Abstract
Background
Ovarian endodermal sinus tumors (ESTs) are rapidly growing and highly malignant tumors that respond well to chemotherapy. They can be difficult to diagnose and delayed diagnosis can worsen prognosis.
Case
We present the case of a 20-year-old woman with an EST initially misdiagnosed as a tubo-ovarian abscess who then experienced rapid progression within weeks of initial presentation and was subsequently found to have unresectable advanced stage disease.
Conclusion
ESTs are extremely aggressive and require prompt referral and early treatment with chemotherapy. Presenting symptoms of pain and a mass can lead to a broad range of differential diagnoses. In such patients, early consideration of tumor markers is warranted. This case report reviews the key aspects for prompt diagnosis and rapid treatment of these tumors, which significantly impacts the prognosis.
Keywords: Germ cell tumor, Endodermal sinus tumor, Differential, Prognosis, Bleomycin-etoposide-cisplatin chemotherapy, Tubo-ovarian abscess
Highlights
-
•
Delayed diagnosis of endodermal sinus tumors can worsen prognosis.
-
•
Certain radiological signs are specific for endodermal sinus tumors.
-
•
Broad differentials and early tumor markers may decrease time to diagnosis.
1. Introduction
Endodermal sinus tumors (ESTs) account for approximately 20% of malignant germ cell tumors [1]. The median age of onset is 19, and the majority of patients present with a combination of abdominal pain and an abdominal mass [1]. Historically, survival for this this type of ovarian tumor has been low but with the utilization of combination chemotherapy, survival has drastically improved. Modern therapy for ESTs consists of bleomycin, etoposide, and cisplatin (BEP). Negative prognostic factors include unsatisfactory decline in serum alpha-fetoprotein (AFP) with chemotherapy, presence of ascites at diagnosis, the presence of residual tumor after debulking, and increased stage at presentation [[2], [3], [4]]. Positive prognostic factors include use of BEP therapy. Here we present the case of a 20-year-old woman who had significant progression of disease within weeks of presentation, highlighting the need for prompt referral of these patients to allow for immediate intervention with chemotherapy. The patient has consented to the discussion of her case.
2. Case
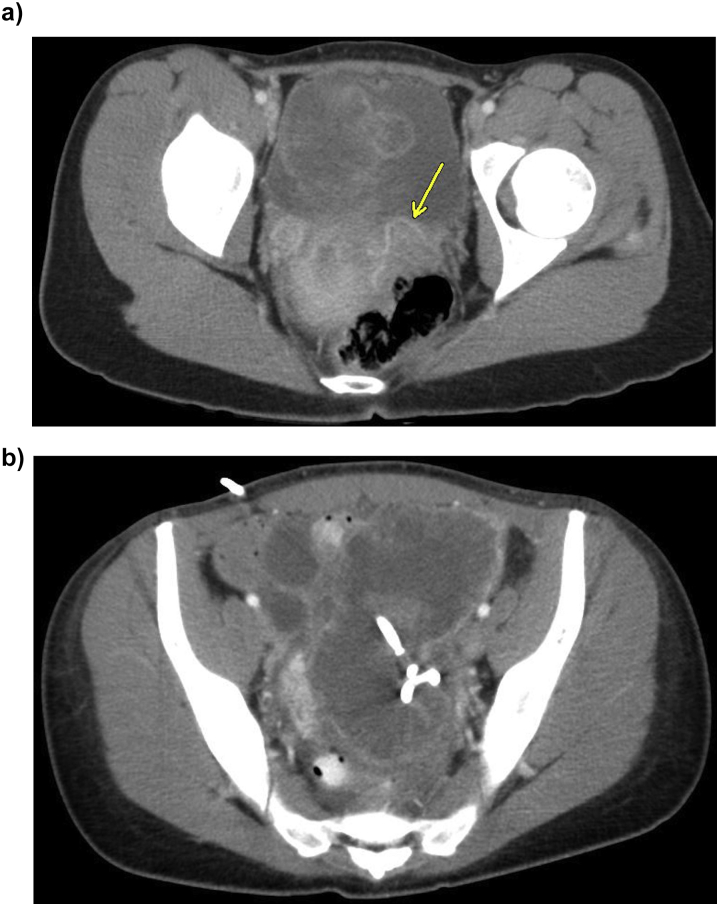
A 20-year-old G1P0010 Caucasian woman with no significant medical history presented to an outside hospital with pelvic pain and fevers to 101.2 °F. Initial imaging demonstrated a 10 cm × 7 cm ovarian mass (Fig. 1a). The patient was treated for a suspected tubo-ovarian abscess (TOA) and was discharged four days after admission on the appropriate antibiotics. During this first admission, the patient never had a leukocytosis (maximum white blood cell count of 11.5 × 109 cells/L) and blood and cervical cultures were negative.
Fig.1.
a. Initial pelvic mass, likely representing primary malignancy, intratumoral vessels visible (arrows). b. Pelvic mass with pigtail drain in place.
The patient presented five days after discharge with worsening pelvic pain, nausea, and fever. Computed tomography (CT) demonstrated a 13 cm × 10.7 cm × 11 cm multi-loculated, cystic pelvic mass which was described as uncharacteristic of a TOA because the cystic structure did not appear to encase the ovary. While the patient had a leukocytosis to 13.4 × 109 cells/L during the admission, blood cultures were negative and no fevers occurred. A pigtail drain was placed and drained approximately two liters of fluid that was negative for malignancy, with only a minimal decrease in mass size (Fig. 1b). Repeat imaging three days after drain insertion showed a mass 11.8 cm × 10.3 cm × 11.2 cm. The patient then underwent a laparoscopic right salpingo-oophorectomy to remove the large cystic mass, suspected to be a ruptured TOA. Pelvic washings were collected and cytology demonstrated inflammatory cells, negative for malignancy, and no frozen section was sent at that time. The patient was discharged on doxycycline and metronidazole.
Pathology from the outside hospital, reported on post-operative day seven, was consistent with a mixed ovarian germ cell tumor with predominant (90%) endodermal sinus tumor characteristics (Fig. 2) and a minor (10%) dysgerminoma component.
Fig. 2.
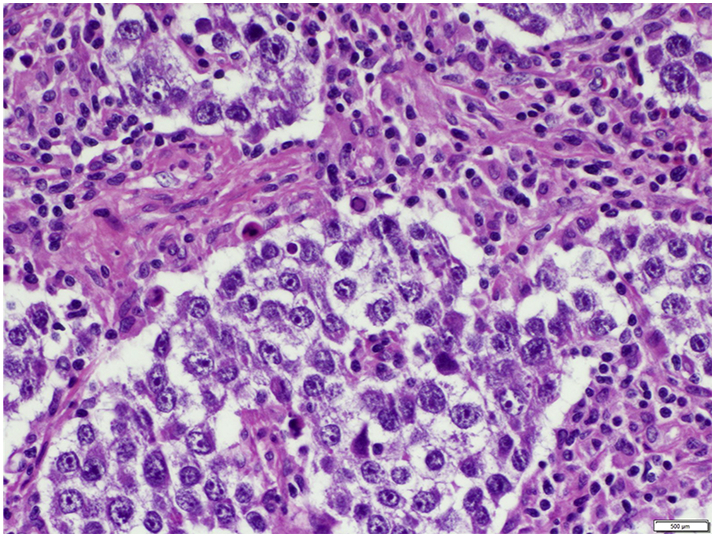
Mixed germ cell tumor, relative proportions determined at the institution the patient was transferred to are 95% EST, 5% dysgerminoma.
On post-operative day nine, the patient was transferred to an urban, academic hospital for pelvic pain and an enlarging palpable abdominal mass. Tumor markers were significant for an AFP of 4155 ng/mL (reference range 0.0–9.0 ng/mL) and the patient underwent an exploratory laparotomy demonstrating hemorrhagic metastasis throughout the abdomen, including encasement of the colon and small bowel. The tumor was unable to be resected completely, though the large abdominal mass was able to be resected. Her uterus had small implants on it and the left ovary was normal. These organs were left in situ. The patient was immediately started on chemotherapy with etoposide and cisplatin on post-operative day two. Bleomycin was held for poor pulmonary function testing in the setting of bilateral pulmonary edema and pleural effusions. The patient's chemotherapeutic regimen is summarized in Table 1. The patient's AFP trended down with each cycle of chemotherapy, as noted in Table 1. She completed 6 cycles of chemotherapy, cycles 4–6 being completed after the normalization of AFP. Prior to cycle 6, she had interval debulking surgery prompted by possible residual pelvic carcinomatosis on imaging which included laparotomy with resection of adhesions, omentectomy, and resection of cul de sac nodules. The pathology of all resected implants revealed no residual disease consistent with a complete pathologic response. Five months after she had completed therapy there was no evidence of disease and a normal AFP (0.9 ng/mL).
Table 1.
Chemotherapeutic regimen with patient's concordant AFP values. BEP chemotherapy is a 21 day chemotherapeutic cycle with etoposide and cisplatin given on days 1–5 (D1–5) and bleomycin given on days 1, 8, and 15 (D1,8,15).
| Chemotherapeutic and Alpha-fetoprotein | Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4a | Cycle 5 | Cycle 6 |
|---|---|---|---|---|---|---|
| Etoposide | 100 mg/m2 IV D1–5 | 100 mg/m2 IV D1–5 | 100 mg/m2 IV D1–5 | 100 mg/m2 IV D1–5 | c75 mg/m2 IV D2–5 | c100 mg/m2 D1–2, 50 mg/m2 D3–4 |
| Cumulative Etoposide Dose | 500 mg/m2 | 1000 mg/m2 | 1500 mg/m2 | 2000 mg/m2 | 2300 mg/m2 | 2600 mg/m2 |
| Bleomycin | b | 30 mg IV D1,8,15 | 30 mg IV D1,8,15 | 30 mg IV D1,8,15 | d | d |
| Cumulative Bleomycin Dose | 90 mg | 180 mg | 270 mg | 270 mg | 270 mg | |
| Cisplatin | 20 mg/m2 IV D1–5 | 20 mg/m2 IV D1–5 | 20 mg/m2 IV D1–5 | 20 mg/m2 IV D1–5 | 20 mg/m2 IV D1–5 | 20 mg/m2 IV D1–5 |
| Alpha-fetoproteine (ng/mL) |
2481.5 | 382 | 35.2 | 8.2 | 2.1 | 1 |
Normalization of AFP.
Bleomycin held for DLCO 46% and bilateral pulmonary edema and effusions.
Etoposide dose reduced for febrile neutropenia with cycle 4.
Bleomycin held for 14% DCLO reduction from 83% to 69%.
Alpha-fetoprotein reference range (0.0–9.0 ng/mL).
3. Discussion
Endodermal sinus tumors are rare and highly malignant ovarian germ cell tumors. It is often agreed among authors in the field that these tumors portend the worst prognosis among germ cell tumors [2,5]. However, their survival rates have drastically improved since the advent of multi-agent BEP chemotherapy [6]. An analysis of 52 patients with ESTs who were treated with BEP therapy had an overall survival and event-free survival of 94% and 90%, respectively [2]. However, there are prognostic factors which contribute to negative outcomes, even with chemotherapy, and several of these factors were present in this case. At the time of cytoreductive surgery, she had International Federation of Gynecology and Obstetrics (FIGO) stage IIIC disease, with macroscopic peritoneal metastasis greater than 2 cm throughout the abdomen. Her debulking was suboptimal, with gross tumor still remaining post-operatively. She additionally exhibited ascites and was unable to receive bleomycin during her first cycle of chemotherapy due to poor baseline pulmonary function testing (attributed to pain from her laparotomy).
At the patient's initial presentation at the outside hospital, she did not have ascites, had no indication of worsening pulmonary function, and had only one pelvic mass seen on imaging - likely representing her primary tumor. Ideally, resection at this time may have resulted in early diagnosis of her malignancy, an earlier stage disease, prompter initiation of chemotherapy and subsequently a better prognosis [2,6]. While there are many challenges to diagnosing ESTs, we see multiple opportunities to increasing the likelihood of diagnosing these uncommon tumors early, when prognosis is very favorable.
First, maintaining a broad differential diagnoses could aid in the early diagnosis of this malignancy. This can be challenging since these tumors predominantly affect women of childbearing age where the differential diagnosis for pelvic pain is vast, including gynecologic, obstetric, gastrointestinal, and urinary etiologies. Interestingly, malignancy is not always listed as part of differential diagnoses in review articles for acute pelvic pain - even while the primary symptom for germ cell tumors is often acute to sub-acute pelvic pain [1]. Therefore, a higher index of suspicion for a malignancy is warranted, including early consideration for tumor markers in patients with a pelvic mass and pain. This should be followed by prompt (within days) referral to a specialist in gynecological oncology if tumor markers are elevated and concerning for an endodermal sinus tumor.
While pelvic masses can be difficult to characterize on imaging, research has demonstrated certain features that are commonly found in ESTs. More common characteristics of ESTs include a mixed solid-cystic appearance upon imaging, hemorrhage and marked enhancement [7]. Most sensitive and specific for EST was intramural vessel enhancement (arrows, Fig. 1a, Fig. 3) [7]. This sign has been called by one research group the “bright dot sign” and it is believed to be caused by dilated vessels, highlighting the vascularity of this tumor. With these characteristics of ESTs in mind, it is possible the diagnosis of EST can be considered early on.
Fig. 3.
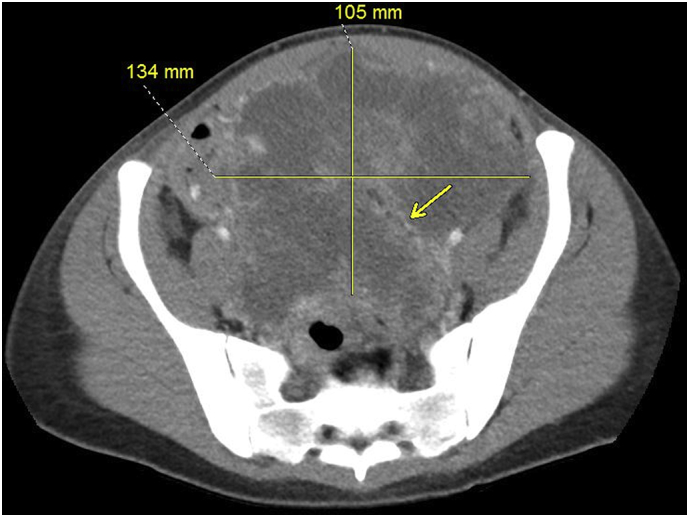
Pelvic mass on arrival to our institution, likely represents metastasis, dimensions present, intratumoral vessels visible (arrows).
Treatment of ESTs includes chemotherapy at all stages and oftentimes surgery. BEP chemotherapy is first line in the treatment of ESTs and is indicated for all stages of EST. [2] It is critical to initiate therapy early in disease process as prognosis worsens with increasing stage of this rapidly growing tumor [2]. While overall survival and disease-free survival have improved with this use of BEP chemotherapy, there are adverse effects of this chemotherapy regimen. Bleomycin is associated with pulmonary toxicity, the most morbid toxicity being pulmonary fibrosis, and a maximum dose of less 400 mg is recommended [8,9]. Pulmonary toxicity is monitored with PFTs prior to initiation of chemotherapy and each subsequent cycle, and evaluation for signs and symptoms of dyspnea. Chest x-rays and high resolution CT can be used for further evaluation for pulmonary toxicities [8]. Etoposide carries the risk of secondary malignancies, particularly leukemias, and a lifetime dose of less than 2000 mg/m2 is recommended. Chemotherapy is recommended for three cycles for patient with favorable prognostic factors (early stage, optimal debulking) and four cycles for unfavorable prognostic factors (advanced stage, suboptimal debulking) and it was the practice of the academic hospital at which the patient was treated to receive one to two additional cycles of BEP therapy after the normalization of tumor markers, as this patient received [10]. It is not recommended to delay therapy for neutropenia; however, dose reductions can be considered (this patient's etoposide dose was decreased due to febrile neutropenia with cycle 4). However, withholding bleomycin for concerning pulmonary function is commonly practiced [8].
Surgical management for unilateral endodermal sinus tumors in patients desiring fertility preservation includes laparotomy with unilateral salpingo-oophorectomy (USO), appropriate staging including staging biopsies, omentectomy and lymphadenopathy, and debulking as indicated [5]. Bilateral tumors are rare. Therefore, fertility conservation with a USO is commonly performed, even at advanced stages of disease [5]. Second look laparotomies are not indicated and laparoscopy is ineffective in dealing with often large endodermal sinus tumors [5]. A second surgery was performed for this patient as imaging was consistent with residual pelvic disease; however, the patient was found to have a complete pathologic response to chemotherapy. Her uterus and residual tube and ovary were free of disease and left in situ for future fertility potential.
Overall, the patient clinically improved with cytoreductive surgery and BEP chemotherapy. While bleomycin was held for the first cycle of chemotherapy for poor pulmonary function (diffusion capacity of carbon monoxide (DLCO) = 46%), it was added to each subsequent cycle after much improved pulmonary function (DLCO = 68%). The patient's AFP steadily declined with treatment; however, time to normalization was not optimal. Time to normalization was 62 days. At 45 days into chemotherapy, AFP was still elevated at 35.2 ng/mL. Time to normalization of AFP helps determine prognosis. An AFP still elevated at 42 days or more after initiation of chemotherapy is considered a poor prognostic factor [11]. Reassuringly, the patient's AFP ultimately did normalize with cycle 4 of chemotherapy and has remained normal since. Ultimately, while the patient is currently without evidence of disease, she still had negative prognostic factors and close surveillance is warranted.
Strengths in treating this case included rapidly initiating first-line therapy for ESTs. This was facilitated by the academic institution having the histological diagnosis of the patient's condition at the time of transfer. A primary limitation to appropriate treatment of ESTs is rapid diagnosis. While the patient did receive a modified treatment regimen in the setting of expected side effects, dose adjustments were based upon stand-of-care practice and, in the setting of these adjustments, the patient is doing well.
In conclusion, ovarian endodermal sinus tumors are aggressive tumors that largely affect young women of reproductive age. Timely diagnosis is crucial for improved prognosis in these patients. While diagnosis can be challenging with an expansive differential for pelvic pain in young women and few specific signs and symptoms, a better understanding of radiographical findings of endodermal sinus tumors and a lower threshold to consider obtaining tumor markers early in evaluation of young women with pain and a pelvic mass could increase the likelihood of correct diagnosis. Early referral to gynecological oncologists is also important so that appropriate surgical and chemotherapeutic treatment may begin.
Acknowledgments
Contributors
All authors made substantial contributions to the conception and design of the study, analysis and interpretation of data, and drafting/revising of the article for important intellectual content. All authors approved the final version of the manuscript being submitted.
Conflict of interest
The authors declare that they have no conflict of interest regarding the publication of this case report.
Funding
No funding from an external source supported the publication of this case report.
Patient consent
Obtained.
Provenance and peer review
This case report was peer reviewed.
Acknowledgements
We would like to thank the patient for her consent in using her case to discuss the difficulties surrounding the diagnosis of endodermal sinus tumors. We also thank Dr. Ady Kendler, Division of Pathology at the University of Cincinnati Medical Center, for his contribution of histology images. Finally, we thank Dr. Tyler McCurdy, Division of Radiology at the University of Cincinnati Medical Center, for his contribution of computed tomography images.
References
- 1.Bidus M.A., Zahn C.M., Rose G.S. Germ cell, stromal, and other ovarian tumors. In: Elsevier, editor. Clin. Gynecol. Oncol. 7th ed. Moby Elsevier; Philadelphia: 2007. pp. 369–395. [Google Scholar]
- 2.De La Motte Rouge T., Pautier P., Duvillard P., Rey A., Morice P., Haie-Meder C., Kerbrat P., Culine S., Troalen F., Lhommé C. Survival and reproductive function of 52 women treated with surgery and bleomycin, etoposide, cisplatin (BEP) chemotherapy for ovarian yolk sac tumor. Ann. Oncol. 2008;19:1435–1441. doi: 10.1093/annonc/mdn162. [DOI] [PubMed] [Google Scholar]
- 3.Nawa A., Obata N., Kikkawa F., Kawai M., Nagasaka T., Goto S., Nichimori K., Nakashima N. Prognostic factors of patients with yolk sac tumors of the ovary. Am. J. Obstet. Gynecol. 2001;184:1182–1188. doi: 10.1067/mob.2001.113323. [DOI] [PubMed] [Google Scholar]
- 4.Wang X., Ma Z., Li Y. Ovarian yolk sac tumor. Int. J. Gynecol. Cancer. 2016;26:884–891. doi: 10.1097/IGC.0000000000000704. [DOI] [PubMed] [Google Scholar]
- 5.Dällenbach P., Bonnefoi H., Pelte M.-F., Vlastos G. 2006. Yolk Sac Tumours of the Ovary: An Update. [DOI] [PubMed] [Google Scholar]
- 6.de Wit R., Stoter G., Kaye S.B., Sleijfer D.T., Jones W.G., ten Bokkel Huinink W.W., Rea L. a, Collette L., Sylvester R. Importance of bleomycin in combination chemotherapy for good-prognosis testicular nonseminoma: a randomized study of the European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group. J. Clin. Oncol. 1997;15:1837–1843. doi: 10.1200/JCO.1997.15.5.1837. [DOI] [PubMed] [Google Scholar]
- 7.Choi H.J., Moon M.H., Kim S.H., Cho J.Y., Jung D.C., Hong S.R. Yolk sac tumor of the ovary: CT findings. Abdom. Imaging. 2008;33:736–739. doi: 10.1007/s00261-007-9355-5. [DOI] [PubMed] [Google Scholar]
- 8.Roncolato F.T., Chatfield M., Houghton B., Toner G., Stockler M., Thomson D., Friedlander M., Gurney H., Rosenthal M., Grimison P. The effect of pulmonary function testing on bleomycin dosing in germ cell tumours. Intern. Med. J. 2016;46:893–898. doi: 10.1111/imj.13158. [DOI] [PubMed] [Google Scholar]
- 9.Blum R.H., Carter S.K., Agre K. A clinical review of bleomycin--a new antineoplastic agent. Cancer. 1973;31:903–914. doi: 10.1002/1097-0142(197304)31:4<903::aid-cncr2820310422>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 10.Plaxe S.C., Chair V., Alvarez R.D., Bakkum-Gamez J.N., Barroilhet L., Behbakht K., DeRosa M., ElNaggar A.C., Gershenson D.M., Hughes N. Jennifer Burns Miranda, Gray H.J., Hakam A., Johnston C., Jones M.B., Leath C.A., III, Lele S., Martin L., Matulonis U.A., R.T. D.M.O., Penson M,.M.A., Powell E., Ratner S.W. Remmenga, Rose P.G., Sabbatini P., Shahabi S., Lurie H. Robert, Werner T.L. NCCN Guidelines Version 2.2018 Ovarian Cancer NCCN Evidence Blocks TM NCCN Guidelines Index Table of Contents Discussion. 2018. www.nccn.org/patients
- 11.De La Motte Rouge T., Pautier P., Rey A., Duvillard P., Kerbrat P., Troalen F., Morice P., Haie-Meder C., Culine S., Lhommé C. Prognostic factors in women treated for ovarian yolk sac tumour: a retrospective analysis of 84 cases. Eur. J. Cancer. 2011;47:175–182. doi: 10.1016/j.ejca.2010.08.012. [DOI] [PubMed] [Google Scholar]