Abstract
Background
Recent studies have suggested association between the ABO blood group and inflammation, which was a crucial pathological process of primary knee osteoarthritis. The aim of this study was to investigate the association between the ABO blood group and primary knee osteoarthritis and the severity of primary knee osteoarthritis evaluated by the Kellgren/Lawrence score, as well as the histopathologic association in a subgroup of patients.
Methods
We performed a retrospective review of patients with primary knee osteoarthritis that served as the case group and a random sampling of healthy blood donors that served as the control group. The severity of knee osteoarthritis at the first outpatient visit was evaluated by the Kellgren/Lawrence scoring system. Further study was performed to investigate the expression of blood group antigens in synovial tissue of the knee in both cases and controls.
Results
A total of 1126 cases and 30299 controls were involved. The proportion of AB blood group was higher in the case group than in the control group (9.7% vs. 7.8%), and logistic regression revealed that the AB blood group was a risk factor of primary knee osteoarthritis (P = 0.025 and 0.048 for univariate and multivariate analysis, respectively), independent of age (P = 0.973) and sex (P = 0.520). Patients of the blood group AB had a higher Kellgren/Lawrence score (P = 0.017). The immunohistochemical study indicated association between LeY antigen and primary knee osteoarthritis (P = 0.029).
Conclusions
This study suggested that the blood group AB was associated with primary knee osteoarthritis, as well as its radiological severity. Further study indicated that LeY antigen, which was related to the blood group, was associated with primary knee osteoarthritis.
Translational potential of this article
This study revealed that blood group AB and LeY antigen was associated with primary knee osteoarthritis, which shed new light on the nature of osteoarthritis, and the development of novel therapy for osteoarthritis.
Keywords: Blood group, Kellgren/Lawrence, Knee, LeY antigen, Osteoarthritis
Abbreviations: OA, osteoarthritis; FUT, fucosyltransferase; IHC, immunohistochemistry; ST, synovial tissue; ACR, American College of Rheumatology; IRB, institutional review board; K/L, Kellgren/Lawrence; ANOVA, analysis of variance; HR, hazard ratio; CI, confidence interval
Background
Osteoarthritis (OA) was believed to be driven by degeneration of cartilage in the past decades [1]. When the chondrocytes fail to repair the injury of articular cartilage, the degeneration process of cartilage is triggered [2]. However, recent studies suggest that inflammatory process is a crucial part in the pathogenesis of OA [3]. Inflammatory cytokines induce the interaction between the synovial membrane, synovial fluid, and cartilage and subchondral bone to accelerate the degeneration of articular cartilage, which implicates an association between inflammatory cytokines and knee OA [4], [5]. Factors influencing the inflammatory process may also be associated with knee OA.
The ABO blood group system was first put forward by Karl Landersteiner at the beginning of the 20th century [6]. It is the most important and most widely used system in clinics. ABO blood group antigens include A antigen, B antigen, and H antigen. H gene exists in each individual and encodes for the H antigen precursor, which eventually becomes H antigen with the help of fucosyltransferase [7]. A gene encodes for α1→3 N-acetylgalactosamyltransferase, which converts H antigen to A antigen. B gene encodes for α1→3-galactosamyltransferase, which converts H antigen to B antigen [8]. Blood group antigens exist not only in erythrocytes but also in many other tissues and cells, which indicates that the antigens may play a role in a wider field [9].
Since blood group A was identified as a risk factor for stomach cancer in 1953 [10], the association between ABO blood group and diseases has been investigated. Various diseases were suggested to be associated with the ABO blood group [11], [12]. Recently, genome-wide studies revealed an association between the ABO gene and tumour necrosis factor α, as well as intercellular adhesion molecule 1 (ICAM-1), which are important proinflammatory cytokines and may have an impact on the systemic inflammatory response [13], [14]. As is widely recognised, an imbalanced level of proinflammatory cytokines and disordered inflammatory process play a vital role in the pathogenesis and progression of primary knee OA [15], [16]. Therefore, we put forward the scientific hypothesis that the ABO blood group may be associated with primary knee OA.
We conducted a retrospective analysis on the medical data of patients who have been hospitalised in our hospital with the diagnosis of primary knee OA to investigate whether there was difference in ABO blood group distribution among patients and healthy controls and further explore the association between ABO blood group and the severity of OA. On the other hand, immunohistochemistry (IHC) staining was applied to synovial tissue (ST) of the knee joint to investigate the expression of ABO blood group antigens and related antigens and further explore the possible mechanism by which the ABO blood group and primary knee OA was related.
Methods
Study population
We performed a retrospective review of medical records in Sun Yat-sen Memorial Hospital from January 1, 1999, to April 1, 2014. Patients with primary knee OA were identified according to diagnostic criteria by the American College of Rheumatology [17]. We excluded those with an age less than 40, history of trauma, other rheumatic diseases, infection or inflammatory diseases, congenital deformity, or tumour. The control group was a representative subset of the local population, constituted by healthy blood donors from the general population of the same geographical area. ABO blood group distribution among the blood donors was kindly provided by Guangzhou Blood Centre.
To investigate the expression of blood group antigens in ST of the knee, we carried out a supplementary study. It involved patients who were admitted to our hospital and underwent knee arthroscopy between November 1, 2014, and February 28, 2015. The protocol of the research project has been approved by the Institutional Review Board of Sun Yat-sen Memorial Hospital and thus has been performed in accordance with the ethical standards laid down in the Declaration of Helsinki.
Determination of the ABO blood group
ABO blood groups were tested by the standard agglutination method. The ABO blood group test is conventional for blood donors and patients of surgical departments and is also performed for patients of other departments when needed. Determination of ABO blood groups for the participants was performed in the clinical laboratory of Sun Yat-sen Memorial Hospital or Guangzhou Blood Centre.
Evaluation of severity of primary knee OA
The anterior–posterior X-ray films of the knee joint at the first outpatient visit was evaluated using the Kellgren/Lawrence (K/L) scoring system, which is the most commonly used scoring system to radiologically assess the severity of primary knee OA [18]. In the K/L system, radiographs are scored from Grade 0 to Grade 4. A higher grade is associated with more severe OA. Knee radiographs at the baseline were read without knowing the participant's clinical status by an orthopaedic surgeon, and the K/L score was defined using the K/L radiographic atlas. To evaluate the intraobserver variability of K/L grading, 100 randomly selected radiographs were scored by the same observer 1 month after the first reading and also scored by another orthopaedic surgeon using the same atlas for interobserver variability [19].
Detection of blood group antigens and related antigens in the synovium
ST of the knee was obtained during arthroscopy and fixed in formalin. IHC staining was applied to the sectioned ST. We used anti–A antibody (Ab) (1:100 diluted, ab2521; Abcam, Cambridge, UK), anti–B Ab (1:100 diluted, ab2524; Abcam, Cambridge, UK), anti–H Ab (1:100 diluted, ab3355; Abcam, Cambridge, UK), and anti–LeY Ab (1:100 diluted, ab3359; Abcam, Cambridge, UK) as the primary Ab and biotinylated anti–mouse IgM as the secondary Ab. A Vector ABC system (PV-6002, Zsbio, Beijing, China) containing avidin-HRP (horseradish peroxidase) was used with 3,3′-diaminobenzidine as a substrate. Slides were counterstained with haematoxylin and eosin.
Both the distribution (the percentage of positive cells) and the intensity of staining were assessed in a semiquantitative way. The distribution of positive cells was graded as follows: none (not stained) = 0, focal (less than one-third of cells of the same kind) = 1, multifocal (less than two-thirds of cells of the same kind) = 2, and diffuse (more than two-thirds of cells of the same kind) = 3. The intensity of staining was graded as follows: none (not stained) = 0, slight (light yellow) = 1, mild (brown) = 2, and strong (dark brown) = 3. The scores of distribution and intensity were added as the final score [20]. In addition, the localisation of staining was recorded.
Statistical analysis
Distribution of ABO blood groups in the patients and controls was summarised. We used the χ2 test for categorical data and the Student t test for quantitative data. Normality of data distribution was tested by the Shapiro–Wilk test with 95% confidence interval (CI), and Levene's test was used to assess homogeneity of variance. Logistic regression was applied for analysis of risk factors. Factors with P value less than 0.10 in the univariate analysis was involved in the multivariate analysis. The intraobserver and interobserver variabilities were tested by kappa analysis. A Kappa value of >0.8 indicates high consistency, 0.6–0.8 indicates moderate consistency, and 0.4–0.6 indicates acceptable consistency [21]. The rank test was used for nonparametric analysis of ranked data. The Wilcoxon test was used for comparison between two groups, and the Kruskal–Wallis test was used for more than two groups. Statistical analysis was performed using SPSS (version 20.0; IBM, Chicago, IL, USA). Generally, P value < 0.05 was considered to indicate statistical significance (α = 0.05).
Results
Basic characteristics
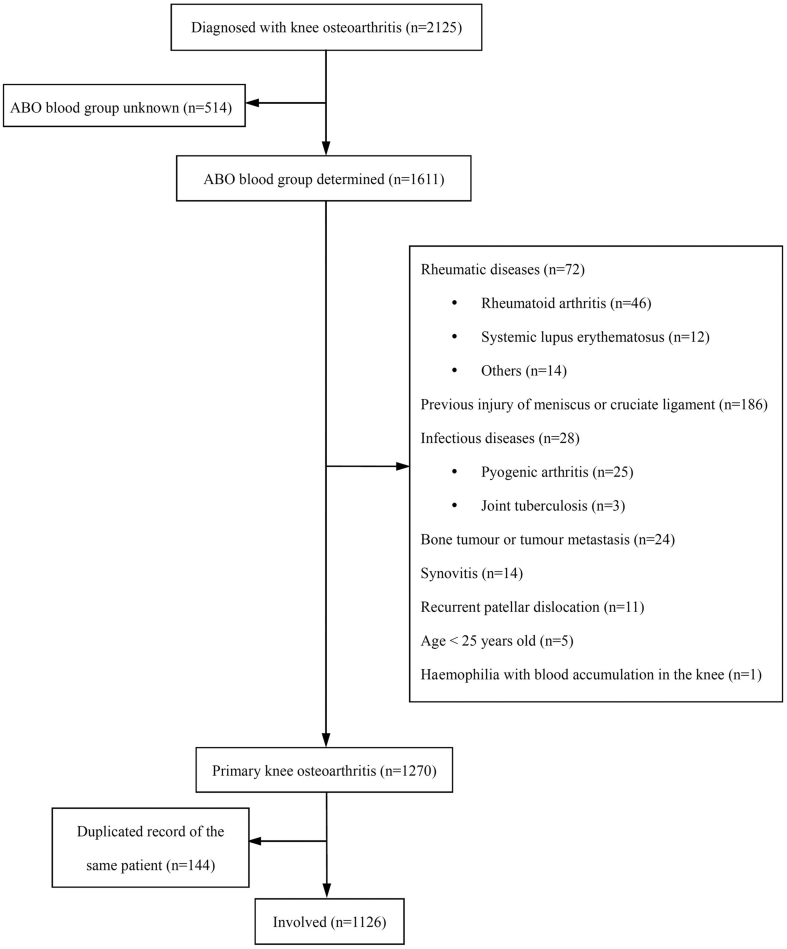
A total of 1126 cases were obtained after careful examination of medical records (Figure 1). Data of 30299 controls were achieved from Guangzhou Blood Centre. Compared with the control group, the Student t test showed that the average age of the case group was older (66.4 ± 11.2 vs. 32.3 ± 9.7, respectively, P < 0.001), whereas the χ2 test showed that the proportion of females in the case group was higher [76.3% (859 in 1126) vs. 30.8% (9346 in 20399), P < 0.001].
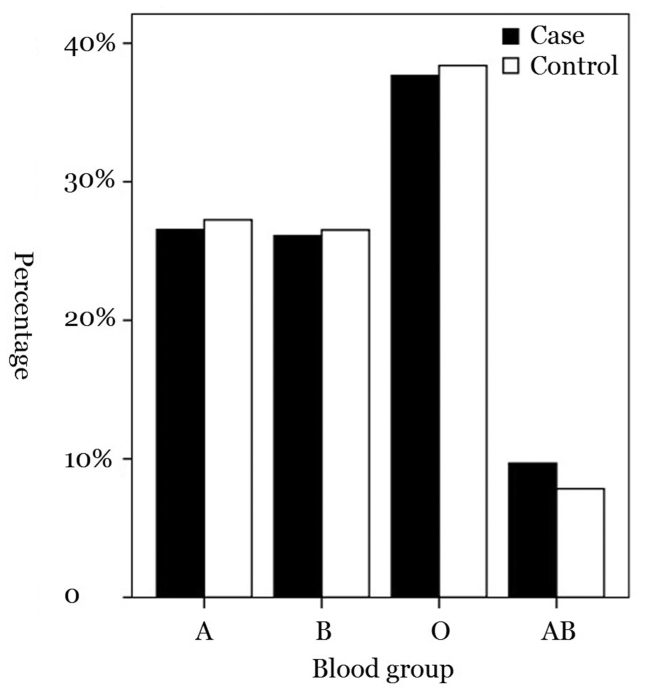
Figure 1.
Distribution of ABO blood groups among the case group and the control group.
ABO blood group distribution among cases and controls
Information on the ABO blood group of the patients and controls were extracted. Of the 30299 healthy blood donors from the general population, 8255 (27.3%) were of group A, 8036 (26.5%) of group B, 116333 (38.4%) of blood group O, and 2375 (7.8%) of group AB. Among the patients, 299 (26.6%) were of group A, 294 (26.1%) of group B, 424 (37.7%) of blood group O, and 109 (9.7%) of group AB (Figure 2). The proportion of those of AB blood group was higher in the case group than in the control group.
Figure 2.
Flow chart of the screening process.
AB blood group as a risk factor of primary knee OA
Logistic regression was applied to explore potential risk factors of primary knee OA. Univariate analysis showed that the AB blood group, as well as age and sex, was a risk factor of primary knee OA, which was confirmed by multivariate analysis (Table 1). Blood antigens A and B were not associated with primary knee OA in univariate analysis (P = 0.319 and 0.246, respectively) and thus were not put into the multivariate analysis.
Table 1.
Logistic regression analysis of risk factors of primary knee osteoarthritis.
| Factor | Univariate |
Multivariate |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age | 1.393 (1.368–1.418) | <0.001 | 1.391 (1.364–1.418) | <0.001 |
| Sex | 7.142 (6.215–8.209) | <0.001 | 8.105 (6.310–10.411) | <0.001 |
| AB blood group | 1.260 (1.030–1.542) | 0.025 | 1.506 (1.004–2.260) | 0.048 |
| A antigen | 1.066 (0.940–1.209) | 0.319 | / | / |
| B antigen | 1.078 (0.950–1.233) | 0.246 | / | / |
HR = hazard ratio; CI = confidence interval.
We further investigated whether there was any interaction between the AB blood group and age or sex. Logistic regression revealed that the AB blood group was a risk factor of primary knee OA independent of age (hazard ratio = 0.999, 95% CI = 0.933–1.069, P = 0.973) or sex (hazard ratio = 1.353, 95% CI = 0.538–3.397, P = 0.520).
AB blood group and radiological severity of primary knee OA
We retrospectively analysed the anterior–posterior radiographs of the knee in weight-bearing condition and obtained radiographs of 801 patients. We evaluated the radiological severity of knee OA using the K/L atlas (Table 2). To evaluate the intraobserver variability of K/L grading, 100 randomly selected radiographs were scored by the same observer (C.L.) 1 month after the first reading and also scored by another orthopaedic surgeon (S.L.) using the same atlas for interobserver variability. We used kappa analysis to test the intraobserver and interobserver variabilities. The consistency parameter kappa was 0.801 and 0.761, indicating high and moderate consistency, respectively.
Table 2.
K/L score of the knee X-ray radiograph in patients with primary knee osteoarthritis.
| Blood group | K/L score |
Total | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| A | 3 | 4 | 68 | 104 | 34 | 213 |
| B | 4 | 4 | 64 | 111 | 28 | 211 |
| O | 4 | 6 | 89 | 160 | 42 | 301 |
| AB | 1 | 1 | 15 | 41 | 18 | 76 |
K/L = Kellgren/Lawrence.
We used the Kruskal–Wallis test to compare the K/L score of patients of different blood groups and found no significant difference (P = 0.124). However, when we further investigated the association between the AB blood group and K/L score, the Wilcoxon rank test revealed that compared with patients of the other blood groups, those in the AB blood group had a higher K/L score (P = 0.017), indicating more significant radiological changes, whereas group A (P = 0.143) or B (P = 0.151) did not show any significant difference in the K/L score.
Histological study of ST from the knee joint
A total of 22 cases and 13 controls were involved. The Student t test revealed that age of the case group was higher than that of the control group (65.9 ± 14.2 vs. 25.9 ± 10.2, respectively, P < 0.001), while the χ2 test revealed no significant difference in sex and ABO blood group distribution between the case group and the control group (P = 0.481 and 0.263, respectively). ST was obtained by arthroscopy, with the acquisition of patients' written informed consent before the surgery.
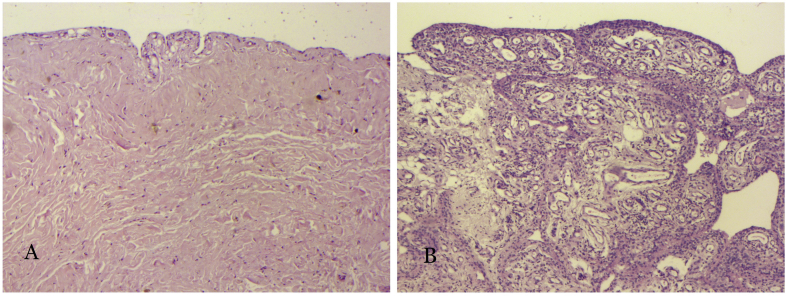
Haematoxylin and eosin staining was applied to the ST from the case group and the control group (Figure 3). ST from the control group was lined by a simple squamous epithelium. Loose connective tissue and collagen fibre could be seen under the epithelial lining. ST from the case group, in contrast, manifested significant signs of inflammation, including villiform hypertrophy of the synovial epithelium, multilayered change of the epithelium, massive neovascularisation, and appearance of fibroblasts and inflammatory cells.
Figure 3.
Haematoxylin staining of the synovium of the knee joint: (A) normal; (B) osteoarthritis.
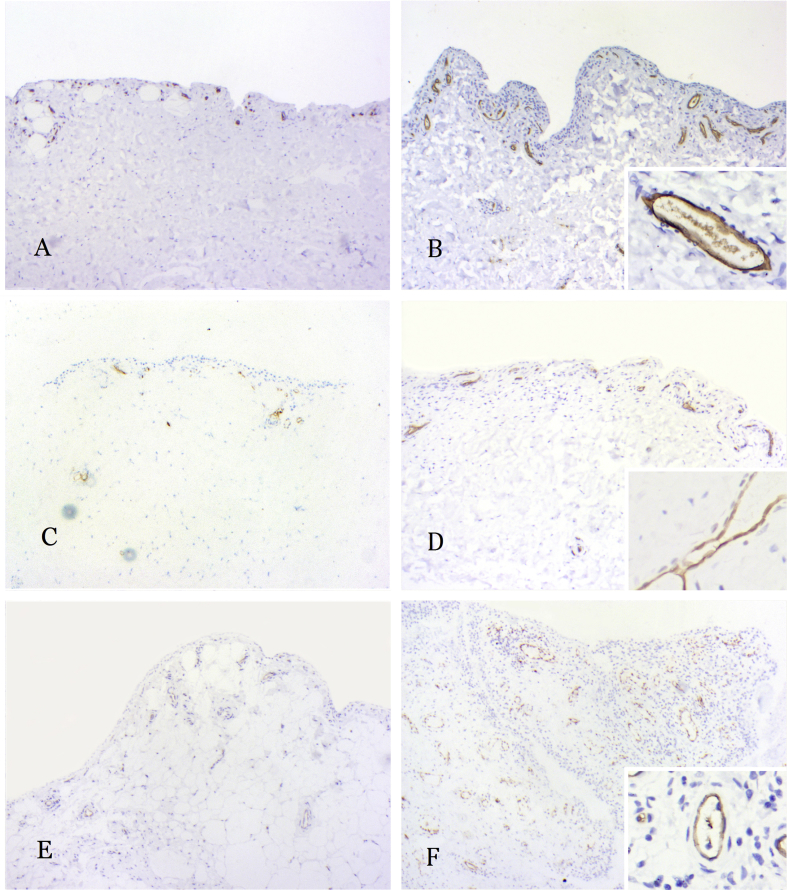
We used IHC staining to investigate the expression of blood group antigens A, B, and H and blood group–related LeY antigen on ST of the knee joint. IHC staining showed that blood group antigens A and B and blood group–related LeY antigen were expressed in the cytoplasm and cell membrane of endothelial cells in ST of both cases and controls (Figure 4). H antigen was not shown in IHC staining. We assessed each IHC-stained section by evaluating the distribution and intensity of staining and gave them each an added score. The Wilcoxon test was used to compare the final score of the case group and that of the control group. Statistical analysis revealed that the case group got a higher score for the LeY antigen than the control group (P = 0.029), whereas there was no significant difference in the IHC staining score for blood antigens A and B between the two groups (P = 0.685 and 0.898, respectively).
Figure 4.
Immunohistochemical staining of the synovium of the knee joint. (A and B) A antigen expression on the normal/OA synovium; (C and D) B antigen expression on the normal/OA synovium; (E and F) LeY antigen expression on the normal/OA synovium. OA, osteoarthritis.
Discussion
This study suggested that the AB blood group was associated with higher risk of primary knee OA and higher radiological severity of the knee joint. However, neither A antigen nor B antigen was found to be associated with primary knee OA. Further study indicated that the blood group–related LeY antigen was associated with primary knee OA.
A previous study by Lourie et al. [12] assessed the association between the ABO blood group and primary hip OA. They performed a retrospective case–control study and found that compared with the control group, the proportion of those of blood group O was lower in the case group [12]. Its sample size was relatively small, with 341 participants in the case group. Besides, the inclusion criteria were patients who underwent total hip arthroplasty owing to severe hip OA and were not representative of the patient population. Therefore, the validity of the previous study was limited. According to literature searching, to date, there is no published article concerning the association between the ABO blood group and primary knee OA.
The K/L classification system developed by Kellgren and Lawrence in 1957 has been the most widely used criteria to evaluate the radiological severity of knee OA. However, it was criticised that it may not be able to distinguish between mild OA. Researchers had investigated the impact of different descriptions of the K/L classification criteria on the diagnosis of knee OA. In conclusion, they recommended the use of the original criteria if you want to distinguish definite/mild OA (K&L ≥ 2) from none/possible OA (K&L < 2) and recommended the use of alternative criteria for distinguishing no OA (K&L = 0) from possible OA (K&L = 1). Considering that we needed to distinguish the difference of severity among the patients with OA, we used the original description of K/L criteria. To minimise the influence of different stages of OA progression, this study focused on the anterior–posterior X-ray film taken at the patients' first visit to our hospital. In this study, we found that patients with blood group AB had a higher K/L score, although the mechanism of this association remains unknown.
The blood group is determined by the blood group antigen A and B expression. However, we found that blood group antigens A and B were not associated with primary knee OA, as was mentioned previously. On the other hand, as is well known, each individual shows various levels of H antigen expression. Expression of H antigen is higher in blood group O individuals and lower in blood group AB individuals [22]. Taking into consideration that the previous study suggested the blood group O to be a protective factor, our study revealed the blood group AB as a risk factor for primary knee OA; we deduced that H antigen might be a protective factor against primary knee OA.
Blood group antigen expression has been investigated since the ABO blood group was put forward. Previous studies have shown expression of antigens A, B, and H on vascular endothelia, mucosa-secreting cells, and some other tissues [23]. Similarly, we demonstrated that the vascular endothelium of ST expressed blood group antigens A and B, but not antigen H. IHC staining showed no association between primary knee OA and expression of blood antigens A or B, which was in accordance with our conclusion from clinical data. Absence of H antigen might be a pseudonegative outcome owing to relatively low sensitivity of IHC compared with immunofluorescence staining or unfavourable affinity between the antibody and H antigen.
Despite the absence of H antigen in IHC staining, we demonstrated the expression of LeY antigen, which was in close relationship with H antigen. Previous studies have indicated that LeY was a proinflammatory factor. Researchers found that LeY was upregulated in ST and synovial fluid of patients with rheumatoid arthritis (RA) compared with normal controls [24], [25], [26]. Further research studies revealed novel functions of LeY as a mediator of angiogenesis and leucocyte-endothelial adhesion [7], [27]. Our results also suggested that LeY was associated with primary knee OA. On the other hand, H antigen and LeY were synthesised from the H antigen precursor under the catalysation by different subtypes of fucosyltransferase [28], which means their biosyntheses were competing for the same substrate. Individuals with blood group AB have lower levels of H antigen, which may thus allow higher levels of LeY antigen to become more vulnerable to inflammatory diseases such as primary knee OA. This may explain why the blood group AB was associated with higher risk of primary knee OA.
To the best of our knowledge, this is the first study to investigate the association between the ABO blood group and primary knee OA. Moreover, well-acknowledged diagnostic criteria and the classification system were applied in this study to ensure the validity of results. Limitation of this study include that it was retrospective, so the data were limited to those in the medical records.
Conclusions
This study suggested that the blood group AB was a risk factor for primary knee OA, independent of age and sex. Blood group–related LeY antigen might play a role in the association between the blood group AB and primary knee OA. Despite our rigorous methodology, the inherent limitations of the retrospective study were inevitable. Prospective cohort studies are expected to confirm the findings of this study.
Ethics approval and consent to participate
The protocol of the research project has been approved by the Institutional Review Board (IRB) of Sun Yat-sen Memorial Hospital and thus has been performed in accordance with the ethical standards laid down in the Declaration of Helsinki. All selected citizens were asked to sign an informed consent form if they wished to participate in the study.
Availability of data and material
The data sets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Conflict of interest
The authors have no conflicts of interest to disclose in relation to this article.
Funding
This study was supported by the National Natural Science Foundation of China (81472100) and Sun Yat-sen University (17YKJC16). The funding bodies had no role in the design of the study and collection, analysis, interpretation of data, and writing of the manuscript.
Acknowledgments
The authors would like to express their sincere thanks to the Information Department of Guangzhou Blood Centre.
References
- 1.Goldring M.B. The role of the chondrocyte in osteoarthritis. Arthritis Rheum. 2000 Sep;43(9):1916–1926. doi: 10.1002/1529-0131(200009)43:9<1916::AID-ANR2>3.0.CO;2-I. PubMed PMID: 11014341. [DOI] [PubMed] [Google Scholar]
- 2.Lee W.Y., Wang B. Cartilage repair by mesenchymal stem cells: clinical trial update and perspectives. J Orthop Transl. 2017 Apr;9:76–88. doi: 10.1016/j.jot.2017.03.005. PubMed PMID: 29662802. Pubmed Central PMCID: 5822962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallo J., Raska M., Kriegova E., Goodman S.B. Inflammation and its resolution and the musculoskeletal system. J Orthop Transl. 2017 Jul;10:52–67. doi: 10.1016/j.jot.2017.05.007. PubMed PMID: 28781962. Pubmed Central PMCID: 5541893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scanzello C.R., Goldring S.R. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012 Aug;51(2):249–257. doi: 10.1016/j.bone.2012.02.012. PubMed PMID: 22387238. Pubmed Central PMCID: 3372675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bijlsma J.W., Berenbaum F., Lafeber F.P. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011 Jun 18;377(9783):2115–2126. doi: 10.1016/S0140-6736(11)60243-2. PubMed PMID: 21684382. [DOI] [PubMed] [Google Scholar]
- 6.Fukumoto K., Taniguchi T., Usami N., Kawaguchi K., Fukui T., Ishiguro F. The ABO blood group is an independent prognostic factor in patients with resected non-small cell lung cancer. J Epidemiol/Japan Epidemiol Assoc. 2015 Feb 5;25(2):110–116. doi: 10.2188/jea.JE20140102. PubMed PMID: 25483106. Pubmed Central PMCID: 4310871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amin M.A., Ruth J.H., Haas C.S., Pakozdi A., Mansfield P.J., Haghshenas J. H-2g, a glucose analog of blood group H antigen, mediates mononuclear cell recruitment via Src and phosphatidylinositol 3-kinase pathways. Arthritis Rheum. 2008 Mar;58(3):689–695. doi: 10.1002/art.23296. PubMed PMID: 18311817. [DOI] [PubMed] [Google Scholar]
- 8.Patenaude S.I., Seto N.O., Borisova S.N., Szpacenko A., Marcus S.L., Palcic M.M. The structural basis for specificity in human ABO(H) blood group biosynthesis. Nat Struct Biol. 2002 Sep;9(9):685–690. doi: 10.1038/nsb832. PubMed PMID: 12198488. [DOI] [PubMed] [Google Scholar]
- 9.Mohandas N., Narla A. Blood group antigens in health and disease. Curr Opin Hematol. 2005 Mar;12(2):135–140. doi: 10.1097/01.moh.0000153000.09585.79. PubMed PMID: 15725904. [DOI] [PubMed] [Google Scholar]
- 10.Aird I., Bentall H.H., Roberts J.A. A relationship between cancer of stomach and the ABO blood groups. Br Med J. 1953 Apr 11;1(4814):799–801. doi: 10.1136/bmj.1.4814.799. PubMed PMID: 13032504. Pubmed Central PMCID: 2015995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xavier J.M., Shahram F., Sousa I., Davatchi F., Matos M., Abdollahi B.S. FUT2: filling the gap between genes and environment in Behcet's disease? Ann Rheum Dis. 2013 Dec 10 doi: 10.1136/annrheumdis-2013-204475. PubMed PMID: 24326010. [DOI] [PubMed] [Google Scholar]
- 12.Lourie J.A. Is there an association between ABO blood groups and primary osteoarthrosis of the hip? Ann Hum Biol. 1983 Jul-Aug;10(4):381–383. doi: 10.1080/03014468300006551. PubMed PMID: 6614864. [DOI] [PubMed] [Google Scholar]
- 13.Pare G., Chasman D.I., Kellogg M., Zee R.Y., Rifai N., Badola S. Novel association of ABO histo-blood group antigen with soluble ICAM-1: results of a genome-wide association study of 6,578 women. PLoS Genet. 2008 Jul;4(7):e1000118. doi: 10.1371/journal.pgen.1000118. PubMed PMID: 18604267. Pubmed Central PMCID: 2432033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melzer D., Perry J.R., Hernandez D., Corsi A.M., Stevens K., Rafferty I. A genome-wide association study identifies protein quantitative trait loci (pQTLs) PLoS Genet. 2008 May;4(5):e1000072. doi: 10.1371/journal.pgen.1000072. PubMed PMID: 18464913. Pubmed Central PMCID: 2362067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haseeb A., Haqqi T.M. Immunopathogenesis of osteoarthritis. Clin Immunol. 2013 Mar;146(3):185–196. doi: 10.1016/j.clim.2012.12.011. PubMed PMID: 23360836. Pubmed Central PMCID: 4015466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Q., Benderdour M., Lavigne P., Ranger P., Fernandes J.C. Evidence for two distinct pathways in TNFalpha-induced membrane and soluble forms of ICAM-1 in human osteoblast-like cells isolated from osteoarthritic patients. Osteoarthritis and cartilage/OARS. Osteoarthr Res Soc. 2007 Mar;15(3):300–308. doi: 10.1016/j.joca.2006.08.010. PubMed PMID: 17161959. [DOI] [PubMed] [Google Scholar]
- 17.Altman R., Asch E., Bloch D., Bole G., Borenstein D., Brandt K. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986 Aug;29(8):1039–1049. doi: 10.1002/art.1780290816. PubMed PMID: 3741515. [DOI] [PubMed] [Google Scholar]
- 18.Kellgren J.H., Lawrence J.S. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957 Dec;16(4):494–502. doi: 10.1136/ard.16.4.494. PubMed PMID: 13498604. Pubmed Central PMCID: 1006995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Atlas of Standard Radiographs of Arthritis. Rheumatology. 2005 Dec:44. Suppl 4:iv46-iv72. PubMed PMID: 16306483. [PubMed] [Google Scholar]
- 20.Shimizu M., Saitoh Y., Itoh H. Immunohistochemical staining of Ha-ras oncogene product in normal, benign, and malignant human pancreatic tissues. Hum Pathol. 1990 Jun;21(6):607–612. doi: 10.1016/s0046-8177(96)90006-4. PubMed PMID: 2161789. [DOI] [PubMed] [Google Scholar]
- 21.Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977 Mar;33(1):159–174. PubMed PMID: 843571. [PubMed] [Google Scholar]
- 22.Hu L. 2013. Clinical tests for blood transfusion Beijing: people's medical publishing house; p. 26. [Google Scholar]
- 23.Szulman A.E. The histological distribution of the blood group substances in man as disclosed by immunofluorescence. IV. The ABH antigens in embryos at the fifth week post fertilization. Hum Pathol. 1971 Dec;2(4):575–585. doi: 10.1016/s0046-8177(71)80071-0. PubMed PMID: 4939265. [DOI] [PubMed] [Google Scholar]
- 24.Halloran M.M., Carley W.W., Polverini P.J., Haskell C.J., Phan S., Anderson B.J. Ley/H: an endothelial-selective, cytokine-inducible, angiogenic mediator. J Immunol. 2000 May 1;164(9):4868–4877. doi: 10.4049/jimmunol.164.9.4868. PubMed PMID: 10779796. [DOI] [PubMed] [Google Scholar]
- 25.Zhu K., Amin M.A., Kim M.J., Katschke K.J., Jr., Park C.C., Koch A.E. A novel function for a glucose analog of blood group H antigen as a mediator of leukocyte-endothelial adhesion via intracellular adhesion molecule 1. J Biol Chem. 2003 Jun 13;278(24):21869–21877. doi: 10.1074/jbc.M213052200. PubMed PMID: 12672794. [DOI] [PubMed] [Google Scholar]
- 26.Dettke M., Palfi G., Pursch E., Fischer M.B., Loibner H. Increased expression of the blood group-related Lewis Y antigen on synovial fluid granulocytes of patients with arthritic joint diseases. Rheumatology. 2001 Sep;40(9):1033–1037. doi: 10.1093/rheumatology/40.9.1033. PubMed PMID: 11561115. [DOI] [PubMed] [Google Scholar]
- 27.Isozaki T., Ruth J.H., Amin M.A., Campbell P.L., Tsou P.S., Ha C.M. Fucosyltransferase 1 mediates angiogenesis, cell adhesion and rheumatoid arthritis synovial tissue fibroblast proliferation. Arthritis Res Ther. 2014 Jan 28;16(1):R28. doi: 10.1186/ar4456. PubMed PMID: 24467809. Pubmed Central PMCID: 3978694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hokke C.H., Neeleman A.P., Koeleman C.A., van den Eijnden D.H. Identification of an alpha3-fucosyltransferase and a novel alpha2-fucosyltransferase activity in cercariae of the schistosome Trichobilharzia ocellata: biosynthesis of the Fucalpha1-->2Fucalpha1-->3[Gal(NAc)beta1-->4]GlcNAc sequence. Glycobiology. 1998 Apr;8(4):393–406. doi: 10.1093/glycob/8.4.393. PubMed PMID: 9499387. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and/or analyzed during the present study are available from the corresponding author on reasonable request.