Abstract
Osteonecrosis of the femoral head (ONFH) is a common and refractory disease in orthopaedic clinics. The number of patients with ONFH is increasing worldwide every year. There are an estimated 8.12 million patients with nontraumatic osteonecrosis in China alone. Treatment of nontraumatic osteonecrosis has always been a clinical challenge for orthopaedic surgeons. To further standardize diagnosis and treatment of ONFH, these guidelines provide not only basic diagnosis, treatment, and evaluation systems for ONFH but also expert advice and standards in many aspects, including epidemiology, aetiology, diagnostic criteria, pathological staging, prevention and treatment options, and postoperative rehabilitation. The aetiological factors of ONFH can currently be divided into two major categories: traumatic and nontraumatic; however, the specific pathological mechanism of ONFH is not completely clear. Currently, the staging system of ONFH formulated by the Association Research Circulation Osseous is widely used in clinical practice. Based on the changes in the intraosseous blood supply at different stages, the corresponding nonsurgical and surgical treatments are recommended, and when there are risk factors for possible ONFH, certain preventive measures to avoid the occurrence of osteonecrosis are recommended. These guidelines provide brief classification criteria and treatment regimen for osteonecrosis. Specification of the aetiology, treatment plan based on comprehensive consideration of the different stages of osteonecrosis, hip function, age, and occupation of the patients are important steps in diagnosis and developing treatment strategies.
Translational potential of this article
New advances in the epidemiology, etiology, pathophysiology, imaging, diagnosis and treatment of ONFH have been renewed in this revision. This guideline can be used for reference by orthopedic professionals and researchers, and for standardized diagnosis and treatment management under the clinical guidance, which is conducive to the prevention, treatment and further research of ONFH, improving the diagnosis and treatment level, making patients' symptoms under good control, and improving their quality of life.
Keywords: Diagnosis, Guideline, Osteonecrosis of the femoral head (ONFH), Treatment
Abbreviations: ARCO, Association Research Circulation Osseous; BMES, Bone marrow oedema syndrome; CT, Computed tomography; DSA, Digital subtraction angiography; MRI, Magnetic resonance imaging; ONFH, Osteonecrosis of the femoral head; PET, Positron emission tomography; RHS, Reconstruction Hip Scores; SPECT, Single-photon emission computed tomography; T1WI, T1-weighted images
General introduction
Osteonecrosis of the femoral head (ONFH), previously also called ischaemic necrosis of the femoral head, mainly affects younger patients. An evidence-based standardized treatment regimen is very important. “Expert Advice on the Diagnosis and Treatment of Osteonecrosis of the Femoral Head (2007 version) [1]”, “Chinese Experts' Consensus on the Diagnosis and Treatment of Osteonecrosis of the Femoral Head in Adults (2012 version) [2]”, and “Chinese Guideline for the Diagnosis and Treatment of Femoral Head Necrosis in Adults (2016 version) [3]” were formulated based on evidence-based medicine and the opinion of a large number of professional specialists and orthopaedists in the discipline. These guidelines have promoted the standardization of classification and improvement in the diagnosis and selection of treatment procedures for patients with ONFH. However, there are still deficiencies in the understanding of staging and the choice of diagnostic and treatment methods. In May 2019, based on the 2016 version guideline [3], professional specialists, scholars, and orthopaedists from Europe, Asia, America, and China jointly develop new guidelines for further standardized and effective clinical diagnosis and treatment of adults with ONFH. New advances in the epidemiology, aetiology, pathophysiology, imaging, diagnosis, and treatment of ONFH have been renewed in this revision.
Inclusion criteria of the references
An extensive literature search was conducted based on the Medline database at the PubMed and China Knowledge Resource Integrated database. Considering an advanced established guideline based on the 2016 version, the recent studies up to November 2019 were included, and the studies lacking evidence-based medicine support, i.e., published in non-journal citation reports (JCR) journals, were excluded in the current guideline version.
Overview
-
(1)
Definition: ONFH is a disease in which local death of osteocytes and the component of the bone marrow occurs owing to venous stasis or arterial blood supply damage or interruption in the femoral head; the subsequent repair process attempts to heal the necrotic area, but structural deterioration and collapse of the femoral head causes pain and dysfunction of the hip joint [2,[4], [5], [6], [7], [8], [9], [10], [11]].
-
(2)
Epidemiology: To date, there is no epidemiological report on ONFH worldwide, although some countries have screened their osteonecrosis population. An estimated 20,000 new cases of osteonecrosis are diagnosed in the United States each year, and the cumulative number of patients with ONFH is 300,000 to 600,000 [12]. In recent years, approximately 12,000–24,000 new cases of osteonecrosis were diagnosed in Japan. In South Korea, the prevalence rate was 20.53 per 100,000 people in 2002; however, in 2006, this number reached 37.96, and the estimated number of new cases reached 14,103 per year on average [13]. The first large-scale epidemiological survey of nontraumatic osteonecrosis in China showed that the estimated cumulative number of patients with nontraumatic ONFH reached 8.12 million; the prevalence rate was significantly higher in men (1.02%) than in women (0.51%); the prevalence rate was higher in northern residents (0.85%) than in southern residents (0.61%) and higher in urban residents than in rural residents. Glucocorticoids, alcohol, high blood lipid level, obesity, certain occupations (e.g., diving), smoking, and diabetes are all high-risk factors for nontraumatic osteonecrosis [14,15].
-
(3)
Aetiology and high-risk populations: ONFH can be divided into two major categories: traumatic and nontraumatic. The main aetiological factors for traumatic ONFH include femoral head and neck fracture, acetabular fracture, hip dislocation, and severe hip sprain or contusion (no fracture, with intraarticular haematoma) [[16], [17], [18], [19], [20], [21]]. The main causes of nontraumatic ONFH in China are use of corticosteroids, chronic alcohol overconsumption, decompression sickness, haemoglobin diseases (sickle cell anaemia, sickle cell-haemoglobin C disease, thalassaemia, sickle cell trait, and so on), autoimmune diseases, and idiopathic diseases [6,15,[22], [23], [24], [25]]. Smoking and obesity increase the risk of ONFH [26,27] and are considered to be correlated with ONFH [[28], [29], [30]].
Diagnostic criteria
Diagnostic criteria have been developed as per the “Chinese Experts' Consensus on the Diagnosis and Treatment of Osteonecrosis of the Femoral Head in Adults(2012 version)” [2], the “Chinese Guideline for the Diagnosis and Treatment of Femoral Head Necrosis in Adults (2016 version) [3]”, and the International Diagnostic Criteria for Osteonecrosis of the Femoral Head [4,6].
-
(1)
Clinical features: Pain is primarily localized in the hip, buttock, or groin area, occasionally accompanied by knee pain and limited hip internal rotation. Patients often have a history of hip trauma, corticosteroid usage, or alcoholism or have a high-risk occupational history, such as being a diver [6,14,15,23].
-
(2)
Magnetic resonance imaging: Magnetic resonance imaging (MRI) examination has a high sensitivity for ONFH [[31], [32], [33], [34]], demonstrated as a limited subchondral linear-shaped low signal intensity in T1-weighted images (T1WIs) or a “double-line sign” in T2-weighted images (T2WIs) [32,35,36].
-
(3)
X-ray imaging: Anteroposterior and frog-leg positioning are the basic X-ray positions used for diagnosis of ONFH, and the X-ray manifestations are typically osteosclerosis, cystic change, and a “crescent sign” [37] in earlier stages. After collapse, there is a loss of sphericity of the femoral head and degenerative arthritis in the late stages.
-
(4)
Computed tomography scanning: Computed tomography (CT) scanning usually reveals zones of osteosclerosis surrounding the necrotic bone and repaired bone or shows subchondral bone fracture [[38], [39], [40]].
-
(5)
Radionuclide examination: An acute-phase bone scan of the femoral head (99Tcm-MDP (Methylene Diphosphonate), 99Tcm-DPD (Dicarboxypropane Diphosphonate), and so on) can reveal cold areas; scanning during the necrosis repair period demonstrates a cold area in the middle of a hot area, in other words a “donut-like” change (cold in hot) [41]. Radionuclide examination has less specificity than MRI, but has the advantage to detect multifocal osteonecrosis (other sites than the hip). Single-photon emission computed tomography may increase the sensitivity of radionuclide examination for diagnosis of ONFH [42,43]. Positron emission tomography may detect signs of ONFH earlier than MRI and single-photon emission computed tomography and can predict progression of ONFH [44].
-
(6)
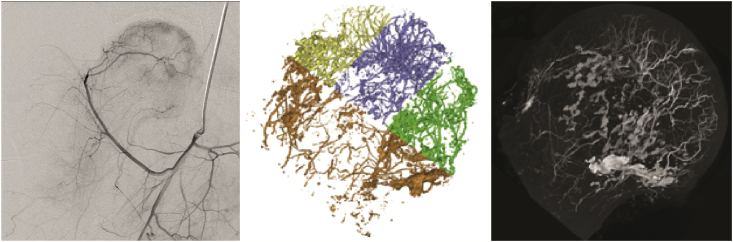
Bone biopsy and pathological manifestations: Pathological morphology is divided into three phases: the early phase of blood supply changes, the midphase of blood supply changes, and the late phase of blood supply changes. During the early phase of blood supply changes, the blood supply to the epiphysis is blocked; some cells show signs of necrosis, the proportion of empty lacunae in trabecular bone is more than 50%, a number of adjacent trabecular bones are involved, and a portion of the bone marrow is necrotic [45]. Stimulated by ONFH risk factors, bone marrow stem cells gradually differentiated into hypertrophic fat cells. The diameter of the marrow fat cells increases by more than 10-μm hypertrophy of the fat cell [46]. Small veins exhibit thrombosis, larger veins are dilated, venous sinuses are congested, the interstitial space shows oedema, and venous stasis and high pressure in the bone develops owing to poor venous return in digital subtraction angiography (DSA) (Figure 1).
Figure 1.
Representative DSA imaging and micro-CT scanning of intraosseous vessels in the early phase of venous stasis (permission obtained from Guoshuang et al [45] and Benjie [47]). CT = computed tomography; DSA = digital subtraction angiography.
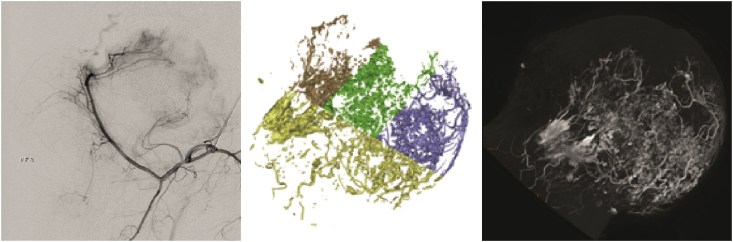
During the midphase of blood supply changes, the venous thrombosis is further aggravated, and arteriovenous stenosis or arterial thrombosis occurs, resulting in insufficient blood supply to the arterial system and subsequent entry into an arterial ischaemic state. In this phase, subchondral bone fracture is observed, the necrotic area is enlarged, local cystic change is visible, part of the femoral head area is collapsed, necrotic bone tissue enters the main repair stage, and new blood vessels and new fibrotic tissue grow into the necrotic area to form granulation tissue (Figure 2).
Figure 2.
Representative DSA imaging and micro-CT scanning of intraosseous vessels in the midphase of arterial ischaemic (permission obtained from Guoshuang et al [45] and Benjie [47]). CT = computed tomography; DSA = digital subtraction angiography.
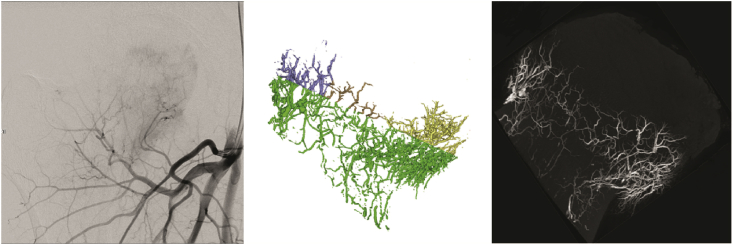
During the late phase of blood supply changes, arterial endothelial hyperplasia occurs, the arterial diameter decreases, and the loss of arterial structure is further aggravated, leading to complete arterial occlusion, which indicates osteoarthritis of the hip joint (Figure 3). Obstruction or interruption of blood flow in the arteries and veins occurs at the beginning of trauma in traumatic ONFH. This ischaemic state is manifested by pathologic and histological changes similar to those that occur in the midphase of blood supply changes which gradually occur and may develop into the late phase of blood supply changes [45]. Biopsy was usually obtained when core decompression was performed using a large-diameter trephine. Actually, when MRI shows typical lesion, biopsy is not necessary.
-
(7)
Digital subtraction angiography: ONFH may manifest as stasis, damage, and interruption of blood supply to the femoral head, which have important guiding significance for preventing the occurrence of osteonecrosis after femoral neck fracture. In nontraumatic ONFH, venous stasis and blockage of blood return appear in the early phase, arterial ischaemia occurs in the midphase, and arterial occlusion develops in the late phase. After diagnosis of osteonecrosis is confirmed, DSA is recommended for patients undergoing hip preservation surgery to provide a basis for development of a surgical plan.
Figure 3.
Representative DSA imaging and micro-CT scanning of intraosseous vessels in the late phase of arterial occlusion (permission obtained from Guoshuang et al [45] and Benjie [47]). CT = computed tomography; DSA = digital subtraction angiography.
Meeting Criterion 1 and 2 can confirm diagnosis of femoral head necrosis; Criteria 3–7 are examination methods for auxiliary diagnosis and treatment.
Differential diagnosis
Attention should be paid to patients with clinical symptoms, X-ray findings, or MRI findings similar to those exhibited in cases of ONFH.
-
(1)
Middle and late stages of hip osteoarthritis: When the joint space is narrowed and degeneration with subchondral cysts occurs, hip osteoarthritis is difficult to be distinguished from ONFH. However, the CT manifestations of ONFH are osteosclerosis and cystic degeneration, and the primary MRI change is low signal intensity, which can be used for differentiation.
-
(2)
Osteoarthritis secondary to acetabular dysplasia: X-ray imaging shows incomplete covering of the femoral head, a narrowed or absent joint space, osteosclerosis and cystic degeneration, and similar changes in the corresponding area of the acetabulum, which are easy to differentiate.
-
(3)
Ankylosing spondylitis involving the hip joint: This condition is common in adolescent men and usually involves both sacroiliac joints. Serum detection of Human Leuc ocyte Antigen (HLA-B27) is positive. X-ray imaging shows that the femoral head remains round and that the joint space narrows, disappears, or even fuses, which is easy to distinguish. Long-term use of corticosteroids in some patients may cause ONFH, in which the femoral head may collapse but not to a serious degree.
-
(4)
Transient osteoporosis or bone marrow oedema syndrome: The onset of this condition can be observed in young and middle-aged people, and it is a temporary, painful bone marrow oedema. X-ray imaging shows a decrease in bone mass in the head, neck, and even the trochanter of the femur; MRI shows a uniform low signal intensity in T1WIs and high signal intensity in T2WIs, with a range that may reach the femoral neck and the trochanter but with no band-like low signal intensity. The lesion may disappear within 3–12 months [[48], [49], [50]].
-
(5)
Chondroblastoma in the femoral head: MRI demonstrates flake-shaped high signal intensity in T2WIs. CT scanning shows irregular osteolytic destruction.
-
(6)
Incomplete fracture in subchondral bone: This is most common in elderly patients older than 60 years who have no obvious history of trauma and manifests as sudden hip pain, inability to walk, and limited joint movement. X-ray imaging shows slight flattening of the upper lateral portion of the femoral head; MRI demonstrates a subchondral low signal intensity line and the surrounding bone marrow oedema in T1WIs and T2WIs, and fat-suppressed T2WIs show a flake-shaped high signal intensity line [[51], [52], [53], [54]]. Meanwhile, it is to be noted that young healthy adults with good bone quality were also found to suffer from subchondral stress fracture (e.g., military recruits during military drills) [55,56].
-
(7)
Pigmented villonodular synovitis: This condition is common in the knee joints, and hip involvement is rare. Patients with hip involvement are characterized by adolescent-onset, mild to moderate hip pain with limp and mild limitation of joint movement during the early stage and midstage. CT and X-ray imaging show cortical erosion of the femoral head and neck or acetabulum and mild to moderate narrowing of the joint space; MRI shows extensive synovial hypertrophy and an even distribution of low or moderate signal intensity.
-
(8)
Synovial herniation: This is a benign lesion in which the synovial tissue overgrows and invades into the femoral neck cortex, usually without clinical symptoms. MRI shows small round lesions with a low signal intensity in T1WIs and high signal intensity in T2WIs in the upper cortex of the femoral neck.
-
(9)Bone infarction of the metaphysis: Bone infarction is osteonecrosis that occurs in the metaphysis or shaft of the long bones. This condition has different MRI manifestations in different phases.
-
1)Acute phase: The lesion center shows equal or slightly higher signal intensity than normal bone marrow in T1WIs and shows high signal intensity in T2WIs, and the lesion edge shows a low signal intensity in T1WIs and a high signal intensity in T2WIs.
-
2)Subacute phase: The lesion center shows equal or slightly lower signal intensity than normal bone marrow in T1WIs and shows equal or slightly higher signal intensity than normal bone marrow in T2WIs, and the lesion edge shows a low signal intensity in T1WIs and a high signal intensity in T2WIs.
-
3)Chronic phase: The lesion shows a low signal intensity on both T1WIs and T2WIs.
-
1)
-
(10)
Femoroacetabular impingement syndrome: Femoroacetabular impingement is divided into three types: the pincer-type, the cam-type, and the mixed-type. X-ray examination in the frog-leg position or lateral hip position shows obvious osteophyte formation in the head and neck of the femur and an increase in the angle α. MRI examination shows a flake-shaped, low signal intensity at the femoral head and neck in T1WIs and a large, sheet-shaped bone marrow oedema signal at the femoral head and neck in T2WIs. CT examination shows obvious hyperplasia of the femoral head and neck, an increase in the angle α in the oblique sagittal view, no obvious cystic degeneration in the femoral head, and no bone destruction. Three-dimensional CT reconstruction can clearly reveal anatomical abnormalities or osteophytosis of the femoral head and neck [[57], [58], [59]].
Staging
The newly released 2019 updated version of Association Research Circulation Osseous (ARCO) staging system is recommended (Table 1).
Table 1.
Association Research Circulation Osseous (ARCO) international classification of osteonecrosis [60].
| ARCO stage | Image findings | Anteroposterior images | Description |
|---|---|---|---|
| 1 | X-ray normal MRI abnormal |
 |
A band lesion of low signal intensity around the necrotic area is seen on MRI. A cold spot is seen on bone scan. No changes are seen on plain radiographs. |
| 2 | X-ray abnormal MRI abnormal |
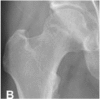 |
Osteosclerosis, focal osteoporosis or cystic changes are seen in the femoral head on plain radiographs or CT scan. Still there is no evidence of subchondral fracture, fracture in the necrotic portion or flattening of the femoral head. |
| 33A(early)3B(late) | Subchondral fracture on X-ray or CT |
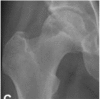 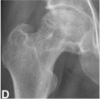
|
Subchondral fracture, fracture in the necrotic portion and/or flattening of the femoral head is seen on plain radiography or CT scan. →3A: Femoral head depression ≤ 2 mm →3B: Femoral head depression >2 mm |
| 4 | X-ray osteoarthritis | 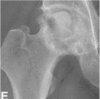 |
Osteoarthritis of the hip joint with joint space narrowing, acetabular changes and destruction are seen on plain radiographs. |
The ARCO staging system was developed by the ARCO committee in 1991 after integration of Ficat staging, Steinberg staging (Table 2) [61], and the staging system of the Japanese Investigation Committee on Osteonecrosis [62]. The 2019 updated version of the ARCO staging system was presented and approved on May 3, 2019, at the 2019 ARCO conference in Dalian, China [60]. ARCO staging is more systematic, comprehensive, and practical than any previous staging method and is of great value in diagnosis and assessment of treatment efficacy and prognosis. In 2015, China developed a Chinese staging system for ONFH (Table 3) [63], which is recommended to be used simultaneously with ARCO staging in clinical practice.
Table 2.
The Steinberg - University of Pennsylvania classification of osteonecrosis [61].
| Stage 0 | Normal or non-diagnostic radiograph, bone scan, and MRI |
| Stage I | Normal radiograph; abnormal bone scan and/or MRI
|
| Stage II | Lucent and sclerotic changes in femoral head
|
| Stage III | Subchondral collapse (crescent sign) without flattening
|
| Stage IV | Flattening of femoral head
|
| Stage V | Joint narrowing and/or acetabular changes
|
| Stage VI | Advanced degenerative changes |
MRI = magnetic resonance imaging.
Table 3.
Chinese staging of osteonecrosis of the femoral head [3].
| Stage | Clinical findings | Radiographic signs | Pathological changes |
|---|---|---|---|
|
1 (pre-clinical, no-collapse) According to size of necrotic: 1a, small <15% 1b, medium 15%–30% 1c, large >30% |
No | MRI Bone scan |
Necrosis of bone marrow Necrosis of osteocytes |
|
2 (early stage, no-collapse) According to size of necrotic: 2 a, small <15% 2 b, medium 15%–30% 2 c, large >30% |
No or slight pain | MRI X-ray CT |
Necrotic area absorbed Bone repair |
|
3 (medium stage, pre-collapse) According to length percentage of crescent in articular facel: 3 a, small <15% 3 b, medium 15%–30% 3 c, large >30% |
On set of pain, Slight claudication, Moderate pain, Limited internal rotation, Pain in internal rotation |
MRI T2-WI: bone marrow edema, CT: subchondral fracture, X-rays: Femoral head contour interrupted Crescent sign | Subchondral fracture or fracture through necrotic bone |
|
4 (middle–late stage, collapse) According to depth of collapse: 4 a, slight (2 mm) 4 b, medium (2–4 mm) 4 c, severe (4 mm) |
Moderate to severe pain, claudication, limited internal rotation, Aggravated pain when strenuous internal rotation, limited abduction and adduction |
X-rays: femoral head collapse with normal joint space | Femoral head collapse |
| 5 (late-stage, osteoarthritis) | Severe pain Severe claudication Limited range of motion |
X-ray: flattening of femoral head, narrow joint space, acetabular cystic changes or sclerosis | Cartilage involved osteoarthritis |
Prevention and treatment of ONFH
-
(1)Prevention of ONFH
-
1)Prevention of traumatic ONFH: DSA or computed tomography angiography combined with X-ray imaging can be used to evaluate the blood supply in the femoral head and to analyze whether blood vessels are present in the supporting region of the femoral head. When there is no blood supply, exploration and anastomosis of blood vessels in the supporting region can be performed, bone flaps with vascular pedicles can be grafted, and blood supply needs to be protected during internal fixation, while damage to the arterial arch should be avoided [64].
- 2)
-
3)Prevention of other types of ONFH: For a population with high-risk factors for osteonecrosis, removal of the aetiologic factors of osteonecrosis can effectively prevent the occurrence of ONFH.
-
1)
-
(2)Nonsurgical treatments
-
1)Protective weight-bearing: Impingement and impact loading activities should be avoided. The use of crutches can effectively relieve pain. Wheelchair use is not recommended.
-
2)Drug treatment: Combined use of anticoagulants, fibrinolysis-enhancing drugs, blood vessel dilatators, and lipid-reducing drugs has been recommended [[67], [68], [69], [70], [71]]. A combination of drugs that inhibit osteoclast formation and drugs that increase osteogenesis can also be used [72]. Medication can be used alone or in combination with hip-preserving surgery.
- 3).
-
1)
-
(3)Surgical treatment: When ONFH progresses rapidly and nonsurgical treatment is not effective, most patients require surgical treatment. Surgical methods can be sorted into two major categories: repair and reconstruction methods that primarily preserve the patient's own femoral head and the hip joint replacement method. Surgeries that preserve the femoral head include core decompression, osteotomy, and (non)vascularized bone transplantation [2,4,6], which are suitable for patients in the early (ARCO 0–1) or midstage (ARCO 2–3 B) of ONFH and with a necrotic volume of more than 15%. If the method is effective, joint replacement can be avoided or delayed.
-
1)Core decompression: This method can be selected when DSA and MRI results suggest that the blood supply demonstrates characteristics of early venous stasis (stage 1–2A ARCO staging). This method has been developed over a long period of time and has beneficial results. At present, it can be divided into core decompression via multiple drilling and core decompression via a thick channel. The difference lies primarily in the diameter of the decompression channel; the channel diameter for multiple drilling is 3, 3.5, or 4 mm [[82], [83], [84], [85]], whereas that of the thick channel is 6 mm or more [[86], [87], [88]]. Core decompression combined with cell transplantation (in vitro culture or concentrated autologous bone marrow containing mononuclear cells) has been administered clinically in qualified medical institutions [[89], [90], [91], [92]].
-
2)Nonvascularized transplantation: The primary methods used include decompression and bone transplantation via the femoral trochanter and bulb decompression and bone grafting via the femoral head and neck [93]. Bone grafting methods include compact bone grafting and strut grafting. The bone transplantation materials include autologous cortical bone and cancellous bone, allogeneic bone, and bone substitute materials [[94], [95], [96], [97]].
-
3)Osteotomy: The purpose of an osteotomy is to move the necrotic zone to the non–weight-bearing area of the femoral head. Osteotomy techniques include varus or valgus osteotomy and rotational osteotomy via the femoral trochanter. The choice of the surgical method is based on the principle that the femoral cavity is not modified [98,99].
-
4)Vascularized transplantation: This method is selected when DSA and MRI results suggest that the blood supply shows arterial ischaemia (stage 2B–3B of ARCO staging). Transplantation of autologous bone is divided into peri-hip bone flap transplantation and fibula transplantation [3,6,100,101]. Transplantation of a peri-hip bone flap with a vascular pedicle includes the following: (1) transposition of an iliac bone (periosteum) flap with the ascending branch of the lateral circumflex femoral artery [102]; (2) transposition of a greater trochanter bone flap with the gluteus medius muscle branch and the ascending branch of the lateral circumflex femoral artery [[103], [104], [105], [106]]; (3) transposition of a greater trochanter bone flap with the transverse branch of the lateral circumflex femoral artery [[103], [104], [105], [106]]; (4) transposition of an iliac bone (periosteum) flap with a deep circumflex iliac vascular pedicle; (5) transplantation of a greater trochanter bone flap with the transverse branch combined with an iliac bone (periosteum) flap with the ascending branch to reconstruct the femoral head (neck) for patients with the entire femoral head and even part of the femoral neck involved [107]; and (6) transplantation of the greater trochanter bone flap with the deep branch of the medial femoral vessel and an iliac bone flap with the deep superior branch of the superior gluteal vessel via the posterior approach to the hip. Surgical techniques involving the peri-hip bone flap with a vascular pedicle are less traumatic, highly effective, and easy to master and are therefore recommended. To enhance mechanical support within the femoral head, application of a peri-hip bone flap with a vascular pedicle can be combined with implantation of a supportive material, which can help avoid collapse of the femoral head after surgery and has demonstrated good short-term to midterm efficacy [108,109]; however, the long-term effects still need to be determined. In addition, transplantation of a vascularized fibula graft via anastomoses is effective [[110], [111], [112], [113], [114]] and is recommended. The choice of the transplantation method using autologous bone with blood supply is considered based on the comprehensive advantages and disadvantages of each method, the proficiency of the surgeon, and other factors.
-
5)Joint replacement: Joint replacement should be selected in cases in which the femoral head is significantly collapsed, late-stage arterial occlusion features (ARCO stage 3C and 4) are present, and severe joint function loss or moderate/severe pain is present [[115], [116], [117], [118], [119], [120], [121]]. Generally, the medium- to long-term effects of cementless or hybrid-type prostheses are believed to be superior to those of all cement-type prostheses. Special attention should be paid to the following in joint replacement for ONFH: (1) If the patient has undergone long-term use of corticosteroids or requires continuous treatment for other basic diseases, the risk of infection increases; (2) If the patient has not borne weight for a long period or has osteoporosis, the acetabular component may penetrate into the acetabulum; (3) Previous femoral head–preserving surgery, e.g., intertrochanteric osteotomies, may cause technical difficulties in joint replacement; (4) Steroid-induced osteonecrosis or alcohol-induced ONFH is a lesion of the femoral head, but it also involves the surrounding and distant bones [122]. Therefore, the long-term effect of joint replacement may not be as good as that for osteoarthritis or traumatic ONFH.
-
1)
-
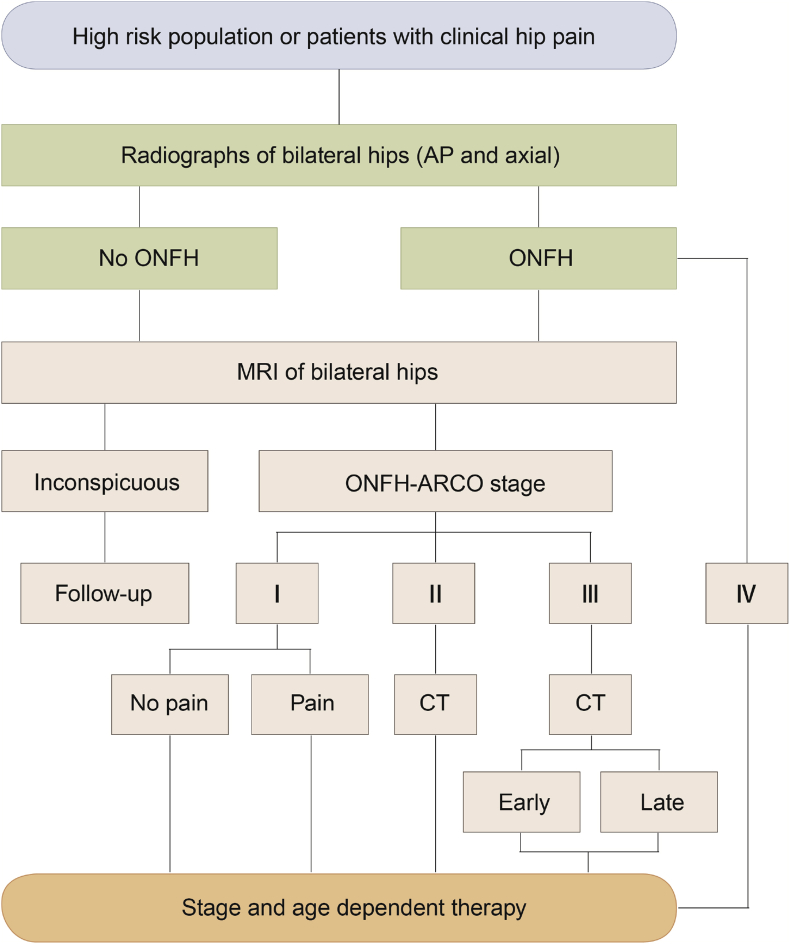
(4)Principles for choosing treatment regimens: The choice of treatment plans for ONFH should consider comprehensive factors, including MRI results, manifestations of blood supply changes in the necrotic femoral head, staging and classification of osteonecrosis, necrosis volume, joint function, and the age, occupation, and compliance with joint preservation treatment of the patient (Figure 4).
-
1)Patients who have no clinical symptoms, necrosis in the non–weight-bearing areas, and a necrosis area <15% can be closely observed, be actively treated to achieve anticoagulation and blood vessel dilatation, avoid high-impact loading, and be followed up regularly. Patients with necrosis in the weight-bearing area who have no clinical symptoms and a necrotic volume >30% should be actively treated and assessed for blood supply, and physicians should not wait for symptoms to appear. Based on the changes in blood supply, core decompression or nonsurgical treatments may be applied in combination [123].
-
2)ARCO stage 0: If one side is diagnosed as nontraumatic ONFH, the other side should be highly suspected. Bilateral MRI should be performed. Follow-up every 3–6 months and use of anticoagulation therapy combined with vasodilator drugs are recommended.
-
3)ARCO stage 1 and 2: For patients with symptoms or a necrosis area of 15–30%, based on the blood supply manifestations, such as venous stasis and arterial ischaemia, performing joint preservation surgery or core decompression alone [[82], [83], [84], [85], [86], [87], [88]] or in combination with stem cell transplantation or transplantation of concentrated autologous bone marrow containing mononuclear cells is recommended. Patients with ARCO stage 2C disease can be treated with (non)vascularized bone graft transplantation (can be combined with supportive materials) [[95], [96], [97],108,[124], [125], [126]] and osteotomy. Nonsurgical treatments, such as lower limb traction and drug treatment, should be actively performed as auxiliary therapeutic treatments.
- 4)
- 5)
-
6)ARCO stage 4: Severe hip function loss or pain appears in this stage, and joint replacement should be selected. If the symptoms are mild and the patient is relatively young (younger than 55 years), joint preservation surgery can be performed. Based on the blood supply changes, the vascularized autologous bone transplantation is recommended, which can be combined with implantation of supportive material. Femoral head preservation surgery typically uses one method or a combination of two or more surgical methods. Nonsurgical treatments should also be included for comprehensive treatment.
-
7)Age factor: Age is another key factor in the choice of treatment options. Young and middle-aged patients are very active, and therefore, a treatment plan that can retain the femoral head without adversely affecting future joint replacement opportunity should be selected. Based on the observed blood supply changes, performing core decompression (stem cell transplantation), transplantation of autologous bone with a blood supply, or transplantation of bone without a blood supply (necrosis range of 15–30%) is recommended. If middle-aged patients are diagnosed with ONFH at an early stage (no collapse), great effort should be made to preserve the femoral head by using core decompression and (non)vascularized bone transplantation. In the middle to late stage, treatment that preserves the femoral head or joint replacement should be chosen considering the patients' subjective wishes and technical conditions. When performing joint replacement, prosthesis selection should fully consider the possibility of secondary revision. For elderly patients, a total hip joint replacement is recommended. For patients older than 75 years, the decision is reached based on factors such as daily activity status, hip bone condition, and prediction of longevity. Total hip joint replacement is recommended.
-
1)
-
(5)Efficacy of evaluation and rehabilitation exercises.
-
1)Efficacy of evaluation: Evaluation approaches to assess the efficacy of treatments for ONFH can be divided into clinical evaluation and imaging evaluation. Clinical evaluation uses hip function scores (such as Harris Hip Score (Table 4, Table 5, Table 6, Table 7), Reconstruction Hip Scores (hip preservation score) [127] (Table 8), the Western Ontario and McMaster Universities Arthritis Index (WOMAC) scores, and the percentage method of the orthopedics branch of the Chinese Medical Association, which are evaluated on a case-by-case basis based on the stage, necrotic area, and treatment method compared with other patients exhibiting similar parameters. Simultaneous gait analysis is recommended. Imaging evaluation is performed using X-ray images, and the concentric circle template is used to observe changes in the femoral head morphology, joint space, and acetabulum. Lesion evaluation of ONFH at stage 2 or lower in the Chinese staging system should use MRI data. DSA should be performed for patients undergoing vascularized bone transplantation to evaluate the recovery of blood supply [105]. Patient documents should be established to evaluate different causes, necrosis stages, age, and the efficacy of treatment methods, which are necessary for standardization of treatment of ONFH [3].
-
2)Rehabilitation exercises: Rehabilitation exercises can prevent inactivity muscle hypotrophy in patients with ONFH and are an effective means to promote early recovery in patients. Functional exercises should be based on active movements, be supplemented by passive movements, start from small movements to large movements, and gradually progress from little exercise to a large amount of exercise. Appropriate exercise methods are determined based on the stage of ONFH, treatments, hip function scores, and gait analysis results [129,130].
-
①Leg lifting in the flat supine position: In the flat supine position, raise the affected limb, bend the hip and the knee, and then flatten the affected limb. This method is used for conservative treatment of ONFH and in the bed rest period after surgical treatment.
-
②Leg opening and closing in the sitting position: Sit in a chair, with hands on the knees and feet at the same width as that of the shoulders, and then fully stretch out and adduct both legs at the same time. This method is used for conservative treatment of ONFH and in the partial weight-bearing period after surgical treatment.
-
③Leg lifting in the standing position: With hands on a fixed support, keep the body straight, raise the affected limb, bend the hip and the knee so that the body and thigh are at a right angle, and let down the affected limb. This method is used for conservative treatment of ONFH and in the partial weight-bearing period after surgical treatment.
-
④Crouching with a support: With hands on a fixed support, keep the body straight, with feet at the same width as that of the shoulders, crouch, and stand up. This method is used in conservative treatment of ONFH and in the full weight-bearing period after surgical treatment.
-
⑤Internal rotation and extension: With hands on a fixed support, the legs are sequentially moved in a full internal rotation, extension, and circular action. This method is used in conservative treatment of ONFH and during the full weight-bearing period after surgical treatment.
-
⑥Walking exercise with a crutch or cycling exercise: This method is used for conservative treatment of ONFH and in the full weight-bearing period after surgical treatment.
-
1)
Figure 4.
Clinical diagnosis flow chart of osteonecrosis of the femoral head (ONFH). ARCO = Association Research Circulation Osseous; CT = computed tomography; MRI = magnetic resonance imaging; AP = Anteroposterior.
Table 4.
Harris hip score system: pain score [128].
| Pain level | Pain manifestations | Score |
|---|---|---|
| No pain | No pain | 44 |
| Minor | Occasional pain or a little pain, no function affected | 40 |
| Mild | General activities not affected, occasional moderate pain after excessive activities | 30 |
| Moderate | Tolerable, daily activities slightly limited, while normal work sustainable, occasional use of analgesics stronger than aspirin | 20 |
| Severe | Sometimes severe pain, not necessarily restricted to bed, severely limited activities, regular use of analgesics stronger than aspirin. | 10 |
| Disabled | Restricted to bed owing to pain, severe pain while resting in bed, limpness due to pain, disabled | 0 |
Table 5.
Harris hip score system: living ability score [128].
| Item | Daily activities | Score |
|---|---|---|
| Climbing stairs | One stair-step per step, with no use of the handrail | 4 |
| One stair-step per step, with use of the handrail | 2 | |
| Can climb stairs in a certain way | 1 | |
| Unable to climb stairs | 0 | |
| Transportation tools | Capable of entering public transportation tools | 1 |
| Sitting | Sitting in any chair for 1 h without discomfort | 5 |
| Sitting in a high chair for an hour and a half without discomfort | 3 | |
| Sitting in any chair with discomfort | 0 | |
| Wearing shoes and socks | Wearing socks and tying shoelaces without any difficulty | 4 |
| Wearing socks and tying shoelaces with difficulty | 2 | |
| Unable to wear socks or tie shoelaces | 0 |
Table 6.
Harris hip score system: walking ability score [128].
| Item | Degree | Score |
|---|---|---|
| Limpness | No limpness | 11 |
| Little limpness | 8 | |
| Moderate limpness | 5 | |
| Severe limpness | 0 | |
| Walking distance | No limitation | 11 |
| 6 blocks | 8 | |
| 2–3 blocks | 5 | |
| Indoor activities | 2 | |
| Restricted to bed or need a chair (wheelchair) | 0 | |
| Aid usage | Not required | 7 |
| Single cane for long distance | 5 | |
| Single cane used most of the time | 3 | |
| Single crutch | 2 | |
| Two crutches | 0 | |
| Completely unable to walk (reason must be explained) | 0 |
Table 7.
Harris hip score system: joint deformity and activity score [128].
| Item | Degree and range | Score |
|---|---|---|
| Deformity | Without the following deformities (A) Fixed flexion contracture of less than 30° (B) Fixed adduction deformity of less than 10° (C) Fixed extension deformity with internal rotation of less than 1° (D) Limb shortening of less than 3.2 cm |
4 |
| Flexion Extension Extension with external rotation Extension with internal rotation Adduction |
0°–45° × 1.0, 45°–90° × 0.6, 90°–110° × 0.3 0°–15° × 0.8, 15°–20° × 0.3, >20° × 0 0°–15° × 0.4, >15° × 0 Any movement × 0 0°–15° × 0.2 |
5 |
Table 8.
Reconstruction Hip Scores (RHSs; hip preservation) [127].
| Pain (30 points) | Walking ability (20 points) | Range of motion (20 points) | X-ray (30 points) | ||||
|---|---|---|---|---|---|---|---|
| 30 | None, or ignores it | 20 | Unlimited | 20 | Flexion 110°–0°, others sum>110° | 30 | Normal (stable) |
| 20 | Slight, no compromise in activity | 15 | Walk within 1000 mm | 15 | Flexion 90°–0°, others sum>90° | 20 | Intact femoral head with cystic and sclerotic bone |
| 10 | Moderate pain in activity, may take medication | 10 | Walk within 500 mm | 10 | Flexion 70°–0°, others sum>70° | 10 | Collapsed < 2 mm |
| 5 | Marked pain, serious limitation of activities, need medication | 5 | Indoors only | 5 | Flexion 50°–0°, others sum>50° | 5 | Collapsed >2 mm |
| 0 | Totally disabled, pain in bed, bedridden | 0 | Bed only | 0 | Flexion 30°–0°, Others sum > 30 | 0 | Osteoarthritis change, subluxation |
RHS, functional = 70 points, RHS, radiographic = 30 points.
Summary
This guideline is the amendment and renewal of “Chinese Guideline for the Diagnosis and Treatment of Femoral Head Necrosis in Adults (2016 version)”. New advances in the epidemiology, aetiology, pathophysiology, imaging, and diagnosis and treatment of ONFH have been renewed in this revision. From the basic science of ONFH to the clinical application, this guide comprehensively introduces the epidemiology, aetiology, pathology, clinical manifestations, imaging diagnosis, staging and classification, differential diagnosis, nonsurgical treatment, and surgical treatment of ONFH. This guideline can be used as a reference by orthopaedic professionals and researchers and for standardized diagnosis and treatment management under clinical guidance, which is conducive to the prevention, treatment, and further research of ONFH, improving the diagnosis at the treatment level, making patients' symptoms under good control, and improving their quality of life.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors, and no material support of any kind was received.
Conflict of Interest
The authors have no conflicts of interest to disclose in relation to this article.
References
- 1.Zhang H., Li Z. Expert advice on the diagnosis and treatment of osteonecrosis of the femoral head. Chin J Orthop. 2007;27(2):146–148. [Google Scholar]
- 2.Zhao Dw, Hu Yc. Chinese experts' consensus on the diagnosis and treatment of osteonecrosis of the femoral head in adults. Orthop Surg. 2012;4(3):125–130. doi: 10.1111/j.1757-7861.2012.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Association MDotOBotCMD Chinese guideline for the diagnosis and treatment of osteonecrosis of the femoral head in adults. Orthop Surg. 2017;9(1):3. doi: 10.1111/os.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mont M.A., Zywiel M.G., Marker D.R., McGrath M.S., Delanois R.E. The natural history of untreated asymptomatic osteonecrosis of the femoral head: a systematic literature review. JBJS. 2010;92(12):2165–2170. doi: 10.2106/JBJS.I.00575. [DOI] [PubMed] [Google Scholar]
- 5.Zhao D.W. To strengthen the understanding of the pathophysiology in osteonecrosis of femoral head. Chin J Joint Surg. 2014;8(5):560–562. [Google Scholar]
- 6.Mont M.A., Cherian J.J., Sierra R.J., Jones L.C., Lieberman J.R. Nontraumatic Osteonecrosis of the femoral head: where do we stand today?: a ten-year update. JBJS. 2015;97(19):1604–1627. doi: 10.2106/JBJS.O.00071. [DOI] [PubMed] [Google Scholar]
- 7.Cooper C., Steinbuch M., Stevenson R., Miday R., Watts N. The epidemiology of osteonecrosis: findings from the GPRD and THIN databases in the UK. Osteoporos Int. 2010;21(4):569–577. doi: 10.1007/s00198-009-1003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang J.S., Park S., Song J.H., Jung Y.Y., Cho M.R., Rhyu K.H. Prevalence of osteonecrosis of the femoral head: a nationwide epidemiologic analysis in Korea. J Arthroplast. 2009;24(8):1178–1183. doi: 10.1016/j.arth.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 9.Mont M.A., Jones L.C., Hungerford D.S. Nontraumatic osteonecrosis of the femoral head: ten years later. JBJS. 2006;88(5):1117–1132. doi: 10.2106/JBJS.E.01041. [DOI] [PubMed] [Google Scholar]
- 10.Powell C., Chang C., Gershwin M.E. Current concepts on the pathogenesis and natural history of steroid-induced osteonecrosis. Clin Rev Allergy Immunol. 2011;41(1):102–113. doi: 10.1007/s12016-010-8217-z. [DOI] [PubMed] [Google Scholar]
- 11.Kang J.S., Moon K.H., Kwon D.G., Shin B.K., Woo M.S. The natural history of asymptomatic osteonecrosis of the femoral head. Int Orthop. 2013;37(3):379–384. doi: 10.1007/s00264-013-1775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hungerford D.S. Osteonecrosis: avoiding total hip arthroplasty. J Arthroplast. 2002;17(4):121–124. doi: 10.1054/arth.2002.33300. [DOI] [PubMed] [Google Scholar]
- 13.Gosling-Gardeniers A., Rijnen W., Gardeniers J. Osteonecrosis. Springer; 2014. The prevalence of osteonecrosis in different parts of the world; pp. 35–37. [Google Scholar]
- 14.Zhao D.W., Yang L., Tian F.D., Wang B.J., Cui D.P., Guo L. Incidence of osteonecrosis of the femoral head in divers: an epidemiologic analysis in Dalian. Chin J Orthop. 2012;32(6):521–525. [Google Scholar]
- 15.Zhao D.-W., Yu M., Hu K., Wang W., Yang L., Wang B.-J. Prevalence of nontraumatic osteonecrosis of the femoral head and its associated risk factors in the Chinese population: results from a nationally representative survey. Chin Med J. 2015;128(21):2843. doi: 10.4103/0366-6999.168017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panteli M., Rodham P., Giannoudis P.V. Biomechanical rationale for implant choices in femoral neck fracture fixation in the non-elderly. Injury. 2015;46(3):445–452. doi: 10.1016/j.injury.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 17.Thompson G.H., Lea E.S., Chin K., Liu R.W., Son-Hing J.P., Gilmore A. Closed bone graft epiphysiodesis for avascular necrosis of the capital femoral epiphysis. Clin Orthop Relat Res. 2013;471(7):2199–2205. doi: 10.1007/s11999-013-2819-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehlinger M., Moser T., Adam P., Bierry G., Gangi A., de Mathelin M. Early prediction of femoral head avascular necrosis following neck fracture. Orthop Traumatol: Surgery & Research. 2011;97(1):79–88. doi: 10.1016/j.otsr.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Bartoníček J., Vávra J., Bartoška R., Havránek P. Operative treatment of avascular necrosis of the femoral head after proximal femur fractures in adolescents. Int Orthop. 2012;36(1):149–157. doi: 10.1007/s00264-011-1272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Palma L., Santucci A., Verdenelli A., Bugatti M., Meco L., Marinelli M. Outcome of unstable isolated fractures of the posterior acetabular wall associated with hip dislocation. Eur J Orthop Surg Traumatol. 2014;24(3):341–346. doi: 10.1007/s00590-013-1200-7. [DOI] [PubMed] [Google Scholar]
- 21.Tannast M., Pleus F., Bonel H., Galloway H., Siebenrock K.A., Anderson S.E. Magnetic resonance imaging in traumatic posterior hip dislocation. J Orthop Trauma. 2010;24(12):723–731. doi: 10.1097/BOT.0b013e3181d76918. [DOI] [PubMed] [Google Scholar]
- 22.Gangji V., Rooze M., De Maertelaer V., Hauzeur J.-P. Inefficacy of the cementation of femoral head collapse in glucocorticoid-induced osteonecrosis. Int Orthop. 2009;33(3):639–642. doi: 10.1007/s00264-008-0518-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y., Yin L., Li Y., Liu P., Cui Q. Preventive effects of puerarin on alcohol-induced osteonecrosis. Clin Orthop Relat Res. 2008;466(5):1059–1067. doi: 10.1007/s11999-008-0178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukisi-Mukaza M., Gomez-Brouchet A., Donkerwolcke M., Hinsenkamp M., Burny F. Histopathology of aseptic necrosis of the femoral head in sickle cell disease. Int Orthop. 2011;35(8):1145–1150. doi: 10.1007/s00264-010-1121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hua L., Zhang J., HE J-w, Kun W., Wang G-s, Jiang N. Symptomatic osteonecrosis of the femoral head after adult orthotopic liver transplantation. Chin Med J. 2012;125(14):2422–2426. [PubMed] [Google Scholar]
- 26.Wessel J.H., Dodson T.B., Zavras A.I. Zoledronate, smoking, and obesity are strong risk factors for osteonecrosis of the jaw: a case-control study. J Oral Maxillofac Surg. 2008;66(4):625–631. doi: 10.1016/j.joms.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi S., Fukushima W., Kubo T., Iwamoto Y., Hirota Y., Nakamura H. Pronounced risk of nontraumatic osteonecrosis of the femoral head among cigarette smokers who have never used oral corticosteroids: a multicenter case–control study in Japan. J Orthop Sci. 2012;17(6):730–736. doi: 10.1007/s00776-012-0293-x. [DOI] [PubMed] [Google Scholar]
- 28.Chen L., Hong G., Fang B., Zhou G., Han X., Guan T. Predicting the collapse of the femoral head due to osteonecrosis: from basic methods to application prospects. J Orthop Trans. 2017;11:62–72. doi: 10.1016/j.jot.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao H., Guan H., Lai Y., Qin L., Wang X. Review of various treatment options and potential therapies for osteonecrosis of the femoral head. J Orthop Trans. 2016;4:57–70. doi: 10.1016/j.jot.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie X.-H., Wang X.-L., Yang H.-L., Zhao D.-W., Qin L. Steroid-associated osteonecrosis: epidemiology, pathophysiology, animal model, prevention, and potential treatments (an overview) J Orthop Trans. 2015;3(2):58–70. doi: 10.1016/j.jot.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinberg D.R., Steinberg M.E. Osteonecrosis. Springer; 2014. The University of Pennsylvania classification of osteonecrosis; pp. 201–206. [Google Scholar]
- 32.Mitchell D., Rao V., Dalinka M., Spritzer C., Alavi A., Steinberg M. Femoral head avascular necrosis: correlation of MR imaging, radiographic staging, radionuclide imaging, and clinical findings. Radiology. 1987;162(3):709–715. doi: 10.1148/radiology.162.3.3809484. [DOI] [PubMed] [Google Scholar]
- 33.Ha Y.-C., Jung W.H., Kim J.-R., Seong N.H., Kim S.-Y., Koo K.-H. Prediction of collapse in femoral head osteonecrosis: a modified Kerboul method with use of magnetic resonance images. JBJS. 2006;88(Suppl. 3):35–40. doi: 10.2106/JBJS.F.00535. [DOI] [PubMed] [Google Scholar]
- 34.Li J.D., Zhao D.W., Cui D.P., Yang L., Liu B.Y. Quantitative analysis and comparison of osteonecrosis extent of alcoholic ONFH using magnetic resonance imaging and pathology. Chin J Bone Jt Inj. 2011;26(8):689⁃91. [Google Scholar]
- 35.Malizos K.N., Karantanas A.H., Varitimidis S.E., Dailiana Z.H., Bargiotas K., Maris T. Osteonecrosis of the femoral head: etiology, imaging and treatment. Eur J Radiol. 2007;63(1):16–28. doi: 10.1016/j.ejrad.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 36.Huang Z.G., Zhang X.Z., Wei H.Y., Hong W., Ren A., Li Z.R. Application of CT and MRI in volumetric measurement of necrotic lesion in patient with avascular necrosis of the femoral head. Chin J Radiol. 2012:820–824. 09. [Google Scholar]
- 37.Sun W., Li Z.R., Yang Y.R., Shi Z.C., Wang B., Liu B. Experimental study on phase-contrast imaging with synchrotron hard X-ray for repairing osteonecrosis of the femoral head. Orthopedics. 2011;32(9):e530–e534. doi: 10.3928/01477447-20110714-07. [DOI] [PubMed] [Google Scholar]
- 38.Dihlmann W. CT analysis of the upper end of the femur: the asterisk sign and ischaemic bone necrosis of the femoral head. Skelet Radiol. 1982;8(4):251–258. doi: 10.1007/BF02219619. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell D., Kressel H., Arger P., Dalinka M., Spritzer C., Steinberg M. Avascular necrosis of the femoral head: morphologic assessment by MR imaging, with CT correlation. Radiology. 1986;161(3):739–742. doi: 10.1148/radiology.161.3.3786725. [DOI] [PubMed] [Google Scholar]
- 40.Conway W.F., Totty W.G., McEnery K.W. CT and MR imaging of the hip. Radiology. 1996;198(2):297–307. doi: 10.1148/radiology.198.2.8596820. [DOI] [PubMed] [Google Scholar]
- 41.Ryu K.N., Jin W., Park J.S. Osteonecrosis. Springer; 2014. Radiography, MRI, CT, bone scan, and PET-CT; pp. 179–195. [Google Scholar]
- 42.Resnick D., Sweet D., Madewell J. Diagnosis of bone and joint disorders. 4th ed. Saunders; Philadelphia, Pa: 2002. Osteonecrosis: pathogenesis, diagnostic techniques, specific situations, and complications; pp. 3599–3685. [Google Scholar]
- 43.Motomura G., Yamamoto T., Karasuyama K., Iwamoto Y. Bone SPECT/CT of femoral head subchondral insufficiency fracture. Clin Nucl Med. 2015;40(9):752–754. doi: 10.1097/RLU.0000000000000886. [DOI] [PubMed] [Google Scholar]
- 44.Dasa V., Adbel-Nabi H., Anders M.J., Mihalko W.M. F-18 fluoride positron emission tomography of the hip for osteonecrosis. Clin Orthop Relat Res. 2008;466(5):1081–1086. doi: 10.1007/s11999-008-0219-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guoshuang z, Dewei z, Xing Q. Paper presented at: 2019 ARCO Biennial Meeting. 2019. Changes in blood supply and bone structure of different stages osteonecrosis of the human femoral head. 2019; [Dalian, China] [Google Scholar]
- 46.Miyanishi K., Yamamoto T., Irisa T., Yamashita A., Jingushi S., Noguchi Y. Bone marrow fat cell enlargement and a rise in intraosseous pressure in steroid-treated rabbits with osteonecrosis. Bone. 2002;30(1):185–190. doi: 10.1016/s8756-3282(01)00663-9. [DOI] [PubMed] [Google Scholar]
- 47.Benjie w. Dalian University of Technology; 2016. Multimodality imaging, pathological change and clinical treatment of osteonecrosis of the femoral head; pp. 51–78. PhD thesis. [Google Scholar]
- 48.Korompilias A.V., Karantanas A.H., Lykissas M.G., Beris A.E. Transient osteoporosis. JAAOS. 2008;16(8):480–489. doi: 10.5435/00124635-200808000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Patel S. Primary bone marrow oedema syndromes. Rheumatology. 2013;53(5):785–792. doi: 10.1093/rheumatology/ket324. [DOI] [PubMed] [Google Scholar]
- 50.Guler O., Ozyurek S., Cakmak S., Isyar M., Mutlu S., Mahirogullari M. Evaluation of results of conservative therapy in patients with transient osteoporosis of hip. Acta Orthop Belg. 2015;81(3):420–426. [PubMed] [Google Scholar]
- 51.Ikemura S., Yamamoto T., Motomura G., Nakashima Y., Mawatari T., Iwamoto Y. The utility of clinical features for distinguishing subchondral insufficiency fracture from osteonecrosis of the femoral head. Arch Orthop Trauma Surg. 2013;133(12):1623–1627. doi: 10.1007/s00402-013-1847-x. [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto T. Subchondral insufficiency fractures of the femoral head. Clin Orthop Surg. 2012;4(3):173–180. doi: 10.4055/cios.2012.4.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma J.H., Guo W.S., Li Z.R., Shi Z.C., Wang W.G., Sun W. Differential diagnosis of lesions in the femoral head that are easily misdiagnosed as femoral head necrosis. Chin J Bone Jt Surg. 2017;10(1):52–57. [Google Scholar]
- 54.Hackney L.A., Lee M.H., Joseph G.B., Vail T.P., Link T.M. Subchondral insufficiency fractures of the femoral head: associated imaging findings and predictors of clinical progression. Eur Radiol. 2016;26(6):1929–1941. doi: 10.1007/s00330-015-3967-x. [DOI] [PubMed] [Google Scholar]
- 55.Yoon P.W., Yoo J.J., Yoon K.S., Kim H.J. Case report: multifocal subchondral stress fractures of the femoral heads and tibial condyles in a young military recruit. Clin Orthop Relat Res. 2012;470(3):944–949. doi: 10.1007/s11999-011-2209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee Y.-K., Yoo J.J., Koo K.-H., Yoon K.S., Min B.W., Kim H.J. Collapsed subchondral fatigue fracture of the femoral head. Orthop Clin N Am. 2009;40(2):259–265. doi: 10.1016/j.ocl.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 57.Xu Z.H., Chen D.Y., Shi D.Q., Dai J., Jiang Q. Summary of 19 cases of femoral acetabular impact syndrome misdiagnosed as femoral head necrosis. Chin J Sports Med. 2015;34(11):1098–1101. [Google Scholar]
- 58.Chen X.D. Road of hip preservation will only be wide. Chin J Joint Surg. 2017;11(3):1–3. [Google Scholar]
- 59.Hampton S., Nakonezny P., Richard H., Wells J. Pain catastrophizing, anxiety, and depression in hip pathology. Bone Joint J. 2019;101(7):800–807. doi: 10.1302/0301-620X.101B7.BJJ-2018-1309.R1. [DOI] [PubMed] [Google Scholar]
- 60.Yoon B-H., Mont M.A., Koo K-H., Chen C-H., Cheng E.Y., Cui Q. The 2019 revised version of Association Research Circulation Osseous staging system of osteonecrosis of the femoral head. J Arthroplast. 2019 doi: 10.1016/j.arth.2019.11.029. in press. [DOI] [PubMed] [Google Scholar]
- 61.Steinberg M.E., Hayken G.D., Steinberg D.R. A quantitative system for staging avascular necrosis. J Bone Jt Surg British. 1995;77(1):34–41. [PubMed] [Google Scholar]
- 62.Gardeniers J.W.M., Gosling-Gardeniers A.C., Rijnen W.H.C. The ARCO staging system: generation and evolution since 1991. In: Koo K.-H., Mont M.A., Jones L.C., editors. Osteonecrosis. Springer Berlin Heidelberg; Berlin, Heidelberg: 2014. pp. 215–218. [Google Scholar]
- 63.Association JSGotOBotCM, Chen Wh, Chen Sb, Cheng Lm, Gao P., Guo Ws. Guideline for diagnostic and treatment of osteonecrosis of the femoral head. Orthop Surg. 2015;7(3):200–207. doi: 10.1111/os.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao D., Qiu X., Wang B., Wang Z., Wang W., Ouyang J. Epiphyseal arterial network and inferior retinacular artery seem critical to femoral head perfusion in adults with femoral neck fractures. Clin Orthop Relat Res. 2017;475(8):2011–2023. doi: 10.1007/s11999-017-5318-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cao F., Liu G., Wang W., Wang B., Wei X., Lu F. Combined treatment with an anticoagulant and a vasodilator prevents steroid-associated osteonecrosis of rabbit femoral heads by improving hypercoagulability. BioMed Res Int. 2017;2017:1624074. doi: 10.1155/2017/1624074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu B.Y., Yang L., Wang B.J., Wang Z.H., Cheng L.L., Xie H. Prevention for glucocorticoid-induced osteonecrosis of femoral head: a long-term clinical follow-up trail. Natl Med J China (Peking) 2017;97(41):3213–3218. doi: 10.3760/cma.j.issn.0376-2491.2017.41.004. [DOI] [PubMed] [Google Scholar]
- 67.Yamaguchi R., Yamamoto T., Motomura G., Ikemura S., Iwasaki K., Zhao G. Effects of an anti-platelet drug on the prevention of steroid-induced osteonecrosis in rabbits. Rheumatology. 2011;51(5):789–793. doi: 10.1093/rheumatology/ker197. [DOI] [PubMed] [Google Scholar]
- 68.Glueck C.J., Freiberg R.A., Sieve L., Wang P. Enoxaparin prevents progression of stages I and II osteonecrosis of the hip. Clin Orthop Relat Res. 2005;435:164–170. doi: 10.1097/01.blo.0000157539.67567.03. (1976–2007) [DOI] [PubMed] [Google Scholar]
- 69.Hsu S.-L., Wang C.-J., Lee M.S.-S., Chan Y.-S., Huang C.-C., Yang K.D. Cocktail therapy for femoral head necrosis of the hip. Arch Orthop Trauma Surg. 2010;130(1):23. doi: 10.1007/s00402-009-0918-5. [DOI] [PubMed] [Google Scholar]
- 70.Peled E., Davis M., Axelman E., Norman D., Nadir Y. Heapranase role in the treatment of avascular necrosis of femur head. Thromb Res. 2013;131(1):94–98. doi: 10.1016/j.thromres.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 71.Sakamoto K., Osaki M., Hozumi A., Goto H., Fukushima T., Baba H. Simvastatin suppresses dexamethasone-induced secretion of plasminogen activator inhibitor-1 in human bone marrow adipocytes. BMC Muscoskelet Disord. 2011;12(1):82. doi: 10.1186/1471-2474-12-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang L.X., Dong T.H., Xie D.H., Xu M. Reliminary observation of clinical results of treatment for early non⁃traumatic osteonecrosis of the femoral head with Madopar. Chin J Orthop. 2010;30(7):641⁃45. [Google Scholar]
- 73.Chen J.-M., Hsu S.-L., Wong T., Chou W.-Y., Wang C.-J., Wang F.-S. Functional outcomes of bilateral hip necrosis: total hip arthroplasty versus extracorporeal shockwave. Arch Orthop Trauma Surg. 2009;129(6):837. doi: 10.1007/s00402-008-0812-6. [DOI] [PubMed] [Google Scholar]
- 74.Vulpiani M.C., Vetrano M., Trischitta D., Scarcello L., Chizzi F., Argento G. Extracorporeal shock wave therapy in early osteonecrosis of the femoral head: prospective clinical study with long-term follow-up. Arch Orthop Trauma Surg. 2012;132(4):499–508. doi: 10.1007/s00402-011-1444-9. [DOI] [PubMed] [Google Scholar]
- 75.Wang C.-J., Wang F.-S., Ko J.-Y., Huang H.-Y., Chen C.-J., Sun Y.-C. Extracorporeal shockwave therapy shows regeneration in hip necrosis. Rheumatology. 2008;47(4):542–546. doi: 10.1093/rheumatology/ken020. [DOI] [PubMed] [Google Scholar]
- 76.Sun W., Gao F.Q., Li Z.R. Application of extracorporeal shock wave in the treatment of osteonecrosis of the femoral head. Chin J Bone Jt. 2014;3(4):314⁃17. [Google Scholar]
- 77.Li J.P., Chen S., Peng H., Fang H.S., Zhou J.L. Effects of low⁃frequency pulsed electromagnetic fields therapy on peroxisome proliferator⁃activated receptor-γ2 and Runt-related transcription factor 2 expression in steroid⁃induced avascular necrosis of femoral head in rats. Chin J Experiment Surg. 2015;32(7):1578–1581. [Google Scholar]
- 78.Massari L., Fini M., Cadossi R., Setti S., Traina G.C. Biophysical stimulation with pulsed electromagnetic fields in osteonecrosis of the femoral head. JBJS. 2006;88(Suppl. 3):56–60. doi: 10.2106/JBJS.F.00536. [DOI] [PubMed] [Google Scholar]
- 79.Pang C.G., Jiang W.X., Jiang C.Q., Xu D.M. Effects of allogenic bone marrow mesenchymal stem cells combining with bionics electromagnetic fields on osteonecrosis of femoral head in rabbits. Chin J Joint Surg. 2012;6(5):756–763. [Google Scholar]
- 80.Cui C., Chang W., Yu A.X., Cheng S.H., Li H.C. Hemorrheologic changes in steroid-induced avascular necrosis of femoral head after hyperbaric oxygen treatment. Med J Wuhan Univ. 2007;28(1):103–106. [Google Scholar]
- 81.Camporesi E.M., Vezzani G., Bosco G., Mangar D., Bernasek T.L. Hyperbaric oxygen therapy in femoral head necrosis. J Arthroplast. 2010;25(6):118–123. doi: 10.1016/j.arth.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 82.Al Omran A. Multiple drilling compared with standard core decompression for avascular necrosis of the femoral head in sickle cell disease patients. Arch Orthop Trauma Surg. 2013;133(5):609–613. doi: 10.1007/s00402-013-1714-9. [DOI] [PubMed] [Google Scholar]
- 83.Song W.S., Yoo J.J., Kim Y.-M., Kim H.J. Results of multiple drilling compared with those of conventional methods of core decompression. Clin Orthop Relat Res. 2007;454:139–146. doi: 10.1097/01.blo.0000229342.96103.73. [DOI] [PubMed] [Google Scholar]
- 84.Bae J.Y., Kwak D.S., Park K.S., Jeon I. Finite element analysis of the multiple drilling technique for early osteonecrosis of the femoral head. Ann Biomed Eng. 2013;41(12):2528–2537. doi: 10.1007/s10439-013-0851-1. [DOI] [PubMed] [Google Scholar]
- 85.Kang P., Pei F., Shen B., Zhou Z., Yang J. Are the results of multiple drilling and alendronate for osteonecrosis of the femoral head better than those of multiple drilling? A pilot study. Jt Bone Spine. 2012;79(1):67–72. doi: 10.1016/j.jbspin.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 86.Helbig L., Simank H., Kroeber M., Schmidmaier G., Grützner P., Guehring T. Core decompression combined with implantation of a demineralised bone matrix for non-traumatic osteonecrosis of the femoral head. Arch Orthop Trauma Surg. 2012;132(8):1095–1103. doi: 10.1007/s00402-012-1526-3. [DOI] [PubMed] [Google Scholar]
- 87.Israelite C., Nelson C.L., Ziarani C.F., Abboud J.A., Landa J., Steinberg M.E. Bilateral core decompression for osteonecrosis of the femoral head. Clin Orthop Relat Res. 2005;441:285–290. doi: 10.1097/01.blo.0000192365.58958.84. (1976–2007) [DOI] [PubMed] [Google Scholar]
- 88.Rajagopal M., Samora J.B., Ellis T.J. Efficacy of core decompression as treatment for osteonecrosis of the hip: a systematic review. Hip Int. 2012;22(5):489–493. doi: 10.5301/HIP.2012.9748. [DOI] [PubMed] [Google Scholar]
- 89.Persiani P., De Cristo C., Graci J., Noia G., Gurzi M., Villani C. Stage-related results in treatment of hip osteonecrosis with core-decompression and autologous mesenchymal stem cells. Acta Orthop Belg. 2015;81(3):406–412. [PubMed] [Google Scholar]
- 90.Zhao D., Cui D., Wang B., Tian F., Guo L., Yang L. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone. 2012;50(1):325–330. doi: 10.1016/j.bone.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 91.Mao Q., Jin H., Liao F., Xiao L., Chen D., Tong P. The efficacy of targeted intraarterial delivery of concentrated autologous bone marrow containing mononuclear cells in the treatment of osteonecrosis of the femoral head: a five year follow-up study. Bone. 2013;57(2):509–516. doi: 10.1016/j.bone.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shi S.H., Li Z.R., Sun W., Wang B.L., Xu S.Q., Wang R.D. Effect of quality and quantity of blood taken from unilateral and bilateral iliac on the concentration of bone marrow mononuclear cells and the efficacy of bone marrow mononuclear cells transplantation for the treatment of early femoral head necrosis. J Clin Rehabilitative Tissue Eng Res. 2012;16(27):5097–5102. [Google Scholar]
- 93.Zhang H.-J., Liu Y.-W., Du Z.-Q., Guo H., Fan K.-J., Liang G.-H. Therapeutic effect of minimally invasive decompression combined with impaction bone grafting on osteonecrosis of the femoral head. Eur J Orthop Surg Traumatol. 2013;23(8):913–919. doi: 10.1007/s00590-012-1141-6. [DOI] [PubMed] [Google Scholar]
- 94.Yang S., Wu X., Xu W., Ye S., Liu X., Liu X. Structural augmentation with biomaterial-loaded allograft threaded cage for the treatment of femoral head osteonecrosis. J Arthroplast. 2010;25(8):1223–1230. doi: 10.1016/j.arth.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 95.Xiao X., Wang W., Liu D., Zhang H., Gao P., Geng L. The promotion of angiogenesis induced by three-dimensional porous beta-tricalcium phosphate scaffold with different interconnection sizes via activation of PI3K/Akt pathways. Sci Rep. 2015;5:9409. doi: 10.1038/srep09409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gao P., Zhang H., Liu Y., Fan B., Li X., Xiao X. Beta-tricalcium phosphate granules improve osteogenesis in vitro and establish innovative osteo-regenerators for bone tissue engineering in vivo. Sci Rep. 2016;6:23367. doi: 10.1038/srep23367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Feng B., Jinkang Z., Zhen W., Jianxi L., Jiang C., Jian L. The effect of pore size on tissue ingrowth and neovascularization in porous bioceramics of controlled architecture in vivo. Biomed Mater. 2011;6(1) doi: 10.1088/1748-6041/6/1/015007. [DOI] [PubMed] [Google Scholar]
- 98.Ito H., Tanino H., Yamanaka Y., Nakamura T., Takahashi D., Minami A. Long-term results of conventional varus half-wedge proximal femoral osteotomy for the treatment of osteonecrosis of the femoral head. J Bone Jt Surg British. 2012;94(3):308–314. doi: 10.1302/0301-620X.94B3.27814. [DOI] [PubMed] [Google Scholar]
- 99.Hamanishi M., Yasunaga Y., Yamasaki T., Mori R., Shoji T., Ochi M. The clinical and radiographic results of intertrochanteric curved varus osteotomy for idiopathic osteonecrosis of the femoral head. Arch Orthop Trauma Surg. 2014;134(3):305–310. doi: 10.1007/s00402-013-1919-y. [DOI] [PubMed] [Google Scholar]
- 100.Zhao D.W. New methods and selection for head preserving therapy in osteonecrosis of femoral head. Natl Med J China. 2011;91(47):3313–3315. [PubMed] [Google Scholar]
- 101.Zhao D.W. Minimally invasive surgery and microscopic repair in osteonecrosis of femoral head. Chin J Microsurg. 2015;38(3):209–210. [Google Scholar]
- 102.Zhao D., Xu D., Wang W., Cui X. Iliac graft vascularization for femoral head osteonecrosis. Clin Orthop Relat Res. 2006;442:171–179. doi: 10.1097/01.blo.0000181490.31424.96. (1976–2007) [DOI] [PubMed] [Google Scholar]
- 103.Zhao D., Wang B., Guo L., Yang L., Tian F. Will a vascularized greater trochanter graft preserve the necrotic femoral head? Clin Orthop Relat Res. 2010;468(5):1316–1324. doi: 10.1007/s11999-009-1159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang B.J., Zhao D.W., Guo L., Yang L., Liu B.Y., Fu W.M. Clinical outcome of vascularized iliac bone flap transplantation for bilateral osteonecrosis of the femoral head. Chin J Bone Jt Surg. 2014;7(1):15–18. [Google Scholar]
- 105.Zhao D., Xiaobing Y., Wang T., Wang B., Liu B., Fengde T. Digital subtraction angiography in selection of the vascularized greater trochanter bone grafting for treatment of osteonecrosis of femoral head. Microsurgery. 2013;33(8):656–659. doi: 10.1002/micr.22179. [DOI] [PubMed] [Google Scholar]
- 106.Wang B., Zhao D., Liu B., Wang W. Treatment of osteonecrosis of the femoral head by using the greater trochanteric bone flap with double vascular pedicles. Microsurgery. 2013;33(8):593–599. doi: 10.1002/micr.22114. [DOI] [PubMed] [Google Scholar]
- 107.Zhao D., Cui D., Lu F., Wang B., Wang W., Tian F. Combined vascularized iliac and greater trochanter graftings for reconstruction of the osteonecrosis femoral head with collapse: reports of three cases with 20 years follow-up. Microsurgery. 2012;32(7):546–551. doi: 10.1002/micr.21995. [DOI] [PubMed] [Google Scholar]
- 108.Zhao D., Zhang Y., Wang W., Liu Y., Li Z., Wang B. Tantalum rod implantation and vascularized iliac grafting for osteonecrosis of the femoral head. Orthopedics. 2013;36(6):789–795. doi: 10.3928/01477447-20130523-26. [DOI] [PubMed] [Google Scholar]
- 109.Zhao D., Liu B., Wang B., Yang L., Xie H., Huang S. Autologous bone marrow mesenchymal stem cells associated with tantalum rod implantation and vascularized iliac grafting for the treatment of end-stage osteonecrosis of the femoral head. BioMed Res Int. 2015;2015 doi: 10.1155/2015/240506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen W., Zhang F., Chang S.-M., Hui K., Lineaweaver W.C. Microsurgical fibular flap for treatment of avascular necrosis of the femoral head. J Am Coll Surg. 2006;202(2):324–334. doi: 10.1016/j.jamcollsurg.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 111.Xue F., Zhang C.Q., Chai L.Z., Chen S.B., Ding L. Free vascularized fibular grafting for osteonecrosis of femoral head: a systemic review. Chin J Bone Jt Injury. 2014;29(4):322–324. [Google Scholar]
- 112.Zhang C.Q., Ding H., Chen S.B. Patients with necrosis of the femoral head and taking hormone for a long time after fibular graft: eight hips receiving total hip replacement. Chin J Tissue Eng Res. 2014;18(53):8547–8552. [Google Scholar]
- 113.Tian L., Wang K.Z., Dang X.Q., Wang C.S. Long⁃term effects of vascularized fibular graft transplantation for avascular necrosis of femoral head. Chin J Jt Surg. 2012;6(6):879⁃887. [Google Scholar]
- 114.Tu Y., Chen Z., Lineaweaver W.C., Zhang F. Different recipient vessels for free microsurgical fibula flaps in the treatment of avascular necrosis of the femoral head: a systematic review and meta-analysis. Ann Plast Surg. 2017;79(6):583–589. doi: 10.1097/SAP.0000000000001178. [DOI] [PubMed] [Google Scholar]
- 115.Nakasone S., Takao M., Sakai T., Nishii T., Sugano N. Does the extent of osteonecrosis affect the survival of hip resurfacing? Clin Orthop Relat Res. 2013;471(6):1926–1934. doi: 10.1007/s11999-013-2833-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Issa K., Johnson A.J., Naziri Q., Khanuja H.S., Delanois R.E., Mont M.A. Hip osteonecrosis: does prior hip surgery alter outcomes compared to an initial primary total hip arthroplasty? J Arthroplast. 2014;29(1):162–166. doi: 10.1016/j.arth.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 117.Li J., He C., Li D., Zheng W., Liu D., Xu W. Early failure of the Durom prosthesis in metal-on-metal hip resurfacing in Chinese patients. J Arthroplast. 2013;28(10):1816–1821. doi: 10.1016/j.arth.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 118.Lim S.-J., Kim J.-H., Moon Y.-W., Park Y.-S. Femoroacetabular cup impingement after resurfacing arthroplasty of the hip. J Arthroplast. 2012;27(1):60–65. doi: 10.1016/j.arth.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 119.Mont M.A. CORR Insights®: does the extent of osteonecrosis affect the survival of hip resurfacing? Clin Orthop Relat Res. 2013;471(6):1935–1936. doi: 10.1007/s11999-013-2925-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bedard N.A., Callaghan J.J., Liu S.S., Greiner J.J., Klaassen A.L., Johnston R.C. Cementless THA for the treatment of osteonecrosis at 10-year follow-up: have we improved compared to cemented THA? J Arthroplast. 2013;28(7):1192–1199. doi: 10.1016/j.arth.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 121.Bergh C., Fenstad A.M., Furnes O., Garellick G., Havelin L.I., Overgaard S. Increased risk of revision in patients with non-traumatic femoral head necrosis: 11,589 cases compared to 416,217 cases with primary osteoarthritis in the NARA database, 1995–2011. Acta Orthop. 2014;85(1):11–17. doi: 10.3109/17453674.2013.874927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tingart M., Beckmann J., Opolka A., Matsuura M., Schaumburger J., Grifka J. Analysis of bone matrix composition and trabecular microarchitecture of the femoral metaphysis in patients with osteonecrosis of the femoral head. J Orthop Res. 2009;27(9):1175–1181. doi: 10.1002/jor.20873. [DOI] [PubMed] [Google Scholar]
- 123.Nam K.W., Kim Y.L., Yoo J.J., Koo K.-H., Yoon K.S., Kim H.J. Fate of untreated asymptomatic osteonecrosis of the femoral head. JBJS. 2008;90(3):477–484. doi: 10.2106/JBJS.F.01582. [DOI] [PubMed] [Google Scholar]
- 124.Sun W., Li Z.R., Gao F.Q., Shi Z.C., Wang B.L., Guo W.S. Porous bioceramic beta⁃tricalcium phosphate for treatment of osteonecrosis of the femoral head. J Clin Rehab Tissue Eng Res. 2014;18(16):2474–2479. [Google Scholar]
- 125.Liu B., Sun W., Yue D., Li Z., Guo W. Combined tantalum implant with bone grafting for the treatment of osteonecrosis of the femoral head. J Investig Surg. 2013;26(3):158–162. doi: 10.3109/08941939.2012.718409. [DOI] [PubMed] [Google Scholar]
- 126.Liu Z., Guo W., Li Z., Cheng L., Zhang Q., Yue D. Porous tantalum rods for treating osteonecrosis of the femoral head. Genet Mol Res. 2014;13(4):8342–8352. doi: 10.4238/2014.October.20.10. [DOI] [PubMed] [Google Scholar]
- 127.Dewei z, Benjie w, Weimin f, Simiao t. Paper presented at: 2019 ARCO Biennial Meeting. 2019. Osteonecrosis of the femoral head: a new function evaluation technique. [Dalian, China] [Google Scholar]
- 128.Harris W.H. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty: an end-result study using a new method of result evaluation. JBJS. 1969;51(4):737–755. [PubMed] [Google Scholar]
- 129.Zeng Z.H., Yu A.X., Yu G.R., Tan J.H., Xiong J. Postoperative rehabilitation treatment in ONFH. Chin J Phys Med Rehab. 2005;27(9):557–558. [Google Scholar]
- 130.Kisner C., Colby L.A., Borstad J. Fa Davis; 2017. Therapeutic exercise: foundations and techniques; pp. 714–762. Chapter 20. [Google Scholar]