Abstract
In eukaryotic cells, protein sorting is a highly regulated mechanism important for many physiological events. After synthesis in the endoplasmic reticulum and trafficking to the Golgi apparatus, proteins sort to many different cellular destinations including the endolysosomal system and the extracellular space. Secreted proteins need to be delivered directly to the cell surface. Sorting of secreted proteins from the Golgi apparatus has been a topic of interest for over thirty years, yet there is still no clear understanding of the machinery that forms the post-Golgi carriers. Most evidence points to these post-Golgi carriers being tubular pleomorphic structures that bud from the trans-face of the Golgi. In this review, we present the background studies and highlight the key components of this pathway, we then discuss the machinery implicated in the formation of these carriers, their translocation across the cytosol, and their fusion at the plasma membrane.
Abbreviations: ATP, adenosine triphosphate; BFA, Brefeldin A; CARTS, CARriers of the TGN to the cell Surface; CI-MPR, cation-independent mannose-6 phosphate receptor; CtBP3/BARS, C-terminus binding protein 3/BFA adenosine diphosphate–ribosylated substrate; ER, endoplasmic reticulum; GlcCer, glucosylceramidetol; GPI-anchored proteins, glycosylphosphatidylinositol-anchored proteins; PAUF, pancreatic adenocarcinoma up-regulated factor; RUSH, retention using selective hooks; SBP, streptavidin-binding peptide; SM, sphingomyelin; SNARE, soluble N-ethylmaleimide sensitive fusion protein attachment protein receptor; SPCA1, secretory pathway calcium ATPase 1; PKD, Protein Kinase D; TGN, trans-Golgi Network; TIRF, total internal reflection fluorescence; ts, temperature sensitive; VSV, vesicular stomatitis virus
Keywords: Secretion, Constitutive Secretion, post-Golgi carriers, Golgi to plasma membrane sorting, pleomorphic tubular carriers
1. Introduction
Cells are internally compartmentalised. This allows proteins and lipids that participate in the same biochemical pathways to have higher local concentrations. Additionally, compartmentalisation segregates proteins that could potentially damage other cellular components (eg. proteases). This system is maintained by the trafficking of lipids and proteins in vesicles within the cell. Vesicles bud from a ‘donor’ compartment and translocate across the cytosol to fuse with a ‘receiver’ compartment, a process orchestrated by the membrane trafficking machinery.
Membrane trafficking allows delivery of nascently biosynthesised proteins and lipids to the plasma membrane. Approximately 11-15% of human proteins are secreted as soluble proteins to the extracellular space [[1], [2], [3]] and an additional 10% localise to the plasma membrane [4]. This represents over 3500 proteins with a vast array of functions, including antibodies, signalling molecules, and integral endomembrane proteins which traffic to the plasma membrane before being internalised to the endolysosomal system. Secreted proteins are not only numerous, but abundant, with the secreted protein collagen family accounting for 30% of total protein mass in mammals [5]. This review will focus on the trafficking route and machinery required to deliver proteins from the Golgi apparatus to the plasma membrane.
2. Background studies on export from the Golgi apparatus
The fundamental principles of protein secretion were established during the early days of protein trafficking, by the pioneering work of George Palade. To track the intracellular biosynthetic routes, Palade used the highly secretory pancreatic exocrine cells, which accumulate proteins in zymogen granules before their regulated secretion [6]. Using analytical centrifugation and pulse-chase autoradiography, Palade demonstrated the fundamental principle that protein sorting is sequential and vectorial.
Later studies on the trafficking of immunoglobulin, a model secreted protein which traffics directly from the Golgi to the plasma membrane, identified the 'smooth vesicles' or 'secretory vacuoles' that contain this protein [7,8]. It was also realised, around the same time, that there were two different forms of secretion: constitutive (i.e. continuous) and regulated (i.e. upon stimulation). Both forms of secretion can occur in the same cell [9,10] and segregation between the two routes happens in the trans-Golgi network [11]. In this review we focus on the constitutive secretory route that is found in the vast majority of eukaryotic cells.
It soon became clear that specialised cells, such as endocrine cells and neurons, are able to secrete a subset of proteins from a preferred portion of the plasma membrane. This process was later defined as polarised secretion [9,12]. This theory was formed from multiple lines of evidence including the polarised distribution of proteins in electron micrographs [[13], [14], [15]], immunoisolation of synaptic vesicles identifying specific proteins localised to polarised carriers [16], imaging studies tracking directional trafficking of organelles in the axons of squids [[17], [18], [19]], and secretion of viral proteins in polarised epithelial cells [[20], [21], [22]].
To identify the fundamental machinery of the secretory process, the laboratory of Randy Schekman performed a genetic screen in yeast [23]. Secretory mutants in yeast accumulate proteins and membrane intracellularly, making the cells denser. These mutant yeast would therefore differentially sediment from wild-type yeast on density gradients. This principle was employed to screen the yeast genome for mutants of the secretory system. To perform this screen they used ‘temperature sensitive’ (ts) mutants. ts mutants result in temperature dependent protein instability. Proteins with ts mutations fold properly in a ‘permissive’ temperature, but when the temperature is shifted they misfold, thus revealing the phenotype of protein loss. This allowed a set of 23 complementation groups (Sec1-23) to be discovered in what was called the Sec screen [23]. One of the major findings from this screen was the COPII protein complex that allows for endoplasmic reticulum (ER) to Golgi transport. Along with Rothman’s work [24,25], this landmark study identified the first genes encoding the machinery involved in protein trafficking and secretion. At this point, however, the route from the early secretory system to the plasma membrane and the machinery involved was still not understood.
In efforts to understand the principle of protein sorting and secretion, the ‘bulk flow’ hypothesis was formally proposed [9,26]. The ‘bulk flow’ model states that secretion from the ER through the Golgi apparatus to the plasma membrane does not require a signal and happens in ‘bulk’ by default. The first hints at this hypothesis came from the secretion of bacterial β-lactamase after microinjection of the RNA into Xenopus oocytes [27]. Subsequently, bulk peptide secretion was demonstrated by monitoring glycosylation and secretion of exogenously added peptides to mammalian cell lines and detecting their arrival at the cell surface [26]. A subset of the secreted peptides were glycosylated indicating they had been transported through the Golgi apparatus. The conclusion was that these peptides were secreted in bulk by default.
In the following years there were a number of studies that questioned the generality of this hypothesis. Most of these studies focussed on the ER export of membrane spanning proteins rather than the Golgi to plasma membrane route. Studies on soluble proteins reported increased concentration of secreted proteins as they are trafficked to the plasma membrane, suggesting that they are actively sorted. Concentrative transport of soluble proteins, however, was thought to be due to selective removal of other cargo rather than active sorting, supporting the idea that there is no selective transport on the soluble cargo itself [28,29]. There are a subset of soluble proteins that are actively sorted from the ER, such as glycosylphosphatidylinositol-anchored proteins (GPI-anchored proteins) which are sorted by the p24 protein family [30] and proteins including cathepsin C [31], cathepsin Z [32], factor V, and factor VIII [33] which require the ERGIC-53/LMAN1 receptor. It is generally agreed, however, that the default fate of soluble proteins without any known signal is to be secreted.
Soluble protein secretion has been demonstrated to be highly efficient. In yeast, induction of secretion of acid phosphatase with a temperature shift indicates a six-fold increase in secreted protein with almost no detectable increase in intracellular levels [34]. Studies in plants demonstrated that 50-90% of synthesised soluble proteins are delivered to the extracellular space [35,36], consistent with studies in mammalian cells [37]. A quantitative study on the secretion of a soluble non-glycosylated protein found that after synthesis and folding the fastest molecules are secreted in about 15 min with the half-life of secretion being 40 min [38]. The resulting consensus for soluble secreted proteins is that transport is not signal-mediated, relatively fast and follows a default bulk flow pathway from the Golgi apparatus to the plasma membrane.
The ‘default’ route of integral membrane proteins is more complicated as there are multiple sorting signals and protein topologies. As discussed above there are two membrane trafficking steps to arrive at the plasma membrane: from the ER to the Golgi and from the Golgi to the plasma membrane. Following the proposal of the bulk-flow hypothesis, a number of groups demonstrated that ER export of transmembrane proteins is at least somewhat signal-mediated [36,[39], [40], [41]]. After arrival at the Golgi, membrane proteins need to sort to their final destination. Transport from the Golgi apparatus was hypothesised to be selectively mediated by clathrin after the discovery of the AP-1 clathrin adaptor complex [[42], [43], [44]]. Studies using a type-I membrane-spanning protein, the cation-independent mannose-6 phosphate receptor (CI-MPR), demonstrated that mutations in the cytoplasmic terminus cause retention in the Golgi apparatus [45]. It was later shown that monomeric GGA clathrin adaptors also play a role at the Golgi apparatus and are essential for export of CI-MPR [46,47]. Therefore, there are two clathrin adaptor complexes at the Golgi which selectively sort cargo in a signal mediated manner.
There are also trafficking mechanisms for integral membrane proteins in the Golgi apparatus, independent of cytoplasmic signals. The mechanism of Golgi retention was investigated by making a series of chimeras made of two integral membrane proteins: a Golgi localised protein (β-1,4-galactosyltransferase (GT)) and an endosomal protein (human invariant chain (Ii)) [48]. A chimera with the transmembrane domain of GT and the cytoplasmic and lumenal domain of Ii is retained in the Golgi, demonstrating that the transmembrane domain alone can mediate Golgi localisation [48]. Similarly, chimeras of the transmembrane domain of the Golgi-localised α-2,6-sialyltransferase (ST) with the cell surface protein dipeptidylpeptidase IV (DPPIV) are Golgi retained [49]. ST fusions with the transmembrane domain replaced with either 17 or 23 leucines localises to the Golgi apparatus and plasma membrane respectively. This effect was only observed, however, when the lumenal domain of ST was also in the chimera [49,50]. Additionally, a type-I membrane protein (CD8) with a transmembrane domain of 23 leucine residues accumulates on the plasma membrane, whereas the same fusion with 17 leucines accumulates on the Golgi apparatus [50]. The relationship between transmembrane domain length and localisation was also analysed using temperature sensitive VSV-G (described in Section 4, below) [51]. A 7 amino acid truncation of the transmembrane domain of VSV-G causes less efficient Golgi export.
The E1 glycoprotein from an avian coronavirus localises to the Golgi apparatus in mammalian cells [52]. The E1 glycoprotein is a triple-pass transmembrane protein (with the C-terminus in the cytoplasm). Replacing the transmembrane domain of VSV-G with the first transmembrane domain of E1 mislocalises VSV-G to the Golgi apparatus [53]. Another example, from plants, is the vacuolar transmembrane protein α-TIP which accumulates on the vacuolar membrane of tobacco leaf cells despite deletion of the cytosolic terminus [54]. Truncations of the transmembrane domain of α-TIP resulted in protein accumulation at the Golgi. This suggests that the length of the transmembrane domain is a determining factor for post-Golgi sorting, a finding supported by other studies in plants [36,55]. These observations were further corroborated in mammals by comparing the length of an extensive list of over 1000 single-pass membrane proteins with a known localisation. Proteins known to reside on the plasma membrane have, on average, a longer hydrophobic section when compared to Golgi localised proteins [56].
In summary, by the early 1990’s the general rules of protein trafficking had been discovered. It was clear that proteins sort in a controlled way to various subcellular localisations. The studies above established a series of fundamental principles including: the binding of cytoplasmic adaptors to transmembrane cargos, the default secretion of soluble proteins and that membrane proteins do not have a ‘default’ route.
3. Navigating through the Early Secretory System
Proteins that enter the secretory system can be identified by a number of signals. Soluble proteins and type-I integral membrane proteins have a signal peptide on their N-terminus, type-II integral membrane proteins have a stop-transfer signal to allow C-terminal insertion into the ER, and multipass proteins have a combination of signal peptide and stop-transfers depending on the topology of the transmembrane domains. Once in the ER, the proteins fold due to an array of ATPase chaperones, the most characterised of which is BiP. The chaperones bind to unfolded hydrophobic portions of the protein and expend ATP to allow proper folding. The last step before export to the Golgi apparatus is the covalent addition of glycans [57].
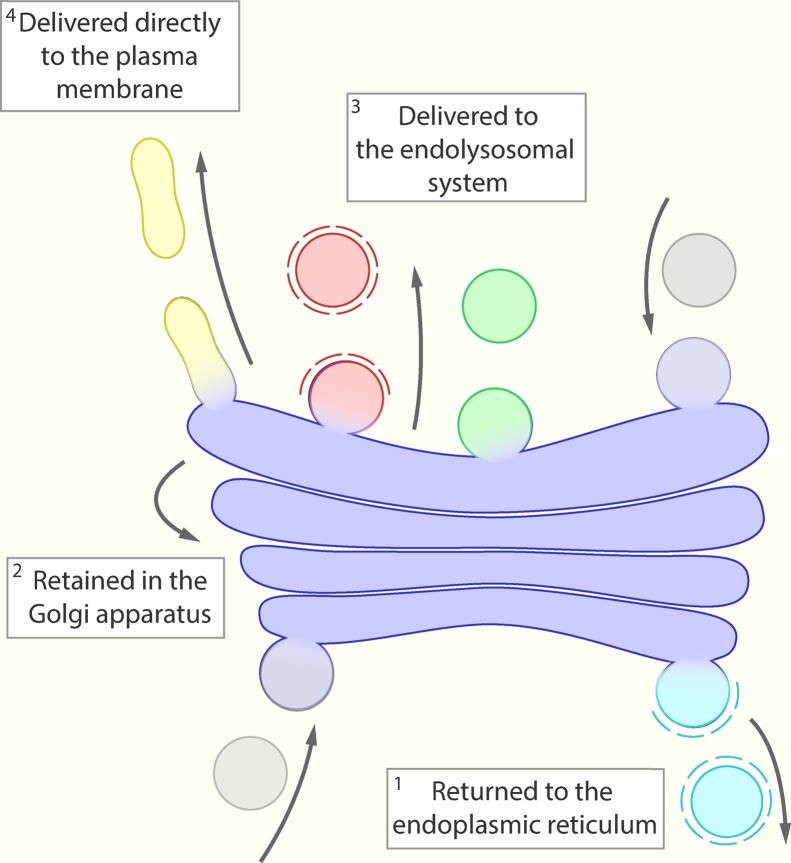
There are two key processes happening in the Golgi stacks: proteins are progressively further glycosylated [58] and then sorted to their steady-state destination in the cell. Within the Golgi apparatus proteins are sorted into different domains [[59], [60], [61], [62]] to be trafficked to at least four distinct locations (Fig.1 ). One subset of proteins return in COPI vesicles back to the ER. This includes the soluble resident ER chaperones that are ‘rescued’ from the cis Golgi stacks by the KDEL receptor, to allow steady-state localisation in the ER [63,64]. A second subset includes the Golgi resident enzymes that are retained in the Golgi apparatus [65]. These Golgi resident enzymes are thought to be retained by a combination of active sorting and oligomerisation (reviewed: [65]). The third subset traffic in clathrin-coated vesicles from the Golgi apparatus. These vesicles traffic directly to the endolysosomal system and contain lysosomal hydrolases and their receptors [59]. This process is mediated by the clathrin monomeric GGA adaptors and AP-1 complex which bind to both a motif in the cytosolic termini of the transmembrane cargos and accessory proteins present on the trans-Golgi [66]. The final subset of proteins leave the Golgi apparatus and traffic directly to the plasma membrane. This pathway and these carriers are the focus of this review.
Fig. 1.
Destinations of proteins from the Golgi apparatus. Proteins arrive into the Golgi apparatus from either the ER (bottom of the schematic) or endolysosomal system (top of the schematic). After arrival proteins can either return to the ER in COPI vesicles (light blue), can be retained in the Golgi apparatus, can be delivered directly to the plasma membrane (yellow) or delivered to the endolysosomal system by either clathrin (red) or other clathrin independent mechanism (eg, AP-4, green).
4. Budding of constitutive cargos from the Golgi Apparatus
The trans-Golgi network was proposed to be the point at which cargos leave the Golgi apparatus [11] based on the observation of budding structures in electron micrographs [67]. To understand what machinery was involved in the formation of these membrane carriers budding from the Golgi, the well-characterised clathrin-mediated transport seemed an obvious candidate to investigate. Clathrin carriers range from the typical spherical 60-100 nm clathrin-coated vesicles to larger, up to 1200 nm in size, grape-like structures displaying several coated buds [68]. The clathrin coat and its accessory protein complex, AP-1, was also shown to drive the formation of Weibel-Palade bodies, large cigar-shaped secretory organelles produced by endothelial cells which can be up to 5 μm long [69,70]. It has been proposed that the diverse array of carrier morphologies could be an adaptive mechanism for a variety cargo size or abundance. In the same line, COPII coat, which mediates cargo transport from the ER to the Golgi apparatus, can adopt more flexible conformations than the conventional 80 nm COPII carriers and assemble into tubular structures [71] or even potentially compartment-to-compartment tunnels [72] to allow the transport of large cargos like procollagen. In addition, cargos could potentially also affect the architecture of the Golgi. For example, overexpression of uroplakins, proteins that follow the conventional trafficking route from Golgi to plasma membrane localising on the apical surface of urothelial cells, triggers the fragmentation of the Golgi apparatus [73]. This effect suggests an adaptation of the cell in response to an increased flux of secretory proteins.
Although some clathrin adaptors such as GGAs and AP-1 associate to the cytosolic termini of transmembrane proteins at the trans-Golgi, it was soon identified that a clathrin-dependent mechanism was unlikely to be involved in the secretory carriers. In fact, the secreted protein invertase was unaffected by deletion of the clathrin heavy chain in S. cerevisiae [74]. It was therefore established relatively early that the secretory carriers are probably not clathrin coated vesicles and perhaps completely independent of clathrin.
In addition to the various clathrin carriers that bud from the Golgi apparatus, there is a second class of carriers often referred to as a ‘pleomorphic tubular-vesicular carriers’. These are lipid tubules and vesicles that bud from the Golgi apparatus, and are heterogeneous in size and shape in contrast to a clathrin-coated vesicle, which are generally geometrically constrained by the clathrin lattice. In contrast, pleomorphic tubular-vesicular carriers that traffic from the Golgi apparatus directly to the plasma membrane seem devoid of coat [75]. Studies on temperature sensitive vesicular stomatitis virus (VSV) mutant strains revealed a subset which had a mutation in the envelope ‘G’ protein of the virus. This protein has incomplete glycosylation at the non-permissive temperature [76], and was shown by cellular fractionation to localise at a different site in the cell depending on the temperature [77]. Cells infected with the temperature sensitive mutant strain retain the G protein in the ER and, upon temperature shift, traffic the protein towards the plasma membrane [78]. This effect is recapitulated with overexpression of a cDNA clone of the mutant protein [79] allowing for an ectopically expressed tsVSV-G that misfolds and accumulates in the ER at the ‘non-permissive’ temperature of 40 °C. Accumulated tsVSV-G is released en masse when the cells are shifted to the permissive temperature. This en masse export allows observation of tsVSV-G as it traffics to the plasma membrane, either biochemically or by imaging fluorophore fusions. Visualisation of tsVSV-G-GFP as it buds from the Golgi revealed tubular carriers that bud from the Golgi apparatus and traffic to the plasma membrane [80,81].
These post-Golgi carriers were described to be large pleomorphic tubular structures which traffic directly to the plasma membrane with an average carrier containing ∼10,000 tsVSV-G–GFP molecules [81,82]. These are likely not artifacts of an overloaded secretory system as they traffic along microtubules and fuse with the plasma membrane, demonstrating that cellular machinery exists to sort these carriers [81,83]. Later studies suggested that tsVSV-G traffics to the plasma membrane indirectly via recycling endosomes [84,85]. Whether tsVSV-G traffics via the indirect or direct route to the plasma membrane is likely to be dependent on cell type, analogous to the indirect and direct trafficking routes of the lysosomal protein LAMP1 [59,86,87].
Correlative light-electron microscopy imaging of the carriers revealed that they are 0.3–1.7 μm [80], a factor larger than typical clathrin coated vesicles, which are approximately between 0.06 and 0.1 μm [88]. These carriers were demonstrated to form en bloc, rather than by fusion of multiple smaller vesicular structures [75]. These initial observations set the stage for understanding the machinery that mediates this process.
5. Machinery associated with Golgi to Plasma Membrane Carriers
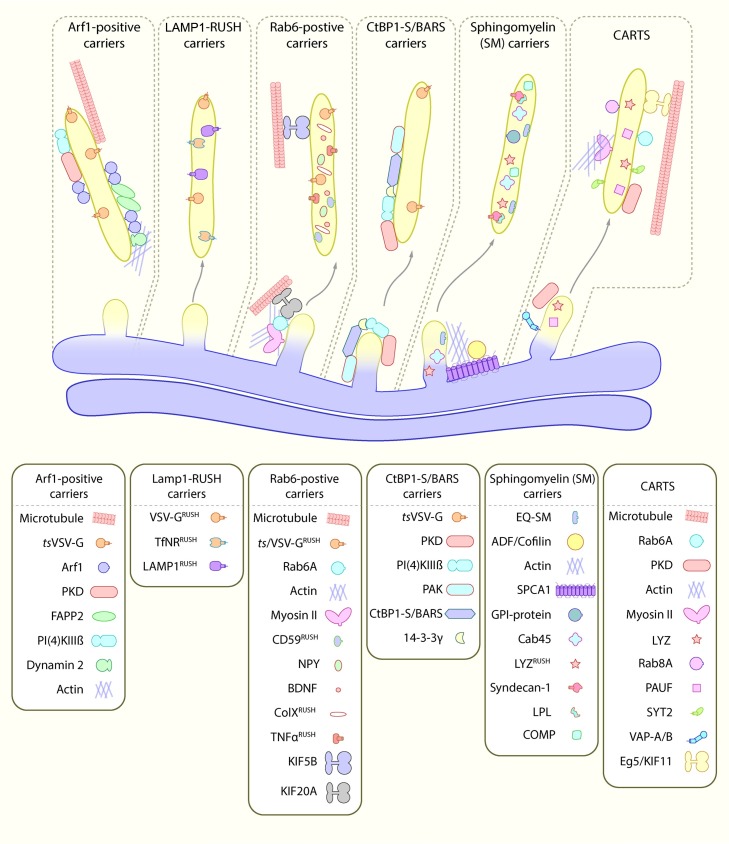
Unlike other cellular pathways, such as clathrin coated vesicles [88], or retromer recycling carriers [89], there is still no clear consensus for the machinery and molecular processes that drive the formation, budding, translocation and fusion of post-Golgi to plasma membrane carriers. There are, however, a number of candidate machineries that play a role in this process. We have detailed these below (see overview in Fig. 2 ).
Fig. 2.
Proteins associated with tubular carriers in mammalian cells. Models from experimental data on different types of secretory tubular carriers that bud towards the plasma membrane in mammalian cells. The carriers have been drawn as separate entities unless there is strong experimental evidence to combine the carriers. Some of the machinery described here might be overlapping on the same carriers, for example the SM carriers and CARTS, and the LAMP1-RUSH carriers, Rab6 carriers and the Arf1 carriers. Protein cartoons are not to scale and do not meaningfully reflect protein structure. LYZ = Lysozyme C.
5.1. Arf1 positive tubular carriers
Arf1, an extensively studied small GTPase of the Arf family, has a well-established role in COPI vesicle biogenesis at the Golgi [90]. Arf1 is also important in the budding of a large variety of other coated vesicles, recruiting adaptor protein complexes like AP-1, AP-3, AP-4 as well as GGA, and exomer complexes [91,92]. Arf1 forms a dimer in its GTP-bound form which is crucial to induce positive membrane curvature in vitro [93]. A mutant of Arf1 that is unable to dimerise is not able to induce COPI vesicle formation. Arf1, therefore, seems to induce membrane bending by forming a dimer in order to generate vesicles. This property of Arf1 prompted an investigation into its role in the generation of Golgi-derived tubules [94]. Halo-tagged Arf1 was imaged at endogenous expression levels with super-resolution imaging and, only under these conditions (not by over-expression of Arf1) Arf1 labels transient tubular carriers emerging from the Golgi. Arf1 GTPase activity is required for the formation of these tubular carriers. There are at least two distinct populations of Arf1-containing tubules. A subset of these tubules, containing VSV-G, traffic along microtubules in an anterograde direction towards the cell periphery. Arf1 positive carriers, however, were not observed fusing directly to the plasma membrane implying that they either lose Arf1 before fusion or there is an additional trafficking step via an endosomal compartment [94].
Arf1, cortactin and dynamin 2 can be detected on isolated Golgi membranes from a sucrose gradient. Incubating these membranes with the Arf1 inhibitor brefeldin A (BFA) causes loss of Arf1, cortactin and dynamin 2 from the membranes, which can be inhibited by supplementation with GTP-loaded Arf1 [95]. Imaging experiments show addition of BFA causes loss of cortactin and dynamin 2 from the Golgi, suggesting that Arf1 is able to recruit actin, cortactin and dynamin 2 to the trans-Golgi. Finally, the expression of truncated cortactin proteins decreases the efficiency of tsVSV-G export from the TGN towards the cell surface. Cortactin promotes the formation of branched actin polymers through interactions with the Arp2/3 complex [96], and dynamin 2 is a small GTPase essential for membrane fission [97]. These findings show the importance of Arf1 in the recruitment of an actin/cortactin/dynamin 2 complex essential for post-Golgi transport.
Two binding partners of Arf1, PKD (Serine/threonine-protein kinase D, human gene isoforms: PRKD1-3) and FAPP2 (encoded by the gene PLEKHA8), are also associated with post-Golgi tubular carriers [98,99]. Addition of the metabolite ilimaquinone causes Golgi fragmentation by activating PKD through stimulation of a heterotrimeric G protein (Gβγ) [100]. An inactive mutant of PKD, expressed at moderate levels in a stable HeLa cell line at 20 °C, inhibits protein transport from the trans-Golgi to the cell surface by causing Golgi tubulation [101]. Cargo destined for the plasma membrane like tsVSV-G are trapped in large tubules that remain attached to the TGN. Similarly, depletion of PRKD2 and PRKD3 (human homologs of PKD) by siRNA also inhibits TGN-to-cell surface transport and shows cargo accumulation in large tubules [102]. These results strongly suggest that PKD is required for membrane fission at the trans-Golgi of carriers destined for the cell surface. PKD has two cysteine-rich domains at the N-terminus, one interacts with diacylglycerol and the second one with Arf1 [103,104]. The direct interaction of PKD2 with Arf1 is enhanced when the small GTPase is in its active conformation [104]. PKD is activated by Gβγ to stimulate specific lipid kinases like the enzyme PI(4)KIIIß [98,100]. This promotes the production of PI(4)P, a crucial lipid that drives vesicle biogenesis at the TGN through a variety of mechanisms [105] - see also Section 7 below.
FAPP1 and FAPP2 were originally identified to bind specifically to PI(4)P through their PH domain [106,107]. Pull-down experiments with isolated Golgi membranes and with recombinant proteins show a direct interaction of FAPP proteins with Arf1-GTP [107]. Binding to both PI(4)P and Arf1 allows FAPP targeting to the trans-Golgi via coincidence detection. It was proposed that FAPP proteins are able to deform membranes [[108], [109], [110]]. Recombinant FAPP2, as well as the isolated PH domain of FAPP1, are able to induce tubulation of membrane sheets in vitro [108,110]. During imaging of synchronised transport of tsVSV-G, FAPP proteins localise on tubular carriers emerging from the TGN [107]. Knockdown of FAPPs by siRNA inhibits cargo delivery to the plasma membrane, indicating that FAPP proteins are essential for the constitutive post-Golgi transport. Furthermore, FAPP2 is specifically involved in apical transport in polarised MDCK cells [111]. FAPP2 has a similar domain structure to FAPP1 aside from an additional glycolipid-transfer-protein-homology domain at its C-terminus. FAPP2 specifically transfers glucosylceramide (GlcCer) in vitro and this transfer is stimulated in the presence of PI(4)P and Arf1 [112]. GlcCer is normally trans-Golgi localised, however in FAPP2 knock-down cells GlcCer is mislocalised to the cis-Golgi. As GlcCer is a precursor for glycosphingolipids which are key components of the plasma membrane, this role of FAPP2 connects tubule formation, PI(4)P generation, and subsequent FAPP2 recruitment at the Golgi with plasma membrane homeostasis.
Another binding partner of Arf1 at the trans-Golgi is the exomer complex [92,113,114]. To date, the exomer complex is the only known cargo adaptor involved in the direct transport of cargo from the TGN to the plasma membrane [92,115,116]. Discovered in yeast, there is no obvious exomer homolog in metazoans. The exomer complex is a heterotetramer composed of two Chs5 subunits and any two members of the four paralogous Chs5-Arf1 binding proteins (ChAPs), Chs6, Bud7, Bch1, and Bch2, which confer cargo specificity through direct binding to the cytoplasmic termini. Structural studies reveal that the exomer complex binds two Arf1 molecules [113]. By inserting a hydrophobic element into the membrane and coordinating the membrane insertion of two Arf1 molecules, exomer amplifies Arf1 membrane remodelling ability. Thus, exomer participates in cargo sorting and membrane fission.
In summary, Arf1 has a key role in the formation of tsVSV-G enriched post-Golgi tubular carriers by inducing membrane curvature and by recruiting a variety of binding partners such as lipid modifying enzymes, lipid transport proteins and fission promoting proteins.
5.2. Biosynthetic LAMP1 carriers
Post-Golgi carriers have also been observed using the 'retention using selective hooks' (RUSH) system [59,117]. By fusing a protein of interest to streptavidin-binding peptide (SBP) and co-expressing an ER localised streptavidin, RUSH allows the protein of interest to accumulate in the ER due to the interaction between the streptavidin and the SBP [118]. Addition of exogenous biotin outcompetes the interaction between the SBP and streptavidin leaving the protein of interest free to traffic along its normal trafficking itinerary. By combining this approach with high-resolution microscopy to track the secretion of different endolysosomal proteins, LAMP1 was shown to sort into distinct Golgi domains when compared to the cation-dependent mannose-6-phosphate receptor or sortillin [59]. LAMP1 leaves the Golgi in tubular carriers depending on the transmembrane and lumenal domains of LAMP1 but independently of sorting signals in the cytosolic tail. Finally, microscopy experiments show that LAMP1 tubular carriers that contain transferrin receptor and VSV-G, fuse directly with the plasma membrane. RUSH of VSV-G shows that VSV-G concentrates in LAMP1 carriers, suggesting that these carriers correspond to the tsVSV-G Golgi-to-plasma membrane carriers discussed above. The route of endogenous LAMP1 from the Golgi remains controversial as immunoelectron microscopy studies suggest that a fraction of these non-clathrin coated LAMP1 carriers travel instead directly to late endosomes and contain the HOPS component Vps41 and the SNARE VAMP7 which are both required for fusion [119].
In summary, the lysosomal protein LAMP1 traffics on VSV-G enriched post-Golgi tubular carriers to reach the cell surface before being recycled to the endolysosomal compartments. However, further investigations on this class of carriers is essential and will help determine if these carriers are the same as the Arf1 positive carriers described above.
5.3. Rab6 associated carriers
Rab6 was also found associated with about 50% of Golgi-derived vesicles enriched with tsVSV-G together with neuropeptide Y and BDNF which are secreted when overexpressed in HeLa cells [120]. TIRF experiments show the direct fusion of these Rab6 secretory vesicles with the plasma membrane. Furthermore, Rab6 knockdown delays the secretion of VSV-G, implying a role of Rab6 in the transport of the carriers to the cell periphery. The microtubule motors kinesin-1 (KIF5B) and dynein are also important for the delivery of the carriers [120]. A few years later, an unexpected role of Rab6 in the fission of these Rab6 secretory carriers was highlighted [121]. Long tubular carriers connected to the Golgi and seemingly unable to detach have been observed by Live cell imaging when cells are depleted of Rab6A, or its isoform Rab6A`. Pulldown experiments show that Rab6 interacts in vivo with myosin II and the direct interaction was observed with purified proteins. Inhibition or depletion of myosin II in different cell types prevents the fission of Rab6 secretory carriers which form long tubules connected to the Golgi apparatus. Therefore, myosin II has an important role at the Golgi in the fission step of secretory carriers. Further investigations show that myosin II works with F-actin filaments for vesicle fission since actin depolymerisation leads to the formation of long tubules [121]. Altogether, this work shows that Rab6 is also associated with a subset tsVSV-G enriched post-Golgi tubular carriers and unravels a novel function of Rab GTPases in vesicle fission.
The kinesin KIF20A which interacts with Rab6 and myosin II, has a key role in the fission of Rab6-positive vesicles [122]. Long Golgi-attached Rab6 positive tubules can be observed by light microscopy upon either addition of a specific chemical inhibitor of KIF20A motor activity or depletion of KIF20A by siRNA. Rab6 participates in the recruitment of KIF20A to the Golgi complex confining it to growing microtubules at ‘Golgi fission hotspots’. Rab6 and KIF20A recruit myosin II which, together with actin, drive fission of Rab6 secretory carriers. The carriers are then transported along microtubules towards the cell surfacedue to the kinesin KIF5B[120,121].
Rab6 secretory carriers transport a large variety of cargos. Using the RUSH system, it was shown that Rab6 secretory carriers contain the anterograde cargos CD59, TNFα, and ColX and Rab6 depletion delays the delivery of these cargos to the plasma membrane [117]. Furthermore, Rab6 depletion in embryonic fibroblast cells reduces global protein secretion by 50%. This together implies that Rab6 is a major regulator of post-Golgi secretion by regulating fission as detailed above. However, Rab6 does not seem to be involved in cargo sorting.
5.4. CtBP1-S/BARS carriers
BFA is a small molecule originally isolated from fungi that has been demonstrated to inhibit the Arf1 exchange factor, GBF1 [123]. As Arf1 is essential to Golgi structure and function, extended incubation with BFA leads to Golgi disassembly [124]. While investigating the molecular factors involved in the Golgi disassembly induced by BFA, the protein CtBP1-S/BARS, also known as CtBP3/BARS (C-terminus binding protein 3/BFA adenosine diphosphate–ribosylated substrate), was identified [125]. Isolated Golgi membranes are fragmented when incubated with either recombinant or rat brain purified CtBP1-S/BARS [126]. Co-incubation of CtBP1-S/BARS on Golgi membranes with various lipids demonstrated that CtBP1-S/BARS acts as a lysophosphatidic acid acyltransferase to promote generation of phosphatidic acid on the membrane of the Golgi. Microinjection of CtBP1-S/BARS increases the number of post-Golgi carriers. tsVSV-G TGN-exit assays show that CtBP1-S/BARS is recruited to the Golgi and associates with tsVSV-G enriched post-Golgi carriers [127]. Microinjection of an anti-CtBP1-S/BARS antibody or siRNA experiments against CtBP1-S/BARS blocks the delivery of tsVSV-G carriers to the plasma membrane, whereas inhibition of dynamin 2 has no detectable effect [128]. Thus, CtBP1-S/BARS is hypothesised to be essential for membrane fission in a dynamin 2 independent mechanism. As the small GTPase dynamin 2 has been shown to be essential for the fission of Arf1 tubular carriers (section 5.1) and clathrin coated vesicles [129], this is a novel cellular fission mechanism.
14-3-3γ and PI(4)KIIIβ co-immunoprecipitate with CtBP1-S/BARS from rat brain cytosol [127]. The resulting complex is stabilised by phosphorylation by the kinases PKD and PAK. CtBP1-S/BARS subsequently activates lysophosphatidic acid acyltransferase δ which produces phosphatidic acid, this process is essential to induce the fission of post-Golgi carriers [130]. In summary, CtBP1-S/BARS is a key component of a protein complex involving 14-3-3γ, PI(4)KIIIβ, PKD and PAK, and has an essential role in the fission of tsVSV-G enriched post-Golgi carriers.
5.5. Sphingomyelin carriers
The importance of the lipid sphingomyelin (SM) for carrier formation was suggested while investigating lipid-raft formation at the Golgi [131], and confirmed by the identification of a new class of TGN-derived vesicles particularly enriched in that type of lipid [132]. SM, a principal component of the plasma membrane, is synthesised in the Golgi apparatus and transported to the cell surface. A non-toxic reporter, EQ-SM was used to monitor intracellular trafficking of SM along the biosynthetic pathway from the lumenal side of the membrane. Proximity biotinylation identified new proteins associated with the SM secretory carriers [133]. EQ-SM (or a control protein that does not bind SM) was fused to the promiscuous biotin ligase APEX2 and expressed in HeLa cells. Cells were permeabilised and incubated with rat liver cytosol and an ATP regenerating system to generate Golgi-derived vesicles. APEX2-mediated biotinylation was then induced on vesicle fractions. Biotin-labelled proteins were isolated and identified by mass spectrometry. SM-rich secretory vesicles contain lysozyme C and GPI-anchored proteins and are highly enriched in Cab45 (calcium-binding protein 45), a lumenal Golgi resident protein important for soluble cargo sorting in a calcium dependent manner [134,135].
The Golgi localised protein SPCA1 (Secretory Pathway Calcium ATPase 1), activated by its direct interaction with cofilin-1 and F-actin, pumps calcium into the TGN [134,136,137]. The lumenal flux of calcium triggers the oligomerisation of the soluble Golgi localised protein, Cab45 [134,138]. As Cab45 binds to certain secreted cargos (eg. lysozyme C), in a calcium dependent manner, the oligomerisation leads to sorting into secretory carriers. Interestingly, siRNA experiments show that Cab45 affects the export of endogenous cargos such as the cartilage oligomeric protein (COMP) [134]. Additionally, deletion of SPCA1 reduced the number of SM carriers [133] and RUSH assays show that secretion of lysozyme C containing vesicles are significantly reduced in SPCA1 null cells, which can be rescued by recovery of SPCA1. Furthermore, SPCA1 associates with SM in Golgi membranes and Golgi calcium influx assays show that depletion of SM impairs the TGN calcium uptake by SPCA1. In summary, SPCA1 links sphingomyelin synthesis to calcium/Cab45-dependent cargo sorting in the trans-Golgi.
Lipoprotein lipase (LPL), identified in the proximity biotinylation experiment described above [133], is also secreted in SM carriers [139]. LPL binds to Syndecan-1 (SDC1) and gets co-secreted on SM-enriched secretory carriers. Therefore, SDC1 acts as a sorting receptor for LPL at the trans-Golgi and directs it into the secretory pathway.
In summary, the packaging of specific secretory proteins into SM rich carriers is mediated by SPCA1 and the subsequent Cab45 oligomerisation. Whether these carriers are the same as others described here requires further investigation and experimentation.
5.6. CARTS
In a series of experiments purifying post-Golgi carriers, a new class of secretory carriers named CARTS (CARriers of the TGN to the cell Surface) was discovered [140]. Partial permeabilisation of HeLa cells and the use of an ATP regenerating system allowed the formation and purification of post-Golgi vesicles. Isolation and analysis of these carriers indicate that CARTS are devoid of collagen and tsVSV-G but enriched in endogenous proteins such as pancreatic adenocarcinoma up-regulated factor (PAUF), lysozyme C, synaptotagmin II (SYT2), Rab6A, and Rab8A [140]. Earlier experiments had shown that PKD was essential for forming tubular carriers at the trans-Golgi [101], as discussed in Section 5.1. Knock-down of PKD resulted in extended CARTS tubules from the Golgi apparatus. Furthermore, vesicle-associated membrane protein–associated proteins (VAP-A/B) regulate CARTS formation upstream of PKD [141]. Interestingly, expression of an OSBP construct (PH-FFAT) which immobilises ER-Golgi contact sites, drastically reduced CARTS production and PAUF secretion [141,142], suggesting a key role of ER-Golgi contact sites in the generation of post-Golgi carriers. Similarly, FAPP1, localised at ER-Golgi contact sites, controls the level of PI(4)P at the trans-Golgi and regulates the secretion of the endogenous cargo ApoB100 [143]. Finally, the kinesin protein Eg5, well known for its role in bipolar spindle assembly during mitosis, has a role in the transport of CARTS toward the cell surface along microtubules [144].
CARTS are distinct from COPI, COPII and clathrin carriers and are pleomorphic structures of 100 to 250 nm in diameter [140]. In summary, CARTS constitute a totally distinct class of carriers destined to the cell surface as they exclude tsVSV-G. However, several cargo proteins sorted by Cab45 (like lysozyme C) are associated with CARTS [145] raising the question: how similar are these carriers to SM secretory carriers discussed above? Further investigation is essential to fully understand what distinguishes these carriers from the other ones.
6. Fusion Machinery
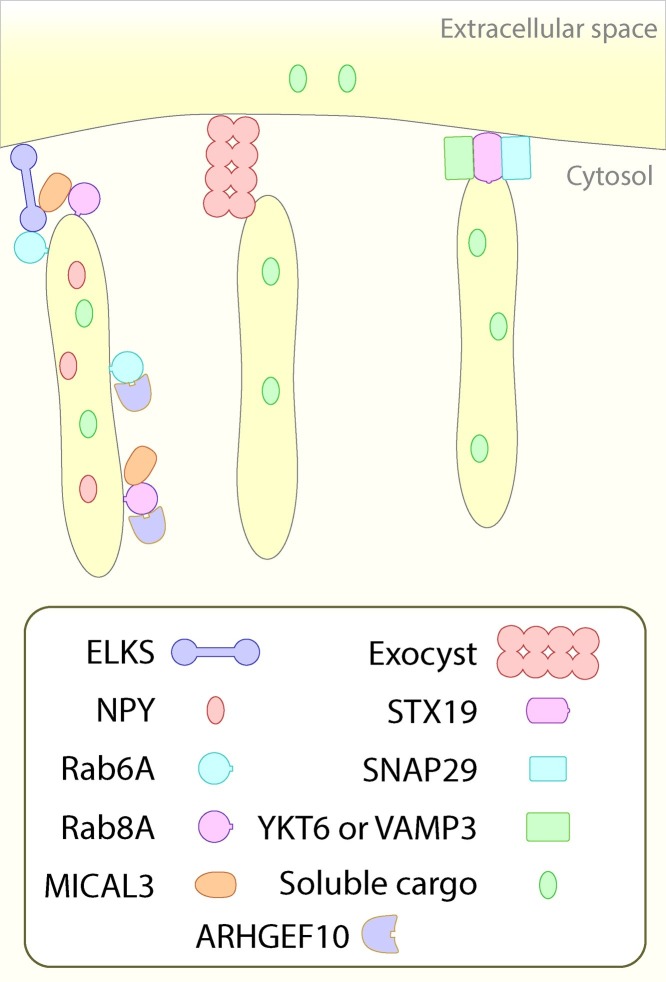
The final event of a protein trafficking pathway is the fusion of the carrier with the distal compartment. This involves membrane tethering (‘recognition’) and membrane fusion to allow soluble content mixing and membrane delivery. In the case of the post-Golgi carrier and the plasma membrane there are a number of candidate tethering factors (see summary in Fig. 3 ), it is currently unclear if the machinery for carrier fusion is distinct for constitutive and regulated secretion.
Fig. 3.
Fusion of post-Golgi carriers in mammalian cells. There are 2 tethering complexes associated with post-Golgi carriers, ELKS (left) and exocyst (center). The SNAREs associated with the final fusion event are shown on the right. YKT6 and VAMP3 appear to work either redundently or as two parallel pathways [171]. Soluble cargo represents bulk flow as assayed by exogenously expressed cargos (eg. [168]).
6.1. ELKS
The large coiled-coil protein ELKS, also called CAST2, Rab6IP2, or ERC1 [[146], [147], [148], [149]], is involved in synaptic vesicle exocytosis based on purification of highly exocytic synaptic junctions [150,151]. TIRF imaging in insulin-producing cells shows ELKS foci on the exocytic fusion pore, supporting a role in regulated secretion [152,153]. ELKS was identified as a Rab6 interactor [147]. An accumulation in Rab6 positive vesicular structures was observed after ELKS depletion [120], later shown to be positive for MICAL3 and Rab8 [154]. MICAL3 and Rab8 interact with each other and MICAL3 with ELKS, hinting at a functional complex for exocytosis. ARHGEF10, an exchange factor for RhoA has been shown to localise to Rab6/Rab8 positive carriers and is required for Rab8 recruitment suggesting that there is a small G protein cascade leading to carrier fusion [155,156]. The soluble biosynthetic cargo neuropeptide Y accumulates in pre-fusion ELKS dependent carriers, suggesting that this is the biosynthetic transport route [120]. ELKS and RAB6 are on plasma membrane ‘hotspots’ which are the fusion points of the secretory carriers [117].
6.2. Exocyst
Another obvious candidate for the fusion of the carriers at the plasma membrane is the exocyst complex. Exocyst is an octameric protein complex, with six of the subunits (Sec6, Sec8, Sec15, Sec3, Sec5 and Sec10) discovered in the original Sec yeast genetic screen and later characterised as a complex [23,[157], [158], [159]]. The remaining subunits (Exo70 and Exo84) were discovered subsequently as bona fide members of the complex [[160], [161], [162]]. Exocyst is important for exocytic transport (hence the name exocyst) based on the localisation in budding yeast and the Sec phenotype.
In non-polarised mammalian cells exocyst components localise to the plasma membrane and Golgi apparatus [163]. tsVSV-G colocalises with exocyst in the Golgi stacks. It is not clear, however, if it colocalises to the post-Golgi tsVSV-G carriers or at the fusion point of the carriers. Exocyst inhibition with antibodies does not affect VSV-G delivery [163,164]. siRNA depletion of Exo70 decreased the efficiency but did not completely stop tsVSV-G delivery [165]. Colocalisation of tsVSV-G and Sec8 on the plasma membrane using TIRF microscopy showed only 20% overlap between the two markers [166]. To-date it is not clear if exocyst is directly involved in fusion of biosynthetic carriers to the plasma membrane.
6.3. SNAREs
The final membrane fusion step of the membrane of pathways is mediated by SNARE proteins. SNAREs interact to form four-helix bundles to promote membrane fusion, with each bundle requiring R-, Qa-, Qb-, and Qc-SNARE motifs [167]. tsVSV-G positive post-Golgi carriers accumulate upon SNARE inhibition by abrogation of the protein NSF [75]. As NSF is essential for SNARE function these experiments demonstrated that SNARE machinery is essential for fusion of the carriers. A targeted siRNA screen identified the specific SNAREs and SM (Sec1/Munc18-like) proteins which help to organise the SNAREs [168]. 38 SNAREs, 4 SNARE‐like proteins and 7 SM proteins were targeted, 9 of which were identified and validated as being strongly inhibitory to secretory exocytosis. These 9 hits included proteins for both the ER to Golgi transport step and Golgi to plasma membrane step. Candidate validation identified the SNAREs SNAP29 and syntaxin-19. SNAP29 has both a Qb- and Qc-SNARE domain [169], and syntaxin-19 is Qa-SNARE [170]. Depletion of both syntaxin-19 and SNAP29 decreased plasma membrane fusion events of secretory vesicles, additionally in SNAP29 depleted cells there was an accumulation of pre-fusion carriers in the cytosol. Surprisingly no R-SNAREs were identified in this screen [168].
In a subsequent targeted screen Drosophila cells were used, which have less genomic redundancy than mammalian cell lines. Results from the screen highlighted the R-SNAREs YKT6 and VAMP3 as important for fusion of post-Golgi secretory carriers, consistent with mammalian cells [171], although YKT6 is a promiscuous SNARE that also has a role in ER to Golgi transport [168]. In summary, one of the SNARE complexes for the secretory route to the plasma membrane requires SNAP29 and syntaxin-19 and perhaps VAMP3 redundantly with YKT6. There are likely multiple SNARE complexes for this route and multiple redundancies.
7. Importance of the lipid PI(4)P in post-Golgi trafficking
Lipids play a central role in the budding of carriers from the trans-Golgi (reviewed in [145]). One of the major lipids in this process is the phosphoinositide PI(4)P [105] see also section 5.1 above. PI(4)P is crucial for post-Golgi vesicle biogenesis including carriers destined directly for the cell surface. Enriched on the cytosolic outer membrane bilayer, PI(4)P can recruit an array of specific-binding proteins.
7.1. PI(4)KIIIβ
Mammals encode four different PI 4-kinases and PI(4)KIIIβ is involved in the formation of tsVSV-G carriers destined for the plasma membrane [107,127]. PI(4)KIIIβ is recruited to the Golgi by different mechanisms [105] including by the action of Arf1 [172] and the Golgi localised ACBD3 [173]. Once at the trans-Golgi, PI(4)KIIIβ generates PI(4)P that recruits further proteins such as GOLPH3 and lipid transport proteins as discussed below. PI(4)KIIIβ is also involved in the recruitment of the small G protein Rab11a [174,175] shown to be important for tsVSV-G trafficking from the trans-Golgi to the plasma membrane [176]. PI(4)KIIIβ was shown to have an important role in mitochondrial scission generating PI(4)P on trans-Golgi derived vesicles after recruitment by Arf1 [177]. These vesicles are targeted to mitochondria-ER contact sites and are thought to drive mitochondrial division downstream of Drp1 (Dynamin-related protein-1).
7.2. GOLPH3
One example of a PI(4)P mediated binding is GOLPH3, discovered via a proteomic lipid-binding screen, which is recruited to the trans-Golgi by interacting with PI(4)P [178]. GOLPH3 binds to the unconventional myosin MYO18A thus connecting the Golgi to F-actin. The model suggests that this complex applies tension to extract the carriers at the trans-Golgi. Knock-down of GOLPH3 or MYO18A or actin depolymerisation impairs trafficking of tsVSV-G from the Golgi to the plasma membrane. Additionally, GOLPH3 induces membrane curvature, by inserting a loop into the proximal leaflet of the bilayer and this property is required for efficient trans-Golgi to plasma membrane trafficking [179]. However, GOLPH3 has also been shown to specifically bind and recycle a subset of Golgi enzymes involved in glycan assembly on sphingolipids [180]. This role of GOLPH3 matches the role of its yeast homolog Vps74 which binds to PI(4)P at the trans-Golgi and interacts with specific Golgi enzymes allowing their retrograde transport [181,182].
7.3. Lipid transport proteins
PI(4)P recruits a range of lipid transport proteins (reviewed in [[183], [184], [185]]), such as FAPP2, oxysterol-binding protein (OSBP), and ceramide transfer protein (CERT). These proteins share a similar domain organisation: an N-terminal PH domain that binds PI(4)P (and simultaneously Arf1-GTP for OSBP and FAPP2), a C-terminal FFAT motif that binds the ER membrane protein VAP and a C-terminal lipid transport domain. FAPP2 transfers GlcCer to the trans-Golgi [112] as described in section 5.1 above. At membrane contact sites, OSBP directs cholesterol transfer from the ER to the trans-Golgi through coupled counter-transport of PI(4)P [142,186]. CERT transports ceramide from the ER to the trans-Golgi [187], thereby promoting SM and diacylglycerol synthesis. Sterols and sphingolipids, which are suggested to assemble into lipid microdomains are important for the formation of secretory carriers [132,188]. In agreement, depletion of VAP inhibits trans-Golgi to plasma membrane transport of tsVSV-G [189]. Similarly, VAP knockdown, double knockdown of CERT/OSBP or the treatment of the cells with D-ceramide-C6, impairs the secretion of the cargo PAUF [141].
Another lipid transfer protein that functions at contact sites between the ER and the trans-Golgi is Nir2 [184]. Nir2 supplies the trans-Golgi with PI in exchange of phosphatidylcholine and thereby participates in PI(4)P synthesis and diacylglycerol homeostasis [190]. As a result, depletion of Nir2 affects the transport of tsVSV-G from the trans-Golgi to the cell surface. Similarly, two other PI transfer proteins, PITPNA and PITPNB, stimulate the production of PI(4)P which promotes the recruitment of GOLPH3 and CERT to the Golgi thus facilitating the apical targeting of membrane trafficking in neural stem cells [191].
Overall, lipid transfer proteins by executing non-vesicular lipid exchange are essential for the remodelling of the lipid landscape and, as a result, ER-Golgi contact sites could represent key sites for secretory cargo sorting and membrane fission.
7.4. Importance of PI(4)P in polarised secretion and cargo sorting in yeast
In yeast, PI(4)P has a key role in the generation of secretory vesicles during polarised growth. The PI(4)-kinase Pik1, responsible for PI(4)P synthesis at the trans-Golgi, regulates several transport events including the Golgi to surface route [192]. Together with the activated Rab GTPase Ypt32, PI(4)P recruits the exchange factor Sec2 to the trans-Golgi which in turn activates another Rab, Sec4 [193,194]. The secretory vesicles subsequently pinch off and Sec4 recruits the exocyst component Sec15 to promote the tethering and fusion of the vesicles with the plasma membrane [195,196]. PI(4)P, Ypt32-GTP, Sec4-GTP and then Sec15 recruit Myo2, a type V myosin, to allow the transport of vesicles along actin cables toward the exocytic site [[197], [198], [199]]. Additionally, Sec2 is able to bind directly to the Sec4 effector Sec15 [200]. Sec15 and Ypt32-GTP compete for binding to Sec2. Also, the association of Sec2 with its alternate binding partners is regulated by PI(4)P [193]. Thus, when secretory vesicles are initially formed and enriched in PI(4)P, Sec2 interacts with its initial binding partner Ypt32-GTP. It was shown that the concentration of PI(4)P on secretory vesicles is reduced by the time of their arrival at the cell surface [193,201] by the action of the protein Osh4 [201]. This suggests that during the maturation of secretory vesicles, as the PI(4)P concentration drops, Ypt32-GTP is replaced on Sec2 by Sec15 leading to a positive feedback loop and thus preparing the vesicles for docking and fusion with the plasma membrane.
Furthermore, an additional regulatory level is accomplished by the phosphorylation of Sec2 by casein kinases Yck1/Yck2 [202,203]. Sec2 phosphorylation inhibits its interaction with its initial binding partners PI(4)P and Ypt32-GTP and promotes its interaction with its downstream effector Sec15. Additionally, Yck2 binds to Sec2 near the PI(4)P binding region and the presence of PI(4)P inhibits phosphorylation of Sec2 by Yck2 in vitro [202,203]. This suggests that phosphorylation of Sec2 is initially prevented by the high level of PI(4)P in nascent secretory vesicles. As the secretory vesicle matures and the PI(4)P level drops, Sec2 gets phosphorylated promoting the switch in Sec2 binding partners and thus pushing the reaction forwards. Altogether, this complex regulatory circuit helps to impose a directionality in vesicular transport and shows the key role of PI(4)P in the secretory route. This Rab cascade is somewhat conserved in mammalian cells and involves the Ypt32-homologue Rab11 recruiting the exchange factor Rabin8 to activate the Sec4-homologue Rab8 [204]. There are some differences, however, for example the lipid phosphatidylserine participating in the Golgi recruitment of Rabin8 in mammalian cells instead of PI(4)P in yeast [205].
Glucose induces a change in intracellular pH that governs the protonation state of PI(4)P at the trans-Golgi [206]. This regulates recruitment of Osh1, a member of the OSBP family of lipid transfer proteins, which promotes the trafficking of the Tat2 amino acid permease to the plasma membrane regulating tryptophan uptake. This study shows that pH biosensing by PI(4)P in response to nutrient availability regulates cargo sorting at the trans-Golgi.
8. Open Questions
At this stage, we still do not have a clear picture of the exact molecular machinery of the pleomorphic sorting carriers that bud from the Golgi apparatus and new types of carriers are emerging [207]. This is complicated by the fact that these carriers are transient and contain low cargo abundance. Accordingly, they have mostly been observed using temporal kinetic assays, such as tsVSV-G and RUSH. Many of the cargos used are exogenous proteins, including non-mammalian proteins such as GFP and HRP fused to a signal peptide and human proteins that are not expressed normally in that cell type. These techniques are essential and have facilitated fundamental discoveries over the years. It is important, although technically challenging, to investigate these carriers using native proteins under more physiological conditions.
Mutations in genes encoding for proteins involved in the trans-Golgi export machinery have been shown to cause severe genetic diseases [66,208]. For example, mutations in different subunits of the AP-1-complex, component of clathrin-coated vesicles and associated with the trans-Golgi and early/recycling endosomes, are linked with X-linked mental retardation and with MEDNIK syndrome (Mental retardation, Enteropathy, Deafness, peripheral Neuropathy, Ichthyosis and Keratodermia). Other genetic diseases have been linked to mutations in AP subunits [66]. Therefore a better understanding of the mechanistic basis of these pathologies is essential.
There are also a number of diseases associated with secreted proteins. One example is transthyretin (TTR) amyloidosis: a rare genetic disease caused by over 80 known mutations in the secreted protein TTR [209], resulting in protein aggregation into amyloid fibrils [210]. Monoclonal gammopathies, caused by an abundance of a particular immunoglobulin into the blood (IgM, IgG, IgE or a combination), are another example of a disease associated with protein secretion [211,212]. Our current understanding of the machinery is still incomplete and it is possible that diseases associated with mutations in this pathway are yet to be genetically characterised.
How many routes are there from the Golgi apparatus to the plasma membrane? It is possible that tsVSV-G takes multiple routes from the Golgi apparatus (fig, 2), which makes the number of routes that leave the Golgi hard to interpret. Similarly, GPI-anchored proteins traffic to the apical surface of most epithelial cells, however, there are some exceptions such as Fischer rat thyroid cells where GPI-anchored proteins are sorted to the basolateral surface [213]. In Fig. 2, the schematic shows the various carriers that have been shown experimentally. It is of course possible, for example, that the Rab6 carriers and LAMP1-RUSH carriers shown in the image are the same. One reason for multiple pathways from the Golgi apparatus to the plasma membrane is due to polarised sorting in some cell types. Examples of polarised cells are epithelial tissues such as the choroid plexus that makes up a portion of the blood-brain barrier and is responsible for the secretion of cerebrospinal fluid (CSF). Neurons, another polarised cell type, are characterised by a long axonal process, reaching a meter in length in humans.
Polarised cells need to maintain differential protein trafficking in the cellular subdomains, for example, the choroid plexus polarised epithelial layer faces fenestrated capillaries on the basolateral side and the ventricular space on the apical side where the CSF is secreted. The generation, maintenance and trafficking of cell polarity has been comprehensively reviewed elsewhere [[214], [215], [216]]. The machinery that mediates the sorting into the two different routes is still being characterised. It seems likely that some of the machinery discussed in this review is essential, for example loss of FAPP2 in MDCK cells causes loss of complete polarisation and defective cilium formation and is important for delivery of apical cargos [111,217]. It is possible that the routes that exist in polarised cells also exist in ‘non-polarised’ cells and act as redundant or alternative routes to the plasma membrane, as demonstrated in comparisons of trafficking non-polarised Fao cells with polarised WIF-B cells [218].
A final outstanding question is the presence of a ‘coat’. All other known carriers characterised to-date have a coat of some sort. In the case of clathrin coated vesicles it is a geometric lattice that directly forms and scaffolds the forming vesicles, in the retromer coat it is a core complex that allows for cargo selectivity and binding to other machinery. Although, as discussed above, there are some candidate proteins, there is still no bona fide coat for the secretory carriers. Perhaps there is no need for a coat in these carriers or perhaps it has yet to be discovered. With the recent advances in genetics and proteomics there is surely more to be discovered on this elusive trafficking pathway.
Acknowledgements
The authors thank Laura Pellegrini (MRC Laboratory of Molecular Biology, Cambridge), Paul Luzio (Cambridge Institute for Medical Research, University of Cambridge), Margaret Robinson (Cambridge Institute for Medical Research, University of Cambridge), Conceição Pereira (Cambridge Institute for Medical Research, University of Cambridge) and Jérôme Cattin-Ortolá (MRC Laboratory of Molecular Biology, Cambridge) for critical readings of the manuscript and useful discussions throughout. D.G. and D.S. are funded by a Sir Henry Dale Fellowship awarded to D.G. from the Wellcome Trust/Royal Society (Grant 210481). The authors apologise for colleagues whose work we have failed to include.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.semcdb.2020.04.001.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Kanapin A., Batalov S., Davis M.J., Gough J., Grimmond S., Kawaji H., Magrane M., Matsuda H., Schönbach C., Teasdale R.D., Yuan Z. RIKEN GER Group, GSL Members, Mouse proteome analysis. Genome Res. 2003;13:1335–1344. doi: 10.1101/gr.978703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uhlén M., Fagerberg L., Hallström B.M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson Å., Kampf C., Sjöstedt E., Asplund A., Olsson I., Edlund K., Lundberg E., Navani S., Szigyarto C.A.-K., Odeberg J., Djureinovic D., Takanen J.O., Hober S., Alm T., Edqvist P.-H., Berling H., Tegel H., Mulder J., Rockberg J., Nilsson P., Schwenk J.M., Hamsten M., von Feilitzen K., Forsberg M., Persson L., Johansson F., Zwahlen M., von Heijne G., Nielsen J., Pontén F. Proteomics. Tissue-based map of the human proteome. Science. 2015;347 doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 3.Uhlén M., Karlsson M.J., Hober A., Svensson A.-S., Scheffel J., Kotol D., Zhong W., Tebani A., Strandberg L., Edfors F., Sjöstedt E., Mulder J., Mardinoglu A., Berling A., Ekblad S., Dannemeyer M., Kanje S., Rockberg J., Lundqvist M., Malm M., Volk A.-L., Nilsson P., Månberg A., Dodig-Crnkovic T., Pin E., Zwahlen M., Oksvold P., von Feilitzen K., Häussler R.S., Hong M.-G., Lindskog C., Ponten F., Katona B., Vuu J., Lindström E., Nielsen J., Robinson J., Ayoglu B., Mahdessian D., Sullivan D., Thul P., Danielsson F., Stadler C., Lundberg E., Bergström G., Gummesson A., Voldborg B.G., Tegel H., Hober S., Forsström B., Schwenk J.M., Fagerberg L., Sivertsson Å. The human secretome. Sci. Signal. 2019;12 doi: 10.1126/scisignal.aaz0274. [DOI] [PubMed] [Google Scholar]
- 4.Thul P.J., Åkesson L., Wiking M., Mahdessian D., Geladaki A., Ait Blal H., Alm T., Asplund A., Björk L., Breckels L.M., Bäckström A., Danielsson F., Fagerberg L., Fall J., Gatto L., Gnann C., Hober S., Hjelmare M., Johansson F., Lee S., Lindskog C., Mulder J., Mulvey C.M., Nilsson P., Oksvold P., Rockberg J., Schutten R., Schwenk J.M., Sivertsson Å., Sjöstedt E., Skogs M., Stadler C., Sullivan D.P., Tegel H., Winsnes C., Zhang C., Zwahlen M., Mardinoglu A., Pontén F., von Feilitzen K., Lilley K.S., Uhlén M., Lundberg E. A subcellular map of the human proteome. Science. 2017;356 doi: 10.1126/science.aal3321. [DOI] [PubMed] [Google Scholar]
- 5.Ricard-Blum S. The collagen family, Cold Spring Harb. Perspect. Biol. 2011;3 doi: 10.1101/cshperspect.a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palade G.E. Intracellular aspects of the process of protein synthesis. Science. 1975;189:347–358. doi: 10.1126/science.189.4206.867-b. [DOI] [PubMed] [Google Scholar]
- 7.Tartakoff A.M., Vassali P., Detraz M. Plasma cell immunoglobulin secretion. Arrest is accompanied by alterations the golgi complex. J. Exp. Med. 1977;146:1332–1345. doi: 10.1084/jem.146.5.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geuze H.J., Slot J.W. The subcellular localization of immunoglobulin in mouse plasma cells, as studied with immunoferritin cytochemistry on ultrathin frozen sections. Am. J. Anat. 1980;158:161–169. doi: 10.1002/aja.1001580206. [DOI] [PubMed] [Google Scholar]
- 9.Kelly R.B. Pathways of protein secretion in eukaryotes. Science. 1985;230:25–32. doi: 10.1126/science.2994224. [DOI] [PubMed] [Google Scholar]
- 10.Gumbiner B., Kelly R.B. Two distinct intracellular pathways transport secretory and membrane glycoproteins to the surface of pituitary tumor cells. Cell. 1982;28:51–59. doi: 10.1016/0092-8674(82)90374-9. [DOI] [PubMed] [Google Scholar]
- 11.Griffiths G., Simons K. The trans Golgi network: sorting at the exit site of the Golgi complex. Science. 1986;234:438–443. doi: 10.1126/science.2945253. [DOI] [PubMed] [Google Scholar]
- 12.Simons K., Fuller S.D. Cell surface polarity in epithelia. Annu. Rev. Cell Biol. 1985;1:243–288. doi: 10.1146/annurev.cb.01.110185.001331. [DOI] [PubMed] [Google Scholar]
- 13.Trifaro´ J.M., Lee R.W.H. Morphological characteristics and stimulus-secretion coupling in bovine adrenal chromaffin cell cultures. Neuroscience. 1980;5:1533–1546. doi: 10.1016/0306-4522(80)90018-4. [DOI] [PubMed] [Google Scholar]
- 14.Melmed R.N., Benitez C.J., Holt S.J. Intermediate cells of the pancreas. I. Ultrastructural characterization. J. Cell Sci. 1972;11:449–475. doi: 10.1242/jcs.11.2.449. https://www.ncbi.nlm.nih.gov/pubmed/4627700 [DOI] [PubMed] [Google Scholar]
- 15.Sips H.J., Brown D., Oonk R., Orci L. Orientation of rat-liver plasma membrane vesicles. A biochemical and ultrastructural study. Biochim. Biophys. Acta. 1982;692:447–454. doi: 10.1016/0005-2736(82)90396-0. [DOI] [PubMed] [Google Scholar]
- 16.Matthew W.D., Tsavaler L., Reichardt L.F. Identification of a synaptic vesicle-specific membrane protein with a wide distribution in neuronal and neurosecretory tissue. J. Cell Biol. 1981;91:257–269. doi: 10.1083/jcb.91.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilbert S.P., Sloboda R.D. Bidirectional transport of fluorescently labeled vesicles introduced into extruded axoplasm of squid Loligo pealei. J. Cell Biol. 1984;99:445–452. doi: 10.1083/jcb.99.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schroer T.A., Brady S.T., Kelly R.B. Fast axonal transport of foreign synaptic vesicles in squid axoplasm. J. Cell Biol. 1985;101:568–572. doi: 10.1083/jcb.101.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brady S.T., Lasek R.J., Allen R.D. Fast axonal transport in extruded axoplasm from squid giant axon. Science. 1982;218:1129–1131. doi: 10.1126/science.6183745. [DOI] [PubMed] [Google Scholar]
- 20.Pfeiffer S., Fuller S.D., Simons K. Intracellular sorting and basolateral appearance of the G protein of vesicular stomatitis virus in Madin-Darby canine kidney cells. J. Cell Biol. 1985;101:470–476. doi: 10.1083/jcb.101.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matlin K.S., Simons K. Sorting of an apical plasma membrane glycoprotein occurs before it reaches the cell surface in cultured epithelial cells. J. Cell Biol. 1984;99:2131–2139. doi: 10.1083/jcb.99.6.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Misek D.E., Bard E., Rodriguez-Boulan E. Biogenesis of epithelial cell polarity: intracellular sorting and vectorial exocytosis of an apical plasma membrane glycoprotein. Cell. 1984;39:537–546. doi: 10.1016/0092-8674(84)90460-4. [DOI] [PubMed] [Google Scholar]
- 23.Novick P.J., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. https://www.ncbi.nlm.nih.gov/pubmed/6996832 [DOI] [PubMed] [Google Scholar]
- 24.Balch W.E., Dunphy W.G., Braell W.A., Rothman J.E. Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell. 1984;39:405–416. doi: 10.1016/0092-8674(84)90019-9. [DOI] [PubMed] [Google Scholar]
- 25.Söllner T., Whiteheart S.W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J.E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 26.Wieland F.T., Gleason M.L., Serafini T.A., Rothman J.E. The Rate of Bulk Flow from the Endoplasmic Reticulum to the Cell Surface. Cell. 1987;50:289–300. doi: 10.1016/0092-8674(87)90224-8. [DOI] [PubMed] [Google Scholar]
- 27.Wiedmann M., Huth A., Rapoport T.A. Xenopus oocytes can secrete bacterial beta-lactamase. Nature. 1984;309:637–639. doi: 10.1038/309637a0. [DOI] [PubMed] [Google Scholar]
- 28.Martínez-Menárguez J.A., Geuze H.J., Slot J.W., Klumperman J. Vesicular tubular clusters between the ER and Golgi mediate concentration of soluble secretory proteins by exclusion from COPI-coated vesicles. Cell. 1999;98:81–90. doi: 10.1016/S0092-8674(00)80608-X. [DOI] [PubMed] [Google Scholar]
- 29.Warren G., Mellman I. Bulk flow redux? Cell. 1999;98:125–127. doi: 10.1016/s0092-8674(00)81006-5. [DOI] [PubMed] [Google Scholar]
- 30.Fujita M., Watanabe R., Jaensch N., Romanova-Michaelides M., Satoh T., Kato M., Riezman H., Yamaguchi Y., Maeda Y., Kinoshita T. Sorting of GPI-anchored proteins into ER exit sites by p24 proteins is dependent on remodeled GPI. J. Cell Biol. 2011;194:61–75. doi: 10.1083/jcb.201012074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vollenweider F., Kappeler F., Itin C., Hauri H.P. Mistargeting of the lectin ERGIC-53 to the endoplasmic reticulum of HeLa cells impairs the secretion of a lysosomal enzyme. J. Cell Biol. 1998;142:377–389. doi: 10.1083/jcb.142.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Appenzeller C., Andersson H., Kappeler F., Hauri H.P. The lectin ERGIC-53 is a cargo transport receptor for glycoproteins. Nat. Cell Biol. 1999;1:330–334. doi: 10.1038/14020. [DOI] [PubMed] [Google Scholar]
- 33.Nichols W.C., Seligsohn U., Zivelin A., Terry V.H., Hertel C.E., Wheatley M.A., Moussalli M.J., Hauri H.P., Ciavarella N., Kaufman R.J., Ginsburg D. Mutations in the ER-Golgi intermediate compartment protein ERGIC-53 cause combined deficiency of coagulation factors V and VIII. Cell. 1998;93:61–70. doi: 10.1016/s0092-8674(00)81146-0. [DOI] [PubMed] [Google Scholar]
- 34.Novick P.J., Schekman R. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 1979;76:1858–1862. doi: 10.1073/pnas.76.4.1858. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=383491&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Denecke J., Botterman J., Deblaere R. Protein secretion in plant cells can occur via a default pathway. Plant Cell. 1990;2:51–59. doi: 10.1105/tpc.2.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gershlick D.C., Lousa C. de M., Foresti O., Lee A.J., Pereira E.A., daSilva L.L.P., Bottanelli F., Denecke J. Golgi-dependent transport of vacuolar sorting receptors is regulated by COPII, AP1, and AP4 protein complexes in tobacco. Plant Cell. 2014;26:1308–1329. doi: 10.1105/tpc.113.122226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trychta K.A., Bäck S., Henderson M.J., Harvey B.K. KDEL Receptors Are Differentially Regulated to Maintain the ER Proteome under Calcium Deficiency. Cell Rep. 2018;25:1829–1840. doi: 10.1016/j.celrep.2018.10.055. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thor F., Gautschi M., Geiger R., Helenius A. Bulk flow revisited: transport of a soluble protein in the secretory pathway. Traffic. 2009;10:1819–1830. doi: 10.1111/j.1600-0854.2009.00989.x. [DOI] [PubMed] [Google Scholar]
- 39.Balch W.E., McCaffery J.M., Plutner H., Farquhar M.G. Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell. 1994;76:841–852. doi: 10.1016/0092-8674(94)90359-x. https://www.ncbi.nlm.nih.gov/pubmed/8124720 [DOI] [PubMed] [Google Scholar]
- 40.Barlowe C. Signals for COPII-dependent export from the ER: what’s the ticket out? Trends Cell Biol. 2003;13:295–300. doi: 10.1016/s0962-8924(03)00082-5. [DOI] [PubMed] [Google Scholar]
- 41.Nishimura N., Balch W.E. A di-acidic signal required for selective export from the endoplasmic reticulum. Science. 1997;277:556–558. doi: 10.1126/science.277.5325.556. [DOI] [PubMed] [Google Scholar]
- 42.Robinson M.S. 100-kD coated vesicle proteins: molecular heterogeneity and intracellular distribution studied with monoclonal antibodies. J. Cell Biol. 1987;104:887–895. doi: 10.1083/jcb.104.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahle S., Mann A., Eichelsbacher U., Ungewickell E. Structural relationships between clathrin assembly proteins from the Golgi and the plasma membrane. EMBO J. 1988;7:919–929. doi: 10.1002/j.1460-2075.1988.tb02897.x. https://www.ncbi.nlm.nih.gov/pubmed/3402440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robinson M.S., Pearse B.M. Immunofluorescent localization of 100K coated vesicle proteins. J. Cell Biol. 1986;102:48–54. doi: 10.1083/jcb.102.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson K.F., Kornfeld S. The cytoplasmic tail of the mannose 6-phosphate/insulin-like growth factor-II receptor has two signals for lysosomal enzyme sorting in the Golgi. J. Cell Biol. 1992;119:249–257. doi: 10.1083/jcb.119.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puertollano R., Aguilar R.C., Gorshkova I., Crouch R.J., Bonifacino J.S. Sorting of mannose 6-phosphate receptors mediated by the GGAs. Science. 2001;292:1712–1716. doi: 10.1126/science.1060750. [DOI] [PubMed] [Google Scholar]
- 47.Braulke T., Bonifacino J.S. Sorting of lysosomal proteins. Biochim. Biophys. Acta. 2009;1793:605–614. doi: 10.1016/j.bbamcr.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 48.Nilsson T., Lucocq J.M., Mackay D., Warren G. The membrane spanning domain of beta-1,4-galactosyltransferase specifies trans Golgi localization. EMBO J. 1991;10:3567–3575. doi: 10.1002/j.1460-2075.1991.tb04923.x. https://www.ncbi.nlm.nih.gov/pubmed/1935889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Munro S. Sequences within and adjacent to the transmembrane segment of alpha-2,6-sialyltransferase specify Golgi retention. EMBO J. 1991;10:3577–3588. doi: 10.1002/j.1460-2075.1991.tb04924.x. https://www.ncbi.nlm.nih.gov/pubmed/1935890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Munro S. An investigation of the role of transmembrane domains in Golgi protein retention. EMBO J. 1995;14:4695–4704. doi: 10.1002/j.1460-2075.1995.tb00151.x. https://www.ncbi.nlm.nih.gov/pubmed/7588599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dukhovny A., Yaffe Y., Shepshelovitch J., Hirschberg K. The length of cargo-protein transmembrane segments drives secretory transport by facilitating cargo concentration in export domains. J. Cell Sci. 2009;122:1759–1767. doi: 10.1242/jcs.039339. [DOI] [PubMed] [Google Scholar]
- 52.Machamer C.E., Mentone S.A., Rose J.K., Farquhar M.G. The E1 glycoprotein of an avian coronavirus is targeted to the cis Golgi complex. Proc. Natl. Acad. Sci. U. S. A. 1990;87:6944–6948. doi: 10.1073/pnas.87.18.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swift A.M., Machamer C.E. A Golgi retention signal in a membrane-spanning domain of coronavirus E1 protein. J. Cell Biol. 1991;115:19–30. doi: 10.1083/jcb.115.1.19. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2289920&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Höfte H., Chrispeels M.J. Protein sorting to the vacuolar membrane. Plant Cell. 1992;4:995–1004. doi: 10.1105/tpc.4.8.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brandizzi F., Frangne N., Marc-martin S., Hawes C., Neuhaus J.M., Paris N. The destination for single-pass membrane proteins is influenced markedly by the length of the hydrophobic domain. Plant Cell. 2002;14:1077–1092. doi: 10.1105/tpc.000620.where. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharpe H.J., Stevens T.J., Munro S. A comprehensive comparison of transmembrane domains reveals organelle-specific properties. Cell. 2010;142:158–169. doi: 10.1016/j.cell.2010.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu C., Ng D.T.W. Glycosylation-directed quality control of protein folding. Nat. Rev. Mol. Cell Biol. 2015;16:742–752. doi: 10.1038/nrm4073. [DOI] [PubMed] [Google Scholar]
- 58.Ohtsubo K., Marth J.D. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 59.Chen Y., Gershlick D.C., Park S.Y., Bonifacino J.S. Segregation in the Golgi complex precedes export of endolysosomal proteins in distinct transport carriers. J. Cell Biol. 2017;216:4141–4151. doi: 10.1083/jcb.201707172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patterson G.H., Hirschberg K., Polishchuk R.S., Gerlich D., Phair R.D., Lippincott-Schwartz J. Transport through the Golgi apparatus by rapid partitioning within a two-phase membrane system. Cell. 2008;133:1055–1067. doi: 10.1016/j.cell.2008.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clermont Y., Rambourg A., Hermo L. Segregation of secretory material in all elements of the Golgi apparatus in principal epithelial cells of the rat seminal vesicle. Anat. Rec. 1992;232:349–358. doi: 10.1002/ar.1092320304. [DOI] [PubMed] [Google Scholar]
- 62.Keller P., Toomre D., Díaz E., White J., Simons K. Multicolour imaging of post-Golgi sorting and trafficking in live cells. Nat. Cell Biol. 2001;3:140–149. doi: 10.1038/35055042. [DOI] [PubMed] [Google Scholar]
- 63.Lewis M.J., Sweet D.J., Pelham H.R.B. The ERD2 gene determines the specificity of the luminal ER protein retention system. Cell. 1990;61:1359–1363. doi: 10.1016/0092-8674(90)90699-f. https://www.ncbi.nlm.nih.gov/pubmed/2194671 [DOI] [PubMed] [Google Scholar]
- 64.Munro S., Pelham H.R.B. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987;48:899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- 65.Tu L., Banfield D.K. Localization of Golgi-resident glycosyltransferases. Cell. Mol. Life Sci. 2010;67:29–41. doi: 10.1007/s00018-009-0126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sanger A., Hirst J., Davies A.K., Robinson M.S. Adaptor protein complexes and disease at a glance. J. Cell Sci. 2019;132 doi: 10.1242/jcs.222992. [DOI] [PubMed] [Google Scholar]
- 67.Griffiths G., Pfeiffer S., Simons K., Matlin K. Exit of newly synthesized membrane proteins from the trans cisterna of the Golgi complex to the plasma membrane. J. Cell Biol. 1985;101:949–964. doi: 10.1083/jcb.101.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Polishchuk R.S., San Pietro E., Di Pentima A., Teté S., Bonifacino J.S. Ultrastructure of long-range transport carriers moving from the trans Golgi network to peripheral endosomes. Traffic. 2006;7:1092–1103. doi: 10.1111/j.1600-0854.2006.00453.x. [DOI] [PubMed] [Google Scholar]
- 69.Lui-Roberts W.W.Y., Collinson L.M., Hewlett L.J., Michaux G., Cutler D.F. An AP-1/clathrin coat plays a novel and essential role in forming the Weibel-Palade bodies of endothelial cells. J. Cell Biol. 2005;170:627–636. doi: 10.1083/jcb.200503054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McCormack J.J., Lopes da Silva M., Ferraro F., Patella F., Cutler D.F. Weibel-Palade bodies at a glance. J. Cell Sci. 2017;130:3611–3617. doi: 10.1242/jcs.208033. [DOI] [PubMed] [Google Scholar]
- 71.McCaughey J., Stephens D.J. ER-to-Golgi Transport: A Sizeable Problem. Trends Cell Biol. 2019;29:940–953. doi: 10.1016/j.tcb.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 72.Raote I., Malhotra V. Protein transport by vesicles and tunnels. J. Cell Biol. 2019;218:737–739. doi: 10.1083/jcb.201811073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Višnjar T., Chesi G., Iacobacci S., Polishchuk E., Resnik N., Robenek H., Kreft M., Romih R., Polishchuk R., Kreft M.E. Uroplakin traffic through the Golgi apparatus induces its fragmentation: new insights from novel in vitro models. Sci. Rep. 2017;7:12842. doi: 10.1038/s41598-017-13103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Payne G.S., Schekman R. A test of clathrin function in protein secretion and cell growth. Science. 1985;230:1009–1014. doi: 10.1126/science.2865811. [DOI] [PubMed] [Google Scholar]
- 75.Polishchuk E.V., Di Pentima A., Luini A., Polishchuk R.S. Mechanism of constitutive export from the golgi: bulk flow via the formation, protrusion, and en bloc cleavage of large trans-golgi network tubular domains. Mol. Biol. Cell. 2003;14:4470–4485. doi: 10.1091/mbc.E03-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Knipe D.M., Baltimore D., Lodish H.F. Maturation of viral proteins in cells infected with temperature-sensitive mutants of vesicular stomatitis virus. J. Virol. 1977;21:1149–1158. doi: 10.1128/jvi.21.3.1149-1158.1977. https://www.ncbi.nlm.nih.gov/pubmed/191642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lafay F. Envelope proteins of vesicular stomatitis virus: effect of temperature-sensitive mutations in complementation groups III and V. J. Virol. 1974;14:1220–1228. doi: 10.1128/jvi.14.5.1220-1228.1974. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=355638&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bergmann J.E., Tokuyasu K.T., Singer S.J. Passage of an integral membrane protein, the vesicular stomatitis virus glycoprotein, through the Golgi apparatus en route to the plasma membrane. Proc. Natl. Acad. Sci. U. S. A. 1981;78:1746–1750. doi: 10.1073/pnas.78.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gallione C.J., Rose J.K. A single amino acid substitution in a hydrophobic domain causes temperature-sensitive cell-surface transport of a mutant viral glycoprotein. J. Virol. 1985;54:374–382. doi: 10.1128/jvi.54.2.374-382.1985. https://www.ncbi.nlm.nih.gov/pubmed/2985803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Polishchuk R.S., Polishchuk E.V., Marra P., Alberti S., Buccione R., Luini A., Mironov A.A. Correlative light-electron microscopy reveals the tubular-saccular ultrastructure of carriers operating between Golgi apparatus and plasma membrane. J. Cell Biol. 2000;148:45–58. doi: 10.1083/jcb.148.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hirschberg K., Miller C.M., Ellenberg J., Presley J.F., Siggia E.D., Phair R.D., Lippincott-Schwartz J. Kinetic analysis of secretory protein traffic and characterization of golgi to plasma membrane transport intermediates in living cells. J. Cell Biol. 1998;143:1485–1503. doi: 10.1083/jcb.143.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luini A., Ragnini-Wilson A., Polishchuck R.S., De Matteis M.A. Large pleiomorphic traffic intermediates in the secretory pathway. Curr. Opin. Cell Biol. 2005;17:353–361. doi: 10.1016/j.ceb.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 83.Toomre D., Keller P., White J., Olivo J.C., Simons K. Dual-color visualization of trans-Golgi network to plasma membrane traffic along microtubules in living cells. J. Cell Sci. 1999;112(Pt 1):21–33. doi: 10.1242/jcs.112.1.21. https://www.ncbi.nlm.nih.gov/pubmed/9841901 [DOI] [PubMed] [Google Scholar]
- 84.Ang A.L., Taguchi T., Francis S., Fölsch H., Murrells L.J., Pypaert M., Warren G., Mellman I. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J. Cell Biol. 2004;167:531–543. doi: 10.1083/jcb.200408165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lock J.G., Stow J.L. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol. Biol. Cell. 2005;16:1744–1755. doi: 10.1091/mbc.e04-10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carlsson S.R., Fukuda M. The lysosomal membrane glycoprotein lamp-1 is transported to lysosomes by two alternative pathways. Arch. Biochem. Biophys. 1992;296:630–639. doi: 10.1016/0003-9861(92)90619-8. [DOI] [PubMed] [Google Scholar]
- 87.Akasaki K., Michihara A., Mibuka K., Fujiwara Y., Tsuji H. Biosynthetic transport of a major lysosomal membrane glycoprotein, lamp-1: convergence of biosynthetic and endocytic pathways occurs at three distinctive points. Exp. Cell Res. 1995;220:464–473. doi: 10.1006/excr.1995.1338. [DOI] [PubMed] [Google Scholar]
- 88.Kirchhausen T., Owen D., Harrison S.C. Molecular structure, function, and dynamics of clathrin-mediated membrane traffic, Cold Spring Harb. Perspect. Biol. 2014;6 doi: 10.1101/cshperspect.a016725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hierro A., Gershlick D.C., Rojas A.L., Bonifacino J.S. Formation of Tubulovesicular Carriers from Endosomes and Their Fusion to the trans-Golgi Network. Int. Rev. Cell Mol. Biol. 2015;318:159–202. doi: 10.1016/bs.ircmb.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 90.Serafini T., Orci L., Amherdt M., Brunner M., Kahn R.A., Rothman J.E. ADP-ribosylation factor is a subunit of the coat of Golgi-derived COP-coated vesicles: a novel role for a GTP-binding protein. Cell. 1991;67:239–253. doi: 10.1016/0092-8674(91)90176-y. https://www.ncbi.nlm.nih.gov/pubmed/1680566 [DOI] [PubMed] [Google Scholar]
- 91.Boehm M., Bonifacino J.S. Adaptins: the final recount. Mol. Biol. Cell. 2001;12:2907–2920. doi: 10.1091/mbc.12.10.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang C.-W., Hamamoto S., Orci L., Schekman R. Exomer: A coat complex for transport of select membrane proteins from the trans-Golgi network to the plasma membrane in yeast. J. Cell Biol. 2006;174:973–983. doi: 10.1083/jcb.200605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Beck R., Sun Z., Adolf F., Rutz C., Bassler J., Wild K., Sinning I., Hurt E., Brügger B., Béthune J., Wieland F. Membrane curvature induced by Arf1-GTP is essential for vesicle formation. Proc. Natl. Acad. Sci. U. S. A. 2008;105:11731–11736. doi: 10.1073/pnas.0805182105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bottanelli F., Kilian N., Ernst A.M., Rivera-Molina F., Schroeder L.K., Kromann E.B., Lessard M.D., Erdmann R.S., Schepartz A., Baddeley D., Bewersdorf J., Toomre D., Rothman J.E. A novel physiological role for ARF1 in the formation of bidirectional tubules from the Golgi. Mol. Biol. Cell. 2017;28:1676–1687. doi: 10.1091/mbc.E16-12-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cao H., Weller S., Orth J.D., Chen J., Huang B., Chen J.-L., Stamnes M., McNiven M.A. Actin and Arf1-dependent recruitment of a cortactin-dynamin complex to the Golgi regulates post-Golgi transport. Nat. Cell Biol. 2005;7:483–492. doi: 10.1038/ncb1246. [DOI] [PubMed] [Google Scholar]
- 96.Schnoor M., Stradal T.E., Rottner K. Cortactin: Cell Functions of A Multifaceted Actin-Binding Protein. Trends Cell Biol. 2018;28:79–98. doi: 10.1016/j.tcb.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 97.Ferguson S.M., De Camilli P. Dynamin, a membrane-remodelling GTPase. Nat. Rev. Mol. Cell Biol. 2012;13:75–88. doi: 10.1038/nrm3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Malhotra V., Campelo F. PKD regulates membrane fission to generate TGN to cell surface transport carriers, Cold Spring Harb. Perspect. Biol. 2011;3 doi: 10.1101/cshperspect.a005280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.D’Angelo G., Rega L.R., De Matteis M.A. Connecting vesicular transport with lipid synthesis: FAPP2. Biochim. Biophys. Acta. 2012;1821:1089–1095. doi: 10.1016/j.bbalip.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jamora C., Yamanouye N., Van Lint J., Laudenslager J., Vandenheede J.R., Faulkner D.J., Malhotra V. Gbetagamma-mediated regulation of Golgi organization is through the direct activation of protein kinase D. Cell. 1999;98:59–68. doi: 10.1016/S0092-8674(00)80606-6. [DOI] [PubMed] [Google Scholar]
- 101.Liljedahl M., Maeda Y., Colanzi A., Ayala I., Van Lint J., Malhotra V. Protein kinase D regulates the fission of cell surface destined transport carriers from the trans-Golgi network. Cell. 2001;104:409–420. doi: 10.1016/s0092-8674(01)00228-8. [DOI] [PubMed] [Google Scholar]
- 102.Bossard C., Bresson D., Polishchuk R.S., Malhotra V. Dimeric PKD regulates membrane fission to form transport carriers at the TGN. J. Cell Biol. 2007;179:1123–1131. doi: 10.1083/jcb.200703166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maeda Y., Beznoussenko G.V., Van Lint J., Mironov A.A., Malhotra V. Recruitment of protein kinase D to the trans-Golgi network via the first cysteine-rich domain. EMBO J. 2001;20:5982–5990. doi: 10.1093/emboj/20.21.5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pusapati G.V., Krndija D., Armacki M., von Wichert G., von Blume J., Malhotra V., Adler G., Seufferlein T. Role of the second cysteine-rich domain and Pro275 in protein kinase D2 interaction with ADP-ribosylation factor 1, trans-Golgi network recruitment, and protein transport. Mol. Biol. Cell. 2010;21:1011–1022. doi: 10.1091/mbc.e09-09-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Waugh M.G. The Great Escape: how phosphatidylinositol 4-kinases and PI4P promote vesicle exit from the Golgi (and drive cancer) Biochem. J. 2019;476:2321–2346. doi: 10.1042/BCJ20180622. [DOI] [PubMed] [Google Scholar]
- 106.Dowler S., Currie R.A., Campbell D.G., Deak M., Kular G., Downes C.P., Alessi D.R. Identification of pleckstrin-homology-domain-containing proteins with novel phosphoinositide-binding specificities. Biochem. J. 2000;351:19–31. doi: 10.1042/0264-6021:3510019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Godi A., Di Campli A., Konstantakopoulos A., Di Tullio G., Alessi D.R., Kular G.S., Daniele T., Marra P., Lucocq J.M., De Matteis M.A. FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P. Nat. Cell Biol. 2004;6:393–404. doi: 10.1038/ncb1119. [DOI] [PubMed] [Google Scholar]
- 108.Lenoir M., Coskun U., Grzybek M., Cao X., Buschhorn S.B., James J., Simons K., Overduin M. Structural basis of wedging the Golgi membrane by FAPP pleckstrin homology domains. EMBO Rep. 2010;11:279–284. doi: 10.1038/embor.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lenoir M., Grzybek M., Majkowski M., Rajesh S., Kaur J., Whittaker S.B.-M., Coskun Ü., Overduin M. Structural basis of dynamic membrane recognition by trans-Golgi network specific FAPP proteins. J. Mol. Biol. 2015;427:966–981. doi: 10.1016/j.jmb.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 110.Cao X., Coskun U., Rössle M., Buschhorn S.B., Grzybek M., Dafforn T.R., Lenoir M., Overduin M., Simons K. Golgi protein FAPP2 tubulates membranes. Proc. Natl. Acad. Sci. U. S. A. 2009;106:21121–21125. doi: 10.1073/pnas.0911789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vieira O.V., Verkade P., Manninen A., Simons K. FAPP2 is involved in the transport of apical cargo in polarized MDCK cells. J. Cell Biol. 2005;170:521–526. doi: 10.1083/jcb.200503078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.D’Angelo G., Polishchuk E., Di Tullio G., Santoro M., Di Campli A., Godi A., West G., Bielawski J., Chuang C.-C., van der Spoel A.C., Platt F.M., Hannun Y.A., Polishchuk R., Mattjus P., De Matteis M.A. Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide. Nature. 2007;449:62–67. doi: 10.1038/nature06097. [DOI] [PubMed] [Google Scholar]
- 113.Paczkowski J.E., Fromme J.C. Structural basis for membrane binding and remodeling by the exomer secretory vesicle cargo adaptor. Dev. Cell. 2014;30:610–624. doi: 10.1016/j.devcel.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Paczkowski J.E., Richardson B.C., Strassner A.M., Fromme J.C. The exomer cargo adaptor structure reveals a novel GTPase-binding domain. EMBO J. 2012;31:4191–4203. doi: 10.1038/emboj.2012.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Trautwein M., Schindler C., Gauss R., Dengjel J., Hartmann E., Spang A. Arf1p, Chs5p and the ChAPs are required for export of specialized cargo from the Golgi. EMBO J. 2006;25:943–954. doi: 10.1038/sj.emboj.7601007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sanchatjate S., Schekman R. Chs5/6 complex: a multiprotein complex that interacts with and conveys chitin synthase III from the trans-Golgi network to the cell surface. Mol. Biol. Cell. 2006;17:4157–4166. doi: 10.1091/mbc.e06-03-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fourriere L., Kasri A., Gareil N., Bardin S., Bousquet H., Pereira D., Perez F., Goud B., Boncompain G., Miserey-Lenkei S. RAB6 and microtubules restrict protein secretion to focal adhesions. J. Cell Biol. 2019 doi: 10.1083/jcb.201805002. jcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Boncompain G., Divoux S., Gareil N., de Forges H., Lescure A., Latreche L., Mercanti V., Jollivet F., Raposo G., Perez F. Synchronization of secretory protein traffic in populations of cells. Nat. Methods. 2012;9:493–498. doi: 10.1038/nmeth.1928. [DOI] [PubMed] [Google Scholar]
- 119.Pols M.S., van Meel E., Oorschot V., ten Brink C., Fukuda M., Swetha M.G., Mayor S., Klumperman J. hVps41 and VAMP7 function in direct TGN to late endosome transport of lysosomal membrane proteins. Nat. Commun. 2013;4:1361. doi: 10.1038/ncomms2360. [DOI] [PubMed] [Google Scholar]
- 120.Grigoriev I., Splinter D., Keijzer N., Wulf P.S., Demmers J., Ohtsuka T., Modesti M., Maly I.V., Grosveld F., Hoogenraad C.C., Akhmanova A. Rab6 regulates transport and targeting of exocytotic carriers. Dev. Cell. 2007;13:305–314. doi: 10.1016/j.devcel.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 121.Miserey-Lenkei S., Chalancon G., Bardin S., Formstecher E., Goud B., Echard A. Rab and actomyosin-dependent fission of transport vesicles at the Golgi complex. Nat. Cell Biol. 2010;12:645–654. doi: 10.1038/ncb2067. [DOI] [PubMed] [Google Scholar]
- 122.Miserey-Lenkei S., Bousquet H., Pylypenko O., Bardin S., Dimitrov A., Bressanelli G., Bonifay R., Fraisier V., Guillou C., Bougeret C., Houdusse A., Echard A., Goud B. Coupling fission and exit of RAB6 vesicles at Golgi hotspots through kinesin-myosin interactions. Nat. Commun. 2017;8:1254. doi: 10.1038/s41467-017-01266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Niu T.-K., Pfeifer A.C., Lippincott-Schwartz J., Jackson C.L. Dynamics of GBF1, a Brefeldin A-sensitive Arf1 exchange factor at the Golgi. Mol. Biol. Cell. 2005;16:1213–1222. doi: 10.1091/mbc.e04-07-0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lippincott-Schwartz J., Yuan L.C., Bonifacino J.S., Klausner R.D. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989;56:801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Spanò S., Silletta M.G., Colanzi A., Alberti S., Fiucci G., Valente C., Fusella A., Salmona M., Mironov A., Luini A., Corda D. Molecular cloning and functional characterization of brefeldin A-ADP-ribosylated substrate. A novel protein involved in the maintenance of the Golgi structure. J. Biol. Chem. 1999;274:17705–17710. doi: 10.1074/jbc.274.25.17705. [DOI] [PubMed] [Google Scholar]
- 126.Weigert R., Silletta M.G., Spanò S., Turacchio G., Cericola C., Colanzi A., Senatore S., Mancini R., Polishchuk E.V., Salmona M., Facchiano F., Burger K.N., Mironov A., Luini A., Corda D. CtBP/BARS induces fission of Golgi membranes by acylating lysophosphatidic acid. Nature. 1999;402:429–433. doi: 10.1038/46587. [DOI] [PubMed] [Google Scholar]
- 127.Valente C., Turacchio G., Mariggiò S., Pagliuso A., Gaibisso R., Di Tullio G., Santoro M., Formiggini F., Spanò S., Piccini D., Polishchuk R.S., Colanzi A., Luini A., Corda D. A 14-3-3γ dimer-based scaffold bridges CtBP1-S/BARS to PI(4)KIIIβ to regulate post-Golgi carrier formation. Nat. Cell Biol. 2012;14:343–354. doi: 10.1038/ncb2445. [DOI] [PubMed] [Google Scholar]
- 128.Bonazzi M., Spanò S., Turacchio G., Cericola C., Valente C., Colanzi A., Kweon H.S., Hsu V.W., Polishchuck E.V., Polishchuck R.S., Sallese M., Pulvirenti T., Corda D., Luini A. CtBP3/BARS drives membrane fission in dynamin-independent transport pathways. Nat. Cell Biol. 2005;7:570–580. doi: 10.1038/ncb1260. [DOI] [PubMed] [Google Scholar]
- 129.Mettlen M., Pucadyil T., Ramachandran R., Schmid S.L. Dissecting dynamin’s role in clathrin-mediated endocytosis. Biochem. Soc. Trans. 2009;37:1022–1026. doi: 10.1042/BST0371022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pagliuso A., Valente C., Giordano L.L., Filograna A., Li G., Circolo D., Turacchio G., Marzullo V.M., Mandrich L., Zhukovsky M.A., Formiggini F., Polishchuk R.S., Corda D., Luini A. Golgi membrane fission requires the CtBP1-S/BARS-induced activation of lysophosphatidic acid acyltransferase δ. Nat. Commun. 2016;7:12148. doi: 10.1038/ncomms12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Duran J.M., Campelo F., van Galen J., Sachsenheimer T., Sot J., Egorov M.V., Rentero C., Enrich C., Polishchuk R.S., Goñi F.M., Brügger B., Wieland F., Malhotra V. Sphingomyelin organization is required for vesicle biogenesis at the Golgi complex. EMBO J. 2012;31:4535–4546. doi: 10.1038/emboj.2012.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Deng Y., Rivera-Molina F.E., Toomre D.K., Burd C.G. Sphingomyelin is sorted at the trans Golgi network into a distinct class of secretory vesicle. Proc. Natl. Acad. Sci. U. S. A. 2016;113:6677–6682. doi: 10.1073/pnas.1602875113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Deng Y., Pakdel M., Blank B., Sundberg E.L., Burd C.G., von Blume J. Activity of the SPCA1 Calcium Pump Couples Sphingomyelin Synthesis to Sorting of Secretory Proteins in the Trans-Golgi Network. Dev. Cell. 2018;47:464–478. doi: 10.1016/j.devcel.2018.10.012. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.von Blume J., Alleaume A.-M., Kienzle C., Carreras-Sureda A., Valverde M., Malhotra V. Cab45 is required for Ca(2+)-dependent secretory cargo sorting at the trans-Golgi network. J. Cell Biol. 2012;199:1057–1066. doi: 10.1083/jcb.201207180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pakdel M., von Blume J. Exploring new routes for secretory protein export from the trans-Golgi network. Mol. Biol. Cell. 2018;29:235–240. doi: 10.1091/mbc.E17-02-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kienzle C., Basnet N., Crevenna A.H., Beck G., Habermann B., Mizuno N., von Blume J. Cofilin recruits F-actin to SPCA1 and promotes Ca2+-mediated secretory cargo sorting. J. Cell Biol. 2014;206:635–654. doi: 10.1083/jcb.201311052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.von Blume J., Alleaume A.-M., Cantero-Recasens G., Curwin A., Carreras-Sureda A., Zimmermann T., van Galen J., Wakana Y., Valverde M.A., Malhotra V. ADF/cofilin regulates secretory cargo sorting at the TGN via the Ca2+ ATPase SPCA1. Dev. Cell. 2011;20:652–662. doi: 10.1016/j.devcel.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 138.Crevenna A.H., Blank B., Maiser A., Emin D., Prescher J., Beck G., Kienzle C., Bartnik K., Habermann B., Pakdel M., Leonhardt H., Lamb D.C., von Blume J. Secretory cargo sorting by Ca2+-dependent Cab45 oligomerization at the trans-Golgi network. J. Cell Biol. 2016;213:305–314. doi: 10.1083/jcb.201601089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sundberg E.L., Deng Y., Burd C.G. Syndecan-1 Mediates Sorting of Soluble Lipoprotein Lipase with Sphingomyelin-Rich Membrane in the Golgi Apparatus. Dev. Cell. 2019;5(1):387–398. doi: 10.1016/j.devcel.2019.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wakana Y., van Galen J., Meissner F., Scarpa M., Polishchuk R.S., Mann M., Malhotra V. A new class of carriers that transport selective cargo from the trans Golgi network to the cell surface. EMBO J. 2012;31:3976–3990. doi: 10.1038/emboj.2012.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wakana Y., Kotake R., Oyama N., Murate M., Kobayashi T., Arasaki K., Inoue H., Tagaya M. CARTS biogenesis requires VAP-lipid transfer protein complexes functioning at the endoplasmic reticulum-Golgi interface. Mol. Biol. Cell. 2015;26:4686–4699. doi: 10.1091/mbc.E15-08-0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mesmin B., Bigay J., Moser von Filseck J., Lacas-Gervais S., Drin G., Antonny B. A four-step cycle driven by PI(4)P hydrolysis directs sterol/PI(4)P exchange by the ER-Golgi tether OSBP. Cell. 2013;155:830–843. doi: 10.1016/j.cell.2013.09.056. [DOI] [PubMed] [Google Scholar]
- 143.Venditti R., Masone M.C., Rega L.R., Di Tullio G., Santoro M., Polishchuk E., Serrano I.C., Olkkonen V.M., Harada A., Medina D.L., La Montagna R., De Matteis M.A. The activity of Sac1 across ER-TGN contact sites requires the four-phosphate-adaptor-protein-1. J. Cell Biol. 2019;218:783–797. doi: 10.1083/jcb.201812021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wakana Y., Villeneuve J., van Galen J., Cruz-Garcia D., Tagaya M., Malhotra V. Kinesin-5/Eg5 is important for transport of CARTS from the trans-Golgi network to the cell surface. J. Cell Biol. 2013;202:241–250. doi: 10.1083/jcb.201303163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.von Blume J., Hausser A. Lipid-dependent coupling of secretory cargo sorting and trafficking at the trans-Golgi network. FEBS Lett. 2019;593:2412–2427. doi: 10.1002/1873-3468.13552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Deguchi-Tawarada M., Inoue E., Takao-Rikitsu E., Inoue M., Ohtsuka T., Takai Y. CAST2: identification and characterization of a protein structurally related to the presynaptic cytomatrix protein CAST. Genes Cells. 2004;9:15–23. doi: 10.1111/j.1356-9597.2004.00697.x. [DOI] [PubMed] [Google Scholar]
- 147.Monier S., Jollivet F., Janoueix-Lerosey I., Johannes L., Goud B. Characterization of novel Rab6-interacting proteins involved in endosome-to-TGN transport. Traffic. 2002;3:289–297. doi: 10.1034/j.1600-0854.2002.030406.x. https://www.ncbi.nlm.nih.gov/pubmed/11929610 [DOI] [PubMed] [Google Scholar]
- 148.Nakata T., Kitamura Y., Shimizu K., Tanaka S., Fujimori M., Yokoyama S., Ito K., Emi M. Fusion of a novel gene, ELKS, to RET due to translocation t(10;12)(q11;p13) in a papillary thyroid carcinoma. Genes Chromosomes Cancer. 1999;25:97–103. doi: 10.1002/(sici)1098-2264(199906)25:2<97::aid-gcc4>3.0.co;2-l. https://doi.org/3.0.co;2-l.">10.1002/(sici)1098-2264(199906)25:2<97::aid-gcc4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 149.Wang Y., Liu X., Biederer T., Südhof T.C. A family of RIM-binding proteins regulated by alternative splicing: Implications for the genesis of synaptic active zones. Proc. Natl. Acad. Sci. U. S. A. 2002;99:14464–14469. doi: 10.1073/pnas.182532999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gundelfinger E.D., Kessels M.M., Qualmann B. Temporal and spatial coordination of exocytosis and endocytosis. Nat. Rev. Mol. Cell Biol. 2003;4:127–139. doi: 10.1038/nrm1016. [DOI] [PubMed] [Google Scholar]
- 151.Ohtsuka T., Takao-Rikitsu E., Inoue E., Inoue M., Takeuchi M., Matsubara K., Deguchi-Tawarada M., Satoh K., Morimoto K., Nakanishi H., Takai Y. Cast: a novel protein of the cytomatrix at the active zone of synapses that forms a ternary complex with RIM1 and munc13-1. J. Cell Biol. 2002;158:577–590. doi: 10.1083/jcb.200202083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ohara-Imaizumi M., Ohtsuka T., Matsushima S., Akimoto Y., Nishiwaki C., Nakamichi Y., Kikuta T., Nagai S., Kawakami H., Watanabe T., Nagamatsu S. ELKS, a protein structurally related to the active zone-associated protein CAST, is expressed in pancreatic beta cells and functions in insulin exocytosis: interaction of ELKS with exocytotic machinery analyzed by total internal reflection fluorescence microscopy. Mol. Biol. Cell. 2005;16:3289–3300. doi: 10.1091/mbc.e04-09-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ohara-Imaizumi M., Aoyagi K., Ohtsuka T. Role of the active zone protein, ELKS, in insulin secretion from pancreatic β-cells. Mol Metab. 2019;27S:S81–S91. doi: 10.1016/j.molmet.2019.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Grigoriev I., Yu K.L., Martinez-Sanchez E., Serra-Marques A., Smal I., Meijering E., Demmers J., Peränen J., Pasterkamp R.J., van der Sluijs P., Hoogenraad C.C., Akhmanova A. Rab6, Rab8, and MICAL3 cooperate in controlling docking and fusion of exocytotic carriers. Curr. Biol. 2011;21:967–974. doi: 10.1016/j.cub.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 155.Shibata S., Teshima Y., Niimi K., Inagaki S. Involvement of ARHGEF10, GEF for RhoA, in Rab6/Rab8-mediating membrane traffic. Small GTPases. 2019;10:169–177. doi: 10.1080/21541248.2017.1302550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shibata S., Kawanai T., Hara T., Yamamoto A., Chaya T., Tokuhara Y., Tsuji C., Sakai M., Tachibana T., Inagaki S. ARHGEF10 directs the localization of Rab8 to Rab6-positive executive vesicles. J. Cell Sci. 2016;129:3620–3634. doi: 10.1242/jcs.186817. [DOI] [PubMed] [Google Scholar]
- 157.Bowser R., Novick P. Sec15 protein, an essential component of the exocytotic apparatus, is associated with the plasma membrane and with a soluble 19.5S particle. J. Cell Biol. 1991;112:1117–1131. doi: 10.1083/jcb.112.6.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bowser R., Müller H., Govindan B., Novick P. Sec8p and Sec15p are components of a plasma membrane-associated 19.5 S particle that may function downstream of Sec4p to control exocytosis. J. Cell Biol. 1992;118:1041–1056. doi: 10.1083/jcb.118.5.1041. http://jcb.rupress.org/content/118/5/1041.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.TerBush D.R., Novick P. Sec6, Sec8, and Sec15 are components of a multisubunit complex which localizes to small bud tips in Saccharomyces cerevisiae. J. Cell Biol. 1995;130:299–312. doi: 10.1083/jcb.130.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.TerBush D.R., Maurice T., Roth D., Novick P. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 1996;15:6483–6494. https://www.ncbi.nlm.nih.gov/pubmed/8978675 [PMC free article] [PubMed] [Google Scholar]
- 161.Hsu S.C., Ting A.E., Hazuka C.D., Davanger S., Kenny J.W., Kee Y., Scheller R.H. The mammalian brain rsec6/8 complex. Neuron. 1996;17:1209–1219. doi: 10.1016/s0896-6273(00)80251-2. [DOI] [PubMed] [Google Scholar]
- 162.Guo W., Grant A., Novick P. Exo84p is an exocyst protein essential for secretion. J. Biol. Chem. 1999;274:23558–23564. doi: 10.1074/jbc.274.33.23558. [DOI] [PubMed] [Google Scholar]
- 163.Yeaman C., Grindstaff K.K., Wright J.R., Nelson W.J. Sec6/8 complexes on trans-Golgi network and plasma membrane regulate late stages of exocytosis in mammalian cells. J. Cell Biol. 2001;155:593–604. doi: 10.1083/jcb.200107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ponnambalam S., Baldwin S.A. Constitutive protein secretion from the trans-Golgi network to the plasma membrane. Mol. Membr. Biol. 2003;20:129–139. doi: 10.1080/0968768031000084172. [DOI] [PubMed] [Google Scholar]
- 165.Liu J., Zuo X., Yue P., Guo W., Phosphatidylinositol 4. 5-bisphosphate mediates the targeting of the exocyst to the plasma membrane for exocytosis in mammalian cells. Mol. Biol. Cell. 2007;18:4483–4492. doi: 10.1091/mbc.e07-05-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rivera-Molina F., Toomre D. Live-cell imaging of exocyst links its spatiotemporal dynamics to various stages of vesicle fusion. J. Cell Biol. 2013;201:673–680. doi: 10.1083/jcb.201212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Südhof T.C., Rothman J.E. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gordon D.E., Bond L.M., Sahlender D.A., Peden A.A. A targeted siRNA screen to identify SNAREs required for constitutive secretion in mammalian cells. Traffic. 2010;11:1191–1204. doi: 10.1111/j.1600-0854.2010.01087.x. [DOI] [PubMed] [Google Scholar]
- 169.Mastrodonato V., Morelli E., Vaccari T. How to use a multipurpose SNARE: The emerging role of Snap29 in cellular health. Cell Stress Chaperones. 2018;2:72–81. doi: 10.15698/cst2018.04.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ampah K.K., Greaves J., Shun-Shion A.S., Asnawi A.W., Lidster J.A., Chamberlain L.H., Collins M.O., Peden A.A. S-acylation regulates the trafficking and stability of the unconventional Q-SNARE STX19. J. Cell Sci. 2018;131 doi: 10.1242/jcs.212498. [DOI] [PubMed] [Google Scholar]
- 171.Gordon D.E., Chia J., Jayawardena K., Antrobus R., Bard F., Peden A.A. VAMP3/Syb and YKT6 are required for the fusion of constitutive secretory carriers with the plasma membrane. PLoS Genet. 2017;13 doi: 10.1371/journal.pgen.1006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Godi A., Pertile P., Meyers R., Marra P., Di Tullio G., Iurisci C., Luini A., Corda D., De Matteis M.A. ARF mediates recruitment of PtdIns-4-OH kinase-beta and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat. Cell Biol. 1999;1:280–287. doi: 10.1038/12993. [DOI] [PubMed] [Google Scholar]
- 173.Chalupska D., Różycki B., Humpolickova J., Faltova L., Klima M., Boura E. Phosphatidylinositol 4-kinase IIIβ (PI4KB) forms highly flexible heterocomplexes that include ACBD3, 14-3-3, and Rab11 proteins. Sci. Rep. 2019;9:567. doi: 10.1038/s41598-018-37158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Burke J.E., Inglis A.J., Perisic O., Masson G.R., McLaughlin S.H., Rutaganira F., Shokat K.M., Williams R.L. Structures of PI4KIIIβ complexes show simultaneous recruitment of Rab11 and its effectors. Science. 2014;344:1035–1038. doi: 10.1126/science.1253397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.de Graaf P., Zwart W.T., van Dijken R.A.J., Deneka M., Schulz T.K.F., Geijsen N., Coffer P.J., Gadella B.M., Verkleij A.J., van der Sluijs P. P.M.P. van Bergen en Henegouwen, Phosphatidylinositol 4-kinasebeta is critical for functional association of rab11 with the Golgi complex. Mol. Biol. Cell. 2004;15:2038–2047. doi: 10.1091/mbc.e03-12-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chen W., Feng Y., Chen D., Wandinger-Ness A. Rab11 is required for trans-golgi network-to-plasma membrane transport and a preferential target for GDP dissociation inhibitor. Mol. Biol. Cell. 1998;9:3241–3257. doi: 10.1091/mbc.9.11.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nagashima S., Tábara L.-C., Tilokani L., Paupe V., Anand H., Pogson J.H., Zunino R., McBride H.M., Prudent J. Golgi-derived PI(4)P-containing vesicles drive late steps of mitochondrial division. Science. 2020;367:1366–1371. doi: 10.1126/science.aax6089. [DOI] [PubMed] [Google Scholar]
- 178.Dippold H.C., Ng M.M., Farber-Katz S.E., Lee S.-K., Kerr M.L., Peterman M.C., Sim R., Wiharto P.A., Galbraith K.A., Madhavarapu S., Fuchs G.J., Meerloo T., Farquhar M.G., Zhou H., Field S.J. GOLPH3 bridges phosphatidylinositol-4- phosphate and actomyosin to stretch and shape the Golgi to promote budding. Cell. 2009;139:337–351. doi: 10.1016/j.cell.2009.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rahajeng J., Kuna R.S., Makowski S.L., Tran T.T.T., Buschman M.D., Li S., Cheng N., Ng M.M., Field S.J. Efficient Golgi Forward Trafficking Requires GOLPH3-Driven, PI4P-Dependent Membrane Curvature. Dev. Cell. 2019;50:573–585. doi: 10.1016/j.devcel.2019.05.038. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rizzo R., Russo D., Kurokawa K., Sahu P., Lombardi B., Supino D., Zhukovsky M., Vocat A., Pothukuchi P., Kunnathully V., Capolupo L., Boncompain G., Vitagliano C., Marino F.Z., Aquino G., Henklein P., Mandrich L., Botti G., Clausen H., Mandel U., Yamaji T., Hanada K., Budillon A., Perez F., Parashuraman S., Hannun Y.A., Nakano A., D’Angelo G., Luini A. bioRxiv; 2019. Retrograde Transport of Golgi Enzymes by GOLPH3 Across Maturing Cisternae Regulates Glycan Assembly on Sphingolipids and Cell Growth. 870477. [DOI] [Google Scholar]
- 181.Tu L., Tai W.C.S., Chen L., Banfield D.K. Signal-mediated dynamic retention of glycosyltransferases in the Golgi. Science. 2008;321:404–407. doi: 10.1126/science.1159411. [DOI] [PubMed] [Google Scholar]
- 182.Schmitz K.R., Liu J., Li S., Setty T.G., Wood C.S., Burd C.G., Ferguson K.M. Golgi localization of glycosyltransferases requires a Vps74p oligomer. Dev. Cell. 2008;14:523–534. doi: 10.1016/j.devcel.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mesmin B., Kovacs D., D’Angelo G. Lipid exchange and signaling at ER-Golgi contact sites. Curr. Opin. Cell Biol. 2019;57:8–15. doi: 10.1016/j.ceb.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 184.Drin G. Topological regulation of lipid balance in cells. Annu. Rev. Biochem. 2014;83:51–77. doi: 10.1146/annurev-biochem-060713-035307. [DOI] [PubMed] [Google Scholar]
- 185.Drin G., Moser von Filseck J., Čopič A. New molecular mechanisms of inter-organelle lipid transport. Biochem. Soc. Trans. 2016;44:486–492. doi: 10.1042/BST20150265. [DOI] [PubMed] [Google Scholar]
- 186.Mesmin B., Bigay J., Polidori J., Jamecna D., Lacas-Gervais S., Antonny B. Sterol transfer, PI4P consumption, and control of membrane lipid order by endogenous OSBP. EMBO J. 2017;36:3156–3174. doi: 10.15252/embj.201796687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hanada K., Kumagai K., Yasuda S., Miura Y., Kawano M., Fukasawa M., Nishijima M. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 2003;426:803–809. doi: 10.1038/nature02188. [DOI] [PubMed] [Google Scholar]
- 188.Proszynski T.J., Klemm R.W., Gravert M., Hsu P.P., Gloor Y., Wagner J., Kozak K., Grabner H., Walzer K., Bagnat M., Simons K., Walch-Solimena C. A genome-wide visual screen reveals a role for sphingolipids and ergosterol in cell surface delivery in yeast. Proc. Natl. Acad. Sci. U. S. A. 2005;102:17981–17986. doi: 10.1073/pnas.0509107102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Peretti D., Dahan N., Shimoni E., Hirschberg K., Lev S. Coordinated lipid transfer between the endoplasmic reticulum and the Golgi complex requires the VAP proteins and is essential for Golgi-mediated transport. Mol. Biol. Cell. 2008;19:3871–3884. doi: 10.1091/mbc.e08-05-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Litvak V., Dahan N., Ramachandran S., Sabanay H., Lev S. Maintenance of the diacylglycerol level in the Golgi apparatus by the Nir2 protein is critical for Golgi secretory function. Nat. Cell Biol. 2005;7:225–234. doi: 10.1038/ncb1221. [DOI] [PubMed] [Google Scholar]
- 191.Xie Z., Hur S.K., Zhao L., Abrams C.S., Bankaitis V.A. A Golgi Lipid Signaling Pathway Controls Apical Golgi Distribution and Cell Polarity during Neurogenesis. Dev. Cell. 2018;44:725–740. doi: 10.1016/j.devcel.2018.02.025. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Walch-Solimena C., Novick P. The yeast phosphatidylinositol-4-OH kinase pik1 regulates secretion at the Golgi. Nat. Cell Biol. 1999;1:523–525. doi: 10.1038/70319. [DOI] [PubMed] [Google Scholar]
- 193.Mizuno-Yamasaki E., Medkova M., Coleman J., Novick P. Phosphatidylinositol 4-phosphate controls both membrane recruitment and a regulatory switch of the Rab GEF Sec2p. Dev. Cell. 2010;18:828–840. doi: 10.1016/j.devcel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ortiz D., Medkova M., Walch-Solimena C., Novick P. Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. J. Cell Biol. 2002;157:1005–1015. doi: 10.1083/jcb.200201003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Guo W., Roth D., Walch-Solimena C., Novick P. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 1999;18:1071–1080. doi: 10.1093/emboj/18.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Donovan K.W., Bretscher A. Tracking individual secretory vesicles during exocytosis reveals an ordered and regulated process. J. Cell Biol. 2015;210:181–189. doi: 10.1083/jcb.201501118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lipatova Z., Tokarev A.A., Jin Y., Mulholland J., Weisman L.S., Segev N. Direct interaction between a myosin V motor and the Rab GTPases Ypt31/32 is required for polarized secretion. Mol. Biol. Cell. 2008;19:4177–4187. doi: 10.1091/mbc.e08-02-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Santiago-Tirado F.H., Legesse-Miller A., Schott D., Bretscher A. PI4P and Rab inputs collaborate in myosin-V-dependent transport of secretory compartments in yeast. Dev. Cell. 2011;20:47–59. doi: 10.1016/j.devcel.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jin Y., Sultana A., Gandhi P., Franklin E., Hamamoto S., Khan A.R., Munson M., Schekman R., Weisman L.S. Myosin V transports secretory vesicles via a Rab GTPase cascade and interaction with the exocyst complex. Dev. Cell. 2011;21:1156–1170. doi: 10.1016/j.devcel.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Medkova M., France Y.E., Coleman J., Novick P. The rab exchange factor Sec2p reversibly associates with the exocyst. Mol. Biol. Cell. 2006;17:2757–2769. doi: 10.1091/mbc.e05-10-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ling Y., Hayano S., Novick P. Osh4p is needed to reduce the level of phosphatidylinositol-4-phosphate on secretory vesicles as they mature. Mol. Biol. Cell. 2014;25:3389–3400. doi: 10.1091/mbc.E14-06-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Stalder D., Mizuno-Yamasaki E., Ghassemian M., Novick P.J. Phosphorylation of the Rab exchange factor Sec2p directs a switch in regulatory binding partners. Proc. Natl. Acad. Sci. U. S. A. 2013;110:19995–20002. doi: 10.1073/pnas.1320029110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Stalder D., Novick P.J. The casein kinases Yck1p and Yck2p act in the secretory pathway, in part, by regulating the Rab exchange factor Sec2p. Mol. Biol. Cell. 2016;27:686–701. doi: 10.1091/mbc.E15-09-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Knödler A., Feng S., Zhang J., Zhang X., Das A., Peränen J., Guo W. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc. Natl. Acad. Sci. U. S. A. 2010;107:6346–6351. doi: 10.1073/pnas.1002401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chiba S., Amagai Y., Homma Y., Fukuda M., Mizuno K. NDR2-mediated Rabin8 phosphorylation is crucial for ciliogenesis by switching binding specificity from phosphatidylserine to Sec15. EMBO J. 2013;32:874–885. doi: 10.1038/emboj.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Shin J.J.H., Liu P., Chan L.J., Ullah A., Pan J., Borchers C.H., Burke J.E., Stefan C., Smits G.J., Loewen C.J.R. pH Biosensing by PI4P Regulates Cargo Sorting at the TGN. Dev. Cell. 2020;52(4):461–476. doi: 10.1016/j.devcel.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 207.Larocque G., La-Borde P.J., Clarke N.I., Carter N.J., Royle S.J. Tumor protein D54 defines a new class of intracellular transport vesicles. J. Cell Biol. 2020;219 doi: 10.1083/jcb.201812044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Di Martino R., Sticco L., Luini A. Regulation of cargo export and sorting at the trans-Golgi network. FEBS Lett. 2019;593:2306–2318. doi: 10.1002/1873-3468.13572. [DOI] [PubMed] [Google Scholar]
- 209.Buxbaum J.N. Transthyretin and the Transthyretin Amyloidoses. In: Uversky V.N., Fink A.L., editors. Protein Misfolding, Aggregation, and Conformational Diseases: Part B: Molecular Mechanisms of Conformational Diseases. Springer US; Boston, MA: 2007. pp. 259–283. [DOI] [Google Scholar]
- 210.Kelly J.W. The alternative conformations of amyloidogenic proteins and their multi-step assembly pathways. Curr. Opin. Struct. Biol. 1998;8:101–106. doi: 10.1016/s0959-440x(98)80016-x. [DOI] [PubMed] [Google Scholar]
- 211.Grunenberg A., Buske C. Monoclonal IgM Gammopathy and Waldenström’s Macroglobulinemia. Dtsch. Arztebl. Int. 2017;114:745–751. doi: 10.3238/arztebl.2017.0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Gertz M.A., Kyle R.A. Amyloidosis with IgM monoclonal gammopathies. Semin. Oncol. 2003;30:325–328. doi: 10.1053/sonc.2003.50060. [DOI] [PubMed] [Google Scholar]
- 213.Paladino S., Lebreton S., Zurzolo C. Chapter Eight - Trafficking and Membrane Organization of GPI-Anchored Proteins in Health and Diseases. In: Kenworthy A.K., editor. Current Topics in Membranes. Academic Press; 2015. pp. 269–303. [DOI] [PubMed] [Google Scholar]
- 214.Cao X., Surma M.A., Simons K. Polarized sorting and trafficking in epithelial cells. Cell Res. 2012;22:793–805. doi: 10.1038/cr.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Rodriguez-Boulan E., Kreitzer G., Müsch A. Organization of vesicular trafficking in epithelia. Nat. Rev. Mol. Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- 216.Guo Y., Sirkis D.W., Schekman R. Protein sorting at the trans-Golgi network. Annu. Rev. Cell Dev. Biol. 2014;30:169–206. doi: 10.1146/annurev-cellbio-100913-013012. [DOI] [PubMed] [Google Scholar]
- 217.Vieira O.V., Gaus K., Verkade P., Fullekrug J., Vaz W.L.C., Simons K. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proc. Natl. Acad. Sci. U. S. A. 2006;103:18556–18561. doi: 10.1073/pnas.0608291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Tuma P.L., Nyasae L.K., Hubbard A.L. Nonpolarized cells selectively sort apical proteins from cell surface to a novel compartment, but lack apical retention mechanisms. Mol. Biol. Cell. 2002;13:3400–3415. doi: 10.1091/mbc.02-04-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.