Abstract
Obesity and elevation of circulating free fatty acids are associated with an accumulation and proinflammatory polarization of macrophages within metabolically active tissues, such as adipose tissue, muscle, liver, and pancreas. Beyond macrophages, neutrophils also accumulate in adipose and muscle tissues during high-fat diets and contribute to a state of local inflammation and insulin resistance. However, the mechanisms by which neutrophils are recruited to these tissues are largely unknown. Here we used a cell culture system as proof of concept to show that, upon exposure to a saturated fatty acid, palmitate, macrophages release nucleotides that attract neutrophils. Moreover, we found that palmitate up-regulates pannexin-1 channels in macrophages that mediate the attraction of neutrophils, shown previously to allow transfer of nucleotides across membranes. These findings suggest that proinflammatory macrophages release nucleotides through pannexin-1, a process that may facilitate neutrophil recruitment into metabolic tissues during obesity.
Keywords: fatty acid, nucleotide, chemotaxis, macrophage, neutrophil, inflammation, obesity, high fat diet, metabolic disorder, pannexin-1
Introduction
Insulin resistance and type 2 diabetes are associated with chronic low-grade inflammation, characterized by a gain in immune cells in metabolic tissues, of which macrophages are the most abundant and well-studied (1–3). Within adipose and muscle tissue of obese individuals and mice, macrophages adopt a proinflammatory phenotype, increasing production of proinflammatory cytokines that interfere with insulin signaling (2, 4). Emulating this phenotypic switch ex vivo, bone marrow-derived macrophages (BMDMs)6 respond to saturated fatty acids by mounting a selective proinflammatory response (5–7).
More recently, neutrophils have also been identified as contributors to metabolic inflammation, with neutrophil infiltration in adipose and muscle tissues occurring early during high-fat feeding in mice (8, 9). Neutrophils are innate immune granulocytes that release proteases, oxidative enzymes, and other factors to combat pathogens, although excessive neutrophil recruitment can cause collateral tissue damage and inflammation (10, 11). Notably, inhibition or genetic depletion of certain components of neutrophil granules, such as elastase and myeloperoxidase, is protective against diet-induced insulin resistance in mice (9, 12, 13). Moreover, expression of these neutrophil-specific genes is elevated in circulating cells of overweight and obese humans (14).
We recently reported an association between adipose tissue neutrophil number and the abundance of proinflammatory macrophages in high-fat diet–fed mice (15). Although, in that study, we observed an elevated presence of the chemokine CXCL1 in adipose tissue macrophages of high-fat diet–fed mice, we did not explore whether these macrophages or CXCL1 are responsible for neutrophil recruitment into adipose tissue. Hence, the mechanisms leading to neutrophil recruitment toward metabolic tissues in the context of obesity remain largely unknown. Here we investigated the hypothesis that proinflammatory macrophages are directly responsible for neutrophil chemoattraction. Specifically, we tested whether saturated fatty acids, such as palmitate, cause macrophages to release chemotactic factors that act on neutrophils. Our findings suggest that palmitate-activated macrophages attract neutrophils through nucleotides and implicate pannexin-1 channels on macrophages as conduits for nucleotide release.
Results
Palmitate-treated macrophages, but not adipocytes, release neutrophil chemotactic factors
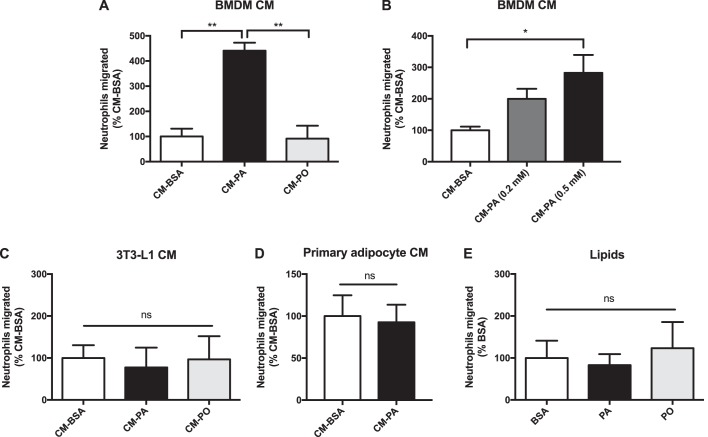
Palmitic acid (PA) is one of the most abundant circulating free fatty acids (16), and we and others have shown that exposure of macrophages to PA induces a proinflammatory shift (5, 17). Conversely, cis-palmitoleic acid (PO), a monounsaturated fatty acid of equal carbon chain length, has opposite effects, conferring a partial anti-inflammatory signature to macrophages (5). Here we used the same dose of PA that activates BMDMs to a proinflammatory state (5) to explore the consequence of this treatment on neutrophil recruitment. In parallel, we used PO as a noninflammatory fatty acid and BSA alone as the control carrier used to conjugate fatty acids to ensure their solubility. Conditioned medium generated from BMDMs exposed previously to PA, but not PO, contained factors that promoted neutrophil migration across the Transwell pores (Fig. 1, A and B). Interestingly, conditioned medium from PA-treated 3T3-L1 adipocytes or from PA-treated primary adipocytes isolated from epididymal white adipose tissue (EWAT) did not show any differences in neutrophil recruitment compared with BSA-treated adipocytes (Fig. 1, C and D). Furthermore, PA alone did not elicit any chemotactic effect on neutrophils (Fig. 1E). These results suggest that the release of neutrophil chemotactic factors after exposure to PA is a cell type–specific response.
Figure 1.
Neutrophils migrate toward palmitate-treated macrophage conditioned medium. A and B, neutrophil migration toward CM from (A) BMDMs pretreated with BSA control, 0.5 mm PA, or 0.5 mm PO, n = 3 or (B) BMDMs pretreated with 0.2 mm or 0.5 mm PA, n = 6. C and D, neutrophil migration toward (C) CM from 3T3-L1 differentiated adipocytes pretreated with BSA control, 0.5 mm PA, or 0.5 mm PO, n = 3 or (D) CM from primary adipocytes isolated from EWAT treated with BSA or 0.5 mm PA, n = 3. E, neutrophil migration toward BSA, 0.5 mm PA, or 0.5 mm PO alone, n = 3. Results expressed as mean ± S.E. *, p < 0.05; **, p < 0.01; ns, not significant.
Nature of the neutrophil chemoattractant released from PA-treated macrophages
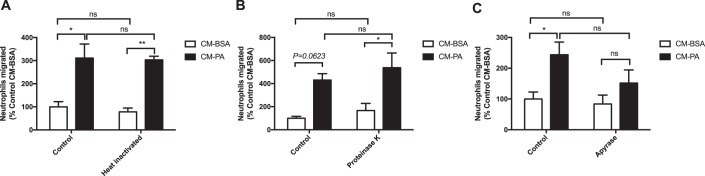
Chemokines or cellular metabolites are common chemical attractants for immune cells. In previous work, we found that lipopolysaccharide (LPS)–treated myotubes chemoattract monocytes via the chemokine CCL2, whereas PA-treated myotubes do so through release of nucleotides (18, 19). Thus, to investigate the nature of the neutrophil chemoattractant released by PA-stimulated macrophages, we first sought to determine whether the chemotactic agents were polypeptides. To denature polypeptides, conditioned media were heated to 95 °C for 20 min. Surprisingly, the chemotactic activity of the conditioned medium from PA-treated BMDMs was insensitive to heat inactivation (Fig. 2A). Moreover, proteinase K digestion of the secreted factors in the conditioned media did not alter neutrophil recruitment (Fig. 2B). As validation of this approach, the heat inactivation and proteinase K digestion protocols were effective in inhibiting the chemotactic effects of CXCL1, a neutrophil chemokine (Fig. S1), reinforcing the notion that the neutrophil chemotactic factors released from PA-treated macrophages are not proteins or polypeptides.
Figure 2.
Nucleotides released from palmitate-treated macrophages attract neutrophils. A–C, neutrophil migration toward BMDM CM-BSA or CM-PA (0.5 mm). Prior to neutrophil migration, CM were treated as follows: (A) heat inactivation (95 °C for 20 min), n = 3; (B) proteinase K (100 μg/ml at 37 °C for 1.5 h), n = 3; or (C) apyrase (0.2 units/ml at 37 °C for 1 h), n = 5. Results are expressed as mean ± S.E. *, p < 0.05; **, p < 0.01; ns, not significant.
Given the ability of nucleotides to not only chemoattract monocytes (as referenced above) but also neutrophils (20), we next examined whether PA-stimulated BMDMs attracted neutrophils through release of nucleotides. First, we treated BMDM conditioned medium with the nucleotidase apyrase, which hydrolyzes nucleotide triphosphates and diphosphates. Apyrase attenuated the ability of conditioned medium from PA-treated BMDMs to attract neutrophils so that the PA effect was no longer significant compared with conditioned medium from BSA-treated BMDMs (Fig. 2C). Similar trends in response to proteinase K and apyrase were observed with conditioned medium from BMDMs treated with a lower concentration (0.2 mm) of PA (Fig. S2). These observations suggest that nucleotides may be the released factors contributing to the chemoattracting property of PA-treated BMDMs.
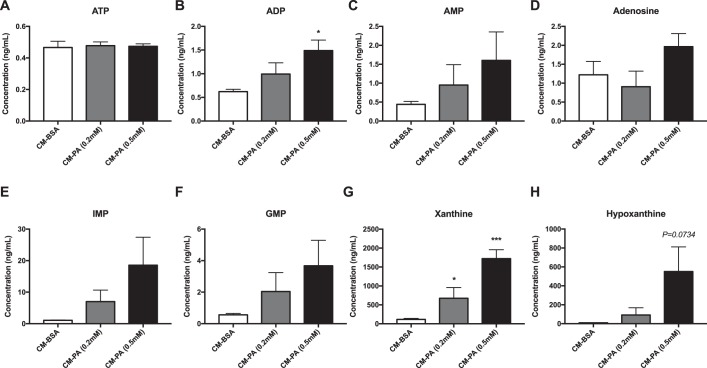
Accordingly, we determined the presence of nucleotides in BMDM conditioned media using UHPLC-MS. A recurring PA dose–dependent trend toward elevated levels of several nucleotides and nucleotide derivatives (ADP, AMP, adenosine, IMP, GMP, xanthine, and hypoxanthine) was observed in conditioned medium from BMDMs exposed to PA compared with BSA, with ADP and xanthine being significantly higher (Fig. 3).
Figure 3.
Nucleotides and nucleotide derivatives released from palmitate-treated macrophages. A–H, concentrations of the following nucleotides and nucleotide derivatives in CM from BMDMs: (A) ATP, (B) ADP, (C) AMP, (D) adenosine, (E) IMP, (F) GMP, (G) xanthine, and (H) hypoxanthine; n = 5. Results are expressed as mean ± S.E. *, p < 0.05; ***, p < 0.001 versus CM-BSA.
Neutrophil migration toward macrophage conditioned medium depends on purinergic signaling
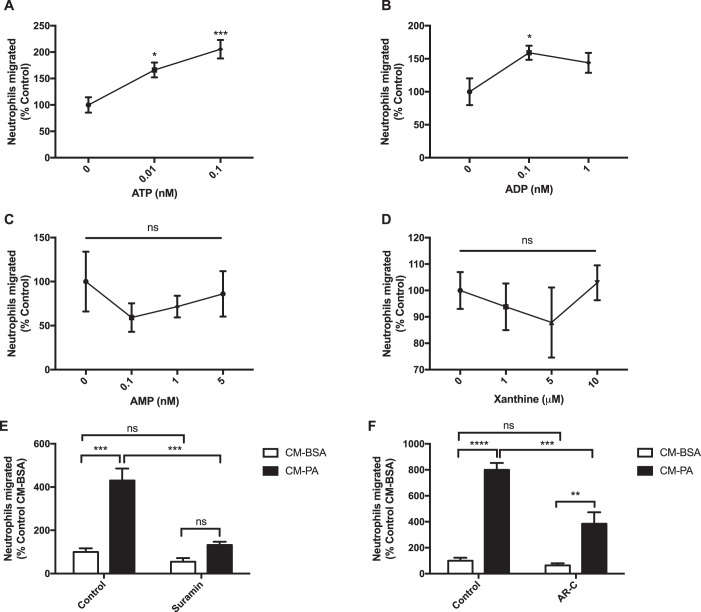
Nucleotides exert chemotactic action by activating purinergic receptors on target cells (21–23). To validate this principle in our experimental model, we first assayed neutrophil migration toward increasing doses of ATP, ADP, and AMP, reflecting the concentrations measured in the conditioned medium from PA-treated BMDMs. Indeed, ATP and ADP attracted neutrophils, whereas AMP, the final product after apyrase digestion of ATP, was not chemotactic (Fig. 4, A–C). Xanthine, a purine base formed as an intermediate in nucleoside degradation, also did not chemoattract neutrophils (Fig. 4D); hence, its elevated release from BMDMs in response to PA may not be responsible for the neutrophil-chemoattracting effect.
Figure 4.
Neutrophil chemotaxis toward macrophage conditioned medium depends on purinergic signaling. A–D, neutrophil migration toward the purine nucleotides and xanthine: (A) ATP, n = 10; (B) ADP, n = 6; (C) AMP, n = 3; and (D) xanthine, n = 3. *, p < 0.05; ***, p < 0.001 versus 0 nm. E, migration of neutrophils preincubated with suramin (100 μm for 30 min) toward BMDM CM-BSA or CM-PA (0.5 mm) that were also supplemented with suramin during neutrophil migration; n = 3. F, migration of neutrophils, preincubated with AR-C (10 μm for 30 min) toward BMDM CM-BSA or CM-PA (0.5 mm) that were also supplemented with AR-C during neutrophil migration; n = 5. Results are expressed as mean ± S.E. **, p < 0.01; ***, p < 0.001; ****, p < 0.0001; ns, not significant.
Next, to determine whether neutrophil chemotaxis toward secreted factors from PA-treated BMDMs depends on sensing purine nucleotides, neutrophils were pretreated with the broad-spectrum purinergic receptor antagonist suramin prior to migration toward the BMDM conditioned medium, which was also supplemented with suramin. Suramin abolished the ability of neutrophils to migrate toward conditioned medium from PA-treated BMDMs (Fig. 4E and Fig. S2), reinforcing the role of purine nucleotides as mediators of neutrophil chemotaxis released by PA-activated macrophages. One of the predominantly expressed purinergic receptors on neutrophils is P2Y2R, which is involved in chemotaxis toward nucleotides (20, 24–26). Thus, we investigated whether P2Y2R is necessary for neutrophil migration toward secreted factors from PA-activated BMDMs. Neutrophils treated with the P2Y2R-selective inhibitor AR-C migrated less toward conditioned medium from PA-treated BMDMs compared with untreated neutrophils, although migration was not completely eliminated (Fig. 4F). These results suggest that P2Y2R is involved in neutrophil chemotaxis toward PA-treated macrophage secreted factors, but other receptors may also be engaged.
Pannexin-1 is up-regulated in macrophages exposed to PA or isolated from high-fat diet–fed mice
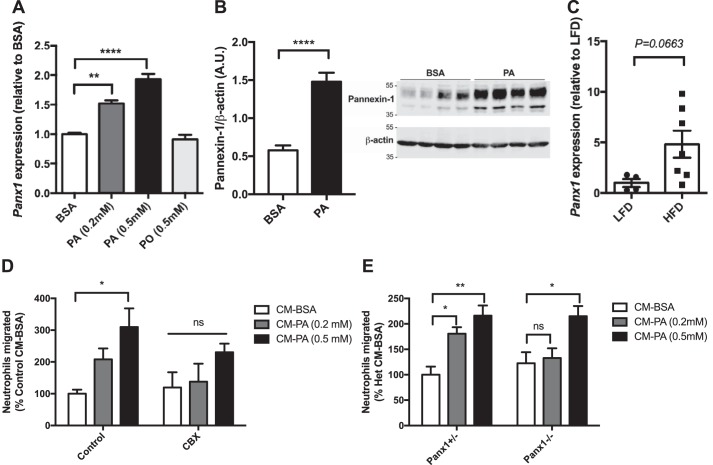
We next aimed to identify the route by which cytosolic nucleotides are released from BMDMs. Pannexins are channel-forming proteins that allow flow of small molecules, including nucleotides such as ATP, ADP, and AMP, between the cytosol and extracellular space (27, 28). The release of nucleotides from pannexin channels has been implicated as a factor contributing to local inflammation in numerous contexts (29). Pannexin-1 channels also regulate fat accumulation and insulin sensitivity (30, 31). Hence, we first measured the expression of pannexin-1 in fatty acid–treated BMDMs and found that Panx1 mRNA expression and pannexin-1 protein levels were up-regulated in BMDMs treated with PA (Fig. 5, A and B, and Fig. S3). Of note, although not achieving statistical significance with the number of samples available (p = 0.0663, n = 4–7), Panx1 expression trended higher in the stromal vascular fraction of mouse EWAT (which includes adipose tissue macrophages) isolated from high-fat diet–fed mice compared with low-fat diet–fed controls (Fig. 5C).
Figure 5.
Pannexin-1 in macrophages mediates neutrophil attraction. A, mRNA expression of Panx1 in Panx1+/− BMDMs treated with fatty acids for 18 h, followed by 24 h in fatty acid-free medium; n = 3. B, protein expression of pannexin-1 in BMDMs treated with 0.5 mm PA for 18 h, followed by 24 h in fatty acid-free medium; n = 7 (representative image of n = 4). C, mRNA expression of Panx1 in the stromal vascular fraction of epididymal white adipose tissue from mice fed a low-fat or high-fat diet for 18 weeks; n = 4–7. D, neutrophil migration toward CM from BMDMs treated with CBX (75 μm) during fatty acid exposure and generation of CM; n = 4. E, neutrophil migration toward CM from Panx1+/− or Panx1−/− BMDMs; n = 6. Results are expressed as mean ± S.E. *, p < 0.05; **, p < 0.01; ****, p < 0.0001; ns, not significant.
Macrophage pannexin-1 mediates neutrophil recruitment
Considering pannexin-1 as a potential conduit for nucleotide release in response to PA, we treated BMDMs with the pannexin-1 channel inhibitor carbenoxolone (CBX) (32) throughout exposure to PA and during the subsequent PA-free incubation to generate conditioned medium. Notably, conditioned medium from CBX-treated BMDMs showed no significant increase in neutrophil chemoattraction when BMDMs were exposed to PA compared with BSA. (Fig. 5D). Furthermore, we generated conditioned media from Panx1−/− and control Panx1+/− BMDMs, which, we verified, have allele dose-dependent expression of Panx1 (Fig. S4). Consistent with our findings using CBX, neutrophils from WT mice had reduced migration toward conditioned medium derived from Panx1−/− BMDMs exposed to 0.2 mm PA compared with BSA, unlike Panx1+/− BMDMs (Fig. 5E). These results suggest a role of pannexin-1 in macrophage-mediated neutrophil attraction.
Discussion
The results of this study reveal the capability of palmitate-activated macrophages to release nucleotides that act as neutrophil chemotactic factors. Our data further support the concept that chemoattractant release in response to palmitate depends on pannexin-1 expression in macrophages.
Macrophages attract neutrophils in response to palmitate
Numerous studies have demonstrated that neutrophils are important contributors to high-fat diet–induced insulin resistance through their granule enzymes elastase and myeloperoxidase (9, 12, 13). Beyond that, there is growing evidence suggesting that neutrophils in a high-fat environment also up-regulate proinflammatory cytokines. Upon contact with adipocytes, neutrophils increase their expression of IL-1β (33). Additionally, circulating neutrophils from obese humans are primed to up-regulate expression of cytokines, including IL-6 and TNFα, as well as elevate production of reactive oxygen species (34). These inflammatory mediators from neutrophils likely potentiate tissue inflammation associated with obesity.
In mice, neutrophil infiltration into adipose tissue is observed within 3–7 days of high-fat feeding, and their population remains elevated throughout long-term high-fat diet (9, 15, 35). Inhibiting this infiltration by depleting neutrophils or by blocking the endothelial leukocyte adhesion molecule ICAM-1 protects against high-fat diet–induced insulin resistance (36). Importantly, although the macrophage population within adipose tissue does not expand during early days of high-fat feeding, tissue-resident macrophages undergo a proinflammatory phenotypic switch (37). Moreover, we observed previously that, when proinflammatory polarization of adipose tissue macrophages is prevented, the high-fat feeding–induced gain of neutrophils in adipose tissue is also abolished (15). That study led to the question of whether macrophages are the cells attracting neutrophils and, if so, through which mediators.
Adipose tissue macrophages comprise resident macrophages embryologically originated from the yolk sac and macrophages differentiated from blood monocytes produced in the bone marrow (38, 39). Upon consumption of fat-rich or hypercaloric diets, the adipose tissue macrophage population expands, largely from influx of blood monocytes in addition to proliferation and lessened apoptosis of resident macrophages (1, 38, 39). With a high-fat diet, conditions within adipose tissue (hypoxia, released fatty acids, and adipocyte hypertrophy) cause macrophages to adopt a proinflammatory polarization, characterized by production of proinflammatory cytokines such as TNFα and IL-6 (38, 39).
On the other hand, BMDMs are generated ex vivo by treating bone marrow monocytic precursors with macrophage colony-stimulating factor. Although cultured BMDMs are not neatly equivalent to bona fide adipose tissue macrophages, BMDMs have been widely used to study metabolic, secretory, and pro- or anti-inflammatory orientations in response to a number of stimuli. In particular, BMDMs exposed to saturated fatty acids, such as PA, produce similar proinflammatory cytokines as those produced by adipose tissue macrophages in high-fat diet–fed mice (5). Thus, PA-activated BMDMs can serve as a useful proof-of-concept model to emulate high-fat diet–activated, proinflammatory adipose tissue macrophages. Using this cell culture model, we show that, when exposed to a saturated fatty acid, bone marrow–derived macrophages, but not adipocytes, recruit neutrophils. These findings support the paradigm that proinflammatory macrophages are responsible for recruitment of neutrophils into adipose tissue.
During an infection, it is commonly observed that neutrophils are the first circulating immune cells recruited to the affected site, initiating the anti-microbial response (40). Thereafter, monocytes are recruited and eventually phagocytose apoptotic neutrophils, contributing to the resolution of inflammation (11, 40). However, under other inflammatory contexts, such as in the presence of LPS in tissues (41), resident macrophages are the first immune cells to contact the foreign agent and mount an inflammatory response that recruits neutrophils from circulation (41, 42). Similarly, based on the findings presented here, we hypothesize that resident macrophages in metabolic tissues may recruit neutrophils from circulation during high-fat feeding and obesity. Notably, mice receiving adipose tissue transplants from obese but not lean mouse donors have elevated circulating neutrophils and increased neutrophil infiltration into the liver. However, this was not observed when the transplanted adipose tissue was first depleted of macrophages (43), further corroborating that proinflammatory macrophages regulate neutrophil recruitment in vivo during a high-fat diet.
Because neutrophils are short-lived immune cells and recognized as key players in acute inflammation (40), the early and sustained elevation of neutrophils in adipose tissue during long-term high-fat feeding and obesity (9) suggests that these cells are continuously recruited and are significant contributors to chronic metabolic inflammation.
Macrophages release nucleotides that attract neutrophils
Nucleotides can act as “find me” signals, often released during infection or tissue injury to promote immune cell recruitment and clearance of infected or dying cells (23, 44). Here we show that, ex vivo, PA evokes an equivalent response, leading to release of nucleotides from macrophages, which act as chemotactic factors promoting neutrophil chemoattraction. In addition, cleavage of nucleotides by apyrase in conditioned medium from PA-treated macrophages diminished its ability to attract neutrophils. Although we did not detect differences in ATP concentrations between conditioned medium from PA-treated and BSA-treated BMDMs, we speculate that ectonucleotidases expressed by macrophages may have cleaved any potential ATP into ADP and AMP (45). Indeed, we observed markedly elevated ADP levels released by PA-treated BMDMs, and we validated that similar levels of ADP were indeed sufficient to chemoattract neutrophils. Other nucleotides that are released may also act synergistically to contribute to neutrophil chemotaxis. In addition to ADP, we also observed significantly elevated levels of the purine base xanthine released from PA-treated BMDMs. Although we found that xanthine alone does not chemoattract neutrophils ex vivo, it has been reported that superoxide products from xanthine–xanthine oxidase reactions promote neutrophil infiltration in vivo (46).
Further emphasizing the role of nucleotide signals, antagonism of the P2X and P2Y family of purinergic receptors on neutrophils by suramin vastly reduced their migration toward secreted factors from BMDMs exposed to PA. Specifically, antagonism of P2Y2R reduced, but did not abolish, migration toward conditioned medium from PA-treated BMDMs, suggesting that P2Y2R, likely along with other receptors, is involved in neutrophil migration toward these secreted factors. From these observations, we suspect that, in vivo, purinergic receptors on neutrophils are instrumental in their migratory response toward local macrophages in tissues experiencing a high-fat environment.
Palmitate and high-fat feeding up-regulate pannexin-1 in macrophages to mediate neutrophil recruitment
During inflammation and apoptosis, pannexin-1 forms pores in the membrane of diverse cell types and mediates the release of nucleotides, including ATP and ADP (28, 47). Along with up-regulation of numerous proinflammatory genes in macrophages (5), we show here that PA also up-regulates the mRNA and protein expression of the pore-forming molecule pannexin-1. Notably, obesity is associated with increased expression of Panx1 mRNA in human whole subcutaneous adipose tissue, and Panx1 expression in human visceral adipose tissue positively correlates with blood glucose and homeostatic model assessment of insulin resistance (HOMA-IR) (30). Here we further found that mice on a prolonged high-fat diet have elevated Panx1 expression in the cell population of the adipose tissue that excludes adipocytes. Although there may be diverse functions for pannexin-1 in adipose tissue, enhanced liberation of nucleotides that chemoattract immune cells toward tissues is a distinct possibility. Moreover, this PA-dependent increased expression of Panx1 in BMDMs is reminiscent of the increase in pannexin-3 expression in PA-treated myotubes that contribute to monocyte recruitment. In myotubes, PA activates the TLR4–NF-κB pathway, leading to transcription of Panx3 (19). It is possible that a similar signaling mechanism occurs in macrophages, leading to PA-induced transcription of Panx1. Collectively, these findings support the concept that pannexin-1 channels may be important regulators of adipose tissue insulin sensitivity, possibly ascribed to adipose tissue–resident macrophages. Pannexin-1 up-regulation in macrophages exposed to saturated fatty acids may serve as a conduit for release of neutrophil chemoattractants.
BMDMs lacking pannexin-1 did not secrete factors that led to neutrophil recruitment when exposed to low doses of PA only. However, carbenoxolone prevented neutrophil recruitment toward conditioned medium from BMDMs treated with low and high doses of PA. These results suggest the possibility that there may be other mechanisms for release of nucleotides in addition to pannexin-1, especially under conditions of high fatty acid availability. One such candidate is connexin 43, a hemichannel-forming protein that is expressed by macrophages and, like pannexins, can also be inhibited by carbenoxolone. Connexin 43 is up-regulated upon stimulation by LPS or inflammatory cytokines such as IFN-γ and TNFα (48, 49); however, we observed no significant changes in expression of connexin 43 following PA treatment (data not shown). PA can also activate the inflammasome to initiate the formation of pyroptotic pores in monocytes (50). These large pores, which facilitate pyroptosis, are formed by gasdermin D oligomers and allow large molecules, but likely also nucleotides, to be released (51, 52). However, this mechanism is unlikely because, unlike monocytes, PA-induced inflammasome activation in macrophages additionally requires a “priming” signal, such as TLR4 activation by LPS (7).
In conclusion, the results of this study demonstrate that, in addition to a proinflammatory polarization, macrophages activated by saturated fats up-regulate pannexin-1 channel expression and release nucleotides that attract neutrophils. These findings provide a potential mechanism for the accumulation of neutrophils in adipose tissue during obesity. Thus, nucleotide release by macrophages and purinergic signaling in neutrophils may be interesting targets to dampen the contribution of neutrophils to tissue inflammation during obesity.
Experimental procedures
Mice
Mouse protocols were approved by the Animal Care Committee at the Hospital for Sick Children, the University of Toronto, and the University of Western Ontario. C57BL/6 mice were obtained from Charles River or bred in-house. Panx1−/− mice were a gift from Genetech Inc. as described previously (53) and backcrossed a minimum of 10 generations with WT C57BL/6N mice from Charles River. Mice were fed a chow diet unless otherwise indicated. Where indicated, 8-week-old mice were fed a low-fat (10% kcal from fat, D12450Ji, Research Diets) or high-fat diet (60% kcal from fat, D12492i, Research Diets) for 18 weeks prior to tissue isolation.
Reagents and fatty acid preparation
PA (C16:0) and PO (C16:1 n-7) (P9767 and P9417, respectively, Sigma-Aldrich) were prepared as 200 mm stock solutions in 50% ethanol heated at 50 °C. Fatty acids were conjugated to BSA at a 5:1 lipid:BSA ratio by dissolving 200 mm of initial stock lipids in serum-free α-minimum Eagle's medium (Wisent) with 10.5% fatty acid–free, low-endotoxin BSA (A8806, Sigma-Aldrich) to a final stock concentration of 8 mm and kept under agitation at 40 °C for 2 h.
For migration experiments, ATP was obtained from Promega (F203A), and ADP, AMP, and xanthine were from Sigma-Aldrich (A5285, A1752, and X3627, respectively). Recombinant murine KC (CXCL1) was from PeproTech (250-11). Proteinase K was from Thermo Scientific (EO0491), apyrase and CBX were from Sigma-Aldrich (A6237 and C4790, respectively), suramin was from Cayman Chemical (11126), and AR-C 118925XX (AR-C) was from Tocris (4890).
Bone marrow isolation and BMDM culture
Bone marrow was isolated from the femora, tibiae, and pelvises of 8- to 16-week-old male chow-fed C57BL/6 mice or from 1-year-old male or female Panx1+/+, Panx1+/−, or Panx1−/− mice. BMDMs were generated by centrifuging bones at 15,000 × g for 10 s to extract bone marrow cells, which were differentiated into BMDMs as described previously (5). Briefly, bone marrow cells were plated at 1 × 106 cells/ml and cultured for 7 days at 37 °C and 5% CO2 in RPMI 1640 medium (RPMI) (Wisent) supplemented with 10% heat-inactivated FBS (Wisent), 1× nonessential amino acids (Wisent), 1 mm sodium pyruvate (Wisent), 1× antibiotic–antimycotic solution (Wisent), 275 μm 2-mercaptoethanol (Life Technologies), and 10% conditioned medium prepared from L929 fibroblasts.
3T3-L1 adipocyte culture and differentiation
3T3-L1 fibroblasts (ATCC) were cultured at 37 °C and 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) (Wisent) with 10% FBS, 1 mm sodium pyruvate, and 1× antibiotic–antimycotic solution. Confluent fibroblasts were differentiated into adipocytes by culturing in medium containing 0.4 μm insulin (Sigma-Aldrich), 0.25 μm dexamethasone (Sigma-Aldrich), 500 μm isobutyl-1-methyl-xanthine (Sigma-Aldrich), and 0.1 μg/ml biotin (Sigma-Aldrich) for 3 days; with medium and 0.4 μm insulin only for 3 more days; and 2 days of regular medium thereafter.
Primary adipocyte culture
EWAT from 10- to 11-week-old male mice was excised and minced, followed by enzymatic digestion by 4 mg/ml collagenase type I (Worthington) while shaking for 1 h. Floating adipocytes were isolated by filtration through a 300-μm cell strainer and centrifugation at 400 × g for 1 min, washed twice, and plated in DMEM with 1% BSA, 0.5% FBS, and 1× antibiotic–antimycotic solution at 37 °C, 5% CO2. For each experiment, adipocytes were pooled from three mice and then evenly separated between each experimental condition.
Generation of conditioned media
BMDMs or 3T3-L1 adipocytes were treated with 0.2 mm or 0.5 mm BSA-conjugated fatty acids or BSA alone for 18 h. Thereafter, cells were washed twice with PBS and replenished with fatty acid–free medium for 24 h to generate conditioned medium (CM) from BSA-, PA-, or PO-treated cells (CM-BSA, CM-PA, or CM-PO, respectively). CM was centrifuged at 15,000 × g for 10 min to remove cell debris. Supernatants were collected and stored at −80 °C. For primary adipocytes, conditioned media were collected immediately after 18 h of fatty acid treatment. Conditioned media were centrifuged at 400 × g to remove floating adipocytes and then stored at −80 °C.
Bone marrow neutrophil isolation
Bone marrow was isolated as described above. Neutrophils were isolated from bone marrow as described previously (54). Briefly, resuspended bone marrow was separated on a Percoll (GE Healthcare Life Sciences) density gradient by layering 80%, 65%, and 55% Percoll solutions and centrifuging at 1000 × g for 30 min. The 65%/80% interface containing neutrophils and red blood cells was collected. Red blood cells were lysed with cold deionized water for 30 s, and then NaCl was added to restore isotonic osmolarity.
Neutrophil migration assay
Bone marrow neutrophils were resuspended at 1 × 107 cells/ml in RPMI with 10% FBS and 1× antibiotic–antimycotic solution. 100 μl of the cell suspension was added to the upper chamber of Transwells with 3.0-μm-diameter pores (Corning), with the conditioned medium or test attractant added to the lower chamber. Cells were allowed to migrate across the Transwell for 1 h at 37 °C, 5% CO2, and then migrated cells in the bottom chamber were detached with EDTA and counted using a Z2 Coulter Counter (Beckman Coulter).
Stromal vascular fraction isolation
Excised EWAT was minced and digested with 0.47 Wünsch units/ml Liberase (Roche) shaking at 200 rpm for 20 min at room temperature. The cell solutions were passed through a 70-μm cell strainer, neutralized with DMEM, and centrifuged at 500 × g for 10 min. Supernatants containing adipocytes were discarded, and the stromal vascular fraction cell pellets were resuspended in red blood cell lysis buffer for 5 min and then washed with PBS.
Quantitative PCR
RNA was isolated from cells using TRIzol (Life Technologies) and used to synthesize cDNA by reverse transcription using the SuperScript VILO cDNA Synthesis Kit (Life Technologies) according to the manufacturer's instructions. TaqMan primers (Life Technologies) were used to perform quantitative PCR reactions with the cDNA on a StepOne Plus Real Time PCR System (Life Technologies). Gene expression values were normalized to that of the housekeeping gene Abt1.
Immunoblotting
BMDMs were lysed with radioimmunoprecipitation assay assay buffer (50 mm Tris, 150 mm NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 5 mm NaF, 1 mm EDTA, 5 mm Na3VO4, and protease inhibitors (Sigma-Aldrich)). Cell lysates were boiled in Laemmli buffer with β-mercaptoethanol, and then equal amounts of protein were separated by SDS-PAGE, transferred to nitrocellulose membranes, and blocked with LI-COR blocking buffer. Membranes were incubated overnight at 4 °C with rabbit anti-Panx1-CT-395 antibodies, generated by Genemed Synthesis (55), and mouse anti-β-actin antibodies (Sigma-Aldrich). Then they were washed with TBS with 0.1% Tween 20, followed by 1 h incubation at room temperature with the fluorescent secondary antibodies anti-mouse IRDye 680LT or anti-rabbit IRDye 800CW (LI-COR). Membranes were washed again, imaged using Odyssey Fc Imager (LI-COR), and quantified with Image Studio 5.0 (LI-COR).
Nucleotide measurement
Quantitation of nucleotides was performed using liquid chromatography electrospray ionization tandem mass spectrometry (LC/ESI/MS/MS) using an AB Sciex 6500 mass spectrometer with an ESI probe and interfaced with an ultra-high performance liquid chromatography (UHPLC) system in positive multiple-reaction monitoring mode. The injection volume was 10 μl for cell culture medium extracts. The extracts were chromatographically resolved using a Hypercarb Javelin high throughput screening column at 55 °C (Thermo Scientific, 35005-022135). Mobile phase A was water with 50 mm ammonium formate adjusted to pH 4.0. Mobile phase B was acetonitrile/methanol (50/50, (v/v)) with 0.5% ammonium hydroxide. The solvent flow rate was 0.5 ml/min. All standards were obtained from Toronto Chemical Research and Cambridge Isotope Laboratories. The calibration curve was fitted by least-squares linear regression with 1/x weighting. All nucleotides and nucleotide derivatives were quantified using the standard curve and ratio of the peak area of analytes to internal standard. Data analysis was performed using MultiQuant 3.0 (AB Sciex).
Statistical analyses
Data are expressed as means ± S.E. For single-variable datasets, an unpaired Student's t test was used to analyze differences between two groups; one-way analysis of variance with Tukey's post hoc test was used to analyze differences between more than two groups. For datasets containing two variables, two-way analysis of variance with Tukey's post hoc test was used. Statistical significance was set as p < 0.05 using GraphPad Prism 7 (GraphPad Software).
Author contributions
T. H. T., K. L. C., and A. K. conceptualization; T. H. T., K. L. C., P. B., Z. L., J. T. B., H. H. B., K. R., and C. B. W. data curation; T. H. T., K. L. C., J. T. B., H. H. B., K. R., and A. K. formal analysis; T. H. T., K. L. C., P. B., Z. L., J. T. B., H. H. B., K. R., C. B. W., P. J. B., and A. K. investigation; T. H. T., K. L. C., P. B., Z. L., J. T. B., H. H. B., K. R., and C. B. W. methodology; T. H. T. and A. K. writing-original draft; T. H. T., K. L. C., P. B., Z. L., J. T. B., H. H. B., K. R., C. B. W., S. P., P. J. B., and A. K. writing-review and editing; S. P. and A. K. resources; S. P., P. J. B., and A. K. supervision; S. P. and A. K. funding acquisition; P. J. B. and A. K. project administration.
Supplementary Material
Acknowledgments
We thank Dr. Michael Glogauer and Dr. Noah Fine for valuable discussions, Rafaela Vas Sousa Pereira for helpful technical support, and Genentech Inc. for providing the Panx1−/− mice.
This study was supported by Foundation Grant FND-143203 (to A. K.) from the Canadian Institutes of Health Research. Panx1−/− mouse studies were supported by Discovery Grant RGPIN-2015-06794 (to S. P.) from the Natural Sciences and Engineering Research Council of Canada. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S4 and Table S1.
- BMDM
- bone marrow–derived macrophage
- PA
- palmitic acid
- PO
- cis-palmitoleic acid
- EWAT
- epididymal white adipose tissue
- LPS
- lipopolysaccharide
- AR-C
- AR-C 118925XX
- CBX
- carbenoxolone
- TNF
- tumor necrosis factor
- CM
- conditioned medium/media
- cDNA
- complementary DNA
- IL
- interleukin
- TLR
- toll-like receptor.
References
- 1. Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., and Ferrante A. W. (2003) Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 10.1172/JCI200319246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lumeng C. N., Bodzin J. L., and Saltiel A. R. (2007) Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 10.1172/JCI29881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saltiel A. R., and Olefsky J. M. (2017) Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Invest. 127, 1–4 10.1172/JCI92035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hotamisligil G. S., Peraldi P., Budavari A., Ellis R., White M. F., and Spiegelman B. M. (1996) IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-α- and obesity-induced insulin resistance. Science 271, 665–668 10.1126/science.271.5249.665 [DOI] [PubMed] [Google Scholar]
- 5. Chan K. L., Pillon N. J., Sivaloganathan D. M., Costford S. R., Liu Z., Théret M., Chazaud B., and Klip A. (2015) Palmitoleate reverses high fat-induced proinflammatory macrophage polarization via AMP-activated protein kinase (AMPK). J. Biol. Chem. 290, 16979–16988 10.1074/jbc.M115.646992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kewalramani G., Fink L. N., Asadi F., and Klip A. (2011) Palmitate-activated macrophages confer insulin resistance to muscle cells by a mechanism involving protein kinase C θ and ϵ. PLoS ONE 6, e26947 10.1371/journal.pone.0026947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wen H., Gris D., Lei Y., Jha S., Zhang L., Huang M. T., Brickey W. J., and Ting J. P. (2011) Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 12, 408–415 10.1038/ni.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Elgazar-Carmon V., Rudich A., Hadad N., and Levy R. (2008) Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J. Lipid Res. 49, 1894–1903 10.1194/jlr.M800132-JLR200 [DOI] [PubMed] [Google Scholar]
- 9. Talukdar S., Oh D. Y., Bandyopadhyay G., Li D., Xu J., McNelis J., Lu M., Li P., Yan Q., Zhu Y., Ofrecio J., Lin M., Brenner M. B., and Olefsky J. M. (2012) Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med. 18, 1407–1412 10.1038/nm.2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sahoo M., Del Barrio L., Miller M. A., and Re F. (2014) Neutrophil elastase causes tissue damage that decreases host tolerance to lung infection with Burkholderia species. PLoS Pathog. 10, e1004327 10.1371/journal.ppat.1004327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beyrau M., Bodkin J. V., and Nourshargh S. (2012) Neutrophil heterogeneity in health and disease: a revitalized avenue in inflammation and immunity. Open Biol. 2, 120134–120134 10.1098/rsob.120134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Q., Xie Z., Zhang W., Zhou J., Wu Y., Zhang M., Zhu H., and Zou M.-H. (2014) Myeloperoxidase deletion prevents high-fat diet-induced obesity and insulin resistance. Diabetes 63, 4172–4185 10.2337/db14-0026 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13. Chai W., Aylor K., Liu Z., Gan L.-M., Michaëlsson E., and Barrett E. (2019) Inhibiting myeloperoxidase prevents onset and reverses established high-fat diet-induced microvascular insulin resistance. Am. J. Physiol. Endocrinol. Metab. 317, E1063–E1069 10.1152/ajpendo.00203.2019 [DOI] [PubMed] [Google Scholar]
- 14. Ali M., Jasmin S., Fariduddin M., Alam S. M. K., Arslan M. I., and Biswas S. K. (2018) Neutrophil elastase and myeloperoxidase mRNA expression in overweight and obese subjects. Mol. Biol. Rep. 45, 1245–1252 10.1007/s11033-018-4279-4 [DOI] [PubMed] [Google Scholar]
- 15. Chan K. L., Tam T. H., Boroumand P., Prescott D., Costford S. R., Escalante N. K., Fine N., Tu Y., Robertson S. J., Prabaharan D., Liu Z., Bilan P. J., Salter M. W., Glogauer M., Girardin S. E., et al. (2017) Circulating NOD1 activators and hematopoietic NOD1 contribute to metabolic inflammation and insulin resistance. Cell Rep. 18, 2415–2426 10.1016/j.celrep.2017.02.027 [DOI] [PubMed] [Google Scholar]
- 16. Ubhayasekera S. J., Staaf J., Forslund A., Bergsten P., and Bergquist J. (2013) Free fatty acid determination in plasma by GC-MS after conversion to Weinreb amides. Anal. Bioanal. Chem. 405, 1929–1935 10.1007/s00216-012-6658-3 [DOI] [PubMed] [Google Scholar]
- 17. Shi H., Kokoeva M. V., Inouye K., Tzameli I., Yin H., and Flier J. S. (2006) TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 116, 3015–3025 10.1172/JCI28898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miyatake S., Bilan P. J., Pillon N. J., and Klip A. (2016) Contracting C2C12 myotubes release CCL2 in an NF-κB-dependent manner to induce monocyte chemoattraction. Am. J. Physiol. Endocrinol. Metab. 310, E160–E170 10.1152/ajpendo.00325.2015 [DOI] [PubMed] [Google Scholar]
- 19. Pillon N. J., Li Y. E., Fink L. N., Brozinick J. T., Nikolayev A., Kuo M.-S., Bilan P. J., and Klip A. (2014) Nucleotides released from palmitate-challenged muscle cells through Pannexin-3 attract monocytes. Diabetes 63, 3815–3826 10.2337/db14-0150 [DOI] [PubMed] [Google Scholar]
- 20. Kukulski F., Ben Yebdri F., Lecka J., Kauffenstein G., Lévesque S. A., Martín-Satué M., and Sévigny J. (2009) Extracellular ATP and P2 receptors are required for IL-8 to induce neutrophil migration. Cytokine 46, 166–170 10.1016/j.cyto.2009.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Erlinge D. (2011) P2Y receptors in health and disease. Adv. Pharmacol. 61, 417–439 10.1016/B978-0-12-385526-8.00013-8 [DOI] [PubMed] [Google Scholar]
- 22. Corriden R., and Insel P. A. (2012) New insights regarding the regulation of chemotaxis by nucleotides, adenosine, and their receptors. Purinergic Signal. 8, 587–598 10.1007/s11302-012-9311-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Idzko M., Ferrari D., and Eltzschig H. K. (2014) Nucleotide signalling during inflammation. Nature 509, 310–317 10.1038/nature13085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang X., and Chen D. (2018) Purinergic regulation of neutrophil function. Front. Immunol. 9, 399 10.3389/fimmu.2018.00399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen Y., Yao Y., Sumi Y., Li A., To U. K., Elkhal A., Inoue Y., Woehrle T., Zhang Q., Hauser C., and Junger W. G. (2010) Purinergic signaling: a fundamental mechanism in neutrophil activation. Sci. Signal. 3, ra45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen Y., Corriden R., Inoue Y., Yip L., Hashiguchi N., Zinkernagel A., Nizet V., Insel P. A., and Junger W. G. (2006) ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314, 1792–1795 10.1126/science.1132559 [DOI] [PubMed] [Google Scholar]
- 27. Velasquez S., and Eugenin E. A. (2014) Role of Pannexin-1 hemichannels and purinergic receptors in the pathogenesis of human diseases. Front. Physiol. 5, 96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boyd-Tressler A., Penuela S., Laird D. W., and Dubyak G. R. (2014) Chemotherapeutic drugs induce ATP release via caspase-gated pannexin-1 channels and a caspase/pannexin-1-independent mechanism. J. Biol. Chem. 289, 27246–27263 10.1074/jbc.M114.590240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Makarenkova H. P., and Shestopalov V. I. (2014) The role of pannexin hemichannels in inflammation and regeneration. Front. Physiol. 5, 63 10.3389/fphys.2014.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Adamson S. E., Meher A. K., Chiu Y. H., Sandilos J. K., Oberholtzer N. P., Walker N. N., Hargett S. R., Seaman S. A., Peirce-Cottler S. M., Isakson B. E., McNamara C. A., Keller S. R., Harris T. E., Bayliss D. A., and Leitinger N. (2015) Pannexin 1 is required for full activation of insulin-stimulated glucose uptake in adipocytes. Mol. Metab. 4, 610–618 10.1016/j.molmet.2015.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee V. R., Barr K. J., Kelly J. J., Johnston D., Brown C. F. C., Robb K. P., Sayedyahossein S., Huang K., Gros R., Flynn L. E., and Penuela S. (2018) Pannexin 1 regulates adipose stromal cell differentiation and fat accumulation. Sci. Rep. 8, 16166 10.1038/s41598-018-34234-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Michalski K., and Kawate T. (2016) Carbenoxolone inhibits Pannexin1 channels through interactions in the first extracellular loop. J. Gen. Physiol. 147, 165–174 10.1085/jgp.201511505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Watanabe Y., Nagai Y., Honda H., Okamoto N., Yanagibashi T., Ogasawara M., Yamamoto S., Imamura R., Takasaki I., Hara H., Sasahara M., Arita M., Hida S., Taniguchi S., Suda T., and Takatsu K. (2019) Bidirectional crosstalk between neutrophils and adipocytes promotes adipose tissue inflammation. FASEB J. 33, 11821–11835 10.1096/fj.201900477RR [DOI] [PubMed] [Google Scholar]
- 34. Roberts H. M., Grant M. M., Hubber N., Super P., Singhal R., and Chapple I. L. C. (2018) Impact of bariatric surgical intervention on peripheral blood neutrophil (PBN) function in obesity. Obes. Surg. 28, 1611–1621 10.1007/s11695-017-3063-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fink L. N., Costford S. R., Lee Y. S., Jensen T. E., Bilan P. J., Oberbach A., Blüher M., Olefsky J. M., Sams A., and Klip A. (2014) Pro-inflammatory macrophages increase in skeletal muscle of high fat-fed mice and correlate with metabolic risk markers in humans: muscle macrophages in obesity and diabetes. Obesity 22, 747–757 10.1002/oby.20615 [DOI] [PubMed] [Google Scholar]
- 36. Hadad N., Burgazliev O., Elgazar-Carmon V., Solomonov Y., Wueest S., Item F., Konrad D., Rudich A., and Levy R. (2013) Induction of cytosolic phospholipase a2α is required for adipose neutrophil infiltration and hepatic insulin resistance early in the course of high-fat feeding. Diabetes 62, 3053–3063 10.2337/db12-1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee Y. S., Li P., Huh J. Y., Hwang I. J., Lu M., Kim J. I., Ham M., Talukdar S., Chen A., Lu W. J., Bandyopadhyay G. K., Schwendener R., Olefsky J., and Kim J. B. (2011) Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes 60, 2474–2483 10.2337/db11-0194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boutens L., and Stienstra R. (2016) Adipose tissue macrophages: going off track during obesity. Diabetologia 59, 879–894 10.1007/s00125-016-3904-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Russo L., and Lumeng C. N. (2018) Properties and functions of adipose tissue macrophages in obesity. Immunology 155, 407–417 10.1111/imm.13002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kolaczkowska E., and Kubes P. (2013) Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175 10.1038/nri3399 [DOI] [PubMed] [Google Scholar]
- 41. De Filippo K., Dudeck A., Hasenberg M., Nye E., van Rooijen N., Hartmann K., Gunzer M., Roers A., and Hogg N. (2013) Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 121, 4930–4937 10.1182/blood-2013-02-486217 [DOI] [PubMed] [Google Scholar]
- 42. Selders G. S., Fetz A. E., Radic M. Z., and Bowlin G. L. (2017) An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen. Biomater. 4, 55–68 10.1093/rb/rbw041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bijnen M., Josefs T., Cuijpers I., Maalsen C. J., van de Gaar J., Vroomen M., Wijnands E., Rensen S. S., Greve J. W. M., Hofker M. H., Biessen E. A. L., Stehouwer C. D. A., Schalkwijk C. G., and Wouters K. (2018) Adipose tissue macrophages induce hepatic neutrophil recruitment and macrophage accumulation in mice. Gut 67, 1317–1327 10.1136/gutjnl-2016-313654 [DOI] [PubMed] [Google Scholar]
- 44. Elliott M. R., Chekeni F. B., Trampont P. C., Lazarowski E. R., Kadl A., Walk S. F., Park D., Woodson R. I., Ostankovich M., Sharma P., Lysiak J. J., Harden T. K., Leitinger N., and Ravichandran K. S. (2009) Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461, 282–286 10.1038/nature08296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Deaglio S., and Robson S. C. (2011) Ectonucleotidases as regulators of purinergic signaling in thrombosis, inflammation, and immunity. Adv. Pharmacol. 61, 301–332 10.1016/B978-0-12-385526-8.00010-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Petrone W. F., English D. K., Wong K., and McCord J. M. (1980) Free radicals and inflammation: superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proc. Natl. Acad. Sci. 77, 1159–1163 10.1073/pnas.77.2.1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chekeni F. B., Elliott M. R., Sandilos J. K., Walk S. F., Kinchen J. M., Lazarowski E. R., Armstrong A. J., Penuela S., Laird D. W., Salvesen G. S., Isakson B. E., Bayliss D. A., and Ravichandran K. S. (2010) Pannexin 1 channels mediate “find-me” signal release and membrane permeability during apoptosis. Nature 467, 863–867 10.1038/nature09413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Eugenín E. A., Brañes M. C., Berman J. W., and Sáez J. C. (2003) TNF plus IFN induce Connexin43 expression and formation of gap junctions between human monocytes/macrophages that enhance physiological responses. J. Immunol. 170, 1320–1328 10.4049/jimmunol.170.3.1320 [DOI] [PubMed] [Google Scholar]
- 49. Li W., Bao G., Chen W., Qiang X., Zhu S., Wang S., He M., Ma G., Ochani M., Al-Abed Y., Yang H., Tracey K. J., Wang P., D'Angelo J., and Wang H. (2018) Connexin 43 hemichannel as a novel mediator of sterile and infectious inflammatory diseases. Sci. Rep. 8, 166 10.1038/s41598-017-18452-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pillon N. J., Chan K. L., Zhang S., Mejdani M., Jacobson M. R., Ducos A., Bilan P. J., Niu W., and Klip A. (2016) Saturated fatty acids activate caspase-4/5 in human monocytes, triggering IL-1β and IL-18 release. Am. J. Physiol. Endocrinol. Metab. 311, E825–E835 10.1152/ajpendo.00296.2016 [DOI] [PubMed] [Google Scholar]
- 51. Liu X., Zhang Z., Ruan J., Pan Y., Magupalli V. G., Wu H., and Lieberman J. (2016) Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158 10.1038/nature18629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Aglietti R. A., Estevez A., Gupta A., Ramirez M. G., Liu P. S., Kayagaki N., Ciferri C., Dixit V. M., and Dueber E. C. (2016) GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc. Natl. Acad. Sci. U.S.A. 113, 7858–7863 10.1073/pnas.1607769113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Qu Y., Misaghi S., Newton K., Gilmour L. L., Louie S., Cupp J. E., Dubyak G. R., Hackos D., and Dixit V. M. (2011) Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation. J. Immunol. 186, 6553–6561 10.4049/jimmunol.1100478 [DOI] [PubMed] [Google Scholar]
- 54. Vong L., Sherman P. M., and Glogauer M. (2013) Quantification and visualization of neutrophil extracellular traps (NETs) from murine bone marrow-derived neutrophils. Methods Mol. Biol. 1031, 41–50 10.1007/978-1-62703-481-4_5 [DOI] [PubMed] [Google Scholar]
- 55. Penuela S., Bhalla R., Gong X.-Q., Cowan K. N., Celetti S. J., Cowan B. J., Bai D., Shao Q., and Laird D. W. (2007) Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J. Cell Sci. 120, 3772–3783 10.1242/jcs.009514 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.