Abstract
Background
Mainland China has experienced five epidemics of human cases of avian influenza A(H7N9) virus infection since 2013. We conducted a prospective study to assess long-term clinical, pulmonary function testing, and chest computed tomography (CT) imaging findings after patients were discharged from hospital.
Methods
A(H7N9) survivors in five provinces and one municipality underwent follow-up visits from August 2013 to September 2018, at three, six, and 12 months after illness onset, and a subset was also assessed at 18 and 64 months after onset. Thirteen patients were enrolled from the first A(H7N9) epidemic in 2013, 36 from the 2013-2014 second epidemic, and 12 from the 2016-2017 fifth epidemic. At each visit, A(H7N9) survivors received a medical examination, including the mMRC (modified Medical Research Council) dyspnea scale assessment, chest auscultation, pulmonary function testing and chest CT scans.
Findings
The median age of 61 A(H7N9) survivors was 50 years. The cumulative rate of pulmonary dysfunction was 38·5% and 78·2% for chest CT scan abnormalities at the end of follow-up. Restrictive ventilation dysfunction was common during follow-up. Mild dyspnea was documented at three to 12-month follow-up visits.
Interpretation
Patients who survived severe illness from A(H7N9) virus infection had evidence of persistent lung damage and long-term pulmonary dysfunction.
Funding
National Science Fund for Distinguished Young Scholars (grant number 81525023); Program of Shanghai Academic/Technology Research Leader (grant number 18XD1400300); National Science and Technology Major Project of China (grant numbers 2017ZX10103009-005, 2018ZX10201001-010).
Keywords: H7N9 subtype, Prognosis, Respiratory function tests, CT scan, Follow-up
Abbreviations: RT-PCR, reverse transcriptase polymerase chain reaction; CT, computed tomography; WHO, World Health Organization; mMRC, modified Medical Research Council; DLCO, diffusion capacity of carbon monoxide; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; SPSS, Statistical Package for Social Sciences; SD, standard deviation; IQR, interquartile range; GGO, ground-glass opacity; ICU, intensive care unit
Research in context.
Evidence before this study
Very few studies have reported the long-term prognosis or medical follow-up of patients with A(H7N9) virus infection after hospital discharge. A study by Tang et al. reported findings of short-term follow-up (5-7 months) of 5 survivors of A(H7N9) virus infection after the first outbreak of human infections in Shanghai in 2013. A single center study by Chen et al. assessed outcomes in 56 A(H7N9) survivors in Zhejiang Province up to two years.
Added value of this study
To further improve understanding of long-term clinical prognosis and recovery of patients infected with avian influenza A(H7N9) viruses in China, we conducted a multi-center prospective medical follow-up study of A(H7N9) patients after their hospital discharge up to 64 months after illness onset. A substantial proportion of these survivors had persistent pulmonary dysfunction, including both ventilation capacity and diffusion capacity abnormalities. Lung abnormalities identified by CT scans also persisted for prolonged periods after hospital discharge.
Implications of all the available evidence
This multi-center study of the long-term clinical prognosis, sequelae, and prolonged recovery period for A(H7N9) patients who survived severe illness highlights the severity of A(H7N9) virus infections after hospital discharge. These findings reinforce the importance of long-term longitudinal studies to monitor the health impact and medical needs of patients who survived severe illness due to infections with avian influenza A viruses.
Alt-text: Unlabelled box
Introduction
During the early spring of 2013, a novel avian influenza A(H7N9) virus emerged to infect humans in eastern China and cause severe lower respiratory tract diseases [1]. Mainland China has experienced five epidemic waves of human cases of avian influenza A(H7N9) virus infection. As of December 2019, a cumulative total of 1568 laboratory-confirmed cases and 616 deaths had been reported, primarily from China [2]. A surge in A(H7N9) cases was observed during the 5th epidemic wave of human infections, raising pandemic concerns [3,4].
The clinical manifestations of acute A(H7N9) virus infection in humans range from asymptomatic infection to mild upper respiratory illness, severe pneumonia, complications including cardiac failure, renal disease, encephalitis, multi organ failure, and disseminated intravascular coagulation. The A(H7N9) case fatality proportion has remained consistently high at approximately 40% but is lower than that observed for patients with avian influenza A(H5N1) virus infection [5], [6], [7], [8], [9].
While the characteristics of patients with A(H7N9) virus infections have been well described during the clinical course, data on the long-term follow-up of survivors are limited. One single center prospective study by Chen et al. assessed outcomes in 56 A(H7N9) survivors in Zhejiang Province for two years [10]. To further improve understanding of long-term clinical prognosis and recovery of patients infected with avian influenza A viruses in China, we conducted a multi-center prospective medical follow-up study of A(H7N9) survivors up to 64 months after illness onset.
Methods
Case definitions and case identification
In China, all laboratory-confirmed A(H7N9) cases are reported through a national influenza surveillance system. The case definitions for confirmed human infection with avian influenza A(H7N9) virus were on the basis of the H7N9 case definitions as recommended by the World Health Organization [11]. All A(H7N9) patients in this study were identified through the national influenza surveillance system and confirmed with A(H7N9) virus infection by RT-PCR.
Study participants and study design
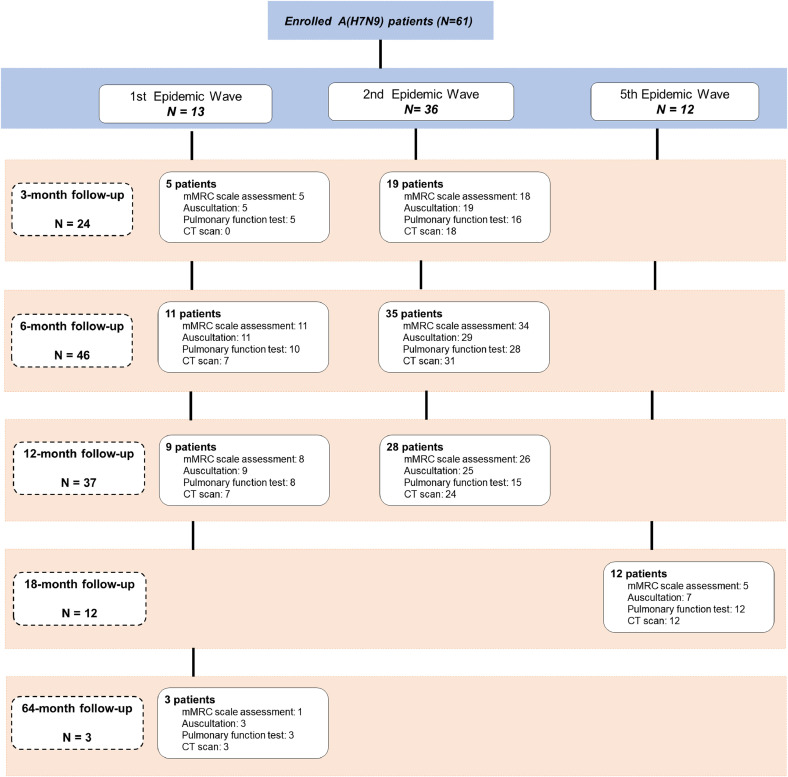
All patients reported in this study were hospitalized and survived. The prospective medical follow-up study period for A(H7N9) patients was August 2013 to September 2018. A flow diagram of the 61 included patients with laboratory-confirmed A(H7N9) virus infection is shown in Fig. 1. Of these, 13 patients were enrolled from the first A(H7N9) epidemic in 2013, 36 from the 2013-2014 second epidemic, and 12 from the 2016-2017 fifth epidemic. All recruited patients from the first and second epidemics agreed to participate. Among patients from fifth epidemic wave in Jiangxi Province, 12 agree to participate and 19 declined. Cumulative incidence of A(H7N9) patients and the locations of patients who participated in the long-term medical follow-up study are shown in Figure S1 (Supplementary materials).
Fig. 1.
Flow chart of A(H7N9) patients’ enrollment and follow-up visits. Flow chart depicting the number of A(H7N9) patients at enrollment and physical assessments performed at the 3-month, 6-month, 12-month, 18-month and 64-month follow-up visits.
A total of 49 A(H7N9) patients from the first two epidemics had follow-up visits at three, six and 12 months after illness onset in five Chinese provinces (Shandong, Jiangxi, Hunan, Fujian, Zhejiang) and one municipality (Beijing). Three A(H7N9) patients in Jiangxi Province also were followed up at 64 months after onset. Twelve A(H7N9) patients from the fifth epidemic were followed up at 18 months after illness onset in Jiangxi Province during July to September 2018 (Figure S2, Supplementary materials). Medical follow-up physical examinations were conducted in 23 tertiary hospitals.
Ethics approval
For subjects younger than 10 years, proxy written informed consent was provided by a parent or legal guardian. For adolescents (10–14 years), verbal assent was obtained and proxy written informed consent was provided by a parent or a legal guardian. For adults (≥15 years), written informed consent was obtained. If an elderly patient lacked the ability to provide informed consent, their legally authorized representative was asked to provide proxy written informed consent. The study protocol, and informed consent were reviewed and approved by the Institutional Review Board of the Chinese Center of Disease Control and Prevention (IRB No. 201326 and 201328) and Institutional Review Board of the School of Public Health, Fudan University (IRB No. 2018-06-0688S).
Clinical data during hospitalization
Clinical and laboratory data for all patients before discharge were collected from hospital medical records or were obtained from patients via standardized questionnaires. Information collected included: demographic data, symptoms and signs at presentation, need for mechanical ventilation, and treatments received.
Medical examination at follow-up visits
At each follow-up visit, survivors received the following clinical assessments: physical examination, pulmonary function testing, and chest CT scans in designated hospitals.
mMRC dyspnea scale and chest auscultation
Dyspnea was evaluated by the modified Medical Research Council (mMRC) five-degree scale at each follow-up visit and classified from Grade 0 to Grade 4 to represent mild to heavy dyspnea [12]. Grade 0: breathless with strenuous exercise; Grade 1: short of breath when hurrying on level ground or walking up a slight hill; Grade 2: walked slower than people of the same age on level ground, and experienced breathlessness or the need to stop to breathe when walking on level ground at their own pace; Grade 3: stop to breathe after walking about 100 yards, or after a few minutes on level ground; and Grade 4: too breathless to leave the house, or breathless when dressing or undressing. Chest auscultation was also performed at each follow-up visit, moist or dry rales were recorded.
Pulmonary function testing
Lung ventilation and diffusion capacity of carbon monoxide (DLCO) testing was performed for A(H7N9) survivors during the follow-up period. Spirometry indicators included forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), and FEV1 to FVC ratio (FEV1/FVC). Pulmonary function testing results were expressed as percentages of predicted normal values. FEV1, FVC, and DLCO were considered abnormal if they were below 80% of the predicted values, FEV1/FVC was considered abnormal if less than 70% [13,14]. If pulmonary function and DLCO testing were within normal ranges, no further testing was performed at the next follow-up visit. Abnormal pulmonary function was interpreted as “restrictive” pattern, “obstructive” pattern or “mixed dysfunction” pattern. Restrictive dysfunction was defined as a reduced FVC with a normal FEV1/FVC; obstructive dysfunction was defined as a reduced FEV1 with a low FEV1/FVC [15].
Chest CT scans and imaging evaluation
The manufacturers and models of CT scanners and instructions used for chest imaging varied across different Chinese hospitals. All scans were performed with the patients in supine position without administration of contrast material. The equipment operating parameters included 0·5 mm or 0·75 mm collimation at 5 mm intervals. Each scan was obtained during breath holds at end inspiration and end expiration. Images were obtained with both mediastinal (width 350–450 HU; level 40–60 HU) and parenchymal (width 1500–2000 HU; level 450–600 HU) window settings. Two chest radiologists reviewed the images independently, reaching a final agreement when there was a discrepancy. The radiologists were blinded to clinical information or prognosis for these patients, except for the knowledge that the images were from A(H7N9) survivors.
Statistical analysis
Data were entered into a Microsoft Excel database and statistical analysis was performed using Statistical Package for Social Sciences (SPSS) version 23.0 for Microsoft Windows, and R programming software package (version 3.5.3; R Development Core Team, 2019). For continuous variables, we calculated mean and standard deviation (SD) or median values and interquartile ranges (IQRs). Since the number of participants with data available at the six month follow-up visit was higher than at other follow-up time points, we analyzed data on mMRC score, chest auscultation, and lung function at six months to compare the effects by the following variables: age group (“0-64” and “≥65”); receipt of antiviral therapy; any underlying comorbidities; and receipt of corticosteroid treatment. Inter-subgroup effect differences were examined by Mann-Whitney U-test for continuous variables with non-normally distributed data. For categorical variables, percentages of survivors in each category were compared using Pearson χ2 test or Fisher's exact test. Kaplan-Meier survival analysis was performed to assess the cumulative rates of pulmonary dysfunction and persistence of chest CT scan abnormalities during the follow-up period. Cox Proportional Hazards (PH) Regression Model was applied to evaluate the relationship between age, gender, ARDS, severe pneumonia, respiratory failure at hospital admission and pulmonary dysfunction, and chest CT abnormalities in A(H7N9) patients for the duration of the follow-up visits. Outcomes in survival analysis were defined as pulmonary function and chest CT scan without abnormalities. Participants’ data were censored due to missing assessments. We used Schoenfeld's global validity test to evaluate the Cox regression model with the null hypothesis defined as “Cox PH assumption valid”. All the statistical tests were two-tailed and the significance level was set as α=0·05.
Results
Patient characteristics
At hospital admission, the median age of 61 A(H7N9) patients was 50 years (range 4 to 80 years), 32·8% were ≥65 years, 68·9% were male, and 46·3% had at least one underlying medical condition (Table 1). The demographic information and underlying comorbidities among A(H7N9) patients with rehabilitation of pulmonary function and chest CT scan were also shown in Table 1. The median duration of hospitalization was 21 days (range 2 to 101 days). Fever and cough were among the most common presenting signs and symptoms, respectively.
Table 1.
Demographic characteristics and underlying comorbidities of A(H7N9) patients at hospital admission.a
| Characteristics | A(H7N9) (N = 61) | Rehabilitation of Pulmonary function (N = 23) | Rehabilitation of Chest CT scan (N = 8) |
|---|---|---|---|
| Demographic characteristic | |||
| Age, median years (range) | 50 (4-80) | 49 (4-78) | 21·5 (4-49) |
| Age group (years) | |||
| 0-4 | 1 (1·6) | 1 (4·3) | 1 (12·5) |
| 5-14 | 4 (6·6) | 1 (4·3) | 2 (25·0) |
| 15-24 | 2 (3·3) | 2 (8·7) | 2 (25·0) |
| 25-49 | 20 (32·8) | 8 (34·8) | 3 (37·5) |
| 50-64 | 14 (23·0) | 5 (21·7) | 0 (0·0) |
| ≥65 | 20 (32·8) | 6 (26·1) | 0 (0·0) |
| Male | 42 (68·9) | 15 (65·2) | 7 (87·5) |
| Underlying comorbiditiesb | |||
| Chronic pulmonary disease | 2 (3·3) | 0 (0·0) | 0 (0·0) |
| Cardiovascular disease | 13 (21·3) | 7 (30·4) | 0 (0·0) |
| Diabetes mellitus | 5 (8·2) | 2 (8·7) | 1 (12·5) |
| Anemia | 1 (1·6) | 0 (0·0) | 0 (0·0) |
| Chronic atrophic gastritis | 1 (1·6) | 0 (0·0) | 0 (0·0) |
| Chronic liver diseasec | 1 (1·6) | 1 (4·3) | 1 (12·5) |
| Schizophrenia | 1 (1·6) | 0 (0·0) | 1 (12·5) |
| Tuberculosis | 1 (1·6) | 0 (0·0) | 0 (0·0) |
| Othersd | 3 (4·9) | 1 (4·3) | 0 (0·0) |
| Any Underlying comorbidities | 27 (44·3) | 10 (43·5) | 3 (37·5) |
Figures are No. (%) unless stated otherwise. NA: Data unavailable. The column of “Rehabilitation of pulmonary function and chest CT scan” represent A(H7N9) patients recovered during follow-up visit.
Comorbidities not mutually exclusive; some patients had multiple chronic comorbid diseases.
Suspected hepatitis B.
Other underlying comorbidities including: hemangioma, uterine fibroid and cerebellar atrophy.
Clinical signs, symptoms, complications and treatment of A(H7N9) patients during hospitalization are shown in Table S1 (Supplementary materials). Of the 61 A(H7N9) patients, 86·3% (44/51) were diagnosed with pneumonia, 45·1% (23/51) were diagnosed with ARDS, 80·8% (42/52) patients were treated with oseltamivir only, 7·7% (4/52) were treated with combined oseltamivir and peramivir, and 64% (32/50) received glucocorticoids during hospitalization. Nearly all (96·6%; 56/58) of the A(H7N9) patients were treated with antibiotics, including ceftazidime, etimicin sulfate, and imipenem.
Dyspnea assessment and chest auscultation
During hospitalization, dyspnea was observed in 52·5% A(H7N9) patients. Mild dyspnea was assessed with mMRC dyspnea grading (mean ± SD) scale of 1·47 ± 0·80, 1·40 ± 0·58 and 1·16 ± 0·38 at the three-month, six-month, and 12-month follow-up visits, respectively, that declined to 1·00 at the 18-month and 64-month visits. On chest auscultation, moist rales were documented in 8·8-15·0% of A(H7N9) patients at the three-month through 12-month visits. Results of dyspnea assessment and chest auscultation for A(H7N9) patients during follow-up are presented in Table 2. The distribution of chest auscultation and pulmonary function finding are shown in Figure S3 (Supplementary materials).
Table 2.
Dyspnea assessment, chest auscultation, pulmonary function, and CT scan findings of A(H7N9) survivors in follow-up visits.a
| Variable | 3-months | 6-months | 12-months | 18-months | 64-months |
|---|---|---|---|---|---|
| Dyspnea assessment | |||||
| No. of patients | 23 | 45 | 34 | 5 | 1 |
| No. of patients with mMRC dyspnea scale ≥1 | 17 | 25 | 18 | 5 | 1 |
| mMRC dyspnea scaleb | 1·47 ± 0·80 | 1·40 ± 0·58 | 1·16 ± 0·38 | 1·00 ± 0·00 | 1·00 ± 0·00 |
| Chest auscultation findings | |||||
| No. of patients | 24 | 40 | 34 | 7 | 3 |
| Moist rales | 3 (12·5) | 6 (15·0) | 3 (8·8) | 0 (0·0) | 0 (0·0) |
| Wheezes | 1 (4·2) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) |
| Rales and wheezes | 1 (4·2) | 1 (2·5) | 0 (0·0) | 0 (0·0) | 0 (0·0) |
| Coarse breath sounds | 1 (4·2) | 1 (2·5) | 5 (14·7) | 0 (0·0) | 0 (0·0) |
| Pulmonary function testing results | |||||
| No. of patients | 21 | 38 | 23 | 12 | 3 |
| Total ventilation dysfunction | 12 (57·1) | 14 (37·8) | 12 (52·1) | 5 (41·7) | 1 (33·3) |
| Obstructive | 3 (14·3) | 5 (13·5) | 4 (17·4) | 0 (0·0) | 0 (0·0) |
| Restrictive | 8 (38·1) | 7 (18·9) | 5 (21·7) | 3 (25·0) | 1 (33·3) |
| Mixed | 1 (4·8) | 2 (5·4) | 3 (13·0) | 2 (16·7) | 0 (0·0) |
| Diffusion dysfunction | 9/12 (75·0) | 17/27 (62·7) | 9/13 (69·2) | 4/7 (57·1) | 3 (100·0) |
| Chest CT scans | |||||
| No. of patients | 18 | 38 | 31 | 12 | 3 |
| Ground-glass opacities | 12 (66·7) | 28 (73·7) | 21 (67·7) | 8 (66·7) | 2 (66·7) |
| Nodule | 4 (22·2) | 2 (5·3) | 3 (9·7) | 1 (8·3) | 0 (0·0) |
| Bullous cysts | 1 (5·6) | 7 (18·4) | 7 (22·6) | 0 (0·0) | 1 (33·3) |
| Fibrosis | 3 (16·7) | 7 (18·4) | 6 (19·4) | 0 (0·0) | 0 (0·0) |
| Liner Fibrosis | 0 (0·0) | 0 (0·0) | 3 (9·7) | 8 (66·7) | 0 (0·0) |
| Pleural thickening | 2 (11·1) | 5 (13·2) | 6 (19·4) | 3 (25·0) | 0 (0·0) |
| Pleural effusion | 1 (5·6) | 2 (5·3) | 0 (0·0) | 0 (0·0) | 0 (0·0) |
| Calcification | 1 (5·6) | 2 (5·3) | 1 (3·2) | 0 (0·0) | 1 (33·3) |
| Increased lung marking | 1 (5·6) | 1 (2·6) | 0 (0·0) | 5 (41·7) | 0 (0·0) |
| Parenchymal opacification | 0 (0·0) | 0 (0·0) | 1 (3·2) | 1 (8·3) | 0 (0·0) |
Figures are No. (%) unless stated otherwise. If Indicates denominators for testing of fewer cases than full group, will be listed. NA, not applicable.
modified Medical Research Council dyspnea scale, mean ± SD, statistical description for mMRC ≥1.
Pulmonary function and chest CT scan abnormalities
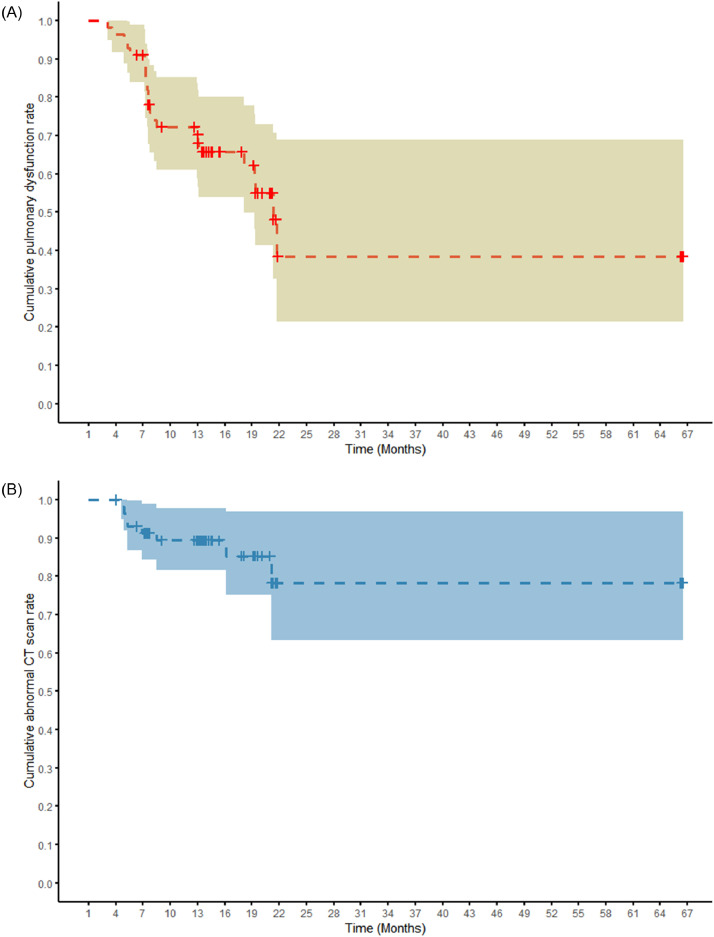
Results of pulmonary function testing and chest CT scans for A(H7N9) patients during follow-up are presented in Table 2. Estimates of the cumulative rates of pulmonary dysfunction and chest CT scan abnormalities for A(H7N9) patients are shown in Fig. 2, Table S2-S3 (Supplementary materials). The cumulative rate of pulmonary dysfunction was 38·5% at 621 days (classified as “18-month follow-up visit” group) and 78·2% for chest CT scan abnormalities at 606 days (classified as “18-month follow-up visit” group) until the last follow-up.
Fig. 2.
Kaplan–Meier analysis of the cumulative risk of pulmonary dysfunction and chest CT scan abnormalities during follow-up of A(H7N9) patients. The horizontal axis is represented by duration (month) from illness onset to follow-up date. The shaded region represents 95% confidence interval. A: Red curve refers to pulmonary dysfunction. B: Blue curve refers to chest CT scan abnormalities.
Cox regression analysis showed that respiratory failure at hospital admission was significantly associated with the risk of persistence of pulmonary dysfunction (adjusted HR = 0·11; 95% CI, 0·02–0·60), and age was significantly associated with the risk of chest CT scan abnormalities (adjusted HR = 0·95, 95% CI, 0·92–0·98) during follow-up (Table S4, Supplementary materials). For the Cox regression evaluation, the p-value of our global test was 0·30 for pulmonary dysfunction and 0·99 for chest CT scans, and we accepted the null hypothesis and the PH assumption as valid.
A total of 56 A(H7N9) patients underwent pulmonary function testing at follow-up visits. Results of pulmonary function testing for A(H7N9) patients are reported in Table 2. A restrictive pattern of lung disease was most prevalent and observed in 38·1% (8/21) of patients at the three-month visit with slow resolution over the follow-up period. An obstructive pattern was noted in 14·3% (3/21) at the three-month visit that generally persisted through 12-months of follow-up, and a mixed pattern was observed from 4·8% (1/21) at the three-month visit to 16·7% (2/12) at the 18-month visit. Abnormal DLCO was detected in 75·0% (9/12) of A(H7N9) patients at the three-month, 57·1% (4/7) at the 18-month, and in all three A(H7N9) patients at the 64-month visits, suggesting persistent impairment in the alveolar diffusion pathway. When pulmonary dysfunction was compared among A(H7N9) patients with and without pneumonia, these were not significantly different by visit duration or types of dysfunction, although ventilation and diffusion dysfunction were more prevalent in pneumonia patients at all follow-up visits (Table 3).
Table 3.
Pulmonary function testing findings of A(H7N9) survivors among pneumonia and non-pneumonia patients.a
| 3-months |
6-months |
12-months |
18-months |
64-months |
p-valuec | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PNEU | Non-PNEU | PNEU | Non-PNEU | Missing datab | PNEU | Non-PNEU | Missing datab | PNEU | Non-PNEU | PNEU | Non-PNEU | ||
| No. of patients | 20 | 1 | 22 | 5 | 10 | 17 | 2 | 3 | 12 | 0 | 3 | 0 | |
| Obstructive dysfunction | 2 (10·0) | 1 (100·0) | 3 (13·6) | 1 (20·0) | 1 (2·7) | 2 (11·8) | 1 (50·0) | 1 (33·3) | 2 (16·7) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 1·00 |
| Restrictive dysfunction | 8 (40·0) | 0 (0·0) | 5 (22·7) | 1 (20·0) | 1 (2·7) | 4 (23·5) | 0 (0·0) | 0 (0·0) | 3 (25·0) | 0 (0·0) | 1 (33·3) | 0 (0·0) | 0·64 |
| Mixed dysfunction | 1 (5·0) | 0 (0·0) | 2 (9·1) | 0 (0·0) | 0 (0·0) | 3 (17·6) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) | 0 (0·0) | NA |
| Diffusion dysfunction | 9 (45·0) | 0 (0·0) | 10 (45·5) | 2 (40·0) | 5 (23·8) | 7 (41·2) | 1 (50·0) | 1 (33·3) | 4 (19·0) | 0 (0·0) | 3 (100·0) | 0 (0·0) | 0·80 |
| p-valued | 0·19 | 1·00 | 0·60 | NA | NA | ||||||||
Figures are No. (%) unless stated otherwise. If indicates denominators for testing of fewer cases than full group, will be listed. Fisher's exact test was used to compare categorical variables. NA, not applicable. PNEU, pneumonia.
Data on pneumonia diagnosis during hospitalization not collected.
The p-values represent the differences of distribution among the time points of follow-up.
The p-values represent the differences of distribution between pneumonia and non-pneumonia patients in a time point of follow-up.
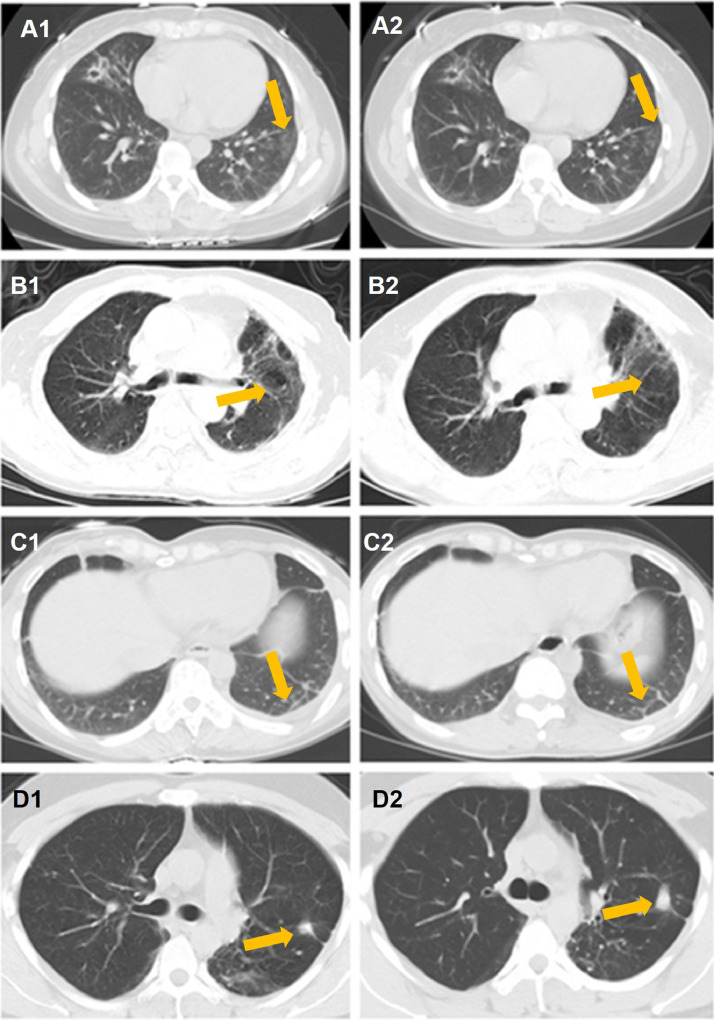
A total of 59 A(H7N9) patients underwent chest CT scans at follow-up visits. The CT scan images were assessed for pattern, extent, and distribution of opacities. The predominant chest CT findings at presentation consisted of bilateral opacities, categorized as fibrosis, nodular opacities, pleural thickening, and bullous cysts. Details of chest radiographic and CT findings for A(H7N9) patients are shown in Table 3. The most common sequelae of A(H7N9)-related pneumonia were bilateral regions of ground-glass opacities (GGO), nodules, or bullous cysts. The pleurae were involved with small fluid accumulation and later pleural thickening. Fibrosis and pleural thickening were also observed through the 12 to 18-month visits. Representative radiographic findings of four A(H7N9) patients are shown in Fig. 3, including GGO and pleural effusions at three, six and 12-month follow-up visits.
Fig. 3.
Chest CT images of four patients who survived severe pneumonia with avian influenza A(H7N9) virus infection during follow-up visits.
A: 47-year-old woman at six (A1) and 12-month (A2) follow-up visits. CT images show mild ground-glass opacities (arrow).
B: 78-year-old man at six (B1) and 12-month (B2) follow-up visits. CT images show pulmonary bullous lesions (arrow).
C: 36-year-old woman at six (C1) and 12-month (C2) follow-up visits. CT images show pleural effusion at six months and improvement at 12-months (arrow).
D: 33-year-old man at three (D1) and 12-months (D2) follow-up visit. CT images show nodule at three months and 12 months (arrow).
In subgroup analyses to compare the effects of mMRC score at 6 months, no statistically significant differences were identified by age group, underlying comorbidities, receipt of antiviral therapy, or corticosteroid treatment. It is possible that the analyses were underpowered due to limited sample size. For chest auscultation and lung function at 6 months, Fisher's exact test was used, and no statistically significant differences were identified (Table S5-S7, Supplementary materials).
Discussion
Few studies have assessed the clinical characteristics of A(H7N9) patients after hospital discharge. Previous studies of A(H7N9) patients who survived hospitalization have mostly concentrated on short-term follow-up after discharge [16,17]. In our prospective evaluation of A(H7N9) patients from five provinces and one municipality, we found that pulmonary function abnormalities, including restrictive, obstructive and mixed patterns, particularly deficits in diffusion capacity, persisted in A(H7N9) patients up to 64-months after illness onset, while abnormalities on chest CT scans persisted after improvement of pulmonary function. Our findings are consistent with those of a follow-up study in Zhejiang Province in which a high percentage of A(H7N9) patients had on-going lower airway abnormalities up to two years of follow-up [10].
At the end of follow-up, 38·5% of A(H7N9) patients had persistent dysfunctional ventilation capacity. Dyspnea assessment and chest auscultation findings improved gradually over 12 months after illness onset. Overall, pulmonary dysfunction appeared to be greater in A(H7N9) patients who were hospitalized with pneumonia than those without pneumonia, although there were no statistically significant differences, perhaps limited by the small sample size. A(H7N9) patients with respiratory failure at hospital admission had increased risk of persistent pulmonary dysfunction.
Chest CT scan findings revealed persistent lung abnormalities during follow-up of A(H7N9) patients. Previous studies demonstrated that bilateral pulmonary infiltrates and multifocal GGOs were the dominant abnormalities associated with influenza viral pneumonia [18,19]. Li et al. compared clinical features of patients with A(H7N9) and A(H1N1)pdm09 virus infections complicated by ARDS. At six-month follow-up, the A(H7N9) group had more changes in pulmonary CT images than the A(H1N1)pdm09 group [20]. Chen et al., reported fibrosis with pulmonary dysfunction up to two years for some A(H7N9) patients [10]. We found persistence of linear fibrosis, nodules, and calcification in some A(H7N9) patients during the entire follow-up period. Our findings reveal that age impact aspects of rehabilitation of CT radiological abnormalities.
The findings of two previous studies of long-term outcomes after avian influenza A(H5N1) virus infection were generally consistent with our findings for A(H7N9) survivors. A follow-up study of two patients with pneumonia caused by A(H5N1) virus infection demonstrated a slow resolution process of lung radiological leisons [21]. A four-year follow-up study of one A(H5N1) patient who survived severe pneumonia reported normal pulmonary function and improved lung compensation function although lung abnormalities persisted in CT scans [22].
The pulmonary abnormalities we identified persisted for longer than has been reported in follow-up of hospitalized patients with pandemic or seasonal influenza. A study of 44 patients hospitalized with influenza A(H1N1)pdm09 virus infection, including 27% diagnosed with pneumonia on admission, reported substantial improvement of respiratory capacity from discharge compared to a six-month follow-up visit [23]. In a study of patients who survived ARDS due to A(H1N1)pdm09 viral pneumonitis, impaired pulmonary function and exercise capacity improved substantially by three months after discharge [24]. Another study of ARDS patients who survived A(H1N1)pdm09 virus infection reported that while 2 of 3 patients had a restrictive pattern at 6 months after ICU admission, all had improvement in CT abnormalities and normal diffusion capacity by six months of follow-up [25]. One study that compared the clinical characteristics of 18 A(H7N9) and 26 A(H1N1)pdm09 patients who were admitted to an intensive care unit reported that the proportion of patients with bronchiectasis, reticular opacities, linear fibrosis, and patchy opacities at six months of follow-up was significantly higher for A(H7N9) than A(H1N1)pdm09 patients [20].
As reported by WHO (13 December 2019), among 1,568 laboratory-confirmed A(H7N9) patients, 33 had HPAI A(H7N9) virus infection, including one patient from Taiwan (the case had visited Guangdong province), and others from Guangxi, Guangdong, Hunan, Shaanxi, Hebei, Henan, Fujian, Yunnan, and Inner Mongolia [26,27]. One study reported that clinical outcomes were similar between hospitalized HPAI and LPAI A(H7N9) patients, except that the time from hospitalization to discharge was longer for HPAI patients [28]. In our study, all 12 patients enrolled from the 2016-17 5th epidemic wave lived in Jiangxi Province. Lu et al. reported that A(H7N9) viruses isolated from humans in Jiangxi province in the 5th epidemic wave belonged to clade C1, while the HPAI A(H7N9) virus clade likely emerged from clade C2 [29]. Therefore, we do not believe that any of the 5th epidemic wave patients included in our study had HPAI A(H7N9) virus infection.
It has been reported that cross-group reactive stalk antibody responses can be boosted after A(H7N9) virus infection, with induction of a broad antibody response to seasonal influenza A viruses [30]. We previously assessed the level of antibodies against seasonal influenza A and A(H7N9) viruses and observed that 17·8% (8/45) of A(H7N9) patients who survived severe disease had 4-fold elevations in HAI titers to seasonal influenza A viruses during 15 months after illness onset of A(H7N9) virus infection. In comparation, levels of A(H1N1)pdm09 and A(H3N2) virus-specific antibodies for the other 37 patients were relatively stable until 15 months after A(H7N9) illness onset, and were not significantly different between A(H7N9) patients and healthy controls [31]. However, these data cannot determine whether survivors of severe illness from A(H7N9) virus infection have a higher or lower risk of infection with A(H1N1)pdm09 or A(H3N2) viruses. We also explored the longevity of A(H7N9) virus-specific antibody titers over time. The main finding is that A(H7N9) patients who survived severe disease mounted higher antibody responses that persisted for longer periods compared with patients who experienced moderate disease. HAI antibody titers of A(H7N9) patients reached 40 on average 11 days after illness onset and peaked at a titer of 290 after three months, and on average HAI antibody titers of ≥80 and ≥40 were present until 11 months and 22 months, respectively [32].
There were several limitations in this study. First, since we only included 61 of 1,568 A(H7N9) cases reported in China to date, our findings may not be representative of all A(H7N9) patients who survived hospitalization in China. Second, data were not available for all clinical and laboratory variables during hospitalization or for all follow-up time points. Baseline pulmonary function testing data and CT scans prior to A(H7N9) illness were not available for comparison. Finally, we lacked a comparison group of patients who survived severe respiratory illness without A(H7N9) virus infection.
In summary, a substantial proportion of patients who were hospitalized in China with A(H7N9) virus infection and survived had persistent pulmonary dysfunction, including both ventilation capacity and diffusion capacity abnormalities, during long-term follow-up. Lung abnormalities identified by CT scans also persisted for prolonged periods after hospital discharge. Our findings add to understanding of the severity of A(H7N9) virus infections and reinforce the importance of long-term longitudinal studies to monitor the health impact of human infections with avian influenza A viruses.
Declaration of Competing Interest
Hongjie Yu reports grants from National Natural Science Foundation of China, grants from Program of Shanghai Academic/Technology Research Leader, grants from National Science and Technology Major Project of China, during the conduct of the study. Hongjie Yu also has received investigator-initiated research funding from Sanofi Pasteur, GlaxoSmithKline, Yichang HEC Changjiang Pharmaceutical Company, and bioMérieux Diagnostic Product (shanghai). None of those research funding is related to human infection with avian influenza virus. Other coauthors: No reported conflicts.
Acknowledgments
Acknowledgments
We thank the A(H7N9) patients for kindly participating in this study, and we thank physicians and nurses in the participating hospitals and local health departments for assistance in medical examination and coordinating data collection. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
This study was funded by the National Science Fund for Distinguished Young Scholars (grant number 81525023); Program of Shanghai Academic/Technology Research Leader (grant number 18XD1400300); National Science and Technology Major Project of China (grant numbers 2017ZX10103009-005, 2018ZX10201001-010).
Disclaimer
The views expressed are those of the authors and do not necessarily represent the official policy of the Centers for Disease Control and Prevention or other institutions with which the authors are affiliated.
Contributors
H. Yu designed and supervised the study. H. Jiang, Y. Xie, T. Zhang, S. Liu, S. Wu, Q. Sun, and S. Song had roles in follow-up management and data collection. Q. Wang, W. Wang, X. Deng, L. Ren, and T. Qin analyzed data. Q. Wang wrote the first draft. T. Uyeki and P. Horby helped to review the data, and contributed to revising the manuscript. All authors contributed to review and revision and have seen and approved the final version.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100282.
Appendix. Supplementary materials
References
- 1.Gao R., Cao B., Hu Y. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013;368(20):1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Recommended composition of influenza virus vaccines for use in the 2020 southern hemisphere influenza season. Available at: https://www.who.int/influenza/vaccines/virus/recommendations/201909_recommendation.pdf?ua=1. Accessed: 23 October2019.
- 3.Wang X., Jiang H., Wu P. Epidemiology of avian influenza A H7N9 virus in human beings across five epidemics in mainland China, 2013-17: an epidemiological study of laboratory-confirmed case series. Lancet Infect Dis. 2017;17(8):822–832. doi: 10.1016/S1473-3099(17)30323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uyeki T.M., Katz J.M., Jernigan D.B. Novel influenza A viruses and pandemic threats. Lancet. 2017;389(10085):2172–2174. doi: 10.1016/S0140-6736(17)31274-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cowling B.J., Jin L., Lau E.H.Y. Comparative epidemiology of human infections with avian influenza A H7N9 and H5N1 viruses in China: a population-based study of laboratory-confirmed cases. Lancet. 2013;382(9887):129–137. doi: 10.1016/S0140-6736(13)61171-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uyeki T.M. Human Infection with Highly Pathogenic Avian Influenza A (H5N1) Virus: Review of Clinical Issues. Clin Infect Dis. 2009;49(2):279–290. doi: 10.1086/600035. [DOI] [PubMed] [Google Scholar]
- 7.Wu Z.Q., Zhang Y., Zhao N. Comparative Epidemiology of Human Fatal Infections with Novel, High (H5N6 and H5N1) and Low (H7N9 and H9N2) Pathogenicity Avian Influenza A Viruses. Int J Environ Res Public Health. 2017;14(3):263. doi: 10.3390/ijerph14030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C., Yu H., Horby P.W. Comparison of patients hospitalized with influenza A subtypes H7N9, H5N1, and 2009 pandemic H1N1. Clin Infect Dis. 2014;58(8):1095–1103. doi: 10.1093/cid/ciu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu H., Cowling B.J., Feng L. Human infection with avian influenza A H7N9 virus: an assessment of clinical severity. Lancet. 2013;382(9887):138–145. doi: 10.1016/S0140-6736(13)61207-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J., Wu J., Hao S. Long term outcomes in survivors of epidemic Influenza A (H7N9) virus infection. Sci Rep. 2017;7(1):17275. doi: 10.1038/s41598-017-17497-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q., Zhou L., Zhou M. Epidemiology of Human Infections with Avian Influenza A(H7N9) Virus in China. N Engl J Med. 2014;370(6):520–532. doi: 10.1056/NEJMoa1304617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahler D.A., Wells C.K. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–586. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 13.Agustí A., Noell G., Brugada J., Faner R. Lung function in early adulthood and health in later life: a transgenerational cohort analysis. Lancet Respir Med. 2017;5(12):935–945. doi: 10.1016/S2213-2600(17)30434-4. [DOI] [PubMed] [Google Scholar]
- 14.Wang C., Xu J., Yang L. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. The Lancet. 2018;391(10131):1706–1717. doi: 10.1016/S0140-6736(18)30841-9. [DOI] [PubMed] [Google Scholar]
- 15.Johnson J.D., Theurer W.M. A Stepwise Approach to the Interpretation of Pulmonary Function Tests. Am Fam Physician. 2014;89(5):359–366. [PubMed] [Google Scholar]
- 16.Lu S., Li T., Xi X. Prognosis of 18 H7N9 avian influenza patients in Shanghai. PLoS One. 2014;9(4):e88728. doi: 10.1371/journal.pone.0088728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang X.J., Xi X.H., Chen C.C. Long-term Follow-up of 5 Survivors after the First Outbreak of Human Infections with Avian Influenza A(H7N9) Virus in Shanghai. China. Chin Med J (Engl) 2016;129(17):2128–2130. doi: 10.4103/0366-6999.189061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao H.N., Lu H.Z., Cao B. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N Engl J Med. 2013;368(24):2277–2285. doi: 10.1056/NEJMoa1305584. [DOI] [PubMed] [Google Scholar]
- 19.Mollura D.J., Asnis D.S., Crupi R.S. Imaging findings in a fatal case of pandemic swine-origin influenza A (H1N1) AJR Am J Roentgenol. 2009;193(6):1500–1503. doi: 10.2214/AJR.09.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H., Weng H., Lan C. Comparison of patients with avian influenza A (H7N9) and influenza A (H1N1) complicated by acute respiratory distress syndrome. Medicine (Baltimore) 2018;97(12):e0194. doi: 10.1097/MD.0000000000010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu P.X., Wang Y.X., Zhou B.P. Radiological features of lung changes caused by avian influenza subtype A H5N1 virus: report of two severe adult cases with regular follow-up. Chin Med J (Engl) 2010;123(1):100–104. [PubMed] [Google Scholar]
- 22.Zhu W., Lu P., Zhou B. Image Features of Severe Pneumonia Caused by Avian Influenza Subtype a H5N1 Virus in Adults and Long-term Follow-up. Chinese Journal of Medical Imaging. 2014;22(8):598–601. 6. [Google Scholar]
- 23.Zarogoulidis P., Kouliatsis G., Papanas N. Long-term respiratory follow-up of H1N1 infection. Virol J. 2011;8(319) doi: 10.1186/1743-422X-8-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsieh M.J., Lee W.C., Cho H.Y. Recovery of pulmonary functions, exercise capacity, and quality of life after pulmonary rehabilitation in survivors of ARDS due to severe influenza A (H1N1) pneumonitis. Influenza Other Respir Viruses. 2018;12(5):643–648. doi: 10.1111/irv.12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toufen Jr C., Costa E.L.V., Hirota A.S., Li H.Y., Amato M.B.P., Carvalho C.R.R. Follow-up after acute respiratory distress syndrome caused by influenza a (H1N1) virus infection. Clinics. 2011;66(6):933–937. doi: 10.1590/S1807-59322011000600002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. Avian Influenza Weekly Update Number 716. Available at: https://www.who.int/docs/default-source/wpro—documents/emergency/surveillance/avian-influenza/ai-20191213. Accessed: 12 December 2019
- 27.Ke C., Mok C.K.P., Zhu W. Human Infection with Highly Pathogenic Avian Influenza A(H7N9) Virus. China. Emerg Infect Dis. 2017;23(8):1332–1340. doi: 10.3201/eid2308.170600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang M., Lau E.H.Y., Guan W. Epidemiology of human infections with highly pathogenic avian influenza A(H7N9) virus in Guangdong, 2016 to 2017. Euro Surveill. 2017;22(27) doi: 10.2807/1560-7917.ES.2017.22.27.30568. pii=30568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu J., Raghwani J., Pryce R. Molecular Evolution, Diversity, and Adaptation of Influenza A(H7N9) Viruses in China. Emerg Infect Dis. 2018;24(10):1795–1805. doi: 10.3201/eid2410.171063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu L., Nachbagauer R., Zhu L. Induction of Broadly Cross-Reactive Stalk-Specific Antibody Responses to Influenza Group 1 and Group 2 Hemagglutinins by Natural H7N9 Virus Infection in Humans. J Infect Dis. 2017;215(4):518–528. doi: 10.1093/infdis/jiw608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao M., Chen J., Tan S. Prolonged Evolution of Virus-Specific Memory T Cell Immunity after Severe Avian Influenza A (H7N9) Virus Infection. J Virol. 2018;92 doi: 10.1128/JVI.01024-18. e01024-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J., Zhu H., Horby P.W. Specificity, Kinetics and Longevity of Antibody Responses to Avian Influenza A(H7N9) Virus Infection in Humans. J Infect. 2020 doi: 10.1016/j.jinf.2019.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.