittal view, (B) Coronal view. (C) Inferior transverse view. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article).
Fig. 6.
Simplified illustration of the mediastinum, thymus, and great vessels associated with thymoma. (A) Anterior (coronal) view. The thymus gland is extending to the IVC and innominate (brachiocephalic) vein. (B) Inferior (transverse) view. Thymoma burden in the anterior mediastinum upon the IVC. (C) Lateral (midsagittal) view of the mediastinum. Thymus/thymoma extrinsically compressing the innominate (brachiocephalic) vein. Helms, Jessica L. Compartments of the Mediastinum and Thymoma. 2019, Private Collection, Victoria, TX. Digital Illustration.
Post-Contrast sagittal images exhibited some irregularity between the interface of the mass and the superior vena cava, suggestive of adherence to the outer aspect of the vessel adventitia. Early invasion of the mass into the blood vessels was possible but not visualized.
Interventional radiology performed a percutaneous core CT guided biopsy of the mediastinal mass. Pathology revealed a predominant T-cell population with very few B cells. The T cells have variable expression of CD3, bright CD45, CD1a, cCD3, and a subset of double CD4/CD8 positive group, and uniformly positivity on CD5 and CD7(Fig. 1, Fig. 2, Fig. 3, Fig. 4). The immunophenotypic features were favorable for Type B2 thymoma with imaging suggestive of a Masaoka-Koga Stage IIb to III [7,8]. Systemic corticosteroid pulse therapy started with dexamethasone 10 mg IV loading dose followed by dexamethasone 4 mg IV every 6 hours as anticipated preoperative induction therapy.
Fig. 1.
H&E. Dense Iymphoid tissue with larger epithelioid cells in nests and single cells.
Fig. 2.
CD3 immunostain. Highlight predominant T cells.
Fig. 3.
TdT coexprression in T cells. Consistent with thymic tissue.
Fig. 4.
Pancytokeratin (AE1/AE3) immunostain highlights he background epithelial cells.
The patient was referred to a tertiary oncological medical center for definitive care and discharged on enoxaparin, 1 mg/kilogram, subcutaneously every 12 hours and dexamethasone 4mg orally every 12 hours for three weeks. On follow up outpatient evaluation, after the three weeks of the above-mentioned pharmacological therapy, the patient had no symptoms of deep venous thrombosis including the absence of pain, numbness, and swelling.
3. Discussion
Risk factors for upper extremity DVT may be endogenous or exogenous, requiring different treatment approaches and management. The incidence of DVT has increased due to the implantation of permanent or temporary devices. A retrospective study revealed that nearly seventy percent of upper extremity deep venous thrombosis is due to mechanical injury causing epithelial damage by the implantation of permanent or temporary devices. The list of devices associated with upper extremity deep vein thrombosis includes intravenous catheters, tunneled dialysis catheters, and pacemakers [9]. Other causes for upper extremity DVT are immobilization from a cast or as a result of strenuous exercise [10].
The patient had no other risk factors for upper extremity DVT other than the thymoma. The upper extremity DVT occurs in five to ten percent of all deep venous thrombosis cases. Invasion of the great vessels of the mediastinum can cause a vascular obstruction that alters the natural flow of blood to the right atrium. The superior vena cava carries venous return from the brain and upper extremities and enters the thoracic outlet directly into the superior mediastinum. The occurrence of upper extremity deep vein thrombosis otherwise was more frequently induced by acquired solid tumor or mass effect upon vessels [11].
The endogenous behavior of thymic tumors can lead to extrinsic compression of the innominate vein causing thrombus formation [12]. Reduced blood flow, increase in venous pressure, and disorders related to increased blood viscosity all contribute to thrombosis formation [21]. Presentation of bilateral upper extremity DVT in the absence of known risk factors is exceedingly rare, according to our investigation (Table 1). The probable mechanism of deep vein thrombosis was secondary to the tumor burden.
Table 1.
Cases of thymoma causing deep vein thrombosis.
| Ref. | Age/Sex | Location | Tobacco Smoking |
Presenting Symptoms | Treatment | Outcome |
|---|---|---|---|---|---|---|
| [19] | unknown | Sunderland, UK | no | Anterior neck lump unilateral DVT of the upper extremity | Surgery, Adjuvant Therapy | Recurrence |
| [20] | Middle age male | New Delhi, India | no | Progressive edema of the upper half of the body dyspnea cough weight loss |
Surgery, Adjuvant Therapy | Remission |
| [4] | 68-year-old male | Bucharest, Romania | Ex-smoker 20 packs/year | Pain in the upper abdomen | Surgery | Remission |
The case reported here presented with a myriad of clinical manifestations with an anterior mediastinal mass effect, and bilateral upper extremity deep vein thrombosis leading to the final diagnosis of thymoma. Bilateral acute extensive deep vein thrombosis at the second emergency visit directed us to the exact etiology and source of the symptoms. Whether or not the extensive bilateral deep vein thrombosis was a direct consequence of the flow obstruction by the thymoma itself, or an additional paraneoplastic syndrome intrinsically to the thymoma, is a matter of debate. We found three other cases with associated unilateral deep vein thrombosis.
Thymoma diagnosis can present in three different scenarios: diagnosis incidentally on imaging, evaluation for insidious symptoms related to mass effect of the tumor, or evaluation of insidious symptoms related to the paraneoplastic syndrome. Anterior mediastinal tumors frequently present with a grossly abnormal plain chest radiograph or chest tomography performed for an unrelated original indication.
The access, availability, improved resolution, and routine use of radiographic studies have resulted in the increased discovery of the tumors, which may have vague symptoms initially. Computed tomography (CT) use during Emergency Department visits increased by almost 60% from 2005 to 2013 [14]. Thus, the increased incidence of thymoma seen in the United States is likely diagnosis biased by these factors and most likely only applies to developed countries.
Alternatively, symptomatic thymoma results in further evaluation. The mass effect upon the neighboring structures and sub partial occlusion of the superior vena cava can be the lead, but can also be misleading due to the many differentials. More rarely, acute deep vein thrombosis can be the primary cause of obstruction of the superior vena cava [15]. Finally, the association of signs and symptoms related to the multiple paraneoplastic syndromes associated with thymoma may result in further investigation and eventual diagnosis; Myasthenia gravis, acquired hypogammaglobulinemia, and pure red cell aplasia are among the three most commonly associated with thymoma. Thymoma and myasthenia gravis demonstrate a similar epidemiologic pattern in the African American population. Thymoma and myasthenia gravis have a higher incidence in African Americans compared to Caucasians with an onset at earlier ages like this case (Engels. et al.).
The thymus gland is an organ of the immune system located in the anterior mediastinum. It plays a role in the endocrine system producing hormones that promote growth and maturation, but its primary function is to facilitate T-cell maturation, which in turn creates cell-mediated immunity.
Thymomas are the most common tumor arising from the anterior mediastinum. They originate from the epithelial cells of the thymus with the usual age distribution of 50–80 years as the mean age at diagnosis [2]. It is fundamental to make a distinction between thymoma and thymic carcinoma, as the nomenclature can lead to confusion.
A clear understanding of the morphology, molecular, and phenotypic characteristics of thymomas and thymic carcinomas are essential to direct treatment decisions. Determining the pathologic classification in these highly heterogeneous tumors is a complex process. Advanced techniques such as next-generation sequencing and comparative genomic hybridization have contributed to implicit understanding [16].
Literature supported the use of preoperative steroid pulse therapy in invasive thymoma for a reduction in tumor size, which correlated significantly in CD4/CD8 positive groups. The mechanism for this response is postulated to be a result of the higher expression of glucocorticoid receptors in this subgroup of cells [17]. Treatment of thymoma may include surgical intervention as the mainstay intervention with radiotherapy and systemic chemotherapy as a consolidative approach [18].
4. Conclusions
Thymomas can present as deep vein thrombosis bilaterally by stasis and compression of central mediastinal vessels. Paraneoplastic myasthenia gravis can be absent despite the positive serology of anti-acetylcholine receptors antibodies in thymomas. Systemic corticosteroids and full anticoagulation are effective in palliating symptoms until the achievement of definite treatment.
Human subjects declaration
The patient was briefed and signed an informed consent form for publication.
Authors contributions
All authors wrote and edited the paper.
Declaration of competing interest
This letter is to declare that none of the authors of the manuscript titled “Thymoma Causing Bilateral Upper Extremity Deep Vein Thrombosis” have any conflict of interest to disclose, including but not circumscribed, to financial, personal relationships with other people or organizations that could inappropriately influence (bias) our work.
Contributor Information
Hoang Bui, Email: Bui_hoang88@yahoo.com.
Jessica L. Helms, Email: jessicahelms79@gmail.com.
Miguel Sierra-Hoffman, Email: msh.xatracho@gmail.com.
Mark L. Stevens, Email: markmd205@gmail.com.
Rafael Deliz-Aguirre, Email: rafael@delizaguirre.com.
Miriams T. Castro-Lainez, Email: mtcastrolainez@gmail.com.
Rafael J. Deliz, Email: rdeliz@uiwtx.edu.
References
- 1.Kazemi S., Kress D.C., Gal R.A., Gupta A. A rare case of intracardiac thymoma. Echocardiography. 2006 Apr;23(4):348–349. doi: 10.1111/j.1540-8175.2006.00217.x. Available from. [DOI] [PubMed] [Google Scholar]
- 2.Engels E.A. Epidemiology of thymoma and associated malignancies. J Thorac Oncol. 2010 Oct;5(10 Suppl 4) doi: 10.1097/JTO.0b013e3181f1f62d. S260–5. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engels E.A., Pfeiffer R.M. Malignant thymoma in the United States: demographic patterns in incidence and associations with subsequent malignancies. Int J Cancer. 2003 Jul 1;105(4):546–551. doi: 10.1002/ijc.11099. Available from. [DOI] [PubMed] [Google Scholar]
- 4.Berbecar V.T., Jurubita R., Paraschiv M., Obrisca B., Sorohan B., Ismail G. Inferior vena cava and renal vein thrombosis associated with thymic carcinoma. Case Rep Med. 2017 Jan 10;2017 doi: 10.1155/2017/1793952. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girard N., Ruffini E., Marx A., Faivre-Finn C., Peters S. ESMO Guidelines Committee. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015 Sep;26(Suppl 5) doi: 10.1093/annonc/mdv277. v40–55. Available from. [DOI] [PubMed] [Google Scholar]
- 6.DeVita V.T., Rosenberg S.A., Lawrence T.S. Lippincott Williams & Wilkins; 2018. DeVita, Hellman, and Rosenberg's Cancer.https://play.google.com/store/books/details?id=QrN7DwAAQBAJ 2432 pp. Available from. [Google Scholar]
- 7.Marx A., Ströbel P., Badve S.S., Chalabreysse L., Chan J.K.C., Chen G. ITMIG consensus statement on the use of the WHO histological classification of thymoma and thymic carcinoma: refined definitions, histological criteria, and reporting. J Thorac Oncol. 2014;9(5):596–611. doi: 10.1097/JTO.0000000000000154. https://www.sciencedirect.com/science/article/pii/S1556086415302756 Available from. [DOI] [PubMed] [Google Scholar]
- 8.Detterbeck F.C., Nicholson A.G., Kondo K., Van Schil P., Moran C. The Masaoka-Koga stage classification for thymic malignancies: clarification and definition of terms. J Thorac Oncol. 2011 Jul;6(7 Suppl 3) doi: 10.1097/JTO.0b013e31821e8cff. S1710–6. Available from. [DOI] [PubMed] [Google Scholar]
- 9.ALKindi S.Y., Chai-Adisaksopha C., Cheah M., Linkins L.-A. Management of cancer-associated upper extremity deep vein thrombosis with and without venous catheters at a tertiary care center. Thromb Res. 2018 Jun;166 doi: 10.1016/j.thromres.2018.03.020. 92–5. Available from. [DOI] [PubMed] [Google Scholar]
- 10.Becker F., Robert-Ebadi H. 2014. Upper Extremity Deep Vein Thrombosis. Current Approaches to Deep Vein Thrombosis. pp. 118–34. Available from. [Google Scholar]
- 11.Kucher N. Deep-vein thrombosis of the upper extremities. N Engl J Med. 2011 Mar 3;364(9):861–869. doi: 10.1056/NEJMcp1008740. Available from. [DOI] [PubMed] [Google Scholar]
- 12.Kim H.J., Cho S.Y., Cho W.H., Lee D.H., Lim D.H., Seo P.W. An unusual case of superior vena cava syndrome caused by the intravascular invasion of an invasive thymoma. Tuberc Respir Dis. 2013 Nov;75(5):210–213. doi: 10.4046/trd.2013.75.5.210. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellolio M.F., Heien H.C., Sangaralingham L.R., Jeffery M.M., Campbell R.L., Cabrera D. Increased computed tomography utilization in the emergency department and its association with hospital admission. West J Emerg Med. 2017 Aug;18(5):835–845. doi: 10.5811/westjem.2017.5.34152. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konstantinov I.E., Saxena P., Koniuszko M., Ghosh S., Low V.H.S., Khor T.S. Superior vena cava obstruction by tumour thrombus in invasive thymoma: diagnosis and surgical management. Heart Lung Circ. 2007 Dec;16(6):462–464. doi: 10.1016/j.hlc.2006.08.010. Available from. [DOI] [PubMed] [Google Scholar]
- 16.Kelly R.J. Thymoma versus thymic carcinoma: differences in biology impacting treatment. J Natl Compr Canc Netw. 2013 May 1;11(5):577–583. doi: 10.6004/jnccn.2013.0073. Available from. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi Y., Fujii Y., Yano M., Sasaki H., Yukiue H., Haneda H. Preoperative steroid pulse therapy for invasive thymoma: clinical experience and mechanism of action. Cancer. 2006 May 1;106(9) doi: 10.1002/cncr.21875. 1901–7. Available from. [DOI] [PubMed] [Google Scholar]
- 18.Falkson C.B., Bezjak A., Darling G., Gregg R., Malthaner R., Maziak D.E. The management of thymoma: a systematic review and practice guideline. J Thorac Oncol. 2009 Jul;4(7):911–919. doi: 10.1097/jto.0b013e3181a4b8e0. https://www.ncbi.nlm.nih.gov/pubmed/19557895 Available from. [DOI] [PubMed] [Google Scholar]
- 19.Ball S.L., Cocks H.C. Thymoma complicated by deep vein thrombosis of the arm. BMJ Case Rep. 2015 Dec 21;2015 doi: 10.1136/bcr-2015-213404. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panda P.K., Wig N., Kumar S., Arava S. Invasive thymoma presenting as classic superior vena cava syndrome: a case of venous spread metastasis. BMJ Case Rep. 2016 Oct 26;2016 doi: 10.1136/bcr-2016-217695. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The link between cancer and venous thromboembolism: a review. American Journal of Oncology. 2009;32(4):S3–S7. doi: 10.1097/COC.0b013e3181b01b17. [DOI] [PubMed] [Google Scholar]