Abstract
Sebaceous gland cells (sebocytes) differentiate to intracellularly accumulate lipid droplets – a phenomenon similar to that found in adipocytes. In the present study, we examined whether the regulation of lipogenesis in sebocytes is the same as that in preadipocytes. When sebocytes and preadipocytes, prepared from auricle and subcutaneous adipose tissues from the inguinal region of hamsters, respectively, were treated with a common differentiation inducer, insulin, intracellular lipid-droplet formation and triacyglycerol (TG) production were dose- and time-dependently augmented in both. Insulin increased the production of perilipin, a differentiation marker in both sebocytes and adipocytes. Insulin-like growth factor 1 (IGF-1) augmented the intracellular level of TG in sebocytes and preadipocytes. In addition, the action of 1α,25-dihydroxyvitamin D3 [1,25(OH2)D3] on TG production was the opposite between sebocytes and preadipocytes. Furthermore, 5α-dihydrotestosterone (5α-DHT) augmented the TG level in sebocytes, whereas it did not alter TG production in preadipocytes. Moreover, insulin-augmented TG production in sebocytes was enhanced by IGF-1 and 5α-DHT, while diminished by 1,25(OH2)D3. In preadipocytes, the insulin-augmented production of TG was decreased by IGF-1, 1,25(OH2)D3, and 5α-DHT. These results suggest that sebocytic lipogenesis is partially similar to but substantially different from adipocyte lipogenesis due to the forementioned hormones and growth factors in the skin under physiological conditions.
Keywords: Sebocytes, Lipogenesis, Preadipocytes, Triacyglycerol, Lipid-droplet formation
Abbreviations: TG, triacylglycerol; 5α-DHT, 5α-dihydrotestosterone; 1,25(OH)2D3, 1α,25-dihydroxyvitamin D3; PG, prostaglandin; IGF-1, insulin-like growth factor 1; DMEM/F12, Dulbecco's modified Eagle's medium/Ham's F12 medium; FBS, fetal bovine serum; Dex, dexamethasone; IBMX, 3-isobutyl-1-methyl-xanthine; PPAR, peroxisome proliferation-activating receptor
Highlights
-
•
Insulin and IGF-1 augmented lipogenesis and perilipin production in hamster preadipocytes and sebocytes.
-
•
The action of 1,25(OH2)D3 and 5a-DHT on lipogenesis differed between sebocytes and preadipocytes
-
•
Insulin-augmented sebaceous lipogenesis was enhanced by IGF-1 and 5α-DHT, while diminished by 1,25(OH2)D3.
-
•
In preadipocytes, the insulin-augmented lipogenesis was decreased by IGF-1, 1,25(OH2)D3, and 5α-DHT.
-
•
Sebocytic lipogenesis is partially similar to but substantially different from adipocyte lipogenesis.
1. Introduction
Sebaceous gland cells (sebocytes) are the cellular manufacturer and reservoir of sebum which mainly consists of triacylglycerols (TG). TG is androgen-dependently synthesized and stored in intracellular lipid droplets. The holocrine secretion of sebum plays an important role for maintaining physiological functions by forming a biological barrier in skin [1,2]. In addition to androgens, such as testosterone and 5α-dihydrotestosterone (5α-DHT), sebocytic differentiation with sebum accumulation has been controlled by various physiological factors such as hormones and growth factors. Insulin is a stimulator of lipid-droplet formation in sebaceous glands in vivo and in vitro. In contrast, retinoic acid, epidermal growth factor, and 1α,25-dihydroxyvitamin D3 [1,25(OH)2D3] have been reported to suppress the differentiation of sebocytes in humans and rodents [3]. Furthermore, an excess secretion of sebum has been reported to cause sebaceous-gland disorders such as acne vulgaris and seborrhea, which are the most common skin diseases [4].
Furthermore, adipocytes accumulate abundant TG within intracellular lipid droplets and supply the primary source of energy for other tissues by enzymic hydrolysis of the accumulated TG [5]. As far as the regulation of adipocyte differentiation is concerned, insulin has been reported to be a principal regulator to cause the maturation of preadipocytes [6,7]. In addition, many investigators have reported that the activation of peroxisome proliferation-activating receptors (PPARs) by their ligands, including dietary fatty acids and eicosanoid metabolits, such as prostaglandin J2 (PGJ2) and PGI2, induces adipocyte differentiation in vivo and in vitro [[8], [9], [10]]. In contrast, androgens have been reported to suppress intracellular lipid accumulation in differentiated adipocytes [11]. Furthermore, other endogenous factors, such as epidermal growth factor and 1,25(OH)2D3, have been reported to be involved in the regulation of lipid metabolism in adipocytes [12].
Although lipogenesis regulator findings have been reported in human and murine sebocyte studies [13,14], it is not well understood whether endogenous sebocyte-differentiation regulators similarly influence the lipogenesis of adipocytes in the skin. In the present study, we have investigated the effect of hormones and growth factors on the accumulation of intracellular lipids in the sebocytes and subcutaneous preadipocytes of hamsters, and demonstrated that the regulation of lipogenesis differs between sebocytes and adipocytes.
2. Materials and methods
2.1. Preparation and treatment of hamster sebocytes
Hamster sebocytes (2.35 × 104 cells per cm2) were treated once every three days for up to nine days with or without insulin, 1,25(OH2)D3 (Sigma Chemical, St Louis, MO), human recombinant insulin-like growth factor 1 (IGF-1) (R&D Systems, Minneapolis, MN), and 5α-DHT (Wako Pure Chemical, Osaka, Japan) in Dulbecco's modified Eagle's medium/Ham's F12 (DMEM/F12) (1:1) (Invitrogen, Carlsbad, CA) supplemented with 6% heat-inactivated fetal bovine serum (FBS) (JRH Bioscience, Tokyo, Japan), 2% human serum (ICN Biochemicals, Costa Mesa, CA), and 0.68 mM L-glutame (Invitrogen) as previously described [13]. In this series of experiments, hamster sebocytes were used up to the third passage.
2.2. Preparation and treatment of hamster preadipocytes
Hamster preadipocytes were prepared from subcutaneous adipose tissues from the inguinal region of five-week-old male golden hamsters according to the method of a previous paper [15] with some modifications. Briefly, minced adipose tissues were soaked in a collagenase solution [100 mM HEPES (pH 7.4)/123 mM NaCl/5 mM KCl/1.3 mM CaCl2/5 mM glucose/1 μg/ml bacterial collagenase (Wako Pure Chemical)/1.5% bovine serum albumin] at 37 °C for 45 min. The digested tissues were filtered with a nylon mesh membrane (pore size: 105 μm), and then centrifuged. The precipitated cells were re-suspended in DMEM/F12 supplemented with 10% FBS, 15 mM HEPES (pH 7.4), 14.3 mM NaHCO3, 16.4 μM biotin, 7.7 μM d-panthotenate, 5 mM l-glutamine, 25 mM glucose, 10 units/ml penicillin G, and 5 μg/ml streptomycin sulfate, and then plated into 60 mm culture dishes (3 × 104 cells/cm2). After the confluence, the preadipocytes were treated with dexamethasone (Dex) (1 μM) and 3-isobutyl-1-methyl-xanthine (IBMX) (500 μM) (Sigma Chemical) for 48 h, and then treated every two days for up to ten days with or without insulin, 1,25(OH2)D3, IGF-1, and 5α-DHT in 7% FBS, 15 mM HEPES (pH 7.4), 14.3 mM NaHCO3, 16.4 μM biotin, 7.7 μM d-panthotenate, 5 mM l-glutamine, 25 mM glucose, 10 units/ml penicillin G, and 5 μg/ml streptomycin sulfate. The present study was approved by the Committee of Animal Care and Welfare of Tokyo University of Pharmacy and Life Sciences.
2.3. Oil red O staining
The cultured sebocytes and preadipocytes were stained with 0.3% oil red O (Sigma Chemical) in isopropanol:distilled H2O (3:2, vol:vol) at 37 °C for 15 min and then viewed with a light microscope furnished with a digital camera (Olympus, Tokyo, Japan) as previously described [13].
2.4. Triacylglycerol measurement
The harvested sebocytes and preadipocytes were subjected to TG quantification using Liquitech TG-II (Roche Diagnostics, Tokyo, Japan) as previously described [13]. The amounts of intracellular TG were calculated using an authentic trioleinate-standard solution (0.6 mg/ml). Intracellular DNA content was measured using salmon sperm DNA (6.25–100 μg/ml) and 3,5-diaminobenzoic acid dihydrochloride (Sigma Chemical) as previously described [13].
2.5. Western blotting
Cells were scraped with 1% Nonidant P-40 and 0.1% SDS (Sigma Chemical) in Ca2+- and Mg2+-free phosphate-buffered saline [PBS(−)], and then homogenated by being passed through a 21-gauge needle. The cell homogenate was then centrifuged at 15,000×g for 20 min at 4 °C, and the resultant supernatant was used for Western blotting. The sample was subjected to SDS-polyacrylamide gel electrophoresis with 10% acrylamide gel for perilipin and PPARγ [16,17]. The proteins separated in the gels were then electrotransferred onto nitrocellulose membranes. The membranes were reacted with rabbit anti-(human perilipin)IgG, which was customized by Operon Biotechnologies (Tokyo, Japan) [16], and rabbit anti-(human PPARγ)IgG (H-100) (Santa Cruz Biotechnology, Santa Cruz, CA) [17]. Next, the membranes were complexed with peroxidase-conjugated goat anti-rabbit IgG (Sigma Chemical). The immunoreactive perilipin and PPARγ were then visualized with enhanced chemiluminescence-western blotting detection reagents (GE Healthcare Bio-Sciences, Tokyo, Japan), according to the manufacturer's instructions, using an Image Analyzer LAS-1000 plus (GE Healthcare Bio-Sciences).
2.6. Statistical analysis
Statistical analyses were performed using one-way ANOVA with Dunnett's posthoc test and Tukey posthoc test (for multiple comparisons) and Student's two-tailed, two-sample t-test (for paired comparisons). P-values <0.05 were considered statistically significant.
3. Results and discussion
3.1. Insulin-induced lipid-droplet formation in hamster preadipocytes and sebocytes
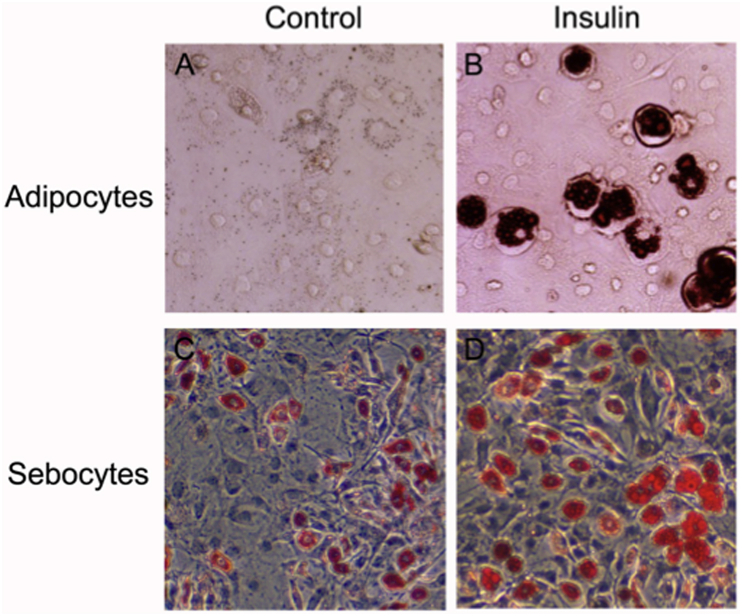
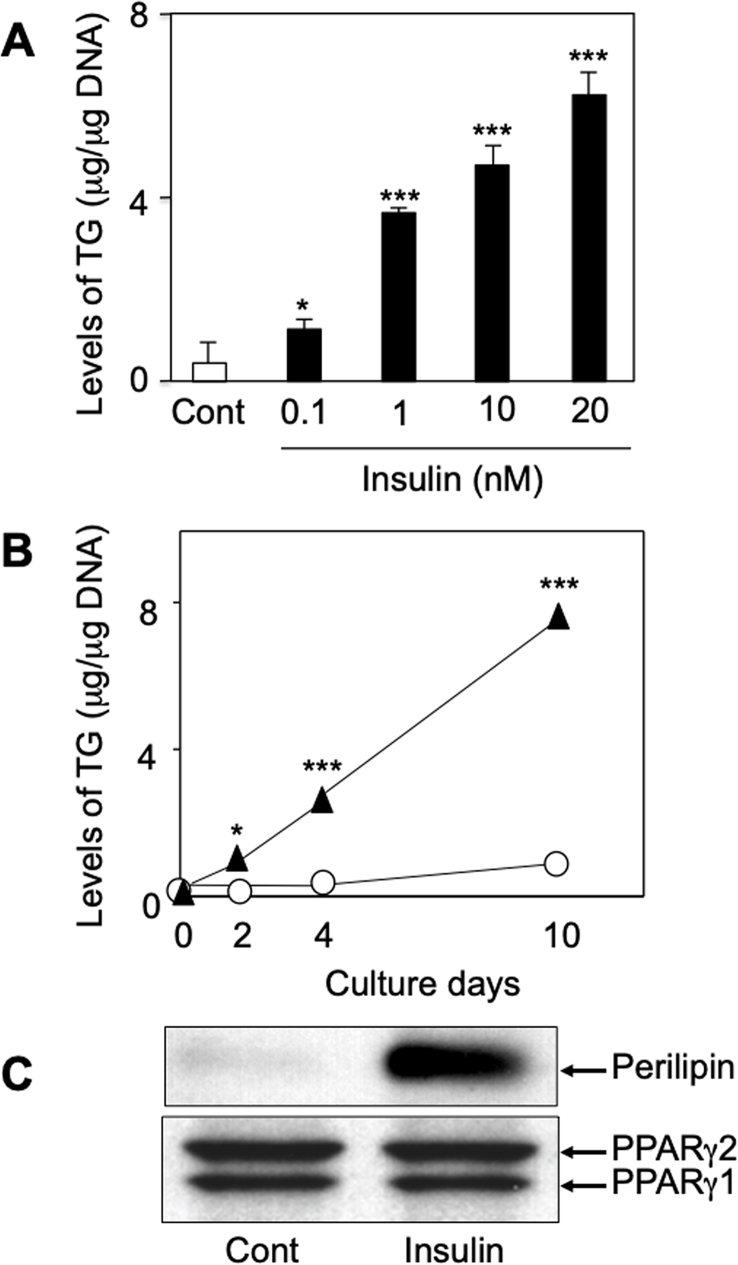
Since insulin is a principal differentiator for both preadipocytes and sebocytes to increase the level of intracellular lipids [3,6,7,13], we first examined the insulin response of the prepared hamster preadipocytes as compared with those of the cultured sebocytes. As shown in Fig. 1, although the lipid-droplet accumulation was negligible in the Dex- and IBMX-treated preadipocytes (panel A), the administration of insulin (20 nM) after the initial stimulation by Dex and IBMX was found to augment the lipid-droplet formation (panel B). In addition, the insulin augmentation was due to the increased level of TG in the hamster preadipocytes, which also occurred dose- and time-dependently (Fig. 2A and B). Furthermore, an adipocyte differentiation marker, perilipin [18], was detected in the insulin-treated preadipocytes but not in the insulin-untreated ones after the initial stimulation with Dex and IBMX (Fig. 2C, upper panel). However, there was no change in the protein levels of PPARγ1 and γ2 under the experimental conditions (Fig. 2C, lower panel). Similar phenomena have been reported in mouse 3T3-L1 preadipocytes [19]. Taken together with the findings of a previous paper [15], hamster preadipocytes are likely to possess the ability of differentiation in response to insulin as in the case of preadipocytes from mice, rats, and humans [3]. Therefore, hamster sebocytes and preadipocytes are likely to be useful to evaluate the regulation of lipogenesis in vitro.
Fig. 1.
Augmentation of intracellular lipid-droplet formation by insulin in preadipocytes and sebocytes from hamsters. Oil-red-O staining shows intracellular lipid-droplet formation in preadipocytes (A and B) and sebocytes (C and D) from hamsters. A and C, untreated cells. B and D, insulin (20 nM)-treated cells. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
Increase of TG, perilipin, and PPARγ production by insulin in hamster preadipocytes. A and B: TG production was increased by insulin (0.1–20 nM) in dose- and time-dependent manners, respectively. C: When hamster preadipocytes, after the initial stimulation with Dex and IBMX were treated every two days for ten days with or without insulin (20 nM), the production of perilipin was augmented by insulin. However, there were no changes in the constitutive expressions of PPARγ1 and PPARγ2 between the insulin-untreated and -treated cells. Three independent experiments were reproducible and typical findings are shown. * and ***, significantly different from untreated cells (Cont) or day 0 (p < 0.05 and 0.001, respectively).
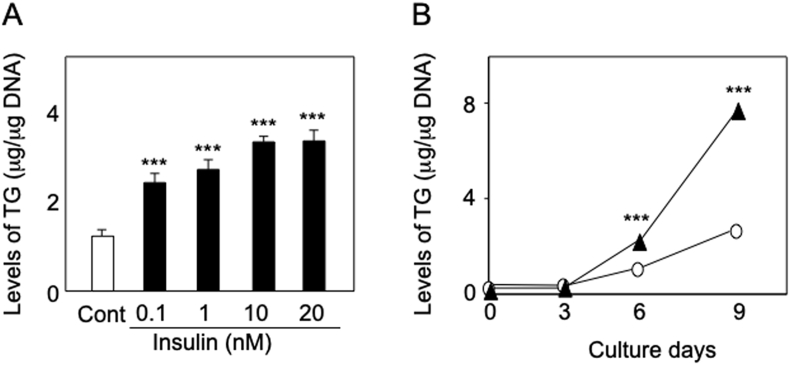
Fig. 1C shows that hamster sebocytes spontaneously differentiated to accumulate intracellular lipid droplets during cultivation similar to the findings of our previous report [13]. In addition, as in the case of rat and human sebocytes [20,21], lipid-droplet accumulation was further augmented by insulin treatment (Fig. 1D). Furthermore, the insulin augmentation of lipid-droplet formation was found to be due to an increase in the level of intracellular TG, which occurred dose- and time-dependently (Fig. 3). Therefore, it is suggested that the insulin-mediated regulation of TG biosynthesis in hamster sebocytes is quite similar to that found in preadipocytes.
Fig. 3.
Upregulation of TG production by insulin in hamster sebocytes. A and B: When hamster sebocytes were treated every three days for nine days with or without insulin (0.1–20 nM), the production of TG was increased in dose- and time-dependent manners, respectively. ***, significantly different from untreated cells (Cont) or day 0 (p < 0.001).
3.2. Regulation of TG production by IGF-1 in hamster preadipocytes and sebocytes
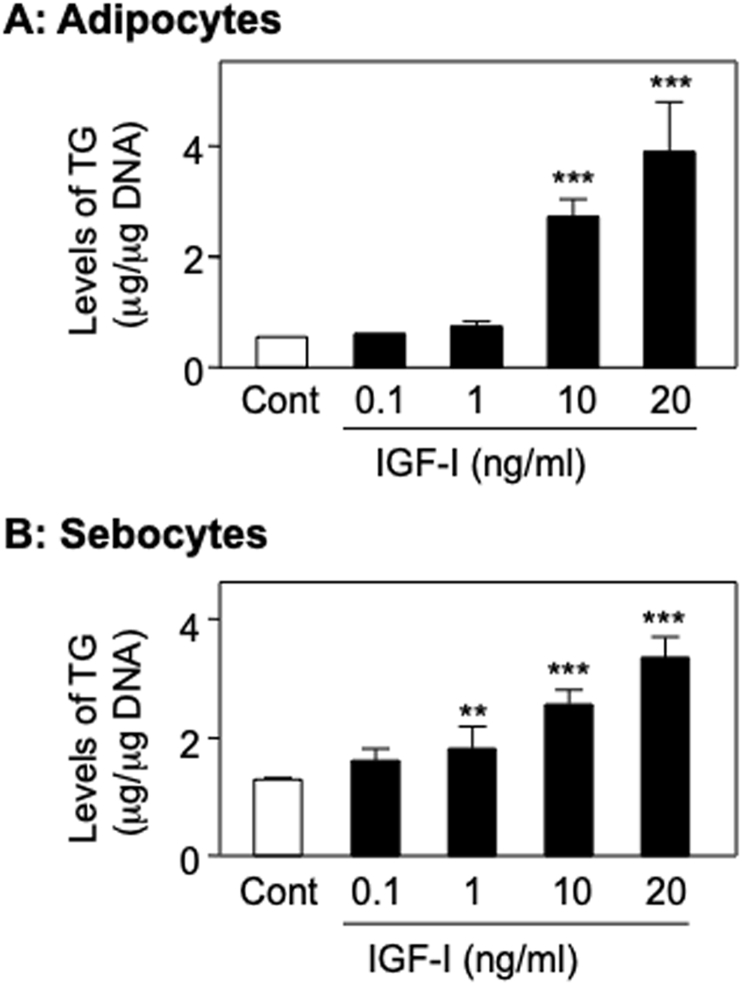
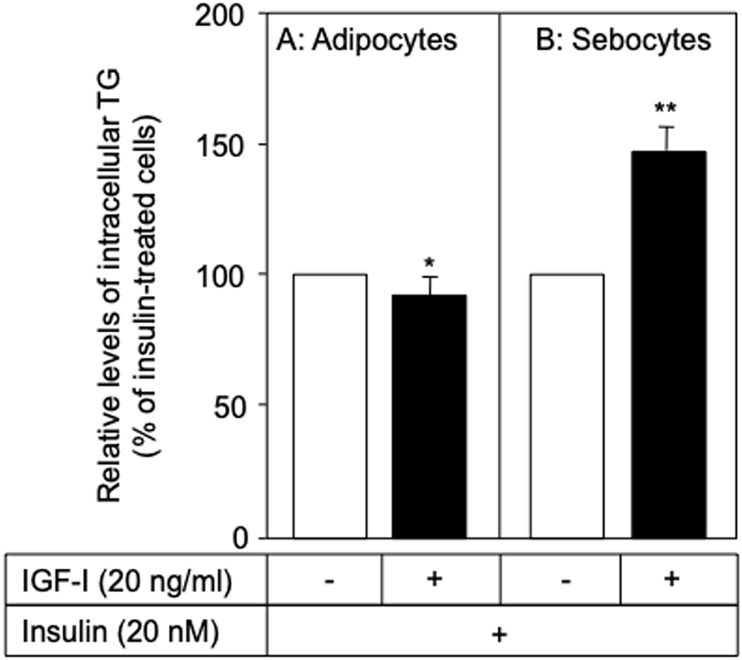
IGF-1 has been reported to cause the differentiation of preadipocytes [7] and an increase in lipid-droplet accumulation in rat and human sebocytes [20,21]. In the present study, IGF-1 (0.1–20 ng/ml) was found to dose-dependently increase the level of intracellular TG in the Dex- and IBMX-treated preadipocytes (Fig. 4A) and hamster sebocytes (Fig. 4B). In addition, as shown in Fig. 5A, IGF-1 (20 ng/ml) slightly decreased the level of TG in the insulin-treated preadipocytes (9.7% inhibition, p < 0.05). In contrast, IGF-1 was found to enhance the production of TG in the insulin-treated sebocytes (1.5-fold, p < 0.001) (Fig. 5B). Therefore, it is suggested that the augmentation of TG production is coordinately regulated by insulin and IGF-1 in sebocytes rather than preadipocytes. This possibility might be supported by previous papers where sebum production is regulated by the signaling via insulin and IGF-1 receptors in the sebaceous glands of rats and humans [22,23].
Fig. 4.
Effect of IGF-1 on the production of TG in hamster preadipocytes and sebocytes. When hamster preadipocytes (A) and sebocytes (B) were treated with IGF-1 (0.1–20 ng/ml) as described in Fig. 2, Fig. 3, TG production increased in a dose-dependent manner. ** and ***, significantly different from untreated cells (Cont) (p < 0.01 and 0.001, respectively).
Fig. 5.
Regulation of TG production by IGF-1 in insulin-differetiated hamster preadipocytes and sebocytes. When hamster preadipocytes (A) and sebocytes (B) were treated with or without IGF-1 (20 ng/ml) in the presence of insulin (20 nM), TG production was enhanced in the sebocytes but slightly decreased in the preadipocytes. * and **, significantly different from the IGF-1-untreated cells (p < 0.05 and 0.01, respectively).
3.3. Effect of 1,25(OH2)D3 on the production of TG in hamster sebocytes and preadipocytes
It has been reported that 1,25(OH2)D3 suppresses lipogensis in the sebocytes of hamsters and humans [13,24]. In addition, Hida et al. [25] have reported that 1,25(OH2)D3 suppresses the differentiation of 3T3-L1 preadipocytes. As such, we examined the effect of 1,25(OH2)D3 on TG production in hamster sebocytes and preadipocytes. As shown in Table 1, 1,25(OH2)D3 was found to decrease intracellular TG levels in hamster sebocytes (72.3% inhibition, p < 0.01). However, TG production was augmented by 1,25(OH2)D3 in the Dex- and IBMX-treated hamster preadipocytes (1.8 fold, p < 0.01), indicating that there is a discrepancy in adipocyte differentiation between mice and hamsters, which may be due to the different culture conditions. On the other hand, as shown in Table 2, 1,25(OH2)D3 was found to suppress TG production in the insulin-differetiated hamster sebocytes (67% inhibition, p < 0.001). Interestingly, 1,25(OH2)D3 inhibited the production of TG in the isulin-treated preadipocytes (33.7% inhibition, p < 0.01). These findings are similar to those of previous reports where 1,25(OH2)D3 was found to suppress lipogenesis in mature adipocytes [25,26]. As such, these results suggest that the physiological actions of 1,25(OH2)D3 in the skin may differ between sebaceous glands and subcutaneous adipose tissues, where lipid metabolism is actively carried out.
Table 1.
Intracellular levels of TG in 1,25(OH)2D3 and 5α-DHT-treated hamster sebocytes and preadipocytes.
| Treatment | Relative level of intracellular TG (% of untreated cells ± SD) |
||
|---|---|---|---|
| Sebocytes | Preadipocytes | ||
| None | 100 | 100 | |
| 1,25(OH)2D3 | 100 nM | 27.7 ± 3.5** | 183.7 ± 63.7** |
| 5α-DHT | 1 μM | 159.1 ± 3.2*** | 111.3 ± 25.9 |
** and ***, significantly different from untreated cells (None) (p < 0.01 and 0.001, respectively).
Table 2.
Effects of 1,25(OH)2D3 and 5α-DHT on TG production in insulin-treated hamster sebocytes and preadipocytes.
| Treatment | Relative levels of intracellular TG (% of control ± SD) |
||
|---|---|---|---|
| Insulin-treated sebocytes | Insulin-treated preadipocytes | ||
| Control | 100 | 100 | |
| 1,25(OH)2D3 | 100 nM | 33.0 ± 1.5*** | 66.3 ± 15.6** |
| 5α-DHT | 1 μM | 173.2 ± 12.4*** | 80.2 ± 19.1* |
*, **, and ***, significantly different from insulin-treated cells (Control) (p < 0.05, 0.01, and 0.001, respectively).
3.4. Effect of 5α-DHT on the production of TG in hamster sebocytes and preadipocytes
Although 5α-DHT augmented the production of TG in sebocytes (1.6 fold, p < 0.001) [13], there was no significant change in the level of TG in the Dex- and IBMX-treated preadipocytes (Table 1). In addition, 5α-DHT was found to enhance the insulin-augmented production of TG in hamster sebocytes (1.7 fold, p < 0.001), whereas it inhibited the level of TG in the insulin-treated preadipocytes (20% inhibition, p < 0.05). Androgens, such as testosterone and 5α-DHT, have been reported to play important roles in the augmentation of lipogenesis and proliferation in sebocytes from humans and rodents [[1], [2], [3]]. In contrast, it has been reported that androgens negatively regulate both differentiation and lipogenesis in adipocytes from humans and mice [[27], [28], [29]]. Taken together, it is strongly suggested that the regulation of lipogenesis by androgen differs between sebocytes and adipocytes.
4. Conclusion
These results suggest that sebocytic lipogenesis is partially similar to but substantially differs from adipocyte lipogenesis due to hormones and growth factors in the skin under physiological conditions. Furthermore, an enhanced understanding of lipogenesis regulation in sebaceous glands and adipose tissues in the skin may provide insights into pathophysiological conditions such as acne and adiposity.
CRediT authorship contribution statement
Takashi Sato: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing - original draft, Writing - review & editing. Fusatoshi Shibata: Data curation, Formal analysis, Investigation, Methodology. Toshikazu Koiwai: Data curation, Formal analysis. Noriko Akimoto: Data curation, Formal analysis, Investigation, Methodology.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported in part by a Grant-in-Aid for Scientific Research (C) (no. 26460633) from the Japan Society for the Promotion of Science (to T. Sato).
References
- 1.Zouboulis C.C., Picardo M., Ju Q., Kurokawa I., Törőcsik D., Bíró T., Schneider M.R. Beyond acne: current aspects of sebaceous gland biology and function. Rev. Endocr. Metab. Disord. 2016;17:319–334. doi: 10.1007/s11154-016-9389-5. [DOI] [PubMed] [Google Scholar]
- 2.Clayton R.W., Göbel K., Niessen C.M., Paus R., van Steensel M.A.M., Lim X. Homeostasis of the sebaceous gland and mechanisms of acne pathogenesis. Br. J. Dermatol. 2019;181:677–690. doi: 10.1111/bjd.17981. [DOI] [PubMed] [Google Scholar]
- 3.Tóth B.I., Oláh A., Szöllosi A.G., Czifra G., Bíró T. Sebocytes' makeup": novel mechanisms and concepts in the physiology of the human sebaceous glands. Pflügers Archiv. 2011;461:593–606. doi: 10.1007/s00424-011-0941-6. [DOI] [PubMed] [Google Scholar]
- 4.Kligman A.M., Wheatley V.R., Mills O.H. Comedogenicity of human sebum. Arch. Dermatol. 1970;102:267–275. [PubMed] [Google Scholar]
- 5.Rogne M., Taskén K. Compartmentalization of cAMP signaling in adipogenesis, lipogenesis, and lipolysis. Horm. Metab. Res. 2014;46:833–840. doi: 10.1055/s-0034-1389955. [DOI] [PubMed] [Google Scholar]
- 6.Bjorntorp P., Karlsson M., Pettersson P., Sypniewska G. Differentiation and function of rat adipocyte precursor cells in primary culture. J. Lipid Res. 1980;21:714–723. [PubMed] [Google Scholar]
- 7.Smith P.J., Wise L.S., Berkowitz R., Wan C., Rubin C.S. Insulin-like growth factor-I is an essential regulator of the differentiation of 3T3-L1 adipocytes. J. Biol. Chem. 1988;263:9402–9408. [PubMed] [Google Scholar]
- 8.Kliewer S.A., Lenhard J.M., Willson T.M., Patel I., Morris D.C., Lehmann J.M. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 9.Sinha D., Addya S., Murer E., Boden G. 15-Deoxy-delta(12,14) prostaglandin J2: a putative endogenous promoter of adipogenesis suppresses the ob gene. Metabolism. 1999;48:786–791. doi: 10.1016/s0026-0495(99)90180-4. [DOI] [PubMed] [Google Scholar]
- 10.Jowsey I.R., Murdock P.R., Moore G.B., Murphy G.J., Smith S.A., Hayes J.D. Prostaglandin D2 synthase enzymes and PPARγ are co-expressed in mouse 3T3-L1 adipocytes and human tissues. Prostag. Other Lipid Mediat. 2003;70:267–284. doi: 10.1016/s0090-6980(02)00134-x. [DOI] [PubMed] [Google Scholar]
- 11.Blouin K., Boivin A., Tchernof A. Androgens and body fat distribution. J. Steroid Biochem. Mol. Biol. 2008;108:272–280. doi: 10.1016/j.jsbmb.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Wood R.J. Vitamin D and adipogenesis: new molecular insights. Nutr. Rev. 2008;66:40–46. doi: 10.1111/j.1753-4887.2007.00004.x. [DOI] [PubMed] [Google Scholar]
- 13.Sato T., Imai N., Akimoto N., Sakiguchi T., Kitamura K., Ito A. Epidermal growth factor and 1α,25-dihydroxyvitamin D3 suppress lipogenesis in hamster sebaceous gland cells in vitro. J. Invest. Dermatol. 2001;117:965–970. doi: 10.1046/j.0022-202x.2001.01516.x. [DOI] [PubMed] [Google Scholar]
- 14.Hinde E., Haslam I.S., Schneider M.R., Langan E.A., Kloepper J.E., Schramm C., Zouboulis C.C., Paus R. A practical guide for the study of human and murine sebaceous glands in situ. Exp. Dermatol. 2013;22:631–637. doi: 10.1111/exd.12207. [DOI] [PubMed] [Google Scholar]
- 15.Klaus S., Cassard-Doulcier A.M., Ricquier D. Development of Phodopus sungorus brown preadipocytes in primary cell culture: effect of an atypical β-adrenergic agonist, insulin, and triiodothyronine on differentiation, mitochondrial development, and expression of the uncoupling protein UCP. J. Cell Biol. 1991;115:1783–1790. doi: 10.1083/jcb.115.6.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato T., Akimoto N., Kitamura K., Kurihara H., Hayashi N., Ito A. Adapalene suppresses sebum accumulation via the inhibition of triacylglycerol biosynthesis and perilipin expression in differentiated hamster sebocytes in vitro. J. Dermatol. Sci. 2013;70:204–210. doi: 10.1016/j.jdermsci.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Akimoto N., Sato T., Iwata C., Koshizuka M., Shibata F., Nagai A., Sumida M., Ito A. Expression of perilipin A on the surface of lipid droplets increases along with the differentiation of hamster sebocytes in vivo and in vitro. J. Invest. Dermatol. 2005;124:1127–1133. doi: 10.1111/j.0022-202X.2005.23718.x. [DOI] [PubMed] [Google Scholar]
- 18.Londos C., Sztalryd C., Tansey J.T., Kimmel A.R. Role of PAT proteins in lipid metabolism. Biochimie. 2005;87:45–49. doi: 10.1016/j.biochi.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Prusty D., Park B.H., Davis K.E., Farmer S.R. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor γ (PPARγ) and C/EBPα gene expression during the differentiation of 3T3-L1 preadipocytes. J. Biol. Chem. 2002;277:46226–46232. doi: 10.1074/jbc.M207776200. [DOI] [PubMed] [Google Scholar]
- 20.Deplewski D., Rosenfield R.L. Growth hormone and insulin-like growth factors have different effects on sebaceous cell growth and differentiation. Endocrinology. 1999;140:4089–4094. doi: 10.1210/endo.140.9.6957. [DOI] [PubMed] [Google Scholar]
- 21.Smith T.M., Cong Z., Gilliland K.L., Clawson G.A., Thiboutot D.M. Insulin-like growth factor-1 induces lipid production in human SEB-1 sebocytes via sterol response element-binding protein-1. J. Invest. Dermatol. 2006;126:1226–1232. doi: 10.1038/sj.jid.5700278. [DOI] [PubMed] [Google Scholar]
- 22.Hansson H.A., Nilsson A., Isgaard J., Billig H., Isaksson O., Skottner A., Andersson I.K., Rozell B. Immunohistochemical localization of insulin-like growth factor I in the adult rat. Histochemistry. 1988;89:403–410. doi: 10.1007/BF00500644. [DOI] [PubMed] [Google Scholar]
- 23.Hodak E., Gottlieb A.B., Anzilotti M., Krueger J.G. The insulin-like growth factor 1 receptor is expressed by epithelial cells with proliferative potential in human epidermis and skin appendages: correlation of increased expression with epidermal hyperplasia. J. Invest. Dermatol. 1996;106:564–570. doi: 10.1111/1523-1747.ep12344044. [DOI] [PubMed] [Google Scholar]
- 24.Krämer C., Seltmann H., Seifert M., Tilgen W., Zouboulis C.C., Reichrath J. Characterization of the vitamin D endocrine system in human sebocytes in vitro. J. Steroid Biochem. Mol. Biol. 2009;113:9–16. doi: 10.1016/j.jsbmb.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Hida Y., Kawada T., Kayahashi S., Ishihara T., Fushiki T. Counteraction of retinoic acid and 1,25-dihydroxyvitamin D3 on up-regulation of adipocyte differentiation with PPARγ ligand, an antidiabetic thiazolidinedione, in 3T3-L1 cells. Life Sci. 1998;62:PL205–211. doi: 10.1016/s0024-3205(98)00059-9. [DOI] [PubMed] [Google Scholar]
- 26.Ishida Y., Taniguchi H., Baba S. Possible involvement of 1α,25-dihydroxyvitamin D3 in proliferation and differentiation of 3T3-L1 cells. Biochem. Biophys. Res. Commun. 1988;151:1122–1127. doi: 10.1016/s0006-291x(88)80482-0. [DOI] [PubMed] [Google Scholar]
- 27.Benvenuti S., Cellai I., Luciani P., Deledda C., Saccardi R., Mazzanti B., Dal Pozzo S., Serio M., Peri A. Androgens and estrogens prevent rosiglitazone-induced adipogenesis in human mesenchymal stem cells. J. Endocrinol. Invest. 2012;35:365–371. doi: 10.3275/7739. [DOI] [PubMed] [Google Scholar]
- 28.Chazenbalk G., Singh P., Irge D., Shah A., Abbott D.H., Dumesic D.A. Androgens inhibit adipogenesis during human adipose stem cell commitment to preadipocyte formation. Steroids. 2013;78:920–926. doi: 10.1016/j.steroids.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang C.K., Lai K.P., Luo J., Tsai M.Y., Kang H.Y., Chen Y., Lee S.O., Chang C. Loss of androgen receptor promotes adipogenesis but suppresses osteogenesis in bone marrow stromal cells. Stem Cell Res. 2013;11:938–950. doi: 10.1016/j.scr.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]