Abstract
Background
Faecal Microbiota Transplant (FMT) has improved outcomes for the treatment of Clostridioides difficile infection (CDI) compared to antibiotic therapy. FMT is classified as a medicinal product in the United Kingdom, similar to the USA and Canada, limiting supply via stool banks without appropriate licencing. In the largest UK cohort to date, we describe the clinical outcomes for 124 patients receiving FMT for recurrent or refractory CDI and present a framework to produce FMT as a licenced medicinal product.
Methods
Anonymous unrelated healthy donors, screened via health assessment and microbiological testing donated stool. In aerobic conditions FMT aliquots were prepared for immediate use or frozen storage, following a production framework developed to comply with Good Manufacturing Practice. Outcome measures were clinical response to FMT defined as resolution of diarrhoea within seven days and clinical cure defined as response without diarrhoea recurrence at 90 days.
Findings
Clinical response was 83·9% (95% CI 76·0%–90·0%) after one treatment. Clinical cure was 78·2% (95% CI 67·4%–89·0%) across the cohort. Refractory cases appeared to have a lower initial clinical response rate compared to recurrent cases, however at day 90 there were no differences observed between these groups.
Interpretation
The methodology developed here enabled successful licencing of FMT by The Medicines and Healthcare products Regulatory Agency as a medicinal product. This has widened the availability of FMT in the National Health Service via a stool bank and can be applied in other centres across the world to improve access to safe and quality assured treatments.
Research in context.
Evidence before this study
FMT has been used mainly for the treatment of recurrent CDI. Small numbers of refractory and severe cases have been successfully treated; however the data in this area is limited. Several different methodologies have been described for FMT production, nonetheless generally comparable efficacy rates have been reported across the literature. Most studies do not discuss important aspects of processing pertaining to successful regulation. Lack of guidance in this area can limit the establishment and supply of FMT.
Added value of this study
The clinical cohort described in this study adds to the evidence of FMT use in antibiotic refractory CDI cases. In addition the detailed methodology and procedures presented provide a proven framework for the production of FMT as a licenced medicinal product to meet regulatory requirements.
Implications of all the available evidence
Combining the presented framework alongside published guidelines and the existing clinical evidence base, will enable the development of licenced FMT services, ensuring the clinical governance of FMT supply. This will allow more equitable access to treatment and support future research for other conditions such as Inflammatory Bowel Disease, Graft versus Host Disease and other non-gastrointestinal disorders associated with gut microbiome disturbance.
Alt-text: Unlabelled box
1. Introduction
Clostridioides difficile infection (CDI) is the leading cause of antibiotic associated gastrointestinal disease causing significant morbidity and mortality [1]. Clinical response to antibiotic treatment of CDI is 80% in the primary episode [2] and rapidly falls to only 30–40% in recurrent disease [3]. Faecal Microbiota Transplant (FMT) is highly successful for the treatment of recurrent CDI with significantly improved efficacy rates compared to antibiotic therapy [4,5]. It has also been used for the treatment of severe CDI [6]. The National Institute for Healthcare and Excellence recommend FMT for the treatment of recurrent CDI, in patients failing to respond to antibiotics and other treatments [7].
Prior to 2015, it was believed that FMT would fall under the Human Tissue (Quality and Safety for Human Application) Regulations 2007 and be regulated by the Human Tissue Authority (HTA). This perspective changed in England in 2015, when FMT was defined as a medicinal product under The Human Medicines Regulations 2012 [8]. In the USA and Canada, FMT is considered a biologic drug, while in Europe, without a formal position from the European Medicines Agency, a mixture of regulatory frameworks exist [9]. As a medicinal product under regulation by the Medicines and Healthcare products Regulatory Agency (MHRA), FMT can be prescribed and produced locally by a medical practitioner for a named patient under a pharmacy exemption. However production and supply to a third party, via a stool bank, requires a Specials licence.
A UK survey showed that only 28% (36/130) of hospitals reported using FMT for CDI. Barriers to uptake were a lack of facilities and understanding of how to establish FMT services [10]. Both European and UK FMT guidelines for good clinical practice and standardisation have been published [11], [12], [13], and there have been commentaries describing the practical and logistical requirements for stool bank operation [9,14], however there is little guidance regarding the specific methodology compliant with the production of a safe and quality assured medicinal product.
We have worked with the HTA and then the MRHA to create a licenced FMT service, using a banked supply of FMT for the treatment of CDI within the National Health Service (NHS). We present outcome data for the first 124 patients treated under HTA approval and present the methodology framework which supported MHRA licencing. Our licenced service is currently supplying FMT to the population of England free of charge under an NHS innovation tariff. The production framework presented will be of value to practitioners in other countries wanting to establish a regulated service for the treatment of CDI and other conditions.
2. Methods
2.1. Preparation of medicinal FMT
The core practical steps used to prepare FMT did not differ between HTA approval and MHRA licencing. However to develop and comply with Good Manufacturing Practice (GMP) required for MHRA licencing, the FMT facility processes and policies were embedded within a Quality Management System, overseen by a Qualified Person and developed to meet the requirements outlined within The Orange Guide [15]. A summary of key steps taken to comply with GMP are presented in Table 1.
Table 1.
Procedures and control measures taken to ensure compliance with the Orange Guide and MHRA licencing.
| FMT production facility | FMT donor screening | FMT preparation | FMT supply | |
|---|---|---|---|---|
| Product quality assurance | Dedicated manufacturing facility Equipment IQ/PQ/OQ1 Environment and equipment temperature monitoring Equipment calibration, maintenance and verification Prospective equipment performance monitoring Controlled facility access Integration into a Quality Management System |
Standardised donor screening UKAS (or equivalent) accredited microbiological testing Independent clinical assessment of donors Regular review of donor screening policy Use of written document controlled SOPs |
Defined donation acceptance criteria (based on visual, consistency and storage time criteria) Consumable supplier validation to establish suitability of consumables Use of written document controlled SOPs Batch release from quarantine by Qualified Person Standardised training and competency assessment of personnel FMT Reference sample storage |
Validation of transport procedures to maintain appropriate storage conditions during transport Detailed instruction for storage and use, with specified expiry date Thawing and re-freezing prohibited Competent transport couriers |
| Cross contamination minimisation | Validation of cleaning methodology Environmental monitoring for enteric indicator organisms, with comparison to Grade D clean room recommended limits Exclusion of materials from the facility which can support microbial growth |
Standard collection protocol utilising sterile containers Specified expiry timescales for storage |
Single batch production2 Single use consumables and excipients Closed filtering and homogenisation Operator personal protective equipment (laboratory coat, gloves, hair net, mask and overshoes) Production within a Class 2 microbiological safety cabinet Direct transfer of filtrate from sterile filer bag into primary container |
Packaging compliant with P650 packing Instruction for biological specimens |
| Traceability | Use of electronic specimen tracking system for donations, batches, lots and reference samples within the FMT facility | Unique anonymous Donor Identifiers | Batch processing records3 Unique and traceable batch and lot numbering |
Supply on named patient basis only Standardised electronic requesting Validation certificate supplied with every FMT for retention in the patients notes4 Product labelling compliant with British Pharmacopoeia 2017 general monograph for unlicensed medicines |
IQ/PQ/OQ: installation qualification, performance qualification, operating qualification.
Batch of FMT defined as all lots prepared from a single faeces donation from an individual donor.
Batch processing records recorded date and time of stool production, unique donor identification number, donation macroscopic appearance, consumable and excipient lot numbers and expiry dates, weight of donor stool used, volume of saline used, volume of glycerol used, date and time of production, unique batch and lot numbers and operator name.
The validation certificate documents unique treatment identification numbers, source of FMT and production location, storage instructions, and statement confirming donor screening testing acceptability.
2.2. FMT donor selection and screening
Donors were healthy un-related anonymous volunteers, ≥18 and <50 years of age, with a Body Mass Index ≥18·5 and ≤25, who had not received antibiotics in the preceding three months. Screening was via health, social and travel questionnaire, clinical assessment (undertaken by an independent clinician) and microbiological testing (Table 2). Health, social and travel exclusion criteria are presented in the supplementary information 1. FMT was quarantined until donor screening results and all health questionnaires were completed (as presented in Table 2) and written confirmation of donor eligibility was received from the independent clinician.
Table 2.
Screening protocol for FMT donors.
| Screening method | Frequency |
|
|---|---|---|
| Fresh FMT | Frozen FMT (MHRA licenced protocol) | |
| Health screening questionnaire 1 | 3 monthly | ≤7 days prior to every 10 day donation period |
| Health screening questionnaire 2 | Day of donation | Final day of donation (day 10) |
| Microbiological testing of blood | 3 monthly | ≤7 days prior to every 10 day donation period |
| HIVa (4th generation immunoassay) | ||
| Hepatitis B Virus (HBsAGb and anti HBcc immunoassay) | ||
| Hepatitis C Virus (anti-HCVd immunoassay) | ||
| Syphilis (total antibody immunoassay) | ||
| Hepatitis A Virus (IgM immunoassay) | ||
| Hepatitis E Virus (IgM immunoassay) | ||
| HTLVe 1 and 2 (IgG immunoassay)j | ||
| Microbiological testing of faeces | 3 monthly | ≤7 days prior to every 10 day donation period lSelected tests repeated on donation day 5 and day 10. |
| Clostridioides difficile (culture and PCRf)l | ||
| Norovirus (PCR)l | ||
| Salmonella species (culture)l | ||
| Shigella species (culture)l | ||
| Escherichia coli O157 (culture)l | ||
| Thermophilic Campylobacter species (culture)l | ||
| Giardia antigen (EIAg) | ||
| Cryptosporidium antigen (EIA) | ||
| Helicobacter pylori stool antigen (EIA)k | ||
| ESBLh producing Enterobacteriaceae (Enrichment culture) | ||
| CPEi (Selective Chromogenic Culture) | ||
| Ova, cysts and parasites (microscopy)m | ||
The donor screening protocol was adapted from the American Gastroenterological Association guidelines for donor screening (Bakken et al 2011)[18] and Annex B of the HTA Guide to Quality and Safety Assurance of Human Tissues and Cells for Patient Treatments. All microbiological tests were performed by a UKAS accredited microbiology laboratory.
Human Immunodeficiency Virus.
Hepatitis B surface antigen.
Hepatitis B core antibody.
Hepatitis C antibodies.
Human T-cell lymphotropic Virus.
Polymerase Chain Reaction.
Enzyme Immunoassay.
Extended spectrum β-lactamase.
Carbapenemase producing Enterobacteriaceae.
Subject to risk assessment.
Screening methodology for H. pylori was modified during the study period from IgG serological testing to stool antigen testing.
Day 5 and day 10 stool testing samples were pooled aliquots of donations 1–5 and donations 6–10 respectively.
Observation of any ova, cysts or parasite structures results in donor exclusion.
2.3. FMT preparation
All FMT was prepared in a single centre in Birmingham and distributed to eleven NHS hospitals for administration. FMT processing was under aerobic conditions in a containment level two laboratory, within a sole use class two microbiological safety cabinet which was decontaminated before and after use using chlorine dioxide-based disinfectant (Tristel Solutions Ltd, UK) and ultra violet light (254 Nm, 30 min). Faeces were processed <6 hours post defecation, Bristol Stool type 2–5, brown in colour and contained no macroscopic blood or mucus. FMT material was prepared by combining 90 ± 5 g of faeces from a single donor with either 150 ml IV grade 0·9% Saline (fresh FMT) or 150 ml IV grade 0·9% Saline containing 10% v/v glycerol (frozen FMT) in a sterile Nasco Whirl-Pak filter bag (Labs UK Ltd, UK). This was homogenised in a Mix-1 stomacher (AES Chemunex, France) for two minutes (Fig. 1). The filtrate was either transferred to an enteral feeding syringe and used immediately (fresh FMT) or stored in Biotite™ Containers (Alpha Laboratories, UK) in 60 ml aliquots at −80 °C for up to 24 weeks (frozen FMT). FMT reference samples from every batch were retained and stored at −80 °C.
Fig. 1.
FMT production in the licenced production facility. (a) weighing out donor faeces into a Nasco-whirl pak filter bag in a class 2 microbiological safety cabinet. (b) homogenisation of stool using a stomacher. (c) aliquoting of prepared FMT into containers for storage. (d) final packaging and labelling of frozen FMT.
2.4. Use of medicinal FMT in the clinical cohort
Patients receiving FMT were treated as they presented to healthcare, as part of routine management of CDI. The cases reported here therefore represent real world consecutive eligible patients offered FMT within the standard care pathway. From March 2013 until October 2014, 31 patients were treated with fresh FMT. From November 2014 to September 2016, 93 patients were treated with frozen FMT. Eleven NHS hospitals within three UK regions (West Midlands, Cambridgeshire and Yorkshire) were supplied with FMT from the Birmingham central stool bank.
2.5. Patient inclusion and exclusion criteria
Patients treated with FMT were >18 years of age, clinically symptomatic with diarrhoea (defined as having as ≥3 type 6/7 stools per day for two consecutive days [16]) and were microbiologically confirmed active cases (defined as C. difficile glutamate dehydrogenase positive and either stool toxin positive by Enzyme Immunoassay (EIA) or C. difficile toxin gene positive by PCR assay within four weeks prior to treatment). Two groups of CDI cases were treated, recurrent CDI (defined as ≥3 episodes of CDI) and CDI refractory to antibiotic treatment (defined as continuing diarrhoea despite antibiotic treatment). FMT was offered for refractory CDI ≥ seven days after the start of the second antibiotic treatment course. Absolute patient exclusion criteria were decompensated liver cirrhosis and significant food allergy. Named patient FMT requests were approved internally by a clinician, via assessment of the clinical information supplied on a standard request form (supplementary material 2) and against the treatment definitions defined above. Informed patient consent was sought prior to FMT treatment according to local hospital policy and a pre-FMT patient serum was stored.
2.6. FMT administration
Prior to FMT all cases were treated with vancomycin, metronidazole or fidaxomicin (varying courses and lengths), which was stopped 12 hours prior to FMT treatment. FMT was predominantly administered via nasogastric (NG) tube, adapted from previously reported protocols [17] and following a standard protocol distributed to hospitals by the Birmingham stool bank (supplementary material 3).
2.7. Follow up and outcomes
Patients were followed up from clinical records, laboratory data and, when necessary, by telephone questionnaire to the physician or patient, using a structured pro-forma distributed to all centres. Outcome measures were, clinical response to FMT defined as resolution of diarrhoea within seven days and clinical cure defined as response without diarrhoea recurrence at 90 days. Total follow up time for each case was 90 days.
2.8. Statistical analysis
Univariate analysis was conducted using the t test for continuous variables and Fisher's test for binary variables. Multivariate analysis was performed using multiple logistic regression analysis. Data were analysed using Stata Statistical Software (Release 15. College Station, TX: StataCorp LLC).
2.9. Ethics
The local research ethics committee reviewed the study plan before data collection and concluded that full ethical approval was not required on the basis that this study was an anonymised retrospective audit of outcomes from routine clinical care.
3. Results
3.1. Clinical outcomes
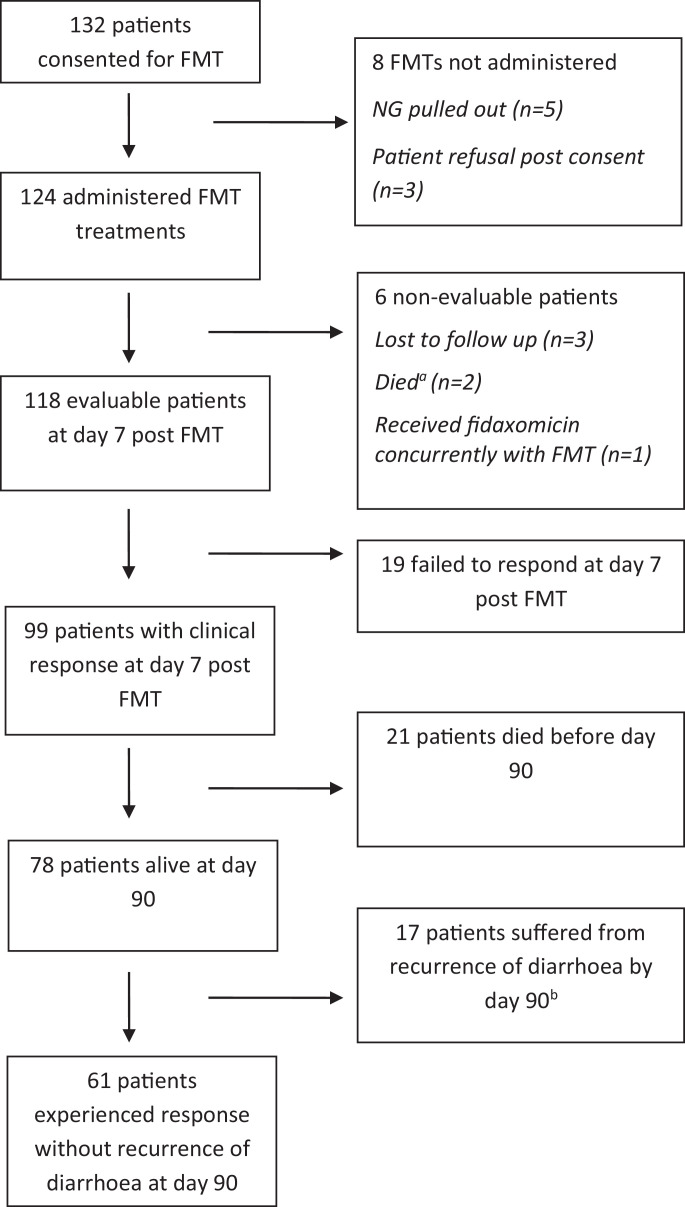
A total of 124 patients (median age 78·5 years) received FMT treatment (Fig. 2), 63% (78/124) of the cohort were female. The majority of patients received FMT via nasogastric tube, alternative routes of administration included colonoscopy (n = 1), PEG (n = 3) and gastroscopy (n = 1). There were 71 and 53 cases of recurrence CDI and refractory CDI respectively. Over half of the cases (75/124) were microbiologically confirmed stool toxin positive by EIA, with the remainder stool toxin negative by EIA, but toxin gene positive by PCR. Patient demographics are presented in Table 3. Six patients received a second FMT due to disease recurrence, between 12 and 106 days. Clinical response to a single FMT in 118 eligible cases (Fig. 2) was 83.9% (95% CI 76·0%–90·0%) and found to be higher for those treated in the recurrence group compared to the refractory group (91·0% vs. 73·0%, p = 0·007) and in those with an increased number of CDI episodes (OR 1·8, p = 0·006). Cases were more likely to be alive at day 90 if they had shown clinical response to FMT at day 7 compared to non-responders (p = 0.05). No significant difference was observed in clinical response when comparing age, gender, FMT preparation (fresh vs. frozen) or CDI microbiology testing result, by multivariate analysis (Table 4).
Fig. 2.
Number of patients receiving FMT and outcomes.
aOne patient died two days post FMT, with perforated viscus and CDI. Second patient died four days post FMT, from bowel cancer.
bSix patients were re-treated with a second FMT, of whom four clinically responded to FMT by day 7 and two of these patients demonstrated clinical response without recurrence of disease at day 90.
Table 3.
Demographics and clinical features of study population.
| Fresh FMT (n = 31) | Frozen FMT (n = 93) | Total (n = 124) | ||
|---|---|---|---|---|
| Sex | Male | 17 (37%) | 29 (63%) | 46 (37%) |
| Female | 14 (18%) | 64 (82%) | 78 (63%) | |
| Age | Median (range) | 81 (44–93) | 76.5 (22–99) | 78 (19–99) |
| Indication | Recurrent CDI | 14 (20%) | 57 (80%) | 71 (57%) |
| Mean prior CDI episodes (range) | 3.0 (3–4) | 4.0 (3–8) | 3.8 (3–8) | |
| Refractory CDI | 17 (32%) | 36 (68%) | 53 (43%) | |
| Mean prior CDI episodes (range) | 1.1 (1–2) | 1.3 (1–2) | 1.2 (1–2) | |
| Clostridioides difficile testing result | C. difficile stool toxin positive by enzyme immunoassay | 21 (28%) | 54 (72%) | 75 (60%) |
| C. difficile stool toxin negative by enzyme immunoassay andstool toxin gene positive by polymerase chain reaction assay | 10 (20%) | 39 (80%) | 49 (40%) |
Table 4.
Comparison of outcome measures across the cohort by multivariate analysis.
| Clinical response at day 7 |
Clinical cure at day 90 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Responder | Non-responder | Total | p value | Responder | Non-responder | Total | p value | |
| Recipient gender | 0·9 | 0.32 | ||||||
| Female | 64 (84%) | 12 (16%) | 76 | 43 (81%) | 10 (19%) | 53 | ||
| Male | 35 (83%) | 7 (17%) | 42 | 23 (72%) | 9 (28%) | 32 | ||
| Stool characteristics | 0·57 | 0·36 | ||||||
| Fresh | 25 (81%) | 6 (19%) | 31 | 21 (84%) | 4 (16%) | 25 | ||
| Frozen | 74 (85%) | 13 (15%) | 87 | 45 (75%) | 15 (25% | 60 | ||
| FMT indication | 0·007 | 0.4 | ||||||
| Recurrence | 64 (91%) | 6 (9%) | 48 | 38 (75%) | 13 (25%) | 51 | ||
| Refractory | 35 (73%) | 13 (27%) | 70 | 28 (82%) | 6 (18%) | 34 | ||
| C. difficile testing result | 0·835 | 0.26 | ||||||
| C. difficile stool toxin EIA positive | 60 (83%) | 12 (17%) | 72 | 44 (81%) | 19 (19%) | 54 | ||
| C. difficile stool toxin EIA negative, stool toxin PCR positive | 39 (85%) | 7 (15%) | 46 | 22 (71%) | 9 (29%) | 31 | ||
Response without recurrence of disease was observed in 78·2% (95% CI 67·4%–89·0%) of those alive at day 90. At day 90 no difference in response without recurrence was observed between groups.
3.2. Adverse events and side effects
All solicited adverse events were investigated with root cause analysis and reported to the HTA/MHRA. Two patients died within 7 days of FMT treatment. The first died two days after FMT from perforated viscus and presumed uncontrolled CDI despite FMT (as assessed by a senior clinician). The second died four days post FMT from underlying bowel cancer. There were two cases of bacteraemia post FMT, both receiving fresh FMT. The first case developed fever 3 hours post FMT, blood cultures grew Escherichia coli and urine analysis from a sample taken on the day of FMT demonstrated a pyuria (1078 WBC/µl) with a scanty growth of bacteria which were not identified. Twenty four hours post FMT the second case was noted to have hypoactive delirium and a rising CRP (increasing to 200), blood cultures were taken and grew Serratia marcescens. The patient remained afebrile and had a normal peripheral white blood cell count. The patient's long term urinary catheter had been replaced on the day of FMT treatment. Urine taken at the time of re-catheterised also grew S. marcescens, with the same antibiogram as the blood culture isolate. Reference FMT samples for the second case were investigated by selective direct and enrichment culture and S. marcescens was not isolated from FMT reference samples. Both cases responded to systemic antibiotic treatment for the acute clinical deterioration.
Minor adverse events reported were constipation (n = 4), bloating (n = 1), flatulence (n = 1), gagging on NG tube (n = 1) and abdominal pain (n = 2).
4. Discussion
This is the largest UK series presenting FMT outcome data for the treatment of CDI and the first of methodology which meets the requirements of a MHRA specials licence. In our uncontrolled retrospective case series, we found using a single FMT protocol clinical response at day 7 (83·9% [95% CI 76·0%–90·0%]) and response without recurrence at 90 days (78·2% [95% CI 67·4%–89·0%]) was broadly similar to that observed previously [4,19,20]. Administration in our cohort was predominantly via the upper gastrointestinal (GI) route, however one patient received FMT by colonoscopy. A specific administration procedure was available to clinicians, but protocol deviation may have occurred in this real world setting, possibly introducing centre specific bias. Meta-analysis comparing FMT administration routes, have observed a trend toward higher efficacy rates with lower GI compared to upper GI administration, but this often does not reach statistical significance [19,20]. The low numbers of lower GI administration precluded analysis here. This series included both fresh FMT and frozen FMT, reflecting the development of the service over time. Similarly to that previously reported [19,21], we observed no significant difference in FMT outcome between these groups, although the study was not powered to assess such differences and fresh FMT comprised only 25% of cases (31/124). Frozen supply was found to be advantageous over fresh FMT on the basis of logistics, turnaround time and minimising the donor screening window, factors which have also been highlighted by others [9,21].
The indication for FMT was recurrent CDI in 57% of cases; the remainder were defined as refractory cases. While other studies have included refractory cases [21], [22], [23], the overall numbers are small and this report presents one of the largest refractory case series to date, particularly in the primary and secondary episode of infection. The use of FMT in refractory CDI is complicated by the absence of accepted standard definitions. In some published studies recurrent and refractory are used interchangeably or poorly defined [22,24,25]. It has been defined as CDI not responding to conventional therapy without specific symptom duration thresholds [26]. Others have defined it as ongoing diarrhoea despite at least five days of oral vancomycin 125 mg given six hourly [27]. In this series the clinical response to FMT, measured at day seven, was higher in recurrent CDI compared to refractory cases (91·0% vs. 73·0%, p = 0·007), however at day 90 no difference was observed in outcome between these groups. A recent meta-analysis found lower cure rates in studies which included both recurrent and refractory patients, compared to studies including only recurrent cases (weighted pooled rate 63.9% vs. 79%; P < .001)(19). Due to the use of FMT in this series within routine clinical care, patients were pre-treated with variable CDI antibiotic course lengths. This uncontrolled variable could have introduced bias in our results, particularly for biased the seven day outcome measure across both refractory and recurrence groups. In addition the retrospective design of our study will limit the strength of conclusions that can be drawn from this finding. Time to resolution of diarrhoea was measured in this study, although not available for all patients, and was not found to be different between recurrence and refractory groups. FMT re-treatment was not applied universally to non-responders here. It has been shown that re-treatment with a second FMT increases clinical response rate [19], particularly in complicated or refractory cases [28].
Only microbiologically confirmed and clinically symptomatic patients were offered FMT, this approach aimed to avoid using FMT in asymptomatic C. difficile carriers. Those who were negative for C. difficile toxin in stool by EIA but positive for toxigenic C. difficile by PCR may have been more likely to have alternative reasons for ongoing diarrhoea, which could have limited our findings; however these patients were being treated clinically as CDI cases at the time of request for FMT. By definition the refractory group had failed to respond to antibiotics for CDI, which may increase the possibility of other contributing causes of diarrhoea in these cases, particularly as the incidence of resistance to vancomycin and fidaxomicin in C. difficile is thought to be low.
Under MHRA regulation in England FMT stool banks are now required to satisfy detailed manufacture requirements which adds complexity and cost of production. Within a Specials licence FMT can only be released for use by the Qualified Person when specified donor screening, production and storage criteria are satisfied. Integration of the service into a Quality Management System (QMS) is critical to successful operation of a licenced FMT facility. The QMS ensures that core components of the service are delivered consistently with appropriate quality assurance activity. It necessitates audit, risk management, thorough facilities management, quality improvement via the investigation of non-conformance, training and competence assessment of personnel, and document control. The minimisation of microbial contamination is a key component of GMP compliant FMT production. We have developed a closed fully disposable system for the preparation of FMT in a class two microbiological safety which minimises the risk of external microbial contamination. Other published methodologies have used external filtering systems and blenders, which may fail to minimise the risk of potential contamination [11,14]. Further to this FMT production is conducted in a dedicated clean non-sterile manufacturing room, which environmental monitoring has shown complies with grade D clean room recommended limits. This level of environmental review has not previously been commented on in international guidelines and is an evolving area for consideration. Control measures utilised to minimise the environmental contamination of the production facility are validated decontamination protocols, physical barriers (Tack-mats), limited personal access, the use of personal protective equipment (laboratory coats, hair nets and overshoes), and limiting material in the facility which supports microbial growth. The aim of the above GMP processes is to standardise and maintain the quality of FMT production as far as practically possible. However, the inherent variability in composition of human faeces between donors and within the same donor over time prevents true consistency and reproducibility control across FMT batches, as would be expected for the production of a drug-based medicinal product. In addition there is currently an absence of accepted profiling standards to quality assure donated faeces. Although it has been shown that higher bacterial diversity in donor faeces is associated with improved FMT clinical outcomes, the precise mechanisms of action of FMT are not fully understood, which limits the adoption of quality assurance testing during production. The selection of infectious agents screened for during FMT donor assessment varies between institutions [29] and until recently has not been expressly specified by regulating agencies, particularly those regulating FMT as a biological drug. However, a general consensus is emerging with the publication of guidelines for FMT production and use [11,12]. These guidelines recommend screening for multidrug resistant organisms (MDRO), in particular those producing Extended Spectrum β-lactamases (ESBL) and Carbapenemase producing Enterobacteriaceae (CPE), although other centre's protocols do not always specify MDRO screening [29]. This topic has become relevant with the recent Food and Drug Administration (FDA) safety alert reporting two immunocompromised patients transplanted from the same donor who became colonised from the FMT with ESBL producing E. coli, with the death of one patient [30]. The FDA has mandated all holders of Investigational New Drug applications for FMT to not only test for ESBL and CPE, but also methicillin resistant Staphylococcus aureus (MRSA) and vancomycin resistant Enterococci (VRE). The diagnostic methods used to screen for MDROs and other pathogens should be validated and demonstrate acceptable sensitivity and specificity, to ensure the accuracy of donor testing. Although many guidelines specify the pathogens to screen for during donor selection, they often do not address which testing methodology to use. To maintain the quality of results, donor testing should be undertaken by accredited diagnostic laboratories following standard methods, such as those described in the UK Standards for Microbiological Investigation. In our own service, the donor screening algorithm continues to develop dynamically. For example to improve the diagnostic sensitivity of testing for enteric pathogens, culture methods will be replaced with Polymerase Chain Reaction (PCR) based tests. This will also enable the detection of pathogens not previously screened for such as all Shiga toxin producing E. coli (STEC) and rotavirus. The repertoire of screening tests for FMT to be given to immunocompromised patients has been discussed [9,12]. The groups concluded that caution should be exercised and the use of Cytomegalovirus (CMV) and Epstein-Barr Virus (EBV) negative donors should be considered for seronegative recipients, the role of other potential pathogens being considered on a case by case basis. This is an evolving area with a need for research, particularly with the potential use of FMT in patients post bone marrow transplantation.
Prior to the re-classification of FMT as a medicine in the UK, stool banks were being developed to widen FMT supply locally and regionally [31,32]. Since the introduction of the revised regulatory position, FMT availability has been limited to intra-institution supply only. The systems described here now enable frozen FMT supply across institutions within a Specials licence, the first service of its kind in the UK. It is notable that encapsulation methods had been described for FMT administration and shown to be non-inferior to liquid FMT delivered via colonoscopy for the treatment of recurrent CDI [33]. The procedures and control measures that have been described here could be applicable to FMT banks preparing liquid or encapsulated FMT to medical product standards in other parts of the world.
Access to pre-screened FMT via larger centres is desired by clinicians in the UK, particularly as many perceive that lack of facilities restrict local production [10]. A move towards FMT stool banks, where FMT is produced to GMP standards under a Specials licence will reduce costs through economy of scale and promote standardisation, safety and quality [9,11]. This approach will also widen access of treatment and reduce inequity of access in the NHS. However, to ensure the resilience of FMT services in the NHS steps need to be taken to develop a networked hub and spoke model of supply under appropriate licencing. Careful management of such a network would enable efficient use of resources across the NHS, while maintaining flexible and adaptable access to this life saving treatment. This will require further engagement with the MHRA to develop this unique regulatory framework appropriately.
Author contributionsVM, PH and MQ had full access to all of the study data and take full responsibility for data analysis. VM and PH were responsible for drafting the manuscript. MQ completed the statistical analysis. VM, SM, PH, TI, and CM planned and delivered the FMT service. CM, TI, MQ, DM, KB, GT and HS oversaw the clinical management of patients. VM, SM, DM, KB, GT, TI, MQ and HS contributed to the data collection. All authors critically revised the manuscript.
Declaration of Competing Interest
We declare no competing interests.
Acknowledgments
Acknowledgments
The authors would like to thank the Shropshire, Staffordshire and Cheshire Blood Bikes Association for their dedicated, professional and speedy voluntary transport of FMT treatments across England.
Funding
No funding was received for this study.
Footnotes
Funding: No funding received.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100301.
Appendix. Supplementary materials
References
- 1.Freeman J., Bauer M.P., Baines S.D., Corver J., Fawley W.N., Goorhuis B. The changing epidemiology of clostridium difficile infections. Clin Microbiol Rev. 2010;23(3):529–549. doi: 10.1128/CMR.00082-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wenisch C., Parschalk B., Hasenhündl M., Hirschl A., Graninger W. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis. 1996;22(5):813–818. doi: 10.1093/clinids/22.5.813. [DOI] [PubMed] [Google Scholar]
- 3.McFarland L.V., Surawicz C.M., Rubin M., Fekety R., Elmer G.W., Greenberg R.N. Recurrent Clostridium difficile disease: epidemiology and clinical characteristics. Infect Control Hosp Epidemiol. 1999;20(1):43–50. doi: 10.1086/501553. [DOI] [PubMed] [Google Scholar]
- 4.van Nood E., Vrieze A., Nieuwdorp M., Fuentes S., Zoetendal E.G., de Vos W.M. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 5.Hvas C.L., Dahl Jørgensen S.M., Jørgensen S.P., Storgaard M., Lemming L., Hansen M.M. Fecal microbiota transplantation is superior to fidaxomicin for treatment of recurrent clostridium difficile infection. Gastroenterology. 2019;156(5):1324–1332. doi: 10.1053/j.gastro.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 6.Hocquart M., Lagier J.-C., Cassir N., Saidani N., Eldin C., Kerbaj J. Early fecal microbiota transplantation improves survival in severe clostridium difficile Infections. Clin Infect Dis. 2018;66(5):645–650. doi: 10.1093/cid/cix762. [DOI] [PubMed] [Google Scholar]
- 7.National Institute for Health and Care Excellence (NICE) Faecal microbiota transplant for recurrent Clostridium difficile infection. NICE Intervent Proc Guid. 2014;485:1–61. [Google Scholar]
- 8.Medicines and Healthcare products Regulatory Agency (MHRA). Faecal microbiota transplantation (FMT) MHRA's position. 2015.
- 9.Terveer E.M., van Beurden Y.H., Goorhuis A., Seegers J.F.M.L., Bauer M.P., van Nood E. How to: fstablish and run a stool bank. Clin Microbiol Infect. 2017;23(12):924–930. doi: 10.1016/j.cmi.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Quraishi M.N., Segal J., Mullish B., McCune V.L., Hawkey P., Colville A. National survey of practice of faecal microbiota transplantation for Clostridium difficile infection in the UK. J Hosp Infect. 2016;95(4):444–445. doi: 10.1016/j.jhin.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 11.Cammarota G., Ianiro G., Tilg H., Rajilić-Stojanović M., Kump P., Satokari R. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66(4):569–580. doi: 10.1136/gutjnl-2016-313017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mullish B.H., Quraishi M.N., Segal J.P., McCune V.L., Baxter M., Marsden G.L. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. J Hosp Infect. 2018;100:S1–31. doi: 10.1016/j.jhin.2018.07.037. [DOI] [PubMed] [Google Scholar]
- 13.Cammarota G., Ianiro G., Kelly C.R., Mullish B.H., Allegretti J.R., Kassam Z. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut. 2019 doi: 10.1136/gutjnl-2019-319548. gutjnl-2019-319548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costello S.P., Tucker E.C., Brooy J.La, Schoeman M.N., Andrews J.M. Establishing a Fecal Microbiota Transplant Service for the Treatment of Clostridium difficile Infection. Clin Infect Dis. 2016;62(7):908–914. doi: 10.1093/cid/civ994. [DOI] [PubMed] [Google Scholar]
- 15.Medicines and Healthcare products Regulatory Agency (MHRA) Pharmaccutical Press; Tenth. London: 2017. Rules and guidance for pharmaceutical manufacturers and distributors (the orange guide) [Google Scholar]
- 16.Louie T.J., Miller M.A., Mullane K.M., Weiss K., Lentnek A., Golan Y. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364(5):422–431. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 17.Aas J., Gessert C.E., Bakken J.S. Recurrent Clostridium difficile Colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis. 2003;36(5):580–585. doi: 10.1086/367657. [DOI] [PubMed] [Google Scholar]
- 18.Bakken J.S., Borody T., Brandt L.J. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9(12):1044–1049. doi: 10.1016/j.cgh.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ianiro G., Maida M., Burisch J., Simonelli C., Hold G., Ventimiglia M. Efficacy of different faecal microbiota transplantation protocols for Clostridium difficile infection: a systematic review and meta-analysis. United Eur Gastroenterol J. 2018;6(8):1232–1244. doi: 10.1177/2050640618780762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tariq R., Pardi D.S., Bartlett M.G., Khanna S. Low cure rates in controlled trials of fecal microbiota transplantation for recurrent clostridium difficile infection: a systematic review and meta-analysis. Clin Infect Dis. 2019;68(8):1351–1358. doi: 10.1093/cid/ciy721. [DOI] [PubMed] [Google Scholar]
- 21.Lee C.H., Steiner T., Petrof E.O., Smieja M., Roscoe D., Nematallah A. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection. Jama. 2016;315(2):142. doi: 10.1001/jama.2015.18098. [DOI] [PubMed] [Google Scholar]
- 22.Bang B.W., Park J.-S., Kim H.K., Shin Y.W., Kwon K.S., Kwon H.Y. Fecal microbiota transplantation for refractory and recurrent clostridium difficile infection: a case series of nine patients. Korean J Gastroenterol. 2017;69(4):226. doi: 10.4166/kjg.2017.69.4.226. [DOI] [PubMed] [Google Scholar]
- 23.Youngster I., Russell G.H., Pindar C., Ziv-Baran T., Sauk J., Hohmann E.L. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA. 2014;312(17):1772–1778. doi: 10.1001/jama.2014.13875. [DOI] [PubMed] [Google Scholar]
- 24.Bamba S., Nishida A., Imaeda H., Inatomi O., Sasaki M., Sugimoto M. Successful treatment by fecal microbiota transplantation for Japanese patients with refractory Clostridium difficile infection: a prospective case series. J Microbiol Immunol Infect. 2017 doi: 10.1016/j.jmii.2017.08.027. [DOI] [PubMed] [Google Scholar]
- 25.Yoon S.S., Brandt L.J. Treatment of refractory/recurrent C. difficile-associated disease by donated stool transplanted via colonoscopy. J Clin Gastroenterol. 2010;44(8):562–566. doi: 10.1097/MCG.0b013e3181dac035. [DOI] [PubMed] [Google Scholar]
- 26.van Beurden Y.H., Nieuwdorp M., van de Berg P.J.E.J., Mulder C.J.J., Goorhuis A. Current challenges in the treatment of severe Clostridium difficile infection: early treatment potential of fecal microbiota transplantation. Therap Adv Gastroenterol. 2017;10(4):373–381. doi: 10.1177/1756283X17690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee C.H., Belanger J.E., Kassam Z., Smieja M., Higgins D., Broukhanski G. The outcome and long-term follow-up of 94 patients with recurrent and refractory Clostridium difficile infection using single to multiple fecal microbiota transplantation via retention enema. Eur J Clin Microbiol Infect Dis. 2014;33(8):1425–1428. doi: 10.1007/s10096-014-2088-9. [DOI] [PubMed] [Google Scholar]
- 28.Weingarden A.R., Hamilton M.J., Sadowsky M.J., Khoruts A. Resolution of severe Clostridium difficile infection following sequential fecal microbiota transplantation. J Clin Gastroenterol. 2013;47(8):735–737. doi: 10.1097/MCG.0b013e31829004ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woodworth M.H., Nolsh E.M., Miller N.S., Dhere T., Burd E.M., Carpentieri C. Laboratory testing of donors and transplantation for recurrent clostridium. J Clin Microbiol. 2017;55(4):1002–1010. doi: 10.1128/JCM.02327-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeFilipp Z., Bloom P.P., Torres Soto M., Mansour M.K., Sater M.R.A., Huntley M.H. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med. 2019;381(21):2043–20500. doi: 10.1056/NEJMoa1910437. [DOI] [PubMed] [Google Scholar]
- 31.Bicknell K., Fogg C., Fowell A., Porter R., Bannister O., Flatt A. OC-014 a regional frozen faecal transplant service for the treatment of chronic recurrent clostridium difficile infection: a review. Gut. 2017;66(Suppl 2):A7–A8. [Google Scholar]
- 32.Quraishi M.N., McCune V.L., Iqbal T., Pathmakanthan S., Struthers J.K., Moran E. Faecal microbiota transplantation via nasogastric route for the treatment of recurrent and antibiotic refractory clostridum difficile infection: the UK experience. Gastroenterology. 2015;(1) PG-S641-S642):S641–2. [Google Scholar]
- 33.Kao D., Roach B., Silva M., Beck P., Rioux K., Kaplan G.G. Effect of oral capsule- vs colonoscopy-delivered fecal microbiota transplantation on recurrent clostridium difficile infection: a randomized clinical trial. JAMA. 2017;318(20):1985–1993. doi: 10.1001/jama.2017.17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.