Abstract
Background
The therapeutic role of methotrexate (MTX) for management of inflammatory bowel disease (IBD) remains unclear.
Methods
We systematically reviewed randomized, controlled trials (RCTs) of MTX for induction and maintenance of remission in IBD until January 2020 in accordance with PROSPERO protocol (#CRD42018115047). Relative risk (RR) of maintenance of remission, induction of remission, endoscopic disease activity, and adverse events were combined in a meta-analysis.
Findings
MTX monotherapy was not superior to placebo for induction of clinical remission in Crohn's disease (CD). However, MTX was superior to placebo in maintaining clinical remission of CD. Concomitant therapy with MTX and the TNF inhibitor infliximab (IFX) was not superior to IFX monotherapy in CD. In ulcerative colitis (UC), MTX monotherapy was not superior to placebo neither for induction of clinical remission, nor for maintenance of clinical remission. MTX did not result in superior endoscopic outcomes during induction or maintenance therapy compared with placebo. Regarding adverse events (AEs), our meta-analysis on CD studies showed a significantly higher risk of AEs when comparing MTX versus placebo in studies investigating induction of remission, but not in maintenance of remission. In UC, no such differences in AEs between MTX or placebo were observed.
Interpretation
Current data support the efficacy of parenteral MTX monotherapy for maintenance of clinical remission in CD. MTX is not confirmed to be effective for treatment of UC or for induction of remission in CD. No evidence supports concomitant MTX to improve efficacy of IFX (no other biologics investigated).
Keywords: Crohn's disease, Inflammatory bowel disease, Methotrexate, Therapy, Ulcerative colitis
Research in context.
Evidence before this study
For long, the role of methotrexate (MTX) to treat flares or to maintain remission in ulcerative colitis (UC) has been uncertain as well as if MTX as concomitant therapy to biologics is associated with greater benefits than placebo or alternative therapeutic agents. Therefore, we searched MEDLINE via PubMed, EMBASE via Ovid, and the Cochrane Central Register of Controlled Trials (CENTRAL), all from inception until January 1, 2020 for randomized, controlled trials evaluating the effectiveness of MTX for inflammatory bowel disease (IBD).
Added value of this study
To our knowledge, this is the first comprehensive meta-analysis to evaluate the efficacy of MTX in IBD in a single paper. The meta-analysis of 10 RCTs with 767 participants showed that parenteral MTX is efficacious for maintenance of remission in Crohn's disease (CD). However, MTX for induction of remission is not effective for CD, and far more potent treatment options are available for treatment of disease flares. In UC, MTX should no longer be considered as a therapeutic option neither for induction or maintenance of remission. MTX as concomitant medication with TNF inhibitors has only been tested with infliximab (IFX) and in CD, and data does not support superiority of MTX-IFX combination therapy over IFX monotherapy.
Implications of all the available evidence
Based on the latest evidence, the use of parenteral MTX monotherapy in the management of patients with IBD should be carefully considered and restricted to situations where there is reliable evidence of benefit, that is in CD as alternative maintenance therapy to thiopurines.
Alt-text: Unlabelled box
1. Introduction
Despite the introduction of a wide range of novel therapeutic agents for the management of inflammatory bowel disease (IBD), including biologics (e.g., tumor necrosis factor [TNF] inhibitors, cell-migration inhibitors, and anti-interleukins) [1,2] and small molecules (e.g., JAK inhibitors), [3] conventional immunomodulators in the form of thiopurines and methotrexate (MTX), along with glucocorticoids, constitute the basic therapeutic armamentarium. European and American guidelines recommend MTX for active Crohn's disease (CD) as adjunctive therapy or as a steroid-sparing agent to maintain remission [4,5]. MTX is an inexpensive, well-characterized analogue of folic acid and aminopterin that targets the enzyme thymidylate synthetase and dihydrofolate reductase [6]. At high doses (i.e., ≥ 500 mg/m2), MTX was originally introduced as a chemotherapeutic agent in the 1940s. Later, lower doses of MTX was discovered to have anti-inflammatory properties and consequently was introduced for a wide range of chronic inflammatory conditions [7].
For decades, MTX has been used in the management of IBD, characteristically as a second-line immunomodulator and steroid-sparing agent in patients intolerant to thiopurines or in whom thiopurine treatment has failed [8,9]. However, current recommendations for the use of MTX in CD build on data from nearly 500 patients included in randomized, controlled trials (RCTs) compared with considerably larger cohorts documenting the effects of the thiopurine family of immunomodulators. In ulcerative colitis (UC), data have been inadequate to recommend MTX, which, nevertheless, has been widely prescribed for patients failing thiopurines equivalently to CD [10,11]. Recent state-of-the-art RCTs evaluating adequate dosages of parenterally administered MTX have, however, shed light on the efficacy of MTX monotherapy in the treatment of UC [12,13].
The objectives of this systematic review of RCTs with a preplanned meta-analysis were to investigate the clinical efficacy of MTX in IBD for induction of remission as well as maintenance of remission (defined by the studies and expressed as a percentage of the total number of patients randomized (i.e. intention-to-treat analysis)), concomitant treatment with biologics, and its side effects separated for CD and UC, respectively.
2. Methods
This systematic review and meta-analysis were performed in accordance with the guidelines from Cochrane and reported following the PRISMA guidelines, and the protocol was registered at the PROSPERO database (Reg. No. CRD42018115047) before initiation of the literature search (eAppendix 1).
2.1. Inclusion and exclusion criteria
Studies were eligible for the systematic review if they included (at least) one treatment group in which MTX was administered for IBD compared with a non-exposed control group or studies where MTX was administered as add-on treatment (e.g., MTX was given in intervention groups in addition to the control treatment). Studies in children and the non-English-language literature were excluded. Secondary publications were checked for additional data before exclusion.
2.2. Search strategy and selection criteria
The following bibliographic databases were searched without restriction on language and publication year: MEDLINE via PubMed, EMBASE via Ovid, and the Cochrane Central Register of Controlled Trials (CENTRAL), all from inception until January 1, 2020. Furthermore, the reference lists of the included studies and systematic reviews from the last five years on MTX therapy for the management of IBD were scrutinized for any further studies of relevance.
The following search strategy was performed in MEDLINE and adjusted to the respective databases: (“inflammatory bowel diseases” [MeSH] OR Crohn* [TIAB] OR ulcerative colitis* [TIAB] OR IBD [TIAB] OR Inflammatory bowel disease* [TIAB] OR proctocolitis* [TIAB] OR proctosigmoiditis* [TIAB] OR rectocolitis* [TIAB] OR rectosigmoiditis* [TIAB] OR proctitis* [TIAB]) AND (“methotrexate” [MeSH] OR methotrexate* [TIAB]) AND (randomized controlled trial [Publication Type] OR controlled clinical trial [Publication Type] OR randomized [TIAB] OR placebo [TIAB] OR drug therapy [Subheading] OR randomly [TIAB] OR trial [TIAB] OR groups [TIAB]).
2.3. Data management
A customized data-extraction form was developed for each of the outcomes (eAppendix 2). Extraction of the following data was considered mandatory: authors of the study, year of publication, design of trial, intervention characteristics, location of the trial (in the case of multicenter studies, primary investigator affiliation), number of patients allocated (to the MTX versus control groups, respectively), average patient age, average disease activity score and score applied, endoscopy score and scoring system applied, number of females within the intention-to-treat population, and duration of the study (presented in weeks).
2.3.1. Primary outcome
Efficacy of MTX was accessed based on established disease activity scores used in IBD. For CD, the Crohn's disease activity index (CDAI) score [14] (150 or below is defined as remission; response ≥70 [Response-70] or response ≥100 [Response-100]) followed by the Harvey and Bradshaw index (HBI) score [15] (remission 0–4, mild 5–7, moderate 8–16, and severe ≥17 [response ≥3 reduction]). For UC, the Mayo score [16] (maximum of 12 points; remission ≤2 [with no item >1], mild 3–5, moderate 6–9, and severe ≥10; response ≥3) or partial score (i.e., without endoscopy [maximum of 9 points]; remission 0–1, mild 2–4, moderate 5–6, and severe 7–9; response ≥2), followed by the simple clinical colitis activity index (SCCAI) [17] (maximum of 15 points including extracolonic manifestations [1 point per manifestation]; remission ≤3, mild 4–6, moderate 7–9, and severe ≥10; response ≥4).
2.3.2. Secondary outcomes
Endoscopic disease activity scores for mucosal healing, defined as an absolute subscore for endoscopy of no more than 1 for the Mayo score [16] (UC); or Crohn's disease endoscopic index of severity (CDEIS) [18], followed by the simple endoscopic score for Crohn's disease (SES-CD) [19] (both CD scores ≤2).
Discontinuation of MTX treatment because of side effects was analyzed and presented as the relative risk (RR). A relative risk larger than 1 indicates a larger risk of withdrawal in the MTX group versus the control group.
2.4. Study selection and bias assessment
Two members of the study team (OHN and CS) independently accessed titles and abstracts for study eligibility, performed data extraction and assessed risk of bias. The full text was obtained if studies were judged eligible by at least one reviewer. The same two reviewers independently judged eligibility of the retrieved full-text studies. Consensus on inclusion was reached by discussion, including CBJ and GR. Risk of bias in included studies was evaluated using the Cochrane risk of bias tool assessing whether each of the following domains were adequate (i.e., low risk of bias), unclear, or inadequate: sequence generation, concealment of allocation, blinding, incomplete outcome data addressed, selective outcome reporting.
2.5. Evidence synthesis
A meta-analysis was applied using the restricted maximum likelihood (REML) method as other methods tend to overestimate the precision of the estimate. Relative risk (RR) and 95% confidence interval (CI) were estimated on remission, relapse, endoscopic disease activity, and adverse events (AEs) in the MTX group versus the placebo (control) group.
The meta-analysis was stratified on oral or parenteral (very low (i.e. ≤ 12.5 mg/week) or low (i.e., 12.5 - 25 mg/week) dosages of MTX applied, if possible. Heterogeneity was examined as the between-study variation and calculated as the I-statistic measuring the proportion of variation (i.e., inconsistency) in the combined estimates owing to between-study variance.
An I²-value of 0% indicates no inconsistency between the results of individual trials, and an I²-value of 100% indicates maximal inconsistency [20]. For outcome measures for which a meta-analysis was not possible, the results of the individual studies was presented.
2.6. Role of the funding source
This systematic review and meta-analysis was not funded.
3. Results
3.1. Included studies into the systematic review
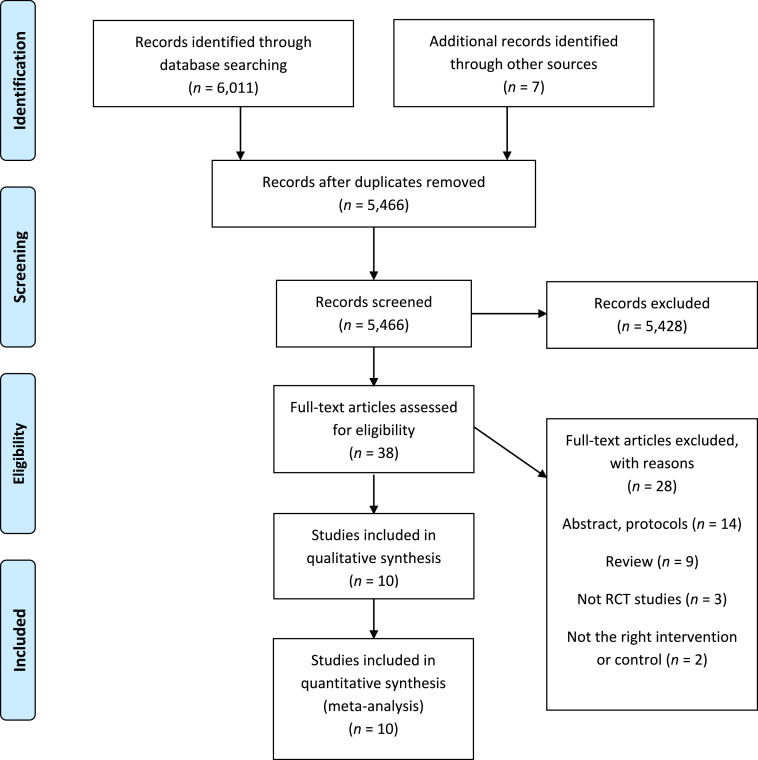
The literature search identified 6,018 papers, of which 10 RCTs were included in the quantitative evaluation (Table 1, Fig. 1). Overall the study quality was high even though the description of the method, especially the sequence generation and the allocation concealment in the older studies was inadequate, and therefore assessed as being unclear (eTable 1). Six studies, including 479 participants, evaluated the effect in CD (two investigated the effect on induction of remission [21,22], one investigated the effect on maintaining clinical remission [23], and three investigated both outcomes) [24], [25], [26]. Further, four trials compared the effect of MTX versus placebo [[21], [22], [23],25], and two examined the effect of concomitant MTX with the TNF inhibitor infliximab (IFX) versus IFX alone [24,26]. Concerning UC, four studies, including 288 participants, were identified. One study investigated induction of remission [12], two investigated maintenance of clinical remission [13,27], and one studied both outcomes [28].
Table 1.
Randomized, Controlled Trials Evaluating MTX for CD or UC.
| Reference and year | MTX dose | Disease | Objective | Severity | No. of patients | Intervention | Duration | Comedication | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Feagan 1995 [22] | 25 mg/wk parenteral | CD | Induction of remission | Active CD (CDAI > 150) for a minimum of 3 months despite a minimum of 12.5 mg prednisolone | 141 | MTX (n = 94) or placebo (n = 47) | 16 weeks | Steroid to be tapered, mesalazine, steroid enemas, antibiotics (perianal disease) | CDAI < 150 without prednisolone at week 16; MTX superior to placebo (p = 0.025) |
| Arora 1999 [21] | 15–22.5 mg/wk orally | CD | Induction of remission | Steroid-dependent CD | 33 | MTX (n = 15) or placebo (n = 18) | 12 months | Prednisolone ≥10 mg/day | MTX not superior to placebo for induction of remission (p > 0.05) |
| Oren 1997 [25] | 12.5 mg/wk orally | CD | Induction and maintenance of remission | Steroid-dependent CD | 84 | MTX (n = 26) or 6-MP (n = 32) or placebo (n = 26) | 9 months | Steroids and mesalazine | The proportions of patients entering first remission (HBI < 3) or experiencing a relapse (HBI ≥ 3) were without statistically significant differences in the three treatment arms |
| Feagan 2014 [24] | 10–25 mg/wk parenteral | CD | Induction and maintenance of remission (combination of MTX with IFX versus IFX alone) | Active CD initiated on prednisolone induction therapy | 126 | MTX + IFX (n = 63) or IFX + placebo (n = 63) | 50 weeks | IFX; prednisolone to be tapered no later than week 14 | Time to treatment failure (i.e., failure of CDAI < 150 at week 14 or failure to maintain this remission through week 50); MTX was not superior to concomitant treatment with placebo (p = 0.63) |
| Feagan 2000 [23] | 15 mg/wk parenteral | CD | Maintenance of remission | CDAI ≤ 150 at inclusion after previous MTX induction therapy | 76 | MTX (n = 40) or placebo (n = 36) | 40 weeks | Hydrocortisone ointment for perianal disease | Increase in CDAI of more than 100 points or use of rescue medication (steroid/antimetabolite) MTX superior to placebo (p = 0.04) |
| Schröder 2006 [26] |
20 mg/wk perenteral for first 5 weeks then orally | CD | Combination with IFX for induction of remission and maintenance | Active CD resistant or intolerant to thiopurines | 19 | MTX + IFX (n = 11) or IFX (n = 8) | 48 weeks | 5-ASA at doses of 4 g or more, prednisolone 40 mg/day or less (sTable 4 weeks before study entry) | CDAI < 150; time to achieve clinical remission and the corticosteroid tapering effect of treatment; MTX + IFX not superior to IFX at week 48 (p = 0.63) |
| Oren 1996 [28] | 12.5 mg/wk orally | UC | Induction and maintenance of remission | Mayo score ≥ 7 | 67 | MTX (n = 30) or placebo (n = 37) | 9 months | Mesalazine and/or steroids | Proportion entering remission/maintenance of remission, no difference (p > 0.73) |
| Carbonnel 2016 [12] |
25 mg/wk parenteral | UC | Induction of remission | Mayo score 0–12 but steroid dependent | 111 | MTX (n = 60) or placebo (n = 51) | 24 weeks | Steroid to be tapered | Mayo score ≤ 2 at week 16 without steroid; no difference (p = 0.15) |
| Onuk 1996 [27] | 15 mg/wk orally | UC | Maintenance of remission | N/A | 26 | MTX + SASP (n = 14) or MTX (n = 12) | 12 months | Sulfasalazine | Symptoms, sigmoidoscopic and histologic activity; no significant difference (p-value not stated) |
| Herfarth 2018 [13] | 25 mg/wk parenteral | UC | Maintenance of remission | Active UC nonresponding to other therapies treated with MTX open label for 16 weeks | 84 | (Only responders to 16-week induction) to placebo (n = 40) or MTX (n = 44) | 32 weeks MTX | Mesalazine 2.4 g/day | Relapse-free and combined clinical and endoscopic remission; no difference (p = 0.78) |
Fig. 1.
Study screening and selection flow diagram.
3.2. Meta-analysis of MTX in Crohn's disease
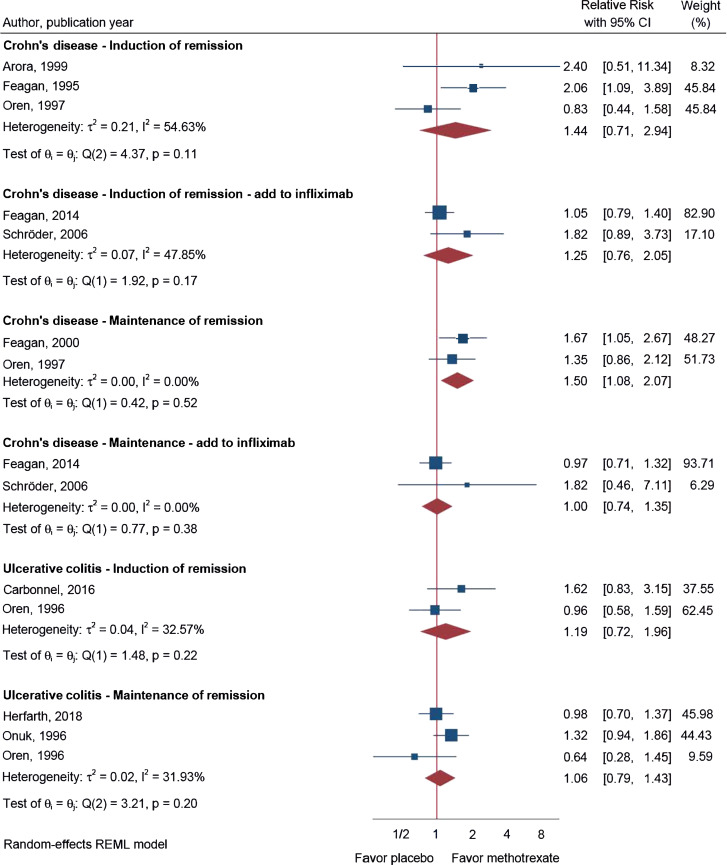
Our meta-analysis of the RCTs, presented in Fig. 2 and Table 1, showed no significant effect of MTX monotherapy in the management of CD in three RCTs investigating induction of clinical remission (primary endpoints) [21,22,25] (RR = 1.44; 95% CI 0.71–2.94; I2 = 55%). However, when investigating maintenance of clinical remission a significant effect in two RCTs was found (RR = 1.50; 95% CI 1.08–2.07; I2 = 0%) [23,25]. Nevertheless, none of the published RCTs in CD assessed endoscopic scores as secondary endpoint. No effect was observed in studies investigating the additional effect of concomitant MTX with IFX versus IFX alone on either induction of clinical remission or maintenance of clinical remission or when assessing mucosal healing by endoscopy [24,26] (Fig. 2). Moreover, the effect of MTX on induction or maintenance of endoscopic healing had not been assessed.
Fig. 2.
Use of MTX for the management of CD and UC in the context of disease activity measured on induction and maintenance of remission (primary outcome). CI, confidence interval.
3.3. Meta-analysis of MTX in ulcerative colitis
In UC, the meta-analysis of the primary endpoints showed no significant effect of MTX monotherapy in data derived from two studies investigating induction of clinical remission [12,28] (RR = 1.19; 95% CI 0.72–1.96; I2 = 33%; Fig. 2), and there was no effect in the three studies investigating maintenance of clinical remission [13,27,28] (RR = 1.06; 95% CI 0.79–1.43; I2 = 32%; Fig. 2). Regarding the secondary endpoints, endoscopic disease activity, MTX for induction (RR = 1.37; 95% CI 0.77–2.46) or maintenance (RR = 0.79; 95% CI 0.43–1.46) of steroid-free endoscopic remission was not superior to placebo. [12,13] Nevertheless, MTX combination therapy with biologics has not yet been investigated in UC.
3.4. Meta-analysis on adverse events of MTX in inflammatory bowel disease
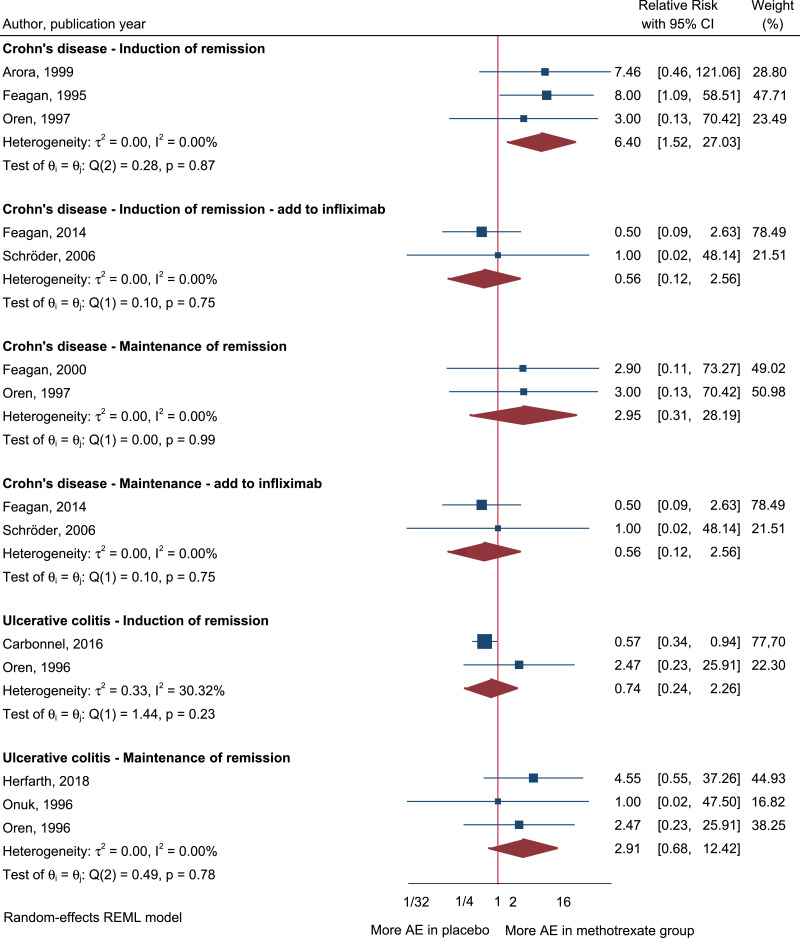
Regarding AEs, our meta-analysis on CD studies showed a significantly higher risk of AEs (defined as MTX withdrawal because of AEs) in three studies investigating induction of remission (RR = 6.40; 95% CI 1.52–27.03; I2 = 0%) [21,22,25], but no statistical differences in two studies investigating maintenance of clinical remission (RR = 2.95; 95% CI 0.31–28.19; I2 = 0%) [23,25] when comparing the risk of AEs in the MTX group versus placebo (Fig. 3). Further, no differences were observed in two studies investing the additional effect of concomitant MTX with IFX versus IFX alone [24,26] (Fig. 3). In studies investigating UC, the meta-analysis showed no differences in the risk of AEs in two studies investigating induction of remission (RR = 0.74; 95% CI 0.24–2.26; I2 = 30%) [12,28] or in three studies investigating maintenance of remission (RR = 2.91; 95% CI 0.68–12.42; I2 = 0%) [13,27,28] when comparing the MTX group with the control group.
Fig. 3.
Adverse events (AEs) reported when using parenteral MTX for the management of CD and UC. AEs were defined as withdrawal because of AEs. CI, confidence interval.
There was no difference in severe adverse events (SAEs, i.e., leading to hospitalization) between MTX and placebo as shown in eFig. 1. However, SAEs were only reported in 6 studies (three out of six studies reported SAEs in CD, and three out of four in UC). Due to the low number of reported SAEs, no significant results were found in any of the analyzed subgroups, and the 95% CI were accordingly broad (eFig. 1).
4. Discussion
This is the first systematic review and meta-analysis of the combined efficacy of MTX for induction of remission and maintenance of remission in both CD and UC, respectively, based on the latest RCTs. In the late 1980s, the first promising pilot studies were published on MTX monotherapy for IBD indicating a steroid-sparing effect and an ability to induce and maintain clinical remission or even mucosal healing in patients with either steroid-refractory or steroid-dependent disease [29,30]. Over the years, several uncontrolled observational studies [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], along with minor RCTs have indicated a benefit of MTX in particular among patients with CD failing thiopurines, but only three state-of-the-art RCTs have supported its clinical efficacy in CD [22], [23], [24].
As concerns dosage used in the 10 RCTs included in the quantitative evaluation, in CD two of the older studies (published before 2000) an oral administration was used [21,25], whereas all studies (both before and after year of 2000) from Feagan et al. [22], [23], [24] used parenteral administration. The dose of MTX ranged from 10 to 25 mg/week with no clear tendency to have changed over time. In the older studies of UC published before 2000 [27, 28] an oral administration was used in contrast to the newer ones (after 2010) [12,13] which used parenteral administration. Here the dose of MTX, however, changed over time from 12.5–15 mg/week in older studies [27,28] to 25 mg/week in the newer ones, including maintenance of remission [12,13].
This meta-analysis shows that MTX monotherapy is not superior to placebo for induction of clinical (i.e., symptomatic) remission in CD when defined as CDAI < 150 (primary outcome). However, the evidence of no effect of MTX for induction of remission may be low due to heterogeneity and sparse number of studies. Nevertheless, more efficient agents with better safety profiles are available for this indication, and most European and American guidelines do not recommend MTX monotherapy to induce remission in CD [4,41], although a recent Canadian guideline for management of luminal CD made a conditional recommendation based on very low-quality evidence that parenteral MTX may be employed in corticosteroid-dependent/resistant CD to induce remission [42].
For maintenance of clinical remission in patients with CD, however, this meta-analysis concludes that MTX monotherapy is superior to placebo. This is also reflected by European and American guidelines, where MTX is advised as a maintenance agent in CD [4,41]. Further, parenteral dosages of MTX below 15 mg have not been shown to be effective for maintenance of clinical remission, although the minimal dose of MTX required to maintain remission has not been adequately investigated [41]. Of note, from the meta-analysis it seems that oral administration of MTX have not proven to be effective for maintenance of remission in CD.
Whereas the efficacy of MTX monotherapy has been reasonably well documented in CD, until recently its effectiveness in UC has been equivocal because of several uncontrolled observational studies characterized by small sample sizes, suboptimal oral doses of MTX, poorly designed inclusion criteria, and overall less encouraging findings [10,27,29,30,33,40,[43], [44], [45]]. Despite such failings, MTX therapy has been applied in UC equivalently to CD under the assumption that MTX inhibits dihydrofolate reductase and thus restricts DNA, RNA, and protein synthesis, which is equally effective in both diseases [10,11]. However, because the mechanism of action of MTX dosages of ≤25 mg/week is more uncertain than the crude cytotoxic effect employed for malignancies with substantially higher doses [46], and because the inflammatory mechanisms differ between UC and CD, this extrapolation may have been unsubstantiated.
Until recently, well-established disease activity indices such as the CDAI [14] (and the Mayo score [16] for UC) have been used as “gold standards” to evaluate therapeutic efficacies of drugs, including MTX. Nonetheless, advances in field of endoscopy have expanded our knowledge of the relatively poor agreement between these partially symptom-driven indices and objective endoscopic findings of the degree of intestinal inflammation. Furthermore, these indices have been shown to be prone to intra- and interindividual variability [47], [48], [49]. This has lately resulted in a paradigm shift in disease monitoring and trial reporting in IBD because regulatory authorities now require efficacy to be documented by healing of the intestinal mucosa, which is considered a more appropriate clinically and prognostically relevant outcome measure [50,51]. Only limited and low-quality data exist on the ability of MTX monotherapy to induce and maintain endoscopic healing in CD, and these data have thus far not been encouraging [30,[52], [53], [54]]. Thus recommendations for the use of MTX in CD are based on symptomatic improvements that have not been backed up by objective endoscopic documentation of treatment efficacy.
Efficacy of MTX in UC was recently assessed in two landmark RCTs including endoscopic assessment of mucosal healing: the METEOR trial [12] and the MERIT-UC trial [13]. Although METEOR provided uncertain results regarding the efficacy of MTX as an induction therapy for UC (the primary endpoint as well as nine of 10 secondary endpoints failed), it could be argued that MTX may be used to provide a bridge to another agent for maintenance therapy by reducing the symptoms of active disease and the need for glucocorticoids. Nevertheless, the results of the MERIT-UC study exclude any role of MTX in maintaining remission in UC [13]. Of note, MTX monotherapy did not provide higher rates of endoscopic healing than placebo in UC.
Three underpowered RCTs of which two used an oral dose of MTX, have compared the efficacy of MTX with that of thiopurines in inducing and maintaining remission in patients with CD. Although these studies indicated a comparable efficacy and a similar time to onset of action [25,44,55], thiopurines have generally been preferred over MTX in the treatment of CD because of their oral administration and better levels of efficacy and safety evidenced.
No data exist on the efficacy of MTX in the postoperative prophylaxis of CD. Preoperative MTX treatment has not been associated with an increased risk of postoperative complications [56]. Apart from case series [26,[57], [58], [59]] and a subgroup analysis of a tiny RCT comparing azathioprine and MTX for induction of fistula closure with similar remission rates observed [55], no data are available on the efficacy of MTX in the treatment of fistulizing CD [60].
Because MTX monotherapy is a keystone in the management of rheumatic disorders, including peripheral arthritis and axial arthropathies [61], it can be tried as a primary immunosuppressive agent in patients with CD [62] in order to target both joints and gut [63], although this has not been investigated in RCTs. Likewise, MTX is not being associated with development of malignancies and may be preferred over thiopurines in patients with CD and a previous history of cancer, whereas thiopurines are well-known to increase risks of lymphomas and skin cancers [64,65].
As concerns the efficacy of MTX as concomitant therapy with TNF inhibitors, the COMMIT RCT investigated treatment outcomes of IFX monotherapy (standard induction of 5 mg/kg at weeks 0, 2, and 6 followed by maintenance therapy of 5 mg/kg every 8 weeks; n = 63) versus MTX-IFX combination therapy (25 mg subcutaneously weekly; n = 63) for 50 weeks and with prednisone tapering no later than week 14 [24]. The primary outcome was time to treatment failure, defined as failure to enter prednisone-free remission (CDAI < 150) at week 14 or failure to maintain this remission through week 50. The actuarial rate of treatment failure was 31% in the combined MTX-IFX therapy group, and 30% in the IFX monotherapy group, and secondary endpoints were all negative as well [24]. Thus, at week 52, 56% of patients in combination treatment maintained remission versus 57% in the IFX group [24]. Endoscopic outcomes were not assessed. However, some lower-quality studies have revealed comparable findings [26,66]. Moreover, a recent retrospective observational real-world study concluded that concomitant MTX-IFX therapy for at least three months was inferior to IFX-thiopurine combined therapy [67]. Finally, a retrospective study found that very low-dose MTX (i.e. ≤ 12.5 mg/week) was equally efficient to the typical anti-inflammatory dose of MTX (12.5–25 mg/week) in combination with TNF inhibitors [68].
Although improved efficacy of combination therapy with MTX and biologics remains to be shown, it appears that MTX to some extent can suppress antibodies against IFX and/or increase IFX trough levels in CD [24,[69], [70], [71], [72], [73]]. This phenomenon has been mechanistically related to a possible interaction between MTX and B-cell activating factor (BAFF) [74]. Initiation of combination therapy with MTX in addition to continued treatment with an existing TNF inhibitor has been reported to potentially reverse antidrug antibody–positive status, increase blood levels, and reestablish clinical efficacy [70], [71], [72]. However, if used for this indication, potential side effects to both MTX and continued IFX therapy in the presence of anti-IFX antibodies should be balanced against switching to another biologic agent. MTX combination therapy has so far not been examined for UC or for other biologics [6,75,76].
The strength of this meta-analysis is the comprehensive search strategy in relevant databases and that study-selection and risk of bias evaluation was performed by two reviewers independently. Moreover, all RCTs on MTX for IBD in adults published up to 2020 were included, and state-of-the-art statistics (i.e. the restricted maximum likelihood (REML) method) has been applied. However, the review at the same time has some limitations due to the design of the underlying RCTs which were eligible for inclusion. First, the intervention period was not consistent among studies (i.e., a variation from 24 weeks to 1 year was observed). Second, risk of bias from random sequence generation, concealment of allocation, blinding, incomplete outcome addressed, and selective outcome reporting varied among the RCTs included (eTable 1). Third, heterogeneity was seen among the results of the individual trials, and few studies reported on disease activity and side effects defined as withdrawal because of AEs. Fourth, concomitant therapy with IFX was reported in only two of 10 studies (and in CD only), and in an attempt to reduce heterogeneity, subgroup analyses were performed with or without combined therapy for induction of remission or maintenance of clinical remission among patients with CD, resulting in very few studies for each meta-analysis. Publications bias (i.e. small study bias) was not investigated due the small number of studies, however, visual inspection of the forest plot did not indicate that small study bias influenced the interpretation of the results.
The sum of available evidence extracted from this meta-analysis supports the effectiveness of MTX monotherapy (parenterally at 15–25 mg/weekly) in maintaining clinical remission of CD, whereas its role in induction of clinical remission is doubtful. However, any future studies on CD would benefit from inclusion of mucosal healing in the scoring systems applied. Moreover, for some special indications such as extraintestinal rheumatic manifestations of peripheral arthritis and axial arthropathies or in case of previous malignancies, MTX may be considered as a useful primary maintenance agent of CD, although RCTs in the field are still missing. Finally, more evidence is needed to assess the potential benefit of MTX as a concomitant agent to improve the clinical efficacy and/or pharmacokinetics of different types of biologic agents in IBD.
In conclusion considering the advent of recent high-quality RCTs on patients with UC, including both symptomatic and endoscopic endpoints as recommended by regulatory agencies on both sides of the Atlantic [77,78], data from this systematic review and meta-analysis indicate that it is time to critically readdress the use of MTX in the management of IBD. Thus, when collectively considering this efficacy in adults (i.e., patients of 18 years or older), including lack of data on mucosal healing, safety, and compliance, it is recommended that MTX is being reserved primarily as an option for maintaining clinical remission in CD as an alternative to thiopurines. Even though it cannot be completely ruled out that MTX may have some limited benefits over placebo in the symptomatic treatment of flaring UC, the availability of far more potent and well-documented agents that can induce mucosal healing, including biologics [79] and novel small-molecule drugs [2], renders MTX—based on this systematic review and meta-analysis—not to be confirmed efficient in the treatment of acute flares of IBD. Furthermore, MTX is not confirmed to be effective in the maintenance of clinical or endoscopic remission in UC.
Declaration of Competing Interest
The authors have no competing interests to declare as concerns MTX. In general, OHN, CS, CBJ have no conflicts of interest to disclose. Gerhard Rogler has consulted to Abbvie, Augurix, BMS, Boehringer, Calypso, Celgene, FALK, Ferring, Fisher, Genentech, Gilead, Janssen, MSD, Novartis, Pfizer, Phadia, Roche, UCB, Takeda, Tillots, Vifor, Vital Solutions and Zeller; received speaker's honoraria from Astra Zeneca, Abbvie, FALK, Janssen, MSD, Pfizer, Phadia, Takeda, Tillots, UCB, Vifor and Zeller; and educational grants and research grants from Abbvie, Ardeypharm, Augurix, Calypso, FALK, Flamentera, MSD, Novartis, Pfizer, Roche, Takeda, Tillots, UCB and Zeller.
Acknowledgments
Author contributions
OHN, CS, CBJ and GR had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. This study was not funded. Study concept and design: All authors. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: OHN. Critical revision of the manuscript for important intellectual content: CS, CBJ, GR. Statistical analysis: CBJ. Study supervision: CS, CBJ, GR. All authors approved the final version before submission.
Acknowledgements
The authors are grateful to Susanne Knygberg Christensen and Lisa Rohbach for skillful secretarial assistance. This study was not funded.
Funding
No funding was received.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100271.
Appendix. Supplementary materials
References
- 1.Nielsen O.H., Ainsworth M.A. Biosimilars for management of Crohn disease. Ann Intern Med. 2019;170:129–130. doi: 10.7326/M18-3060. [DOI] [PubMed] [Google Scholar]
- 2.Coskun M., Vermeire S., Nielsen O.H. Novel targeted therapies for inflammatory bowel disease. Trends Pharmacol Sci. 2017;38:127–142. doi: 10.1016/j.tips.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Soendergaard C., Bergenheim F.H., Bjerrum J.T., Nielsen O.H. Targeting JAK-STAT signal transduction in IBD. Pharmacol Ther. 2018;192:100–111. doi: 10.1016/j.pharmthera.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Gomollon F., Dignass A., Annese V. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. 2017;11:3–25. doi: 10.1093/ecco-jcc/jjw168. [DOI] [PubMed] [Google Scholar]
- 5.Lichtenstein G.R., Loftus E.V., Isaacs K.L., Regueiro M.D., Gerson L.B., Sands B.E. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol. 2018;113:481–517. doi: 10.1038/ajg.2018.27. [DOI] [PubMed] [Google Scholar]
- 6.Coskun M., Steenholdt C., de Boer N.K., Nielsen O.H. Pharmacology and optimization of thiopurines and methotrexate in inflammatory bowel disease. Clin Pharmacokinet. 2016;55:257–274. doi: 10.1007/s40262-015-0316-9. [DOI] [PubMed] [Google Scholar]
- 7.Weinstein G.D. Methotrexate. Ann Intern Med. 1977;86:199–204. doi: 10.7326/0003-4819-86-2-199. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen O.H., Bjerrum J.T., Herfarth H., Rogler G. Recent advances using immunomodulators for inflammatory bowel disease. J Clin Pharmacol. 2013;53:575–588. doi: 10.1002/jcph.2. [DOI] [PubMed] [Google Scholar]
- 9.Kozarek R.A., Patterson D.J., Gelfand M.D., Botoman V.A., Ball T.J., Wilske K.R. Methotrexate induces clinical and histologic remission in patients with refractory inflammatory bowel disease. Ann Intern Med. 1989;110:353–356. doi: 10.7326/0003-4819-110-5-353. [DOI] [PubMed] [Google Scholar]
- 10.Harbord M., Eliakim R., Bettenworth D. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. part 2: current management. J Crohns Colitis. 2017;11:769–784. doi: 10.1093/ecco-jcc/jjx009. [DOI] [PubMed] [Google Scholar]
- 11.Kornbluth A., Sachar D.B. Ulcerative colitis practice guidelines in adults: American college of gastroenterology, practice parameters committee. Am J Gastroenterol. 2010;105:501–523. doi: 10.1038/ajg.2009.727. [DOI] [PubMed] [Google Scholar]
- 12.Carbonnel F., Colombel J.F., Filippi J. Methotrexate is not superior to placebo for inducing steroid-free remission, but induces steroid-free clinical remission in a larger proportion of patients with ulcerative colitis. Gastroenterology. 2016;150:380–388. doi: 10.1053/j.gastro.2015.10.050. [DOI] [PubMed] [Google Scholar]
- 13.Herfarth H., Barnes E.L., Valentine J.F. Methotrexate is not superior to placebo in maintaining steroid-free response or remission in ulcerative colitis. Gastroenterology. 2018;155:1098–1108. doi: 10.1053/j.gastro.2018.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Best W.R., Becktel J.M., Singleton J.W., Kern F., Jr. Development of a Crohn’s disease activity index. National cooperative Crohn’s disease study. Gastroenterology. 1976;70:439–444. [PubMed] [Google Scholar]
- 15.Harvey R.F., Bradshaw J.M. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder K.W., Tremaine W.J., Ilstrup D.M. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–1629. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- 17.Walmsley R.S., Ayres R.C., Pounder R.E., Allan R.N. A simple clinical colitis activity index. Gut. 1998;43:29–32. doi: 10.1136/gut.43.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mary J.Y., Modigliani R. Development and validation of an endoscopic index of the severity for Crohn’s disease: a prospective multicentre study. Groupe d’Etudes Therapeutiques des Affections Inflammatoires du Tube Digestif (GETAID) Gut. 1989;30:983–989. doi: 10.1136/gut.30.7.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daperno M., D’Haens G., Van A.G. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60:505–512. doi: 10.1016/s0016-5107(04)01878-4. [DOI] [PubMed] [Google Scholar]
- 20.Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med. 2000;19:3127–3131. doi: 10.1002/1097-0258(20001130)19:22<3127::aid-sim784>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 21.Arora S., Katkov W., Cooley J. Methotrexate in Crohn’s disease: results of a randomized, double-blind, placebo-controlled trial. Hepatogastroenterology. 1999;46:1724–1729. [PubMed] [Google Scholar]
- 22.Feagan B.G., Rochon J., Fedorak R.N. Methotrexate for the treatment of Crohn’s disease. The North American Crohn’s study group investigators. N Engl J Med. 1995;332:292–297. doi: 10.1056/NEJM199502023320503. [DOI] [PubMed] [Google Scholar]
- 23.Feagan B.G., Fedorak R.N., Irvine E.J. A comparison of methotrexate with placebo for the maintenance of remission in Crohn’s disease. North American Crohn’s study group investigators. N Engl J Med. 2000;342:1627–1632. doi: 10.1056/NEJM200006013422202. [DOI] [PubMed] [Google Scholar]
- 24.Feagan B.G., McDonald J.W., Panaccione R. Methotrexate in combination with infliximab is no more effective than infliximab alone in patients with Crohn’s disease. Gastroenterology. 2014;146:681–688. doi: 10.1053/j.gastro.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 25.Oren R., Moshkowitz M., Odes S. Methotrexate in chronic active Crohn’s disease: a double-blind, randomized, Israeli multicenter trial. Am J Gastroenterol. 1997;92:2203–2209. [PubMed] [Google Scholar]
- 26.Schroder O., Blumenstein I., Stein J. Combining infliximab with methotrexate for the induction and maintenance of remission in refractory Crohn’s disease: a controlled pilot study. Eur J Gastroenterol Hepatol. 2006;18:11–16. doi: 10.1097/00042737-200601000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Onuk M.D., Kaymakoglu S., Demir K. Low-dosage weekly methotrexate therapy in remission maintenance in ulcerative colitis. Gut. 1996;39(suppl. 3):A75. [Google Scholar]
- 28.Oren R., Arber N., Odes S. Methotrexate in chronic active ulcerative colitis: a double-blind, randomized, Israeli multicenter trial. Gastroenterology. 1996;110:1416–1421. doi: 10.1053/gast.1996.v110.pm8613046. [DOI] [PubMed] [Google Scholar]
- 29.Baron T.H., Truss C.D., Elson C.O. Low-dose oral methotrexate in refractory inflammatory bowel disease. Dig Dis Sci. 1993;38:1851–1856. doi: 10.1007/BF01296109. [DOI] [PubMed] [Google Scholar]
- 30.Kozarek R.A., Patterson D.J., Gelfand M.D., Botoman V.A., Ball T.J., Wilske K.R. Methotrexate induces clinical and histologic remission in patients with refractory inflammatory bowel disease. Ann Intern Med. 1989;110:353–356. doi: 10.7326/0003-4819-110-5-353. [DOI] [PubMed] [Google Scholar]
- 31.Chong R.Y., Hanauer S.B., Cohen R.D. Efficacy of parenteral methotrexate in refractory Crohn’s disease. Aliment Pharmacol Ther. 2001;15:35–44. doi: 10.1046/j.1365-2036.2001.00908.x. [DOI] [PubMed] [Google Scholar]
- 32.Domenech E., Manosa M., Navarro M. Long-term methotrexate for Crohn’s disease: safety and efficacy in clinical practice. J Clin Gastroenterol. 2008;42:395–399. doi: 10.1097/MCG.0b013e31802e6875. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Lama Y., Taxonera C., Lopez-Sanroman A. Methotrexate in inflammatory bowel disease: a multicenter retrospective study focused on long-term efficacy and safety. The Madrid experience. Eur J Gastroenterol Hepatol. 2012;24:1086–1091. doi: 10.1097/MEG.0b013e3283556db5. [DOI] [PubMed] [Google Scholar]
- 34.Hausmann J., Zabel K., Herrmann E., Schroder O. Methotrexate for maintenance of remission in chronic active Crohn’s disease: long-term single-center experience and meta-analysis of observational studies. Inflamm Bowel Dis. 2010;16:1195–1202. doi: 10.1002/ibd.21166. [DOI] [PubMed] [Google Scholar]
- 35.Lemann M., Chamiot-Prieur C., Mesnard B. Methotrexate for the treatment of refractory Crohn’s disease. Aliment Pharmacol Ther. 1996;10:309–314. doi: 10.1111/j.0953-0673.1996.00309.x. [DOI] [PubMed] [Google Scholar]
- 36.Lemann M., Zenjari T., Bouhnik Y. Methotrexate in Crohn’s disease: long-term efficacy and toxicity. Am J Gastroenterol. 2000;95:1730–1734. doi: 10.1111/j.1572-0241.2000.02190.x. [DOI] [PubMed] [Google Scholar]
- 37.Nathan D.M., Iser J.H., Gibson P.R. A single center experience of methotrexate in the treatment of Crohn’s disease and ulcerative colitis: a case for subcutaneous administration. J Gastroenterol Hepatol. 2008;23:954–958. doi: 10.1111/j.1440-1746.2007.05006.x. [DOI] [PubMed] [Google Scholar]
- 38.Seinen M.L., Ponsioen C.Y., de Boer N.K. Sustained clinical benefit and tolerability of methotrexate monotherapy after thiopurine therapy in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2013;11:667–672. doi: 10.1016/j.cgh.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 39.Suares N.C., Hamlin P.J., Greer D.P., Warren L., Clark T., Ford A.C. Efficacy and tolerability of methotrexate therapy for refractory Crohn’s disease: a large single-centre experience. Aliment Pharmacol Ther. 2012;35:284–291. doi: 10.1111/j.1365-2036.2011.04925.x. [DOI] [PubMed] [Google Scholar]
- 40.Wahed M., Louis-Auguste J.R., Baxter L.M. Efficacy of methotrexate in Crohn’s disease and ulcerative colitis patients unresponsive or intolerant to azathioprine /mercaptopurine. Aliment Pharmacol Ther. 2009;30:614–620. doi: 10.1111/j.1365-2036.2009.04073.x. [DOI] [PubMed] [Google Scholar]
- 41.Dassopoulos T., Sultan S., Falck-Ytter Y.T., Inadomi J.M., Hanauer S.B. American Gastroenterological Association Institute technical review on the use of thiopurines, methotrexate, and anti-TNF-alpha biologic drugs for the induction and maintenance of remission in inflammatory Crohn’s disease. Gastroenterology. 2013;145:1464–1478. doi: 10.1053/j.gastro.2013.10.046. [DOI] [PubMed] [Google Scholar]
- 42.Panaccione R., Steinhart A.H., Bressler B. Canadian association of gastroenterology clinical practice guideline for the management of luminal Crohn’s disease. Clin Gastroenterol Hepatol. 2019;17:1680–1713. doi: 10.1016/j.cgh.2019.02.043. [DOI] [PubMed] [Google Scholar]
- 43.Khan N., Abbas A.M., Moehlen M., Balart L. Methotrexate in ulcerative colitis: a nationwide retrospective cohort from the veterans affairs health care system. Inflamm Bowel Dis. 2013;19:1379–1383. doi: 10.1097/MIB.0b013e31828133e8. [DOI] [PubMed] [Google Scholar]
- 44.Mate-Jimenez J., Hermida C., Cantero-Perona J., Moreno-Otero R. 6-mercaptopurine or methotrexate added to prednisone induces and maintains remission in steroid-dependent inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2000;12:1227–1233. doi: 10.1097/00042737-200012110-00010. [DOI] [PubMed] [Google Scholar]
- 45.Paoluzi O.A., Pica R., Marcheggiano A. Azathioprine or methotrexate in the treatment of patients with steroid-dependent or steroid-resistant ulcerative colitis: results of an open-label study on efficacy and tolerability in inducing and maintaining remission. Aliment Pharmacol Ther. 2002;16:1751–1759. doi: 10.1046/j.1365-2036.2002.01340.x. [DOI] [PubMed] [Google Scholar]
- 46.Chan E.S., Cronstein B.N. Methotrexate–how does it really work. Nat Rev Rheumatol. 2010;6:175–178. doi: 10.1038/nrrheum.2010.5. [DOI] [PubMed] [Google Scholar]
- 47.Colombel J.F., Panaccione R., Bossuyt P. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2017;390:2779–2789. doi: 10.1016/S0140-6736(17)32641-7. [DOI] [PubMed] [Google Scholar]
- 48.Levesque B.G., Sandborn W.J., Ruel J., Feagan B.G., Sands B.E., Colombel J.F. Converging goals of treatment of inflammatory bowel disease from clinical trials and practice. Gastroenterology. 2015;148:37–51. doi: 10.1053/j.gastro.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 49.Peyrin-Biroulet L., Sandborn W., Sands B.E. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110:1324–1338. doi: 10.1038/ajg.2015.233. [DOI] [PubMed] [Google Scholar]
- 50.Bojic D., Bodger K., Travis S. Patient reported outcome measures (PROMs) in inflammatory bowel disease: new data. J Crohns Colitis. 2017;11:S576–S585. doi: 10.1093/ecco-jcc/jjw187. [DOI] [PubMed] [Google Scholar]
- 51.Singh S. PROMises made, PROMises to be kept: patient-Reported outcome measures in inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2018;16:624–626. doi: 10.1016/j.cgh.2018.01.032. [DOI] [PubMed] [Google Scholar]
- 52.Laharie D., Reffet A., Belleannee G. Mucosal healing with methotrexate in Crohn’s disease: a prospective comparative study with azathioprine and infliximab. Aliment Pharmacol Ther. 2011;33:714–721. doi: 10.1111/j.1365-2036.2010.04569.x. [DOI] [PubMed] [Google Scholar]
- 53.Rouiller-Braunschweig C., Fournier N., Pittet V., Dudler J., Michetti P. Efficacy, safety and mucosal healing of methotrexate in a large longitudinal cohort of inflammatory bowel disease patients. Digestion. 2017;96:220–227. doi: 10.1159/000482007. [DOI] [PubMed] [Google Scholar]
- 54.Manosa M., Naves J.E., Leal C. Does methotrexate induce mucosal healing in Crohn’s disease? Inflamm Bowel Dis. 2010;16:377–378. doi: 10.1002/ibd.21015. [DOI] [PubMed] [Google Scholar]
- 55.Ardizzone S., Bollani S., Manzionna G., Imbesi V., Colombo E., Bianchi P.G. Comparison between methotrexate and azathioprine in the treatment of chronic active Crohn’s disease: a randomised, investigator-blind study. Dig Liver Dis. 2003;35:619–627. doi: 10.1016/s1590-8658(03)00372-4. [DOI] [PubMed] [Google Scholar]
- 56.Afzali A., Park C.J., Zhu K. Preoperative use of methotrexate and the risk of early postoperative complications in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016;22:1887–1895. doi: 10.1097/MIB.0000000000000780. [DOI] [PubMed] [Google Scholar]
- 57.Mahadevan U., Marion J.F., Present D.H. Fistula response to methotrexate in Crohn’s disease: a case series. Aliment Pharmacol Ther. 2003;18:1003–1008. doi: 10.1046/j.1365-2036.2003.01790.x. [DOI] [PubMed] [Google Scholar]
- 58.Soon S.Y., Ansari A., Yaneza M., Raoof S., Hirst J., Sanderson J.D. Experience with the use of low-dose methotrexate for inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2004;16:921–926. doi: 10.1097/00042737-200409000-00018. [DOI] [PubMed] [Google Scholar]
- 59.Scharl M., Rogler G., Biedermann L. Fistulizing Crohn’s disease. Clin Transl Gastroenterol. 2017;8:e106. doi: 10.1038/ctg.2017.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sandborn W.J., Fazio V.W., Feagan B.G., Hanauer S.B. AGA technical review on perianal Crohn’s disease. Gastroenterology. 2003;125:1508–1530. doi: 10.1016/j.gastro.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 61.Larsen S., Bendtzen K., Nielsen O.H. Extraintestinal manifestations of inflammatory bowel disease: epidemiology, diagnosis, and management. Ann Med. 2010;42:97–114. doi: 10.3109/07853890903559724. [DOI] [PubMed] [Google Scholar]
- 62.Lopez-Olivo M.A., Siddhanamatha H.R., Shea B., Tugwell P., Wells G.A., Suarez-Almazor M.E. Methotrexate for treating rheumatoid arthritis. Cochrane Database Syst Rev. 2014 doi: 10.1002/14651858.CD000957.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vavricka S.R., Schoepfer A., Scharl M., Lakatos P.L., Navarini A., Rogler G. Extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:1982–1992. doi: 10.1097/MIB.0000000000000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khan N., Lee H., Trivedi C. Mortality associated with development of squamous cell cancer in patients with inflammatory bowel disease receiving treatment with thiopurines. Clin Gastroenterol Hepatol. 2019;17:2262–2268. doi: 10.1016/j.cgh.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 65.Lemaitre M., Kirchgesner J., Rudnichi A. Association between use of thiopurines or tumor necrosis factor antagonists alone or in combination and risk of lymphoma in patients with inflammatory bowel disease. JAMA. 2017;318:1679–1686. doi: 10.1001/jama.2017.16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sokol H., Seksik P., Carrat F. Usefulness of co-treatment with immunomodulators in patients with inflammatory bowel disease treated with scheduled infliximab maintenance therapy. Gut. 2010;59:1363–1368. doi: 10.1136/gut.2010.212712. [DOI] [PubMed] [Google Scholar]
- 67.Vasudevan A., Raghunath A., Anthony S. Higher mucosal healing with tumor necrosis factor inhibitors in combination with thiopurines compared to methotrexate in Crohn’s disease. Dig Dis Sci. 2019;64:1622–1631. doi: 10.1007/s10620-018-5422-8. [DOI] [PubMed] [Google Scholar]
- 68.Borren N.Z., Luther J., Colizzo F.P., Garber J.G., Khalili H., Ananthakrishnan A.N. Low-dose methotrexate has similar outcomes to high-dose methotrexate in combination with anti-TNF therapy in inflammatory bowel diseases. J Crohns Colitis. 2019;13:990–995. doi: 10.1093/ecco-jcc/jjz020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kennedy N.A., Heap G.A., Green H.D. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. 2019;4:341–353. doi: 10.1016/S2468-1253(19)30012-3. [DOI] [PubMed] [Google Scholar]
- 70.Ben-Horin S., Waterman M., Kopylov U. Addition of an immunomodulator to infliximab therapy eliminates antidrug antibodies in serum and restores clinical response of patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:444–447. doi: 10.1016/j.cgh.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 71.Strik A.S., van den Brink G.R., Ponsioen C., Mathot R., Lowenberg M., D'Haens G.R. Suppression of anti-drug antibodies to infliximab or adalimumab with the addition of an immunomodulator in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2017;45:1128–1134. doi: 10.1111/apt.13994. [DOI] [PubMed] [Google Scholar]
- 72.Ungar B., Kopylov U., Engel T. Addition of an immunomodulator can reverse antibody formation and loss of response in patients treated with adalimumab. Aliment Pharmacol Ther. 2017;45:276–282. doi: 10.1111/apt.13862. [DOI] [PubMed] [Google Scholar]
- 73.Vermeire S., Noman M., Van Assche G., Baert F., D’Haens G., Rutgeerts P. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn’s disease. Gut. 2007;56:1226–1231. doi: 10.1136/gut.2006.099978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bitoun S., Nocturne G., Ly B. Methotrexate and BAFF interaction prevents immunization against TNF inhibitors. Ann Rheum Dis. 2018;77:1463–1470. doi: 10.1136/annrheumdis-2018-213403. [DOI] [PubMed] [Google Scholar]
- 75.Bots S., Gecse K., Barclay M., D’Haens G. Combination immunosuppression in IBD. Inflamm Bowel Dis. 2018;24:539–545. doi: 10.1093/ibd/izx065. [DOI] [PubMed] [Google Scholar]
- 76.Herfarth H.H., Kappelman M.D., Long M.D., Isaacs K.L. Use of methotrexate in the treatment of inflammatory bowel diseases. Inflamm Bowel Dis. 2016;22:224–233. doi: 10.1097/MIB.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ulcerative Colitis: Clinical trial endpoints guidance for industry. 2019: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/ulcerative-colitis-clinical-trial-endpoints-guidance-industry (Assessed Jan 10, 2020).
- 78.European Medicines Agency (EMA)Guideline on the development of new medicinal products for the treatment of ulcerative colitis. 2018: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_001026.jsp&mid=WC0b01ac0580032ec6 (Assessed Jan 10, 2020).
- 79.Nielsen O.H., Ainsworth M.A. Tumor necrosis factor inhibitors for inflammatory bowel disease. N Engl J Med. 2013;369:754–762. doi: 10.1056/NEJMct1209614. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.