Abstract
Evidence suggests that adolescent pregnancies are at increased risk of adverse neonatal outcomes compared to adult pregnancies; however, there are significant inconsistencies in the literature, particularly in studies conducted in developed countries.
The objective of this study therefore is to systematically review the current literature with regard to the relationship between adolescent pregnancy and neonatal outcomes.
A literature search was conducted in eight electronic databases (AMED, ASSIA, Child Development and Adolescent Studies, CINAHL, Cochrane Library, Health Source: Nursing, Maternity and Infant Care, MEDLINE and Scopus. The reference lists of included studies were also hand searched.
Studies were included if: they were conducted in countries with very high human development according to the United Nations Human Development Index; reported at least one comparison between adolescents (19 years or under) and adult mothers (20–34 years); and were published between January 1998 and March 2018.
Studies were screened for inclusion and data extracted by one reviewer. A second reviewer independently reviewed a sub-set of studies. Disagreements were resolved by consensus. Meta-analysis was performed using RevMan 5.3 using crude counts reported in the included studies. Sub-group analyses of adolescents aged 17 and under and 18–19 were conducted. Pooled analysis of adjusted odds ratios was also undertaken in order to consider the effect of confounding factors. Meta-analysis effect estimates are reported as risk ratios (RR) and pooled association as adjusted odds ratios (aORs). Point estimates and 95% confidence intervals are presented.
After removal of duplicates a total of 1791 articles were identified, of which 20 met the inclusion criteria.
The results of the meta-analysis showed adolescents to have increased risk of all primary adverse outcomes investigated. Sub-group analysis suggests an increased risk of perinatal death and low birthweight for children born to adolescent mothers; 17 and under (perinatal death: RR 1.50, CI 1.32–1.71: low birthweight RR 1.43, CI 1.20–1.70); 18–19 (perinatal death RR 1.21, CI 1.06–1.37: low birthweight RR 1.10, CI 1.08–1.57). Mothers aged 17 and under were also at increased risk of preterm delivery (RR 1.64, CI 1.54–1.75). Analysis adjusted for confounders showed increased risk of preterm delivery (aOR 1.23, CI 1.09–1.38), very preterm delivery (aOR 1.22, CI 1.03–1.44) and neonatal death (aOR 1.31, CI 1.14–1.52).
Findings show that young maternal age is a significant risk factor for adverse neonatal outcomes in developed countries. Adolescent maternal age therefore should be considered as a potential cause for concern in relation to neonatal health and it is recommended that health care professionals respond accordingly with increased support and monitoring.
Keywords: Adolescent pregnancy, Neonatal outcomes, Systematic review, Meta-analysis
Introduction
Adolescent pregnancies are defined as pregnancies occurring in women aged 19 years or under at the time of conception. Adolescent pregnancy is a global issue with approximately 2 million girls under the age of 16, and 16 million between the ages of 15 and 19 becoming pregnant annually. Adolescent pregnancies are associated with socio-economic deprivation, on both a global and local level [1]. Young women who are living in poverty, with low levels of education and in marginalised communities are most likely to become pregnant at an early age and continue to experience high levels of deprivation.
Globally rates of adolescent pregnancy have reduced from 56.3 conceptions per 1,000 females aged 15–19 in 2000 to 42.5 per 1,000 in 2017. Rates in high income countries are reducing faster from 23.4–12.5 in the same time period. This said, even amongst countries with the highest levels of human development according to the UN human development index there is significant variation in the adolescent fertility rate from 2.7 per 1,000 in Hong Kong to 19.9 per 1,000 in the United States, suggesting that social and cultural factors play an important part in the prevalence of adolescent childbearing [2].
Adolescent age may be an independent risk factor for adverse pregnancy outcomes. One robust systematic review of studies of young adolescents conducted in low and middle income countries found that maternal age <15 years or less than 2 years after menarche had a negative effect on maternal and foetal growth, and infant survival, and a moderate relationship between young maternal age and anaemia, premature birth and neonatal mortality. [3].
There are suggestions that because some adolescents will still be growing during pregnancy they may compete with the developing embryo to satisfy their own growth needs. A prospective cohort study examining the relationship between maternal growth and outcomes found that continued maternal growth in adolescents affected nutrient partitioning between mother and child, and that this impacted negatively upon foetal growth and prematurity [4]. This is however contested by a UK based study [5] which found that the average birthweight of babies born to adolescent women who were still growing was in fact higher than those of young women who had finished growing. Average infant birthweight in the non-growing adolescent group was however significantly lower than adult controls.
There exists a reasonable amount of evidence to indicate that outcomes for adolescent mothers compare less favourably with adults. However, there is considerable inconsistency in these findings particularly between those studies conducted in developed countries. For example, one Canadian study found that while adolescent women were at increased risk of very preterm birth there was no difference in risk of foetal death between adolescent and adult mothers [6]; however a further Australian study identified adolescent maternal age as a risk factor for stillbirth and neonatal death [7]. There has been no recent systematic review creating an overall picture of differences in birth outcomes for adolescent women compared to an adult control group, particularly focused on high income countries. This study therefore aims to review the evidence to address the question
"Is there a higher risk of adverse perinatal outcomes among babies born to adolescent mothers (age 19 years or younger) compared to those born to older mothers in developed countries?"
Materials and methods
A protocol for this systematic review is registered with Prospero (number CRD42018092182). The study was exempt from ethics approval because the research was not conducted with humans or animals and used publicly available data. There was no patient or public (PPI) involvement in the study.
Search strategy
The following electronic databases were searched: AMED, ASSIA, Child Development and Adolescent Studies, CINAHL, Cochrane Library, Health Source: Nursing, Maternity and Infant Care, MEDLINE and Scopus from Jan 1998 to 1 March 2018. Primary studies using any design were included where they reported data for at least one of the outcomes of interest for an adolescent group (≤19 years) alongside an adult control group (20–34 years) or groups within these age brackets. A detailed report of the search strategy employed is available in Appendix 1. Bibliographies of papers selected for inclusion in the review were also hand searched to identify any further relevant references. Identified citations were entered into a RefWorks database and duplicates removed. One researcher screened papers; initially by title and abstract followed by a full text review of papers whose abstracts appeared to fulfil the inclusion criteria, papers selected after full text review were then screened by a second reviewer. Where uncertainties regarding inclusion arose the article was reviewed by a third researcher and a consensus decision made.
Study selection
Primary studies were included if: they were conducted in countries with very high human development according to the United Nations Human Development Index; reported a comparrison of at least one of the prespecified outcomes of interest between adolescent (19 years and under) and adult mothers (20–34 years); and were published between January 1998 and March 2018. Inclusion criteria were not limited with respect to study design [8 All types of non-peer reviewed literature including editorials, letters and newspaper articles were excluded. The AXIS tool [9] for the critical appraisal of the quality of cross-sectional studies was used.Where there were serious concerns such as inadequate or inconsistent reporting the paper was excluded following unsuccessful attempts to contact the authors.
Outcomes
The prespecified outcomes of interest in this study as outlined in the published protocol were: low birthweight (<2500 g); pre-term delivery (<37 completed weeks gestation); small for gestational age (<5th percentile); APGAR score at 1 min and 5 min <7; stillbirth (death of the foetus before or during birth); perinatal death (death of the foetus or neonate between 22 completed weeks of gestation and 7 days after birth); and neonatal death (deaths up to 28 days following birth). The APGAR score is a value from 0 to 10 which is derived from the sum of scores out of 2 for each of the five components (Appearance, Pulse, Grimace, Activity, Respiration). Assessments are usually made at 1 and 5 min following birth and scores below 7 indicate cause for concern [10]. Where stratified data are reported in the included papers (e.g. very low birthweight, very preterm delivery) these strata were also considered as secondary outcomes in the analysis. No core outcome set was used as none were available concerning maternal age. Core outcome sets for pre-term delivery, stillbirth and Intrauterine Growth Restriction (IUGR) were reviewed and the present study is considered to contribute to the wider understanding of these outcomes.
Data synthesis
Data from the included studies were extracted and collated using RevMan 5.3 by one researcher and checked by a second. Where data are expressed as percentages or rates, crude counts were imputed to enable inclusion in the analysis. Following a sensitivity analysis of the included studies, no sub-groups based on characteristics of included studies were considered to be appropriate, therefore heterogeneity of included studies was assessed statistically. Meta-analytical estimates were reported using a fixed effects model if I2 statistic was less than 50% (indicating low-moderate heterogeneity between study results), otherwise a random effects model was assumed. Estimates for dichotomous outcomes were expressed as risk ratios (RR) with 95% confidence intervals (CI).
Sub-group analyses consisting of adolescents aged 17 and under versus those aged 18–19 (both compared with an adult group) was undertaken where data allowed.
In order to account for potential confounders inverse-variance weighting was used to obtain meta-analytical estimates of adjusted odds ratios (aORs) on the natural logarithmic scale; random effects were assumed. There were insufficient data to perform a sub-group analysis of adjusted odds ratios.
Results
Participants
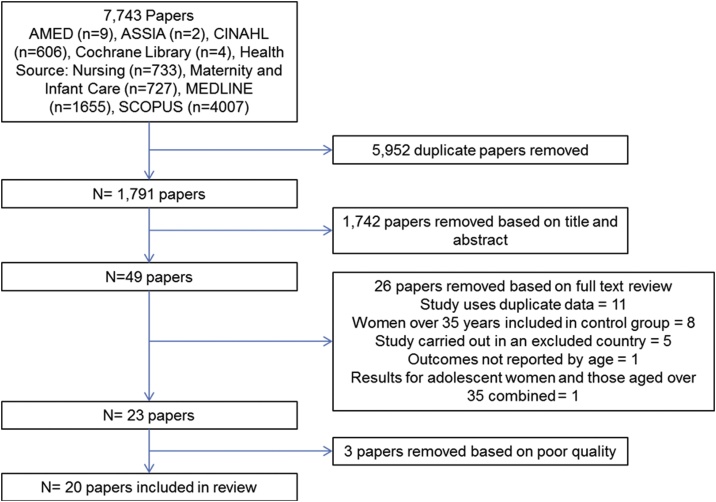
Following a comprehensive search of relevant databases a total of 1,791 unique studies were identified after removal of duplicates. Following screening of the papers and application of the inclusion and exclusion criteria 20 papers remained for inclusion in the review. A PRISMA flowchart of study selection is shown in Fig. 1. Characteristics of included studies are given in Table 1.
Fig. 1.
PRISMA Flow chart.
Table 1.
Characteristics of included studies.
| Study information |
Participants |
Outcomes | Quality | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Country | Design | Time period of data collection | Number in adolescent group(s) | Age | Number in adult group | Age | Inclusion criteria | Exclusion criteria | Outcomes reported | Quality rating |
| Bai 1999 | Australia | Retrospective cohort | March 1996–June 1998 | 128 | <18 | 5898 | 20–34 | Singletons born at Liverpool Hospital, New South Wales, during the study period | Less than 20 weeks gestation or birth weight less than 500 grams | Preterm delivery (<37 weeks) | +/− |
| Stillbirth | |||||||||||
| 313 | 18–19 | Low birth weight (<2500 g) | |||||||||
| Average birth weight | |||||||||||
| Buschman 2001 | UK | Retrospective cohort | NR | 104 | <16 | 150 | 25–30 | Random sample from medical case notes of babies born at Tayside Hospital, Dundee | Last pregnancy body weight recorded before 36 weeks gestation, age over 30 years | Mean gestation at delivery | + |
| Mean birth weight | |||||||||||
| Low birth weight (<2500 g) | |||||||||||
| El-Gilany 2012 | Saudi Arabia | Retrospective cohort | Jan 2010 – Dec 2010 | 404 | <20 | 3691 | 20–34 | Women accessing maternity care across 40 primary health care centres in the Northern region of Saudi Arabia | Files with incomplete data | Preterm delivery (<37 weeks) | + |
| Stillbirth | |||||||||||
| Low birth weight (<2500 g) | |||||||||||
| Fayed 2018 | Saudi Arabia | Retrospective cohort | Nov 2013 - Mar 2015 | 296 | <20 | 6994 | 20–29 | Women recruited for the RAHMA multi-centre cohort study | None | Preterm delivery (<37 weeks) | + |
| Preterm delivery (<34 weeks) | |||||||||||
| Stillbirth | |||||||||||
| Low birth weight (<2500 g) | |||||||||||
| Mean birth weight | |||||||||||
| APGAR <7 | |||||||||||
| Fleming 2013 | Canada | Retrospective cohort | Jan 2006 – Dec 2010 | 23,810 | <20 | 523,721 | 20–35 | Women registered on the Better Outcomes Registry and Network Ontario database | Age over 35 years, stillborn, multiple births | Mean gestation at delivery | + |
| Mean birth weight | |||||||||||
| Small for gestational age | |||||||||||
| Low birth weight (<2500 g) | |||||||||||
| Preterm delivery (<37 weeks) | |||||||||||
| Preterm delivery (<32 weeks) | |||||||||||
| Gupta 2008 | UK | Retrospective cohort | Jan 1990 – Dec 1999 | 587 | <17 | 17,615 | 20–34 | Primiparous women recruited for the Cardiff Births Survey | Age over 34 years | Preterm delivery (<37 weeks) | + |
| Preterm delivery (29–32 weeks) | |||||||||||
| Preterm delivery (24–28 weeks) | |||||||||||
| 4,126 | <20 | Low birth weight (<2500 g) | |||||||||
| Stillbirth | |||||||||||
| Neonatal death | |||||||||||
| Perinatal death | |||||||||||
| Haldre 2007 | Estonia | Retrospective cohort | Jan 1992 – Dec 2002 | 4,248 | <18 | 35,266 | 20–24 | Primiparous women recorded on the Estonian Medical Birth Registry | Age over 24 years | Preterm delivery (<37 weeks) | + |
| Low birth weight (<2500 g) | |||||||||||
| Stillbirth | |||||||||||
| 12,376 | 18–19 | Neonatal death | |||||||||
| Perinatal death | |||||||||||
| Jolly 2000 | UK | Retrospective cohort | Jan 1988 – Dec 1997 | 5,245 | <18 | 336,462 | 18–34 | Singleton births recorded in the St Mary's Maternity Information System Database | Age over 34 years | Small for gestational age | + |
| Preterm delivery (<37 weeks) | |||||||||||
| Preterm delivery (<32 weeks) | |||||||||||
| Stillbirth | |||||||||||
| APGAR <7 | |||||||||||
| Kawakita 2016 | USA | Retrospective cohort | Jan 2002 – Dec 2008 | 1,189 | <16 | 27,645 | 20–24 | Primiparous women with singleton pregnancies recorded in the Consortium of Safe Labor | Women aged over 24 | Preterm delivery (<37 weeks) | + |
| Preterm delivery (<34 weeks) | |||||||||||
| Preterm delivery (<28 weeks) | |||||||||||
| Low birth weight (<2500 g) | |||||||||||
| 14,703 | 16–19 | Very low birth weight (<1500 g) | |||||||||
| APGAR <7 | |||||||||||
| Perinatal death | |||||||||||
| Korencan 2017 | Slovenia | Retrospective cohort | Jan 2008 – Dec 2012 | 318 | <18 | 12,112 | 20–24 | Primiparous women recorded on the National Perinatal Information System in Slovinia | Women aged over 24 | Preterm delivery (<32 weeks) | + |
| Preterm delivery (32–36 weeks) | |||||||||||
| Small for gestational age | |||||||||||
| Low birth weight (<2500 g) | |||||||||||
| 1,413 | <20 | Very low birth weight (<1500 g) | |||||||||
| APGAR <7 | |||||||||||
| Stillbirth | |||||||||||
| Lao 2012 | Hong Kong | Retrospective cohort | Jan 1998 – June 2008 | 1,505 | <20 | 10,320 | 20–24 | Primiparous women with singleton pregnancies delivering at Prince of Wales Hospital, Shatin, during the study period | Women aged over 24 | Mean gestation at delivery | + |
| Mean birth weight | |||||||||||
| Preterm delivery (<34 weeks) | |||||||||||
| Preterm delivery (34–36 weeks) | |||||||||||
| Low birth weight (<2500 g) | |||||||||||
| APGAR <7 | |||||||||||
| Stillbirth | |||||||||||
| Neonatal death | |||||||||||
| Perinatal death | |||||||||||
| Leppalahti 2013 | Finland | Retrospective cohort | Jan 2006 – Dec 2011 | 84 | 13–15 | 51,142 | 25–29 | Primiparous women with singleton pregnancies recorded on the Finish national Medical Birth Register | Cases of major congenital abnormalities, women aged 20–24 or over 29 years | Preterm delivery (<37 weeks) | + |
| 1,234 | 16–17 | Preterm delivery (<28 weeks) | |||||||||
| 5,987 | 18–19 | Small for gestational age | |||||||||
| Stillbirth/neonatal death | |||||||||||
| Marvin-Dowle 2018 | UK | Retrospective cohort | Mar 2007 – Dec 2010 | 68 | <16 | 3,951 | 20–34 | Primiparous women with singleton pregnancies in the Born in Bradford cohort study | Women aged over 34 years | Preterm delivery (<37 weeks) | + |
| Preterm delivery (<32 weeks) | |||||||||||
| Preterm delivery (<28 weeks) | |||||||||||
| Low birth weight (<2500 g) | |||||||||||
| Very low birth weight (<1500 g) | |||||||||||
| Extremely low birth weight (<1000 g) | |||||||||||
| 640 | <20 | Small for gestational age | |||||||||
| APGAR <7 | |||||||||||
| Stillbirth | |||||||||||
| Mean gestation at delivery | |||||||||||
| Mean birth weight | |||||||||||
| Mohsin 2006 | Australia | Retrospective cohort | Jan 1998 – Dec 2002 | 19,648 | <20 | 336,826 | 20–34 | Births recorded in the NSW Midwives data Collection | Births at less than 20 weeks gestation or with a birthweight of less than 400g | Stillbirth | + |
| O'Leary 2007 | Australia | Retrospective cohort | Jan 1994 – Dec 2003 | 14,725 | <20 | 43,810 | 20–24 | Births recorded in the MCHRDB database | Births at less than 20 weeks gestation or with a birthweight of less than 400g | Stillbirth | + |
| Neonatal death | |||||||||||
| Otterblad Olausson 1999 | Sweden | Retrospective cohort | Jan 1973 – Dec 1989 | 831 | 13–15 | 259,494 | 20–24 | Primiparous women with singleton pregnancies recorded on the Swedish Medical Birth Register | Women aged over 24 years | Stillbirth | + |
| 11,379 | 16–17 | Neonatal death | |||||||||
| Post-neonatal death | |||||||||||
| 48,470 | 18–19 | Preterm delivery (33–36 weeks) | |||||||||
| Preterm delivery (23–32 weeks) | |||||||||||
| Papamichael 2009 | UK | Retrospective case-control | Jan 2004 – Dec 2007 | 35 | <16 | 35 | 20–30 | The index group (all women aged <16) was draw from the North Middlesex University Hospital database and the consecutive birth to a women in the other two age groups, mached by parity and ethnicity, selected for the two control groups | Not reported | Small for gestational age | +/− |
| 35 | 16–19 | Stillbirth | |||||||||
| Smith 2001 | UK | Retrospective cohort | Jan 1992 – Dec 1998 | 9699 | 15–19 | 59,315 | 20–29 | Primiparous women recorded on the Scottish morbidity record 2 | Not reported | Preterm delivery (33–36 weeks) | +/− |
| Preterm delivery (24–32 weeks) | |||||||||||
| Small for gestational age | |||||||||||
| Stillbirth | |||||||||||
| Neonatal death | |||||||||||
| Socolov 2017 | Romania | Retrospective cohort | Jan 2007–Dec 2014 | 1,276 | 12–17 | 9,479 | 20–24 | Women delivering a singleton at Cuza Voda Hospital, Iasi, during the study period | Women aged over 24 years, delivery at less than 24 weeks gestation | Mean gestation at delivery | + |
| Mean birth weight | |||||||||||
| Preterm delivery (24–36 weeks) | |||||||||||
| Preterm delivery (24–34 weeks) | |||||||||||
| 2,615 | 18–19 | Preterm delivery (24–28 weeks) | |||||||||
| Low birth weight (<2500 g) | |||||||||||
| Very low birth weight (<1500 g) | |||||||||||
| APGAR <7 | |||||||||||
| Suzuki 2018 | Japan | Retrospective cohort | Jan 2002 – Dec 2016 | 325 | <18 | 2,029 | 28–30 | Women delivering a singleton at Katsushika Maternity Hospital during the study period | Delivery at less than 22 weeks gestation | Preterm delivery (<37 weeks) | +/− |
| Low birth weight (<2500 g) | |||||||||||
| Perinatal death | |||||||||||
The twenty studies included [6,7,[11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28]] in the review reported data on 191,091 adolescent mothers aged under 20, 25,655 adolescent mothers aged 17 and under and 69,761 adolescent mothers aged 18−19 years as well as 1,745,955 adult women. Sample sizes ranged from 35 to 60,680 in the adolescent groups and from 35 to 523,721 in the adult groups. Women included in the adult group were all aged 20–34 years in order to control for the effect of advanced maternal age; however there was some variation between studies within this bracket. With the exception of one retrospective case-control study [25], all included studies used a retrospective cohort design.
Sensitivity analysis was undertaken to examine potential reasons for high levels of heterogeneity observed for some outcomes however no clear sub-groups based on study quality or design or inconsistencies in populations were evident. Four of the included studies were assessed as having a moderate risk of bias with the remaining 16 considered to have low risk of bias. There were sufficient data to perform meta-analysis for nine outcomes in all adolescents vs. control. Sub-group analyses of adolescents aged 17 and under and 18–19 were completed for four outcomes.
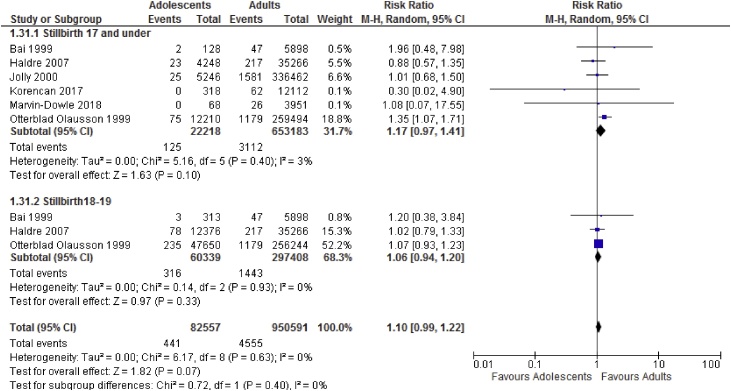
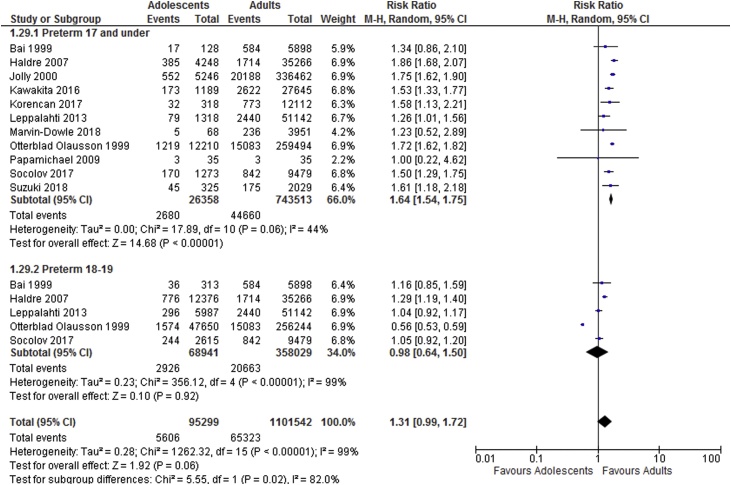
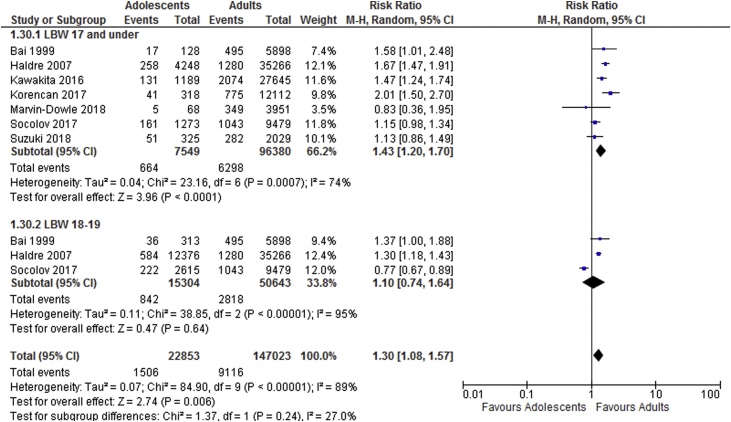
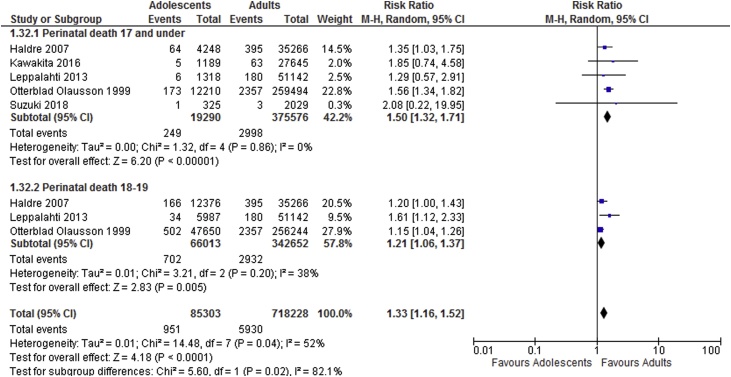
Meta-analysis results suggest adolescents to be at increased risk of adverse neonatal outcomes compared to the adult groups. Sub-group analyses showed increased risk of perinatal death, low birthweight and preterm delivery in women aged 17 and under and increased risk of perinatal death and low birthweight in those ages 18–19. Results of the meta-analyses are shown in Table 2. Forest plots showing sub-group analyses are provided in Fig. 2, Fig. 3, Fig. 4, Fig. 5.
Table 2.
Results of meta-analysis.
| Number of Studies | Participants |
Relative Risk [95% CI] | I2 (%) | ||
|---|---|---|---|---|---|
| Adolescents | Adults | ||||
| Adolescents aged 19 and under vs. control | |||||
| Stillbirth | 14 | 152,129 | 1,534,098 | 1.27 [1.11, 1.45] | 54 |
| Neonatal death | 6 | 142,563 | 1,153,273 | 1.44 [1.33, 1.56] | 43 |
| Perinatal death | 10 | 161,426 | 1,363,367 | 1.29 [1.22, 1.36] | 45 |
| Low birthweight <2500 g | 11 | 65,229 | 641,031 | 1.28 [1.15, 1.43] | 86 |
| Very low birthweight <1500 g | 5 | 38,460 | 88,453 | 1.35 [1.08, 1.70] | 65 |
| Preterm delivery <37 weeks | 17 | 153,580 | 1,405,088 | 1.30 [1.12, 1.50] | 97 |
| Very preterm delivery <32 weeks | 7 | 62,783 | 1,028,436 | 1.69 [1.48, 1.93] | 64 |
| APGAR score <7 at 1 min | 3 | 5,944 | 25,542 | 1.31 [1.17, 1.47] | 17 |
| APGAR score <7 at 5 min | 6 | 23,637 | 70,501 | 1.39 [1.06, 1.82] | 67 |
| Sub-group analysis according to age categories (very young and young versus adult women) | |||||
| Adolescents aged 17 and under vs. control | |||||
| Stillbirth | 7 | 22,253 | 653,218 | 1.15 [0.97, 1.38] | 0 |
| Perinatal death | 5 | 19,290 | 375,576 | 1.50 [1.32, 1.71] | 0 |
| Low birthweight <2500 g | 7 | 7,549 | 96,380 | 1.43 [1.20, 1.70] | 74 |
| Preterm delivery <37 weeks | 11 | 26,358 | 743,513 | 1.64 [1.54, 1.75] | 44 |
| Adolescents aged 18–19 vs. control | |||||
| Stillbirth | 3 | 60,339 | 297,408 | 1.06 [0.94, 1.20] | 0 |
| Perinatal death | 3 | 66,013 | 342,652 | 1.21 [1.06, 1.37] | 38 |
| Low birthweight <2500 g | 3 | 15,304 | 50,643 | 1.10 [1.08, 1.57] | 95 |
| Preterm delivery <37 weeks | 5 | 68,941 | 358,029 | 0.98 [0.64, 1.50] | 99 |
Fig. 2.
Stillbirth sub-group analysis based on age categories.
Fig. 3.
Preterm delivery sub-group analysis based on age categories.
Fig. 4.
Low birthweight sub-group analysis based on age categories.
Fig. 5.
Perinatal death sub-group analysis based on age categories.
Further analysis of adjusted outcomes
Meta-analysis of adjusted odds ratios was carried out for all outcomes where the authors of the included studies had reported an adjusted odds ratio calculated using a multiple logistic regression model. Odds ratios were included where any confounding variables had been included in the model. All studies either only included primiparas and singletons or adjusted for parity and multiple births. Other variables commonly controlled for included measures of socioeconomic status, education, marital status, smoking and maternal body mass index. Full details of the specific variables included in regression models by each study are given in Table 3. Where studies reported more than one adjusted odds ratio for a given outcome (for example one adjusted odds ratio for mothers aged 17 and under and another for mothers aged 18–19) all of these data points were included in the analysis. Care was taken to ensure that no participants were double counted so as to avoid unit of analysis errors.
Table 3.
Variables adjusted for in regression models in studies included in the meta-analysis of adjusted odds ratios.
| Study ID | Number of data points | Restricted to primiparas | Restricted to singletons | Variables adjusted for in regression model |
|---|---|---|---|---|
| Fayed 2018 | 1 | No | Yes | BMI, parity, gestational age, smoke exposure, hypertension, diabetes |
| Haldre 2007 | 2 | Yes | Yes | Ethnicity, marital status, place of residence, calendar year, adequacy of prenatal care, smoking |
| Jolly 2000 | 1 | No | Yes | Ethnicity, parity, BMI, hypertension, diabetes, preclampsia, smoking |
| Kawakita 2016 | 2 | Yes | Yes | Ethnicity, marital status, insurance type, substance abuse, BMI, hospital type, gestational age, diabetes, hypertension |
| Leppalahti 2013 | 3 | Yes | Yes | Cohabitation status, type of residence, smoking, adequacy of prenatal care, alcohol or drug misuse, BMI, diabetes, hypertension, placental abruption, chorioamnionitis, pre-eclampsia, eclampsia, anaemia, history of spontaneous abortions |
| Marvin-Dowle 2018 | 1 | Yes | Yes | Index of multiple deprivation score, ethnicity |
| Mohsin 2006 | 2 | |||
| Model 1 | 1 | No | No | Infant sex, maternal age, country of birth, smoking behaviour during pregnancy, parity, maternal hypertension, birth weight, gestational age |
| Model 2 | 1 | No | No | Infant sex, maternal age, country of birth, smoking behaviour during pregnancy, parity, maternal hypertension, birth weight |
| O'Leary 2007 | 2 | No | No | Birth year, parity, marital status, race, multiple bith, socio-economic disadvantage, region |
| Otterblad-Olausson 1999 | 3 | Yes | Yes | Education, birth year |
| Smith 2001 | 1 | Yes | Yes | Maternal height category, deprivation, previous spontaneous and theraputic abortions, year |
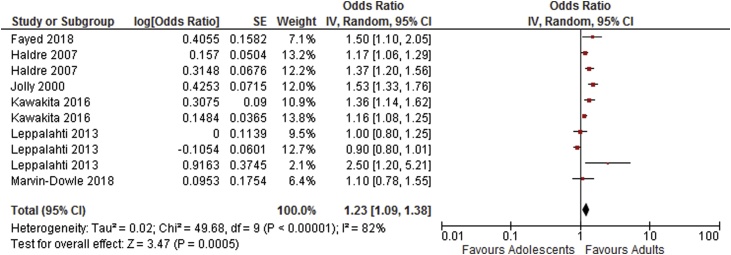
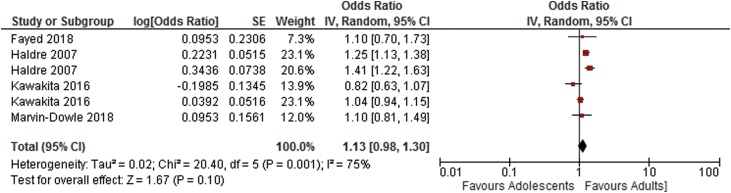
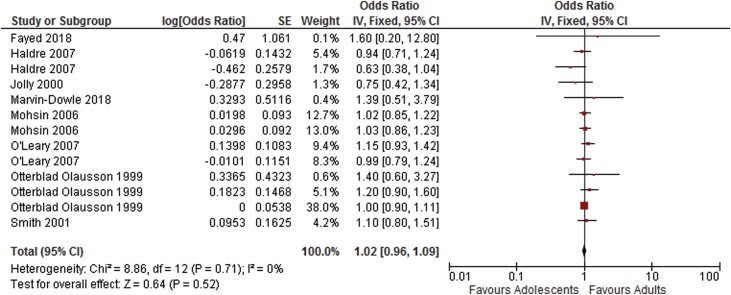
As shown in Table 4, results of the meta-analysis of adjusted odds ratios indicates an increased risk of preterm delivery, very preterm delivery and neonatal death in adolescents compared to adults. Odds ratios for low birthweight, stillbirth and APGAR score <7 at 5 min were not statistically significant. Forest plots showing the results of the meta-anlysis of adjusted odds ratios are shown in Fig. 6, Fig. 7, Fig. 8, Fig. 9, Fig. 10.
Table 4.
Results of meta-analysis of adjusted odds ratios.*
| Number of Studies | Number of Data Points¥ | Odds Ratio [95% CI] | I2 (%) | |
|---|---|---|---|---|
| Preterm delivery <37 weeks [14,[16], [17], [18]] [21,29], | 6 | 10 | 1.23 [1.09–1.38] | 82 |
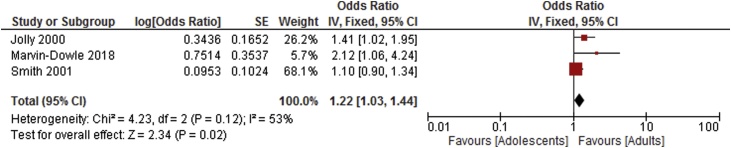
| Very preterm delivery <32 weeks [17,29,30] | 3 | 3 | 1.22 [1.03–1.44] | 53 |
| Low birthweight <2500 g [14,16,18,29] | 4 | 6 | 1.13 [0.98–1.30] | 75 |
| Stillbirth [7,14,16,17,24,[29], [30], [31]] | 8 | 13 | 1.02 [0.96–1.09] | 0 |
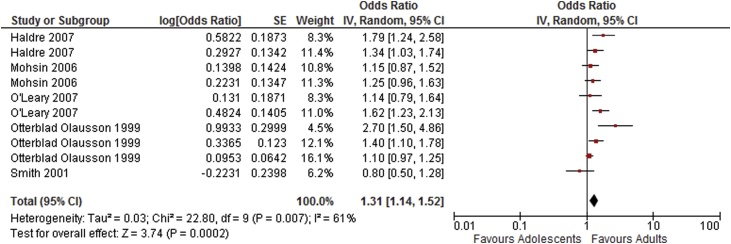
| Neonatal death [7,16,24,30,31] | 5 | 10 | 1.31 [1.14–1.52] | 61 |
| APGAR score <7 at 5 min [14,18,29] | 3 | 4 | 0.97 [0.83–1.14] | 0 |
Details of variables included in the adjusted analysis by each study are shown in Table S2.
Where studies reported more than one adjusted odds ratio (data point) for an outcome, e.g. for sub-groups based on adolescent age, all of the reported results were included in the pooled estimate.
Fig. 6.
Summary odds ratio - Preterm delivery.
Fig. 7.
Summary odds ratio - Low birthweight.
Fig. 8.
Summary odds ratio – Stillbirth.
Fig. 9.
Summary odds ratio - Neonatal death.
Fig. 10.
Summary odds ratio - Very preterm delivery.
Mortality
The main meta-analysis of crude counts showed a higher risk of stillbirth for adolescent vs. adult mothers; however this was not the case in the sub-group or adjusted analyses. The results showed a higher risk of perinatal death, including neonatal death than adult controls consistently for all adolescent age groups and for the subgroups. Analysis of neonatal death in the adjusted analysis also showed increased risk.
Pre-term delivery
The results of the meta-analysis showed that adolescent women had higher risk of both pre-term birth and very pre-term birth (less than 32 completed weeks of gestation) in the adolescent group overall compared to adult women. Sub-group analysis showed an increased risk of preterm birth in those young women aged 17 and under. The adjusted analysis also showed an increased risk of preterm birth in adolescents.
Low birthweight
Unfortunately, the studies reporting small for gestational age (<10th percentile) used wide-ranging definitions meaning that meta-analysis of this outcome was not possible.
Analyses of both low (<2500 g) and very low birthweight (<1500 g) showed a higher risk of these adverse outcomes in all adolescents and in the sub-groups compared to adults. However, the increased risk of low birthweight in adolescents was not statistically significant in the adjusted analysis.
APGAR score
The risk of having a baby with an APGAR score below 7 at both 1 and 5 min was higher in the adolescent group. There were insufficient data to examine APGAR score in sub-groups or with adjusted odds ratios.
Comment
Main findings
The findings showed an increased risk of adverse neonatal outcomes for babies born to adolescent mothers when compared to an adult mothers. The crude meta-analysis showed higher risk for all adverse outcomes in the adolescent group overall, as well as for perinatal death and low birthweight in both sub-group analyses and preterm birth in young women aged 17 and under. The results of the adjusted analysis showed mediated effects; however there is still evidence to suggest that risk of preterm delivery and neonatal death is higher in adolescent mothers compared to adult mothers.
Strengths and limitations
A significant strength of this study is the large number of participants included in the meta-analysis. This made it possible to examine the risk of rarer outcomes such as very low birthweight, very preterm delivery and perinatal mortality with a reasonable degree of precision regarding the reliability of results.
There were some issues with heterogeneity in the main meta-analysis; however these issues appear largely resolved in the sub-group analyses suggesting that the issues are not major methodological concerns and are more likely due to the variation in study size.
While included studies were limited based on the UN human development index, selected due to its multi-dimensional nature, there remains significant differences between the study countries of origin in terms of culture, traditions and health systems which may influence results. Sub-group analysis by country of origin was not possible due to most countries contributing only one study, however this factor should be considered when interpreting the results.
Although limited data were available for some of the outcomes in the sub-groups the analysis returned significant results for three important outcomes; low birthweight, preterm delivery and perinatal death. The lack of significant results for some outcomes is likely to be due to the small number of events despite large numbers of participants, meaning the sub-group analysis was underpowered.
The analysis of adjusted odds ratios was undertaken in order to consider the impact of confounding factors known to effect birth outcomes. This said, there was some variation in the confounding variables included in the multiple logistic regression models used in the original studies, therefore conclusions regarding the relative impact of confounding variables should be interpreted with caution.
Interpretation
The outcomes explored in this review were designed to reflect the main indicators which may affect health and wellbeing both neonatally and in the longer term.
Foetal growth and development has a significant influence on the health and wellbeing of individuals, affecting neonatal outcomes and infant survival as well as the health of the individual throughout their life course. The first 1,000 days of life, from conception to age 2 have been identified as a crucial time period for development and for laying the foundations for a healthy life. Nutrition [32], social support, relationships and environments [33] have been identified as the key components which shape future outcomes.
Babies born with extremely low birthweight and those who are extremely preterm are at significantly higher risk of dying within the first few months of life [34]. Mortality rates of babies born prematurely in the UK decrease rapidly with each additional week of gestation [35] therefore understanding the causes of extremely preterm delivery is a significant factor in reducing perinatal deaths. Longer term, outcomes for children born very preterm and/or with very low birthweight have been shown to include difficulties at school with both behaviour and achievement [36] and low birthweight has been linked to a number of chronic conditions in adulthood such as ischaemic heart disease, hypertension and central adiposity [37]. The results of this review and meta-analysis suggest that young maternal age is associated with perinatal mortality, low birthweight and preterm delivery. This may be an important modifiable factor for reducing the burden of disease in the population.
Analysis of neonatal and perinatal death showed a significant increased risk in adolescents. There is some evidence to suggest that mortality may be higher among babies born to adolescent women due in part to the relationship between these types of death and pre-term, low birthweight babies, which it has already been established are more common in this population. Chen et al. [38] found that the odds of neonatal death were higher in all adolescent age groups studied (10–15 years, 16–17 years and 18−19 years) but that maternal age was no longer predictive of neonatal death once gestational age and birthweight were included in the regression model. Due to the limited availability of reported data it was not possible to consider the influence of birthweight or gestational age on mortality in this review, suggesting that this may be an avenue for further work.
The APGAR score is the most commonly used method of assessing the condition of a new-born at birth. Evidence of low APGAR scores in babies born to adolescent mothers reported to date is mixed; while one large study reports greater relative risk of scores under 7 and under 4 in babies born to young women aged under 17 [38], a number of other similar studies failed to detect a significant difference between groups for this variable [11,31,39]. Using meta-analysis therefore to examine APGAR score in this population is helpful in drawing conclusions regarding the wellbeing of babies at birth. The results reported here show a higher risk of low APGAR score in babies born to adolescent women; however there were insufficient data to include this variable in the sub-group or adjusted analyses.
In addition to the evidence presented here of the impact of young maternal age, there is significant evidence in the literature of the impact of other factors on adverse outcomes. Factors such as socio-economic status [40], cigarette smoking [41] and lower gestational weight gain [42] have all been shown to increase the likelihood of adverse outcomes; particularly preterm delivery and low birthweight. These factors have also been independently associated with adolescent pregnancy [43,44], suggesting that not only are babies born to adolescents potentially affected by the mother's biological immaturity [4,45], they may also be at higher risk of exposure to other detrimental environmental factors. These factors were addressed to some extent in the analysis of adjusted odds ratios, a strategy which was also employed by a previous review [3] which excluded studies which did not control for parity and SES. The previous review only assessed outcomes in women aged under 16 years and did not restrict inclusion by study country of origin. The sub-group analysis conducted in the current review showed a significantly higher risk of preterm birth in those aged under 17 which was not present in the analysis of 18−19 year olds. This suggests that younger age is associated with higher risk of preterm birth even within the adolescent cohort. The results of the two reviews overall are however largely consistent suggesting that higher risk of adverse outcomes is still relevant to older adolescents and those living in countries with high levels of human development.
Although in recent years significant reductions in adolescent pregnancies have been achieved, both globally [46] and locally [47], well-designed studies are required to understand the aetiology of such observed poor outcomes and what appropriate maternity care pathways should be put in place for those adolescent mothers who are pregnant to enhance the health and survival of the new-borns in these vulnerable groups.
Conclusion
Young maternal age is a significant risk factor for adverse neonatal outcomes in developed countries. Adolescent maternal age therefore should be considered as a potential cause for concern in relation to neonatal health and it is recommended that health care professionals respond accordingly with increased support and monitoring.
Further research into the mechanisms underlying differences due to maternal age would be advantageous.
Contribution to authorship
The concept and design of the review was developed jointly by KMD and HS. Acquisition, analysis and interpretation of data was conducted by KMD with HS carrying out checks for quality and accuracy at each stage. The article was drafted by KMD and revised critically by HS, with both authors approving the final version for publication. Both authors agree to be accountable for all aspects of the work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank Dr Lale Say, Co-Ordinator for the Adolescents and At Risk Populations Team, WHO, Geneva, for her assistance with the review and for hosting the student internship which made the work possible.
We would also like to thank Dr Christopher Rose for his expert statistical input and Ghazaleh Oshaghi for her assistance with checking data extraction and data input.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.eurox.2020.100109.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Blum R.W., Gates W., Sr . 2015. Girlhood not motherhood. Preventing adolescent pregnancy. [Google Scholar]
- 2.United Nations Population Division . 2019. Adolescent fertility rate (births per 1,000 women ages 15-19)https://databank.worldbank.org/reports.aspx?source=2&series=SP.ADO.TFRT&country=# Updated, Accessed 12/02, 2019. [Google Scholar]
- 3.Gibbs C.M., Wendt A., Peters S., Hogue C.J. The impact of early age at first childbirth on maternal and infant health. Paediatr Perinat Epidemiol. 2012;26:259–284. doi: 10.1111/j.1365-3016.2012.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scholl T.O., Hediger M.L. Weight gain, nutrition, and pregnancy outcome: Findings from the Camden study of teenage and minority gravidas. Semin Perinatol. 1995;19(3):171–181. doi: 10.1016/s0146-0005(05)80023-0. June. [DOI] [PubMed] [Google Scholar]
- 5.Jones R.L., Cederberg H.M., Wheeler S.J., Poston C.J., Hutchinson J., Seed P.T. Relationship between maternal growth, infant birthweight and nutrient partitioning in teenage pregnancies. BJOG. 2010;117(2):200–211. doi: 10.1111/j.1471-0528.2009.02371.x. [DOI] [PubMed] [Google Scholar]
- 6.Fleming N., Ng N., Osborne C., Biederman S., Yasseen A.S., Dy J. Adolescent pregnancy outcomes in the province of Ontario: a cohort study. J Obstet Gynaecol Can. 2013;35(3):234–245. doi: 10.1016/S1701-2163(15)30995-6. [DOI] [PubMed] [Google Scholar]
- 7.Mohsin M., Bauman A., Jalaludin B. The influence of antenatal and maternal factors on stillbirths and neonatal deaths in New South Wales, Australia. J Biosoc Sci. 2006;38(05):643–657. doi: 10.1017/S002193200502701X. [DOI] [PubMed] [Google Scholar]
- 8.Jāhāna S. United Nations Publications; 2016. Human development report 2016: human development for everyone. [Google Scholar]
- 9.Downes M.J., Brennan M.L., Williams H.C., Dean R.S. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS) BMJ Open. 2016;6(12) doi: 10.1136/bmjopen-2016-011458. e011458-2016-011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apgar V. A proposal for a new method of evaluation of the newborn infant. Anesth Analg. 2015;120(5):1056–1059. doi: 10.1213/ANE.0b013e31829bdc5c. [DOI] [PubMed] [Google Scholar]
- 11.Bai J., Wong F., Stewart Helen. The obstetric and neonatal performance of teenage mothers in an Australian community. J Obstet Gynaecol (Lahore) 1999;19(4):345–348. doi: 10.1080/01443619964607. [DOI] [PubMed] [Google Scholar]
- 12.Buschman N.A., Foster G., Vickers P. Adolescent girls and their babies: achieving optimal birthweight. Gestational weight gain and pregnancy outcome in terms of gestation at delivery and infant birth weight: a comparison between adolescents under 16 and adult women. Child Care Health Dev. 2001;27(2):163–171. doi: 10.1046/j.1365-2214.2001.00164.x. March. [DOI] [PubMed] [Google Scholar]
- 13.El-Gilany A., Hammad S. Obstetric outcomes of teenagers and older mothers: Experience from Saudi Arabia. Int J Collab Res Intern Med Public Health. 2012;4(6):901. [Google Scholar]
- 14.Fayed A.A., Wahabi H., Mamdouh H., Kotb R., Esmaeil S. Demographic profile and pregnancy outcomes of adolescents and older mothers in Saudi Arabia: Analysis from Riyadh mother (RAHMA) and baby cohort study. BMJ Open. 2017;7(9) doi: 10.1136/bmjopen-2017-016501. e016501–e016501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta N., Kiran U., Bhal K. Teenage pregnancies: obstetric characteristics and outcome. Eur J Obstet Gynecol Reprod Biol. 2008;137(2):165–171. doi: 10.1016/j.ejogrb.2007.06.013. April. [DOI] [PubMed] [Google Scholar]
- 16.Haldre K., Rahu K., Karro H., Rahu M. Is a poor pregnancy outcome related to young maternal age? A study of teenagers in Estonia during the period of major socio-economic changes (from 1992 to 2002) Eur J Obstet Gynecol Reprod Biol. 2007;131(1):45–51. doi: 10.1016/j.ejogrb.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Jolly M.C., Sebire N., Harris J., Robinson S., Regan L. Obstetric risks of pregnancy in women less than 18 years old. Obstet Gynecol. 2000;96(6):962–966. doi: 10.1016/s0029-7844(00)01075-9. [DOI] [PubMed] [Google Scholar]
- 18.Kawakita T., Wilson K., Grantz K.L., Landy H.J., Huang C., Gomez-Lobo V. Adverse maternal and neonatal outcomes in adolescent pregnancy. J Pediatr Adolesc Gynecol. 2016;29(2):130–136. doi: 10.1016/j.jpag.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korenčan S., Pinter B., Grebenc M., Verdenik I. The outcomes of pregnancy and childbirth in adolescents in Slovenia. Zdr Varst. 2017;56(4):268–275. doi: 10.1515/sjph-2017-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lao T.T., Suen S.S.H., Sahota D.S. Has improved health care provision impacted on the obstetric outcome in teenage women? J Matern Neonatal Med. 2012;25(8):1358–1362. doi: 10.3109/14767058.2011.634460. [DOI] [PubMed] [Google Scholar]
- 21.Leppälahti S., Gissler M., Mentula M., Heikinheimo O. Is teenage pregnancy an obstetric risk in a welfare society? A population-based study in Finland, from 2006 to 2011. BMJ Open. 2013;3(8) doi: 10.1136/bmjopen-2013-003225. e003225–e003225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marvin-Dowle K., Kilner K., Burley V.J., Soltani H. Impact of adolescent age on maternal and neonatal outcomes in the born in Bradford cohort. BMJ Open. 2018;8(3) doi: 10.1136/bmjopen-2017-016258. e016258-2017-016258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’leary C.M., Bower C., Knuiman M., Stanley F.J. Changing risks of stillbirth and neonatal mortality associated with maternal age in Western Australia 1984–2003. Paediatr Perinat Epidemiol. 2007;21(6):541–549. doi: 10.1111/j.1365-3016.2007.00849.x. [DOI] [PubMed] [Google Scholar]
- 24.Olausson P.O., Cnattingius S., Haglund B. Teenage pregnancies and risk of late fetal death and infant mortality. BJOG: Int J Obstet Gynaecol. 1999;106(2):116–121. doi: 10.1111/j.1471-0528.1999.tb08210.x. [DOI] [PubMed] [Google Scholar]
- 25.Papamichael E., Pillai R., Yoong W. Children having children: outcome of extreme teenage pregnancies (13-15 years) Acta Obstet Gynecol Scand. 2009;88(11):1283–1286. doi: 10.3109/00016340903229427. [DOI] [PubMed] [Google Scholar]
- 26.Smith G.C., Pell J.P. Teenage pregnancy and risk of adverse perinatal outcomes associated with first and second births: population based retrospective cohort study. BMJ. 2001;323(7311):476. doi: 10.1136/bmj.323.7311.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Socolov D., Iorga M., Carauleanu A., Ilea A., Bildaru I., Boiculese L. Pregnancy during adolescence and associated risks: An 8-year hospital-based cohort study (2007–2014) in Romania, the country with the highest rate of teenage pregnancy in Europe. Biomed Res Int. 2017:1–8. doi: 10.1155/2017/9205016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki S. Clinical significance of pregnancy in adolescence in japan. J Matern Fetal Neonatal Med. 2018:1–5. doi: 10.1080/14767058.2017.1421928. [DOI] [PubMed] [Google Scholar]
- 29.Marvin-Dowle K., Kilner K., Burley V.J., Soltani H. Impact of adolescent age on maternal and neonatal outcomes in the born in Bradford cohort. BMJ Open. 2018;8(3) doi: 10.1136/bmjopen-2017-016258. e016258-2017-016258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith G.C., Pell J.P. Teenage pregnancy and risk of adverse perinatal outcomes associated with first and second births: population based retrospective cohort study. BMJ. 2001;323(7311):476. doi: 10.1136/bmj.323.7311.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’leary C.M., Bower C., Knuiman M., Stanley F.J. Changing risks of stillbirth and neonatal mortality associated with maternal age in Western Australia 1984–2003. Paediatr Perinat Epidemiol. 2007;21(6):541–549. doi: 10.1111/j.1365-3016.2007.00849.x. [DOI] [PubMed] [Google Scholar]
- 32.Wrottesley S., Lamper C., Pisa P. Review of the importance of nutrition during the first 1000 days: Maternal nutritional status and its associations with fetal growth and birth, neonatal and infant outcomes among African women. J Dev Orig Health Dis. 2016;7(2):144–162. doi: 10.1017/S2040174415001439. [DOI] [PubMed] [Google Scholar]
- 33.Black M.M., Walker S.P., Fernald L.C.H., Andersen C.T., DiGirolamo A.M., Lu C. Early childhood development coming of age: science through the life course. Lancet. 2017;389(10064):77–90. doi: 10.1016/S0140-6736(16)31389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saugstad O.D., Aune D. Optimal oxygenation of extremely low birth weight infants: a meta-analysis and systematic review of the oxygen saturation target studies. Neonatology. 2014;105(1):55–63. doi: 10.1159/000356561. [DOI] [PubMed] [Google Scholar]
- 35.Tommy’s . 2017. Premature birth statistics.https://www.tommys.org/our-organisation/why-we-exist/premature-birth-statistics Updated. [Google Scholar]
- 36.Aarnoudse-Moens C.S., Weisglas-Kuperus N., van Goudoever J.B., Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124(2):717–728. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- 37.Valdez R., Athens M., Thompson G., Bradshaw B., Stern M. Birthweight and adult health outcomes in a Biethnic population in the USA. Diabetologia. 1994;37(6):624–631. doi: 10.1007/BF00403383. [DOI] [PubMed] [Google Scholar]
- 38.Chen C., Tsai C., Sung F., Lee Y.Y., Lu T.H., Li C.Y. Adverse birth outcomes among pregnancies of teen mothers: Age-specific analysis of national data in Taiwan. Child Care Health Dev. 2010;36(2):232–240. doi: 10.1111/j.1365-2214.2009.01039.x. [DOI] [PubMed] [Google Scholar]
- 39.Tyrberg R.B., Blomberg M., Kjølhede P. Deliveries among teenage women–with emphasis on incidence and mode of delivery: A Swedish national survey from 1973 to 2010. BMC Pregnancy Childbirth. 2013;13(1):204. doi: 10.1186/1471-2393-13-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kramer M.S., Seguin L., Lydon J., Goulet L. Socio‐economic disparities in pregnancy outcome: Why do the poor fare so poorly? Paediatr Perinat Epidemiol. 2000;14(3):194–210. doi: 10.1046/j.1365-3016.2000.00266.x. [DOI] [PubMed] [Google Scholar]
- 41.Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res. 2004;6(Suppl_2):S125–S140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- 42.Goldstein R.F., Abell S.K., Ranasinha S., Misso M., Boyle J.A., Black M.H. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA. 2017;317(21):2207–2225. doi: 10.1001/jama.2017.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scholl T.O., Hediger M.L., Belsky D.H. Prenatal care and maternal health during adolescent pregnancy: a review and meta-analysis. J Adolesc Health. 1994;15(6):444–456. doi: 10.1016/1054-139x(94)90491-k. [DOI] [PubMed] [Google Scholar]
- 44.McCall S.J., Bhattacharya S., Okpo E., Macfarlane G.J. Evaluating the social determinants of teenage pregnancy: A temporal analysis using a UK obstetric database from 1950 to 2010. J Epidemiol Community Health. 2015;69(1):49–54. doi: 10.1136/jech-2014-204214. [DOI] [PubMed] [Google Scholar]
- 45.Scholl T.O., Stein T.P., Smith W.K. Leptin and maternal growth during adolescent pregnancy. Am J Clin Nutr. 2000;72(6):1542–1547. doi: 10.1093/ajcn/72.6.1542. [DOI] [PubMed] [Google Scholar]
- 46.UN D . UN Department of Economic and Social Affairs; 2013. World population prospects: The 2012 revision. [Google Scholar]
- 47.Office for National Statistics . 2018. Conception statistics, England and Wales.https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/conceptionandfertilityrates/datasets/conceptionstatisticsenglandandwalesreferencetables Updated. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.