Abstract
Background
In December 2019, the coronavirus disease 2019 (COVID-19) outbreak occurred in Wuhan. Data on the clinical characteristics and outcomes of patients with severe COVID-19 are limited.
Objective
We sought to evaluate the severity on admission, complications, treatment, and outcomes of patients with COVID-19.
Methods
Patients with COVID-19 admitted to Tongji Hospital from January 26, 2020, to February 5, 2020, were retrospectively enrolled and followed-up until March 3, 2020. Potential risk factors for severe COVID-19 were analyzed by a multivariable binary logistic model. Cox proportional hazard regression model was used for survival analysis in severe patients.
Results
We identified 269 (49.1%) of 548 patients as severe cases on admission. Older age, underlying hypertension, high cytokine levels (IL-2R, IL-6, IL-10, and TNF-α), and high lactate dehydrogenase level were significantly associated with severe COVID-19 on admission. The prevalence of asthma in patients with COVID-19 was 0.9%, markedly lower than that in the adult population of Wuhan. The estimated mortality was 1.1% in nonsevere patients and 32.5% in severe cases during the average 32 days of follow-up period. Survival analysis revealed that male sex, older age, leukocytosis, high lactate dehydrogenase level, cardiac injury, hyperglycemia, and high-dose corticosteroid use were associated with death in patients with severe COVID-19.
Conclusions
Patients with older age, hypertension, and high lactate dehydrogenase level need careful observation and early intervention to prevent the potential development of severe COVID-19. Severe male patients with heart injury, hyperglycemia, and high-dose corticosteroid use may have a high risk of death.
Key words: COVID-19, SARS-CoV-2, risk factors, severity, mortality
Abbreviations used: ACE 2, Angiotensin-converting enzyme 2; ARDS, Acute respiratory distress syndrome; COVID-19, Coronavirus disease 2019; CT, Computed tomography; HR, Hazard ratio; LDH, Lactate dehydrogenase; OR, Odds ratio; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
In December 2019, an outbreak caused by coronavirus disease 2019 (COVID-19) occurred in Wuhan, Hubei Province, China. As of March 22, 2020, a total of 306,506 COVID-19 cases were reported in more than a hundred countries worldwide. More than 12,000 patients died from infection of this new virus (named severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]), urging early identification and intervention for severe cases.
SARS-CoV-2, as a betacoronavirus, shares 88% of 2 bat-derived SARS-like coronaviruses and distances from SARS-CoV (around 79%) and Middle East respiratory syndrome coronavirus (around 50%).1 SARS and Middle East respiratory syndrome epidemics posed threats to global health due to high mortality rates of 9.6% for SARS-CoV and 34.4% for Middle East respiratory syndrome coronavirus globally.2 , 3 Epidemiological data released by the Chinese Center for Disease Control and Prevention showed that 50,005 confirmed cases have been identified in Wuhan and 31,513 in mainland China except Wuhan as of March 22, 2020. The mortality rate of patients with COVID-19 was 5.0% in Wuhan, which was close to that in the world (4.2%) and much higher than that in mainland China except Wuhan (2.4%). This study aimed to describe and compare the epidemiologic, demographic, clinical, laboratory, and radiological characteristics as well as the complications, treatment, and outcomes of hospitalized patients with nonsevere and severe COVID-19. Potential risk factors for severe COVID-19 and factors associated with death in severe cases were analyzed to provide scientific data for relief in severe cases and reduce mortality.
Methods
Data source
This study was an ambispective cohort study of consecutive hospitalized patients with COVID-19 enrolled at Sino-French New City Branch of Tongji Hospital, Huazhong University of Science and Technology in Wuhan from January 26, 2020, to February 5, 2020. The final date of follow-up was March 3, 2020. The Sino-French New City Branch of Tongji Hospital is one of the major nationally designated hospitals only providing medical care for adult patients with COVID-19 in Wuhan. All cases with COVID-19 enrolled in this study were diagnosed on the basis of World Health Organization interim guidance4 and the diagnostic and treatment guideline for COVID-19 issued by the Chinese National Health Committee (version 5). Detection of SARS-CoV-2 nucleic acids is described in text in this article’s Online Repository at www.jacionline.org.5 This study was approved by the Institutional Review Board of Tongji Hospital, Huazhong University of Science and Technology. Written informed consent was waived in light of the urgent need to collect data.
The epidemiologic and demographic data were obtained by face-to-face or telephonic interview. Clinical symptoms, laboratory, and radiological findings on admission as well as the complications, treatment, and outcomes during hospitalization were extracted from electronic medical records. Serum cytokine levels (IL-1β, IL-2R, IL-6, IL-8, IL-10, and TNF-α) were measured on admission. Patient data were cross-checked for consistency before final data entry and then entered into a computerized database.
The presence of underlying comorbidities was identified on the basis of International Classification of Diseases, Revision 10 diagnostic codes. The complications of COVID-19 after admission were assessed, and the definitions are described in text in this article’s Online Repository at www.jacionline.org. Cardiac injury was one of the complications, which was defined as a serum hypersensitive cardiac troponin I level higher than 15.6 pg/mL without acute coronary symptoms or abnormal electrocardiogram. The clinical outcomes were classified into discharge from hospital, in-hospitalization, and death.
Severe COVID-19 was defined according to the 2019 clinical practice guideline from the Infectious Diseases Society of America and the American Thoracic Society for diagnosis and treatment of adults with community-acquired pneumonia.6 On the basis of whether or not requiring ventilatory support on admission, severe cases upon admission were divided into 2 cohorts, severely ill and critically ill cases.
Statistical analysis
The descriptive statistics are median and interquartile range for continuous data. The statistics for categorical variables are counts and percentages. Mann-Whitney U test was performed for continuous variables, and the χ2 test and Fisher exact test were used for categorical variables as appropriate. Kruskal-Wallis test with Dunn’s multiple comparison was used to compare across groups.
Multivariable binary logistic regression analyses were used to assess the association between age, sex, source of infection, underlying comorbidity, number of hospital visits, time from onset to hospitalization, days of fever preadmission, abnormal laboratory findings, and the dependent variable of severity of disease. The odds ratio (OR) along with the 95% CI were reported. Univariable and multivariable analyses to identify factors associated with death from COVID-19 in severe patients were performed by Cox proportional hazards regression model. Considering the total number of deaths (n = 87) in our study, 9 variables were chosen for multivariate Cox model on the basis of univariable analysis (P < .05), previous findings, and clinical importance, including sex, age, laboratory findings (blood leukocyte count and lactose dehydrogenase [LDH]) on admission, the complications (cardiac injury and hyperglycemia), and drug therapy (corticosteroid, lopinavir/ritonavir, and umifenovir) during hospitalization. The hazard ratio (HR) along with the 95% CI were reported. A P value of less than .05 was regarded as statistically significant. All statistical analyses were performed using SPSS 25.0 for Windows (SPSS, Inc, Chicago, Ill). Detailed statistical analyses are presented in text and Table E6 in this article’s Online Repository at www.jacionline.org.
Results
Epidemiologic and demographic characteristics
A total of 549 patients with COVID-19 were enrolled, of whom 548 cases were included in the study. One case not meeting inclusion criteria was excluded because of inclusion criteria. Almost half the patients (49.1%, 269 of 548) were identified as severe cases and 50.9% (279 of 548) were nonsevere cases on admission; 68.7% (347 of 505) of cases were positive for SARS-CoV-2 nucleic acid test preadmission. Comparison of findings between nonsevere and severe cases in the patients with positive viral nucleic acid test preadmission showed essentially the similar differences to those in the total patients (see Table E1 in this article’s Online Repository at www.jacionline.org).
The epidemiologic and demographic characteristics are presented in Table I . Fifty-two (9.5%) of 546 patients got the infection in hospital. Forty-five (8.2%) of 547 patients were health care workers, and 67 (12.2%) patients were family members of health care workers. Nonsevere cases had a higher proportion of health care workers and family members than severe cases (P < .001). The date of onset of the first reported case with COVID-19 was December 1, 2019.7 The median time from December 1, 2019, to the onset of COVID-19 was 54 days, ranging from 19 days to 63 days.
Table I.
Epidemiologic, demographic, and clinical characteristics of hospitalized patients with COVID-19
| Characteristic | All patients (n = 548) | Nonsevere (n = 279) | Severe (n = 269) | P value |
|---|---|---|---|---|
| Age (y) | 60 (48-69) | 56 (44-66) | 65 (54-72) | .000 |
| 0-44 | 107 of 548 (19.5%) | 75 of 279 (26.9%) | 32 of 269 (11.9%) | .000 |
| 45-64 | 231 of 548 (42.2%) | 129 of 279 (46.2%) | 102 of 269 (37.9%) | |
| ≥65 | 210 of 548 (38.3%) | 75 of 279 (26.9%) | 135 of 269 (50.2%) | |
| Male | 279 of 548 (50.9%) | 126 of 279 (45.2%) | 153 of 269 (56.9%) | .006 |
| Body mass index (kg/m2) | 24.7 (22.4-26.7) | 24.5 (22.4-26.0) | 25.3 (22.4-27.6) | .257 |
| Source of infections | ||||
| Household contact | 494 of 546 (90.5%) | 245 of 278 (88.1%) | 249 of 268 (92.9%) | .060 |
| Hospital-acquired infections | 52 of 546 (9.5%) | 33 of 278 (11.9%) | 19 of 268 (7.1%) | |
| Disease risk | ||||
| Health care workers | 45 of 547 (8.2%) | 36 of 279 (12.9%) | 9 of 268 (3.4%) | .000 |
| Family member of health care workers | 67 of 547 (12.2%) | 42 of 279 (15.1%) | 25 of 268 (9.3%) | |
| Not health care workers or their family members | 435 of 547 (79.5%) | 201 of 279 (72.0%) | 234 of 268 (87.3%) | |
| Time of onset (d)∗ | 54 (51-56) | 54 (52-56) | 54 (51-56) | .394 |
| Time from onset to outpatient visit (d) | 3 (1-6) | 3 (1-5) | 4 (1-7) | .018 |
| 0-3 | 283 of 522 (54.2%) | 158 of 270 (58.5%) | 125 of 252 (49.6%) | .044 |
| >3 | 239 of 522 (45.8%) | 112 of 270 (41.5%) | 127 of 252 (50.4%) | |
| Time from onset to hospitalization (d) | 10 (7-12) | 9 (7-12) | 10 (7-12) | .035 |
| No. of hospital visits ≥2 | 307 of 548 (56.0%) | 144 of 279 (51.6%) | 163 of 269 (60.6%) | .039 |
| Smoking history | ||||
| Never smokers | 452 of 544 (83.1%) | 238 of 279 (85.3%) | 214 of 265 (80.8%) | .051 |
| Former smokers | 51 of 544 (9.4%) | 18 of 279 (14.7%) | 33 of 265 (12.5%) | |
| Current smokers | 41 of 544 (7.5%) | 23 of 279 (8.2%) | 18 of 265 (6.8%) | |
| Underlying comorbidity | ||||
| Chronic obstructive pulmonary disease | 17 of 548 (3.1%) | 4 of 279 (1.4%) | 13 of 269 (4.8%) | .026 |
| Asthma | 5 of 548 (0.9%) | 2 of 279 (0.7%) | 3 of 269 (1.1%) | .681 |
| Tuberculosis | 9 of 548 (1.6%) | 5 of 279 (1.8%) | 4 of 269 (1.5%) | 1.000 |
| Diabetes | 83 of 548 (15.1%) | 31 of 279 (11.1%) | 52 of 269 (19.3%) | .009 |
| Hypertension | 166 of 548 (30.3%) | 62 of 279 (22.2%) | 104 of 269 (38.7%) | .000 |
| Coronary heart disease | 34 of 548 (6.2%) | 6 of 279 (2.2%) | 28 of 269 (10.4%) | .000 |
| Hepatitis B | 5 of 548 (0.9%) | 3 of 279 (1.1%) | 2 of 269 (0.7%) | 1.000 |
| Chronic kidney disease | 10 of 547 (1.8%) | 4 of 278 (1.4%) | 6 of 269 (2.2%) | .539 |
| Tumor | 24 of 513 (4.7%) | 10 of 256 (3.9%) | 14 of 257 (5.5%) | .531 |
| Previous drugs use | ||||
| ACEI/ARB | 42 of 545 (7.7%) | 23 of 279 (8.2%) | 19 of 266 (7.1%) | .748 |
| Systemic corticosteroids | 6 of 548 (1.1%) | 4 of 279 (1.4%) | 2 of 269 (0.7%) | .686 |
| Inhaled corticosteroids | 5 of 548 (0.9%) | 3 of 279 (1.1%) | 2 of 269 (0.7%) | 1.000 |
| Antibiotics | 7 of 548 (1.3%) | 3 of 279 (1.1%) | 4 of 269 (1.5%) | .720 |
| Anticoagulants | 16 of 547 (2.9%) | 5 of 278 (1.8%) | 11 of 269 (4.1%) | .132 |
| Immunosuppressant drugs | 5 of 548 (0.9%) | 3 of 279 (1.1%) | 2 of 269 (0.7%) | 1.000 |
| Antiviral drugs | 2 of 548 (0.4%) | 1 of 279 (0.4%) | 1 of 269 (0.4%) | 1.000 |
| Symptoms | ||||
| Fever at preadmission | 476 of 500 (95.2%) | 248 of 260 (95.4%) | 228 of 240 (95.0%) | 1.000 |
| Highest temperature (°C) | 38.8 (38.2-39) | 38.8 (38-39) | 38.8 (38.4-39) | .416 |
| Duration (d) | 9 (6-11) | 8.5 (6-11) | 10 (7-12) | .031 |
| Fatigue | 258 of 548 (47.1%) | 128 of 279 (45.9%) | 130 of 269 (48.3%) | .608 |
| Sore throat | 28 of 548 (5.1%) | 17 of 279 (6.1%) | 11 of 269 (4.1%) | .335 |
| Cough | 415 of 548 (75.7%) | 212 of 279 (76.0%) | 203 of 269 (75.5%) | .921 |
| Chest pain | 41 of 548 (7.5%) | 25 of 279 (9.0%) | 16 of 269 (6.0%) | .197 |
| Dyspnea | 310 of 548 (56.6%) | 112 of 279 (40.1%) | 198 of 269 (73.6%) | .000 |
| Chest tightness | 162 of 425 (38.1%) | 86 of 245 (42.2%) | 76 of 180 (38.1%) | .157 |
| Dizziness | 56 of 548 (10.2%) | 29 of 279 (10.4%) | 27 of 269 (10.0%) | 1.000 |
| Confusion | 17 of 548 (3.1%) | 1 of 279 (0.4%) | 16 of 269 (6.0%) | .000 |
| Headache | 62 of 548 (11.3%) | 37 of 279 (13.3%) | 25 of 269 (9.3%) | .177 |
| Myalgia | 111 of 548 (20.3%) | 62 of 279 (22.2%) | 49 of 269 (18.2%) | .288 |
| Vomiting | 45 of 548 (8.2%) | 25 of 279 (9.0%) | 20 of 269 (7.4%) | .537 |
| Diarrhea | 179 of 548 (32.7%) | 94 of 279 (33.7%) | 85 of 269 (31.6%) | .649 |
| Abdominal pain | 16 of 548 (2.9%) | 4 of 279 (1.4%) | 12 of 269 (4.5%) | .043 |
| Administration of systemic corticosteroids preadmission | 64 of 540 (11.9%) | 22 of 274 (8.0%) | 42 of 266 (15.8%) | .007 |
| Duration (d) | 1 (0-3) | 0 (0-1) | 2.5 (1-4) | .000 |
| Cumulative dose† (mg) | 50 (0-150) | 0 (0-66.7) | 100 (50-187.5) | .000 |
| Administration of antiviral drugs preadmission | ||||
| Lopinavir/ritonavir | 13 of 541 (2.4%) | 10 of 276 (3.6%) | 3 of 265 (1.1%) | .089 |
| Umifenovir | 177 of 538 (32.9%) | 113 of 274 (41.2%) | 64 of 264 (24.2%) | .000 |
| Oseltamivir | 189 of 538 (35.1%) | 112 of 274 (40.9%) | 77 of 264 (29.2%) | .005 |
| Ribavirin | 8 of 538 (1.5%) | 2 of 274 (0.7%) | 6 of 264 (2.3%) | .169 |
Data are expressed as median (IQR), n (%), or n of N (%), where N is the total number of patients with available data. P values comparing nonsevere cases and severe cases are from χ2 test, Fisher exact test, or Mann-Whitney U test.
ACEI/ARB, Angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker; IQR, interquartile range.
Days from December 1, 2019, to the date of onset.
Equivalent doses of prednisone.
The median age of study population was 60 years (interquartile range, 48-69), ranging from 18 years to 95 years, of whom 210 (38.3%) were aged 65 years or older. The patients aged 65 years or older in severe cases were almost twice as nonsevere cases of the same age (50.2% vs 26.9%; P < .001). Slightly more than half (50.9%) of all patients were male, and the proportion of males in severe cases was higher than in nonsevere cases (56.9% vs 45.2%; P = .006).
Clinical characteristics on admission
About 19.2% of patients with severe COVID-19 were smokers (Table I). Compared with nonsevere cases, severe cases exhibited more comorbidities, including chronic obstructive pulmonary disease (4.8% vs 1.4%; P = .026), coronary heart disease (10.4% vs 2.2%; P < .001), hypertension (38.7% vs 22.2%; P < .001), and diabetes (19.3% vs 11.1%; P = .009), respectively. Only 5 cases of asthma (0.9%) were identified in the total population. Forty-two (7.7%) of 545 patients regularly took angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers; no significant difference was found between nonsevere and severe cases.
Most patients reported at least 1 of the following symptoms: fever (95.2%), fatigue (47.1%), sore throat (5.1%), cough (75.5%), chest pain (7.5%), dyspnea (56.6%), chest tightness (38.1%), dizziness (10.2%), confusion (3.1%), headache (11.3%), myalgia (20.3%), vomiting (8.2%), diarrhea (32.7%), and abdominal pain (2.9%). Six patients were asymptomatic and diagnosed by computed tomography (CT) screening. Duration of fever preadmission was significantly longer among severe cases than among nonsevere cases (P = .031). Severe cases experienced longer duration from onset to outpatient visit and longer duration from onset to hospitalization compared with nonsevere cases (P = .018 and P = .035, respectively). Sixty-four (11.9%) of 540 patients were treated with corticosteroids delivered by oral or intravenous preadmission. A total of 304 (56.5%) of 538 patients had received at least 1 of the following antiviral medications: umifenovir (32.9%), oseltamivir (35.1%), lopinavir/ritonavir (2.4%), and ribavirin (1.5%).
Radiographic and laboratory findings on admission
CT scans for 461 patients were evaluated preadmission, and showed multilobar pulmonary infiltrates in 436 patients (Table II ). The median time from onset to pneumonia diagnosed by CT scan was 4 days. On admission, oxygen saturation less than 93.1% on room air presented in 33.3% of all patients, of whom 163 (89%) were severe cases; 90.2% of all patients experienced lymphopenia (<1500 cells/mm3), and 29.1% of all patients had thrombocytopenia (<150,000 cells/mm3). Compared with nonsevere cases, inflammation-related marker levels (high sensitivity C-reactive protein, erythrocyte sedimentation rate, and ferritin) were significantly higher in severe cases. The levels of procalcitonin, globulin, LDH, NT-proB-type natriuretic peptide, d-dimer, alanine aminotransferase, aspartate aminotransferase, total bilirubin, conjugated bilirubin, blood urea nitrogen, and creatinine were elevated in 9.5%, 40.4%, 73.6%, 27.5%, 67.4%, 23.1%, 33.1%, 4.4%, 9.2%, 15.8%, and 27.1% of all patients, respectively. Serum cytokine levels of IL-2R, IL-6, IL-10, and TNF-α were significantly higher in severe patients than in nonsevere patients (all P < .01).
Table II.
Radiographic and laboratory findings of patients with COVID-19
| Findings | All patients (n = 548) | Nonsevere (n = 279) | Severe (n = 269) | P value |
|---|---|---|---|---|
| CT findings preadmission | ||||
| Negative | 4 of 461 (0.9%) | 4 of 228 (1.8%) | 0 | .032 |
| Unilobar lesion | 21 of 461 (4.6%) | 14 of 228 (6.1%) | 7 of 233 (3.0%) | |
| Multilobar lesion | 436 of 461 (94.6%) | 210 of 228 (92.1%) | 226 of 233 (97.0%) | |
| Time from onset to pneumonia diagnosed by CT scan (d) | 4 (2-7) | 4 (2-6) | 4 (2-7) | .258 |
| SARS-CoV-2 nucleic acid test∗ | ||||
| Positive | 347 of 505 (68.7%) | 180 of 270 (66.7%) | 167 of 235 (71.1%) | .503 |
| Suspected positive | 41 of 505 (8.1%) | 22 of 270 (8.1%) | 19 of 235 (8.1%) | |
| Negative | 117 of 505 (23.2%) | 68 of 270 (25.2%) | 49 of 235 (20.9%) | |
| Oxygen saturation (%) | ||||
| ≤93 | 182 of 546 (33.3%) | 19 of 278 (6.8%) | 163 of 268 (60.8%) | .000 |
| >93 | 364 of 546 (66.7%) | 259 of 278 (93.2%) | 105 of 268 (39.2%) | |
| Blood leukocyte count (×10⁹/L) | ||||
| >10 | 63 of 542 (11.6%) | 8 of 275 (2.9%) | 55 of 267 (20.6%) | .000 |
| <4 | 130 of 542 (24.0%) | 84 of 275 (30.5%) | 46 of 267 (17.23%) | .000 |
| Neutrophil count (×10⁹/L) | ||||
| >6.5 | 118 of 542 (21.8%) | 22 of 275 (8.0%) | 96 of 267 (36.0%) | .000 |
| ≤2.0 | 67 of 542 (12.4%) | 50 of 275 (18.2%) | 17 of 267 (6.4%) | .000 |
| Lymphocyte count (×10⁹/L) | ||||
| <1.5 | 489 of 542 (90.2%) | 234 of 275 (85.1%) | 255 of 267 (95.5%) | .000 |
| ≤0.5 | 85 of 542 (15.7%) | 21 of 275 (7.6%) | 64 of 267 (24.0%) | .000 |
| Platelet count <150 × 10⁹/L | 157 of 539 (29.1%) | 68 of 274 (24.8%) | 89 of 265 (33.6%) | .029 |
| High sensitive C-reactive protein (mg/L) | ||||
| >10 | 460 of 540 (85.2%) | 205 of 272 (75.4%) | 255 of 268 (95.2%) | .000 |
| >100 | 138 of 540 (25.6%) | 40 of 272 (14.7%) | 98 of 268 (36.6%) | .000 |
| Procalcitonin >0.5 ng/mL | 46 of 486 (9.5%) | 3 of 249 (1.43%) | 43 of 237 (18.9%) | .000 |
| Erythrocyte sedimentation rate >20 mm/h | 377 of 518 (72.8%) | 179 of 264 (67.8%) | 198 of 254 (78.0%) | .010 |
| Ferritin >500 μg/L | 211 of 313 (67.4%) | 95 of 171 (55.9%) | 116 of 142 (81.7%) | .000 |
| D-dimer >1 mg/L | 227 of 501 (45.3%) | 78 of 254 (31.1%) | 149 of 247 (56.4%) | .000 |
| NT-proB-type natriuretic peptide >500 pg/L | 92 of 335 (27.5%) | 17 of 136 (13.3%) | 75 of 199 (37.9%) | .000 |
| LDH (U/L) | ||||
| >250 | 393 of 534 (73.6%) | 162 of 272 (59.6%) | 231 of 262 (88.2%) | .000 |
| >445 | 133 of 534 (24.9%) | 25 of 272 (9.2%) | 108 of 262 (41.2%) | .000 |
| Globulin >35 g/L | 218 of 540 (40.4%) | 88 of 275 (32.0%) | 130 of 265 (49.1%) | .000 |
| Albumin ≤35 g/L | 320 of 541 (59.1%) | 126 of 275 (45.8%) | 194 of 266 (72.9%) | .000 |
| Alanine aminotransferase >40 U/L | 125 of 541 (23.1%) | 61 of 275 (22.3%) | 64 of 266 (24.1%) | .683 |
| Aspartate aminotransferase >40 U/L | 179 of 540 (33.1%) | 64 of 275 (23.3%) | 115 of 265 (43.4%) | .000 |
| Total bilirubin >21 μmol/L | 24 of 541 (4.4%) | 7 of 275 (2.3%) | 17 of 266 (6.4%) | .036 |
| Conjugated bilirubin >8 μmol/L | 50 of 541 (9.2%) | 17 of 275 (6.3%) | 33 of 266 (12.6%) | .017 |
| Blood urea nitrogen >7.5 mmol/L | 85 of 539 (15.8%) | 18 of 273 (6.6%) | 67 of 266 (25.2%) | .000 |
| Creatinine >85 μmol/L | 146 of 539 (27.1%) | 61 of 273 (22.3%) | 85 of 266 (32.0%) | .015 |
| IL-1β >5 ng/L | 51 of 306 (16.7%) | 34 of 170 (20.0%) | 17 of 136 (12.5%) | .091 |
| IL-2R >710 U/mL | 164 of 309 (53.1%) | 73 of 171 (42.7%) | 91 of 138 (65.9%) | .000 |
| IL-6 >7 ng/L | 221 of 312 (70.8%) | 107 of 175 (61.1%) | 114 of 137 (83.2%) | .000 |
| IL-8 >62 ng/L | 24 of 309 (7.8%) | 10 of 171 (5.9%) | 14 of 137 (10.1%) | .200 |
| IL-10 >9.1 ng/L | 83 of 307 (27.0%) | 34 of 170 (20.0%) | 49 of 170 (35.8%) | .003 |
| TNF-α >8.1 ng/L | 182 of 309 (58.9%) | 89 of 171 (52.1%) | 93 of 138 (67.4%) | .008 |
| Proteinuria | 200 of 330 (60.6%) | 98 of 193 (50.8%) | 102 of 137 (74.5%) | .000 |
Data are expressed as median (IQR), n (%), or n of N (%), where N is the total number of patients with available data. P values comparing nonsevere cases and severe cases are from χ2 test, Fisher exact test, or Mann-Whitney U test.
IQR, Interquartile range.
SARS-CoV-2 nucleic acid test was performed preadmission.
Subgroup analysis
Of the 269 severe cases, 46 were classified as critically ill for requiring respiratory support. Compared with severely ill cases, the time from December 1, 2019, to onset was shorter and the time from onset to outpatient visit was longer in critically ill cases (see Table E2 in this article’s Online Repository at www.jacionline.org). There were no significant differences in age and underlying comorbidities between severely ill and critically ill cases. More abnormal laboratory findings (such as high leukocyte, high procalcitonin, high NT-proB-type natriuretic peptide, high LDH, high d-dimer, low albumin, and high creatinine) were observed in critically ill cases compared with severely ill cases (all P < .05). About 34.1% of critically ill patients received systemic corticosteroids preadmission, which was significantly higher than that in severely ill cases (12.2%; P < .001).
Compared with nonsevere cases, systemic corticosteroid use preadmission was more common in severe cases, with larger cumulative dose and longer duration (P = .007, P < .001, P < .001, respectively). Stratification of patients by corticosteroid exposure is presented in Table E3 in this article’s Online Repository at www.jacionline.org. Severe patients treated with corticosteroids had higher LDH level compared with severe patients without corticosteroid use preadmission (P < .05).
Nonsevere cases were more likely to receive antiviral drugs preadmission, including umifenovir and oseltamivir (P < .001 and P = .005, respectively). In the severe case subgroup, the patients receiving umifenovir were younger than those without umifenovir use (P < .05). A comparison of baseline demographic and clinical characteristics between patients with and without antiviral drug use revealed no marked difference in oxygen saturation or laboratory findings in both nonsevere and severe case subgroups (see Tables E4 and E5 in this article’s Online Repository at www.jacionline.org).
Risk factors for severe cases on admission
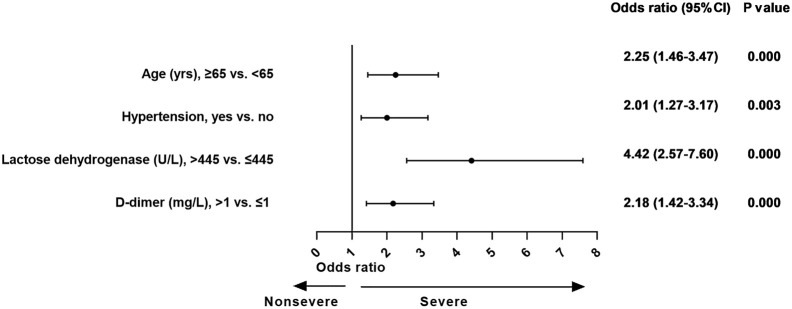
In the final logistic regression model, variables such as age 65 years or more (OR, 2.2; 95% CI, 1.5-3.5), hypertension (OR, 2.0; 95% CI, 1.3-3.2), LDH more than 445 U/L (OR, 4.4; 95% CI, 2.6-7.6), and d-dimer more than 1 mg/L (OR, 2.2; 95% CI, 1.4-3.3) were significantly associated with cases with severe COVID-19 (Fig 1 ).
Fig 1.
The effect of various potential risk factors on patients with severe COVID-19 at admission.
Complications, treatment, and clinical outcomes during hospitalization and follow-up
In the follow-up period, the complications of COVID-19 were assessed, including acute respiratory distress syndrome (ARDS) (38.3%), cardiac injury (21.7%), liver dysfunction (19.3%), acute kidney injury (17.3%), bacteremia (7.7%), diffuse intravascular coagulation (7.7%), and hyperglycemia (33.2%) (Table III ). All the above-mentioned complications were more common in severe cases, compared with nonsevere cases (all P < .05).
Table III.
Complications and treatment during hospitalization and clinical outcomes of patients with COVID-19
| Complications and treatment | All patients (n = 548) | Nonsevere (n = 279) | Severe (n = 269) | P value |
|---|---|---|---|---|
| Complications | ||||
| ARDS | 210 of 548 (38.3%) | 27 of 279 (9.7%) | 183 of 269 (68.0%) | .000 |
| Cardiac injury | 119 of 548 (21.7%) | 25 of 279 (9.0%) | 94 of 269 (34.9%) | .000 |
| Liver dysfunction | 106 of 548 (19.3%) | 44 of 279 (15.8%) | 62 of 269 (23.0%) | .040 |
| Acute kidney injury | 95 of 548 (17.3%) | 33 of 279 (11.8%) | 62 of 269 (23.0%) | .001 |
| Bacteremia | 42 of 548 (7.7%) | 4 of 279 (1.4%) | 38 of 269 (14.1%) | .000 |
| DIC | 42 of 548 (7.7%) | 5 of 279 (1.8%) | 37 of 269 (13.8%) | .000 |
| Hyperglycemia | 182 of 548 (33.2%) | 60 of 279 (21.5%) | 122 of 269 (45.4%) | .000 |
| Administration of systemic corticosteroids | 341 of 548 (62.2%) | 145 of 279 (52.0%) | 196 of 269 (72.9%) | .000 |
| Duration (d) | 4 (0-11) | 1 (0-10) | 5 (0-12) | .000 |
| Cumulative dose (mg) | 200 (0-450) | 50 (0-400) | 295 (0-575) | .000 |
| Administration of antiviral drugs | ||||
| Lopinavir/ritonavir | 164 of 548 (29.9%) | 91 of 279 (32.6%) | 73 of 269 (27.1%) | .163 |
| Umifenovir | 401 of 548 (73.2%) | 222 of 279 (79.6%) | 179 of 269 (66.5%) | .001 |
| Oseltamivir | 221 of 548 (40.3%) | 127 of 279 (45.5%) | 94 of 269 (34.9%) | .015 |
| Ribavirin | 29 of 548 (5.3%) | 8 of 279 (2.9%) | 21 of 269 (7.8%) | .012 |
| IFN-α nebulization | 168 of 548 (30.7%) | 97 of 279 (34.8%) | 71 of 269 (26.4%) | .041 |
| Intravenous immunoglobulin | 213 of 548 (38.9%) | 103 of 279 (36.9%) | 110 of 269 (40.9%) | .381 |
| Vasopressor | 79 of 548 (14.4%) | 5 of 279 (1.8%) | 74 of 269 (27.5%) | .000 |
| Oxygen therapy | 355 of 548 (64.8%) | 131 of 279 (47.0%) | 224 of 269 (83.3%) | .000 |
| Nasal cannula or mask | 228 of 548 (41.6%) | 118 of 279 (42.3%) | 110 of 269 (40.9%) | .000 |
| High-flow oxygen therapy | 24 of 548 (4.4%) | 2 of 279 (0.7%) | 22 of 269 (8.2%) | |
| Noninvasive mechanical ventilation | 78 of 548 (14.2%) | 10 of 279 (3.6%) | 68 of 269 (25.3%) | |
| Invasive mechanical ventilation | 25 of 548 (4.6%) | 1 of 279 (0.4%) | 24 of 269 (8.9%) | |
| Continuous renal replacement therapy | 2 of 548 (0.4%) | 0 | 2 of 269 (99.3%) | .241 |
| Clinical outcomes | ||||
| Discharge from hospital | 287 of 545 (52.7%) | 202 of 277 (72.9%) | 85 of 268 (31.7%) | .000 |
| In-hospitalization | 168 of 545 (30.8%) | 72 of 277 (26.0%) | 96 of 268 (35.8%) | |
| Death | 90 of 545 (16.5%) | 3 of 277 (1.1%) | 87 of 268 (32.5%) |
Data are expressed as median (IQR), n (%), or n of N (%), where N is the total number of patients with available data. P values comparing nonsevere cases and severe cases are from χ2 test, Fisher exact test, or Mann-Whitney U test.
DIC, Diffuse intravascular coagulation; IQR, interquartile range.
Antiviral drugs were used specifically to treat COVID-19 during hospitalization, including lopinavir/ritonavir (29.9%), umifenovir (73.2%), oseltamivir (40.3%), ribavirin (5.3%), and IFN-α nebulization (30.7%). Antiviral drug use was more common in nonsevere cases than in severe cases except for ribavirin. A total of 341 (62.2%) of 548 patients were administered systemic corticosteroids, with a medium duration of 4 days and medium cumulative dose equivalent to 200 mg prednisone. Of the 548 patients, 355 (64.8%) required oxygen support during hospitalization, including nasal cannula or mask (41.6%), high-flow oxygen therapy (4.4%), noninvasive mechanical ventilation (14.2%), and invasive mechanical ventilation (4.6%).
Mortality rates for COVID-19 were estimated to be 1.1% (3 of 277) in nonsevere patients and 32.5% (87 of 268) in severe cases during the average 32 days of follow-up; 72.9% of nonsevere cases and 31.7% of severe cases were discharged from hospital.
Factors associated with death in severe cases
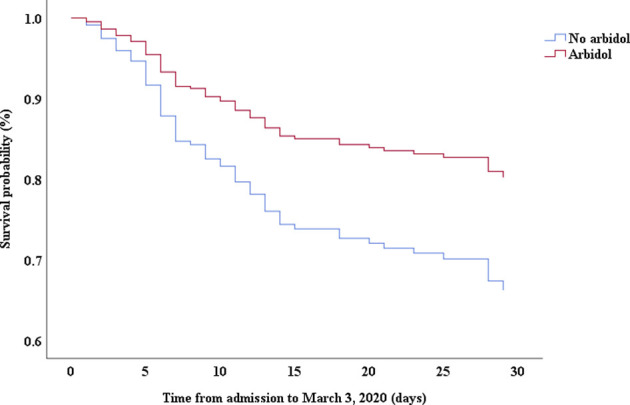
Multivariable Cox proportional hazards regression analysis revealed that male sex (adjusted HR, 1.7; 95% CI, 1.0-2.8), age 65 years or more (adjusted HR, 1.7; 95% CI, 1.1-2.7), blood leukocyte count more than 10 cells/mm3 (adjusted HR, 2.0; 95% CI, 1.3-3.3), and LDH more than 445 U/L (adjusted HR, 2.0; 95% CI, 1.2-3.3) at admission, cardiac injury (adjusted HR, 2.9; 95% CI, 1.8-4.8), hyperglycemia (adjusted HR, 1.8; 95% CI, 1.1-2.8), and administration of high-dose corticosteroids (adjusted HR, 3.5; 95% CI, 1.8-6.9) during hospitalization were significant risk factors associated with death in cases with severe COVID-19 (Table IV). Lopinavir/ritonavir (adjusted HR, 0.4; 95% CI, 0.2-0.9) and umifenovir (adjusted HR, 0.5; 95% CI, 0.3-0.8) were associated with lower death in patients with severe COVID-19. The adjusted Kaplan-Meier estimates of survival for sex, age, leukocyte, LDH, corticosteroid use, lopinavir/ritonavir use, and umifenovir use are shown in Figs E1-E7 in this article’s Online Repository at www.jacionline.org.
Table IV.
Unadjusted and adjusted Cox proportional hazards regression model for death among patients with severe COVID-19
| Variable | Unadjusted HR | 95% CI | P value | Adjusted HR | 95% CI | P value |
|---|---|---|---|---|---|---|
| Sex, male vs female | 1.96 | 1.24-3.11 | .004 | 1.72 | 1.05-2.82 | .032 |
| Age, ≥65 y vs <65 y | 1.69 | 1.09-2.59 | .018 | 1.72 | 1.09-2.73 | .021 |
| Blood leukocyte count, >10 × 10⁹/L vs ≤10 × 10⁹/L | 3.85 | 2.50-5.93 | .000 | 2.04 | 1.26-3.31 | .004 |
| LDH, > 445 U/L vs ≤445 U/L | 3.94 | 2.48-6.28 | .000 | 2.00 | 1.21-3.30 | .007 |
| Complications | ||||||
| Cardiac injury | 3.89 | 2.52-6.01 | .000 | 2.92 | 1.80-4.76 | .000 |
| Hyperglycemia | 2.49 | 1.61-3.87 | .000 | 1.77 | 1.11-2.84 | .017 |
| Treatment | ||||||
| Corticosteroids | .000 | .000 | ||||
| No steroid (reference) | ||||||
| Low dose∗ | 1.07 | 0.57-2.01 | .825 | 1.26 | 0.61-2.580 | .534 |
| High dose† | 3.32 | 1.85-5.97 | .000 | 3.50 | 1.79-6.86 | .000 |
| Lopinavir/ritonavir | 0.26 | 0.13-0.52 | .000 | 0.43 | 0.21-0.89 | .022 |
| Umifenovir | 0.46 | 0.30-0.71 | .000 | 0.54 | 0.34-0.84 | .007 |
P values are from Cox proportional hazards regression model. The final model was adjusted for sex, age, blood leukocyte count, LDH, cardiac injury, hyperglycemia, and administration of corticosteroids, lopinavir/ritonavir, and umifenovir.
Low dose of steroid indicates that the maximum dose was <1 mg/kg/d prednisone.
High dose of steroid indicates that the maximum dose was equivalent to or more than 1 mg/kg/d prednisone.
Discussion
This study provided comprehensive data on the epidemiologic, demographic, clinical, laboratory, and radiological characteristics as well as the complications, treatment, and outcomes of hospitalized patients with nonsevere and severe COVID-19 in Wuhan. Almost half the patients in this study were identified as severe cases, which may differ from the results of the previous studies.8 The proportion of patients aged 65 years or more was higher in our study than in Nanshan Zhong’s study (38.8% vs 15.1%, respectively).9 The time from December 1, 2019, to the onset of disease in most patients was longer than 50 days. During mid-January to early February, Wuhan experienced the highest peak of COVID-19 outbreak, with a family cluster and high prevalence of COVID-19 in older adults. Longer wait for access to medical care was observed in severe cases compared with nonsevere cases. More than half the patients experienced at least 2 hospital visits, which may have increased the risk of nosocomial transmission events. Diagnosis and treatment may have been delayed because of the long wait for access to medical care. Patients with severe COVID-19 likely developed ARDS and died of respiratory failure. Although there are currently no effective antiviral drugs for SARS-CoV-2, prompt identification and early respiratory support would provide relief in severe cases and reduce mortality. The severity of disease in patients with initial positive nucleic acid test result was similar to that of all patients with COVID-19. We thus propose that urgent timely diagnosis is crucial, and that early intervention should not be delayed on the basis of the nucleic acid test.
There are 6 coronavirus species currently known to cause human infection. SARS-CoV-2 was most closely related to SARS-CoV through phylogenetic analysis and was revealed to share a similar receptor, angiotensin-converting enzyme 2 (ACE 2), to SARS-CoV.1 This fact hints that COVID-19 may partly mimic SARS infection. The autopsy results of patients with SARS showed that high levels of proinflammatory cytokines were expressed in ACE 2–expressing cells infected by SARS-CoV.10 Plasma cytokine profiles of patients with SARS showed TH1-dominated responses with markedly elevated proinflammatory cytokine levels (INF-γ, IL-1β, IL-6, IL-8, IL-12, and TNF-α) and were associated with the development of ARDS.11, 12, 13 In our study, patients with severe COVID-19 had significantly higher levels of TH1 cytokines (IL-6 and TNF-α) and a higher incidence rate of ARDS, compared with nonsevere cases. Interestingly, the prevalence of asthma in patients with COVID-19 (0.9%) in our study was markedly lower than that reported in the adult population of Wuhan (6.4%).14, 15, 16 We thus speculate that TH2 immune response in patients with asthma may counter the inflammation process induced by SARS-CoV-2 infection. Further studies are required to characterize the immune response and inflammation features of COVID-19.
Most severe patients showed rapid progression and multiple organ dysfunction. The median time from onset to pneumonia diagnosed by CT scan was only 4 days. Approximately one-third of the patients experienced gastrointestinal symptoms. During hospitalization, a substantial proportion of patients presented cardiac injury, liver and kidney dysfunction, and hyperglycemia. It was proved that the fecal and urine samples and rectal swabs of patients with COVID-19 were positive for SARS-CoV-2 nucleic acids.9 ACE 2 was reported to be expressed in small intestinal epithelial cells, cholangiocytes, and the pancreas,17, 18, 19 indicating that SARS-CoV-2 infection may induce the multiorgan injury in patients with COVID-19. The shorter duration from December 1, 2019, to onset in critically ill cases than that in severely ill cases may reflect a higher virulence of SARS-CoV-2, or earlier onset of COVID-19.
The risk factors for severity identified in this study included age, high LDH level, and high d-dimer level, consistent with those in previous reports.15 , 20 However, different from the findings of previous studies,21 hypertension was the only comorbidity associated with the severity of COVID-19 after adjustment for age, sex, and smoking status. The distinct features of pneumonia and high severity in patients with COVID-19 in this study may lead to this difference from previous reports. ACE 2, a gateway to SARS, was reported to be a protective factor against SARS-CoV–induced lung injury.22 , 23 The association between ACE 2 expression and hypertension was confirmed in a previous study.24 This fact may partly explain the high prevalence of severe COVID-19 in patients with hypertension. LDH has been recognized as a marker for severe prognosis in various diseases, including cancer and infection.25 The high LDH level in COVID-19 in severe cases suggested that LDH may be associated with lung injury and tissue damage, warranting an investigation for the potential mechanism.
This study evaluated preadmission medications for patients with severe COVID-19. Although the proportion of nonsevere cases in patients receiving oseltamivir was higher than that in patients without oseltamivir use, stratification analysis showed that there was no significant difference in hypoxia between patients with and without oseltamivir use either in the severe case subgroup or in the nonsevere case subgroup. Therefore, oseltamivir use may just be an indicator of disease severity. The patients receiving umifenovir were younger than those without umifenovir use, indicating that younger patients may have easier access to drugs or prefer umifenovir.
Older age, leukocytosis, and high LDH level were reported to be risk factors associated with in-hospital death in previous studies.20 , 26 , 27 The present study also revealed that hyperglycemia was related with increased mortality in patients with COVID-19. The prevalence of hyperglycemia may be associated with underlying diabetes and corticosteroid therapy. However, the localization of ACE 2 expression in the pancreas in patients with SARS was reported to damage islets, resulting in hyperglycemia19; this finding suggested that hyperglycemia may also be an indicator of severe COVID-19.
This study indicated that corticosteroid use was more common in severe cases than in nonsevere cases and that high-dose corticosteroid use was related to high risk of death in patients with severe COVID-19. High-dose steroid use may be an indicator of disease severity rather than a predisposing factor. In a previous study, treatment with methylprednisolone was shown to be beneficial for patients with COVID-19 who developed ARDS.20 However, critically ill cases had more signs of infection and abnormal laboratory findings, including high leukocyte, high procalcitonin, high d-dimer, low albumin, and high creatinine levels. High-dose corticosteroids should be used with caution in critically ill patients to avoid aggravating complications.
A recent study by Cao et al28 showed that lopinavir/ritonavir treatment offered no significant benefit over standard care for hospitalized adult patients with COVID-19. Cao et al’s study also reported that lopinavir/ritonavir led to a shorter median time to clinical improvement than standard care (HR, 1.39; 95% CI, 1.00-1.91) in a modified intention-to-treat analysis. Compared with Cao et al’s study, the severity of patients was more serious and lopinavir/ritonavir treatment was associated with a lower risk of death in patients with severe COVID-19 in this study. However, our study was an observational study; thus, the benefit of lopinavir/ritonavir for patients with severe COVID-19 needs to be further confirmed.
There were limitations to the current study. First, epidemiological data were collected respectively and recall bias might have occurred. Second, missing data on some variables, such as detailed information of CT scan, may cause bias in the estimation and reduce the representativeness of the samples. Third, laboratory findings were measured on admission and may indicate the severity of COVID-19. The causal relationship between abnormal laboratory findings and severity could not be determined. Fourth, this study was an observational study with limitations in terms of evaluating the efficacy of corticosteroids and antiviral drugs. Finally, the absence of comparative data from patients with COVID-19 not admitted or from other critically ill patients was a limitation of this study.
Conclusions
The COVID-19 outbreak has caused widespread concern and has threatened the global public health security. Recent evidence of possible fecal-oral transmission of the SARS-CoV-2 infection, asymptomatic infection,8 , 29 , 30 and positive result for SARS-CoV-2 test in recovered patients31 warrant aggressive measures to suppress and prevent the pandemic from spreading, such as hygiene maintenance, early screening and intervention, and self-isolation after recovery. As a major transportation hub of China, Wuhan faced increased difficulties in outbreak control. Efforts to control COVID-19 need to take into account globalization processes.32 Severe male patients with heart injury, hyperglycemia, and high-dose corticosteroid use may have a high risk of death.
Clinical implications.
Male sex, older age, leukocytosis, high LDH level, cardiac injury, and hyperglycemia may be associated with the fatal outcome of patients with severe COVID-19. High-dose corticosteroid use was related to a high risk of death.
Acknowledgments
We respectfully and sincerely thank all front-line medical staff for their hard work and sacrifice.
Footnotes
This project was supported by grants from the Science and Technology Program of Hubei Province (grant no. 2020FCA026).
Disclosure of potential conflict of interest: H. Renz is a member of and receives funding from the German Lung Centre (Deutsches Zentrum für Lungenforschung [DZL]) and of the Universities Giessen and Marburg Lung Centre. The rest of the authors declare that they have no relevant conflicts of interest.
Supplementary data
Figure E1.
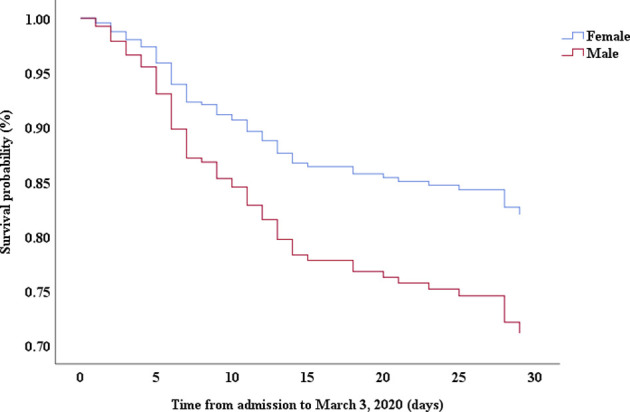
Figure E2.
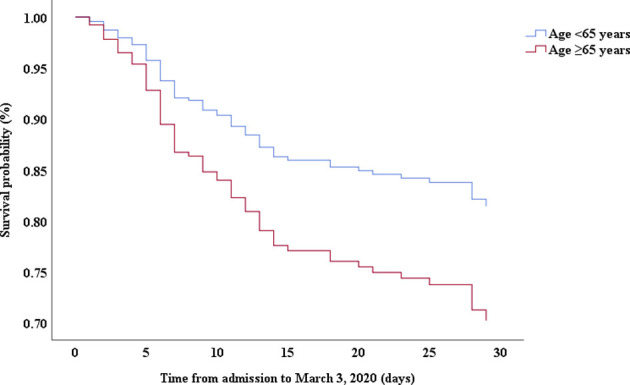
Figure E3.
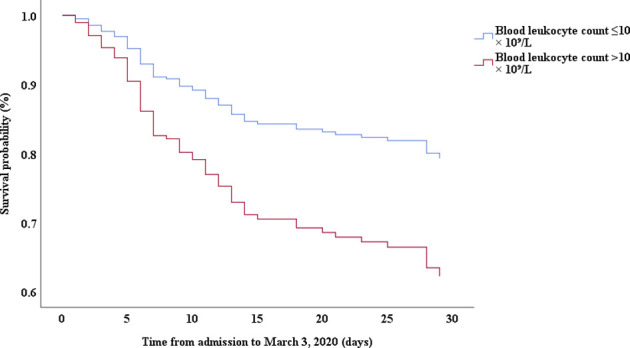
Figure E4.
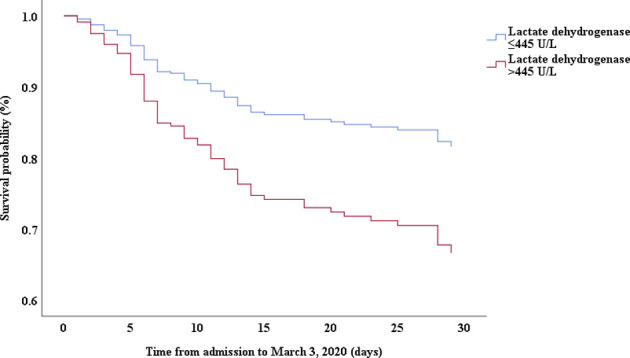
Figure E5.
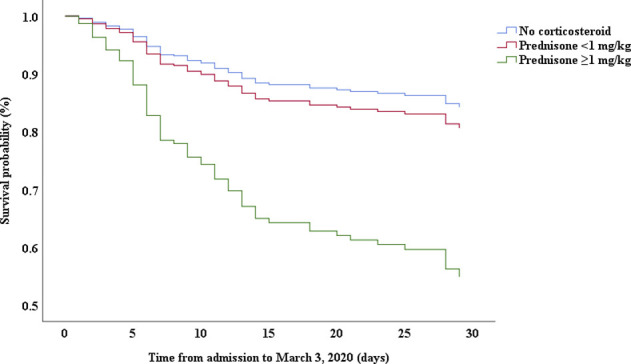
Figure E6.
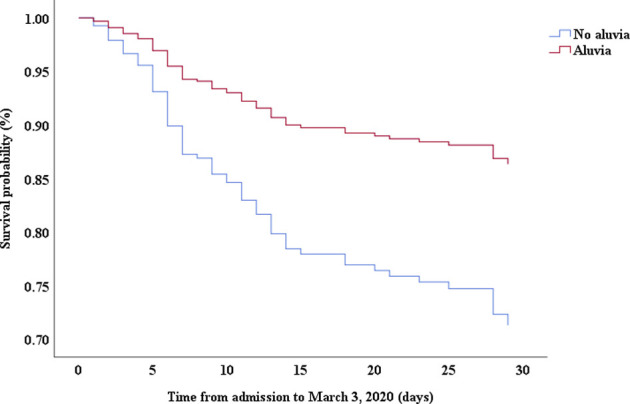
Figure E7.
References
- 1.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. https://www.who.int/csr/sars/country/table2004_04_21/en/ Available at: Accessed March 22, 2020.
- 3.World Health Organization Middle East respiratory syndrome coronavirus (MERS-CoV) http://www.who.int/emergencies/mers-cov/en/ Available at: Accessed March 22, 2020.
- 4.World Health Organization Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. https://www.who.int/internal-publications-detail/clinical-managementof-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected Available at: Accessed March 22, 2020.
- 5.World Health Organization Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases. https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117 Available at: Accessed March 22, 2020.
- 6.Metlay J.P., Waterer G.W., Long A.C., Anzueto A., Brozek J., Crothers K. Diagnosis and treatment of adults with community-acquired pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention [published online ahead of print February 24, 2020]. JAMA. https://doi.org/10.1001/jama.2020.2648. [DOI] [PubMed]
- 9.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China [published online ahead of print February 28, 2020]. N Engl J Med. https://doi.org/10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed]
- 10.He L., Ding Y., Zhang Q., Che X., He Y., Shen H. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J Pathol. 2006;210:288–297. doi: 10.1002/path.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lam C.W., Chan M.H., Wong C.K. Severe acute respiratory syndrome: clinical and laboratory manifestations. Clin Biochem Rev. 2004;25:121–132. [PMC free article] [PubMed] [Google Scholar]
- 12.Sheng W.H., Chiang B.L., Chang S.C., Ho H.N., Wang J.T., Chen Y.C. Clinical manifestations and inflammatory cytokine responses in patients with severe acute respiratory syndrome. J Formos Med Assoc. 2005;104:715–723. [PubMed] [Google Scholar]
- 13.Zhu M. SARS immunity and vaccination. Cell Mol Immunol. 2004;1:193–198. [PubMed] [Google Scholar]
- 14.Huang K., Yang T., Xu J., Yang L., Zhao J., Zhang X. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. Lancet. 2019;394:407–418. doi: 10.1016/S0140-6736(19)31147-X. [DOI] [PubMed] [Google Scholar]
- 15.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China [published online ahead of print February 19, 2020]. Allergy. https://doi.org/10.1111/all.14238. [DOI] [PubMed]
- 16.Wang X.D., Zheng M., Lou H.F., Wang C.S., Zhang Y., Bo M.Y. An increased prevalence of self-reported allergic rhinitis in major Chinese cities from 2005 to 2011. Allergy. 2016;71:1170–1180. doi: 10.1111/all.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashimoto T., Perlot T., Rehman A., Trichereau J., Ishiguro H., Paolino M. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chai X., Hu L., Zhang Y., Han W., Lu Z., Ke A. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv. 2020 2020.02.03.931766. [Google Scholar]
- 19.Yang J.K., Lin S.S., Ji X.J., Guo L.M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu C, Chen X, Cai Y, Xia Ja, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China [published online ahead of print March 13, 2020]. JAMA Intern Med. https://doi.org/10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed]
- 21.Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis [published online ahead of print March 26, 2020]. Eur Respir J. https://doi.org/10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed]
- 22.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crackower M.A., Sarao R., Oudit G.Y., Yagil C., Kozieradzki I., Scanga S.E. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 25.Erez A., Shental O., Tchebiner J.Z., Laufer-Perl M., Wasserman A., Sella T. Diagnostic and prognostic value of very high serum lactate dehydrogenase in admitted medical patients. Isr Med Assoc J. 2014;16:439–443. [PubMed] [Google Scholar]
- 26.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study [published online ahead of print February 24, 2020]. Lancet Respir Med. https://doi.org/10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed]
- 28.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19 [published online ahead of print March 18, 2020]. N Engl J Med. https://doi.org/10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed]
- 29.Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lan L, Xu D, Ye G, Xia C, Wang S, Li Y, et al. Positive RT-PCR test results in patients recovered from COVID-19 [published online ahead of print February 27, 2020]. JAMA. https://doi.org/10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed]
- 32.Peeri NC, Shrestha N, Rahman MS, Zaki R, Tan Z, Bibi S, et al. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? [published online ahead of print February 22, 2020] Int J Epidemiol. https://doi.org/10.1093/ije/dyaa033. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.