Coronavirus disease 2019 (COVID-19) emerged from China in December 2019 and is now a pandemic. Lung transplantation may increase susceptibility to severe acute respiratory syndrome–coronavirus 2 (SARS-CoV-2) infection because of the highly immunosuppressed state, with lungs being the predominantly affected organ. No specific recommendations exist for patients after solid organ transplantation. Currently, it is unclear how immunosuppression affects the incubation period and presentation of symptoms. One report describes a potential replication inhibition of several types of coronavirus by FK5061.
We report on a 59-year-old woman 13 months after bilateral lung transplantation who presented with SARS-CoV-2 infection. She underwent bilateral lung transplantation because of chronic thromboembolic pulmonary hypertension in February 2019. Alemtuzumab was administered as induction therapy, and a dual immunosuppressive regimen consisting of tacrolimus and prednisolone was used as maintenance therapy. Following a complicated post-transplant course, she was discharged 3 months post-operatively. The highest forced expiratory volume at 1 second value was reached in December 2019 with 1.60 liters (72% predicted). On the day of hospitalization (Day 0), she presented to the outpatient clinic for a routine follow-up visit with no apparent symptoms. Lung function testing showed a drop in forced expiratory volume at 1 second to 1.28 liters (57% predicted). Upon questioning, she expressed mild exercise dyspnea and a dry cough but no fever or diarrhea. The patient had no concerning travel history. Immunosuppression consisted of tacrolimus 0.6 mg twice daily (trough level was 5–7 ng/dl owing to alemtuzumab induction and history of osteomyelitis), and 5 mg prednisolone daily. Leukocyte count was 11,360 cells/μl (6% lymphocytes), C-reactive protein was 1.0 mg/dl, lactate dehydrogenase was 297 units/liter, and creatine kinase was 32 units/liter. There was no thrombocytopenia. Arterial blood had a partial pressure of oxygen of 55 mm Hg without supplementation. Real-time reverse transcriptase–PCR (RT-PCR; SARS-CoV-2 RT-PCR Kit 1.0 from Altona Diagnostics) of nasal and pharyngeal swabs showed evidence of SARS-CoV-2 RNA, thereby establishing a diagnosis of COVID-19. She was hospitalized, and a chest X-ray showed chronic post-operative dystelectasis on the left side with some increase in density (Figure 1 ) compared with December 2019. A chest computerized tomography scan showed ground glass opacities mainly in the left lower lobe with left-sided parenchymal consolidation or partial atelectasis (Figure 2 ). Oxygen supplementation with 1 to 2 liters/minute was administered. Antibacterial therapy was empirically started based on suspected bacterial superinfection. Otherwise, no major changes in medication were performed. A detailed medication plan is shown in Table 1 . She remained asymptomatic and remained stable. RT-PCR testing was performed on a weekly basis and remained positive on Day 7 and Day 14. Cycle threshold levels of SARS-CoV-2 E- and S-gene are provided in Table 2 . Cycle threshold values increased at every sample time point, indicating a decrease in virus levels over time. On nasopharyngeal swabs on Day 21, no SARS-CoV-2-RNA could be detected by RT-PCR. No oxygen was required from Day 17 until discharge. The patient was discharged home on Day 21.
Figure 1.
Chest X-ray at admission showing chronic post-operative dystelectasis with minor increase in density on the left side.
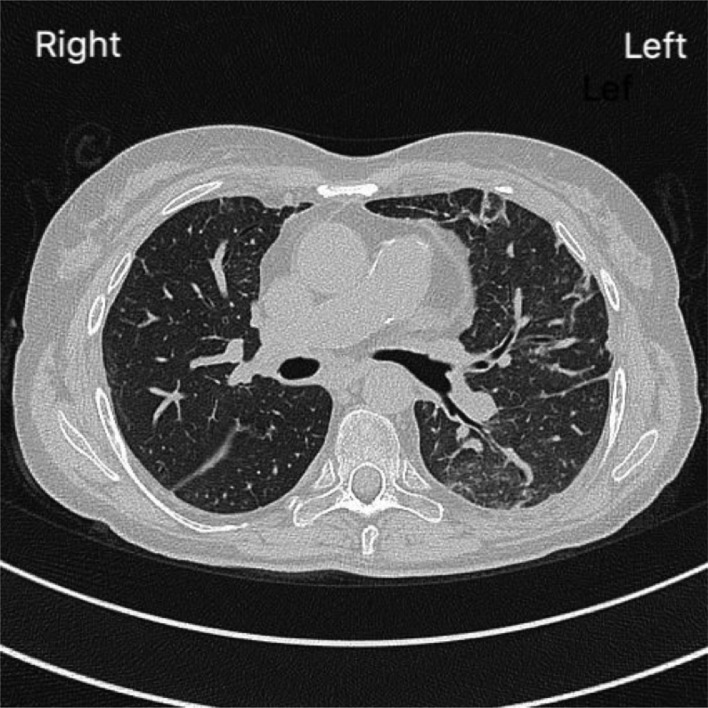
Figure 2.
Chest computerized tomography scan at admission with ground glass opacity and dystelectatic areas in the left lung.
Table 1.
Detailed Medication Plan of Immunosuppression and Changes in Existing Medication
| Medication | Dose | Baseline Day 0 | Midpoint Day 10 | Discharge Day 21 |
|---|---|---|---|---|
| Tacrolimus | mg/day orally | 4 | 5 | 6 |
| Prednisolone | mg/day orally | 5 | 5 | 5 |
| Trimethoprim/sulfamethoxazole | mg/day orally/twice weekly | 960 | 960 | 960 |
| Metamizole | mg/day orally | — | 500 | — |
| Salbutamol | inhalation dose 4 times/day | — | 100 µg | 100 µg |
| Meropenem | g/day intravenously | 4 | 4 | — |
Table 2.
Ct Levels in SARS-CoV-2 RT-PCR
| Date | Ct E-gene | Ct S-gene |
|---|---|---|
| Day 0 | 23.59 | 22.07 |
| Day 7 | 34.34 | 33.96 |
| Day 14 | 35.23 | 35.28 |
| Day 21 | target not detected (≥40) | target not detected (≥40) |
Abbreviations: Ct, cycle threshold; RT-PCR, reverse transcriptase–PCR; SARS-CoV-2, severe acute respiratory syndrome–coronavirus 2.
Ct levels ranged from 20 to 39.
In a report based on data collected in China from 1,099 patients during the 2 first months of COVID-19 outbreaks, 5% of the patients were admitted to the intensive care unit, 2.3% underwent invasive mechanical ventilation, and 1.4% died.2 Although older patients (>50 years old) and patients presenting with coexisting disorders are more prone to suffer from severe disease, data on disease presentation and evolution in immunocompromised patients are scarce. In the first report of the COVID-19 outbreak, 2 patients (0.2%) with COVID-19 and a not-otherwise-specified immunodeficiency were reported. Both patients had non-severe disease, and neither was admitted to the intensive care unit, underwent invasive mechanical ventilation, or died. In a 52-year-old patient who had kidney transplantation 12 years earlier and confirmed COVID-19, successful recovery was achieved following reduction of immunosuppressant therapy coupled with low-dose methylprednisolone-based therapy.3 In 2 heart transplant recipients, both recovered after supportive therapy with antibiotics and antiviral therapy coupled to reduction of immunosuppressive therapy.4 From these first very early experiences with COVID-19 in renal, heart, and lung transplant recipients, disease presentation seemed to be similar to the general population. Whether COVID-19 is more severe or probably mitigated owing to the effects of the immunosuppression on virus replication in patients after solid organ transplantation is still unknown, but recovery was so far possible in most reported cases, although some anecdotal unpublished reports from Italy suggest a higher morbidity in older transplant recipients. Based on this experience, a higher clinical suspicion is warranted, and early testing is recommended, because COVID-19 can be present even in relatively asymptomatic patients after lung transplantation.
References
- 1.Carbajo-Lozoya J, Müller MA, Kallies S, Thiel V, Drosten C, von Brunn A. Replication of human coronaviruses SARS-CoV, HCoV-NL63 and HCoV-229E is inhibited by the drug FK506. Virus Res. 2012;165:112–117. doi: 10.1016/j.virusres.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China [e-pub ahead of print]. N Engl J Med. doi: 10.1056/NEJMoa2002032, accessed April 18, 2020. [DOI] [PMC free article] [PubMed]
- 3.Zhu L, Xu X, Ma K, et al. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression [e-pub ahead of print]. Am J Transplant. doi: 10.1111/ajt.15869, accessed April 18, 2020. [DOI] [PMC free article] [PubMed]
- 4.Li F, Cai J, Dong N. First cases of COVID-19 in heart transplantation from China. J Heart Lung Transplant, in press. [DOI] [PMC free article] [PubMed]