Abstract
Introduction
Although carbapenem-resistant Enterobacteriaceae (CRE) have been thoroughly investigated as the pathogens most commonly associated with clinical infections, data on Serratia marcescens are inadequate and superficial.
Methods
In this study, we characterized 36 carbapenem-resistant Serratia marcescens (CRSM) isolates in our hospital from April 2018 to March 2019 by analysing whole-genome sequencing (WGS) data. The molecular typing of the isolates was performed using both pulsed-field gel electrophoresis (PFGE) and core genome multilocus sequence typing (cgMLST).
Results
Thirty-three of the 36 isolates showed carbapenem resistance conferred by a blaKPC-2-harbouring plasmid, while the remaining three isolates were characterized by overexpression of beta-lactamase combined with porin loss. The blaKPC-2 genes in all the isolates were located on a plasmid of ~103 kb, except one, which was on a plasmid of ~94 kb. The gene structure surrounding blaKPC-2 in the plasmids was confirmed by integration of a partial Tn4401 structure and an intact IS26 as previously reported. Most of the plasmids also contained a mobile genetic element (MGE) comprising qnr and ISKpn19, which provided evidence of horizontal transfer of antibiotic resistance genes.
Conclusion
The thirty-six CRSM isolates were mainly clonally disseminated with a blaKPC-2-harbouring plasmid in our hospital. The gene structure surrounding blaKPC-2 as an MGE, as well as the qnr segment, might be acquired by horizontal gene transfer, and it could aggravate the infection and increase the difficulty of clinical treatment.
Keywords: Serratia marcescens, carbapenem resistance, blaKPC-2-harbouring plasmid
Introduction
Carbapenem-resistant Enterobacteriaceae are a major class of bacterial pathogens globally, but studies have focused on the organisms most commonly associated with clinical infections in the United States: Klebsiella spp., Enterobacter spp., and Escherichia coli.1,2 A report from the United States in 2014–2017 described Serratia marcescens as a less commonly encountered species that could also show carbapenem resistance by harbouring carbapenem resistance genes such as blaKPC-2 and blaVIM.3 Similarly, in China, little attention has been paid to S. marcescens, as it is not often encountered in clinical infection. S. marcescens has intrinsic resistance to ampicillin and cephalosporins but not to carbapenems.4 However, carbapenem-resistant S. marcescens (CRSM) was found harbouring a plasmid-mediated Klebsiella pneumoniae carbapenemase (KPC), which potentially increased the complexity of clinical infections in China in 2007.5,6
As a member of Enterobacteriaceae that was first described in 1819, S. marcescens is a gram-negative bacillus and was initially considered a non-pathogenic organism for years. It is characterized by red pigmentation, and the function of this red pigment (prodigiosin) remains unclear because clinical isolates are rarely pigmented.7 In the 19th century, this species was used as a tracer organism and as a biological warfare test agent in the military and was studied in medical experiments.8 However, as reports of clinical infections have emerged and increased, S. marcescens is now thought to be an opportunistic pathogen.9 It is an important cause of every conceivable kind of infection, including respiratory tract infection, urinary tract infection (UTI), septicaemia, meningitis, conjunctivitis, endocarditis and wound infections.10–16 Although S. marcescens displayed relatively low virulence, it caused nosocomial infections in immunocompromised patients, both adults and neonates.17,18
S. marcescens often shows carbapenem resistance conferred by blaKPC-harbouring plasmids of different types.5,19,20 As a result of the resistance of S. marcescens to most beta-lactams, clonal dissemination is aggravated, and the difficulty of clinical treatment increases. In this study, we investigated the clonal dissemination and resistance mechanism of 36 non-duplicated CRSM isolates in our hospital between April 2018 and March 2019. The whole-genome sequencing (WGS) method was used for molecular typing of isolates, which was rarely performed before analysing the structure of carbapenemase-producing plasmids. We aimed to clarify the dissemination and carbapenem resistance mechanism of S. marcescens in our hospital, which may contribute to clinical treatment and monitoring for infection control.
Materials and Methods
Isolate Collection
Thirty-six CRSM isolates were collected from 36 different patients in Sir Run Run Shaw Hospital, Hangzhou, from April 2018 to March 2019. Species identification was performed by MALDI-TOF mass spectrometry. All CRSM isolates were collected from various wards, including the Department of General Surgery (n=29), ICUs (n=4), the Department of Infectious Diseases (n=1), the Department of Cardiology (n=1), and the Department of Head and Neck Surgery (n=1). Three kinds of specimens were collected, namely, body fluid (n=29), sputum (n=5) and blood (n=2). Most patients were exposed to broad-spectrum antibiotics before the isolate was collected. All isolates were obtained more than 2 days after the patients were admitted to the hospital.
Antimicrobial Susceptibility Testing
The minimal inhibitory concentrations (MICs) of the CRSM isolates were determined by the broth microdilution method. Carbapenem resistance was defined as resistance to any carbapenems, including meropenem, imipenem and ertapenem, in accordance with 2019 Clinical and Laboratory Standards Institute (CLSI) guidelines.4 The susceptibility of tigecycline was determined according to US Food and Drug Administration breakpoints for Enterobacteriaceae. Escherichia coli ATCC 25922 was used as a quality control strain. The efflux pump inhibitor test was performed as a broth microdilution experiment with or without the inhibitors in combination with meropenem.21
Pulsed-Field Gel Electrophoresis (PFGE)
Genomic DNA was prepared as described previously with some modifications.22 Isolated colonies were harvested from Mueller-Hinton agar plates after overnight incubation at 37°C, and the suspension was adjusted to a concentration of 109 CFU/mL in cell suspension buffer (100 mM Tris-HCl, 100 mM EDTA, pH=8). After a short incubation of approximately 5–10 mins at 37°C, the bacterial suspension was mixed with an equal volume of 1% Gold Agarose (Lonza, USA) and allowed to solidify in a 100-µL plug mould. The DNA block was incubated overnight at 54°C in 1 mL of cell lysis buffer (50 mM Tris-HCl, 50 mM EDTA, 1% sarcosyl, 100 µg/mL proteinase K, pH=8). To eliminate the lysed bacterial material and inactivate proteinase K activity, the DNA blocks were washed four times at 50°C in 4 mL of Tris-EDTA buffer (100 mM Tris-HCl, 1 mM EDTA, pH=8). A slice of each plug was cut and incubated with SpeI (Takara, Japan). Restriction fragments of DNA were separated by pulsed-field gel electrophoresis (PFGE) with a CHEF MAPPER apparatus (Bio-Rad, USA) through 1% Gold Agarose. Electrophoresis was performed at 6 V/cm and 14°C. The run time was 18 h, with the pulse time ramping from 5 to 60 s. XbaI-digested DNA of Salmonella enterica serotype Braenderup H9812 was electrophoresed as the size marker.
Whole Genome Sequencing (WGS) and Resistance Gene Analysis
Thirty-six CRSM isolates were cultured overnight in Mueller-Hinton broth at 37°C for genomic DNA extraction using the QIAamp DNA Mini Kit (QIAGEN, Germany). All the genomic DNAs were sequenced using paired-end 500-bp insert libraries on an Illumina HiSeq X Ten, and the resulting 150-bp Illumina reads were assembled using CLC Genomics Workbench with default settings. To obtain complete genome assemblies, five isolates (C110, 1140, 2838, 3024, and 4201) representing five clone clusters in PFGE were sequenced on the Nanopore MinIon platform. Antibiotic resistance genes were identified by resFinder3.2 (https://cge.cbs.dtu.dk/services/ResFinder/) with the default threshold.
Core Genome Multilocus Sequence Typing (cgMLST)
To improve the accuracy of the PFGE typing method, a local core genome multilocus sequence typing (cgMLST) scheme was established following the guide for Ridom SeqSphere+ software (http://www.ridom.de/seqsphere/tutorials/index.shtml). A task template of 4847 targets for cgMLST and 400 targets as accessories was established by importing assembly files of all isolates except isolates 3024 and 4201. Then, all assembly files of the 36 isolates were imported into the created database, and a comparison table was generated. Finally, a minimum spanning tree was generated by ignoring missing values.
Conjugation Experiment
After incubation for 4–6 h, NaN3-resistant E. coli J53 was used as the recipient strain (100 µL) mixed with the recipient strain (100 µL), and filter mating was performed for 16–18 h at 37°C. Then, the mixture on the filter membrane was inoculated into plates containing NaN3 (300 µg/mL, Sangon, China) supplemented with ampicillin (100 µg/mL, Sangon, China) for 24 h at 37°C. The colonies that grew on the selection plates were picked and identified by PCR and MALDI-TOF mass spectrometry.
Analysis of the Genetic Structure Surrounding blaKPC-2
The related blaKPC-2-harbouring plasmids were identified by BLAST in NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The open reading frames (ORFs) were predicted by the RAST system (http://rast.nmpdr.org/). The mobile genetic elements (MGEs) were determined by ISfinder (https://www-is.biotoul.fr/blast.php), and figures were drawn with CLC Genomics Workbench. The sequencing data for the 36 isolates have been deposited at DDBJ/ENA/GenBank under the accession numbers WUUW00000000-WUWF00000000 and CP047679-CP047693.
Results
Dissemination and Distribution of CRSM
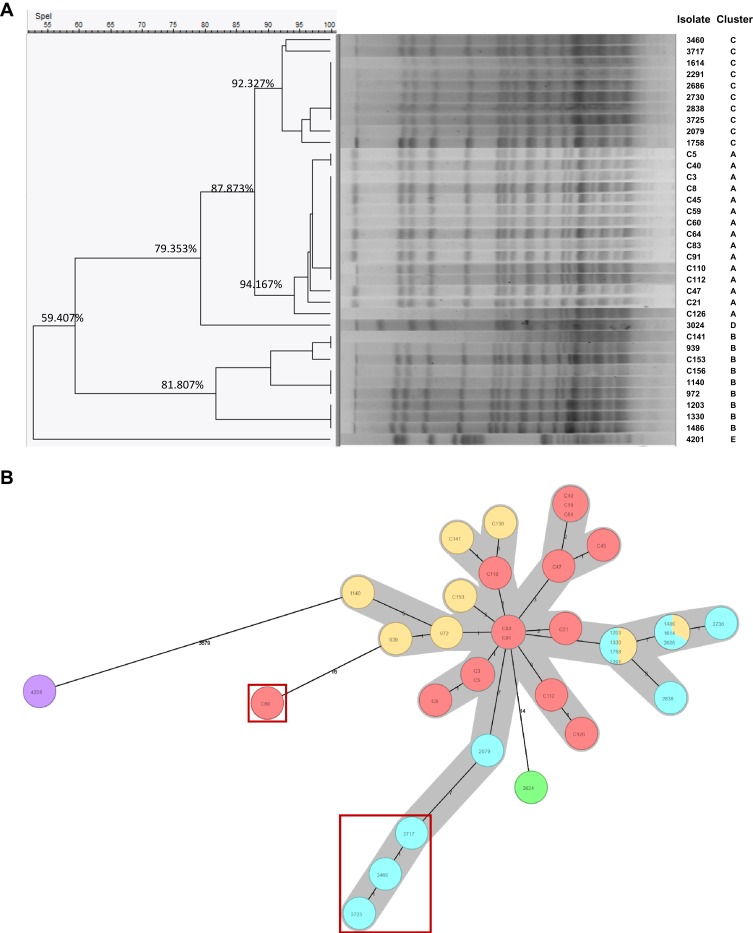
There were a total of 36 nonduplicated CRSM isolates collected from our hospital from April 2018 to March 2019. All isolates were divided into 5 clusters by the PFGE typing method and named from cluster A to cluster E (Figure 1A). Thirty-four of the 36 CRSM isolates were divided into clusters A, B and C with similarity values of 94.167%, 81.807% and 92.327%, respectively. There was only one isolate each in cluster D (isolate 3024) and cluster E (isolate 4201), and the similarity values of isolates 3024 and 4201 were significantly lower than those of the other three clusters. As seen in the minimum spanning tree generated by a local cgMLST scheme (Figure 1B), isolate 3024 (in green) and especially isolate 4201 (in purple) were quite distant from the other isolates, as predicted by the PFGE typing method. In addition, 4 isolates (in red box) differed from isolates C83 and C91 (in red) with no less than 14 allelic genes, similar to isolate 3024. Furthermore, the collection times of all isolates could be divided into 5 periods, which corresponded to the 5 clusters.
Figure 1.
The Molecular typing of 36 carbapenem-resistant Serratia marcescens (CRSM) isolates by two methods. (A). Pulsed-field gel electrophoresis (PFGE) typing. The isolates were divided into 5 cluster types including cluster A, B, C, D and E based on the cutoff values. (B). The local ad hoc scheme of Core genome multilocus sequence typing (cgMLST). Isolates with no less than 14 different allelic genes were identified as follows: C60, 3717, 3460 and 3725 (in red boxes), 3024 (in green), 4201 (in purple).
Upon additionally considering clinical information, 33 of the 36 isolates from the same clone were collected from the Department of General Surgery and ICU, which were the main regions of clonal dissemination and distribution. Notably, the isolate with the most significant difference in molecular typing, isolate 4201, was derived from a patient once transferred from the Department of Cardiac Surgery to the ICU after valve replacement surgery. Since it was the only case found in the ICU, we suggest that it was a special clone type that originated from the Department of Cardiac Surgery.
Antibiotic Resistance Mechanism of CRSM
All 36 CRSM isolates were highly resistant to carbapenems, including meropenem, imipenem and ertapenem, but were almost susceptible to amikacin and especially tigecycline (Table 1). PCR and a modified carbapenem inactivation method (mCIM)4 revealed that 33 of the 36 CRSM isolates were KPC-producing isolates. The WGS data were used to investigate the diversity of the antibiotic resistance genes carried by the 36 CRSM isolates (Table 2). It was demonstrated that blaKPC-2 was the only carbapenemase gene in 33 of the isolates. In 3 carbapenem-resistant isolates without any carbapenemase genes, the outer membrane proteins ompF and ompC were not detected, while the beta-lactamases SRT-1 and CTX-M-14 were overexpressed (Supplementary Figure S1). Moreover, the efflux pump inhibitor test based on antimicrobial susceptibility testing was performed for the 36 isolates. This test demonstrated that the MICs of meropenem for most of the isolates decreased by one- or two-fold after the inhibitor was added.
Table 1.
The MIC Distribution of 36 CRSM
| Antibiotic (μg/mL) | MEM | IPM | ETP | CAZ | FOX | CTX | FEP | ATM | CIP | AK | TGC | CAV/AVI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC50 | 512 | 1024 | 512 | 32 | 512 | 2048 | 1024 | >2048 | 16 | 4 | 1 | 1/4 |
| MIC90 | 512 | 1024 | 1024 | 64 | 512 | 2048 | >2048 | >2048 | 32 | 8 | 2 | 2/4 |
| MIC range | 64–1024 | 128–1024 | 64–1024 | 8–64 | 64–1024 | 512–2048 | 32->2048 | 128->2048 | <0.125–64 | 2–32 | 0.5–16 | 0.5–2/4 |
Abbreviations: CRSM, carbapenem-resistant Serratia marcescens, MIC, minimum inhibitory concentration; MEM, meropenem; IPM, imipenem; ETP, ertapenem; CAZ, ceftazidime; FOX, cefoxitin; CTX, cefotaxime; FEP, cefepime; ATM, aztreonam; CIP, ciprofloxacin; AK, amikacin; TGC, tigecycline; CAV/AVI, ceftazidime avibactam.
Table 2.
The Distribution of Antibiotic Resistance Genes in 36 CRSM Isolates
| resGene(s) | Beta-Lactamases | Quinolone | Aminoglycoside | Tetracycline | |||
|---|---|---|---|---|---|---|---|
| blaKPC-2 | blaCTX-M-14 | blaSRT-1(blaSST-1) | qnrS1(qnrD1) | aac(3)-IId | aac(6ʹ)-Ic | tet(41) | |
| NO. of isolates | 33 | 21 | 35(1) | 34(1) | 35 | 36 | 1 |
| Location | P | C | C | P+C | C | C | C |
Abbreviations: CRSM, carbapenem-resistant Serratia marcescens, P, plasmid; C, chromosome.
Resistance to carbapenems was successfully transferred from 33 blaKPC-2-harbouring isolates to the E.coli J53 recipient, except isolates C112 and 4201, by conjugation experiments. The meropenem, imipenem and ertapenem MICs of the transconjugants were in the range of 1–8 µg/mL, which indicated a slight increase compared to E. coli J53. Meanwhile, all transconjugants remained susceptible to amikacin and tigecycline (Table 3). In addition, the transconjugants were also resistant to ceftazidime but remained susceptible to ciprofloxacin (CIP), with a slight increase in the MIC.
Table 3.
Antibiotic Susceptibilities of Transconjugants and E. coli J53
| MIC Range (μg/mL) | 31 Transconjugants | J53 |
|---|---|---|
| MEM | 1–8 | <0.5 |
| IPM | 1–4 | <0.25 |
| ETP | 2–8 | <0.25 |
| CAZ | 8–32 | <0.25 |
| AK | 4–8 | 4 |
| TGC | 0.25–0.5 | 0.25 |
| CIP | 0.125–0.5 | <0.015625 |
Abbreviations: MIC, minimum inhibitory concentration; MEM, meropenem; IPM, imipenem; ETP, ertapenem; CAZ, ceftazidime; CIP, ciprofloxacin; AK, amikacin; TGC, tigecycline.
General Characteristics of blaKPC-2-Harbouring Plasmids
The blaKPC-2 gene was found in 33 isolates and localized on an ~78–104 kb plasmid by a S1 Southern blot (Supplementary Figure S2). Long-read sequencing data of five isolates were assembled, and the characteristics of the chromosomes and plasmids are listed in Table 4. Each isolate contained a large plasmid (blaKPC-2 harbouring) and a small plasmid (no resistance gene found) in addition to a chromosomal genome with a length of ~5.4 Mbp. The reads were mapped to the reference genome in CLC Genomics Workbench, and 31 blaKPC-2-harbouring plasmids of the same size had the same sequence as the blaKPC-2-harbouring plasmid from isolate C110 (subsequently named pC110-KPC), which was 103.167 kb in size. The blaKPC-2-harbouring plasmids from isolate 3024 and isolate 4201 (subsequently named p3024-KPC and p4201-KPC) had lengths of 103.175 kb and 94.056 kb, respectively.
Table 4.
Characteristics of the Nanopore-Sequenced Genome Assembly of Five CRSM
| Isolates | Genome | Plasmid 1 | Plasmid 2 | |||||
|---|---|---|---|---|---|---|---|---|
| Length (bp) | resGene | Length (bp) | Replicon | resGene | Length (bp) | Replicon | resGene | |
| C110 | 5427741 | blaCTX-M-14, blaSRT-1, qnrS1, aac(3)-Iid,aac(6ʹ)-Ic | 103167 | ‒ | qnrS1, blaKPC‒2 | 2953 | ‒ | ‒ |
| 1140 | 5429004 | blaCTX-M-14, blaSRT-1, qnrS1, aac(3)-Iid,aac(6ʹ)-Ic | 103167 | ‒ | qnrS1, blaKPC‒2 | 2953 | ‒ | ‒ |
| 2838 | 5416416 | blaSRT-1, qnrS1, aac(3)-Iid,aac(6ʹ)-Ic | 103167 | ‒ | qnrS1, blaKPC‒2 | 2953 | ‒ | ‒ |
| 3024 | 5428813 | blaCTX-M-14, blaSRT-1, qnrS1, aac(3)-Iid,aac(6ʹ)-Ic | 103175 | ‒ | qnrS1, blaKPC‒2 | 2953 | ‒ | ‒ |
| 4201 | 5331789 | blaSST-1, aac(6ʹ)-Ic,tet(41) | 94056 | ‒ | blaKPC‒2 | 2953 | ‒ | ‒ |
Abbreviations: CRSM, carbapenem-resistant Serratia marcescens, bp, base pair; ‒, not found by plasmidFinder and resFinder on CGE website.
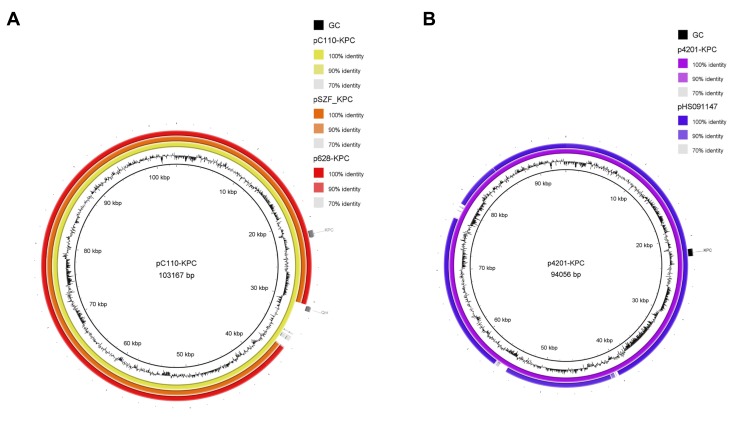
pC110-KPC was a closed circular DNA with a size of 103167 bp and belonged to incompatibility group F. It contained 138 predicted ORFs with an average G+C content of 54.2%. A BLAST search in NCBI revealed that plasmid pC110-KPC shares high identity with the previously reported IncF plasmids pSZF_KPC (accession no. MH917122.1)23 and p628-KPC (accession no. KP987218.1),24 both of which were from a K. pneumoniae isolate found in China, with 94% query coverage and >99% nucleotide identity (Figure 2A). The blaKPC-2-harbouring plasmids from isolates 1140 and 2838 were exactly the same as pC110-KPC. Plasmid p3024-KPC was 8 bp longer than pC110-KPC as a result of a short base segment (TATCTTGT) inserted into position 2476–2477 of pC110-KPC. Plasmid p4201-KPC was 94056 bp in length and contained 122 predicted ORFs with an average G+C content of 53.1%. It was similar (95% query coverage and >99% nucleotide identity) to pHS091147 (accession no. KX236178.1)25 from a K. pneumoniae isolate found in China (Figure 2B).
Figure 2.
The circular maps of pC110-KPC and p4201-KPC. (A). The circle in yellow represented plasmid pC110-KPC, in orange represented pSZF_KPC, in red represented p628-KPC. (B). The circle in purple represented p4201-KPC, in blue represented pHS091157. The peak map in (A) and (B) represented the GC content of plasmid pC110-KPC and p4201-KPC respectively. Arcs in grey indicate the position of blaKPC and blaqnr in plasmids pC110-KPC and p4201-KPC. The maps were created by Brig v0.95.
Genetic Environment of the blaKPC-2 Gene
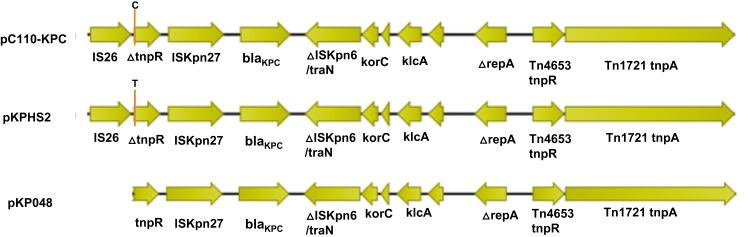
All blaKPC-2-harbouring plasmids in this study belonged to the non-Tn4401 structure type, which was defined as blaKPC-bearing non-Tn4401 elements (NTEKPC) as previously reported.26 In fact, the surrounding structure of blaKPC-2 was considered to result from the integration of a partial Tn4401 structure and an intact IS26. The ORFs are ordered as follows (Figure 3): IS26, Tn3-resolvase, ISKpn27, the blaKPC-2 gene, and the ISKpn6-like element. This structure was the same as that in plasmid pKPHS2 (accession no. CP003224),27 except for a point mutation (C→T) (Figure 3). Plasmid pKPHS2 was from a K. pneumoniae isolate and had a similar backbone as another K. pneumoniae plasmid, pKP048 (accession no. FJ628167),28 and both of these plasmids were found in China. The surrounding structure of blaKPC-2 in plasmid pKP048 lacked IS26 with an incomplete tnpR gene compared to plasmid pC110-KPC, which we considered to be a result of gene recombination.
Figure 3.
A schematic diagram of the genetic structure surrounding blaKPC-2. The genetic structure surrounding blaKPC-2 in the plasmid pC110-KPC represented all plasmids harbouring blaKPC-2 in this study. The positions indicated by orange lines are the point mutation positions at which C and T are in plasmids pC110-KPC and pKPHS2, respectively. The direction of the arrow represents the direction of transcription.
Coexistence of the Quinolone Resistance Gene qnrS1 with blaKPC-2
Other types of resistance genes were identified from the genome sequences, including blaCTX-M-14, blaSRT-1 (blaSST-1), qnrS1 (qnrD1), aac(3)-IId, aac(6ʹ)-Ic, and tet(41), among which qnrS1 was the only resistance gene located on a blaKPC-2-harbouring plasmid (Table 2). There was an ~6-kb region comprising a quinolone resistance gene (qnrS1) and an intact ISKpn19 ~8 kb downstream of blaKPC-2 on plasmids (Figure S3). In addition, gene qnrS1 was also located in the chromosome of most isolates.
Discussion
Carbapenem-resistant S. marcescens strains are distributed and disseminated globally, and the positivity rate has increased since China has been the epidemic region for carbapenem-resistant Enterobacteriaceae (CRE). Several studies on the clonal dissemination of S. marcescens were previously reported.17,29,30 However, this was the first study that utilized WGS to gain insight into antibiotic resistance genes and MGEs in CRSM isolated from different patients in China.
WGS has emerged as an ultimate typing tool suitable for any bacterial species, study type, and laboratory.31 cgMLST has been proposed as a very useful and practical method based on WGS to distinguish isolates within epidemic settings and between epidemic and unrelated specimens.32 As there was no public database of cgMLST for S. marcescens, we established a local cgMLST scheme using Ridom SeqSphere software. By cgMLST, four isolates were distinguished from PFGE typing. The results of cgMLST differed from those of PFGE typing, which improved the accuracy of molecular typing and was attributed to the high sensitivity of cgMLST.
It was reported33 that the mechanisms of beta-lactam resistance in S. marcescens include the production of beta-lactamases, diminished outer membrane permeability, modification of the target penicillin-binding proteins (PBPs), overexpression of active efflux systems, synthesis of aminoglycoside-modifying enzymes, and structural alteration of the GyrA protein. The mechanism of carbapenem resistance in the CRSM isolates was divided into four types:34 carbapenemase production, functional efflux pump systems, diminished permeability of the outer membrane and alteration of PBP targets. Since carbapenemase production was the dominant type referred to in most in related reports,29,30,35 KPC production was the most frequent type observed in our study. The resistance mechanism of the efflux pump in 3 isolates without blaKPC-2 did not play the main role. In addition, we also evaluated the outer membrane porins (ompF and ompC), which are related to antimicrobial resistance. Based on the results (Figure S1) and a previous report,36 it was suggested that the carbapenem resistance of the 3 isolates without blaKPC-2 was predominantly mediated by the overexpression of SRT-1 and CTX-M-14 combined with porin loss.
In China, S. marcescens-harboured blaKPC-2 was first described on a conjugative plasmid in 2007, and since then, related reports have emerged. The sizes of plasmids harbouring blaKPC-2 in S. marcescens varied from 50 kb to 118 kb, and the plasmid types were IncF, IncK, IncL/M and IncX, most of which were conjugative.19,20,23,29 In this study, we found that most of the plasmids were ~103 kb in length and belonged to the IncF incompatibility group, which was not previously reported. Through WGS analysis against the NCBI database, we hypothesized that all of the plasmids were derived from K. pneumoniae isolates in China. All of the blaKPC-2-harbouring plasmids were successfully transferred except plasmids from isolates C112 and 4201. The carbapenem MICs of the transconjugants increased but not significantly, probably because of the low copy numbers of the plasmids. The slight increase in the CIP MIC was due to the qnrS gene carried by the blaKPC-2-harbouring plasmids.
Since blaKPC-2 could be transferred frequently either by plasmids or by integrative and conjugative elements (ICEs) and insertion elements (ISs), confirming the genetic structure surrounding blaKPC-2 can contribute to monitoring the horizontal transfer of blaKPC-2 and the evolution process of blaKPC-2-harbouring plasmids. The most common blaKPC-2-containing mobile element is a Tn3-based transposon, Tn4401. All CRSM isolates harbouring blaKPC-2 in this study possessed the same structure surrounding the blaKPC-2 gene as pC110-KPC, as shown in Figure 3. This structure was defined as the NTEKPC-Ib group, which was represented by plasmid pKPHS2.26 There was only one base difference between pC110-KPC and pKPHS2. Based on the timeline, it was suggested that the former was probably derived from the latter by point mutation.
As WGS showed, qnrS1 was the only resistance gene located on the blaKPC-2-harbouring plasmids. The qnrS1-harbouring segment was widely distributed in plasmids from K. pneumoniae; however, this segment did not occur in plasmid p4201-KPC or in plasmids pSZF_KPC and p628-KPC. The combination of the similar genes qnrS1 and ISKpn19 was also found in E. coli as previously reported.37 It was indicated that the segment could have been transferred among blaKPC-2-harbouring plasmids and chromosomes from different strains, and this was probably the way the multi-resistant plasmid evolved.
All isolates collected in this study were confirmed to be resistant to most beta-lactams, including carbapenems, but were susceptible to tigecycline and amikacin (Table 1). Although most of the patients with CRSM could not be clearly diagnosed with infection or colonization by CRSM because of the complex clinical situation, they recovered after administration of tigecycline and amikacin, which were often combined with beta-lactams. Furthermore, all isolates were susceptible to ceftazidime avibactam (CAZ/AVI), as determined by the broth microdilution method, which provides a strong possibility of combination therapy as previously reported.38,39
Characterization of the dissemination and carbapenem resistance mechanism of S. marcescens in our hospital showed that there were still some limitations in this study. All isolates were collected from the patients of a single center and none was collected from the ward environment in the hospital, so the sample size was limited for a more detailed investigation. Because a public MLST or cgMLST database has not been established to date, the isolates in our study cannot be officially defined and compared among different laboratories.
Conclusion
In this study, CRSM isolates were mainly clonally disseminated with a blaKPC-2-harbouring plasmid in a nosocomial environment. The gene structure surrounding blaKPC-2, as well as the qnr segment, might be acquired by horizontal gene transfer, and it could aggravate the infection and increase the difficulty of clinical treatment. Since MGEs containing carbapenem resistance genes are commonly associated with horizontal gene transfer, further study is required.
Funding Statement
This work was supported by the National Natural Science Foundation of China (grant number 81830069) and the Key Research and Development Programme of Zhejiang (grant number 2015C03046).
Ethics Approval
The collection of CRSM was part of the routine hospital laboratory procedure.
Disclosure
The authors report no conflicts of interest associated with this work.
References
- 1.Guh AY, Bulens SN, Mu Y, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae in 7 US communities, 2012–2013. JAMA. 2015;314(14):1479–1487. doi: 10.1001/jama.2015.12480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiner LM, Webb AK, Limbago B, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the national healthcare safety network at the centers for disease control and prevention, 2011–2014. Infect Control Hosp Epidemiol. 2016;37(11):1288–1301. doi: 10.1017/ice.2016.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walters MS, Witwer M, Lee Y-K, et al. Carbapenemase-producing carbapenem-resistant Enterobacteriaceae from less common Enterobacteriaceae Genera — United States, 2014–2017. MMWR Morb Mortal Wkly Rep. 2018;67(23):668–669. doi: 10.15585/mmwr.mm6723a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. 29th CLSI supplement M100 Wayne, PA: CLSI; 2019 [Google Scholar]
- 5.Zhang R, Zhou HW, Cai JC, et al. Plasmid-mediated carbapenem-hydrolysing β-lactamase KPC-2 in carbapenem-resistant Serratia marcescens isolates from Hangzhou, China. J Antimicrob Chemother. 2007;59(3):574–576. doi: 10.1093/jac/dkl541 [DOI] [PubMed] [Google Scholar]
- 6.Cai JC, Zhou HW, Zhang R, et al. Emergence of Serratia marcescens, Klebsiella pneumoniae, and Escherichia coli isolates possessing the plasmid-mediated carbapenem-hydrolyzing beta-lactamase KPC-2 in intensive care units of a Chinese hospital. Antimicrob Agents Chemother. 2008;52(6):2014–2018. doi: 10.1128/AAC.01539-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hejazi A, Falkiner FR. Serratia marcescens. J Med Microbiol. 1997;46(11):903–912. doi: 10.1099/00222615-46-11-903 [DOI] [PubMed] [Google Scholar]
- 8.Mahlen SD. Serratia infections: from military experiments to current practice. Clin Microbiol Rev. 2011;24(4):755-+. doi: 10.1128/cmr.00017-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yokota M, Okazawa A, Tanaka T. Serratia marcescens as an opportunistic human pathogen. Nihon saikingaku zasshi. Jpn J Bacteriol. 2001;56(3):527–535. doi: 10.3412/jsb.56.527 [DOI] [PubMed] [Google Scholar]
- 10.Sakai S, Nishio A, Kumamoto Y. [Clinical studies on urinary tract infections caused by Serratia marcescens. II. Epidemiological studies on onset of Serratia marcescens urinary tract infections in the urological ward]. Jpn j Urol. 1983;74(4):485–502. doi: 10.5980/jpnjurol1928.74.4_485. Japanese. [DOI] [PubMed] [Google Scholar]
- 11.Theccanat G, Hirschfield L, Isenberg H. SERRATIA-MARCESCENS MENINGITIS. J Clin Microbiol. 1991;29(4):822–823. doi: 10.1128/JCM.29.4.822-823.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogman CF, Fritz H, Sandberg L. Posttransfusion Serratia-Marcescens Septicemia. Transfusion. 1993;33(3):189–191. doi: 10.1046/j.1537-2995.1993.33393174441.x [DOI] [PubMed] [Google Scholar]
- 13.Byrne AH, Boyle B, Herra CM, et al. Serratia marcescens causing hospital-acquired lower respiratory tract infection. J Hosp Infect. 2000;45(3):242–244. doi: 10.1053/jhin.2000.0761 [DOI] [PubMed] [Google Scholar]
- 14.Pak KY, Kim SI, Lee JS. Neonatal bacterial conjunctivitis in Korea in the 21st century. Cornea. 2017;36(4):415–418. doi: 10.1097/ICO.0000000000001122 [DOI] [PubMed] [Google Scholar]
- 15.Us E, Kutlu HH, Tekeli A, et al. Wound and soft tissue infections of Serratia marcescens in patients receiving wound care: a health care-associated outbreak. Am J Infect Control. 2017;45(4):443–447. doi: 10.1016/j.ajic.2016.11.015 [DOI] [PubMed] [Google Scholar]
- 16.Schechter MC, Spicer JO, Del Mar Aldrete S, et al. Serratia marcescens infectious endocarditis: injection drug use, left-sided heart disease, and poor outcomes. Infect Dis Clin Pract. 2018;26(4):216–219. doi: 10.1097/IPC.0000000000000614 [DOI] [Google Scholar]
- 17.Dessi A, Puddu M, Testa M, et al. Serratia marcescens infections and outbreaks in neonatal intensive care units. J Chemother. 2009;21(5):493–499. doi: 10.1179/joc.2009.21.5.493 [DOI] [PubMed] [Google Scholar]
- 18.Ruiz-Sada P, Escalante M, Lizarralde E. Severe acute infection due to Serratia marcescens causing respiratory distress in an immunocompetent adult. Rom J Intern Med. 2016;54(2):134–136. doi: 10.1515/rjim-2016-0013 [DOI] [PubMed] [Google Scholar]
- 19.Bryant KA, Van Schooneveld TC, Thapa I, et al. KPC-4 is encoded within a truncated Tn4401 in an IncL/M plasmid, pNE1280, isolated from Enterobacter cloacae and Serratia marcescens. Antimicrob Agents Chemother. 2013;57(1):37–41. doi: 10.1128/AAC.01062-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gona F, Caio C, Iannolo G, et al. Detection of the IncX3 plasmid carrying bla(KPC-3) in a Serratia marcescens strain isolated from a kidney–liver transplanted patient. J Med Microbiol. 2017;66(10):1454–1456. doi: 10.1099/jmm.0.000592 [DOI] [PubMed] [Google Scholar]
- 21.Kern WV, Steinke P, Schumacher A, et al. Effect of 1-(1-naphthylmethyl)-piperazine, a novel putative efflux pump inhibitor, on antimicrobial drug susceptibility in clinical isolates of Escherichia coli. J Antimicrob Chemother. 2006;57(2):339–343. doi: 10.1093/jac/dki445 [DOI] [PubMed] [Google Scholar]
- 22.Ligozzi M, Fontana R, Aldegheri M, et al. Comparative evaluation of an automated repetitive-sequence-based PCR instrument versus pulsed-field gel electrophoresis in the setting of a Serratia marcescens nosocomial infection outbreak. J Clin Microbiol. 2010;48(5):1690–1695. doi: 10.1128/JCM.01528-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miao M, Wen H, Xu P, et al. Genetic diversity of carbapenem-resistant Enterobacteriaceae (CRE) clinical isolates from a tertiary hospital in Eastern China. Front Microbiol. 2019;9. doi: 10.3389/fmicb.2018.03341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Fang H, Feng J, et al. Complete sequences of KPC-2-encoding plasmid p628-KPC and CTX-M-55-encoding p628-CTXM coexisted in Klebsiella pneumoniae. Front Microbiol. 2015;6. doi: 10.3389/fmicb.2015.00838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang Y, Shen P, Liang W, et al. A putative multi-replicon plasmid co-harboring beta-lactamase genes bla(KPC-2), bla(CTX-M-14) and bla(TEM-1) and trimethoprim resistance gene dfrA25 from a Klebsiella pneumoniae sequence type (ST) 11 strain in China. PLoS One. 2017;12(2). doi: 10.1371/journal.pone.0186097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L, Mathema B, Chavda KD, et al. Carbapenemase-producing Klebsiella pneumoniae: molecular and genetic decoding. Trends Microbiol. 2014;22(12):686–696. doi: 10.1016/j.tim.2014.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu P, Li P, Jiang X, et al. Complete genome sequence of Klebsiella pneumoniae subsp pneumoniae HS11286, a multidrug-resistant strain isolated from human sputum. J Bacteriol. 2012;194(7):1841–1842. doi: 10.1128/JB.00043-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang Y, Yu D, Wei Z, et al. Complete nucleotide sequence of Klebsiella pneumoniae multidrug resistance plasmid pKP048, carrying bla(KPC-2), bla(DHA-1), qnrB4, and armA. Antimicrob Agents Chemother. 2010;54(9):3967–3969. doi: 10.1128/AAC.00137-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.da Costa Guimaraes AC, Almeida ACS, Nicoletti AG, et al. Clonal spread of carbapenem-resistant Serratia marcescens isolates sharing an IncK plasmid containing bla(KPC-2). Int J Antimicrob Agents. 2013;42(4):369–370. doi: 10.1016/j.ijantimicag.2013.05.017 [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez C, Brengi S, Cáceres MA, et al. Polyclonal dissemination of KPC-2 in Serratia marcescens, including a clone with epidemic behaviour in the nosocomial niche. Int J Antimicrob Agents. 2017;49(5):657–658. doi: 10.1016/j.ijantimicag.2017.03.003 [DOI] [PubMed] [Google Scholar]
- 31.Kluytmans-van den Bergh MFQ, Rossen JWA, Bruijning-Verhagen PCJ, et al. Whole-genome multilocus sequence typing of extended-spectrum-beta-lactamase-producing Enterobacteriaceae. J Clin Microbiol. 2016;54(12):2919–2927. doi: 10.1128/JCM.01648-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu -Y-Y, Chen -C-C, Chiou C-S. Construction of a pan-genome allele database of Salmonella enterica serovar enteritidis for molecular subtyping and disease cluster identification. Front Microbiol. 2016;7. doi: 10.3389/fmicb.2016.02010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang H, Cheng J, Hu L, et al. Mechanisms of antimicrobial resistance in Serratia marcescens. Afr J Microbiol Res. 2012;6(21):4427–4437. doi: 10.5897/AJMR11.1545 [DOI] [Google Scholar]
- 34.Sun Y, Zhang H, Liu J, et al. The mechanism of carbapenems resistance in Serratia marcescens strains. Chin J Microbiol Immunol. 2014;34(10):774–779. [Google Scholar]
- 35.Guo P, Qiao Y, Zhang H, et al. Profile and mechanism of imipenem resistance in clinical isolates of Serratia marcescens. Chin J Infect Chemother. 2017;17(2):187–191. [Google Scholar]
- 36.Suh B, Bae IK, Kim J, et al. Outbreak of meropenem-resistant Serratia marcescens comediated by chromosomal AmpC beta-Lactamase overproduction and outer membrane protein loss. Antimicrob Agents Chemother. 2010;54(12):5057–5061. doi: 10.1128/AAC.00768-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu X-Q, Wang J, Li W, et al. Distribution of cfr in Staphylococcus spp. and Escherichia coli strains from pig farms in China and Characterization of a Novel cfr-Carrying F43: A-:B-Plasmid. Front Microbiol. 2017;8:329. doi: 10.3389/fmicb.2017.00329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez C, Brengi S, Cáceres MA, et al. Successful management with fosfomycin + ceftazidime of an infection caused by multiple highly-related subtypes of multidrug-resistant and extensively drug-resistant KPC-producing Serratia marcescens. Int J Antimicrob Agents. 2018;52(5):737–739. doi: 10.1016/j.ijantimicag.2018.07.020 [DOI] [PubMed] [Google Scholar]
- 39.Gaudereto JJ, Perdigão Neto LV, Leite GC, et al. Synergistic effect of ceftazidime-avibactam with meropenem against panresistant, carbapenemase-harboring acinetobacter baumannii and Serratia marcescens investigated using time-kill and disk approximation assays. Antimicrob Agents Chemother. 2019;63(5). doi: 10.1128/AAC.02367-18. [DOI] [PMC free article] [PubMed] [Google Scholar]