Abstract
Background
The COVID-19 has now been declared a global pandemic by the World Health Organization. There is an emergent need to search for possible medications.
Method
Utilization of the available sequence information, homology modeling, and in slico docking a number of available medications might prove to be effective in inhibiting the SARS-CoV-2 two main drug targets, the spike glycoprotein, and the 3CL protease.
Results
Several compounds were determined from the in silico docking models that might prove to be effective inhibitors for SARS-CoV-2. Several antiviral medications: Zanamivir, Indinavir, Saquinavir, and Remdesivir show potential as and 3CLPRO main proteinase inhibitors and as a treatment for COVID-19.
Conclusion
Zanamivir, Indinavir, Saquinavir, and Remdesivir are among the exciting hits on the 3CLPRO main proteinase. It is also exciting to uncover that Flavin Adenine Dinucleotide (FAD) Adeflavin, B2 deficiency medicine, and Coenzyme A, a coenzyme, may also be potentially used for the treatment of SARS-CoV-2 infections. The use of these off-label medications may be beneficial in the treatment of the COVID-19.
Keywords: SARS-CoV-2, Coronavirus, Molecular docking, Approved drugs, Medications
Highlights
-
•
Molecular docking has been used for the search of possible medications that fall under the approved bioactive that may inhibit the SARS-CoV-2 spike protein and the 3CLPRO protease.
-
•
Several exciting hits on the 3CLPRO main proteinase are Zanamivir, Indinavir, Remdesivir, and Saquinavir.
-
•
Flavin Adenine Dinucleotide (FAD) Adeflavin, a B2 Deficiency medicine, and Coenzyme A, a coenzyme, may also be potentially used for the treatment of COVID-19.
1. Introduction
The World Health Organization has now declared a global emergency and pandemic for the coronavirus disease (COVID-19) that has been actively spreading around the globe. COVID-19 which is caused by the virus SARS-CoV-2; can cause symptoms such as fever, cough, pneumonia, nausea, and fatigue. As of now SARS-CoV-2 has reached 24 countries around the globe, with more than 190,000 cases confirmed as of March 18, 2020[1].
The epidemiological background of the virus was thought to stem from a seafood market in Wuhan, China [2]. However, the true epicenter of the initial transfer to humans is still unknown. Currently, there are >100 complete genome sequences known in the NCBI GenBank, from over 10 countries. The variation between these sequences is less than 1%.
This virus is closely related to the SARS-CoV and this allows utilization of the known protein structures to quickly build a model for drug discovery on this new SARS-CoV-2 [3]. While traditional methods of drug discovery could take years, the approach taken here to search for possible medications for the SARS-CoV-2 is in silico docking models from the most variable proteins in the SARS-CoV-2, the spike glycoprotein, and the SARS-CoV-2 3CL main protease.
The CoV spike protein binds to a host cell membrane through a receptor-mediated interaction which allows entrance to the host cell. It has been computationally determined that the SARS-CoV-2 has similar mechanism to that of the SARS virus and the receptor to which it has the highest affinity is ACE2 (angiotensin-converting enzyme 2) [4]. While there are structural similarities between the SARS-CoV-2 spike protein and the SARS spike protein, the conservation is only 73% with most of the variability being in the host cell interaction region of the protein. Currently, there is no crystal structure available for the SARS-CoV-2 spike protein, so we employed homology modeling of the SARS-CoV-2 utilizing the SARS spike protein (PDB: 2GHV) as a template.
The second in silico docking model is the 3CLPRO main protease, which is responsible for controlling several major functions of the virus and has a highly conserved catalytic domain from the SARS virus [5]. Some of its functions include the replication processes of the virus which makes it an ideal target for drug development [6]. The SARS-CoV-2 main protease was determined by Ref. [7] (PDB: 6LU7).
Both these proteins, spike and protease, are essential to the transmission and virulence of the virus. By inhibiting anyone of these two proteins or both for a higher active therapy, the severity of the infection will be reduced. Our efforts have been placed in competitively inhibiting the binding of its natural substrates. A library of known bioactive compounds has been run against several sites on the spike protein and the catalytic site of the SARS-CoV-2 main protease.
By utilizing an approved compound database, quick trials of these compounds, with minimal effort of approval by food and drug agencies, could be carried out. We have chosen to run the Zinc15 database which is classified by Zinc15 [8] as “Approved drugs in major jurisdictions, including the FDA, i.e DrugBank approved”. This database covers all major bioactive pharmaceutical compounds utilized around the globe, and currently has 3447 entries.
2. Methods
2.1. Molecular docking
Molecular docking calculations were completed using Schrodinger® docking suits (Schrödinger Maestro, New York, NY, USA. Version 11.9.011, MMshare Version 4.5.011, Release 2019–1, Platform Windows-x64) using a virtual screening workflow. This workflow utilized three docking precisions, HTVS, SP, and XP, which yielded the top 10% of hits for each binding site. Both proteins were prepared by restrained minimization using force field OPLS3e. The grid sites were created using Glide® receptor grid generator with docking length of 20 Å. Grids centers were determined from active resides on target protein. Ligands were prepared using force field OPLS3e and possible states were generated from pH 7.0±2.0. Docking scores are reported in kcal/mol, the more negative the number, the better binding.
2.2. Homology modeling of spike protein
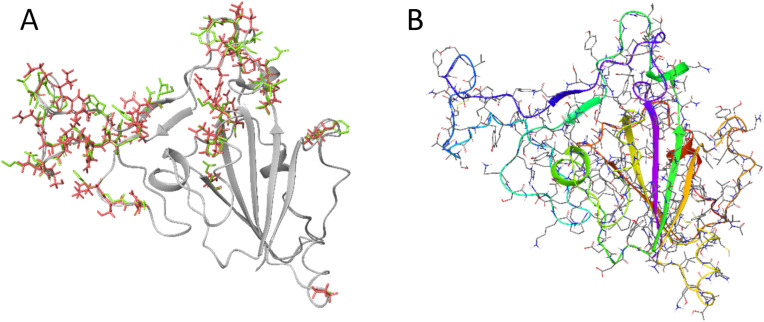
The surface glycoprotein [Wuhan seafood market pneumonia virus] (Sequence ID: YP_009724390.1) structure was modeled using ModBase [9] which utilized Modeller [10] for the structural modeling. The sequence (NCBI Accession: YP_009724390) was uploaded to the ModBase interface and was run with the template being SARS spike protein receptor binding domain (PDB: 2GHV, Chain E). The sequence identity was found to be 73% (Fig. 1 A). The calculation was completed and imported into Schrödinger Maestro®. The structure was then minimized using the force field OPLS3e, the overlay of the pre and post minimized structure can be seen in Fig. S2.
Fig. 1.
A) Modeled SARS-CoV-2 Spike Glycoprotein overlaid with the SARS-CoV (PDB: 2GHV) unique amino acids are shown. Variable amino acid residue side chains are shown: Green: SARS-CoV Red: SARS-CoV-2. B) Minimized final structure of modeled SARS-CoV-2 spike glycoprotein. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3. Results
3.1. Spike glycoprotein
Sequencing has revealed that the SARS-CoV-2 is similar to that of the SARS-CoV virus which allows for genomic and proteomic homology comparison. Using the homology modeling we have been able to develop a model of the Spike glycoprotein (Fig. 1). This model has allowed us to perform docking calculations utilizing a database of known bioactive and approved compounds.
The MODELLER and ModBase programs were able to use a homologue SARS spike protein (PDB: 2GHV) and the original SARS-CoV-2 sequence (GenBank: MN908947) and construct the SARS-CoV-2 spike protein. The protein was then run through a restriction minimization process utilizing Schrodinger Docking Suits® Protein Preparation which allows side chains to be placed in the most energetically favorable conformation (Fig. 1B).
In an effort to stop the Spike-ACE2 interaction, several sites have been determined and targeted on the Spike protein for docking studies. Three of these sites are located at the interaction points specifically where hydrogen bonding is calculated as the main intermolecular force of the Spike-ACE2 interaction and a fourth allosteric site has been determined by surface mapping of the protein.
The locations of the binding sites have been chosen as these would cause the most destruction in ACE2 interactions. The sites are labeled as site 1–4 and information on the sites can be seen in Supplemental (Table S1, Fig. S1). The results from the SARS-CoV-2 spike glycoprotein are reported in Table 1 .
Table 1.
Highest scoring molecules for SARS-CoV-2 Spike Glycoprotein.
| Site # | DrugBank ID | Docking Score (kcal/mol) | Name | Indication |
|---|---|---|---|---|
| 1 | DB06441 | −7.234 | Cangrelor | P2Y12 Inhibitor |
| 1 | DB00157 | −7.038 | Dpnh (NADH) | Supplement Mental Health |
| 2 | DB03147 | −11.089 | Flavin Adenine Dinucleotide (FAD) Adeflavin | B2 Deficiency |
| 2 | DB11705 | −7.687 | Iomeprol | Contrast Medium |
| 3 | DB01992 | −11.555 | Coenzyme A | Supplement |
| 4 | DB01133 | −9.364 | Tiludronate | Paget's disease |
| 4 | DB03147 | −9.353 | Flavin Adenine Dinucleotide (FAD) Adeflavin | B2 Deficiency |
3.2. 3CLPRO main protease
Structural alignments have revealed that the SARS-CoV-2 protease is highly conserved for that of the SARS (PDB: 1LVO) main protease at 98% ID [11]. The 3CLPRO main protease was run through a restriction minimization process utilizing Schrodinger Docking Suits® ( Fig. S3A). Previous studies have revealed in the SARS protease mutation of the residue His162 renders the enzyme inactive. The SARS-CoV-2 homologous residue is His163 (Site 1 center: x = −17.59, y = 15.81, z = 63.53) (Fig. S3B) which has been used as the central point for molecular docking calculations. The active site also revealed a second Histidine (center: His41 Site 2 center: x = −13.81, y = 19.72, z = 71.91) (Fig. S3C) that seems to play a role in the interactions of the bound ligand in the 6LU7 structure, so this was targeted as a second center point for the molecular docking calculations. The results from the SARS-CoV-2 3CL protease are reported in Table 2 .
Table 2.
Highest scoring molecules for SARS-CoV-2 3CLPRO Main Protease.
| Site # | DrugBank ID | Docking Score (kcal/mol) | Name | Indication |
|---|---|---|---|---|
| 1 | DB00157 | −11.016 | Dpnh (NADH) | Supplement Mental Health |
| 1 | DB00558 | −8.843 | Zanamivir | Antiviral Drug |
| 1 | DB00188 | −8.654 | Bortezomib | Anti-Cancer |
| 1 | DB01232 | −7.285 | Saquinavir | HIV Protease Inhibitor |
| 2 | DB03147 | −10.339 | Flavin Adenine Dinucleotide (FAD) Adeflavin | B2 Deficiency |
| 2 | DB06441 | −10.269 | Cangrelor | P2Y12 Inhibitor |
| 2 | DB08889 | −8.924 | Carfilzomib | Anti-Cancer |
| 2 | DB00224 | −8.199 | Indinavir | HIV Protease Inhibitor |
| 2 | DB14761 | −7.215 | Remdesivir | Antiviral |
4. Conclusion
Molecular docking has been employed for the search of possible medications that are contained in the approved bioactive compound database. The hit compounds reported here have potential to inhibit the SARS-CoV-2 spike protein and the 3CLPRO main protease but are not guaranteed to have any activity; however, this lays the groundwork for computational drug discovery for new compounds to reduce transmission and symptoms of SARS-CoV-2. We have used structural homology modeling to determine a dock-able target for the SARS-CoV-2 spike protein and have utilized the newly characterized 3CLPRO main protease in our docking models.
We have several exciting hits on the 3CLPRO main proteinase. Zanamivir is an approved medication for the treatment of influenza A and B viruses [12]. Indinavir and Saquinavir have been shown to treat and prevent HIV. Remdesivir is an antiviral compound in experimental stages that has shown activity against the SARS-coronavirus, Ebola virus, and possibly the SARS-CoV-2 [[13], [14], [15]]. It is also exciting to uncover that Flavin Adenine Dinucleotide (FAD) Adeflavin, B2 Deficiency medicine, and Coenzyme A, a coenzyme, may also be potentially used for the treatment of SARS-CoV-2 infections.
CRediT authorship contribution statement
Donald C. Hall: Writing - original draft. Hai-Feng Ji: Writing - review & editing, Writing - original draft.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tmaid.2020.101646.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Novel coronavirus(2019-nCoV) situation report - 58. Organization, W.H; March 18, 2020. 2020. [Google Scholar]
- 2.Chen, N., Zhou, M., Dong, X., Qu, J., Gong, F., Han, Y., Qiu, Y., Wang, J., Liu, Y., Wei, Y., Xia, J.a., Yu, T., Zhang, X., Zhang, L., Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. [DOI] [PMC free article] [PubMed]
- 3.Hui D.S., E I.A., Madani T.A., Ntoumi F., Kock R., Dar O., Ippolito G., McHugh T.D., Memish Z.A., Drosten C., Zumla A., Petersen E. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis : Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. official publication of the International Society for Infectious Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu, X., Chen, P., Wang, J., Feng, J., Zhou, H., Li, X., Zhong, W., Hao, P., Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci(1674-7305). [DOI] [PMC free article] [PubMed]
- 5.Anand K., Ziebuhr J., Wadhwani P., Mesters J.R., Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. 2003;300(5626):1763–1767. doi: 10.1126/science.1085658. [DOI] [PubMed] [Google Scholar]
- 6.Bacha U., Barrila J., Velazquez-Campoy A., Leavitt S.A., Freire E. Identification of novel inhibitors of the SARS coronavirus main protease 3CLpro. Biochemistry. 2004;43(17):4906–4912. doi: 10.1021/bi0361766. [DOI] [PubMed] [Google Scholar]
- 7.Liu X., Zhang B., Jin Z., Yang H., Rao Z. The crytal structure of 2019-nCoV main protease in complex with an inhibitor N3. http://www.rcsb.org/structure/6LU7
- 8.Sterling T., Irwin J.J. ZINC 15--ligand discovery for everyone. J Chem Inf Model. 2015;55(11):2324–2337. doi: 10.1021/acs.jcim.5b00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pieper U., Webb B.M., Dong G.Q., Schneidman-Duhovny D., Fan H., Kim S.J., Khuri N., Spill Y.G., Weinkam P., Hammel M., Tainer J.A., Nilges M., Sali A. ModBase, a database of annotated comparative protein structure models and associated resources. Nucleic Acids Res. 2014;42(Database issue):D336–346. doi: 10.1093/nar/gkt1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eswar N., Eramian D., Webb B., Shen M.-Y., Sali A. Springer; 2008. Protein structure modeling with MODELLER, Structural proteomics; pp. 145–159. [DOI] [PubMed] [Google Scholar]
- 11.Anand K., Palm G.J., Mesters J.R., Siddell S.G., Ziebuhr J., Hilgenfeld R. Structure of coronavirus main proteinase reveals combination of a chymotrypsin fold with an extra alpha-helical domain. EMBO J. 2002;21(13):3213–3224. doi: 10.1093/emboj/cdf327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eiland L.S., Eiland E.H. Zanamivir for the prevention of influenza in adults and children age 5 years and older. Therapeut Clin Risk Manag. 2007;3(3):461–465. [PMC free article] [PubMed] [Google Scholar]
- 13.Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., Leist S.R., Pyrc K., Feng J.Y., Trantcheva I., Bannister R., Park Y., Babusis D., Clarke M.O., Mackman R.L., Spahn J.E., Palmiotti C.A., Siegel D., Ray A.S., Cihlar T., Jordan R., Denison M.R., Baric R.S. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9(396) doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tchesnokov E.P., Feng J.Y., Porter D.P., Götte M. Mechanism of inhibition of Ebola virus RNA-dependent RNA polymerase by remdesivir. Viruses. 2019;11(4):326. doi: 10.3390/v11040326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020 doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.