Abstract
Protein prenylation is an essential posttranslational modification and includes protein farnesylation and geranylgeranylation using farnesyl diphosphate or geranylgeranyl diphosphate as substrates, respectively. Geranylgeranyl diphosphate synthase is a branch point enzyme in the mevalonate pathway that affects the ratio of farnesyl diphosphate to geranylgeranyl diphosphate. Abnormal geranylgeranyl diphosphate synthase expression and activity can therefore disrupt the balance of farnesylation and geranylgeranylation and alter the ratio between farnesylated and geranylgeranylated proteins. This change is associated with the progression of nonalcoholic fatty liver disease (NAFLD), a condition characterized by hepatic fat overload. Of note, differential accumulation of farnesylated and geranylgeranylated proteins has been associated with differential stages of NAFLD and NAFLD-associated liver fibrosis. In this review, we summarize key aspects of protein prenylation as well as advances that have uncovered the regulation of associated metabolic patterns and signaling pathways, such as Ras GTPase signaling, involved in NAFLD progression. Additionally, we discuss unique opportunities for targeting prenylation in NAFLD/hepatocellular carcinoma with agents such as statins and bisphosphonates to improve clinical outcomes.
Keywords: protein isoprenylation, liver metabolism, glucose metabolism, lipid metabolism, cell signaling, metabolic pattern, nonalcoholic fatty liver disease, prenylation, signaling pathway, geranylgeranyl diphosphate synthase (GGPPS), posttranslational modification, lipid modification, hepatocellular carcinoma (HCC), liver fibrosis, metabolic disorder
Introduction
Prenylation is a type of lipid modification wherein a farnesyl (15-carbon) or a geranylgeranyl (20-carbon) side chain is added to a C-terminal cysteine residue of a CaaX or CaaX-like motif, dependent on the characteristics of X (where C is cysteine, a is any aliphatic amino acid, and X is another amino acid); these modifications are called farnesylation and geranylgeranylation, respectively (1). Given the hydrophobicity of the lipids involved, prenylated proteins are anchored to cellular membranes in proximity to downstream signaling pathways involved in numerous cellular processes, including cell proliferation and differentiation, cell metabolism, and intracellular protein trafficking (2). Geranylgeranyl diphosphate synthase (GGPPS)2 is the branch point enzyme in the mevalonate (MVA) pathway that is responsible for synthesizing GGPP from its substrate FPP, and abnormal expression of this enzyme affects the ratio of FPP to GGPP, disrupting the balance of protein farnesylation and geranylgeranylation (3–5).
The existence of imbalances in this system has a high correlation with the development of many diseases, including nonalcoholic fatty liver disease (NAFLD) and NAFLD-associated fibrosis. NAFLD refers to a clinical condition characterized by hepatic fat overload without alcoholism (6). It is strongly associated with obesity, diabetes, and insulin resistance and is considered a metabolic syndrome (7). NAFLD is classified into nonalcoholic fatty liver (NAFL, simple steatosis) and nonalcoholic steatohepatitis (NASH) (8). The simple steatosis in NAFL represents a state of imbalance where triglyceride deposition overwhelms its consumption. Prolonged lipid accumulation and inflammation can progress to NASH, advanced liver fibrosis, cirrhosis, and, ultimately, hepatocellular carcinoma (HCC).
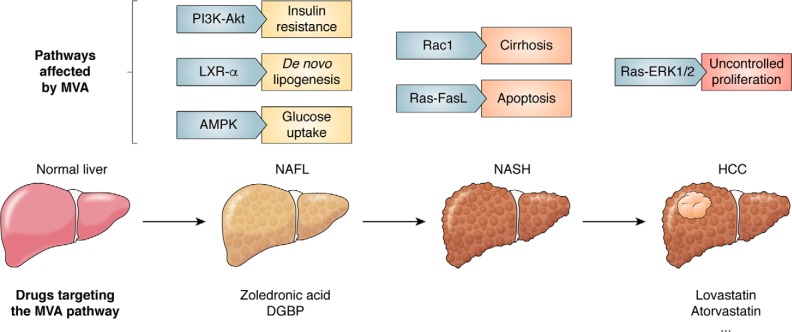
Although the pathogenesis of NAFLD has been investigated through extensive research and clinical studies, the molecular mechanism involved in the progression from NAFLD to HCC remains to be elucidated. Several central molecules/pathways related to the MVA pathway, including Ras-ERK1/2, PI3K-Akt, sterol regulatory element–binding protein 1 (SREBP), Rac, and AMPK, are activated during the progression of NAFLD to HCC. These changes give the cell features of proliferation, genomic instability, and immortalization, eventually promoting progression to HCC (Fig. 1).
Figure 1.
Several signaling pathways affected by metabolites in the MVA pathway involved in the progression from NAFLD to HCC. The progression of NAFLD to HCC is classified into four phases: normal liver, NAFL (simple steatosis), NASH, and HCC. When NAFLD develops, insulin resistance occurs as PI3K-Akt is activated in the liver. Simultaneously, LXR-α and AMPK, sensors of metabolic state dysfunction, promote DNL and glucose uptake. Activation of Rac1 and Ras-FasL is involved in the development of NASH by promoting cirrhosis and apoptosis. Then the Ras-ERK1/2 axis mediates proliferation, leading to the onset of HCC. All of the above pathways are regulated by metabolites in the MVA pathway, and corresponding targeted therapies have been developed.
Interestingly, the accumulation of differential amounts of farnesylated and geranylgeranylated proteins regulated by GGPPS has been associated with differential stages of NAFLD and NAFLD-associated fibrosis (4, 9). Statins, a class of compounds widely used to lower cholesterol, are inhibitors of HMG-CoA reductase (HMGCR, the upstream enzyme in the MVA pathway) and consequently alter the ratio of FPP/GGPP followed by the balance of protein prenylation (2). Considering the effects of several inhibitors targeting MVA pathway enzymes on immune control (66), metabolic disease (10), and cancer progression (11), protein prenylation can also affect the progression of NAFLD through processes such as metabolic reprogramming and signaling pathway activation. More importantly, identifying a drug targeting the prenylation balance can provide insights for prospective therapeutic strategies for NAFLD and HCC.
Protein prenylation
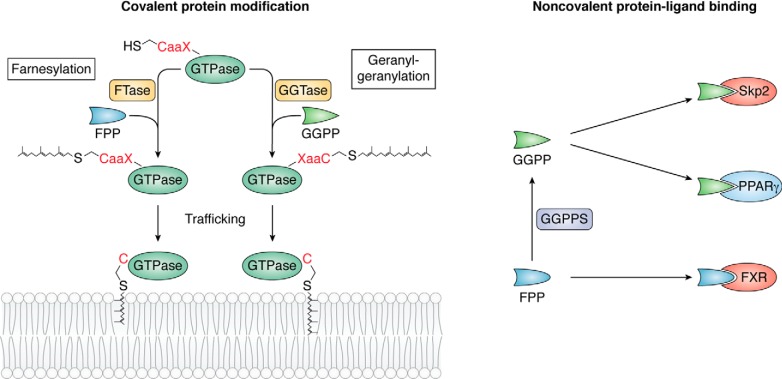
Anchorage to cellular membranes is a prerequisite for the biological function of many regulatory proteins, which can be located on the membrane surface or embedded in the lipid bilayer. Many peripheral proteins are targeted to membranes as a result of posttranslational modification with lipid moieties. Two types of isoprenoid lipids, FPP and GGPP, which are intermediates in the MVA pathway for cholesterol, terpene and terpenoid synthesis, are utilized for such modification (Fig. 2, left). Proteins with cysteine residues typically found in the CaaX motif can be farnesylated with FPP or geranylgeranylated with GGPP. Either of these biochemical reactions depends upon the nature of the X residue. If X is serine, methionine, alanine, or glutamine, the protein is farnesylated; if X refers to leucine or isoleucine, the protein is geranylgeranylated (12).
Figure 2.
Two functions of FPP and GGPP. Left, FPP and GGPP can be covalently attached to proteins, especially GTPases, by the isoprenyltransferases FTase and GGTase in processes called farnesylation and geranylgeranylation, respectively. Right, FPP and GGPP can directly interact with other proteins as ligands via noncovalent binding to regulate their activities. For example, GGPP can interact with Skp2 and PPARγ, whereas FPP interacts with FXR.
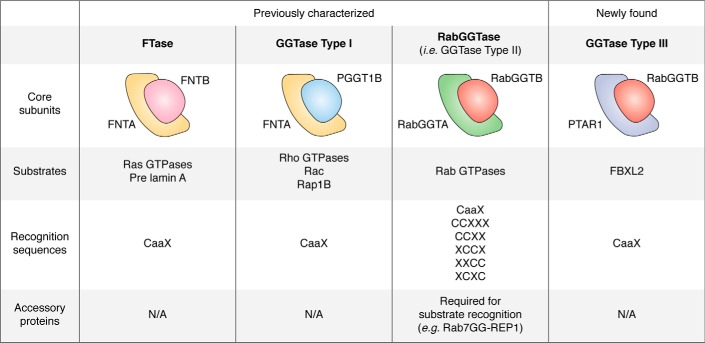
Protein prenylation depends on the activity of prenyltransferases. There are three prenyltransferases, all of which are heterodimeric enzymes containing α and β subunits. Farnesyltransferase (FTase) and geranylgeranyltransferase 1 (GGTase1) share the same α subunit but contain different β subunits. Both transferases recognize substrates with a CaaX sequence, the site where lipid modification occurs. Another prenyltransferase, GGTase2, is formed by RabGGTA (the α subunit) and RabGGTB (the β subunit). GGTase2 prenylates sites in additional C-terminal motifs, including CCXXX, CCXX, XCCX, XXCC, and XCXC. Unlike FTase and GGTase1, the prenylation by GGTase2 requires the participation of the Rab escort protein, an accessory protein involved in the recognition of Rab by GGTase2 (12). Distinct substrates have been identified for FTase (H-Ras, K-Ras, N-Ras, Ras2, Rap2, pre-Lamin A, Lamin B, RhoB, RhoE, and Rheb), GGTase1 (RhoA, RhoB, RhoC, Rab8, Rab11, Rab13, Rac1, Rac2, RalA, Rap1B, and Cdc42), and GGTase2 (Rab GTPases) (13–17). In addition, recently, a new prenyltransferase, GGTase3, which consists of the α subunit PTAR1 and the β subunit of GGTase2 (RabGGTB), was identified. This enzyme geranylgeranylates FBXL2, a ubiquitin ligase, allowing it to associate with cell membranes (18) (Fig. 3). After recognition, the aaX residues at the C terminus can be further removed by Ras-converting CaaX endopeptidase 1 (RCE1), and isoprenylcysteine carboxylmethyltransferase adds a methyl group to the isoprenoid-modified cysteine residue (12).
Figure 3.
Prenyltransferases and their substrates. Shown are the four classes of human prenyltransferases with α (PTAR1, FNTA (PTAR2), and RabGGTA (PTAR3)) and β (FNTB, PGGT1B, and RabGGTB) subunits and their substrates, recognition sequences, and accessory proteins. N/A, not applicable.
A large number of prenylated peptides in living cells without metabolic perturbation have been reported in a newly developed proteome-scale analysis (19). Hundreds of prenylated candidates have been identified by the development of isoprenoid analogues YnF and YnGG in combination with quantitative chemical proteomics, such as Ganab, K-Ras, N-Ras, Nos2, Nos3, Rab, Rac, and Rheb. Among these candidates, Ganab, Nos2, and Nos3 are involved in metabolic pathways, and K-Ras and N-Ras participate in thermogenesis, the insulin signaling pathway, and choline metabolism in cancer. In addition, Ras, Rheb, Rab, Rac, and liver kinase B1 (LKB1) are involved in the MAPK, PI3K/Akt, AMPK, and other signaling pathways, which are also engaged in metabolic regulation. These metabolism-related candidates give a hint that protein prenylation may influence metabolic state.
The balance of protein farnesylation and geranylgeranylation and its effect on altered metabolic states
The balance of protein farnesylation and geranylgeranylation is highly related to the activation state of GGPPS, the branch point enzyme in the MVA pathway. When GGPPS is activated, the GGPP/FPP ratio increases; consequently, protein geranylgeranylation is enhanced or vice versa, because the expression level and activity of FTase and GGTase normally do not change significantly. However, some proteins can alternatively undergo either type of prenylation under extreme conditions; for example, when either GGPP or FPP is unavailable, or X is phenylalanine, the proteins can be either farnesylated or geranylgeranylated (15). In addition, both geranylgeranylation and farnesylation of H-Ras can occur when GGPPS is knocked out (3), and K-Ras can be geranylgeranylated in cells treated with farnesyltransferase inhibitors (FTIs) (17). Furthermore, several studies about these alternative prenylation patterns have been published (13–17), although the molecular mechanisms remain unclear.
Another possible effect by alteration of FPP/GPP ratio is the direct change of metabolic pathways in the cell. FPP is a key intermediate in cholesterol metabolism (2), whereas GGPP is crucial in the metabolism of dolichols (20), which are essential for protein glycosylation (21, 22). Considering the different Km of the enzymes for FPP or GGPP destination, the FPP/GGPP ratio change might also influence the synthesis of cholesterol, heme A, dolichol, etc. by FPP and/or ubiquinone, etc. by GGPP. Therefore, FPP and GGPP, as metabolites, may regulate cell functions with a different mechanism to maintain the metabolic homeostasis apart from protein prenylation.
GGPPS is highly abundant in the liver, adipose tissue, and muscle of mice with obesity and insulin resistance but is expressed at a relatively low level under normal conditions (23). Previous studies have shown that insulin can induce Rab geranylgeranylation by activating GGTase enzymes in 3T3-L1 preadipocytes and that abrogation of GGTase activity inhibits the phosphorylation of MAPK pathway components in 3T3-L1 preadipocytes (24). GGPP stimulates PPARγ expression and adipogenesis (25). Inhibition of GGPPS by digeranyl bisphosphonate (DGBP) leads to reduced GGPP levels but accumulation of FPP, impairing protein geranylgeranylation in MC3T3-E1 preosteoblast cells (26). Our studies have also revealed that the MAPK/Egr-1/GGPPS/Ras axis plays an essential role in certain metabolic states, such as type 2 diabetes. Long-term insulin stimulation activates GGPPS and further activates K-Ras by enhancing its geranylgeranylation. Ras/MAPK/Erk1/2 signaling results in insulin receptor substrate-1 (IRS-1) phosphorylation, contributing to insulin resistance (27). Furthermore, we observed increased GGPPS expression in the skeletal muscles of mice with insulin resistance, and specific knockout of GGPPS in skeletal muscle improved systemic insulin sensitivity and glucose homeostasis by enhancing glucose uptake in skeletal muscle. These metabolic alterations mediated by ggpps knockout were achieved through decreased geranylgeranylation of RhoA, which further induced the phosphorylation of IRS-1 (28). Thus, the GGPPS/RhoA/Rho kinase/IRS-1 pathway mediates lipid-induced systemic insulin resistance in obese mice (29). In summary, GGPPS is crucial for the balance of protein farnesylation and geranylgeranylation in the regulation of metabolic states, which may influence other metabolic diseases, such as NAFLD/HCC.
Metabolic states mediated by the prenylation balance in NAFLD/HCC progression
Proliferative cancer cells often exploit nutrients, such as glucose, to support their energy demand and biomass synthesis. They tend to convert most glucose to lactate regardless of whether oxygen is present (aerobic glycolysis), referred to as the Warburg effect (30, 31). Although compared with fatty acid β-oxidation, glycolysis is an ineffective way to generate energy from glucose, hepatocytes rely more on glycolysis than on β-oxidation for energy during NAFLD/HCC progression (31). This alteration in metabolic control may eventually alter existing cell metabolism in a way that supports cell growth (32).
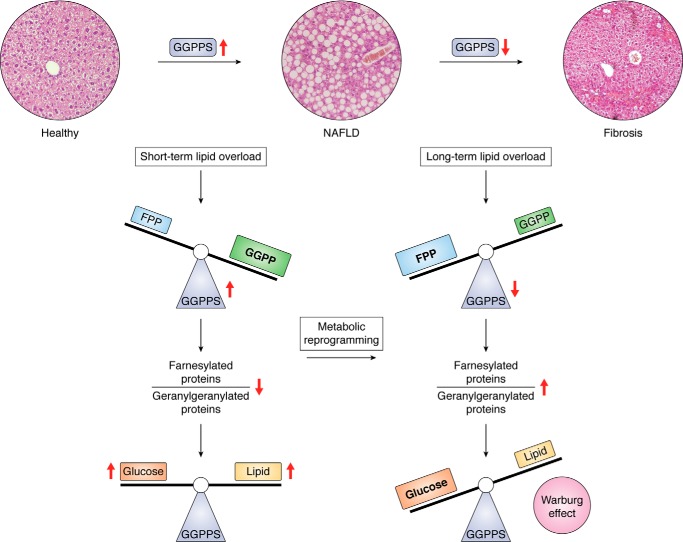
Our previous work revealed that GGPPS was highly expressed in the livers of NAFLD patients but down-regulated in HCC patients (33). Additionally, GGPPS was first up-regulated in the livers of mice with high-fat diet (HFD)-induced NAFLD and was then down-regulated after long-term HFD overload in NAFLD, which was associated with fibrosis, suggesting that the GGPPS-dependent protein prenylation balance mediates metabolic alterations during the development of NAFLD-associated fibrosis (Fig. 4). The balance of prenylation regulated by the two-phase change in GGPPS expression is associated with differential stages of NAFLD progression to HCC by influencing the activity of metabolic enzymes and signal transduction through the related signaling pathways.
Figure 4.
GGPPS regulates NAFLD-HCC progression by determining the hepatic glucose/fatty acid preference under fat overload. Short-term HFD overload increases GGPPS expression and triggers a change in the prenylation pattern favoring GGPP synthesis (9), leading to increased glycolysis and DNL. However, long-term HFD overload decreases GGPPS expression and disrupts the FPP and GGPP balance so as to unbalance the ratio of farnesylated proteins and geranylgeranylated proteins, which drives the Warburg effect through metabolic reprogramming favoring glycolysis to exacerbate fibrosis (4).
During NAFLD progression, an HFD enhances hepatic lipid oxidation and glycolysis. Additionally, an HFD accelerates lipid accumulation by up-regulating the expression of GGPPS (4, 9), which alters the relative ratio of FPP to GGPP, thereby influencing SREBP activation. However, GGPPS is down-regulated during the progression of NAFLD to HCC, which drives NAFLD-associated fibrosis by promoting glycolysis and suppressing oxidative phosphorylation via the LKB1/AMPK axis (4). Hence, these factors promote the Warburg effect to support glycolysis and result in metabolic reprogramming during NAFLD progression, thus driving HCC progression.
FPP/GGPP and SREBP activation
SREBP is a critical regulator for maintaining lipid homeostasis (34). SREBP precursors form a complex with SREBP cleavage–activating protein, which is retained by the insulin-induced gene-1/2 proteins in the endoplasmic reticulum in the presence of increased cellular sterol levels. Downstream effectors of SREBP are primarily encoded by genes involved in regulating lipid metabolism, particularly those associated with de novo lipogenesis (DNL), including FAS (35), ACC1 (35), and SCD1 (36). Moreover, these genes can mediate the inflammatory reaction in NAFLD, which is essential for HCC progression. Hence, SREBP down-regulation can reduce the inflammatory reaction and prevent the progression from NAFLD to NASH, cirrhosis, and even HCC. A recent study revealed that geranylgeranylated RhoA-dependent actomyosin contraction inhibits SREBP1 activation. FPP accumulation resulting from GGPPS deficiency inhibits DNL in hepatocytes, suppressing SREBP-1 expression and LXR activation by activating FXR/SHP signaling (37). Moreover, a recent report indicated that SREBP-1 couples mechanical cues and lipid metabolism via protein geranylgeranylation, indicating the role of isoprenoids in regulating SREBP activation in lipid metabolism (38). Moreover, our study showed that zoledronic acid, an inhibitor of FPPS, inhibits hepatic DNL and liver steatosis by suppressing RhoA prenylation-dependent SREBP-1c activation (39). Considering the alteration of the prenylation balance by GGPPS in NAFLD, this finding indicates that up-regulation of SREBP mediates inflammatory reactions and increases hepatic total cholesterol and triglyceride levels, consequently correlating with HCC progression (4).
LKB farnesylation and AMPK activation
As the LKB1-AMPK pathway is important for cells to maintain metabolic homeostasis by sensing the AMP/ATP levels (40), it is thought to control the GGPPS-regulated metabolic reprogramming process. Mechanistically, as we reported, Ggpps deficiency enhances the farnesylation of LKB1 and promotes metabolic reprogramming by regulating AMPK activity (4). AMPK activation turns off ATP-consuming pathways and switches on ATP-producing pathways. Such metabolic alterations further induce hepatic inflammation through elevated macrophage infiltration and proinflammatory cytokine production. In addition, insulin resistance is frequently detected in patients with NAFLD; this state decreases AMPK activity and produces a hyperuricemic environment, resulting in hepatic ATP depletion and further favoring glycolysis (41). Thus, the GGPPS-regulated protein prenylation balance is a metabolic controller of fat overload–induced NAFLD and fibrosis development (4).
Signaling pathways mediated by prenylation in NAFLD/HCC progression
The progression from NAFLD to HCC is related to several signaling pathways involved in steatogenic, fibrogenic, proliferative, and proinflammatory signaling (42), such as the Ras/PI3K/AKT and Hippo-Yes–associated protein (YAP)/YAZ pathways. These signaling pathways have been reported to be regulated by the prenylation balance. Thus, an abnormal balance of protein prenylation may contribute to HCC progression from NAFLD via signal transduction through related signaling pathways.
Ras signaling pathway
The intracellular GTP-binding proteins involved in signal transduction comprise the largest family of prenylated proteins. The Ras protein is the most extensively studied small GTPase (11). As malignancy is associated with Ras mutation, Ras is a potential target for cancer therapy. For example, Ras mutations have been found in pancreatic cancer (90%) (43), thyroid cancer (50%) (44), acute myeloid leukemia (44%) (45), colon cancer (47%) (46), melanoma (36%) (47), and lung cancer (30%) (48). To date, three forms of mutated Ras have been identified: H-Ras, K-Ras, and N-Ras. K-Ras mutations occur more commonly in cancer than do N-Ras mutations, which are usually found in hematologic malignancies (49). These three different types of mutated Ras share over 90% sequence homology but vary in their association with the inner plasma membrane (50), which accounts for their differing oncogenic potential (51).
Differences in the balance of prenylation and membrane-anchored Ras isoforms can explain differences in the activation of K-Ras, N-Ras, and H-Ras. K-Ras and N-Ras can be geranylgeranylated when FTase is inhibited, whereas H-Ras can only be farnesylated by FTase (17). After prenylation, K-Ras bypasses the Golgi complex, yet N-Ras and H-Ras encounter palmitoyl acyltransferases on the cytoplasmic surface of the Golgi (52). The locations of the three isoforms at the cell membrane also differ; K-Ras and N-Ras are not associated with lipid rafts, although the GDP-bound form of H-Ras binds to lipid rafts (53).
Once activated by prenylation, Ras acts as an upstream master regulator, directly activating downstream pathways involved in various cellular functions. Two pathways, the PI3K/AKT pathway and the Raf/MEK/MAPK/ERK pathway, mediate tumor cell proliferation, migration, and metastasis. Activation of PI3K/AKT signaling by growth factors increases the expression of SREBP1 and SREBP2 (54–58), which results in elevated lipid and cholesterol production and progression of NAFLD (59–61). Moreover, PI3K activity may be decreased with inhibition of the MVA pathway, possibly through decreased Ras prenylation (62).
Several studies have revealed that prenylation inhibition may be an efficient therapeutic strategy for cancer via inhibition of the Ras signaling pathway. Simultaneous knockout of prenyltransferase β subunits (both Fntb and Pggt1b) suppresses K-Ras–induced lung tumor progression more efficiently than deletion of either subunit alone (63). In addition, conditional Fntb or Pggt1b deficiency reduces K-Ras-G12D–induced lung cancer formation in mice (63), suggesting that FTase and GGTase1 are targets for cancer therapy.
Other Ras GTPase superfamily signaling pathways
All proteins in the Ras GTPase superfamily are localized to the membrane by prenylation, and several studies have shown that statin treatment decreases the prenylated and membrane-associated forms of Ras, Rho, Rac, Rap, and Rab subfamily proteins (64). In pancreatic cancer with K-Ras mutation (90%) (49), pathways mediated by geranylgeranylated RalA and RalB correlate much more strongly with malignancy onset than do MEK or AKT pathways (65). In addition, geranylgeranylated RhoC performs an essential function in tumor metastasis (65, 66). A recent study reported that GGPPS inhibition therapy can be a novel strategy for the treatment of pancreatic ductal adenocarcinoma by disrupting Rab geranylgeranylation to induce the unfolded protein response pathway (67), which also occurs in HCC (68).
Furthermore, the Rho subfamily GTPase Rac1 is required for the induction of K-Ras–driven lung cancer in mice (69). Importantly, geranylgeranylated cell division cycle 42 (Cdc42) and Rac are downstream targets of Ras in mediating fibroblast transformation (70). Atorvastatin, which blocks FPP and GGPP production by inhibiting HMGCR, prevents HCC development by decreasing Rac1 prenylation to inhibit MYC phosphorylation (71). As MYC is a potent oncogene that causes transformation in various cancer types, inhibition of MYC phosphorylation can induce sustained regression of HCC (72). Thus, the MVA pathway is important in this MYC-driven HCC model.
Another Ras GTPase superfamily protein, RhoB, is both geranylgeranylated (70%) and farnesylated (30%) under physiological conditions. It has been shown that geranylgeranylated RhoB suppresses Ras-induced transformation and that inhibition of RhoB farnesylation contributes to FTI-induced apoptosis (73). However, both geranylgeranylated RhoB and farnesylated RhoB suppress tumor activity in several human epithelial cancer cells (74). A previous study also showed that the oncogene YAP and transcriptional coactivator with PDZ-binding motif (TAZ) require GGPP-mediated RhoA prenylation to be functional (75). Mechanistically, the balance of prenylation maintains GGPP levels, regulating the nuclear localization of YAP and TAZ through RhoA prenylation (76). Another study demonstrated that treatment with atorvastatin or geranylgeranyltransferase inhibitors (GGTIs) increased the levels of Lats1 and Lats2, two important tumor suppressors involved in the Hippo-YAP/YAZ pathway, which further controls cell proliferation and metabolism (77). Therefore, the balance of prenylation participates in signal transduction through pathways involved in NAFLD/HCC progression.
Other factors involved in protein prenylation in NAFLD/HCC progression
Proinflammatory cytokine release regulated by protein prenylation
Inflammation plays a key role in extracellular matrix deposition and fibrosis, which further leads to HCC (78). The progression from NAFLD to fibrosis requires glucose, lipid, and amino acid metabolic reprogramming in response to a stressful microenvironment (79). Such metabolic changes further contribute to hepatic inflammation via enhanced proinflammatory cytokine production and macrophage infiltration. In a previous study, we illustrated a glycolysis-inflammation regulatory network in hepatocytes, referring to reprogrammed metabolism and inflammation in hepatocytes, with macrophages collectively accelerating progression from NAFLD to fibrosis (4). Further evidence indicates that proinflammatory cytokines, such as interleukin-6, C-reactive protein, C-peptide, and adiponectin, are essential for the development of fibrosis from NASH (31). Previous work has shown that statins can reduce C-reactive protein production stimulated by interleukin-6 (80), further inhibiting inflammatory reactions and hepatic tissue fibrosis. Overall, proinflammatory cytokine release regulated by altered protein prenylation is essential for the progression of NAFLD to fibrosis.
Rab geranylgeranylation and mitochondrial function
Mitochondrial quality control (QC) plays an important role in HCC progression, as mitochondrial dysfunction is commonly found in HCC (81); moreover, by eliminating damaged mitochondria, mitophagy is required for mitochondrial QC (82). Therefore, accumulation of damaged mitochondria is attributed to defective mitophagy, a metabolic defect in patients with NASH (83). Defective mitophagy and mitochondrial biosynthesis can promote fibrosis, which is one of the most important factors during HCC progression. Rab has a crucial role in membrane trafficking, particularly vesicle formation, transport, fusion, cargo selection, and sorting, which are regulated by prenylation. Indeed, as a mitophagy effector downstream of the ubiquitin E3 ligase Parkin, Rab7 is fundamental in mitophagy (84). In our study, Ggpps deletion impaired Rab7 geranylgeranylation and mitophagy, consequently causing defects in mitophagy in hepatocytes, suggesting that dysregulation of Rab7 geranylgeranylation is a susceptibility factor for NAFLD/fibrosis progression. Thus, GGPPS-mediated prenylation in mitochondrial QC is important during HCC progression (4). Furthermore, we analyzed the CaaX motifs in mitochondria-related proteins of mice and humans and found several potential mitochondrial prenyltransferase substrates, including Rab24 (85), Rab32 (86), and Rab35 (87), which may also influence mitochondrial function.
FPP/GGPP as ligands promoting the progression of NAFLD to HCC
The metabolic changes that occur in tumors not only provide energy for cell division but also generate metabolites for downstream signaling. The isoprenoid and sterol metabolites produced by the MVA pathway also perform signaling functions.
FPP and GGPP, as metabolites of the MVA pathway, can act as ligands and directly bind to proteins, thus acting as individual molecules rather than prenylation agents. FPP can directly interact with FXR to control the activity of downstream pathways (88), whereas GGPP binds with Skp2 (28) and PPARγ (25) (Fig. 2, right). Currently, we are working to identify some proteins to which GGPP/FPP bind, which may predict metabolic properties and NAFLD progression.
Drugs targeting the MVA pathway in NAFLD/HCC progression
As mentioned above, the balance of prenylation determines the progression from NAFLD to HCC. Thus, identifying a drug-targeting mediator of the prenylation balance involved in the MVA pathway can provide insight into prospective targeted therapies for NAFLD and HCC.
The MVA pathway is one of the most commonly clinically targeted biochemical pathways in human diseases, and inhibitors of the MVA pathway, such as statins and bisphosphonates, are extensively used in clinical trials (90). Statins, including lovastatin and atorvastatin, inhibit HMGCR, lowering the plasma cholesterol level in the treatment of hypercholesterolemia and atherosclerosis (91, 92). HCC is also responsive to statins (93), perhaps due to the hepatotropic pharmacology of these drugs (94). In addition, reports indicate that statins can act as adjuvants to stimulate the immune system and boost immunity as potent cancer therapeutics targeting immune activation (95, 96). Statins can also reduce cancer stemness, targeting cancer stem cells without impairing the function of innate immunity. Mechanistically, statins attenuate the prenylation of Ras family proteins, which is also highly clinically significant in HCC (97, 98). In addition, simvastatin decreases the synthesis of FPP and GGPP and exhibits anticancer effects against HCC by inducing G0/G1 arrest through up-regulation of p27 (99).
On the other hand, GGTase1 and FTase are required for tumor progression and maintenance (69, 100). GGTIs have been shown to be efficient in treating several cancers and inflammatory diseases (101), and one such agent, GGTI-2418, has been assessed in Phase I clinical trials (102). Two FTIs that have advanced to Phase III clinical trials are lonafarnib (102) and tipifarnib (103–105). However, these drugs could not improve the outcomes of advanced pancreatic cancer (103), advanced colon cancer (104), advanced non-small-cell lung cancer (102), or acute myeloid leukemia (105). More importantly, as H-, N-, and K-Ras require prenylation for their transforming activities (106), inhibition of prenylation exhibits significant therapeutic efficacy in ∼30% of human cancers driven by Ras mutations (102, 107). The results of these collective drug studies indicate that the MVA pathway can be considered a druggable target for HCC therapy.
Prospective applications
Although statins exhibit therapeutic efficacy in NAFLD/HCC, long-term use of statins is associated with adverse effects, such as myopathy (108) and liver injury (109). These effects might develop because statins suppress the MVA pathway from its initiation, thus leading to isoprenoid depletion, which was later demonstrated to be essential for cellular functions. Approximately 300 proteins related to cellular functions, such as membrane trafficking and signal transduction, in the human proteome are modified by prenyltransferases (110). Therefore, to prevent these side effects, other therapies combining different types and doses of MVA pathway inhibitors should be evaluated to achieve the best treatment effect without influencing protein prenylation. In addition, other inhibitors, such as nitrogenous bisphosphonates (NBPs) and DGBP, hold great promise for the treatment of NAFLD by targeting the balance of protein farnesylation and geranylgeranylation.
NBPs, including zoledronic acid, as potent inhibitors of FPPS, are widely used to treat osteoporosis and metastatic bone disease and may also be applied in cancer (111). Similar to statins, zoledronic acid reduces the intracellular levels of both FPP and GGPP, leading to inhibition of protein prenylation (112). Intriguingly, it depletes GGPP more efficiently than FPP (113). This effect might be explained by the observation that GGPPS contains three NBP-binding sites that result in its inhibition by NBP treatment (114); alternatively, it might arise due to the suppression of squalene synthase by NBPs (114, 115), which may ultimately restore the FPP level. Researchers have also demonstrated the cellular effects of NBPs in promoting apoptosis and autophagy, which are mainly mediated via downstream GGPP depletion and decreased protein geranylgeranylation (114, 116). In our previous study, zoledronic acid, an NBP, inhibited FPPS and reduced lipid deposition in the liver, as demonstrated in both mice with HFD-induced hepatic steatosis and ob/ob mice (39). Considering the changes observed in NAFLD progression, such inhibitors can be used in clinical trials on HCC prevention and treatment.
In addition to developing inhibitors of HMGCR and FPPS, some research groups have recently developed several dialkyl bisphosphonate inhibitors of GGPPS, including DGBP, a potent inhibitor of GGPP synthesis (IC50 = 200 nm) (117). Through depletion of intracellular GGPP, DGBP specifically impairs protein geranylgeranylation without changing farnesylation (118). However, other studies have shown that DGBP administration causes intracellular FPP accumulation (26, 89). In addition, another study demonstrated that RAM2061, a potent GGPPS inhibitor, is efficient against pancreatic ductal adenocarcinoma (67). In summary, invention with GGPPS inhibitors provides a NAFLD-HCC therapy with improved selectivity. More studies investigating the balance of farnesylation and geranylgeranylation in NAFLD progression are required to promote the development of NAFLD-HCC therapies.
Author contributions
Y. Z. and C.-J. L. conceptualization; Y. Z., T.-Y. W., and M.-F. Z. resources; Y. Z. data curation; Y. Z. and T.-Y. W. software; Y. Z. formal analysis; Y. Z. supervision; Y. Z. funding acquisition; Y. Z. validation; Y. Z. investigation; Y. Z. visualization; Y. Z. methodology; Y. Z. writing-original draft; Y. Z. and C.-J. L. project administration; Y. Z. and C.-J. L. writing-review and editing.
This work was supported by Chinese National Natural Science Foundation Grant 31601153 (to Y. Z.), Nature Science Foundation of Jiangsu Province Grant BK20160619 (to Y. Z.), Fundamental Research Funds for the Central Universities Grants 14380269 and 14380343 (to Y. Z.), and China Postdoctoral Science Foundation Grant 2016M601778 (to Y. Z.). The authors declare that they have no conflicts of interest with the contents of this article.
- GGPPS
- geranylgeranyl diphosphate synthase
- NAFLD
- non alcoholic fatty liver disease
- NASH
- non alcoholic steatohepatitis
- HCC
- hepatocellular carcinoma
- FPPS
- farnesyl diphosphate synthase
- GGPP
- geranylgeranyl diphosphate
- FPP
- farnesyl diphosphate
- MVA
- mevalonate
- FTase
- farnesyltransferase
- GGTase
- geranylgeranyltransferase
- FTI
- farnesyltransferase inhibitor
- DNL
- de novo lipogenesis
- GGTI
- geranylgeranyltransferase inhibitor
- HMGCR
- HMG-CoA reductase
- DGBP
- digeranyl bisphosphate
- IRS
- insulin receptor substrate
- SREBP
- sterol-regulatory element–binding protein
- NBP
- nitrogenous bisphosphate
- QC
- quality control
- NAFL
- nonalcoholic fatty liver
- ERK
- extracellular signal–regulated kinase
- PI3K
- phosphatidylinositol 3-kinase
- AMPK
- AMP-activated protein kinase
- LKB1
- liver kinase B1
- MAPK
- mitogen-activated protein kinase
- HFD
- high-fat diet
- YAP
- Yes-associated protein
- MEK
- mitogen-activated protein kinase/extracellular signal-regulated kinase kinase.
References
- 1. Zhang F. L., and Casey P. J. (1996) Protein prenylation: molecular mechanisms and functional consequences. Annu. Rev. Biochem. 65, 241–269 10.1146/annurev.bi.65.070196.001325 [DOI] [PubMed] [Google Scholar]
- 2. Wang M., and Casey P. J. (2016) Protein prenylation: unique fats make their mark on biology. Nat. Rev. Mol. Cell Biol. 17, 110–122 10.1038/nrm.2015.11 [DOI] [PubMed] [Google Scholar]
- 3. Wang X. X., Ying P., Diao F., Wang Q., Ye D., Jiang C., Shen N., Xu N., Chen W. B., Lai S. S., Jiang S., Miao X. L., Feng J., Tao W. W., Zhao N. W., et al. (2013) Altered protein prenylation in Sertoli cells is associated with adult infertility resulting from childhood mumps infection. J. Exp. Med. 210, 1559–1574 10.1084/jem.20121806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu J., Jiang S., Zhao Y., Sun Q., Zhang J., Shen D., Wu J., Shen N., Fu X., Sun X., Yu D., Chen J., He J., Shi T., Ding Y., et al. (2018) Geranylgeranyl diphosphate synthase (GGPPS) regulates non-alcoholic fatty liver disease (NAFLD)-fibrosis progression by determining hepatic glucose/fatty acid preference under high-fat diet conditions. J. Pathol. 246, 277–288 10.1002/path.5131 [DOI] [PubMed] [Google Scholar]
- 5. Chen Z., Xu N., Chong D., Guan S., Jiang C., Yang Z., and Li C. (2018) Geranylgeranyl pyrophosphate synthase facilitates the organization of cardiomyocytes during mid-gestation through modulating protein geranylgeranylation in mouse heart. Cardiovasc. Res. 114, 965–978 10.1093/cvr/cvy042 [DOI] [PubMed] [Google Scholar]
- 6. Masuoka H. C., and Chalasani N. (2013) Nonalcoholic fatty liver disease: an emerging threat to obese and diabetic individuals. Ann. N.Y. Acad. Sci. 1281, 106–122 10.1111/nyas.12016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marengo A., Rosso C., and Bugianesi E. (2016) Liver cancer: connections with obesity, fatty liver, and cirrhosis. Annu. Rev. Med. 67, 103–117 10.1146/annurev-med-090514-013832 [DOI] [PubMed] [Google Scholar]
- 8. Takahashi Y., Soejima Y., and Fukusato T. (2012) Animal models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 18, 2300–2308 10.3748/wjg.v18.i19.2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao Y., Zhao M. F., Jiang S., Wu J., Liu J., Yuan X. W., Shen D., Zhang J. Z., Zhou N., He J., Fang L., Sun X. T., Xue B., and Li C. J. (2020) Liver governs adipose remodelling via extracellular vesicles in response to lipid overload. Nat. Commun. 11, 719 10.1038/s41467-020-14450-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miersch S., Sliskovic I., Raturi A., and Mutus B. (2007) Antioxidant and antiplatelet effects of rosuvastatin in a hamster model of prediabetes. Free Radic. Biol. Med. 42, 270–279 10.1016/j.freeradbiomed.2006.10.045 [DOI] [PubMed] [Google Scholar]
- 11. Swanson K. M., and Hohl R. J. (2006) Anti-cancer therapy: targeting the mevalonate pathway. Curr. Cancer Drug Targets 6, 15–37 10.2174/156800906775471743 [DOI] [PubMed] [Google Scholar]
- 12. Perez-Sala D. (2007) Protein isoprenylation in biology and disease: general overview and perspectives from studies with genetically engineered animals. Front. Biosci. 12, 4456–4472 10.2741/2401 [DOI] [PubMed] [Google Scholar]
- 13. James G. L., Goldstein J. L., and Brown M. S. (1995) Polylysine and CVIM sequences of K-RasB dictate specificity of prenylation and confer resistance to benzodiazepine peptidomimetic in vitro. J. Biol. Chem. 270, 6221–6226 10.1074/jbc.270.11.6221 [DOI] [PubMed] [Google Scholar]
- 14. Baron R., Fourcade E., Lajoie-Mazenc I., Allal C., Couderc B., Barbaras R., Favre G., Faye J. C., and Pradines A. (2000) RhoB prenylation is driven by the three carboxyl-terminal amino acids of the protein: evidenced in vivo by an anti-farnesyl cysteine antibody. Proc. Natl. Acad. Sci. U.S.A. 97, 11626–11631 10.1073/pnas.97.21.11626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carboni J. M., Yan N., Cox A. D., Bustelo X., Graham S. M., Lynch M. J., Weinmann R., Seizinger B. R., Der C. J., and Barbacid M. (1995) Farnesyltransferase inhibitors are inhibitors of Ras but not R-Ras2/TC21, transformation. Oncogene 10, 1905–1913 [PubMed] [Google Scholar]
- 16. Rowell C. A., Kowalczyk J. J., Lewis M. D., and Garcia A. M. (1997) Direct demonstration of geranylgeranylation and farnesylation of Ki-Ras in vivo. J. Biol. Chem. 272, 14093–14097 10.1074/jbc.272.22.14093 [DOI] [PubMed] [Google Scholar]
- 17. Whyte D. B., Kirschmeier P., Hockenberry T. N., Nunez-Oliva I., James L., Catino J. J., Bishop W. R., and Pai J. K. (1997) K- and N-Ras are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors. J. Biol. Chem. 272, 14459–14464 10.1074/jbc.272.22.14459 [DOI] [PubMed] [Google Scholar]
- 18. Kuchay S., Wang H., Marzio A., Jain K., Homer H., Fehrenbacher N., Philips M. R., Zheng N., and Pagano M. (2019) GGTase3 is a newly identified geranylgeranyltransferase targeting a ubiquitin ligase. Nat. Struct. Mol. Biol. 26, 628–636 10.1038/s41594-019-0249-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Storck E. M., Morales-Sanfrutos J., Serwa R. A., Panyain N., Lanyon-Hogg T., Tolmachova T., Ventimiglia L. N., Martin-Serrano J., Seabra M. C., Wojciak-Stothard B., and Tate E. W. (2019) Dual chemical probes enable quantitative system-wide analysis of protein prenylation and prenylation dynamics. Nat. Chem. 11, 552–561 10.1038/s41557-019-0237-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sakaihara T., Honda A., Tateyama S., and Sagami H. (2000) Subcellular fractionation of polyprenyl diphosphate synthase activities responsible for the syntheses of polyprenols and dolichols in spinach leaves. J. Biochem. 128, 1073–1078 10.1093/oxfordjournals.jbchem.a022835 [DOI] [PubMed] [Google Scholar]
- 21. Wild R., Kowal J., Eyring J., Ngwa E. M., Aebi M., and Locher K. P. (2018) Structure of the yeast oligosaccharyltransferase complex gives insight into eukaryotic N-glycosylation. Science 359, 545–550 10.1126/science.aar5140 [DOI] [PubMed] [Google Scholar]
- 22. Elharar Y., Podilapu A. R., Guan Z., Kulkarni S. S., and Eichler J. (2017) Assembling glycan-charged dolichol phosphates: chemoenzymatic synthesis of a Haloferax volcanii N-glycosylation pathway intermediate. Bioconjug. Chem. 28, 2461–2470 10.1021/acs.bioconjchem.7b00436 [DOI] [PubMed] [Google Scholar]
- 23. Vicent D., Maratos-Flier E., and Kahn C. R. (2000) The branch point enzyme of the mevalonate pathway for protein prenylation is overexpressed in the ob/ob mouse and induced by adipogenesis. Mol. Cell Biol. 20, 2158–2166 10.1128/MCB.20.6.2158-2166.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Solomon C. S., Leitner J. W., and Goalstone M. L. (2003) Dominant negative α-subunit of farnesyl- and geranylgeranyl-transferase I inhibits insulin-induced differentiation of 3T3-L1 pre-adipocytes. Int. J. Obes. Relat. Metab. Disord. 27, 40–47 10.1038/sj.ijo.0802189 [DOI] [PubMed] [Google Scholar]
- 25. Weivoda M. M., and Hohl R. J. (2012) Geranylgeranyl pyrophosphate stimulates PPARγ expression and adipogenesis through the inhibition of osteoblast differentiation. Bone 50, 467–476 10.1016/j.bone.2011.09.056 [DOI] [PubMed] [Google Scholar]
- 26. Weivoda M. M., and Hohl R. J. (2011) The effects of direct inhibition of geranylgeranyl pyrophosphate synthase on osteoblast differentiation. J. Cell Biochem. 112, 1506–1513 10.1002/jcb.23087 [DOI] [PubMed] [Google Scholar]
- 27. Shen N., Yu X., Pan F. Y., Gao X., Xue B., and Li C. J. (2011) An early response transcription factor, Egr-1, enhances insulin resistance in type 2 diabetes with chronic hyperinsulinism. J. Biol. Chem. 286, 14508–14515 10.1074/jbc.M110.190165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen M. C., Tsai Y. C., Tseng J. H., Liou J. J., Horng S., Wen H. C., Fan Y. C., Zhong W. B., and Hsu S. P. (2017) Simvastatin inhibits cell proliferation and migration in human anaplastic thyroid cancer. Int. J. Mol. Sci. 18, E2690 10.3390/ijms18122690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tao W., Wu J., Xie B. X., Zhao Y. Y., Shen N., Jiang S., Wang X. X., Xu N., Jiang C., Chen S., Gao X., Xue B., and Li C. J. (2015) Lipid-induced muscle insulin resistance is mediated by GGPPS via modulation of the RhoA/Rho kinase signaling pathway. J. Biol. Chem. 290, 20086–20097 10.1074/jbc.M115.657742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harrison S. A., Torgerson S., and Hayashi P. H. (2003) The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am. J. Gastroenterol. 98, 2042–2047 10.1111/j.1572-0241.2003.07659.x [DOI] [PubMed] [Google Scholar]
- 31. Cohen J. C., Horton J. D., and Hobbs H. H. (2011) Human fatty liver disease: old questions and new insights. Science 332, 1519–1523 10.1126/science.1204265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vander Heiden M. G., Cantley L. C., and Thompson C. B. (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yu D. C., Liu J., Chen J., Shao J. J., Shen X., Xia H. G., Li C. J., Xue B., and Ding Y. T. (2014) GGPPS1 predicts the biological character of hepatocellular carcinoma in patients with cirrhosis. BMC Cancer 14, 248 10.1186/1471-2407-14-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gong Y., Lee J. N., Lee P. C., Goldstein J. L., Brown M. S., and Ye J. (2006) Sterol-regulated ubiquitination and degradation of Insig-1 creates a convergent mechanism for feedback control of cholesterol synthesis and uptake. Cell Metab. 3, 15–24 10.1016/j.cmet.2005.11.014 [DOI] [PubMed] [Google Scholar]
- 35. Wang S., Moustaid-Moussa N., Chen L., Mo H., Shastri A., Su R., Bapat P., Kwun I., and Shen C. L. (2014) Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 25, 1–18 10.1016/j.jnutbio.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yao D., Luo J., He Q., Shi H., Li J., Wang H., Xu H., Chen Z., Yi Y., and Loor J. J. (2017) SCD1 alters long-chain fatty acid (LCFA) composition and its expression is directly regulated by SREBP-1 and PPARγ 1 in dairy goat mammary cells. J. Cell Physiol. 232, 635–649 10.1002/jcp.25469 [DOI] [PubMed] [Google Scholar]
- 37. Watanabe M., Houten S. M., Wang L., Moschetta A., Mangelsdorf D. J., Heyman R. A., Moore D. D., and Auwerx J. (2004) Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Invest. 113, 1408–1418 10.1172/JCI21025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bertolio R., Napoletano F., Mano M., Maurer-Stroh S., Fantuz M., Zannini A., Bicciato S., Sorrentino G., and Del Sal G. (2019) Sterol regulatory element binding protein 1 couples mechanical cues and lipid metabolism. Nat. Commun. 10, 1326 10.1038/s41467-019-09152-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tang Q., Jiang S., Jia W., Shen D., Qiu Y., Zhao Y., Xue B., and Li C. (2017) Zoledronic acid, an FPPS inhibitor, ameliorates liver steatosis through inhibiting hepatic de novo lipogenesis. Eur. J. Pharmacol. 814, 169–177 10.1016/j.ejphar.2017.08.010 [DOI] [PubMed] [Google Scholar]
- 40. Shackelford D. B., and Shaw R. J. (2009) The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat. Rev. Cancer 9, 563–575 10.1038/nrc2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Abdelmalek M. F., Suzuki A., Guy C., Unalp-Arida A., Colvin R., Johnson R. J., Diehl A. M., and Nonalcoholic Steatohepatitis Clinical Research Network (2010) Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology 51, 1961–1971 10.1002/hep.23535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Polyzos S. A., Kountouras J., and Mantzoros C. S. (2019) Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metabolism 92, 82–97 10.1016/j.metabol.2018.11.014 [DOI] [PubMed] [Google Scholar]
- 43. Almoguera C., Shibata D., Forrester K., Martin J., Arnheim N., and Perucho M. (1988) Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell 53, 549–554 10.1016/0092-8674(88)90571-5 [DOI] [PubMed] [Google Scholar]
- 44. Lemoine N. R., Mayall E. S., Wyllie F. S., Williams E. D., Goyns M., Stringer B., and Wynford-Thomas D. (1989) High frequency of ras oncogene activation in all stages of human thyroid tumorigenesis. Oncogene 4, 159–164 [PubMed] [Google Scholar]
- 45. Janssen J. W., Steenvoorden A. C., Lyons J., Anger B., Böhlke J. U., Bos J. L., Seliger H., and Bartram C. R. (1987) RAS gene mutations in acute and chronic myelocytic leukemias, chronic myeloproliferative disorders, and myelodysplastic syndromes. Proc. Natl. Acad. Sci. U.S.A. 84, 9228–9232 10.1073/pnas.84.24.9228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., and Bos J. L. (1988) Genetic alterations during colorectal-tumor development. N. Engl. J. Med. 319, 525–532 10.1056/NEJM198809013190901 [DOI] [PubMed] [Google Scholar]
- 47. Ball N. J., Yohn J. J., Morelli J. G., Norris D. A., Golitz L. E., and Hoeffler J. P. (1994) Ras mutations in human melanoma: a marker of malignant progression. J. Invest. Dermatol. 102, 285–290 10.1111/1523-1747.ep12371783 [DOI] [PubMed] [Google Scholar]
- 48. Rodenhuis S., Slebos R. J., Boot A. J., Evers S. G., Mooi W. J., Wagenaar S. S., van Bodegom P. C., and Bos J. L. (1988) Incidence and possible clinical significance of K-ras oncogene activation in adenocarcinoma of the human lung. Cancer Res. 48, 5738–5741 [PubMed] [Google Scholar]
- 49. Bos J. L. (1989) ras oncogenes in human cancer: a review. Cancer Res. 49, 4682–4689 [PubMed] [Google Scholar]
- 50. Niv H., Gutman O., Kloog Y., and Henis Y. I. (2002) Activated K-Ras and H-Ras display different interactions with saturable nonraft sites at the surface of live cells. J. Cell Biol. 157, 865–872 10.1083/jcb.200202009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Drosten M., Simón-Carrasco L., Hernández-Porras I., Lechuga C. G., Blasco M. T., Jacob H. K. C., Fabbiano S., Potenza N., Bustelo X. R., Guerra C., and Barbacid M. (2017) H-Ras and K-Ras oncoproteins induce different tumor spectra when driven by the same regulatory sequences. Cancer Res. 77, 707–718 10.1158/0008-5472.CAN-16-2925 [DOI] [PubMed] [Google Scholar]
- 52. Apolloni A., Prior I. A., Lindsay M., Parton R. G., and Hancock J. F. (2000) H-ras but not K-ras traffics to the plasma membrane through the exocytic pathway. Mol. Cell Biol. 20, 2475–2487 10.1128/MCB.20.7.2475-2487.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Prior I. A., Harding A., Yan J., Sluimer J., Parton R. G., and Hancock J. F. (2001) GTP-dependent segregation of H-ras from lipid rafts is required for biological activity. Nat. Cell Biol. 3, 368–375 10.1038/35070050 [DOI] [PubMed] [Google Scholar]
- 54. Demoulin J. B., Ericsson J., Kallin A., Rorsman C., Rönnstrand L., and Heldin C. H. (2004) Platelet-derived growth factor stimulates membrane lipid synthesis through activation of phosphatidylinositol 3-kinase and sterol regulatory element-binding proteins. J. Biol. Chem. 279, 35392–35402 10.1074/jbc.M405924200 [DOI] [PubMed] [Google Scholar]
- 55. Zhou R. H., Yao M., Lee T. S., Zhu Y., Martins-Green M., and Shyy J. Y. (2004) Vascular endothelial growth factor activation of sterol regulatory element binding protein: a potential role in angiogenesis. Circ. Res. 95, 471–478 10.1161/01.RES.0000139956.42923.4A [DOI] [PubMed] [Google Scholar]
- 56. Fleischmann M., and Iynedjian P. B. (2000) Regulation of sterol regulatory-element binding protein 1 gene expression in liver: role of insulin and protein kinase B/cAkt. Biochem. J. 349, 13–17 10.1042/0264-6021:3490013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Luu W., Sharpe L. J., Stevenson J., and Brown A. J. (2012) Akt acutely activates the cholesterogenic transcription factor SREBP-2. Biochim. Biophys. Acta 1823, 458–464 10.1016/j.bbamcr.2011.09.017 [DOI] [PubMed] [Google Scholar]
- 58. Porstmann T., Griffiths B., Chung Y. L., Delpuech O., Griffiths J. R., Downward J., and Schulze A. (2005) PKB/Akt induces transcription of enzymes involved in cholesterol and fatty acid biosynthesis via activation of SREBP. Oncogene 24, 6465–6481 10.1038/sj.onc.1208802 [DOI] [PubMed] [Google Scholar]
- 59. Ricoult S. J., Yecies J. L., Ben-Sahra I., and Manning B. D. (2016) Oncogenic PI3K and K-Ras stimulate de novo lipid synthesis through mTORC1 and SREBP. Oncogene 35, 1250–1260 10.1038/onc.2015.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yamauchi Y., Furukawa K., Hamamura K., and Furukawa K. (2011) Positive feedback loop between PI3K-Akt-mTORC1 signaling and the lipogenic pathway boosts Akt signaling: induction of the lipogenic pathway by a melanoma antigen. Cancer Res. 71, 4989–4997 10.1158/0008-5472.CAN-10-4108 [DOI] [PubMed] [Google Scholar]
- 61. Calvisi D. F., Wang C., Ho C., Ladu S., Lee S. A., Mattu S., Destefanis G., Delogu S., Zimmermann A., Ericsson J., Brozzetti S., Staniscia T., Chen X., Dombrowski F., and Evert M. (2011) Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology 140, 1071–1083 10.1053/j.gastro.2010.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kusama T., Mukai M., Iwasaki T., Tatsuta M., Matsumoto Y., Akedo H., Inoue M., and Nakamura H. (2002) 3-Hydroxy-3-methylglutaryl-coenzyme a reductase inhibitors reduce human pancreatic cancer cell invasion and metastasis. Gastroenterology 122, 308–317 10.1053/gast.2002.31093 [DOI] [PubMed] [Google Scholar]
- 63. Liu M., Sjogren A. K., Karlsson C., Ibrahim M. X., Andersson K. M., Olofsson F. J., Wahlstrom A. M., Dalin M., Yu H., Chen Z., Yang S. H., Young S. G., and Bergo M. O. (2010) Targeting the protein prenyltransferases efficiently reduces tumor development in mice with K-RAS-induced lung cancer. Proc. Natl. Acad. Sci. U.S.A. 107, 6471–6476 10.1073/pnas.0908396107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tsubaki M., Mashimo K., Takeda T., Kino T., Fujita A., Itoh T., Imano M., Sakaguchi K., Satou T., and Nishida S. (2016) Statins inhibited the MIP-1α expression via inhibition of Ras/ERK and Ras/Akt pathways in myeloma cells. Biomed. Pharmacother. 78, 23–29 10.1016/j.biopha.2015.12.017 [DOI] [PubMed] [Google Scholar]
- 65. Hamad N. M., Elconin J. H., Karnoub A. E., Bai W., Rich J. N., Abraham R. T., Der C. J., and Counter C. M. (2002) Distinct requirements for Ras oncogenesis in human versus mouse cells. Genes Dev. 16, 2045–2057 10.1101/gad.993902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Clark E. A., Golub T. R., Lander E. S., and Hynes R. O. (2000) Genomic analysis of metastasis reveals an essential role for RhoC. Nature 406, 532–535 10.1038/35020106 [DOI] [PubMed] [Google Scholar]
- 67. Haney S. L., Varney M. L., Chhonker Y. S., Shin S., Mehla K., Crawford A. J., Smith H. J., Smith L. M., Murry D. J., Hollingsworth M. A., and Holstein S. A. (2019) Inhibition of geranylgeranyl diphosphate synthase is a novel therapeutic strategy for pancreatic ductal adenocarcinoma. Oncogene 38, 5308–5320 10.1038/s41388-019-0794-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wu H., Wei L., Fan F., Ji S., Zhang S., Geng J., Hong L., Fan X., Chen Q., Tian J., Jiang M., Sun X., Jin C., Yin Z.-Y., Liu Q., et al. (2015) Integration of Hippo signalling and the unfolded protein response to restrain liver overgrowth and tumorigenesis. Nat. Commun. 6, 6239–6239 10.1038/ncomms7239 [DOI] [PubMed] [Google Scholar]
- 69. Sjogren A. K., Andersson K. M., Liu M., Cutts B. A., Karlsson C., Wahlstrom A. M., Dalin M., Weinbaum C., Casey P. J., Tarkowski A., Swolin B., Young S. G., and Bergo M. O. (2007) GGTase-I deficiency reduces tumor formation and improves survival in mice with K-RAS-induced lung cancer. J. Clin. Invest. 117, 1294–1304 10.1172/JCI30868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Qiu R. G., Abo A., McCormick F., and Symons M. (1997) Cdc42 regulates anchorage-independent growth and is necessary for Ras transformation. Mol. Cell Biol. 17, 3449–3458 10.1128/MCB.17.6.3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cao Z., Fan-Minogue H., Bellovin D. I., Yevtodiyenko A., Arzeno J., Yang Q., Gambhir S. S., and Felsher D. W. (2011) MYC phosphorylation, activation, and tumorigenic potential in hepatocellular carcinoma are regulated by HMG-CoA reductase. Cancer Res. 71, 2286–2297 10.1158/0008-5472.CAN-10-3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Meyer N., and Penn L. Z. (2008) Reflecting on 25 years with MYC. Nat. Rev. Cancer 8, 976–990 10.1038/nrc2231 [DOI] [PubMed] [Google Scholar]
- 73. Mazières J., Tillement V., Allal C., Clanet C., Bobin L., Chen Z., Sebti S. M., Favre G., and Pradines A. (2005) Geranylgeranylated, but not farnesylated, RhoB suppresses Ras transformation of NIH-3T3 cells. Exp. Cell Res. 304, 354–364 10.1016/j.yexcr.2004.10.019 [DOI] [PubMed] [Google Scholar]
- 74. Chen Z., Sun J., Pradines A., Favre G., Adnane J., and Sebti S. M. (2000) Both farnesylated and geranylgeranylated RhoB inhibit malignant transformation and suppress human tumor growth in nude mice. J. Biol. Chem. 275, 17974–17978 10.1074/jbc.C000145200 [DOI] [PubMed] [Google Scholar]
- 75. Sorrentino G., Ruggeri N., Specchia V., Cordenonsi M., Mano M., Dupont S., Manfrin A., Ingallina E., Sommaggio R., Piazza S., Rosato A., Piccolo S., and Del Sal G. (2014) Metabolic control of YAP and TAZ by the mevalonate pathway. Nat. Cell Biol. 16, 357–366 10.1038/ncb2936 [DOI] [PubMed] [Google Scholar]
- 76. Santinon G., Pocaterra A., and Dupont S. (2016) Control of YAP/TAZ activity by metabolic and nutrient-sensing pathways. Trends Cell Biol. 26, 289–299 10.1016/j.tcb.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 77. Aylon Y., Gershoni A., Rotkopf R., Biton I. E., Porat Z., Koh A. P., Sun X., Lee Y., Fiel M. I., Hoshida Y., Friedman S. L., Johnson R. L., and Oren M. (2016) The LATS2 tumor suppressor inhibits SREBP and suppresses hepatic cholesterol accumulation. Genes Dev. 30, 786–797 10.1101/gad.274167.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wang X., Zheng Z., Caviglia J. M., Corey K. E., Herfel T. M., Cai B., Masia R., Chung R. T., Lefkowitch J. H., Schwabe R. F., and Tabas I. (2016) Hepatocyte TAZ/WWTR1 promotes inflammation and fibrosis in nonalcoholic steatohepatitis. Cell Metab. 24, 848–862 10.1016/j.cmet.2016.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sookoian S., and Pirola C. J. (2014) NAFLD. Metabolic make-up of NASH: from fat and sugar to amino acids. Nat. Rev. Gastroenterol. Hepatol. 11, 205–207 10.1038/nrgastro.2014.25 [DOI] [PubMed] [Google Scholar]
- 80. Arnaud C., Burger F., Steffens S., Veillard N. R., Nguyen T. H., Trono D., and Mach F. (2005) Statins reduce interleukin-6-induced C-reactive protein in human hepatocytes: new evidence for direct antiinflammatory effects of statins. Arterioscler. Thromb. Vasc. Biol. 25, 1231–1236 10.1161/01.ATV.0000163840.63685.0c [DOI] [PubMed] [Google Scholar]
- 81. Begriche K., Massart J., Robin M. A., Bonnet F., and Fromenty B. (2013) Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology 58, 1497–1507 10.1002/hep.26226 [DOI] [PubMed] [Google Scholar]
- 82. Scherz-Shouval R., and Elazar Z. (2011) Regulation of autophagy by ROS: physiology and pathology. Trends Biochem. Sci. 36, 30–38 10.1016/j.tibs.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 83. Sanyal A. J. (2005) Mechanisms of disease: pathogenesis of nonalcoholic fatty liver disease. Nat. Clin. Pract. Gastroenterol. Hepatol. 2, 46–53 10.1038/ncpgasthep0084 [DOI] [PubMed] [Google Scholar]
- 84. Wong Y. C., Ysselstein D., and Krainc D. (2018) Mitochondria-lysosome contacts regulate mitochondrial fission via RAB7 GTP hydrolysis. Nature 554, 382–386 10.1038/nature25486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ortiz Sandoval C., and Simmen T. (2012) Rab proteins of the endoplasmic reticulum: functions and interactors. Biochem. Soc. Trans. 40, 1426–1432 10.1042/BST20120158 [DOI] [PubMed] [Google Scholar]
- 86. Haile Y., Deng X., Ortiz-Sandoval C., Tahbaz N., Janowicz A., Lu J. Q., Kerr B. J., Gutowski N. J., Holley J. E., Eggleton P., Giuliani F., and Simmen T. (2017) Rab32 connects ER stress to mitochondrial defects in multiple sclerosis. J. Neuroinflammation 14, 19 10.1186/s12974-016-0788-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Minowa-Nozawa A., Nozawa T., Okamoto-Furuta K., Kohda H., and Nakagawa I. (2017) Rab35 GTPase recruits NDP52 to autophagy targets. EMBO J. 36, 2790–2807 10.15252/embj.201796463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Weinberger C. (1996) A model for farnesoid feedback control in the mevalonate pathway. Trends Endocrinol. Metab. 7, 1–6 10.1016/1043-2760(95)00180-8 [DOI] [PubMed] [Google Scholar]
- 89. Weivoda M. M., and Hohl R. J. (2011) Effects of farnesyl pyrophosphate accumulation on calvarial osteoblast differentiation. Endocrinology 152, 3113–3122 10.1210/en.2011-0016 [DOI] [PubMed] [Google Scholar]
- 90. Buhaescu I., and Izzedine H. (2007) Mevalonate pathway: a review of clinical and therapeutical implications. Clin. Biochem. 40, 575–584 10.1016/j.clinbiochem.2007.03.016 [DOI] [PubMed] [Google Scholar]
- 91. Maron D. J., Fazio S., and Linton M. F. (2000) Current perspectives on statins. Circulation 101, 207–213 10.1161/01.CIR.101.2.207 [DOI] [PubMed] [Google Scholar]
- 92. Rosenson R. S. (2004) Statins in atherosclerosis: lipid-lowering agents with antioxidant capabilities. Atherosclerosis 173, 1–12 10.1016/S0021-9150(03)00239-9 [DOI] [PubMed] [Google Scholar]
- 93. Graf H., Jüngst C., Straub G., Dogan S., Hoffmann R. T., Jakobs T., Reiser M., Waggershauser T., Helmberger T., Walter A., Walli A., Seidel D., Goke B., and Jüngst D. (2008) Chemoembolization combined with pravastatin improves survival in patients with hepatocellular carcinoma. Digestion 78, 34–38 10.1159/000156702 [DOI] [PubMed] [Google Scholar]
- 94. Mullen P. J., Yu R., Longo J., Archer M. C., and Penn L. Z. (2016) The interplay between cell signalling and the mevalonate pathway in cancer. Nat. Rev. Cancer 16, 718–731 10.1038/nrc.2016.76 [DOI] [PubMed] [Google Scholar]
- 95. Robert C., and Vagner S. (2018) Boosting immunity by targeting post-translational prenylation of small GTPases. Cell 175, 901–902 10.1016/j.cell.2018.10.032 [DOI] [PubMed] [Google Scholar]
- 96. Xia Y., Xie Y., Yu Z., Xiao H., Jiang G., Zhou X., Yang Y., Li X., Zhao M., Li L., Zheng M., Han S., Zong Z., Meng X., Deng H., et al. (2018) The mevalonate pathway is a druggable target for vaccine adjuvant discovery. Cell 175, 1059–1073.e21 10.1016/j.cell.2018.08.070 [DOI] [PubMed] [Google Scholar]
- 97. Gruenbacher G., and Thurnher M. (2018) Mevalonate metabolism in cancer stemness and trained immunity. Front. Oncol. 8, 394 10.3389/fonc.2018.00394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Likus W., Siemianowicz K., Bieńk K., Pakuła M., Pathak H., Dutta C., Wang Q., Shojaei S., Assaraf Y. G., Ghavami S., Cieślar-Pobuda A., and Łos M. J. (2016) Could drugs inhibiting the mevalonate pathway also target cancer stem cells? Drug Resist. Updat. 25, 13–25 10.1016/j.drup.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 99. Chen Q., Rong P., Xu D., Zhu S., Chen L., Xie B., Du Q., Quan C., Sheng Y., Zhao T. J., Li P., Wang H. Y., and Chen S. (2017) Rab8a deficiency in skeletal muscle causes hyperlipidemia and hepatosteatosis by impairing muscle lipid uptake and storage. Diabetes 66, 2387–2399 10.2337/db17-0077 [DOI] [PubMed] [Google Scholar]
- 100. Mijimolle N., Velasco J., Dubus P., Guerra C., Weinbaum C. A., Casey P. J., Campuzano V., and Barbacid M. (2005) Protein farnesyltransferase in embryogenesis, adult homeostasis, and tumor development. Cancer Cell 7, 313–324 10.1016/j.ccr.2005.03.004 [DOI] [PubMed] [Google Scholar]
- 101. Khan O. M., Ibrahim M. X., Jonsson I. M., Karlsson C., Liu M., Sjogren A. K., Olofsson F. J., Brisslert M., Andersson S., Ohlsson C., Hultén L. M., Bokarewa M., and Bergo M. O. (2011) Geranylgeranyltransferase type I (GGTase-I) deficiency hyperactivates macrophages and induces erosive arthritis in mice. J. Clin. Invest. 121, 628–639 10.1172/JCI43758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Berndt N., Hamilton A. D., and Sebti S. M. (2011) Targeting protein prenylation for cancer therapy. Nat. Rev. Cancer 11, 775–791 10.1038/nrc3151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Van Cutsem E., van de Velde H., Karasek P., Oettle H., Vervenne W. L., Szawlowski A., Schoffski P., Post S., Verslype C., Neumann H., Safran H., Humblet Y., Perez Ruixo J., Ma Y., and Von Hoff D. (2004) Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J. Clin. Oncol. 22, 1430–1438 10.1200/JCO.2004.10.112 [DOI] [PubMed] [Google Scholar]
- 104. Rao S., Cunningham D., de Gramont A., Scheithauer W., Smakal M., Humblet Y., Kourteva G., Iveson T., Andre T., Dostalova J., Illes A., Belly R., Perez-Ruixo J. J., Park Y. C., and Palmer P. A. (2004) Phase III double-blind placebo-controlled study of farnesyl transferase inhibitor R115777 in patients with refractory advanced colorectal cancer. J. Clin. Oncol. 22, 3950–3957 10.1200/JCO.2004.10.037 [DOI] [PubMed] [Google Scholar]
- 105. Harousseau J. L., Martinelli G., Jedrzejczak W. W., Brandwein J. M., Bordessoule D., Masszi T., Ossenkoppele G. J., Alexeeva J. A., Beutel G., Maertens J., Vidriales M. B., Dombret H., Thomas X., Burnett A. K., Robak T., et al. (2009) A randomized phase 3 study of tipifarnib compared with best supportive care, including hydroxyurea, in the treatment of newly diagnosed acute myeloid leukemia in patients 70 years or older. Blood 114, 1166–1173 10.1182/blood.V114.22.1166.1166, 10.1182/blood-2009-01-198093 [DOI] [PubMed] [Google Scholar]
- 106. Wright L. P., and Philips M. R. (2006) Thematic review series: lipid posttranslational modifications. CAAX modification and membrane targeting of Ras. J. Lipid Res. 47, 883–891 10.1194/jlr.R600004-JLR200 [DOI] [PubMed] [Google Scholar]
- 107. Cox A. D., Der C. J., and Philips M. R. (2015) Targeting RAS membrane association: back to the future for anti-RAS drug discovery? Clin. Cancer Res. 21, 1819–1827 10.1158/1078-0432.CCR-14-3214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Thompson P. D., Clarkson P., and Karas R. H. (2003) Statin-associated myopathy. JAMA 289, 1681–1690 10.1001/jama.289.13.1681 [DOI] [PubMed] [Google Scholar]
- 109. Chalasani N. (2005) Statins and hepatotoxicity: focus on patients with fatty liver. Hepatology 41, 690–695 10.1002/hep.20671 [DOI] [PubMed] [Google Scholar]
- 110. Hougland J. L., and Fierke C. A. (2009) Getting a handle on protein prenylation. Nat. Chem. Biol. 5, 197–198 10.1038/nchembio0409-197 [DOI] [PubMed] [Google Scholar]
- 111. Fritz G. (2009) Targeting the mevalonate pathway for improved anticancer therapy. Curr. Cancer Drug Targets 9, 626–638 10.2174/156800909789057033 [DOI] [PubMed] [Google Scholar]
- 112. Reszka A. A., and Rodan G. A. (2004) Nitrogen-containing bisphosphonate mechanism of action. Mini Rev. Med. Chem. 4, 711–719 [PubMed] [Google Scholar]
- 113. Goffinet M., Thoulouzan M., Pradines A., Lajoie-Mazenc I., Weinbaum C., Faye J. C., and Séronie-Vivien S. (2006) Zoledronic acid treatment impairs protein geranyl-geranylation for biological effects in prostatic cells. BMC Cancer 6, 60 10.1186/1471-2407-6-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Amin D., Cornell S. A., Gustafson S. K., Needle S. J., Ullrich J. W., Bilder G. E., and Perrone M. H. (1992) Bisphosphonates used for the treatment of bone disorders inhibit squalene synthase and cholesterol biosynthesis. J. Lipid Res. 33, 1657–1663 [PubMed] [Google Scholar]
- 115. Amin D., Cornell S. A., Perrone M. H., and Bilder G. E. (1996) 1-Hydroxy-3-(methylpentylamino)-propylidene-1,1-bisphosphonic acid as a potent inhibitor of squalene synthase. Arzneimittelforschung 46, 759–762 [PubMed] [Google Scholar]
- 116. Wasko B. M., Dudakovic A., and Hohl R. J. (2011) Bisphosphonates induce autophagy by depleting geranylgeranyl diphosphate. J. Pharmacol. Exp. Ther. 337, 540–546 10.1124/jpet.110.175521 [DOI] [PubMed] [Google Scholar]
- 117. Shull L. W., Wiemer A. J., Hohl R. J., and Wiemer D. F. (2006) Synthesis and biological activity of isoprenoid bisphosphonates. Bioorg. Med. Chem. 14, 4130–4136 10.1016/j.bmc.2006.02.010 [DOI] [PubMed] [Google Scholar]
- 118. Wiemer A. J., Tong H., Swanson K. M., and Hohl R. J. (2007) Digeranyl bisphosphonate inhibits geranylgeranyl pyrophosphate synthase. Biochem. Biophys. Res. Commun. 353, 921–925 10.1016/j.bbrc.2006.12.094 [DOI] [PubMed] [Google Scholar]