Abstract
Viral hepatitis can cause a wide spectrum of clinical presentations from a benign form with minimal or no symptoms to acute liver failure or death. Hepatitis D coinfection and superinfection have distinct clinical courses, with the latter more likely leading to chronic infection. Management of chronic hepatitis D virus is individualized because of the paucity of treatment options and significant side effect profile of currently available treatments. Sporadic cases of hepatitis E caused by contaminated meats are becoming increasingly prevalent in immunocompromised hosts. Human herpesviruses are an important cause of disease also in immunocompromised individuals.
Keywords: Hepatitis D, Hepatitis E, Hepatotropic viruses, Human herpesvirus, Viral hepatitis
Key points
-
•
Hepatitis D virus (HDV) requires hepatitis B virus for replication and is more likely to cause chronic infection in the setting of HDV superinfection in hepatitis B surface antigen–positive individuals. Treatment of HDV remains limited, with ongoing need for new therapies.
-
•
Hepatitis E is an increasingly recognized cause of chronic infection in immunocompromised individuals and is more common in genotypes 3 and 4, with sporadic cases occurring worldwide.
-
•
Human herpesviruses are commonly benign infections in immunocompetent individuals but cause significant morbidity and mortality in immunocompromised hosts.
Introduction
The term viral hepatitis refers to liver inflammation that occurs because of a viral infection. There are 5 hepatotropic viruses (hepatitis A, B, C, D, and E) that selectively infect the liver. Acute hepatitis caused by these viruses may resolve without intervention or may develop into chronic infection in some instances. Nonhepatotropic viruses target different organs in the body but are also known to cause hepatitis, although these infections are typically mild in immunocompetent hosts. The significance of nonhepatotropic viruses is most notable in immunocompromised hosts, particularly in transplant recipients.
Hepatitis D
Hepatitis D virus (HDV), also called delta virus, was first described in 1977 in a group of patients infected with hepatitis B virus (HBV) who were found to have more severe hepatitis than their counterparts.1 The hepatitis D virion consists of the hepatitis D RNA genome, hepatitis D antigen (HDAg), and a lipoprotein envelope containing HBV surface antigen (HBsAg) proteins.2 Thus, HDV requires HBV in addition to cellular RNA polymerases for replication and cannot infect individuals without the presence of HBsAg, which is required for cell entry, virion assembly and export. Since the widespread availability of the hepatitis B vaccine with worldwide implementation of vaccination programs, a concomitant decrease in HDV alongside HBV would be expected. However, the prevalence of HDV seems to be increasing and may be attributed to the higher prevalence of HDV infection in human immunodeficiency virus (HIV) coinfected individuals and intravenous drug users.3 , 4 The global burden of disease is estimated to be 62 million to 72 million, affecting nearly 1% of the general population.4
Clinical Presentation
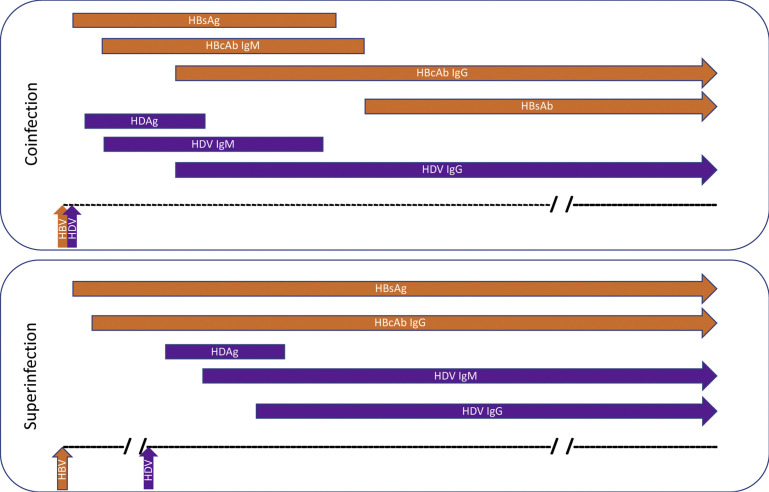
Infection with hepatitis D can occur under 2 circumstances (Fig. 1 ). Coinfection occurs when an individual is exposed to both hepatitis B and D viruses simultaneously with a similar presentation to acute HBV infection and potential risk of acute liver failure (ALF). Superinfection occurs when an individual with established chronic hepatitis B infection (defined by the presence of HBsAg) is exposed to an acute hepatitis D infection. Although superinfection with HDV is more likely to develop into chronic infection, 95% of individuals with HBV-HDV coinfection ultimately have viral clearance.5 Chronic HDV infection is the most aggressive form of viral hepatitis with greater rates of hepatocellular carcinoma and more rapid progression to cirrhosis compared with HBV monoinfection.6, 7, 8, 9
Fig. 1.
Typical pattern of HBV and HDV serologies in HDV infection. Coinfection leads to clearance of both viruses in 95% of patients. Superinfection in a patient with preexisting chronic HBV infection most often leads to chronic HDV infection. HBcAb, hepatitis B core antibody; HBsAb, hepatitis B surface antibody; IgG, immunoglobulin G; IgM, immunoglobulin M.
Screening for HDV is recommended for individuals with chronic HBV (HBsAg positivity) and presence of 1 or more of the following risk factors10:
-
•
Individuals with HIV or hepatitis C virus (HCV) coinfection
-
•
Current or past intravenous drug users
-
•
Men who have sex with men
-
•
Individuals with high-risk sexual behavior
-
•Immigrants from high-prevalence areas11
-
○Africa (central and West Africa)
-
○Asia (central and northern Asia, Vietnam, Mongolia, Pakistan, Japan, and Chinese Taipei)
-
○Pacific Islands (Kiribati, Nauru)
-
○Middle East (all countries)
-
○Eastern Europe (eastern Mediterranean regions, Turkey)
-
○South America (Amazon basin)
-
○Greenland
-
○
-
•
Individuals with high transaminase levels despite low or undetectable HBV DNA levels
Diagnosis
HDV infection is diagnosed with serum-based tests (Table 1 ). HDV antigen is typically only detected in the blood during the early acute phase of infection and is not a reliable test for diagnosis.12 Acute HDV infection leads to both innate and adaptive immune responses with production of HDV immunoglobulin M (IgM) and immunoglobulin G (IgG), respectively. HDV IgM is detectable 1 to 3 weeks after exposure and remains positive in chronic active infection with levels reflective of disease activity.13 HDV IgG is a marker of either current active or prior resolved infection. Thus, HDV IgG is checked first for chronic HDV screening, and HDV RNA is used to confirm active infection and to follow response to therapy. Because there remains ongoing work to standardize and improve HDV RNA assays, HDV IgM can be tested in patients with high clinical suspicion of HDV infection but an undetected RNA level.14
Table 1.
Serologies in hepatitis B virus and hepatitis D virus infection
| HBsAg | Anti-HBc IgM | Anti-HBc IgG | HBV DNA | HDAg | Anti-HDV IgM | Anti-HDV IgG | HDV RNA | |
|---|---|---|---|---|---|---|---|---|
| Acute HBV infection | + | + | +a | + | − | − | − | − |
| Chronic HBV infection | + | − | + | + | − | − | − | − |
| Acute HBV-HDV coinfection | + | + | +a | + | ±b | + | +a | + |
| Acute HBV-HDV superinfection | + | − | + | + | ±b | + | +a | + |
| Chronic HBV-HDV infection | + | − | + | + | ±b | +c | + | + |
| Resolved HBV and HDVd | − | − | + | − | − | − | + | − |
Abbreviations: anti-HBc, hepatitis B core antibody; HDAg, hepatitis D antigen; anti-HDV, hepatitis D antibody; IgG, immunoglobulin G; IgM, immunoglobulin M.
May not be present yet in early infection.
Present transiently, often not detected.
Typically remains persistently increased.
Occurs rarely in superinfection, more common in coinfection.
Noninvasive markers for fibrosis, including the FIB-4 score, have not been reliable in patients with chronic HDV infection.15 The greater degree of inflammation in HDV compared with HBV monoinfection likely alters elastography measurement. A recent study showed that vibration-controlled transient elastography may have reasonable accuracy to detect cirrhosis16 but remains to be validated and has not yet been studied for grading lesser degrees of fibrosis. Thus, liver biopsy is typically still required for accurate grading of inflammation and staging of fibrosis.
Treatment
Interferon alfa (IFN-α) is currently the only available treatment for chronic HDV infection. The goal of HDV therapy is to achieve viral suppression with sustained clearance of HDV after treatment completion. Thus far, no study has been able to achieve this in the majority of patients treated.17 The Hep-Net International Delta Hepatitis Intervention Trial (HIDIT), a large multicenter initiative, treated patients with peginterferon α-2a and/or adefovir for 48 weeks of therapy. Six months after treatment completion, 28% of patients treated with interferon alone had continued undetectable HDV RNA with no additional benefit derived in those who also received adefovir and no response in individuals treated with adefovir alone.18 In the follow-up study, HIDIT-II, treating with peginterferon α-2a with or without tenofovir, only 23% of patients had undetectable HDV RNA 24 weeks after completing a 96-week course of therapy with interferon and no additional benefit from concomitant tenofovir therapy.19
The international societies, including the American Association for the Study of Liver Diseases (AASLD), European Association for the Study of the Liver (EASL), and Asian Pacific Association for the Study of the Liver (APASL), do not provide specific guidelines on indications for chronic HDV treatment.10 , 20 , 21 The decision to treat with interferon must be balanced between the suspected degree of inflammation and fibrosis and whether the trajectory of disease warrants the potential side effects from interferon therapy and expected low response rates. Although the presence of HDV typically suppresses HBV replication,22 treatment with a nucleoside/nucleotide analogue (entecavir or tenofovir) is generally recommended for co-infected patients with HBV DNA levels greater than 2000 IU/mL and all patients with cirrhosis regardless of HBV replication status (Table 2 ).
Table 2.
Treatment recommendations in chronic hepatitis B virus and hepatitis D virus coinfection
| HDV RNA | ALT | HBV DNA | Cirrhosis | Treatment |
|---|---|---|---|---|
| + | + | <2000 IU/mL | No | IFN alone |
| + | + | >2000 IU/mL | No | IFN + NA |
| + | + | <2000 IU/mL | Yes | IFN + NA |
| + | + | >2000 IU/mL | Yes | IFN + NA |
IFN treatment for 48 weeks: Peg-IFN-α-2a (Pegasys) 180 μg weekly; Peg-IFN-α-2b (PegIntron) 1.5 μg/kg weekly.
NA treatment: entecavir (Baraclude) 0.5 to 1 mg daily; tenofovir dipovoxil fumarate (Viread) 300 mg daily; tenofovir alafenamide (Vemlidy) 25 mg daily.
Abbreviations: ALT, alanine transaminase; NA, nucleotide or nucleoside analogue.
The ability to achieve sustained virologic response (SVR) in the treatment of HDV remains uncertain given the high rates of late relapse. Follow-up of the HIDIT-I study participants at a median time of 4.5 years found detectable HDV RNA levels in half of the patients who had met the initial SVR definition with undetectable HDV RNA 24 weeks after treatment.23 Likelihood of response may be predicted by HDV RNA and HBsAg kinetics during treatment.24 Earlier decline of HDV RNA levels by more than 2 log copies per milliliter and HBsAg level less than 1000 IU/mL by week 24 of therapy indicate a higher likelihood of virologic response after treatment completion.25 , 26 Because of the high rates of relapse, ongoing surveillance for HDV RNA is needed, particularly in the setting of increased transaminase levels after completion of prior therapy. However, loss of HBsAg after treatment of HDV with IFN is considered a marker of cure for both HBV and HDV.
In patients with chronic HDV infection who are decompensated and unable to tolerate IFN because of its side effects, liver transplant may be considered. As with all patients with cirrhosis, ongoing screening is needed for esophageal varices and hepatocellular carcinoma. For patients who undergo liver transplant, hepatitis B immune globulin is administered similar to patients with HBV monoinfection, which results in clearance of HBsAg and HDV RNA.27
Given the paucity of treatment options, high relapse rates, and poor side effect profile, there remains a need and ongoing investigation for a treatment option that may be more efficacious. Novel treatments with promising early data under investigation include myrcludex, an entry inhibitor that blocks both HDV and HBV hepatocyte entry; the prenylation inhibitor lonafarnib, which inhibits farnesyltransferase, a key enzyme required for HDV replication; and pegylated interferon lambda, a type 3 interferon.28
Hepatitis E
Hepatitis E virus (HEV) is the most common cause of acute viral hepatitis worldwide. The original hepatitis E outbreak likely occurred in New Delhi in 1955, involving 29,000 individuals based on analysis of stored serum. The virus was initially isolated from the stool of Soviet soldiers experiencing hepatitis outbreaks during the military conflict in Afghanistan during the 1980s. HEV was subsequently named in 1990 to distinguish it from hepatitis A virus, an additional source of waterborne hepatitis epidemics at the time.29 There are 4 known HEV genotypes. Infections with genotypes 1 and 2 are limited to humans and cause disease via consumption of contaminated water. HEV genotypes 3 and 4 cause zoonotic infections, with human disease attributed to consumption of raw or undercooked meat, particularly pork and wild game.30 Thus, HEV endemic outbreaks are related to genotypes 1 and 2 typically in Asia, Africa, and Mexico, and sporadic cases caused by genotype 3 and 4 have been observed in nations worldwide.
Clinical Presentation
Clinical presentation in acute HEV infection depends on the exposed person’s risk factors. Most healthy individuals are either asymptomatic or have a self-limited course of acute hepatitis with nonspecific symptoms and spontaneous resolution after 4 to 6 weeks.31 More severe clinical courses are observed in infants, pregnant women, and individuals with excessive alcohol consumption or other chronic preexisting liver diseases.30 , 32 Mortality from acute HEV genotype 1 and 2 infections in developing countries has been largely attributed to ALF in pregnant women.33 In addition, HEV has been recognized as a cause of acute-on-chronic liver failure worldwide.34
Chronic HEV, defined by chronic hepatitis with increased aminotransferase levels and persistent detection of HEV RNA for 6 months after exposure, is rare in immunocompetent individuals but has been increasingly recognized with genotype 3 HEV infections in immunocompromised hosts, particularly in those with solid organ transplants (SOTs), stem cell transplants (SCTs), or HIV. This condition is likely caused by the impaired and/or insufficient immune T-cell response with an inability to control the virus in the immunocompromised state.35 An estimated 60% of SOT recipients infected with HEV do not clear the virus and develop chronic infection with increased risk of rapid progression to cirrhosis.36 Unlike immunocompetent individuals, HEV infection in immunocompromised patients typically presents with lower transaminase and bilirubin levels and minimal symptoms. Both acute and chronic HEV have been associated with numerous extrahepatic manifestations (Table 3 ), which may be the only sign or symptom at presentation.37, 38, 39, 40, 41
Table 3.
Hepatitis E infection in immunocompetent and immunocompromised individuals
| Immunocompetent | Immunocompromised | |
|---|---|---|
| Presentation | ||
| Symptoms | Self-limited, nonspecific symptoms | Typically asymptomatic |
| ALT Level | High >1000 IU/L | Moderate 100–300 IU/L |
| Extrahepatic Manifestations | Neurologic: Guillain-Barré syndrome, radiculoneuropathy, amyotrophy, encephalitis Renal: membranous and membranoproliferative glomerulonephritis, IgA nephropathy Hematologic: aplastic anemia, autoimmune hemolytic anemia, cryoglobulinemia, thrombocytopenia Pancreatic: pancreatitis Rheumatologic: polyarthritis Cardiac: myocarditis Endocrine: thyroiditis |
|
| Diagnosis | ||
| Serologies | HEV IgM and/or HEV RNA | HEV RNA |
| Liver Biopsy | Varies: mixed inflammatory infiltrate, interface hepatitis, cholestasis, apoptotic bodies | Varies: minimal inflammation, mild acute cellular rejection |
| Differential Diagnoses | Acute viral hepatitis (HAV, HBV, HCV, HEV, CMV, EBV) Autoimmune hepatitis Drug-induced liver injury |
Acute cellular rejection (liver transplant patients) Graft-versus-host disease (SOT or SCT patients) Drug-induced liver injury Chronic viral hepatitis (HBV, HCV, HDV) EBV and CMV hepatitis (reactivation) |
| Treatment | None Consider treatment if extrahepatic manifestations or high risk; ie, pregnancy, chronic liver disease |
Reduction of immunosuppression (avoidance of calcineurin inhibitors) Ribavirin 600 mg for 3 mo, dose adjusted for weight and renal function |
| Prevention | Universal access to clean drinking water Avoidance of undercooked pork, wild game, and shellfish Screening of blood donors and/or products Vaccination (currently not available in most countries) |
|
Abbreviations: CMV, cytomegalovirus; EBV, Epstein-Barr virus; HAV, hepatitis A virus; HCV, hepatitis C virus; IgA, immunoglobulin A; SOT, solid organ transplant; SCT, stem cell transplant.
Diagnosis
After initial exposure and an incubation period of 2 to 8 weeks, HEV RNA may be detectable in the stool and serum for 1 to 2 weeks after onset of symptoms. The diagnostic window is narrow because patients typically present after the peak viremic period has concluded. Anti-HEV IgM is produced early after infection, coinciding with peak alanine transaminase levels, and may last 4 to 6 months. Anti-HEV IgG is first present at low titers and increases incrementally over time. Thus, patients who present early may only have detectable HEV RNA, whereas many patients do not present until the early viremic period has already subsided.42 In immunocompetent hosts, diagnosis of acute HEV infection may require anti-HEV IgM or HEV RNA.
In immunocompromised hosts, levels of anti-HEV immunoglobulin are lower and frequently undetectable, so diagnosis often requires testing for HEV RNA by polymerase chain reaction (PCR) for confirmation. The World Health Organization has developed an international standard for nucleic acid amplification techniques to improve HEV RNA detection and quantification.43 To increase diagnostic rates of HEV, use of at least 2 of the 3 markers mentioned earlier is suggested to increase yield, particularly because accuracy and reliability of anti-HEV immunoglobulin assays differ widely in laboratories and among the particular individuals being tested.42 , 44 Although there are distinct HEV genotypes, the body’s immune response and production of anti-HEV IgG antibodies are cross-reactive to all 4 known genotypes.45
Liver biopsy in acute HEV infection may show a wide range of features, including mixed inflammatory infiltrate, interface hepatitis, cholestasis, and apoptotic bodies, which may have similar overlapping features with other viral hepatitis, autoimmune hepatitis, or drug-induced liver injury.46 Because most cases of acute HEV are self-limited in immunocompetent hosts, liver biopsy is often not necessary. By contrast, liver biopsy is often obtained before chronic HEV infection is suspected in immunosuppressed hosts because unexplained increase in transaminase levels is the typical presentation with no other clinical symptoms. In patients with known infection, liver biopsy may be beneficial for staging of fibrosis given the potential risk for accelerated progression to cirrhosis. HEV RNA may be detected in the liver biopsy specimen and histopathology ranges from minimal inflammation to clinical features suggestive of mild acute cellular rejection.47 Given the nonspecific findings on histologic examination, HEV RNA should be tested in transplant recipients if a liver biopsy shows chronic hepatitis of uncertain cause or a nondiagnostic biopsy in the setting of persistently abnormal liver chemistries.48
Treatment of Chronic Hepatitis E Virus Infection
The initial step in management of chronic HEV is reduction of immunosuppression, if possible, particularly using medications with an effect on T cells (ie, calcineurin inhibitors and mammalian target of rapamycin inhibitors), which has been shown to be a sufficient strategy to allow clearance of the virus in one-third of patients.49 The optimal immunosuppressive regimen still requires further studies, with current recommendations to minimize immunosuppression as much as possible and favoring use of mycophenolate rather than calcineurin or mammalian target of rapamycin inhibitors.50 Interferon has been used for treatment of hepatitis B and C, so it has similarly been investigated in use for treatment of chronic HEV.51 However, its long list of potential side effects, including the potential for graft rejection, makes it a poor treatment option. Ribavirin is used and tolerated well for treatment of chronic HEV in SOT recipients at a median dose of 600 mg daily (8 mg/kg) for 3 months and longer treatment courses for 6 to 12 months in those with partial response or relapse after treatment.52 There is currently 1 licensed vaccine for hepatitis E (HEV 239, Hecolin) available in China, which is derived from a 26-KDa protein coded by ORF2 of HEV1.53
Human herpesviruses
There are 8 viruses in the Herpesviridae family that can cause disease in humans, including viral hepatitis (Table 4 ). Initial infections with these viruses are typically self-limited. The viruses then become latent infections with the ability to reactivate when there is an immunocompromised or immunosuppressed state.
Table 4.
Human herpesviruses
| Alternate Name | Immunocompetent Host | Immunocompromised Hostsc | Liver Histology | |
|---|---|---|---|---|
| HHV-1 | HSV-1 | Oral and genital ulcers | ALF, encephalitis | Hepatocyte necrosis, intranuclear inclusions, multinucleated giant cells |
| HHV-2 | HSV-2 | Oral and genital ulcers | ALF, encephalitis | Hepatocyte necrosis, intranuclear inclusions, multinucleated giant cells |
| HHV-3 | VZV | Chickenpox, shingles | ALF, encephalitis | Hepatocyte necrosis, intranuclear inclusions, multinucleated giant cells |
| HHV-4 | EBV | Infectious mononucleosisa | Hepatitis, PTLD, lymphoma | Sinusoidal lymphocytic infiltration |
| HHV-5 | CMV | Mononucleosislike syndromea | Multisystemic organ involvementb | Mononuclear portal and sinusoidal infiltration, owl’s eye nuclear inclusions |
| HHV-6 | Roseola | Rare | Nonspecific | |
| HHV-7 | Pityriasis rosea | Rare | Nonspecific | |
| HHV-8 | KSHV | Fever, rash, lymphadenopathy | Kaposi sarcoma, Castleman disease | Proliferation of spindle-shaped cells |
Abbreviations: CMV, cytomegalovirus; EBV, Epstein-Barr virus; HHV, human herpesvirus; HSV, herpes simplex virus; KSHV, Kaposi sarcoma–associated herpes virus; PTLD, posttransplant lymphoproliferative disease; VZV, varicella zoster virus.
Mononucleosis syndrome is the classic triad of fever, pharyngitis, and lymphadenopathy.
Hepatitis, pneumonitis, colitis, myocarditis, retinitis, encephalitis, cytopenias.
Mild hepatitis may occur with all HHV infections but severe hepatitis and ALF typically only occur in immunocompromised hosts.
Herpes Simplex Virus
Herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) are common infections that cause both oral and genital vesicular lesions. Although immunocompetent individuals can develop disseminated HSV with hepatic involvement, it is more common in immunocompromised states, including pregnancy, HIV infection, and use of immunosuppressant medications. HSV hepatitis, more commonly caused by HSV-2, is less likely to manifest with characteristic mucocutaneous vesicular lesions and typically presents with fever and ALF leading to death or liver transplant in most cases.54 Diagnosis should be made with HSV DNA by PCR rather than serologies (HSV IgG or IgM) because of the latter’s inaccuracies in acute hepatitis.55 Liver biopsy may be needed for definitive diagnosis and shows hepatocellular necrosis with intranuclear inclusions and immunostaining for HSV. However, immediate initiation of empiric treatment with intravenous acyclovir is recommended given the severity and potentially rapid progression of disease, including death if treatment is delayed.54
Varicella Zoster Virus
Varicella zoster virus (VZV) is commonly known for causing chickenpox in children at the time of initial infection, and later becoming latent in the dorsal root ganglia with reactivation causing shingles in adults. Transmission occurs via aerosolized nasopharyngeal secretions or direct contact with fluid from vesicular lesions. VZV-associated hepatitis has been rarely reported in the literature but can present similarly to HSV hepatitis.56 Liver biopsy typically looks similar to HSV hepatitis, although diagnosis is made by checking serum VZV PCR. Similar to HSV, treatment with acyclovir is recommended. In immunocompromised hosts, varicella zoster immune globulin may be considered if known exposure occurs.57 An inactivated zoster vaccine (Shingrix) is now available and recommended for posttransplant and other immunocompromised patients.58
Epstein-Barr Virus
Epstein-Barr virus (EBV) is a common infection that causes infectious mononucleosis with fevers, pharyngitis, and lymphadenopathy. More than 90% of the population has evidence of prior exposure by 20 years of age.59 Unlike the other herpesviruses, mild hepatitis with hepatomegaly and increased transaminase level typically occurs with EBV infection. However, ALF caused by EBV is less common, accounting for 1 in 500 cases of the Acute Liver Failure Study Group and may occur in young and immunocompetent individuals, unlike the other herpesviruses that typically only lead to ALF in the immunocompromised host.60 After primary infection, the virus becomes latent in the memory B cells.61 EBV PCR and in situ hybridization of liver tissue can be used to identify the presence of virus, although confirmation of EBV-related hepatitis also requires the appropriate clinical features, including increased transaminase levels with serologies (viral capsid IgG/IgM and Epstein-Barr nuclear antigen antibody and EBV DNA).62 Virtually all cases of EBV hepatitis are self-limited, but rare cases of severe hepatitis or ALF may require liver transplant.
EBV infection after liver transplant has been associated with posttransplant lymphoproliferative disorder (PTLD), particularly in cases that occur in the first 18 months after transplant.63 Risk factors for PTLD within the first year of transplant include primary EBV infection, use of antilymphocyte antibodies, younger age at transplant, and transplant of the intestine, lung, or heart. Risk factors for PTLD after the first year of transplant include longer duration of immunosuppression and older age at transplant.64 Symptoms of PTLD are similar to other lymphoproliferative disorders, including malaise, fevers, weight loss, and lymphadenopathy. Diagnosis requires biopsy of the affected organ, which is typically an excisional biopsy of an enlarged lymph node. Treatment of PTLD first requires the reduction of immunosuppression, but use of anti-CD20 (anti–cluster of differentiation 20) monoclonal antibodies (ie, rituximab) or other therapies may be needed in more refractory cases.64
Cytomegalovirus
Cytomegalovirus (CMV) infection may be asymptomatic or lead to a mononucleosislike syndrome, with an estimated 64% of adults having evidence of prior CMV infection by 50 years of age.65 Mild transaminase level increases are common and may persist for months after infection.66 In solid organ transplant recipients, CMV infection is associated with increased death and graft loss, particularly within the first year of transplant.67 In these cases, CMV hepatitis may be difficult to differentiate from graft rejection. CMV may also lead to a multisystemic disease with end-organ involvement including cytopenias, pneumonitis, colitis, retinitis, myocarditis, and encephalitis. Although CMV IgM may be checked as a marker of acute infection in immunocompetent individuals, serologies are not reliable in immunocompromised hosts. CMV PCR or immunostaining of liver tissue is needed for diagnosis. Preemptive antiviral therapy with valganciclovir has been recommended for SOT recipients at risk, particularly those with no evidence of prior CMV exposure (ie, CMV IgG is negative) who receive allografts from CMV IgG-positive donors.68 Oral valganciclovir or intravenous ganciclovir may be used to treat CMV hepatitis depending on the severity of illness.
Human Herpes Viruses 6 and 7
Human herpesvirus 6 (HHV-6) and 7 (HHV-7) are typically subclinical infections that may present as roseola or pityriasis rosea, respectively. Reactivation in transplant recipients has been reported to cause hepatitis, graft rejection, and liver failure alongside extrahepatic manifestations including colitis, pneumonitis, encephalitis, and bone marrow suppression.69 Tissue biopsy with viral PCR is available but not standardized, and positive results do not necessarily confirm causation of clinical disease.70
Kaposi Sarcoma–Associated Herpesvirus
Human herpesvirus 8 (HHV-8), also called Kaposi sarcoma–associated herpesvirus, is a known cause of Kaposi sarcoma, lymphoma, and multicentric Castleman disease. Although Kaposi sarcoma is more commonly reported in association with acquired immunodeficiency syndrome, there have also been reported cases in transplant recipients, particularly in liver transplant recipients, who may have graft involvement with hepatitis.70 Reduction of immunosuppression, including conversion to mammalian target of rapamycin inhibitors, leads to response in most patients, whereas chemotherapy is reserved for those with severe disease with visceral involvement.71
Miscellaneous viruses
Additional viruses have been reported to cause a range of clinical presentations, from mild to severe acute hepatitis and ALF, including:72
-
•
Adenoviridae
-
•
Arenaviridae: Lassa virus
-
•
Coronaviridae: severe acute respiratory syndrome virus
-
•
Filoviridae: Ebola virus
-
•
Flaviviridae: Dengue virus, West Nile virus, yellow fever virus, Zika virus
-
•
Orthomyxoviridae: influenza virus
-
•
Paramyxoviridae: measles morbillivirus
-
•
Parvoviridae: parvovirus B19
-
•
Picornaviridae: Coxsackie virus, echovirus, poliovirus
-
•
Retroviridae: HIV
-
•
Togaviridae: chikungunya virus
Summary
Both HDV and HEV are causes of disease worldwide and diagnosis requires high clinical suspicion to test for disease presence. HDV remains difficult to treat with the current available therapies and typically leads to chronic disease after superinfection with an accelerated course to cirrhosis or related complications. HEV leading to chronic hepatitis is more common in immunocompromised hosts. Although the hepatotropic viruses (HAV, HBV, HCV, HDV, HEV) may cause disease in all exposed individuals, the nonhepatotropic viruses (ie, HSV-1, HSV-2, VZV, EBV, CMV) typically have self-limited courses that may include a mild hepatitis caused by the immune system’s response to the virus at the time of primary infection. For immunocompromised hosts, the risk of clinical disease from the nonhepatotropic viruses is typically at the time of reactivation, with the potential for significant morbidity and mortality.
Acknowledgments
Disclosure
A. Cheung has nothing to disclose. P. Kwo has received grant support from Eiger.
References
- 1.Rizzetto M., Canese M.G., Aricò S. Immunofluorescence detection of new antigen-antibody system (delta/anti-delta) associated to hepatitis B virus in liver and in serum of HBsAg carriers. Gut. 1977;18(12):997–1003. doi: 10.1136/gut.18.12.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heller T., Koh C., Glenn J.S. Hepatitis D. In: Sanyal A., Boyer T., Lindor K., editors. Zakim and Boyer's hepatology: a textbook of liver disease. 7th edition. Elsevier; Philadelphia: 2018. pp. 501–511. [Google Scholar]
- 3.Lin H.H., Lee S.S., Yu M.L. Changing hepatitis D virus epidemiology in a hepatitis B virus endemic area with a national vaccination program. Hepatology. 2015;61(6):1870–1879. doi: 10.1002/hep.27742. [DOI] [PubMed] [Google Scholar]
- 4.Chen H.Y., Shen D., Ji D. Prevalence and burden of hepatitis D virus infection in the global population: a systematic review and meta-analysis. Gut. 2019;68:512–521. doi: 10.1136/gutjnl-2018-316601. [DOI] [PubMed] [Google Scholar]
- 5.Caredda F., Rossi E., d'Arminio Monforte A. Hepatitis B virus-associated coinfection and superinfection with delta agent: indistinguishable disease with different outcome. J Infect Dis. 1985;151(5):925–928. doi: 10.1093/infdis/151.5.925. [DOI] [PubMed] [Google Scholar]
- 6.Fattovich G., Boscaro S., Noventa F. Influence of hepatitis delta virus infection on progression to cirrhosis in chronic hepatitis type B. J Infect Dis. 1987;155(5):931–935. doi: 10.1093/infdis/155.5.931. [DOI] [PubMed] [Google Scholar]
- 7.Romeo R., Del Ninno E., Rumi M. A 28-year study of the course of hepatitis Delta infection: a risk factor for cirrhosis and hepatocellular carcinoma. Gastroenterology. 2009;136(5):1629–1638. doi: 10.1053/j.gastro.2009.01.052. [DOI] [PubMed] [Google Scholar]
- 8.Ji J., Sundquist K., Sundquist J. A population-based study of hepatitis D virus as potential risk factor for hepatocellular carcinoma. J Natl Cancer Inst. 2012;104(10):790–792. doi: 10.1093/jnci/djs168. [DOI] [PubMed] [Google Scholar]
- 9.Mahale P., Aka P., Chen X. Hepatitis D virus infection, cirrhosis and hepatocellular carcinoma in the Gambia. J Viral Hepat. 2019;26(6):738–749. doi: 10.1111/jvh.13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terrault N.A., Lok A.S.F., McMahon B.J. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 Hepatitis B guidance. Clin Liver Dis (Hoboken) 2018;12(1):33–34. doi: 10.1002/cld.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization Hepatitis D (fact sheet) 2019. https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-d Available at: Accessed November 22, 2019.
- 12.Aragona M., Macagno S., Caredda F. Serological response to the hepatitis delta virus in hepatitis D. Lancet. 1987;1(8531):478–480. doi: 10.1016/s0140-6736(87)92090-3. [DOI] [PubMed] [Google Scholar]
- 13.Wranke A., Heidrich B., Ernst S. Anti-HDV IgM as a marker of disease activity in hepatitis delta. PLoS One. 2014;9(7):e101002. doi: 10.1371/journal.pone.0101002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Gal F., Brichler S., Sahli R. First international external quality assessment for hepatitis delta virus RNA quantification in plasma. Hepatology. 2016;64(5):1483–1494. doi: 10.1002/hep.28772. [DOI] [PubMed] [Google Scholar]
- 15.Takyar V., Surana P., Kleiner D.E. Noninvasive markers for staging fibrosis in chronic delta hepatitis. Aliment Pharmacol Ther. 2017;45(1):127–138. doi: 10.1111/apt.13834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Da B.L., Surana P., Takyar V. Vibration-controlled transient elastography for the detection of cirrhosis in chronic Hepatitis D infection. J Viral Hepat. 2020;27(4):428–436. doi: 10.1111/jvh.13235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes S.A., Wedemeyer H., Harrison P.M. Hepatitis delta virus. Lancet. 2011;378(9785):73–85. doi: 10.1016/S0140-6736(10)61931-9. [DOI] [PubMed] [Google Scholar]
- 18.Wedemeyer H., Yurdaydìn C., Dalekos G.N. Peginterferon plus adefovir versus either drug alone for hepatitis delta. N Engl J Med. 2011;364(4):322–331. doi: 10.1056/NEJMoa0912696. [DOI] [PubMed] [Google Scholar]
- 19.Wedemeyer H., Yurdaydin C., Hardtke S. Peginterferon alfa-2a plus tenofovir disoproxil fumarate for hepatitis D (HIDIT-II): a randomised, placebo controlled, phase 2 trial. Lancet Infect Dis. 2019;19(3):275–286. doi: 10.1016/S1473-3099(18)30663-7. [DOI] [PubMed] [Google Scholar]
- 20.European Association for the Study of the Liver EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 21.Sarin S.K., Kumar M., Lau G.K. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zachou K., Yurdaydin C., Drebber U. Quantitative HBsAg and HDV-RNA levels in chronic delta hepatitis. Liver Int. 2010;30(3):430–437. doi: 10.1111/j.1478-3231.2009.02140.x. [DOI] [PubMed] [Google Scholar]
- 23.Heidrich B., Yurdaydın C., Kabaçam G. Late HDV RNA relapse after peginterferon alpha-based therapy of chronic hepatitis delta. Hepatology. 2014;60(1):87–97. doi: 10.1002/hep.27102. [DOI] [PubMed] [Google Scholar]
- 24.Guedj J., Rotman Y., Cotler S.J. Understanding early serum hepatitis D virus and hepatitis B surface antigen kinetics during pegylated interferon-alpha therapy via mathematical modeling. Hepatology. 2014;60(6):1902–1910. doi: 10.1002/hep.27357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keskin O., Wedemeyer H., Tüzün A. Association between level of Hepatitis D virus RNA at week 24 of pegylated interferon therapy and outcome. Clin Gastroenterol Hepatol. 2015;13(13):2342–2349.e1-2. doi: 10.1016/j.cgh.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 26.Niro G.A., Smedile A., Fontana R. HBsAg kinetics in chronic hepatitis D during interferon therapy: on-treatment prediction of response. Aliment Pharmacol Ther. 2016;44(6):620–628. doi: 10.1111/apt.13734. [DOI] [PubMed] [Google Scholar]
- 27.Samuel D., Zignego A.L., Reynes M. Long-term clinical and virological outcome after liver transplantation for cirrhosis caused by chronic delta hepatitis. Hepatology. 1995;21(2):333–339. [PubMed] [Google Scholar]
- 28.Yurdaydin C., Abbas Z., Buti M. Treating chronic hepatitis delta: the need for surrogate markers of treatment efficacy. J Hepatol. 2019;70(5):1008–1015. doi: 10.1016/j.jhep.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 29.Reyes G.R., Huang C.C., Yarbough P.O. Hepatitis E virus. Comparison of 'new and Old World' isolates. J Hepatol. 1991;13(Suppl 4):S155–S161. doi: 10.1016/0168-8278(91)90050-l. [DOI] [PubMed] [Google Scholar]
- 30.Kamar N., Bendall R., Legrand-Abravanel F. Hepatitis E. Lancet. 2012;379(9835):2477–2488. doi: 10.1016/S0140-6736(11)61849-7. [DOI] [PubMed] [Google Scholar]
- 31.Scobie L., Dalton H.R. Hepatitis E: source and route of infection, clinical manifestations and new developments. J Viral Hepat. 2013;20(1):1–11. doi: 10.1111/jvh.12024. [DOI] [PubMed] [Google Scholar]
- 32.Kumar Acharya S., Kumar Sharma P., Singh R. Hepatitis E virus (HEV) infection in patients with cirrhosis is associated with rapid decompensation and death. J Hepatol. 2007;46(3):387–394. doi: 10.1016/j.jhep.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 33.Navaneethan U., Al Mohajer M., Shata M.T. Hepatitis E and pregnancy: understanding the pathogenesis. Liver Int. 2008;28(9):1190–1199. doi: 10.1111/j.1478-3231.2008.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarin S.K., Choudhury A., Sharma M.K. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13(4):353–390. doi: 10.1007/s12072-019-09946-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suneetha P.V., Pischke S., Schlaphoff V. Hepatitis E virus (HEV)-specific T-cell responses are associated with control of HEV infection. Hepatology. 2012;55(3):695–708. doi: 10.1002/hep.24738. [DOI] [PubMed] [Google Scholar]
- 36.Kamar N., Garrouste C., Haagsma E.B. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology. 2011;140(5):1481–1489. doi: 10.1053/j.gastro.2011.02.050. [DOI] [PubMed] [Google Scholar]
- 37.Fernandes B., Dias E., Mascarenhas-Saraiva M. Rheumatologic manifestations of hepatic diseases. Ann Gastroenterol. 2019;32(4):352–360. doi: 10.20524/aog.2019.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamar N., Marion O., Abravanel F. Extrahepatic manifestations of hepatitis E virus. Liver Int. 2016;36(4):467–472. doi: 10.1111/liv.13037. [DOI] [PubMed] [Google Scholar]
- 39.Haffar S., Bazerbachi F., Garg S. Frequency and prognosis of acute pancreatitis associated with acute hepatitis E: a systematic review. Pancreatology. 2015;15(4):321–326. doi: 10.1016/j.pan.2015.05.460. [DOI] [PubMed] [Google Scholar]
- 40.Cheung M.C., Maguire J., Carey I. Review of the neurological manifestations of hepatitis E infection. Ann Hepatol. 2012;11(5):618–622. [PubMed] [Google Scholar]
- 41.Kamar N., Weclawiak H., Guilbeau-Frugier C. Hepatitis E virus and the kidney in solid-organ transplant patients. Transplantation. 2012;93(6):617–623. doi: 10.1097/TP.0b013e318245f14c. [DOI] [PubMed] [Google Scholar]
- 42.Huang S., Zhang X., Jiang H. Profile of acute infectious markers in sporadic hepatitis E. PLoS One. 2010;5(10):e13560. doi: 10.1371/journal.pone.0013560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baylis S.A., Wallace P., McCulloch E. Standardization of nucleic acid tests: the approach of the World Health Organization. J Clin Microbiol. 2019;57(1) doi: 10.1128/JCM.01056-18. [pii:e01056-18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Norder H., Karlsson M., Mellgren Å. Diagnostic performance of five assays for anti-hepatitis E virus IgG and IgM in a large cohort study. J Clin Microbiol. 2016;54(3):549–555. doi: 10.1128/JCM.02343-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Emerson S.U., Clemente-Casares P., Moiduddin N. Putative neutralization epitopes and broad cross-genotype neutralization of Hepatitis E virus confirmed by a quantitative cell-culture assay. J Gen Virol. 2006;87(Pt 3):697–704. doi: 10.1099/vir.0.81545-0. [DOI] [PubMed] [Google Scholar]
- 46.Malcolm P., Dalton H., Hussaini H.S. The histology of acute autochthonous hepatitis E virus infection. Histopathology. 2007;51(2):190–194. doi: 10.1111/j.1365-2559.2007.02756.x. [DOI] [PubMed] [Google Scholar]
- 47.Protzer U., Böhm F., Longerich T. Molecular detection of hepatitis E virus (HEV) in liver biopsies after liver transplantation. Mod Pathol. 2015;28(4):523–532. doi: 10.1038/modpathol.2014.147. [DOI] [PubMed] [Google Scholar]
- 48.Te H., Doucette K. Viral hepatitis: guidelines by the American society of transplantation infectious disease community of practice. Clin Transplant. 2019;33(9):e13514. doi: 10.1111/ctr.13514. [DOI] [PubMed] [Google Scholar]
- 49.Unzueta A., Rakela J. Hepatitis E infection in liver transplant recipients. Liver Transpl. 2014;20(1):15–24. doi: 10.1002/lt.23764. [DOI] [PubMed] [Google Scholar]
- 50.Behrendt P., Steinmann E., Manns M.P. The impact of hepatitis E in the liver transplant setting. J Hepatol. 2014;61(6):1418–1429. doi: 10.1016/j.jhep.2014.08.047. [DOI] [PubMed] [Google Scholar]
- 51.Haagsma E.B., Riezebos-Brilman A., van den Berg A.P. Treatment of chronic hepatitis E in liver transplant recipients with pegylated interferon alpha-2b. Liver Transpl. 2010;16(4):474–477. doi: 10.1002/lt.22014. [DOI] [PubMed] [Google Scholar]
- 52.Kamar N., Izopet J., Tripon S. Ribavirin for chronic hepatitis E virus infection in transplant recipients. N Engl J Med. 2014;370(12):1111–1120. doi: 10.1056/NEJMoa1215246. [DOI] [PubMed] [Google Scholar]
- 53.Li S.W., Zhao Q., Wu T. The development of a recombinant hepatitis E vaccine HEV 239. Hum Vaccin Immunother. 2015;11(4):908–914. doi: 10.1080/21645515.2015.1008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Norvell J.P., Blei A.T., Jovanovic B.D. Herpes simplex virus hepatitis: an analysis of the published literature and institutional cases. Liver Transpl. 2007;13(10):1428–1434. doi: 10.1002/lt.21250. [DOI] [PubMed] [Google Scholar]
- 55.Levitsky J., Duddempudi A.T., Lakeman F.D. Detection and diagnosis of herpes simplex virus infection in adults with acute liver failure. Liver Transpl. 2008;14(10):1498–1504. doi: 10.1002/lt.21567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patti M.E., Selvaggi K.J., Kroboth F.J. Varicella hepatitis in the immunocompromised adult: a case report and review of the literature. Am J Med. 1990;88(1):77–80. doi: 10.1016/0002-9343(90)90133-x. [DOI] [PubMed] [Google Scholar]
- 57.Kusne S., Pappo O., Manez R. Varicella-zoster virus hepatitis and a suggested management plan for prevention of VZV infection in adult liver transplant recipients. Transplantation. 1995;60(6):619–621. doi: 10.1097/00007890-199509270-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Danziger-Isakov L., Kumar D., Practice AICo. Vaccination of solid organ transplant candidates and recipients: guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. 2019;33(9):e13563. doi: 10.1111/ctr.13563. [DOI] [PubMed] [Google Scholar]
- 59.Kofteridis D.P., Koulentaki M., Valachis A. Epstein Barr virus hepatitis. Eur J Intern Med. 2011;22(1):73–76. doi: 10.1016/j.ejim.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 60.Mellinger J.L., Rossaro L., Naugler W.E. Epstein-Barr virus (EBV) related acute liver failure: a case series from the US Acute Liver Failure Study Group. Dig Dis Sci. 2014;59(7):1630–1637. doi: 10.1007/s10620-014-3029-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Babcock G.J., Decker L.L., Volk M. EBV persistence in memory B cells in vivo. Immunity. 1998;9(3):395–404. doi: 10.1016/s1074-7613(00)80622-6. [DOI] [PubMed] [Google Scholar]
- 62.Suh N., Liapis H., Misdraji J. Epstein-Barr virus hepatitis: diagnostic value of in situ hybridization, polymerase chain reaction, and immunohistochemistry on liver biopsy from immunocompetent patients. Am J Surg Pathol. 2007;31(9):1403–1409. doi: 10.1097/PAS.0b013e31802ffdd5. [DOI] [PubMed] [Google Scholar]
- 63.Kremers W.K., Devarbhavi H.C., Wiesner R.H. Post-transplant lymphoproliferative disorders following liver transplantation: incidence, risk factors and survival. Am J Transplant. 2006;6(5 Pt 1):1017–1024. doi: 10.1111/j.1600-6143.2006.01294.x. [DOI] [PubMed] [Google Scholar]
- 64.Allen U.D., Preiksaitis J.K., Practice AIDCo Post-transplant lymphoproliferative disorders, epstein-barr virus infection, and disease in solid organ transplantation: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9):e13652. doi: 10.1111/ctr.13652. [DOI] [PubMed] [Google Scholar]
- 65.Bate S.L., Dollard S.C., Cannon M.J. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clin Infect Dis. 2010;50(11):1439–1447. doi: 10.1086/652438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cohen J.I., Corey G.R. Cytomegalovirus infection in the normal host. Medicine (Baltimore) 1985;64(2):100–114. doi: 10.1097/00005792-198503000-00003. [DOI] [PubMed] [Google Scholar]
- 67.Bosch W., Heckman M.G., Diehl N.N. Association of cytomegalovirus infection and disease with death and graft loss after liver transplant in high-risk recipients. Am J Transplant. 2011;11(10):2181–2189. doi: 10.1111/j.1600-6143.2011.03618.x. [DOI] [PubMed] [Google Scholar]
- 68.Kotton C.N., Kumar D., Caliendo A.M. The third international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2018;102(6):900–931. doi: 10.1097/TP.0000000000002191. [DOI] [PubMed] [Google Scholar]
- 69.Abdel Massih R.C., Razonable R.R. Human herpesvirus 6 infections after liver transplantation. World J Gastroenterol. 2009;15(21):2561–2569. doi: 10.3748/wjg.15.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pellett Madan R., Hand J., Practice AIDCo Human herpesvirus 6, 7, and 8 in solid organ transplantation: guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. 2019;33(9):e13518. doi: 10.1111/ctr.13518. [DOI] [PubMed] [Google Scholar]
- 71.Delyon J., Rabate C., Euvrard S. Management of Kaposi sarcoma after solid organ transplantation: a European retrospective study. J Am Acad Dermatol. 2019;81(2):448–455. doi: 10.1016/j.jaad.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 72.Mrzljak A., Tabain I., Premac H. The role of emerging and neglected viruses in the etiology of hepatitis. Curr Infect Dis Rep. 2019;21(12):51. doi: 10.1007/s11908-019-0709-2. [DOI] [PubMed] [Google Scholar]