Highlights
-
•
The immune status is significantly different between severe and non-severe COVID-19.
-
•
The decrease of T lymphocyte correlated with the course of patients with COVID-19.
-
•
The level of T lymphocyte is an indicator for severity and prognosis of COVID-19.
Keywords: SARS-CoV-2, COVID-19, immunity, lymphocyte subsets
Abstract
Objectives
To explore the clinical course and its dynamic features of immune status in COVID-19 patients and find predictors correlated with severity and prognosis of COVID-19.
Methods
The electronic medical records of 204 patients with COVID-19 pneumonia confirmed by nucleic acid testing were retrospectively collected and analyzed.
Results
All patients were divided into severe (69) and non-severe group (135). Lymphocyte subsets count, including CD3+ T cell, CD4+ T cell, CD8+ T cell, B cell (CD19+) and NK cell (CD16+ 56+), were significantly lower in severe group (P<0.001). The dynamic levels of T lymphocyte in severe group were significantly lower from disease onset, but in the improved subgroup the value of T lymphocyte began to increase after about 15-day treatment and finally returned to the normal level. The cut-off value of the counts of CD3+ (576), CD4+ (391) and CD8+ (214) T cell were calculated and indicated significantly high sensitivity and specificity for severity of COVID-19.
Conclusion
Our results shown that the decrease of CD3+, CD4+ and CD8+ T lymphocyte correlated with the course of patients with COVID-19 pneumonia, especially in severe cases. The level of T lymphocyte could be used as an indicator for prediction of severity and prognosis of patients with COVID-19 pneumonia. The application of glucocorticoid should be cautious in severe cases.
1. Introduction
In December 2019, the acute viral respiratory diseases named as Coronavirus Disease 2019 (COVID-19) was reported in Wuhan, China. COVID-19 is caused by a novel coronavirus, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). The World Health Organization (WHO) has listed the novel coronavirus pneumonia epidemic as a public health emergency of international concern. As of April 1, 2020, over 700,000 SARS-CoV-2 infection had been confirmed all over the world.
The clinical presentation of COVID-19 varies from mild to severe, but most elder patients or patients with known comorbidities, such as hypertension, diabetes, cardiomyopathy, chronic renal failure, chronic obstructive pulmonary disease (COPD) and decreased immunity appear to be at a higher risk of developing severe disease, resulting in a high case-fatality rate1 , 2. A retrospective study showed the mortality was 61.5% in severe patients with COVID-193. Early identification and intervention for the underlying severe patients was crucial for reduction of mortality.
Due to the lack of specific antiviral drugs and vaccine, own immune status become one of the most crucial factors affecting disease progression and prognosis. The clinical laboratory data of patients revealed that most COVID-19 cases displayed significantly low circulating lymphocyte counts, especially severe patients admitted to ICU1 , 2 , 4.
We aimed to characterize the immune status between the severe and non-severe patients with COVID-19 pneumonia, review the correlation of the clinical course and dynamic changes of T lymphocyte subsets in those patients, and attempt to find an indicator for early identification of the underlying severe patients with COVID-19 pneumonia.
2. Methods
2.1. Study design and Participants
This is a retrospective study. All patients confirmed by fluorescence reverse-transcriptase-polymerase chain reaction (fRT-PCR) assay for SARS-CoV-2 at Renmin Hospital of Wuhan University between January 10.2020 and February 13,2020 were enrolled. All patients were divided into severe and non-severe groups according to "Pneumonia diagnosis and treatment program for novel coronavirus infection (trial version 5)" issued by National Health Commission of the People’s Republic of China (http://www.nhc.gov.cn/). The classification criteria were summarized as Table S1. The end of follow-up was February 13, 2020.
2.2. Data Collection
The electronic medical records, including the epidemiological, clinical, laboratory, and radiological findings of patients were collected and analyzed. The laboratory results were obtained within the early stage of the disease. The cases with incomplete data were excluded.
2.3. Statistical Analysis
Statistical analysis was performed using SPSS 23.0 software and GraphPad prism 8.0 software. Percentages and frequency rates were used to describe categorical variables, mean, median, interquartile range (IQR) and standard deviation (SD) were used to describe continue variables. T test or Mann-Whitney test was used to compare mean for continuous variables. χ2 test or Fisher exact test was used to compare proportions of categorical variables. Pearson correlation tests were also performed. The Receiver operating characteristics (ROC) curve was used to assess the sensitivity and specificity of lymphocyte subsets for classification of COVID-19.
3. Results
3.1. Clinical Characteristics
A total of 204 patients were enrolled in the study. The median age of 204 patients was 49 (IQR, 34-62), and 79 (38.73%) patients were male. There were 57 (57, 27.94%) patients with comorbidities, including hypertension, diabetes, malignancy, chronic lung disease and other diseases. The clinical manifestations of COVID-19 pneumonia were listed in Table 1
Table 1.
Clinical characteristics of all patients
| No. (%) |
P value | |||
|---|---|---|---|---|
| Total (N = 204) | non-severe (n = 135) | severe (n = 69) | ||
| Age, median (IQR), year | 49 (34-62) | 43 (31-53) | 61 (52-74) | <0.001 |
| Gender | ||||
| Male | 79 (38.73) | 42 (31.11) | 37 (53.62) | 0.002 |
| Female | 125 (61.27) | 93 (68.89) | 32 (46.38) | |
| Comorbidities | ||||
| Hypertension | 36 (17.65) | 10 (7.41) | 26 (37.68) | <0.001 |
| Cardiovascular disease | 5 (2.45) | 0 | 5 (7.25) | 0.04 |
| Malignancy | 5 (2.45) | 1 (0.74) | 4 (5.80) | 0.083 |
| Diabetes | 16 (7.84) | 8 (5.93) | 8 (11.59) | 0.154 |
| Chronic liver disease | 2 (0.98) | 2 (1.48) | 0 | 0.55 |
| Chronic lung disease | 3 (1.47) | 2 (1.48) | 1 (1.45) | 1.000 |
| Cerebral aneurysm | 8 (3.92) | 1 (0.74) | 7 (10.14) | 0.004 |
| Urologic disease | 7 (3.43) | 4 (2.96) | 3 (4.35) | 0.914 |
| Endocrine disease (except diabetes) | 20 (9.80) | 9 (6.67) | 11 (15.94) | 0.35 |
| Signs and symptoms | ||||
| Fever | 146 (71.57) | 90 (66.67) | 56 (81.16) | 0.300 |
| Cough | 84 (41.18) | 51 (37.78) | 33 (47.83) | 0.168 |
| Expectoration | 42 (20.59) | 32 (23.70) | 10 (14.49) | 0.124 |
| Pharyngalgia | 24 (11.76) | 19 (14.07) | 5 (7.25) | 0.152 |
| Chest congestion | 44 (21.57) | 30 (22.22) | 14 (20.29) | 0.751 |
| Dyspnea | 70 (34.31) | 21 (15.56) | 49 (68.12) | <0.001 |
| Diarrhea | 19 (9.31) | 14 (10.37) | 5 (7.25) | 0.486 |
| Headache | 14 (6.86) | 11 (8.15) | 3 (4.35) | 0.47 |
| Myalgia | 20 (9.80) | 14 (10.37) | 6 (8.70) | 0.704 |
| Fatigue | 46 (22.55) | 33 (24.44) | 13 (18.84) | 0.365 |
| Anorexia | 12 (5.88) | 7 (5.19) | 5 (7.25) | 0.554 |
| Radiological characteristics | ||||
| Unilateral | 49 (24.02) | 43 (31.85) | 6 (8.70) | <.001 |
| Bilateral | 155(75.98) | 92 (68.15) | 63 (91.30) | |
| Single lung lobe | 45 (22.06) | 39 (28.89) | 6 (8.70) | 0.001 |
| Multiple lung lobe | 159 (77.94) | 96 (71.11) | 63 (91.30) | |
| Ground glass opacity | 130 (63.73) | 90 (66.67) | 40 (57.97) | 0.222 |
| Patchy shadows | 74 (36.27) | 45 (33.33) | 29 (42.03) | |
Abbreviations: IQR, interquartile range
Patients were divided into non-severe (135, 66.18%) and severe group (69, 33.82%). There was significant difference in age between non-severe (43, IQR, 31-53) and severe (61, IQR, 52-74) group. Compared to non-severe patients, the proportion of some comorbidities, including hypertension, cardiovascular diseases and cerebral aneurysm, were significantly higher in severe group. The proportion of patients with dyspnea (68.12%) was also significant higher in severe group.
3.2. Radiological and laboratory data
Chest CT was the important method of diagnosis for COVID-19 pneumonia. There was significant difference between non-severe and severe group. The bilateral and multiple lobe lesions were seen in the most patients with COVID-19 (all P<0.01).
Significant difference in laboratory findings were observed between non-severe and severe group (Table 2 ), including median white blood cell count (P = 0.021), neutrophil count (P<0.001), lymphocyte count (P<0.001), platelet count (P<0.001), D-dimer (P = 0.018), aspartate aminotransferase (P = 0.002), urea (P<0.001), lactate dehydrogenase (P<0.001) and C-reactive protein (P<0.001).
Table 2.
The laboratory findings of all patients
| Normal range | Media (IQR) |
P value |
|||
|---|---|---|---|---|---|
| Total (N = 204) | non-severe (n = 135) | severe (n = 69) | |||
| White blood cell count, ×109 /L | 3.5-9.5 | 4.75 (3.98-5.94) | 4.74 (4.04-5.76) | 4.84 (3.83-7.85) | 0.021 |
| Neutrophil count, ×109 /L | 1.8-6.3 | 2.93 (2.14-4.05) | 2.69 (2.03-3.61) | 3.87 (2.49-6.11) | <0.001 |
| Lymphocyte count, ×109 /L | 1.1-3.2 | 1.17 (0.84-1.61) | 1.43 (1.12-1.88) | 0.76 (0.55-0.93) | <0.001 |
| Platelet count, ×109 /L | 125-350 | 189 (153-240) | 200 (167-261) | 171 (138-217) | <0.001 |
| Prothrombin time, s | 9-13 | 11.7 (11.1-12.4) | 11.6 (11.0-12.3) | 11.8 (11.2-12.7) | 0.081 |
| D-dimer, mg/L | 0-0.55 | 0.53 (0.23-1.24) | 0.32 (0.20-0.70) | 0.95 (0.41-3.10) | 0.018 |
| Alanine aminotransferase, U/L | 9-50 | 18.5 (14.0-30.0) | 16.5 (11.3-26.5) | 22.5 (16.8-33.3) | 0.137 |
| Aspartate aminotransferase, U/L | 15-40 | 24.0 (19.0-32.0) | 21.0 (17.0-28.8) | 29.0 (22.8-37.0) | 0.002 |
| Urea, mmol/L | 3.1-8.0 | 4.09 (3.21-5.26) | 3.83 (3.02-4.60) | 5.40 (3.95-7.33) | <0.001 |
| Creatinine, μmol/L | 57-97 | 60 (48-69) | 54 (47-63) | 89 (51-86) | 0.109 |
| Lactate dehydrogenase, U/L | 120-250 | 211 (169-271) | 188 (161-228) | 276 (214-365) | <0.001 |
| Creatine kinase, U/L | 50-310 | 60 (39-101) | 54 (38-86) | 89 (43-129) | 0.152 |
| C-reactive protein, mg/L | 0-5 | 7.4 (0.5-35.0) | 3.45 (0.5-17.1) | 42.7 (11.5-72.5) | <0.001 |
| Procalcitonin, ng/mL | <0.1 | 0.05 (0.02-0.13) | 0.023 (0.02-0.05) | 0.102 (0.04-0.28) | 0.017 |
| Hypersensitive troponin I, ng/mL (86)a | 0-0.04 | 0.0065 (0.006-0.025) | 0.006 (0.006-0.006) | 0.017 (0.006-0.043) | 0.369 |
| Lymphocyte subpopulation | |||||
| CD3+ count, /μL | 723-2737 | 801 (496-1154) | 1066 (804-1321) | 305 (198-525) | <0.001 |
| CD4+ count, /μL | 404-1612 | 461 (269-701) | 645 (461-794) | 184 (103-293) | <0.001 |
| CD8+ count, /μL | 220-1129 | 291 (164-424) | 366 (274-482) | 121 (54-197) | <0.001 |
| CD4/CD8 | 0.9-2.0 | 1.66 (1.27-2.20) | 1.66 (1.37-2.16) | 1.57 (1.10-2.36) | 0.342 |
| CD19+ count, /μL | 80-616 | 155 (105-229) | 190 (139-268) | 91 (54-139) | <0.001 |
| CD16+ 56+ count, /μL | 84-724 | 127 (81-209) | 144 (93-231) | 105 (66-168) | <0.001 |
| Humoral immune function (189)b | |||||
| IgG, g/L | 8-16 | 12.1 (10.0-14.7) | 11.6 (9.9-13.8) | 13.4 (10.5-16.5) | 0.024 |
| IgM, g/L | 0.4-3.45 | 1.04 (0.75-1.34) | 1.11 (0.86-1.38) | 0.94 (0.66-1.22) | 0.009 |
| IgA, g/L | 0.76-3.9 | 1.91 (1.45-2.53) | 1.84 (1.45-2.44) | 2.10 (1.43-2.85) | 0.281 |
| IgE, IU/mL | <100 | 23.5 (17.3-80.8) | 21.9 (17.3-84.3) | 30.85 (17.3-68.9) | 0.707 |
| Complement C3, g/L | 0.81-1.6 | 0.85 (0.74-0.98) | 0.82 (0.73-0.96) | 0.91 (0.78-1.00) | 0.045 |
| Complement C4, g/L | 0.1-0.4 | 0.24 (0.18-0.31) | 0.23 (0.17-0.30) | 0.26 (0.20-0.32) | 0.275 |
| Cytokines (28)c | |||||
| IL-2, pg/ml | ≤11.4 | 3.65 (3.42-4.06) | 3.55 (3.38-3.65) | 4.06 (3.28-4.09) | 0.249 |
| IL-4, pg/ml | ≤12.9 | 3.95 (3.74-4.36) | 3.75 (3.70-3.85) | 4.30 (4.01-4.60) | 0.005 |
| IL-5, pg/ml | ≤20.0 | 2.33 (2.22-2.56) | 2.39 (2.30-3.05) | 2.27 (2.12-2.35) | 0.062 |
| IL-6, pg/ml | ≤20.0 | 14.1 (7.5-14.0) | 14.0 (7.2-15.3) | 14.3 (7.8-11.6) | 0.953 |
| IL-10, pg/ml | ≤5.9 | 6.48 (6.07-7.65) | 6.37 (5.71-6.67) | 7.25 (6.20-8.05) | 0.147 |
| TNF, pg/ml | ≤5.5 | 2.72 (2.47-3.00) | 2.50 (2.44-2.73) | 2.98 (2.63-3.11) | 0.004 |
| γ-interferon, pg/ml | ≤18 | 3.8 (3.57-4.46) | 3.93 (3.51-4.61) | 3.8 (3.8-3.93) | 0.334 |
Abbreviations, IQR, interquartile range.
The number of patients whose data of Hypersensitive troponin I was available were 86, including 37 non-severe patients and 49 severe patients.
The number of patients whose data of humoral immune function was available were 189, including 125 non-severe patients and 64 severe patients.
The number of patients whose data of cytokines was available were 28, including 12 non-severe patients and 16 severe patients.
Lymphocyte subsets count, including CD3+ T cell, CD4+ T cell, CD8+ T cell and B cell (CD19+) and NK cell (CD16+56+), were significantly lower in severe group than those in non-severe group (all P<0.001). For the humoral immune function, the significantly higher level of IgG (P = 0.024) and Complement C3 (P = 0.045) and lower IgM (P = 0.009) were detected in patients of severe group. The level of IL-4 (P = 0.005) and TNF-α (P = 0.004) were significantly higher in severe group.
Patients also were divided into age<60 and age ≥ 60 group. There were significant differences in count of lymphocyte subsets between two groups (Fig. S1a). Compared to patients without comorbidities, the count of T lymphocyte and value of humoral immune, such as IgM, IgA and C4, were significant lower in patients with comorbidities (Fig. S1g, j).
3.3. Dynamic profile of lymphocyte subsets
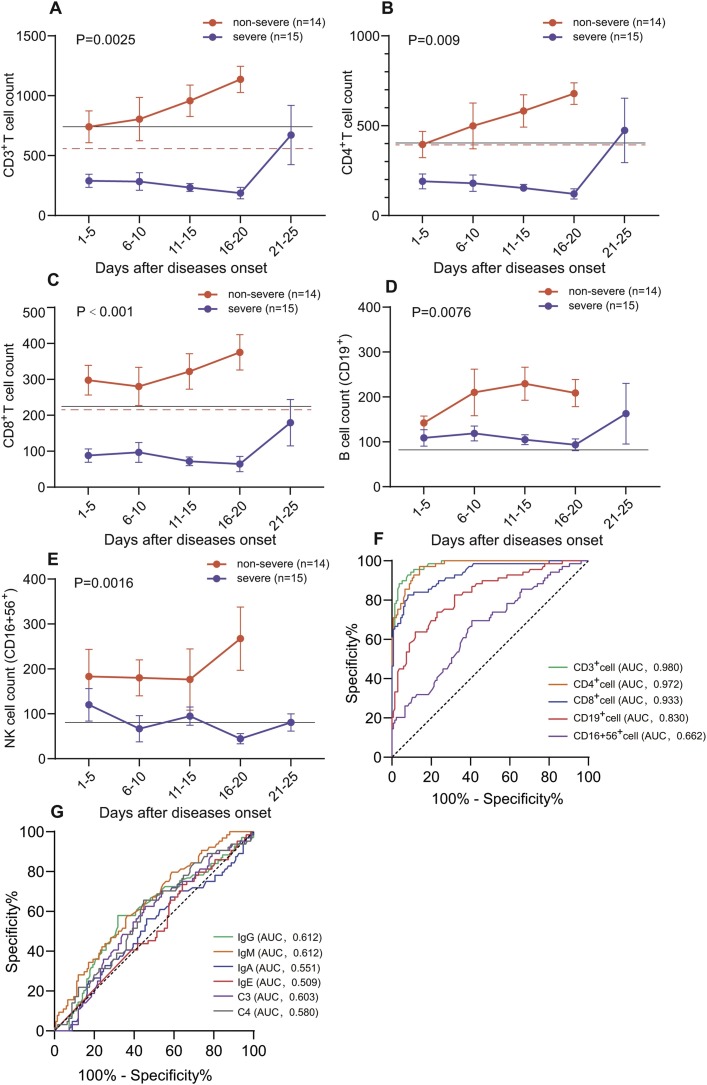
The results shown that the level of all lymphocyte subsets was normal during hospitalization in non-severe group (Fig. 1 ). The level of T lymphocyte was significant lower in patients of COVID-19 pneumonia in severe group (Fig. 1a–c). To further investigate the relationship between level of lymphocyte subsets with clinical course of COVID-19, according to the results of treatment, 15 patients in severe group were divided into improved subgroup (7 patients) and dead subgroup (8 patients).
Fig. 1.
Dynamic changes of lymphocyte subsets count between non-severe and severe group. The count of CD3+ T cell (A), CD4+ T cell (B), CD8+ T cell (C), B cell (D) and NK cell (E) were illustrated in chronological order. The solid lines (black) show the lower normal limit of each parameter. The dotted line (red) show the cut-off value calculated by ROC analysis. The sensitivity and specificity of lymphocyte subsets count (F) and level of humoral immune function (G) for classification of COVID-19. Error bars, mean ± sem.
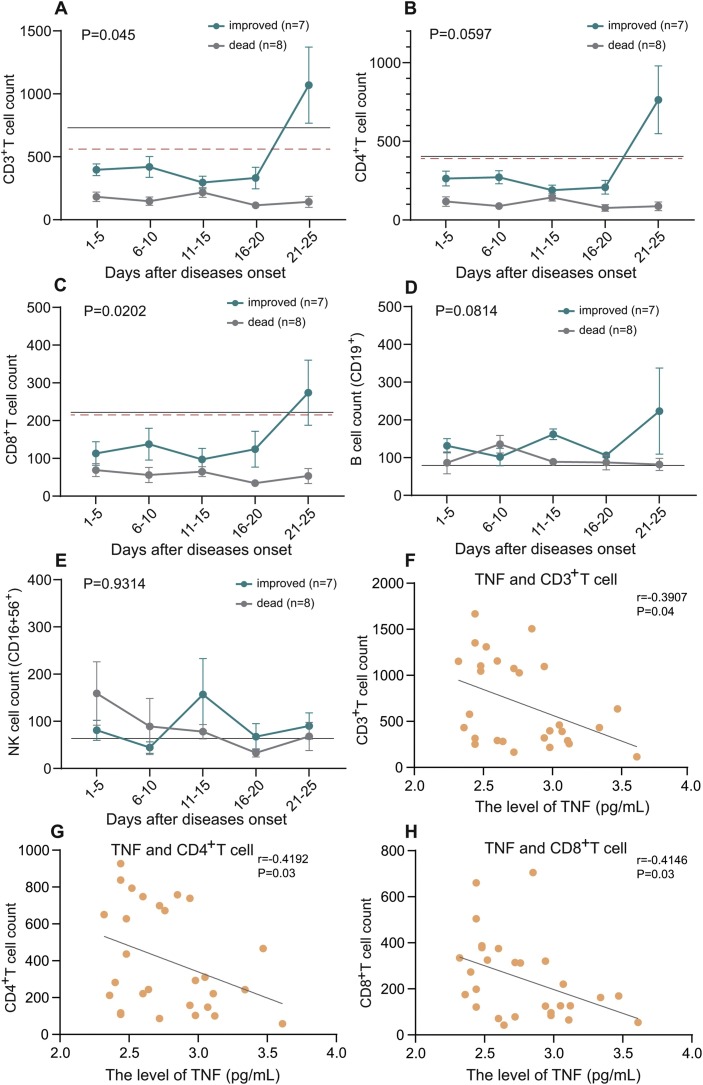
The dynamic results indicated that the level of T lymphocyte in dead subgroup continued to decrease until death. However, the count of T lymphocyte began to increase after 15-day treatment, finally returned to the normal level after 25-day treatment in patients of improved subgroup (Fig. 2 a–c). The time of recovery of lymphocyte count was approximately consistent with the time point of improvement of clinical course. The levels of B cell and NK cell were close to normal range and there was no significant difference in both groups.
Fig. 2.
Dynamic changes of lymphocyte subsets count between improved and dead group. The severe patients were divided into improved and dead groups according to the outcomes as of February 13. The count of CD3+ T cell (A), CD4+ T cell (B), CD8+ T cell (C), B cell (D) and NK cell (E) were illustrated in chronological order. The solid lines (black) show the lower normal limit of each parameter. The dotted line (red) show the cut-off value calculated by ROC analysis. Scatter plot described the correlation between TNF with CD3+ T cell (F), CD4+ T cell (G) and CD8+ T cell (H), respectively.
Our results shown that the levels of TNF-α and IL-4 were negatively correlated with the counts of T cell respectively in severe patients with COVID-19 pneumonia (Figs. 2f–h, S2a). The level of IgG (Fig. S2b) and C3 (Fig. S2d) were also negatively correlated with the counts of T cell, but IgM shown positive correlation (Fig. S2c).
3.4. Treatment and prognosis
All patients were isolated for standardized treatment followed ‘Pneumonia diagnosis and treatment program for novel coronavirus infection’ updated in real time issued by National Health Commission of the People’s Republic of China. Treatment mainly included antiviral therapy (98.53%, oseltamivir, Arbidol, Ganciclovir and Lopinavir and Ritonavir Tablets), antibiotic therapy (97.02%, moxifloxacin, levofloxacin, cephalosporins), albumin infusion (25.98%). Glucocorticoid (25.00%) was used at appropriate time. The ventilator was required in 27 patients (13.24%). Invasive mechanical ventilation was required in 10 patients (4.90%). As of February 13, 134 (65.69%) patients were discharged, 44 (21.57%) patients were hospitalization and 26 (12.75%) patients were dead.
3.5. The ROC curve (AUC) in severe patients
Our results have indicated that the level of lymphocyte subsets was strongly correlated with the course of COVID-19 patients. ROC curve and area under ROC curve (AUC) were used to assess the diagnostic value of them for identification of severe COVID-19. Our results shown that the count of CD3+, CD4+ and CD8+ T cell had significantly high sensitivity and specificity (Fig. 1f), and the AUC were 0.980 (95%CI, 0.966-0.995), 0.972 (95%CI, 0.954-0.990) and 0.933 (95%CI, 0.896-0.969), respectively in severe patients with COVID-19 pneumonia. Simultaneously, the cut-off value was calculated according to ROC curve, including CD3+ T cell (576), CD4+ T cell (391), CD8+ T cell (214), B cell (111) and NK cell (128). The sensitivity and specificity of humoral immune parameters were not excellent and the AUC of them were at the range from 0.5 to 0.612 and lower than AUC of T lymphocytes (Fig. 1g). In addition, our results shown that the cut-off value of T lymphocytes can excellently identify the severe and non-severe groups in 29 patients with dynamic data. The 7 patients in severe group improved and transformed into non-severe type after 20-day treatment, and the cut-off value of T lymphocytes also excellently predicted the dynamic trends of diseases.
4. Discussion
SARS-CoV-2 has high infectivity and pathogenicity. The basic reproductive number R0 of SARS-Cov-2 was estimated to be 3.77, much higher than SARS-CoV(R0 = 2.7) and MERS-CoV (R0<1)[5], [6], [7]. The affinity between SARS-CoV-2 and ACE2 receptor is 10-20 times higher than that of SARS-CoV8. Without a therapeutic vaccine or specific antiviral drugs, early detection and intervention for the underlying severe patients was crucial for reduction of mortality.
Consistent with previous studies1 , 2, the average age of patients and its comorbidities in severe group were significantly higher than those in non-severe group. The distribution of gender was significantly different between two groups. In severe groups, the mortality of male patients was higher, suggesting that the male patient may be more tend to develop into severe illness. The epidemiological data from Chinese Center for Disease Control and Prevention (CCDC) also indicated that the mortality of male patients was higher than the female. The sex-based immunological differences also contribute to variations in the susceptibility to infectious diseases and responses to virus9.
There were significant increases of white blood cell and neutrophil, decrease of lymphocyte in severe patients, which were consistent with other COVID-19 studies. The further analysis about lymphocyte subsets also indicated that the count of all lymphocyte subsets significantly declined in severe patients than those in non-severe group. CD3 as the biomarker of mature T lymphocyte, helps to activate the CD4+ T cell and CD8+ T cell10. CD4+ T cell and CD8+ T cell have an important role in regulating and maintaining the stability of the internal immune environment11. Some research about SARS shown that a dramatic loss of CD4+ T (90–100% of patients) and CD8+ T cells (80–90% patients) was characterized in patients with the acute phase of SARS12. The mechanism of the reduction of T lymphocytes may be associated with the direct invasion of SRAS-COV-2, which have been confirmed in MERS-CoV13. In addition, the development of autoimmune antibodies triggered by viral infection may lead to growth inhibition and apoptosis of hematopoiesis14, which can decline the production and differentiation of T lymphocytes and other lymphocytes. Severe SARS-CoV infected patients presented the delayed development of the adaptive immune response and prolonged virus clearance15. SARS-CoV-2 may have the ability to activates the extrinsic and intrinsic apoptosis pathways and promote apoptosis of T lymphocytes as well as that in cases with MERS-CoV13. The level of T lymphocytes also regulated by antigen presenting cell (APC) and dendritic cell (DC) cells, and SARS-CoV-2 may alter function of APC and impair DC migration, which can reduce priming of T lymphocytes16 , 17. In addition to being affected by SARS-CoV-2 and other immune cell, the function of T lymphocytes was also regulated by cytokines. TNF-α is a pro-inflammatory cytokine which can promote T lymphocytes apoptosis through interacting with TNFR1, which mainly expressed on aged T cell18. The production of IL-4 can block regulatory T cell function19.
The results of the dynamic changes of lymphocyte subsets in the course of COVID-19 further confirmed the correlation between the level of T lymphocytes with severity of COVID-19 pneumonia. From the onset of COVID-19 pneumonia, the level of T lymphocytes gradually increased during the treatment in non-severe patients, and always significantly higher than those in severe group. In the improved subgroup of severe patients, the value of T lymphocytes began to increase after 15-day treatment, finally returned to the normal level after 25-day treatment. The time of recovery of T lymphocyte count was approximately consistent with the clinical course. In contrast, the level of T lymphocytes in dead subgroup of severe patients continued to fall until death. Compared to young patients with COVID-19 pneumonia, the elderly patients with comorbidities had a worse prognosis was associated with the poor immunity, especially the lower function of T lymphocytes. However, there were no obvious decrease of B cell and NK cell between improved and dead subgroup of severe patients, most of them kept in normal level. It is because that the cellular immune, T lymphocytes plays a crucial role in virus clearance after virus infection, while humoral immune is primarily mediated by the production of antibodies, neutralization of virus to play a role. T lymphocytes directly dissolve and destroy infected cells to eliminate the virus, and secret cytokines which enhance the immune response of T lymphocytes and other immunologically active cells such as macrophages and B lymphocytes20.
The complement system was a critical part of host defense to many bacterial, viral, and fungal infections. But the complement system was also strictly regulated through a number of inhibiting proteins in the serum, because of its potential to damage host tissues21. Our results shown that complement C3 were significantly higher in severe patients with COVID-19 pneumonia than that in non-severe patients. It had been found that complement activation regulates a systemic proinflammatory response to SARS-CoV infection and the inhibition of complement C3 can reduced systemic inflammation22. The anaphylatoxins C3a, C4a, and C5a, which were produced during activation of complement signaling, had potent proinflammatory properties and can aggravate the viral infection and acute lung injury23. The complement system may play an essential role in COVID-19 pneumonia. The inhibition of complement C3 in patients may reduce the proinflammation response and acute lung injury induced by SARS-CoV-2 infection, which may provide a new therapy for COVID-19.
The results of ROC curve further confirmed that the levels of CD3+, CD4+ and CD8+ T cell performed excellent sensitivity and specificity for severity of COVID-19. The cut-off value of T lymphocytes also excellently predicted the dynamic trends of diseases, facilitate to detect and intervene the underlying severe patients earlier, which was crucial for reduction of mortality.
The results of our study also provided some new perspectives for treatment of severe COVID-19. Glucocorticoids which can suppress cell-mediated immunity, promote the decrease of T lymphocyte and delay the virus clearance24, may aggravate the impaired function of T lymphocytes in patients with SARS-CoV-2. Therefore, the use of glucocorticoid should be cautious in severe patients with COVID-19 pneumonia, and the timing of glucocorticoid application deserves further exploration. Improvement of T lymphocyte function maybe a promising therapy for severe patients with COVID-19 pneumonia.
5. Conclusions
In conclusion, we characterized and analyzed the clinical course and its correlated immune function of patients with COVID-19 pneumonia. The decrease of CD3+, CD4+ and CD8+ T lymphocyte correlated with the course of patients with COVID-19 pneumonia, especially in severe cases. The level of T lymphocyte could be used as an indicator for prediction of severity and prognosis of patients with COVID-19 pneumonia. The use of glucocorticoid should be cautious in severe patients, the timing of glucocorticoid application deserves further exploration. Improvement of T lymphocytes function maybe a potential therapeutic strategy for severe patients with COVID-19 pneumonia.
Author Contributions
Conceptualization, Ruyuan He, Zilong Lu and Qing Geng; Data curation, Qing Geng; Formal analysis, Ruyuan He and Zilong Lu; Funding acquisition, Qing Geng; Methodology, Ruyuan He, Zilong Lu, Tao Fan, Rui Xiong and Xiaokang Shen; Project administration, Ruyuan He and Qing Geng; Supervision, Ruyuan He and Qing Geng; Validation, Zilong Lu, Lin Zhang, Wenyang Jiang and Qing Geng; Visualization, Ruyuan He; Writing – original draft, Ruyuan He; Writing – review & editing, Lin Zhang, Haojie Feng, Heng Meng, Weichen Lin and Qing Geng. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the National Natural Science Foundation of China (grant number 81770095)
Ethics approval and consent to participate
The study was a retrospective analysis and was approved by the Ethics Committee of Renmin Hospital of Wuhan University (approved number: WDRY2020-K009).
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgment
We acknowledge health-care workers involved in the diagnosis and treatment of COVID-19 and all patients in our study.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jcv.2020.104361.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D., Hu B., Hu C. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA. 2020;(February) doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;(February) doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y., Lu Q., Liu M. Epidemiological and clinical features of the 2019 novel coronavirus outbreak in China. medRxiv. 2020 doi: 10.1101/2020.02.10.20021675. 2020.02.10.20021675. [DOI] [Google Scholar]
- 6.Breban R., Riou J., Fontanet A. Interhuman transmissibility of Middle East respiratory syndrome coronavirus: estimation of pandemic risk. The Lancet. 2013;382(9893):694–699. doi: 10.1016/S0140-6736(13)61492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riley S., Fraser C., Donnelly C.A. Transmission Dynamics of the Etiological Agent of SARS in Hong Kong: Impact of Public Health Interventions. Science. 2003;300(5627):1961. doi: 10.1126/science.1086478. [DOI] [PubMed] [Google Scholar]
- 8.Wrapp D., Wang N., Corbett K.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020 doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 10.Dong D., Zheng L., Lin J. Structural basis of assembly of the human T cell receptor-CD3 complex. Nature. 2019;573(7775):546–552. doi: 10.1038/s41586-019-1537-0. [DOI] [PubMed] [Google Scholar]
- 11.St. John A.L., Rathore A.P.S. Adaptive immune responses to primary and secondary dengue virus infections. Nat Rev Immunol. 2019;19(4):218–230. doi: 10.1038/s41577-019-0123-x. [DOI] [PubMed] [Google Scholar]
- 12.Wong RSM Wu A, To K.F. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. BMJ. 2003;326(7403):1358. doi: 10.1136/bmj.326.7403.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu H., Zhou J., Wong B.H.-Y. Middle East Respiratory Syndrome Coronavirus Efficiently Infects Human Primary T Lymphocytes and Activates the Extrinsic and Intrinsic Apoptosis Pathways. J Infect Dis. 2015;213(6):904–914. doi: 10.1093/infdis/jiv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang M., Li C., Li K. Hematological findings in SARS patients and possible mechanisms (Review) Int J Mol Med. 2004;(August) doi: 10.3892/ijmm.14.2.311. [DOI] [PubMed] [Google Scholar]
- 15.Cameron M.J., Bermejo-Martin J.F., Danesh A., Muller M.P., Kelvin D.J. Human immunopathogenesis of severe acute respiratory syndrome (SARS) Virus Res. 2008;133(1):13–19. doi: 10.1016/j.virusres.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshikawa T., Hill T., Li K., Peters C.J., Tseng C.-T.K. Severe Acute Respiratory Syndrome (SARS) Coronavirus-Induced Lung Epithelial Cytokines Exacerbate SARS Pathogenesis by Modulating Intrinsic Functions of Monocyte-Derived Macrophages and Dendritic Cells. J Virol. 2009;83(7):3039–3048. doi: 10.1128/JVI.01792-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao J., Zhao J., Van Rooijen N., Perlman S. Evasion by Stealth: Inefficient Immune Activation Underlies Poor T Cell Response and Severe Disease in SARS-CoV-Infected Mice. PLoS Pathog. 2009;5(10) doi: 10.1371/journal.ppat.1000636. Gale M, ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aggarwal S., Gollapudi S., Gupta S. Increased TNF-α-Induced Apoptosis in Lymphocytes from Aged Humans: Changes in TNF-α Receptor Expression and Activation of Caspases. J Immunol. 1999;162(4):2154. http://www.jimmunol.org/content/162/4/2154.abstract [PubMed] [Google Scholar]
- 19.Noval Rivas M., Burton O.T., Oettgen H.C., Chatila T. IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function. J Allergy Clin Immunol. 2016;138(3):801–811. doi: 10.1016/j.jaci.2016.02.030. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Channappanavar R., Zhao J., Perlman S. T cell-mediated immune response to respiratory coronaviruses. Immunol Res. 2014;59(1-3):118–128. doi: 10.1007/s12026-014-8534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merle N.S., Church S.E., Fremeaux-Bacchi V., Roumenina L.T. Complement System Part I – Molecular Mechanisms of Activation and Regulation. Front Immunol. 2015;6:262. doi: 10.3389/fimmu.2015.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gralinski L.E., Sheahan T.P., Morrison T.E. Complement Activation Contributes to Severe Acute Respiratory Syndrome Coronavirus Pathogenesis. mBio. 2018;9(5):e01753–18. doi: 10.1128/mBio.01753-18. Subbarao KE Luis Schultz-Cherry, Stacey, ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang R., Xiao H., Guo R., Li Y., Shen B. The role of C5a in acute lung injury induced by highly pathogenic viral infections. Emerg Microbes Infect. 2015;4(1):1–7. doi: 10.1038/emi.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cain D.W., Cidlowski J.A. Immune regulation by glucocorticoids. Nat Rev Immunol. 2017;17(4):233–247. doi: 10.1038/nri.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.