Abstract
Purpose
Aimed to characterize the CT imaging and clinical course of asymptomatic cases with COVID-19 pneumonia.
Methods
Asymptomatic cases with COVID-19 pneumonia confirmed by SARS-COV-2 nucleic acid testing in Renmin Hospital of Wuhan University were retrospectively enrolled. The characteristics of CT imaging and clinical feature were collected and analyzed.
Results
58 asymptomatic cases with COVID-19 pneumonia admitted to our hospital between Jan 1, 2020 and Feb 23, 2020 were enrolled. All patients had history of exposure to SARS-CoV-2. On admission, patients had no symptoms and laboratory findings were normal. The predominant feature of CT findings in this cohort was ground glass opacity (GGO) (55, 94.8%) with peripheral (44, 75.9%) distribution, unilateral location (34, 58.6%) and mostly involving one or two lobes (38, 65.5%), often accompanied by characteristic signs. After short-term follow-up, 16 patients (27.6%) presented symptoms with lower lymphocyte count and higher CRP, mainly including fever, cough and fatigue. The evolution of lesions on CT imaging were observed in 10 patients (17.2%). The average days of hospitalization was19.80±10.82 days, and was significantly longer in progression patients (28.60±7.55 day).
Conclusion
CT imaging of asymptomatic cases with COVID-19 pneumonia has definite characteristics. Since asymptomatic infections as “covert transmitter”, and some patients can progress rapidly in the short term. It is essential to pay attention to the surveillance of asymptomatic patients with COVID-19. CT scan has great value in screening and detecting patients with COVID-19 pneumonia, especially in the highly suspicious, asymptomatic cases with negative nucleic acid testing.
Keywords: SARS-CoV-2, Coronavirus Disease 2019(COVID-19), Asymptomatic, Computed Tomography, Ground Glass Opacity
Introduction
In December 2019, a novel coronavirus, currently designated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), formerly called 2019-nCoV, was first identified in Wuhan, China. SARS-CoV-2 caused a respiratory disease called Coronavirus 2019 (COVID-19) which was officially named by the World Health Organization (WHO) on February 11, 2020. COVID-19 had caused severe illness and death in China and had spread to countries around the world.1, 2, 3, 4 By March 30, 2020, a total of 82,447 confirmed cases have been reported in China.5 Moreover, 552,479 cases have been confirmed in other countries, mainly in America, Italy and Spain.5 The main symptoms of COVID-19 are fever, dry cough, fatigue, sputum production, shortness of breath, and sore throat.6 Asymptomatic infected individuals, called “asymptomatic carrier or transmitter”, may also become the contagious source of SARS-CoV-2, and some of them progress rapidly, even resulting in acute respiratory distress syndrome (ARDS) with a high case-fatality rate.7, 8, 9, 10 However, the positive rate of SARS-CoV-2 nucleic acid testing by reverse transcription-polymerase chain reaction (RT-PCR), which is the golden standard for the diagnosis of COVID-19, is only about 30–50%. The high false-negative result rate of SARS-CoV-2 nucleic acid testing inevitably increases the difficulty in managing the current COVID-19 outbreak as the misdiagnosed patients might miss the best timing for proper treatment and cause the spread of disease.11 The chest computed tomography (CT), especially high-resolution CT (HRCT) is also the main diagnostic method followed by "Pneumonia diagnosis and treatment guideline for SARS-CoV-2 infection (trial version 5)" issued by National Health Commission of the People's Republic of China (http://www.nhc.gov.cn/). CT findings plays an important role in detecting lung abnormalities, facilitate the early identification of the diseases.
We aimed to characterize the CT imaging features and clinical course of asymptomatic patients with COVID-19 pneumonia admitted to our hospital, in order to have a more comprehensive understanding of asymptomatic patients with COVID-19, facilitate detection and isolation of patients with COVID-19 pneumonia, especially in the highly suspicious, asymptomatic cases with negative nucleic acid testing.
Materials and methods
Patients
The study was conducted in accordance with the principles of the Declaration of Helsinki. The study was a retrospective analysis and approved by the Ethics Committee of Renmin Hospital of Wuhan University (approved number: WDRY 2020-K009), which waived the requirement for patients’ informed consent referring to the CIOMS guideline. Asymptomatic patients with COVID-19 pneumonia confirmed by fluorescence reverse-transcriptase-polymerase chain reaction (fRT-PCR) assay for SARS-CoV-2 in Renmin Hospital of Wuhan University between January 1, 2020 and February 23, 2020 were enrolled. All patients rechecked every 3–7 days. The clinical data including gender, age, course, comorbidities, epidemiologic characteristics, laboratory results and CT findings were collected and analyzed retrospectively.
Image acquisition and analysis
All CT scans were obtained using one of the following scanners: Optima CT680, Revolution CT, Bright Speed (GE Healthcare System, Milwaukee, WI, USA & GEHW Healthcare System, Beijing, China). Scans were done from the level of the thoracic entrance to the inferior level of the costophrenic angle with patients in a supine, head-first position and breath-holding manner, and the following parameters were used: slice thickness 1.0∼1.25 mm, tube voltage 120 kV. The tube current was regulated by an automatic exposure control system (ASiR, GE, Healthcare). Images were reconstructed with a slice thickness of 0.625 mm and an interval of 0.625 mm, respectively, using GE AW workstation (AW 4.6 and AW4.7). The reconstructed CT images were transmitted to the workstation and picture archiving and communication systems (PACS) for multiplanar reconstruction post-processing.
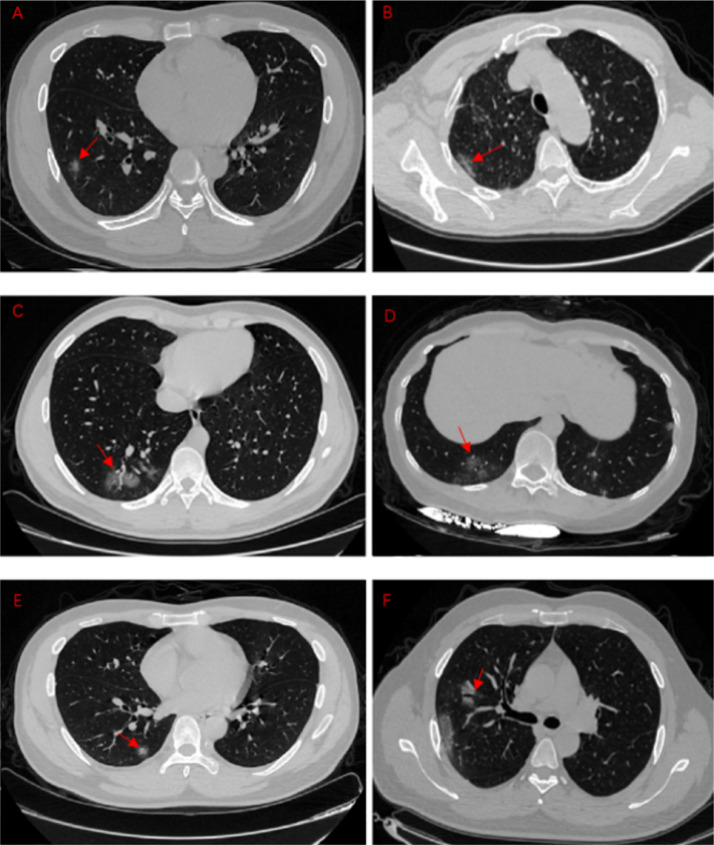
All CT images were independently reviewed by two experienced thoracic radiologists blinded to the clinical data. All discrepancies were resolved by consensus. The features of CT imaging were focused in the following aspects: (a) lesion distribution: left, right lung or bilateral lung; (b) lesion location: peripheral, central or both; (c) lobes involved; (d) lesion characteristics: Ground Glass Opacity(GGO), consolidation, margins; (e) other signs in GGO: reticulation, air bronchogram, and vascular enlargement; (f) other findings: subpleural curviliner line, halo sign, interlobular septal thickening, craze-paving pattern, pleural thickening and pleural effusion, etc. (see Fig. 1 ). GGO was defined as hazy opacity that did not obscure underlying bronchial and vascular margins; consolidation was the opacity with obscuration of bronchial and vascular structures; reticulation is a collection of innumerable small linear opacities; halo sign is a ground-glass opacity surrounding a nodule or mass; interlobular septal thickening is thin linear opacities at right angles to and in contact with the lateral pleural surfaces near the lung bases; crazy-paving pattern was defined as thickened interlobular septa and intralobular lines superimposed on a background of GGO, resembling irregularly shaped paving stones.12
Fig. 1.
The different GGO manifestations in COVID-19 pneumonia patient.
A: single, pure GGO. B: Pleural parallel sign. C: Vascular thickening sign. D: Fine reticulation. E: Halo sign. F: Air bronchogram.
Results
The clinical characteristics and laboratory results of this cohort are presented in Table 1 . 58 patients (26 male and 32 female) were enrolled. All patients had a clear exposure to COVID-19. All 58 patients were asymptomatic at admission. The average age of patients was 42.60±16.56 years old. Based on the CT findings of first reexamination after admission, 58 patients were divided into four groups: 1. Absorption group (22 cases, 37.9%), 2. Improvement group (23 cases, 39.7%),3. No change group (3 cases, 5.2%), 4. Progression group (10 cases, 17.2%) (see Fig. 2 ). Among these asymptomatic patients, 16 patients (27.6%) presented symptoms after admission, including fever (8 cases, 50%), cough (9 cases, 56.3%), fatigue (8 cases, 50%), shortness of breath (2 cases, 12.5%) and diarrhea (1 case, 6.3%). In these 16 patients, 10 patients (62.5%) were in lesion progression group, 3 patients (18.75%) were in lesion improvement group and 3 patients (18.75%) were in no change group. The average days before symptoms onset is 3.71±2.86 days, patients in lesion progression group have shorter days before symptoms onset (3.25±2.77 day). CT findings at admission showed abnormalities in all patients (seen in Table 2 and Fig. 1). Less than half of the patients (24, 41.4%) presented bilateral lesions; 34 (58.6%) patients showed unilateral lung distribution, including 14 (24.1%) in left lung and 20 (34.5%) in right lung. The lesions mostly located in peripheral (44, 75.9%), and 14 (24.1%) patients presented central distribution. The involvement of lower lobes (right 68.9% vs left 62.1%) was a bit more than that of the upper lobes (right 51.7% vs left 53.4%). 24 (41.4%) patients had single lobe involved, 14 (24.1%) patients had involved of two lobes, and 20 (34.5%) patients had three or more lobes involved. The most common pattern of CT findings was GGO (55,94.8%), including simple GGO in 30 cases (51.7%), GGO with fine reticulation in 7 cases (12.1%), GGO with subpleural curviliner line in 6 cases (10.3%), GGO with air bronchogram in 5 cases (8.6%), GGO with halo sign in 5 cases (8.6%) and GGO with vascular enlargement in 2 cases (3.5%) (seen in Table 3 ). Another CT feature, consolidation was presented in 3 cases (5.2%). Compared with the CT images at admission, there were evolution of CT manifestations in 10 patients at the first reexamination after admission. Lesions were fused to form patchy, crazy-paving signs, or diffusion pattern, distributed in multiple lung lobes or bilateral, and a few patients showed consolidation in CT imaging. In these patients, there were significantly lower lymphocyte count(P = 0.009), higher C-reactive protein (P = 0.023) than that in other cases. The average days of hospitalization is 19.80±10.82 days, and the hospitalization of patients (28.60±7.55 day) in progression group was significantly longer than others (P = 0.016).
Table 1.
Clinical characteristics and laboratory results of asymptomatic cases with COVID-19 pneumonia.
| Lesion absorption | Lesion improvement | No change | Lesion progression | All patients | |
|---|---|---|---|---|---|
| Characteristics | |||||
| Cases | 22(37.9%) | 23(39.7%) | 3(5.2%) | 10(17.2%) | 58 |
| Age, years | 40.00±14.87 | 33.90±19.42 | 34.50±13.65 | 57.40±22.54 | 42.60±16.56 |
| Gender | |||||
| Male | 9(40.9%) | 11(47.8%) | 2(66.7%) | 4(40%) | 26(44.8%) |
| Female | 13(59.1%) | 12(52.2%) | 1(33.3%) | 6(60%) | 32(55.2%) |
| Comorbidities | |||||
| Diabetes | 1(1.7%) | 2(3.5%) | 1(1.7%) | 2(3.5%) | 6(10.3%) |
| Hypertension | 3(5.2%) | 2(3.5%) | 0 | 2(3.5%) | 7(12.1%) |
| Cardiovascular disease | 2(3.5%) | 3(5.2%) | 0 | 1(3.5%) | 6(10.3%) |
| COPD | 0 | 0 | 0 | 1(1.7%) | 1(1.7%) |
| Epidemiological history | |||||
| Yes | 22(100%) | 23(100%) | 3(100%) | 10(100%) | 58(100%) |
| No | 0 | 0 | 0 | 0 | 0 |
| Subsequent symptoms | / | 3 (18.75%) | 3 (18.75%) | 10(62.5%) | 16(27.6%) |
| Days before symptoms onset | / | 4.10±3.38 | 4.50±3.50 | 3.25±2.77 | 3.71±2.86 |
| Fever | 0 | 0 | 1(6.3%) | 7(43.7%) | 8(50%) |
| Cough | 0 | 2(12.5%) | 1(6.3%) | 6(37.5%) | 9(56.3%) |
| Fatigue | 0 | 2(12.5%) | 2(12.5%) | 4(25%) | 8(50%) |
| Shortness of breath | 0 | 0 | 0 | 2(12.5%) | 2(12.5%) |
| Diarrhea | 0 | 0 | 0 | 1(6.3%) | 1(6.3%) |
| Laboratory test | |||||
| Red blood cell count, × 109/L | 3.96±0.19 | 4.14±0.52 | 4.26±0.39 | 3.84±0.58 | 4.02±0.45 |
| Leukocyte count, × 109/L | 5.52±1.47 | 5.98±1.60 | 5.47±1.77 | 3.91±1.18 | 5.38±1.63 |
| Neutrophil count, × 109/L | 3.37±1.19 | 3.71±1.66 | 2.81±1.12 | 2.38±0.83 | 3.29±1.42 |
| Lymphocyte count, × 109/L | 1.61±0.37 | 1.68±0.63 | 1.88±0.71 | 1.05±0.36 | 1.54±0.55 |
| Platelet count, × 109/L | 228.89±76.11 | 221.80±50.89 | 249±70.35 | 163.40±53.95 | 215.76±67.86 |
| C-reactive protein, mg/L | 1.54±2.20 | 1.39±1.18 | 1.48±1.52 | 26.58±24.39 | 9.06±21.67 |
| ALT, U/L | 26.50±29.96 | 21.00±13.80 | 24.28±14.52 | 20.20±19.17 | 22.79±21.97 |
| AST, U/L | 21.50±10.33 | 24.60±11.65 | 28.56±13.37 | 28.40±15.01 | 24.50±12.33 |
| Urea, mmol/L | 4.64±1.46 | 3.63±0.96 | 5.37±2.24 | 6.64±4.86 | 4.66±3.15 |
| Creatinine, μmol/L | 49.38±4.90 | 47.90±14.07 | 50.78±21.45 | 97.60±87.57 | 58.83±46.63 |
| LDH, U/L | 184.50±33.65 | 148.00±50.35 | 178.63±59.52 | 259.00±74.94 | 188.20±70.09 |
| Creatine kinase, U/L | 63.33±22.34 | 62.38±47.88 | 58.82±26.52 | 213.00±90.67 | 99.60±171.57 |
| PT, s | 10.65±0.25 | 10.76±0.43 | 11.47±1.13 | 11.77±1.08 | 11.04±0.87 |
| APTT, s | 27.05±1.45 | 28.80±2.16 | 30.52±4.84 | 32.17±5.11 | 29.46±3.97 |
| D-dimer, mg/L | 0.54±0.29 | 0.86±1.26 | 0.73±0.58 | 0.75±0.77 | 0.76±1.05 |
| CD3 cell count | 1170.00±327.12 | 1157.89±322.29 | 1075±289.15 | 937.50±314.01 | 1113.30±326.61 |
| CD4 cell count | 669.50±187.17 | 646.44±222.68 | 684.06±205.43 | 403.25±64.64 | 606.60±210.07 |
| CD8 cell count | 431.67±155.98 | 421.22±122.13 | 391.63±133.48 | 453.00±200.91 | 427.20±150.10 |
| CD19 cell count | 189.67±73.35 | 254.44±173.80 | 240.25±108.19 | 218.25±106.65 | 232.05±137.36 |
| CD16/CD56 cell count | 166.50±39.88 | 175.11±116.98 | 170.35±88.35 | 208.75±180.79 | 174.95±117.44 |
| Days of hospitalization | 16.00±12.06 | 15.40±7.89 | 16.50±6.45 | 28.60±7.55 | 19.80±10.82 |
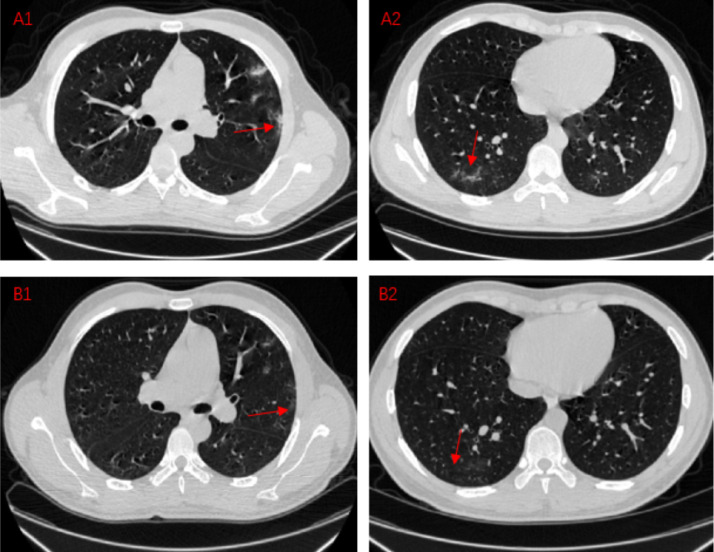
Fig. 2.
Comparison between recheck chest CT results and first CT results in COVID-19 pneumonia patients.
Situation1:Lesion absorption
A1,A2:A COVID-19 pneumonia patient, CT images on Feb 20,2020
B1,B2:A COVID-19 pneumonia patient, CT images on Feb 25,2020
These four CT images mainly shows the absorption of the lesion during the course of the disease. A1 shows the lesions parallel to the pleura under the pleura of the patient's left lung. A2 shows an ill-defined, irregularly shaped lesion. After 5 days, we recheck chest CT and find out the lesion at the original location is reduced or even disappeared. The lung tissue in the original position is gradually restored and returns to normal.
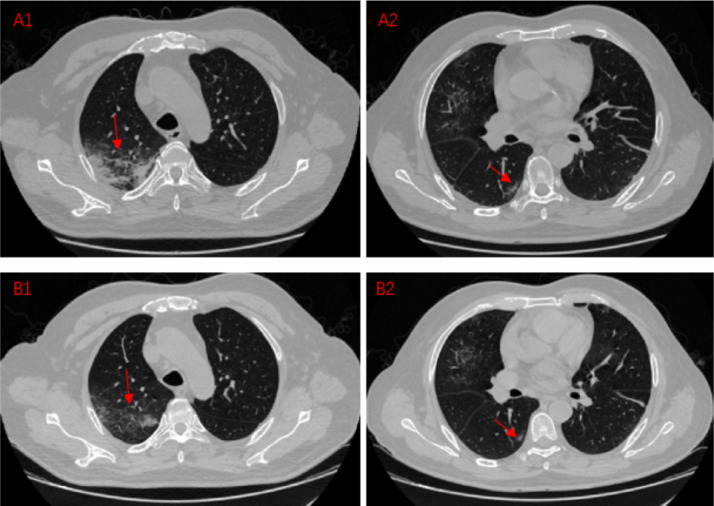
Situation2:Lesion improvement
A1,A2:A COVID-19 pneumonia patient, CT images on Feb 13,2020
B1,B2:A COVID-19 pneumonia patient, CT images on Feb 19,2020
These four CT images mainly shows the improvement of the lesion during the course of the disease. A1 shows a large, fused lesion in the patient's right lung, and the lesions are extensive. A2 shows a small lesion under the pleura in right lung. After 6 days, we recheck chest CT and find out the lesion at the original location is reduced. The expansion of the lesion is contained. The scope of the lesion is gradually reduced. Lung lesions are getting better after treatment.
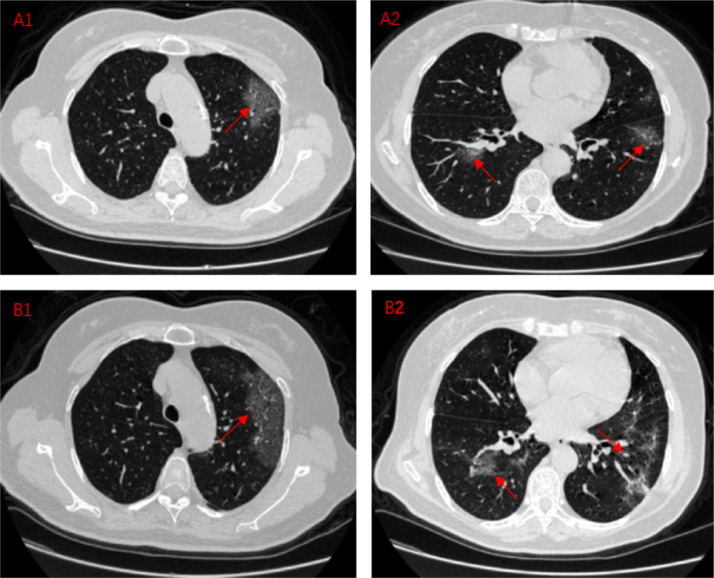
Situation3:Lesion progression
A1,A2:A COVID-19 pneumonia patient, CT images on Feb 9,2020
B1,B2:A COVID-19 pneumonia patient, CT images on Feb 16,2020
These four CT images mainly shows the improvement of the lesion during the course of the disease. A1 shows a large, fused lesion in the patient's left lung. A2 shows multiple lesions in both lungs. After 7 days, we recheck chest CT and find out the lesion at the original location is expanded. The expansion of the lesion is larger than before. The scope of the lesion is out of control. Diffuse or scattered ground glass-like shadows, superimposed with thin grid-like shadows, named as paving stone sign. This patient gradually showed clinical symptoms during this period.
Table 2.
Distribution characteristics of CT lesions in asymptomatic cases with COVID-19.
| Lesion | COVID-19 group(n = 58) | |
|---|---|---|
| number | ||
| Single | 22(37.9%) | |
| Multiple(≧2) | 36(62.1%) | |
| Distribution | ||
| unilateral | Left | 14 (24.1%) |
| Right | 20 (34.5%) | |
| bilateral | 24(41.4%) | |
| Transverse Distribution | ||
| central | 14(24.1%) | |
| peripheral | 44(75.9%) | |
| Numbers of lobes invloved | ||
| one | 24(41.4%) | |
| two | 14(24.1%) | |
| three | 6(10.3%) | |
| four | 7(12.1%) | |
| five | 7(12.1%) | |
| Location of lobes involved | ||
| left upper lobe | 31(53.4%) | |
| left lower lobe | 36(62.1%) | |
| right upper lobe | 30(51.7%) | |
| right middle lobe | 12(20.7%) | |
| right lower lobe | 40(68.9%) | |
Table 3.
Different GGO manifestations in asymptomatic cases with COVID-19 patients.
| Characteristics | Numbers |
|---|---|
| Pure GGO | 30(51.7%) |
| GGO accompanied signs | |
| fine reticulation | 7(12.1%) |
| subpleural curviliner line | 6(10.3%) |
| halo sign | 5(8.6%) |
| air bronchogram | 5(8.6%) |
| vascular enlargement | 2(3.5%) |
| consolidation | 3(5.2%) |
Discussion
Six previously known coronaviruses, including the SARS coronavirus (SARS CoV), which first appeared in China in 2003, and the Middle East respiratory syndrome coronavirus (MERS CoV), which first appeared in the Middle East in September 2012, are zoonoses and highly pathogenic coronaviruses. The novel coronavirus SARS-CoV-2, as the 7th coronavirus discovered, has high contagion and pathogenicity.
So far, more than 700,000 thousand cases with COVID-19 have been confirmed in the world. With the global outbreak of SARS-CoV-2, more and more facts show that many patients with COVID-19 pneumonia have no symptoms or only mild symptoms, but they can transmit SARS-CoV-2 to others.7, 8, 9, 10, 11 The latest research showed that 30% - 60% of patients with SARS-CoV-2 infected had no symptoms or mild symptoms, but their ability to spread the virus was not low, and these covert SARS-CoV-2 infections could be seeding new outbreaks.7 It is essential to fully understand the asymptomatic or mild symptomatic cases (covert SARS-CoV-2 infections) to control the epidemic of SARS-CoV-2.
The diagnosis of COVID-19 is mainly based on the epidemiological history, symptoms, laboratory results, imaging findings and virus nucleic acid detection according to the Chinese Guidelines for the Diagnosis and Treatment Plan of SARS-CoV-2 Infection by the National Health Commission (Trial Version 5),13 Although SARS-CoV-2 nucleic acid testing is the golden standard for the diagnosis of COVID-19, due to qualification of the specimen sampling from nasopharyngeal swab and the detection kit, the positive rate of SARS-CoV-2 nucleic acid testing is just about 30–50%.11 Furthermore, the supply of the detection kit limits its widespread use in the outbreak of COVID-19, especially in those areas with limited health care resources. Chest CT scan is of great value in the diagnosis of COVID-19 in the outbreak of COVID-19.
In this cohort, all 58 asymptomatic cases with COVID-19 pneumonia confirmed by SARS-CoV-2 nucleic acid testing and CT scan had history of exposure to SARS-CoV-2. At the time of admission, all patients had not any symptoms, but all had abnormal CT findings. GGO was the main CT manifestation in this cluster of patients (55, 94.8%), consolidation was present in other 3 patients (5.2%). The lesions of CT imaging in asymptomatic patients with COVID-19 pneumonia were predominantly located in the peripheral and subpleural area of the lung (44, 75.9%), mostly involving one or two lung lobes (38, 65.5%). GGO lesions were inclined to distribute in the lower lobes (left 62.1% vs right 68.9%). In addition to pure GGO, it is often accompanied by other characteristic imaging, including fine reticuticulatio, subpleural curviliner line, halo sign, air bronchogram and vascular enlargement.14, 15, 16, 17 The CT manifestations of COVID-19 pneumonia had its pathophysiological basis.18 Since the diameter of SARS-CoV-2 is about 60–140 nm, and the size of the alveolar pores is about 10–15 μm, after inhaled through the respiratory tract, SARS-CoV-2 invades the bronchioles, mainly involving the interstitium around bronchioles at the end of lobular bronchioles, causing bronchiolitis and peribronchitis, and spreads to the distal end. Therefore, the lesion originates from a round-like nodule in the core of the secondary lung lobule, which were usually shown as round ground-glass opacity at first, and then extends to the whole secondary pulmonary lobules, forming lobular patchy imaging. SARS-CoV-2 mainly invades the interlobular interstitium, resulting in the appearance of fine reticulation sign. Halo sign is the image that the lesion infiltrates from central nodule of lobule core to surrounding interstitium and accumulation of inflammatory cells in interstitium. The virus spreads along both sides of reticular structure of the interlobular septa, leading to the sign that the long axis of the lesion is parallel to the pleura. Inflammatory stimulation leads to thickening of blood vessels in the lesion, which results in a corresponding alteration on imaging.
Compared with the CT manifestation at admission, there were significant changes presented in CT findings after short-term treatment, including lesion absorption (22 cases, 37.9%), lesion improvement (23 cases, 39.7%), lesion no change (3 cases, 5.2%) and lesion progression (10 cases, 17.2%). After admission, 16 patients (27.6%) presented symptoms, predominantly including fever (8 cases, 50%), cough (9 cases, 56.3%), fatigue (8 cases, 50%). The average days before symptoms onset is 3.71±2.86 days, patients in lesion progression group have shorter days than other group (3.25±2.77 day). In these 16 patients, 10 patients (17.2%) were in lesion progression group. The results indicated in asymptomatic patients with COVID-19 pneumonia, with the disease progress, about 25% cases present symptoms, and the evolution of CT findings were shown in 17% patients in the short-term follow-up. The average age of patients in lesion progression group was 57.40±22.54, much older than other patients (P = 0.016). This may indicate that younger patients with pneumonia have strong immunity and recover quickly. With the progress of the inflammation, scatttered lesion gradually fused to form patchy, crazy-paving pattern, even diffusion appearance in CT manifestation. All the asymptomatic patients with COVID-19 pneumonia discharged after treatment. The mean days of hospitalization was 19.80±10.82 days and the hospitalization of patients in progression group was 28.60±7.55 day, significantly longer than other cases (P = 0.016).
The cases recruited in this study were all asymptomatic patients with COVID-19 pneumonia, which were confirmed by positive results of SARS-COV-2 nucleic acid testing and abnormal findings in CT imaging. The predominant feature of CT findings in asymptomatic patients with COVID-19 pneumonia was ground glass opacity with peripheral distribution, unilateral location and, mostly involving one or two lobes, often combined with subpleural curviliner line, fine reticulation, air bronchogram, halo sign or vascular enlargement signs. After a short-term follow-up, a few asymptomatic patients presented clinical symptom, mainly fever, cough and fatigue. And the evolution of CT findings was shown in patients with the progression of disease.
In summary, CT images of asymptomatic cases with COVID-19 pneumonia have definite characteristics. Since the asymptomatic patients with COVID-19 is called “covert transmitter”, covert SARS-CoV-2 infections could be seeding new outbreaks, and some patients can progress rapidly in the short term. Therefore, it is essential to pay attention to the surveillance of asymptomatic patients with COVID-19. CT examination plays a vital role in managing the current COVID-19 outbreak, for early detection of COVID-19 pneumonia, especially in the highly suspicious, asymptomatic cases with negative SARS-CoV-2 nucleic acid testing.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Acknowledgments
Acknowledgment
We thank for all patients and their families involved in the study. We thank all the authors for their contributions.
Funding
This work was supported by National Natural Science Foundation of China (81770095, 81700093)
Ethics Approval
The study was approved by the Ethics Committee of Renmin Hospital of Wuhan University (approved number: WDRY2020-K009), which waived the requirement for patients’ informed consent referring to the CIOMS guideline.
Consent to Participate
Written informed consent was waived in light of the urgent need to collect data.
Consent for Publication
All authors have confirmed this article and agree to publication.
References
- 1.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, china. JAMA. 2020 doi: 10.1001/jama.2020.1585. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorbalenya A.E., Baker S.C., Baric R.S. Severe acute respiratory syndrome-related coronavirus: the species and its viruses-a statement of the coronavirus study group. bioRxiv. 2020 doi: 10.1101/2020.02.07.937862. 02.07.937862. [DOI] [Google Scholar]
- 4.World Health Organization. WHO director-general's remarks at the media briefing on 2019-nCoV on 11Feb 11, 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020 (Accessed 1 March 2020).
- 5.Chinese Center for Disease Control and Prevention. Coronavirus disease (COVID-2019) situation reports. March 30, 2020. http://2019ncov.chinacdc.cn/2019-nCoV/global.html (Accessed 30 March 2020).
- 6.Qiu J. Covert coronavirus infections could be seeding new outbreaks. Nature. 2020 doi: 10.1038/d41586-020-00822-x. Correction 20 MARCH. [DOI] [PubMed] [Google Scholar]
- 7.C. Wang, L. Liu, X. Hao, H. Guo, Q. Wang, J. Huang, N. He, H. Yu, X. Lin, A. Pan, S. Wei, T. Wu. Evolving epidemiology and impact of non-pharmaceutical interventions on the outbreak of coronavirus disease 2019 in Wuhan, China. medRxiv. doi:10.1101/2020.03.03.20030593
- 8.Nishiura H., Kobayashi T., Suzuki A., Jung S.-M., Hayashi Katsuma, Kinoshita R., Yang Y., Yuan B., Akhmetzhanov A.R., Linton N.M, Miyama T. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19) Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.R. Woelfel, V. M. Corman, W. Guggemos, M. Seilmaier, etc. Clinical presentation and virological assessment of hospitalized cases of coronavirus disease 2019 in a travel-associated transmission cluster. medRxiv. doi: 10.1101/2020.03.05.20030502
- 10.Hansell Fleischner society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 11.Li J., Ye G.M., Chen L.J. Analysis of false-negative results for 2019 novel coronavirus nucleic acid test and related countermeasures. Chin J Lab Med. 2020;43 doi: 10.3760/cma.j.issn.1009-9158.2010.0006. [Epub ahead of print] [DOI] [Google Scholar]
- 12.Liu K., Fang Y.Y., Deng Y., Liu W., Wang M.F. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei province. Chin Med J (Engl.) 2020 doi: 10.1097/CM9.0000000000000744. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin L., Li T.S. Interpretation of "Guidelines for the diagnosis and treatment of novel coronavirus (2019-nCoV) infection by the national health commission (Trial version 5)". Natl Med J China. 2020;100(00):E001. doi: 10.3760/cma.j.issn.0376-2491.2020.0001. [DOI] [PubMed] [Google Scholar]
- 14.Huang C., Wang Y., Li X., Ren L., Zhao J. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chong S., Kim T.S., Cho E.Y. Herpes simplex virus pneumonia: high-resolution ct findings. Br. J. Radiol. 2010;83(991):585–589. doi: 10.1259/bjr/51409455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koo H.J., Lim S., Choe J. Radiographic and ct features of viral pneumonia. Radiographics. 2018;38(3):719–739. doi: 10.1148/rg.2018170048. [DOI] [PubMed] [Google Scholar]
- 17.Chung M., Bernheim A., Mei X. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020 doi: 10.1148/radiol.2020200230. 200230 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicholls J.M., Poon L.L., Lee K.C. Lung pathology of fatal severe acute respiratory syndrome. The Lancet. 2003;361(9371):1773–1778. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]