Abstract
Total hip arthroplasty is a common surgical technique, yet it has severe complications, such as loosening and repeated revision. Thus, hip-preserving surgical options should be considered first to treat cartilage defects in the femoral head, especially for younger patients. Current surgical options for chondral repair of the femoral head include microfracture, trapdoor procedure, transplantation of osteochondral allografts and autografts, and autologous chondrocyte implantation. Each of these techniques has unique advantages and limitations; however, none of them have been consented as the best practice for cartilage defects. In this review article, we also introduced a novel technique for repairing osteochondral defects of the femoral head using autologous costal cartilage grafts that may have good translational potential for cost-effective and safe applications.
The translational potential of this article
This review updates current surgical options for reparing articular cartilage defects in the femoral head. We also introduce a novel technique for repairing osteochondral defects of the femoral head using autologous costal cartilage grafts.
Keywords: Articular cartilage, Costal cartilage, Femoral head, Transplantation
Introduction
Articular cartilage is hyaline cartilage that covers the surface of the joint and disperses the joint load with an interface that exhibits an ultralow friction coefficient. The hips are major load-bearing joints, and when the articular surface is damaged in the femoral head, patients often exhibit narrowed and/or stiff hip joints, which greatly impacts daily physical activities and quality of life. The causative factors of osteochondral injuries of the femoral head include (1) mechanical damage, including either acute violent injury or chronic damage caused by long-time and high-load sports that affect femoral head cartilage; (2) rheumatic diseases, such as ankylosing spondylitis and hip infection, which lead to pathological osteochondral damage of the femoral head; and (3) osteonecrosis of the femoral head (ONFH), which can cause degeneration of articular cartilage and peeling from the collapsed necrotic area [1], [2], [3], [4]. The joint space then becomes narrowed and ultimately develops into osteoarthritis if no treatment is applied [5].
Nonreparative surgical options for extensive osteochondral injuries in the hip include rotation osteotomy and hip joint replacement. Intertrochanteric rotation osteotomy is a surgical strategy to rotate the damaged cartilage surface away from weight-bearing areas. However, only a narrow limited rotatable angle is often achieved, and the blood supply to the femoral head may be further interrupted. Moreover, changes in the proximal femoral structure contribute to difficulty in matching the stem prosthesis for potential hip joint replacement. Instead of changing the weight-bearing articular area, total hip arthroplasty seems a more thorough option, which comprises removal of the entire involved femoral head. Notably, total hip arthroplasty is one of the most successful orthopaedic surgical procedures, which allows the patient to walk on the first postoperative day; however, there are increasing concerns of severe complications, such as infection, prosthesis loosening, and dislocation [6]. The limited lifespan of the replacement femoral head causes young patients to enter an endless cycle of repetitive revision once hip arthroplasty is chosen. Therefore, particularly for young patients, hip-preserving treatment should be attempted first to reconstruct the damaged articular cartilage surface.
As there are no nerves or blood vessels inside the adult cartilage tissue and the chondrocytes are confined in the lacuna, when the articular cartilage is damaged and the injury gradually accumulates without self-healing, osteoarthritis may then be the consequence. Therefore, treatment of osteochondral injuries of the femoral head is a particular challenge in the field of orthopaedics. Compared with the knee joint, the femoral head articular surface has a higher ratio of cartilage coverage, reduced extent of weight-bearing area, vulnerable blood supply, and deeper location; hence, it is much more difficult to repair.
Current reparative surgical techniques for osteochondral injuries in the femoral head include microfracture, trapdoor procedure, transplantation of osteochondral allografts and autografts, and autologous chondrocyte implantation. These methods have been frequently used in the knee joint with favourable outcomes, but the evidence for their use in the hip joint remains limited, and none of these methods has been consented as the best clinical surgical technique indicated for osteochondral defects.
Surgically induced microfracture
Surgically induced microfracture is a marrow-stimulating multiple-drilling technique that is well established for knee surgery; this approach resurfaces chondral defects with fibrocartilage tissue [7], [8]. On the basis of similar indications and technology, its application has been extended to the hip [9], [10], [11], [12], [13] and is mostly performed through hip arthroscopy [14], [15]. Microfracture is indicated for focal (<2 cm in size) [16], full-thickness chondral lesions with minor arthritis [15], based on the data from knee surgery. When the lesion is identified by arthroscopy, unstable cartilage detached from the bone bed should be debrided to a normal cartilage edge, using a ringed curette. Multiple holes (3–4 mm in depth, 2–3 mm apart) are then made perpendicularly on the subchondral bone, using microfracture awls [16]. The bone marrow blood, containing intrinsic mesenchymal stem cells and growth factors, should be confirmed to emanate from the holes to help to fill the chondral defect space with fibrocartilage [17]. Fibrocartilage is histologically quite different from hyaline cartilage and is mechanically weaker [18], so its application for extensive chondral defects over a large area is limited, especially when subchondral bone is also involved.
Karthikeyan et al. [19] reported that 19 of 20 minor chondral defects (mean size of 1.54 cm2) were filled with fibrocartilage at second-look arthroscopy. However, a matched cohort–controlled study showed patients undergoing microfracture (79 hips) during hip arthroscopy had similar patient-reported outcome scores, compared with the control group (158 hips), at 2 years postoperatively [20]. Moreover, the hip function score showed greatest improvement in the first 3 months after surgery. Another case series study of 70 patients by Trask and Keene [10] showed that only 60% of full-thickness chondral lesions treated by microfracture had good/excellent results after 2 years. Indeed, if the subchondral bone is also involved, the efficiency is lowered. In addition, further evidence is needed to establish specific criteria for microfracture in the hip joint because the structural and mechanical environment of the hip is quite different from that of the knee. Although clinical results are presently variable, microfracture remains a first-line surgical strategy, especially for minor cartilage lesions.
Autologous chondrocyte implantation
Although microfracture is suitable for treatment of small chondral lesions, autologous chondrocyte implantation (ACI) has been developed for treatment of cartilage defects in the knee joint; clinical results have shown promise for treatment of full-thickness chondral lesions of larger areas [21], [22]. The ACI procedure typically comprises two stages. In the first stage, a small amount of articular cartilage tissue is harvested from the non–weight-bearing area of the knee joint, generally via minimally invasive methodology. The tissue (containing viable chondrocytes) is then sent to a qualified institution for cell amplification. During the second stage, the cultured chondrocytes are implanted into the osteochondral defect and covered by a patch (e.g., periosteum). At this point, chondrocytes can also be incorporated into biodegradable scaffolds [21], [22], [23], [24], such as hydrogel and decellularized type I collagen, to facilitate the maintenance of chondrocytes in the defect. This modified ACI procedure is also known as matrix-assisted ACI (MACI) [23], [24], [25], [26]. The indications of ACI and MACI for the hip have not been well established. According to current evidence, ACI and MACI perform well for focal full-thickness chondral defects with larger areas (≥3 cm2) and minimal arthritis [25], [27], [28].
Akimau et al. [29] treated a 31-year-old patient with posttraumatic ONFH using ACI. Two hundred forty milligrams of hyaline cartilage was taken from the non–weight-bearing area of the knee and sent for culturing for 3 weeks. After hip surgical dislocation, the necrotic tissue in the femoral head was debrided. After bone grafting, the extensive cartilage defect area was wrapped with a type I collagen membrane. Cultured chondrocytes were then injected into the lesion area under the membrane. Importantly, hip function improved after surgery. Fifteen months postoperatively, biopsy taken during an arthroscopic examination showed that the reparative tissue was primarily fibrocartilage. Fontana et al. [25] treated 30 patients with hip chondral lesions (mean size of 2.6 cm2) using ACI. After a mean follow-up of 74 months, the ACI group had better clinical outcomes than the debridement-only group. Presently, ACI seems to be more often used for chondral defects on the acetabular side than for those on the femoral side [25], [28].
Because both the cellular component and the matrix lack mechanical strength, similar to the situation during microfracture treatment, the integrity of subchondral bone is important for achievement of good clinical results in both ACI and MACI procedures. Therefore, caution must be exercised if ACI or MACI is to be used for patients with extensive cartilage defects, particularly when subchondral defects have occurred during femoral head necrosis. Concerns also exist regarding the dedifferentiation of chondrocytes during in vitro culture, which might reduce the efficiency of treatment; moreover, there remains a lack of direct evidence that the implanted chondrocytes form hyaline cartilage tissue in the defect. The high cost and demanding conditions of the cell-processing facility limit the widespread use of this approach. Moreover, most optimistic clinical results have been demonstrated by application in the knee; notably, the curvature of the articular surface in the femoral head is much larger than that of the knee, and this difference in the mechanical environment might reduce the likelihood of converting the protocol and indications directly from knee to hip. A one-stage procedure may comprise the next generation of ACI technology in the future.
Replantation of laminated cartilage
It is difficult for both microfracture and ACI to restore chondral lesions of hyaline cartilage [7], [29]. The reparative tissue, similar to fibrocartilage, is mechanically much weaker than articular hyaline cartilage tissue. Delaminated cartilage lesion is full-thickness cartilage separated from the underlying subchondral bone primarily because of trauma or femoroacetabular impingement. It is reasonable to replant the detached cartilage to repair the defect. Lim et al. [30] screwed the chondral flap directly back onto the femoral head in a young patient with severe delamination of cartilage caused by traumatic hip dislocation in an open procedure. Fibrin adhesive can be used to secure the delaminated cartilage back to the subchondral bone through hip arthroscopy [31], [32]. It is generally recommended to perform microfracture on subchondral bone before resuturing the detached chondral flap to improve biological integration of the osteochondral interface [33]. However, the early mechanical bonding strength of fibrin adhesive is still weak [34]. Present reports primarily focus on the treatment of chondral lesions on the acetabulum, instead of the femoral head, and further histological studies are needed to determine the fate of the replanted cartilage and the interface.
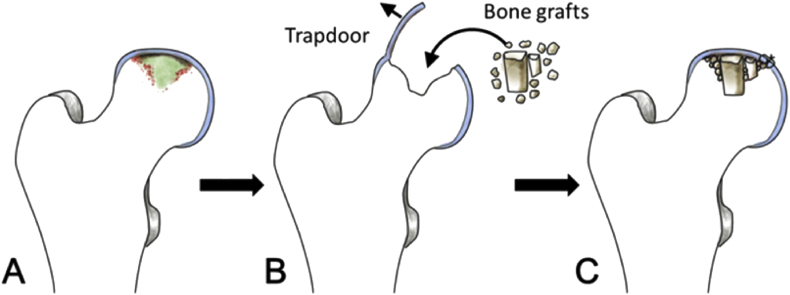
When ONFH reaches Stage III and early Stage IV, subchondral bone often collapses and articular cartilage peels off over the necrotic area [35]. Thus, it appears impossible to simply replant the detached cartilage without bone management. The trapdoor procedure [36], [37], [38], [39], [40], [41], [42] is an approach to reconstruct subchondral bone using bone grafting and then replant the cartilage flap on top of the subchondral bone. Although the cartilage is not directly repaired, this procedure aims to repair the bone bed, which enables the replanted cartilage to heal. After the osteochondral lesion of the femoral head is exposed by surgical dislocation, a cartilage trapdoor flap is made using a scalpel or an osteotome along the edge of the detached area, which is then elevated to enable debridement of necrotic bone beneath the cartilage; subsequently, it is hinged back to recover the articular surface. The trapdoor flap is between 10% and 30% of the femoral head cartilage surface area (Fig. 1). Mont et al. [36] reviewed the outcomes of 24 patients with osteochondral defect due to Stage III-IV femoral head necrosis who were treated with this method (mean follow-up of 4.7 years); 22 of these patients had excellent results, as determined by the Harris hip-scoring system. However, the cartilage in the necrotic area, which is the intended area of replantation, is often denatured or degenerated. Occasionally, the cartilage is too fragile for complete preservation for the trapdoor procedure and is easily disrupted during fixation [43]. Moreover, histological evidence is not available regarding the fate of the replanted cartilage or formation of the interface between the replanted cartilage and grafted bone. Xu et al. [43] also used the ligamentum teres as a trapdoor for resuturing to fill the osteochondral defect that remained after treatment of chondroblastoma in the femoral head. After a mean follow-up period of 66 months, only two of 13 patients experienced postoperative complications.
Figure 1.
Illustration of a trapdoor technique (A) Delaminated cartilage in the necrotic femoral head (B) chondral flap used as a trapdoor in the necrotic area. After debridement, bone grafts are packed into the bone defect (C) the chondral flap is rehinged and repaired.
Osteochondral autograft transplantation—mosaicplasty
Articular osteochondral autograft transplantation is also known as mosaicplasty. The procedure uses multiple autologous osteochondral cylindrical grafts harvested from non–weight-bearing locations of the knee or hip joint to reconstruct osteochondral defects over large areas [46], [48]. Because osteochondral grafts contain hyaline cartilage with an original interface and subchondral bone structure, this approach may constitute a reliable strategy to repair extensive osteochondral defects; thus, it is used in the knee. According to the evidence from performance in the knee, the recommended indications are patient age <45 years and localized osteochondral defects <3 cm2 [44], [45]. Mosaicplasty has been used in the hip for the management of osteochondral defects on the femoral head, but the clinical results are varied and have thus far been limited to case reports or case series.
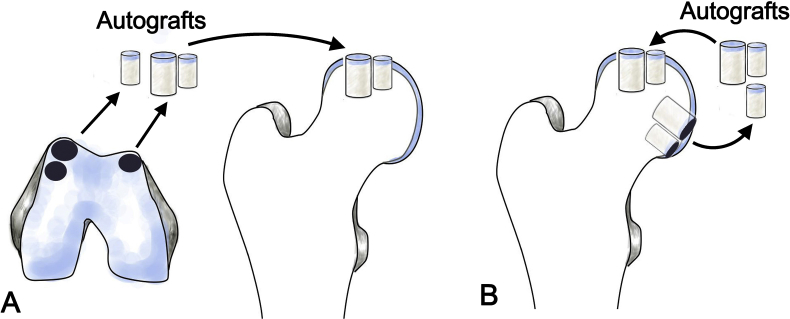
As a traditional method of knee repair, osteochondral cylindrical grafts can be harvested from the non–weight-bearing area of the knee joint and transplanted to the osteochondral lesion on the femoral head. After hip surgical dislocation, the osteochondral lesion is identified and debrided. The difference in curvature between the femoral head and knee joint must be considered in advance. If the debridement is excessive, there may be difficulty in securing the grafts firmly within the defect. An osteochondral harvesting system is used to harvest multiple osteochondral grafts of suitable diameter (e.g., 6 or 8 mm) from the non–weight-bearing area of the knee joint, based on the lesion area in the femoral head [46]. The grafts are packed into the defects in the femoral head to reconstruct the articular surface (Fig. 2A). Fotopoulos et al. [46] reconstructed a necrotic osteochondral lesion in the femoral head of a 19-year-old female using multiple autologous osteochondral grafts obtained from the non–weight-bearing area of the knee joint. Five donor plugs were transplanted in separate holes made in the 2 cm × 3 cm necrotic area; these formed a smooth articular surface. Three years postoperatively, the hip function of the patient was restored (Harris Hip Score = 96), and radiographic analysis showed good joint space and articular surface. Anthonissen et al. [47] used osteochondral grafts from ipsilateral knee to reconstruct a traumatic osteochondral lesion (5 cm2 in size). Two years postoperatively, the patient was able to partially return to the previous activity level. Güngör et al. [48] reported two cases of repairing osteochondral defects in the femoral head, which were due to femoroacetabular impingement. The sizes of defects were 2.7 and 3.6 cm2; three to four osteochondral grafts from the knee were press-fitted into the defect to reconstruct the articular surface. After 1 year of follow-up, the patients returned to the previous level of daily activity [48]. Kılıçoğlu et al. [49] reported a case in which a large osteochondral defect was treated by this method and demonstrated excellent results (Harris Hip Score = 96) over 8 years of follow-up. Other clinical results achieved with this method have been variable. Rittmeister et al. [50] used autologous osteochondral grafts from the knee to resurface five severely necrotic femoral heads; the clinical results were unsatisfactory. After an average of 4.8 years of follow-up, one femoral head had remained successful for 31 months, while the others had undergone total hip replacement. Owing to the considerable difference in curvature between the femoral head and knee joint, the contour of the transplanted grafts from the knee is difficult to match with the surrounding cartilage in the femoral head and difficult to repair in a manner similar to that of the knee. Therefore, caution must be exercised when the indications and protocols of mosaicplasty are translated from the knee to the hip. Moreover, Andrade et al. [51] reviewed 21 studies, including 1726 cases of mosaicplasty; they reported that osteochondral harvesting in mosaicplasty often resulted in considerable donor-site morbidity, such as patellofemoral disturbances. The donor-site knee-to-ankle morbidity was 16.9%; knee-to-knee morbidity was 5.9%. Although donor-site morbidity for knee to hip is not known, hip-to-hip mosaicplasty has been performed to avoid disturbing healthy knee joints to repair osteochondral defects in the femoral head.
Figure 2.
Illustrations of osteochondral allograft transplantation (A) Autografts are harvested from non–weight-bearing portion of the knee (B) autografts are harvested from non–weight-bearing region of the femoral head.
Osteochondral grafts from the non–weight-bearing region of the femoral head have been used to repair osteochondral defects in the femoral head [52], [53], [54]. Sotereanos et al. [52] used this method for treatment of a 36-year-old patient with a 1.5-cm osteochondral defect in a necrotic femoral head. After grafting cancellous bone from the greater trochanter into the debrided defect in the femoral head, three osteochondral cylindrical grafts (6 or 8 mm in diameter) were harvested in the inferolateral portion of the femoral head and press-fitted into the defect (Fig. 2B). After 66 months of follow-up, the patient was pain-free with improved hip function (Harris Hip Score = 96). Won et al. [55] reported a case in which a similar method was used to repair a traumatic osteochondral defect (2.5 × 1 cm) in the weight-bearing region of the femoral head. An osteochondral graft from the non–weight-bearing portion of the femoral head was harvested and transplanted. One year postoperatively, arthroscopic second-look surgery showed mild softening and fraying on the repaired surface of the femoral head; however, the hip joint function remained good (modified Harris Hip Score = 82). However, osteochondral harvesting in the non–weight-bearing region of the femoral head may further impair the articular surface and blood supply of the damaged femoral head, particularly when a large osteochondral defect is repaired. Because the donor site is located on the inferior portion of the femoral head, the accessible portion for graft harvesting is very limited. Presently, the relevant literature is limited to a single case report.
Osteochondral allograft transplantation
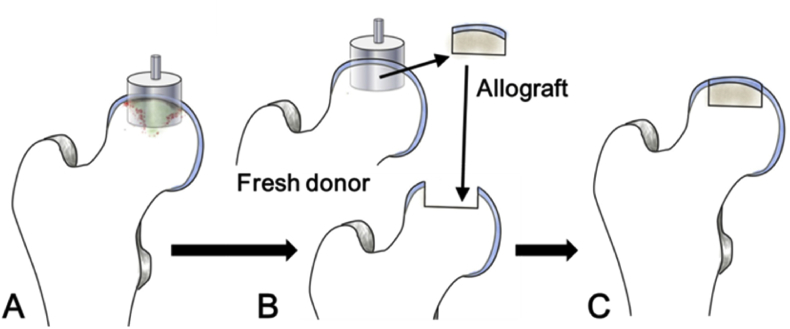
Concerning the donor-site morbidity of autologous grafting, osteochondral allograft transplantation (OAT) is another option for repair of osteochondral defects over large areas. Fresh osteochondral allografts contain viable chondrocytes, hyaline cartilage matrix, and intact subchondral bone. Cryopreserved allogeneic osteochondral grafts can be transplanted and press-fitted into osteochondral defects that cover large areas. Studies have shown that optimal clinical results can be achieved by using fresh allogeneic osteochondral grafts stored for <28 days because their chondrocyte activity rates are >70% [56], [57]. Viable articular surfaces with good mechanical and histological properties can be rapidly achieved using this method (Fig. 3). In a long-term study of using fresh OAT in 63 knees, Raz et al. [58] showed that graft survival rates were 84% and 59% at 10 and 25 years, respectively. Notably, patients with viable grafts had good knee function. Furthermore, >75% of grafted patients could return to sports after OAT [59], [60]. Although OAT showed good clinical outcomes in the knee, there are few reports of OAT for the hip, and the clinical results are variable. Khanna et al. [61] reported that 13 of 17 patients had fair to good outcomes after OAT for the hip, with a follow-up of 3–74 months. Mei et al. [62] reviewed 22 patients who underwent OAT for osteochondral defects in the femoral head. Sixteen of 22 patients had good hip functions (modified Harris Hip Scores ≥ 70), and five of 22 patients underwent total hip replacement with a minimum of 2 years of follow-up. In a retrospective study with a mean follow-up of 1.4 years [63], only seven of 10 patients who underwent OAT for hip osteochondral injuries were successful; three of 10 patients converted to total hip replacement. Delamination of cartilage was also reported in two patients who underwent decellularized OAT at 6 months postoperatively [64]. The disadvantages of OAT involve the use of demanding techniques and protocols, as well as the need for prompt and sustainable supply of fresh donor tissue. Moreover, allogeneic transplantation also carries the risk of immunological rejection and potential transmission of disease.
Figure 3.
Illustrations of osteochondral allograft transplantation (A) Femoral head with osteochondral defect (B) removal of damaged osteochondral portion and transplantation with fresh allograft from the same level (C) reconstructed articular surface of the femoral head.
Autologous costal cartilage transplantation
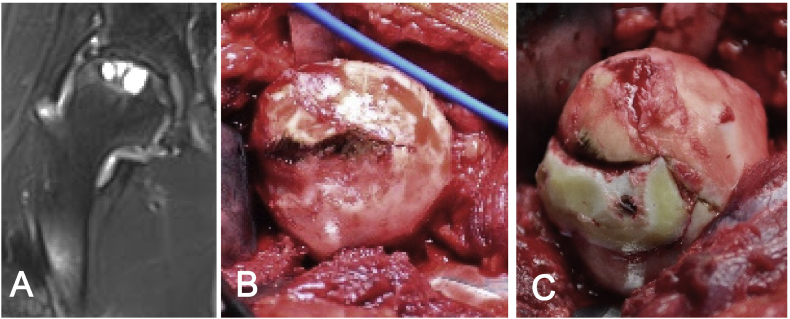
Because articular cartilage is located only in synovial joints with important functions, the donor sites are very limited. Another reservoir of hyaline cartilage is costal cartilage, which has histological and mechanical properties similar to those of articular cartilage; it can be a reliable source of cartilage for articular surface reconstruction. Since the 1920s, costal osteochondral conjunction has been used as osteochondral graft to reconstruct the temporomandibular joint [65]. Twenty-three patients with posttraumatic finger joint ankyloses were treated by costal osteochondral graft transplantation [66]; finger function was significantly improved at follow-up of up to 11 years. Obert et al. [67] reconstructed the radiocarpal joint in patients with posttraumatic arthritis using costal osteochondral grafts (bony portion towards the debrided bone bed; chondral portion facing the radiocarpal joint space). After 10 years of follow-up, two-thirds of the patients had good or excellent clinical outcomes. Costal osteochondral grafts have also been used to treat osteochondral defects in larger joints, such as the elbow. Sato et al. [68] described 72 patients with an osteochondral defect of the humeral capitulum, all of whom were treated using one or two cylindrical grafts from the costal osteochondral conjunction. After follow-up of >3 years, 69 of 72 patients had good or excellent results, and 70 patients returned to sports. Because each rib has only one osteochondral junction, it is difficult to use the costal osteochondral conjunction to repair cartilage defects in the femoral head, which typically exhibits a larger defect area. Our previous study [69] showed that after grafting into the cartilage defect, costal cartilage could form a reliable biological interface with the bone bed, which did not considerably change the morphology of hyaline cartilage. This suggested that it is reasonable to repair the large areas of osteochondral defects in the femoral head using autologous costal cartilage if the graft is sliced and transplanted in a mosaicplasty manner. As a proof of concept, we have used this method to treat a 30-year-old male patient with an osteochondral defect in the femoral head (Fig. 4). Nearly one-sixth of the total femoral head surface on the weight-bearing area was resurfaced by a sliced segment of costal cartilage. After 1 year of follow-up, the patient could walk with a good walking gait and did not experience pain. More evidence with longer follow-up periods, as well as design of clinical trials, is required to further verify the effectiveness of this promising innovative method. This surgical strategy has the potential to preserve a hip joint by using only one segment of costal cartilage, especially desirable for young patients. Moreover, it has no negative impact on any other normal joint or subsequent treatment based on our observation.
Figure 4.
An innovative autologous costal cartilage transplantation (A) Preoperative MRI examination indicated detached femoral head cartilage, multiple cystic changes, and joint space narrowing (B) extensive osteochondral defect remained after debridement (C) reconstructed articular surface of the femoral head using costal cartilage. MRI = magnetic resonance imaging.
Conclusion
Articular cartilage defects in the femoral head can lead to hip arthritis if proper treatment is not performed. Surgical management of severe articular defects may especially benefit young patients to preserve hip joint and avoid replacement. We have described current surgical options for repairing cartilage defects in the femoral head in this article, including microfracture, cartilage replantation, ACI/MACI, and autograft/allograft transplantation. Most of these techniques were originally established for management of the knee joint. The adoption of these methods in the femoral head is however limited, explained by the fact that the articular surface femoral head exhibits greater curvature, deeper location, and more problematic blood supply, than the other joints such as knee joint. Clinical evidence available is limited to single case reports or case series, and the clinical outcomes are not satisfactory as compared with those in the knee. Therefore, additional efficient solutions or options are desirable to treat cartilage defects in the femoral head. Here, we also described an innovative costal cartilage transplantation technology to repair large osteochondral defects in the femoral head and described promising early outcomes. The comparison of current surgical management methods for repairing articular cartilage defects in the femoral head is listed in Table 1. Further investigations are needed to develop and formulate generally acceptable surgical principles for the treatment of articular cartilage defects in the femoral head.
Table 1.
Comparison of current surgical options for repairing articular cartilage defects in the femoral head.
| Technique | Cartilage lesions |
Surgical approach (1-stage/2-stage) | Additional joint injury | Repair formulation |
Cost | ||||
|---|---|---|---|---|---|---|---|---|---|
| Size | Depth | Subchondral bone | Source | Repair tissue | Osteochondral interface | ||||
| Microfracture | <2 cm2 | Full thickness | Intact | Arthroscopy (1-stage) | None | Bone marrow cells | Fibrocartilage | N/A | Minimal |
| ACI | 2–8 cm2 | Full thickness | Intact | Arthroscopy or open surgery (2-stage) | Minimal | Expanded autologous chondrocytes | Hyaline-like cartilage/fibrocartilage | N/A | Significant |
| Replantation | N/A | Delaminated | Intact/impaired | Open surgery (1-stage) | None | Autologous articular cartilage | Hyaline cartilage | Biological integration | Moderate |
| Mosaicplasty | 2–6 cm2 | Full thickness | Intact/impaired | Open surgery (1-stage) | Moderate | Autologous articular cartilage | Hyaline cartilage | Natural | Moderate |
| OAT | 2–6 cm2 | Full thickness | Intact/impaired | Open surgery (1-stage) | None | Allogeneic articular cartilage | Hyaline cartilage | Natural | Significant |
| ACCT | 2–6 cm2 | Full thickness | Intact/impaired | Open surgery (1-stage) | None | Autologous costal cartilage | Hyaline cartilage | Biological integration | Moderate |
ACCT = autologous costal cartilage transplantation; ACI = autologous chondrocyte implantation; Microfracture = surgically induced microfracture; Mosaicplasty = osteochondral autograft transplantation; N/A = not applicable; OAT = osteochondral allograft transplantation; Replantation = replantation of laminated cartilage.
Conflicts of interest
The authors have no conflicts of interest to disclose in relation to this article.
Acknowledgements
This work was supported by the Funds for International Cooperation and Exchange of the National Natural Science Foundation of China (Grant No. 8181001137).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2019.06.002.
Contributor Information
Dajiang Du, Email: dudajiang@sjtu.edu.cn.
Peichun Hsu, Email: eryhsu@outlook.com.
Zhenzhong Zhu, Email: zzz1129@gmail.com.
Changqing Zhang, Email: zhangcq@sjtu.edu.cn.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Sonoda K., Motomura G., Kawanami S., Takayama Y., Honda H., Yamamoto T. Degeneration of articular cartilage in osteonecrosis of the femoral head begins at the necrotic region after collapse: a preliminary study using T1 rho MRI. Skelet Radiol. 2017;46:463–467. doi: 10.1007/s00256-017-2567-z. [DOI] [PubMed] [Google Scholar]
- 2.Magnussen R.A., Guilak F., Vail T.P. Articular cartilage degeneration in post-collapse osteonecrosis of the femoral head: radiographic staging, macroscopic staging, and histologic changes. J Bone Joint Surg Am. 2005;87:1272–1277. doi: 10.2106/JBJS.D.01936. [DOI] [PubMed] [Google Scholar]
- 3.Ruch D.S., Sekiya J., Dickson Schaefer W., Koman L.A., Pope T.L., Poehling G.G. The role of hip arthroscopy in the evaluation of avascular necrosis. Orthopedics. 2001;24:339–343. doi: 10.3928/0147-7447-20010401-15. [DOI] [PubMed] [Google Scholar]
- 4.Li Z.R., Cheng L.M., Wang K.Z., Yang N.P., Yang S.H., He W. XLGB herbal Fufang prevents corticosteroid-induced osteonecrosis of the femoral head - a multicenter, randomized, double-blind, placebo-controlled clinical trial. J Orthop Transl. 2018;12:36–44. doi: 10.1016/j.jot.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glimcher M.J., Kenzora J.E. The biology of osteonecrosis of the human femoral head and itsclinical implications: II. The pathological changes in the femoral head as an organ and in the hip joint. Clin Orthop Relat Res. 1979;139:283–312. [PubMed] [Google Scholar]
- 6.Lee J.H., Lee B.W., Lee B.J., Kim S.Y. Midterm results of primary total hip arthroplasty using high cross-linked polyethylene: minimum 7 year follow-up study. J Arthroplast. 2011;26:1014–1019. doi: 10.1016/j.arth.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Steadman J.R., Briggs K.K., Rodrigo J.J., Kocher M.S., Gill T.J., Rodkey W.G. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19:477–484. doi: 10.1053/jars.2003.50112. [DOI] [PubMed] [Google Scholar]
- 8.Steadman J.R., Miller B.S., Karas S.G., Schlegel T.F., Briggs K.K., Hawkins R.J. The microfracture technique in the treatment of full-thickness chondral lesions of the knee in National Football League players. J Knee Surg. 2003;16:83–86. [PubMed] [Google Scholar]
- 9.Marquez-Lara A., Mannava S., Howse E.A., Stone A.V., Stubbs A.J. Arthroscopic management of hip chondral defects: a systematic review of the literature. Arthroscopy. 2016;32:1435–1443. doi: 10.1016/j.arthro.2016.01.058. [DOI] [PubMed] [Google Scholar]
- 10.Trask D.J., Keene J.S. Analysis of the current indications for microfracture of chondral lesions in the hip joint. Am J Sports Med. 2016;44:3070–3076. doi: 10.1177/0363546516655141. [DOI] [PubMed] [Google Scholar]
- 11.McDonald J.E., Herzog M.M., Philippon M.J. Return to play after hip arthroscopy with microfracture in elite athletes. Arthroscopy. 2013;29:330–335. doi: 10.1016/j.arthro.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 12.McDonald J.E., Herzog M.M., Philippon M.J. Performance outcomes in professional hockey players following arthroscopic treatment of FAI and microfracture of the hip. Knee Surg Sport Traumatol Arthrosc. 2014;22:915–919. doi: 10.1007/s00167-013-2691-9. [DOI] [PubMed] [Google Scholar]
- 13.El Bitar Y.F., Lindner D., Jackson T.J., Domb B.G. Joint-preserving surgical options for management of chondral injuries of the hip. J Am Acad Orthop Surg. 2014;22:46–56. doi: 10.5435/JAAOS-22-01-46. [DOI] [PubMed] [Google Scholar]
- 14.Domb B.G., Redmond J.M., Dunne K.F., Finch N.A., Dunne K.F., Domb B.G. A matched-pair controlled study of microfracture of the hip with average 2-year follow-up: do full-thickness chondral defects portend an inferior prognosis in hip arthroscopy? Arthroscopy. 2015;31:628–634. doi: 10.1016/j.arthro.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 15.MacDonald A.E., Bedi A., Horner N.S., de Sa D., Simunovic N., Philippon M.J. Indications and outcomes for microfracture as an adjunct to hip arthroscopy for treatment of chondral defects in patients with femoroacetabular impingement: a systematic review. Arthroscopy. 2016;32:190–200.e2. doi: 10.1016/j.arthro.2015.06.041. [DOI] [PubMed] [Google Scholar]
- 16.Makhni E.C., Stone A.V., Ukwuani G.C., Zuke W., Garabekyan T., Mei-Dan O. A critical review: management and surgical options for articular defects in the hip. Clin Sports Med. 2017;36:573–586. doi: 10.1016/j.csm.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Kong L., Zheng L.Z., Qin L., Ho K.W. Role of mesenchymal stem cells in osteoarthritis treatment. J Orthop Transl. 2017;9:89–103. doi: 10.1016/j.jot.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shapiro F., Koide S., Glimcher M.J. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75:532–553. doi: 10.2106/00004623-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Karthikeyan S., Roberts S., Griffin D. Microfracture for acetabular chondral defects in patients with femoroacetabular impingement: results at second-look arthroscopic surgery. Am J Sports Med. 2012;40:2725–2730. doi: 10.1177/0363546512465400. [DOI] [PubMed] [Google Scholar]
- 20.Domb B.G., Gupta A., Dunne K.F., Gui C., Chandrasekaran S., Lodhia P. Microfracture in the hip: results of a matched-cohort controlled study with 2-year follow-up. Am J Sports Med. 2015;43:1865–1874. doi: 10.1177/0363546515588174. [DOI] [PubMed] [Google Scholar]
- 21.Knutsen G., Drogset J., Engebretsen L., Grøntvedt T., Isaksen V., Ludvigsen T.C. A randomized trial comparing autologous chondrocyte implantation with microfracture: findings at five years. J Bone Joint Surg Am. 2007;89:2105–2112. doi: 10.2106/JBJS.G.00003. [DOI] [PubMed] [Google Scholar]
- 22.Gooding C.R., Bartlett W., Bentley G., Skinner J.A., Carrington R., Flanagan A. A prospective, randomised study comparing two techniques of autologous chondrocyte implantation for osteochondral defects in the knee: periosteum covered versus type I/III collagen covered. The Knee. 2006;13:203–210. doi: 10.1016/j.knee.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Bartlett W., Skinner J.A., Gooding C.R., Carrington R.W., Flanagan A.M., Briggs T.W. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87:640–645. doi: 10.1302/0301-620X.87B5.15905. [DOI] [PubMed] [Google Scholar]
- 24.Zeifang F., Oberle D., Nierhoff C., Richter W., Moradi B., Schmitt H. Autologous chondrocyte implantation using the original periosteum-cover technique versus matrix-associated autologous chondrocyte implantation: a randomized clinical trial. Am J Sports Med. 2010;38:924–933. doi: 10.1177/0363546509351499. [DOI] [PubMed] [Google Scholar]
- 25.Fontana A., Bistolfi A., Crova M., Rosso F., Massazza G. Arthroscopic treatment of hip chondral defects: autologous chondrocyte transplantation versus simple debridement- A pilot study. Arthroscopy. 2012;28:322–329. doi: 10.1016/j.arthro.2011.08.304. [DOI] [PubMed] [Google Scholar]
- 26.Marcacci M., Zaffagnini S., Kon E., Visani A., Iacono F., Loreti I. Arthroscopic autologous chondrocyte transplantation: technical note. Knee Surg Sport Traumatol Arthrosc. 2002;10:154–159. doi: 10.1007/s00167-001-0275-6. [DOI] [PubMed] [Google Scholar]
- 27.Fickert S., Schattenberg T., Niks M., Weiss C., Thier S. Feasibility of arthroscopic 3-dimensional, purely autologous chondrocyte transplantation for chondral defects of the hip: a case series. Arch Orthop Trauma Surg. 2014;134:971–978. doi: 10.1007/s00402-014-1997-5. [DOI] [PubMed] [Google Scholar]
- 28.Körsmeier K., Claßen T., Kamminga M., Rekowski J., Jäger M., Landgraeber S. Arthroscopic three-dimensional autologous chondrocyte transplantation using spheroids for the treatment of full-thickness cartilage defects of the hip joint. Knee Surg Sport Traumatol Arthrosc. 2016;24:2032–2037. doi: 10.1007/s00167-014-3293-x. [DOI] [PubMed] [Google Scholar]
- 29.Akimau P., Bhosale A., Harrison P.E., Roberts S., McCall I.W., Richardson J.B. Autologous chondrocyte implantation with bone grafting for osteochondral defect due to posttraumatic osteonecrosis of the hip: a case report. Acta Orthop. 2006;77:333–336. doi: 10.1080/17453670610046208. [DOI] [PubMed] [Google Scholar]
- 30.Lim B.H., Jang S.W., Park Y.S., Lim S.J. Open repair and arthroscopic follow-up of severely delaminated femoral head cartilage associated with traumatic obturator fracture-dislocation of the hip. Orthopedics. 2011;34:199. doi: 10.3928/01477447-20110427-26. [DOI] [PubMed] [Google Scholar]
- 31.Stafford G.H., Bunn J.R., Villar R.N. Arthroscopic repair of delaminated acetabular articular cartilage using fibrin adhesive. Results at one to three years. Hip Int. 2011;21:744–750. doi: 10.5301/HIP.2011.8843. [DOI] [PubMed] [Google Scholar]
- 32.Tzaveas A.P., Villar R.N. Arthroscopic repair of acetabular chondral delamination with fibrin adhesive. Hip Int. 2010;20:115–119. doi: 10.1177/112070001002000117. [DOI] [PubMed] [Google Scholar]
- 33.Sekiya J.K., Martin R.L., Lesniak B.P. Arthroscopic repair of delaminated acetabular articular cartilage in femoroacetabular impingement. Orthopedics. 2009;32 doi: 10.3928/01477447-20090728-44. [DOI] [PubMed] [Google Scholar]
- 34.Cassar-Gheiti A.J., Byrne D.P., Kavanagh E., Mulhall K.J. Comparison of four chondral repair techniques in the hip joint: a biomechanical study using a physiological human cadaveric model. Osteoarthr Cartil. 2015;23:1018–1025. doi: 10.1016/j.joca.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 35.Xie X.Y., Wang X.L., Yang H.L., Zhao D.W., Qin L. Steroid-associated osteonecrosis: epidemiology, pathophysiology, animal model, prevention and potential treatments (An overview) J Orthop Transl. 2015;3:58–70. doi: 10.1016/j.jot.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mont M.A., Einhorn T.A., Sponseller P.D., Hungerford D.S. The trapdoor procedure using autogenous cortical and cancellous bone grafts for osteonecrosis of the femoral head. J Bone Joint Surg Br. 1998;80:56–62. doi: 10.1302/0301-620x.80b1.7989. [DOI] [PubMed] [Google Scholar]
- 37.Ganz R., Büchler U. Overview of attempts to revitalize the dead head in aseptic necrosis of the femoral head--osteotomy and revascularization. Hip. 1983:296–305. [PubMed] [Google Scholar]
- 38.Itoman M., Yamamoto M. Pathogenesis and treatment of idiopathic aseptic necrosis of the femoral head. Clin Immunol. 1969;21:713–725. [Google Scholar]
- 39.Judet R., Judet J., Launois B., Gubler J.P. Trial of experimental revascularization of the femoral head. Rev Chir Orthop Reparatrice Appar Mot. 1966;52:277–303. [PubMed] [Google Scholar]
- 40.Merle D'Aubigné R., Postel M., Mazabraud A., Massias P., Gueguen J., France P. Idiopathic necrosis of the femoral head in adults. J Bone Joint Surg [Br] 1965;47-B:612–633. [PubMed] [Google Scholar]
- 41.Meyers M.H., Convery F.R. Grafting procedures in osteonecrosis of the hip. Semin Arthroplast. 1991;2:189–197. [Google Scholar]
- 42.Mont M.A., Jones L.C., Elias J.J., Inoue N., Yoon T.R., Chao E.Y. Strut-autografting with and without osteogenic protein-1 : a preliminary study of a canine femoral head defect model. J Bone Joint Surg Am. 2001;83-A:1013–1022. [PubMed] [Google Scholar]
- 43.Xu H., Niu X., Li Y., Binitie O.T., Letson G.D., Cheong D. What are the results using the modified trapdoor procedure to treat chondroblastoma of the femoral head? Clin Orthop Relat Res. 2014;472:3462–3467. doi: 10.1007/s11999-014-3771-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kellgren J.H., Lawrence J.S. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marcacci M., Kon E., Zaffagnini S., Filardo G., Delcogliano M., Neri M.P. Arthroscopic second generation autologous chondrocyte implantation. Knee Surg Sport Traumatol Arthrosc. 2007;15:610–619. doi: 10.1007/s00167-006-0265-9. [DOI] [PubMed] [Google Scholar]
- 46.Fotopoulos V.C., Mouzopoulos G., Floros T., Tzurbakis M. Steroid-induced femoral head osteonecrosis in immune thrombocytopenia treatment with osteochondral autograft transplantation. Knee Surg Sport Traumatol Arthrosc. 2015;23:2605–2610. doi: 10.1007/s00167-014-3239-3. [DOI] [PubMed] [Google Scholar]
- 47.Anthonissen J., Rommens P.M., Hofmann A. Mosaicplasty for the treatment of a large traumatic osteochondral femoral head lesion: a case report with 2 year follow-up and review of the literature. Arch Orthop Trauma Surg. 2016;136:41–46. doi: 10.1007/s00402-015-2352-1. [DOI] [PubMed] [Google Scholar]
- 48.Güngör H.R., Kıter E., Ök N., Çatak A. Osteochondral mosaicplasty along with osteochondroplasty of the femoral head in femoroacetabular impingement: a case report. Eklem Hastalik Cerrahisi. 2015;26:181–184. doi: 10.5606/ehc.2015.37. [DOI] [PubMed] [Google Scholar]
- 49.Kılıçoğlu Ö.İ., Polat G., Erşen A., Birişik F. Long-term result of mosaicplasty for femoral head osteochondral lesion: a case report with 8 years follow-up. Hip Int. 2015;25:589–592. doi: 10.5301/hipint.5000244. [DOI] [PubMed] [Google Scholar]
- 50.Rittmeister M., Hochmuth K., Kriener S., Richolt J. Five year results following autogenous osteochondral transplantation to the femoral head. Der Orthopäde. 2005;34(320):322–326. doi: 10.1007/s00132-005-0776-y. [DOI] [PubMed] [Google Scholar]
- 51.Andrade R., Vasta S., Pereira R., Pereira H., Papalia R., Karahan M. Knee donor-site morbidity after mosaicplasty - a systematic review. J Exp Orthop. 2016;3:31. doi: 10.1186/s40634-016-0066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sotereanos N.G., DeMeo P.J., Hughes T.B., Bargiotas K., Wohlrab D. Autogenous osteochondral transfer in the femoral head after osteonecrosis. Orthopedics. 2008;31:177. doi: 10.3928/01477447-20080201-33. [DOI] [PubMed] [Google Scholar]
- 53.Girard J., Roumazeille T., Sakr M., Migaud H. Osteochondral mosaicplasty of the femoral head. Hip Int. 2011;21:542–548. doi: 10.5301/HIP.2011.8659. [DOI] [PubMed] [Google Scholar]
- 54.Nam D., Shindle M.K., Buly R.L., Kelly B.T., Lorich D.G. Traumatic osteochondral injury of the femoral head treated by mosaicplasty: a report of two cases. HSS J. 2010;6:228–234. doi: 10.1007/s11420-010-9159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Won Y., Lee G.S., Kim S.B., Kim S.J., Yang K.H. Osteochondral autograft from the ipsilateral femoral head by surgical dislocation for treatment of femoral head fracture dislocation: a case report. Yonsei Med J. 2016;57:1527–1530. doi: 10.3349/ymj.2016.57.6.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nuelle C.W., Nuelle J.A., Cook J.L., Stannard J.P. Patient factors, donor age, and graft storage duration affect osteochondral allograft outcomes in knees with or without comorbidities. J Knee Surg. 2017;30:179–184. doi: 10.1055/s-0036-1584183. [DOI] [PubMed] [Google Scholar]
- 57.Cook J.L., Stannard J.P., Stoker A.M., Bozynski C.C., Kuroki K., Cook C.R. Importance of donor chondrocyte viability for osteochondral allografts. Am J Sports Med. 2016;44:1260–1268. doi: 10.1177/0363546516629434. [DOI] [PubMed] [Google Scholar]
- 58.Raz G., Safir O.A., Backstein D.J., Lee P.T., Gross A.E. Distal femoral fresh osteochondral allografts: follow-up at a mean of twenty-two years. J Bone Joint Surg Am. 2014;96:1101–1107. doi: 10.2106/JBJS.M.00769. [DOI] [PubMed] [Google Scholar]
- 59.Krych A.J., Robertson C.M., Williams R.J., III Cartilage Study Group. Return to athletic activity after osteochondral allograft transplantation in the knee. Am J Sports Med. 2012;40:1053–1059. doi: 10.1177/0363546511435780. [DOI] [PubMed] [Google Scholar]
- 60.Nielsen E.S., McCauley J.C., Pulido P.A., Bugbee W.D. Return to sport and recreational activity after osteochondral allograft transplantation in the knee. Am J Sports Med. 2017;45:1608–1614. doi: 10.1177/0363546517694857. [DOI] [PubMed] [Google Scholar]
- 61.Khanna V., Tushinski D.M., Drexler M., Backstein D.B., Gross A.E., Safir O.A. Cartilage restoration of the hip using fresh osteochondral allograft: resurfacing the potholes. Bone Joint Lett J. 2014;96-B:11–16. doi: 10.1302/0301-620X.96B11.34734. [DOI] [PubMed] [Google Scholar]
- 62.Mei X.Y., Alshaygy I.S., Safir O.A., Gross A.E., Kuzyk P.R. Fresh osteochondral allograft transplantation for treatment of large cartilage defects of the femoral head: a minimum two-year follow-up study of twenty-two patients. J Arthroplast. 2018;33:2050–2056. doi: 10.1016/j.arth.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 63.Oladeji L.O., Cook J.L., Stannard J.P., Crist B.D. Large fresh osteochondral allografts for the hip: growing the evidence. Hip Int. 2018;28:284–290. doi: 10.5301/hipint.5000568. [DOI] [PubMed] [Google Scholar]
- 64.Degen R.M., Tetreault D., Mahony G.T., Williams R.J. Acute delamination of commercially available decellularized osteochondral allograft plugs: a report of two cases. Cartilage. 2016;7:316–321. doi: 10.1177/1947603515626973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blair V.P. The consideration of contour as well as functions for organic ankylosis of the lower jaw. Surg Gynecol Obstet. 1928;46:167–179. [Google Scholar]
- 66.Sato K., Iwamoto T., Matsumura N., Suzuki T., Nishiwaki Y., Nakamura T. Total finger joint arthroplasty with a costal osteochondral autograft: up to 11 years of follow-up. J Hand Surg Eur. 2019;44(2):167–174. doi: 10.1177/1753193418806195. 1753193418806195. [DOI] [PubMed] [Google Scholar]
- 67.Obert L., Lepage D., Ferrier M., Tropet Y. Rib cartilage graft for posttraumatic or degenerative arthritis at wrist level: 10-year results. J Wrist Surg. 2013;2:234–238. doi: 10.1055/s-0033-1351787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sato K., Iwamoto T., Matsumura N., Suzuki T., Nishiwaki Y., Oka Y. Costal osteochondral autograft for advanced osteochondritis dissecans of the humeral capitellum in adolescent and young adult athletes: clinical outcomes with a mean follow-up of 4.8 years. J Bone Joint Surg Am. 2018;100:903–913. doi: 10.2106/JBJS.17.01035. [DOI] [PubMed] [Google Scholar]
- 69.Du D., Sugita N., Liu Z., Moriguchi Y., Nakata K., Myoui A. Repairing osteochondral defects of critical size using multiple costal grafts: an experimental study. Cartilage. 2015;6:241–251. doi: 10.1177/1947603515591628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.