Abstract
Preeclampsia (PE) is new onset hypertension during pregnancy associated with increased uterine artery resistance (UARI) and an imbalance among CD4+ T lymphocytes and natural killer (NK) cells. We have shown an important role for 17-hydroxyprogesterone caproate (17-OHPC) to improve hypertension and fetal demise in the RUPP rat model of PE. However we have not examined a role for 17-OHPC to improve NK cells and CD4+TH2 cells as possible mechanisms for improved fetal weight and hypertension. Therefore, we hypothesized that 17-OHPC lowers NK cells while improving the T cell ratio in the RUPP rat. RUPP was surgically induced on gestational day 14 in pregnant rats. 17-OHPC (3.32mg/kg) was administered intraperitoneal on day 15, UARI was measured on day 18. Blood pressure (MAP), blood and tissues were collected on GD 19. MAP in NP rats (n=9) was 100 ± 2, 104±6 in Sham rats (n=8), 128 ± 2 in RUPP (n=11) and 115±3 mmHg in RUPP+17-OHPC (n=10), p <0.05. Pup weight and UARI were improved after 17-OHPC. Total and cytolytic placental NK cells were 38 ±5, and 12 ±2% gate in RUPP rats which decreased to 1.6 ± 0.5 and 0.4 ± 0.2 % gate in RUPP+17OHPC rats. CD4+ T cells were 40±3 in RUPP rats, which significantly decreased to 7±1 RUPP+17-OHPC rats. Circulating and placental TH2 cells were 6.0 ± 1, 0.3±0.1% gate in RUPP rats and 12±1 %, 2±0.5% gate in RUPP+17-OHPC rats, p <0.05 This study identifies new mechanisms whereby 17-OHPC improves outcomes in response to placental ischemia.
Keywords: Preeclampsia, natural killer cells, 17-hydroxyprogesterone caproate, progesterone
Introduction
Preeclampsia (PE) is a commonly encountered, pregnancy specific multisystem hypertensive disorder and a major contributor to maternal-fetal and perinatal morbidity and mortality. Complicating approximately 10% of pregnancies, PE is a leading cause of iatrogenic preterm births and is responsible for around 60,000 pregnancy-related deaths per year worldwide.(1–7) Traditionally it is associated with new onset hypertension and proteinuria. Recent changes to the diagnostic criteria have also made it possible to diagnose preeclampsia in the absence of proteinuria. (1) Other hallmark characteristics of PE include chronic immune activation, uterine artery resistance, fetal growth restriction (IUGR), increased inflammatory cytokines, decreased vasodilators such as nitric oxide (NO), maternal endothelial dysfunction, and other systemic disturbances. (8–14) Currently, the only known definitive cure for preeclampsia is delivery. This makes any intervention that can positively alter the pathophysiology of preeclampsia and ameliorate preterm delivery highly desirable.
Along with other sex hormones, progesterone is of paramount importance in the maintenance and well-being of pregnancy. In addition to its anti-inflammatory properties, prior studies have shown vasodilatory effects. (15–17) Kiprono, et al. showed that patients with severe PE have significantly lower serum progesterone concentrations when compared to gestational age and race-matched non-PE control pregnant women. (28) More clinically applicable data from our laboratory demonstrate that 17-hydroxyprogesterone caproate (17-OHPC), which is a synthetic hormone commonly used as early as 16 weeks gestation for prevention of preterm delivery in pregnancies not complicated by PE, (18–21) significantly lowers blood pressure and proinflammatory cytokines. (22) More recent studies by Amaral, et al. have shown that supplementation of 17-OHPC in the RUPP model increased NO bioavailability while decreasing sFlt-1, an antiangiogenic factor notoriously associated with intrauterine growth restriction (IUGR) and PE. (9) The utilization of 17-OHPC in patients at risk of preterm PE or for those developing preterm PE has been debated, and the benefit of 17-OHPC to reduce placental ischemia is still unclear. (18, 23)
Evidence for a shift towards proinflammatory CD4+TH1 cells and away from CD4+Treg and CD4+TH2 of normal pregnancy exists in PE.(24–27) Similarly, there are greater numbers of cytolytic NK when compared to uterine NK cells during PE.(28, 29) Cytolytic NK cells destroy unwanted cells by releasing lysis proteins such as perforin and granzymes causing apoptosis. During normal pregnancies, TH2, T regs and the uterine NK cells, a non cytolytic cell, predominate in the uterus and facilitate cytotrophoblast invasion and proliferation.(30)However, in pathological pregnancies such as PE and miscarriage, there are less TH2, T regs and uterine NK cells and more effector TH 1, TH17 cells and cytolytic NK cells within the circulation and placentas. (29, 31–33) The importance of this paradigm has been established through a series of papers by our lab demonstrating the importance of NK cells, T regs TH 1, TH17 cells to cause hypertension during in response to placental ischemia.(34–36)
Clinical studies have observed that preeclamptic woman when compared to pregnant controls manifest an alteration in the population of NK cells that may be influenced by progesterone levels.(28, 29, 37) Progesterone exerts some influence on NK cell differentiation and activity. Peripherally, progesterone decreases NK cell number, activation, and cytotoxicity either directly or indirectly.(38) In the endometrium, uterine NK cell proliferation and differentiation are influenced by progesterone with impact on placental and trophoblast development. (38–41) This altered population of NK cells may be a culprit of some of the adverse outcomes associated with preeclampsia such as decreased fetal weight and elevated blood pressure. Therefore in this study we sought to determine a role for 17-OHPC to reduce cytolytic NK cells while improving TH2 cells, hypertension and fetal weight in response to placental ischemia in RUPP rats, thereby illustrating yet another mechanism whereby 17-OHPC could prove beneficial in managing PE in the clinical setting.
2. Materials and Methods
Pregnant Sprague-Dawley rats purchased from Envigo (Indianapolis, IN) were used in this study. Animals were housed in a temperature-controlled room (23°C) with a 12:12-h light-dark cycle with free access to standard rat chow and water. All experimental procedures executed in this study were in accordance with the National Institutes of Health guidelines for use and care of animals. All protocols were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center.
2.1. Reduction in Uterine Perfusion Pressure
Surgical procedures were carried out under appropriate anesthesia and analgesics were given post-operatively as needed. Pregnant rats at gestational day 14 were exposed to 2.0% isoflurane in a humidified 100% oxygen carrier gas chamber. Rat dams weighting approximately 200–250g were randomly assigned to either RUPP or normal pregnant (NP) control groups. Via a vertical midline incision RUPP surgery was performed on a subset of normal pregnant rates. A constrictive silver clip (0.203 mm) was placed on the aorta superior to the iliac bifurcation, while ovarian collateral circulation to the uterus was reduced with restrictive clips (0.100 mm) to the bilateral uterine arcades at the ovarian end. Rats were excluded from the study when the clipping procedure resulted in total reabsorption of all fetuses. (42, 43) Sham surgery was performed in the same manner as the RUPP surgery without the placement of clips. NP rats did not undergo any anesthetized surgical procedure prior to gestation day 18. Animals were administered carprofen (5mg/kg) for 2 days to control post-surgical pain. Rats were excluded from the study when the clipping procedure resulted in total reabsorption of all fetuses.
2.2. Administration of 17-hydroxyprogesterone caproate
A subset of RUPP rats were injected with 17-hydroxyprogesterone caproate (17-OHPC) diluted in sterile normal saline at day 15 of gestation. The 17-OHPC (Marty’s Compounding Pharmacy, Jackson, MS) was diluted in normal saline and administered intraperitoneally as 0.5 cm3 solution of 3.32 mg/kg 17-OHPC to pregnant rats. (44) We have previously shown that 17-OHPC had no effect on NP rats and therefore 17-OHPC was not administered to NP rats in this study.(45) There were no differences between NP and SHAM operated controls therefore 17-OHPC was not administered to this group.
2.3. Measurement of Uterine Artery Resistance Index (UARI)
Power Doppler velocimetry measurements of the uterine artery were performed at an imaging station with a Vevo 770 unit (Visual sonics) using a 30 Hz transducer and an insonating angle <30°. The peak systolic flow velocity (PSV) and end diastolic flow velocity (EDV) were recorded using the uterine artery Doppler waveform and the index was calculated using the following formula: UARI= (PSV-EDV)/PSV. (46) There were no differences between NP and SHAM operated controls therefore UARI was not analyzed to this group.
2.4. Measurement of Mean Arterial Pressure
On day 18 of gestation, using isoflurane anesthesia carotid arterial catheters were inserted for blood pressure measurements. The catheters inserted were V3 tubing (Scientific Commodities, Inc., Lake Havasu City, AZ), which is tunneled to the back of the neck and exteriorized. On day 19 of gestation, arterial blood pressure was analyzed after placing the rats in individual restraining cages. Arterial pressure was monitored with a pressure transducer (Cobe III tranducer CDX Sema) and recorded continuously for 45 min after a 30-min stabilization period. Subsequently, blood and urine samples were collected; placentas were harvested; and pup weights were obtained. There were no differences between NP and NP+17-OHPC as published previously.(45) Therefore 17-OHPC was not administered to this group.
2.5. Determination of placental NK cell populations using Flow Cytometry
The placental populations of NK cells isolated on day 19 of gestation from all groups were quantified by flow cytometry. At the time of harvest, placentas were collected and placental lymphocytes were isolated by centrifugation on a cushion of Ficoll-Hypaque (Lymphoprep, Accurate Chemical & Scientific Corp., Westbury, NY) according to the instructions of the manufacturer. For flow cytometric analysis, 1 X 106 cells were incubated for 10 minutes at 4 °C with antibodies against rat CD4 and Anti-Natural Killer Cell Activation Structures (ANK61) or rat Anti-Natural Killer Cell antibody (ANK44) (AbCam, Cambridge, MA). ANK61 binds to the killer cell activation structure that is expressed on all NK cells, while ANK44 is only expressed on stimulated, cytotoxic NK cells. (47) After washing, cells were labeled with secondary Fluorescein isothiocyanate (FITC; AbCam) antibody for 10 minutes at 4 °C. As a negative control for each individual rat, cells were treated exactly as described above except they were incubated with isotype controls antibodies conjugated to FITC alone. Subsequently, cells were washed, fixed, and resuspended in 500 μL of Rosswell Park Memorial Institute medium (RPMI) and analyzed for single staining a MACSQuantify Flow Cytometer (Miltenyi Biotec, San Diego, CA)Lymphocytes were gated in the forward and side scatter plot. Cells that stained as ANK61+ were designated as NK cells. Cells that stain as ANK44+ were designated as activated NK cells. The percent of positive stained cells above the negative control was collected for individual rats and the mean values for each experimental group were calculated.(48)
2.6. Determination of CD4+ T cells and TH2 cells using Flow Cytometry
The circulating and placental populations of CD4+T cells and TH2 cells were quantified by flow cytometry from peripheral blood mononuclear cells (PBMCs) and placental immune cells isolated on day 19 of gestation from all groups. PBMCs and placental lymphocytes were isolated by centrifugation on a cushion of Ficoll-Hypaque (Lymphoprep, Accurate Chemical & Scientific Corp., Westbury, NY) according to the instructions of the manufacturer. For flow cytometric analysis, 1 X 106 cells were incubated for 10 minutes at 4 °C with antibodies against rat CD4 (BD Biosciences, San Jose, CA). After washing, cells were labeled with secondary Fluorescein isothiocyanate (FITC; BD Biosciences, San Jose, CA), phycoerythrin (PE- BD Biosciences, San Jose, CA) and Alexa647 (BD Biosciences, San Jose, CA) antibody for 10 minutes at 4 °C. Cells were washed, permeabilized, and stained with anti-rat IL-4 conjugated to PE and anti-mouse GATA-3 conjugated to Alexa Fluor (BD Biosciences, San Jose, CA) for 10 minutes at 4°C. As a negative control for each individual rat, cells were treated exactly as described above except they were incubated with anti-FITC, anti-PE, and anti-Alexa secondary antibodies alone. Subsequently, cells were washed and resuspended in 500 μL FACS Buffer analyzed for single and double staining on a MACSQuantify Flow Cytometer (Miltenyi Biotec, San Diego, CA). The percent of positive stained cells in the gated lymphocyte population were collected for individual rats and the mean values for each experimental group were calculated.
2.7. Determination of Perforin and Granzyme B levels
Placental protein was isolated from all pregnant rats to measure perforin and granzyme B levels using ELISA assays from MyBiosource (City, State). The minimal detectable value for Perforin was 31.2 pg/mL, with maximum being 1000 pg/mL with an intra-assay/inter-assay precision of < 15%. The minimal detectable value for Granzyme B was 15 pg/mL, with maximum being 1000 pg/mL with an intra-assay/inter-assay precision of <% 8 and <%, 10 respectively.
Statistical Analysis
All of the data are expressed as mean ± SEM. Comparisons of controls (NP and SHAMS) with experimental groups (RUPPS and RUPPS+ 17-OHPC) analyzed by Student’s t test (2 groups, figures 5C and 5D) or one-way ANOVA with Bonferroni multiple comparisons test as post hoc analysis (>2 groups, Figures 1,2, 3, 4, 5A and 5B ). A value of p < 0.05 was considered statistically significant.
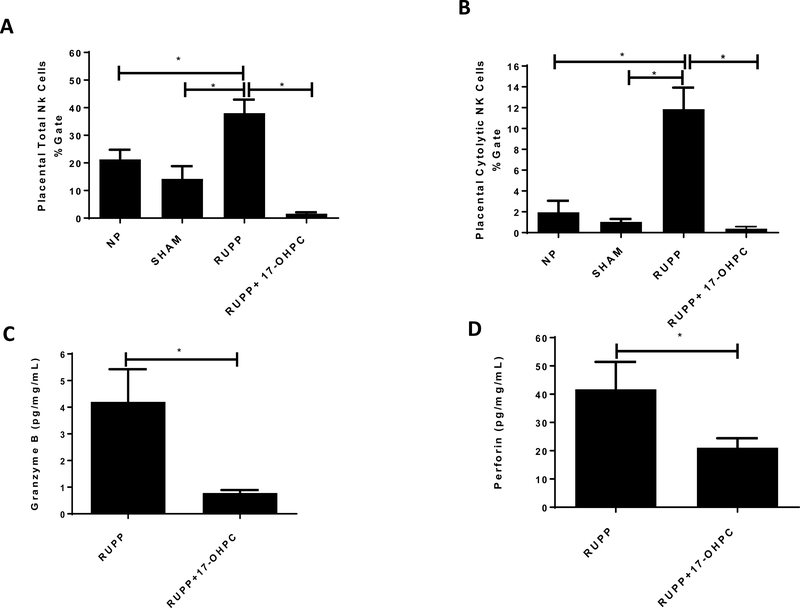
Figure 5.
Early administration of 17-OHPC improves total and cytolytic placental NK cells (A and B) and placental perforin, grazyme B (C and D) levels in RUPP rats. Data are shown as means ± S.E.M (C and D, n=5–6/group). One-way ANOVA with Bonferroni multiple comparisons test as post hoc analysis was performed to generate p values (Figures 5A and B, *p <0.05). Student’s t test was performed to generate p values (, Figures 5C and D, *p <0.05).
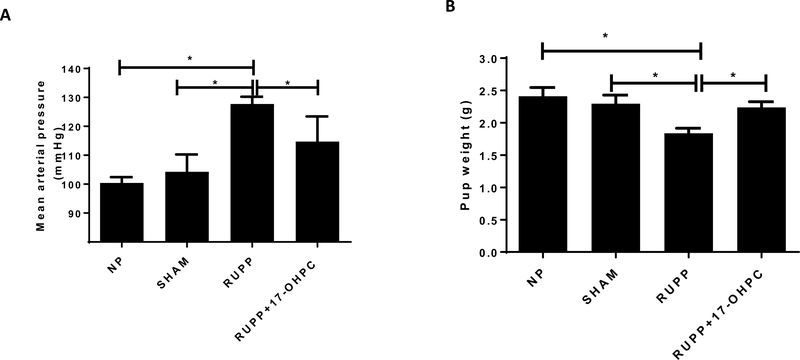
Figure 1.
Administration of 17-OHPC blunts hypertension (A) and improves pup weight (B) in RUPP rats. Data are shown as means ± S.E.M. (n=8–11/group). One-way ANOVA with Bonferroni multiple comparisons test as post hoc analysis was performed to generate p values (*p <0.05).
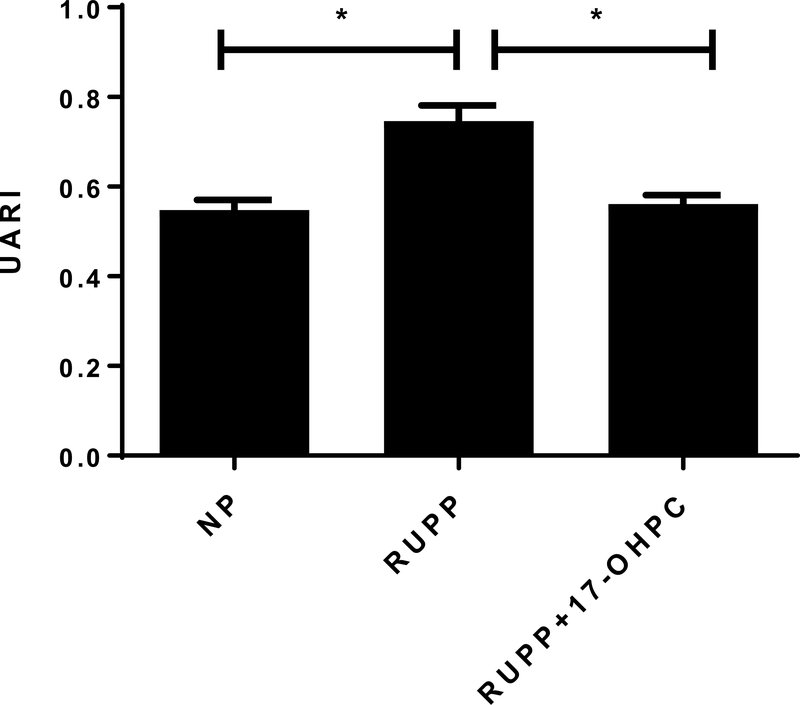
Figure 2.
Early administration of 17-OHPC lowers Uterine Arterial Resistance (B, n=4–7/group) in RUPP rats. Data are shown as means ± S.E.M. One-way ANOVA with Bonferroni multiple comparisons test as post hoc analysis was performed to generate p values (*p <0.05).
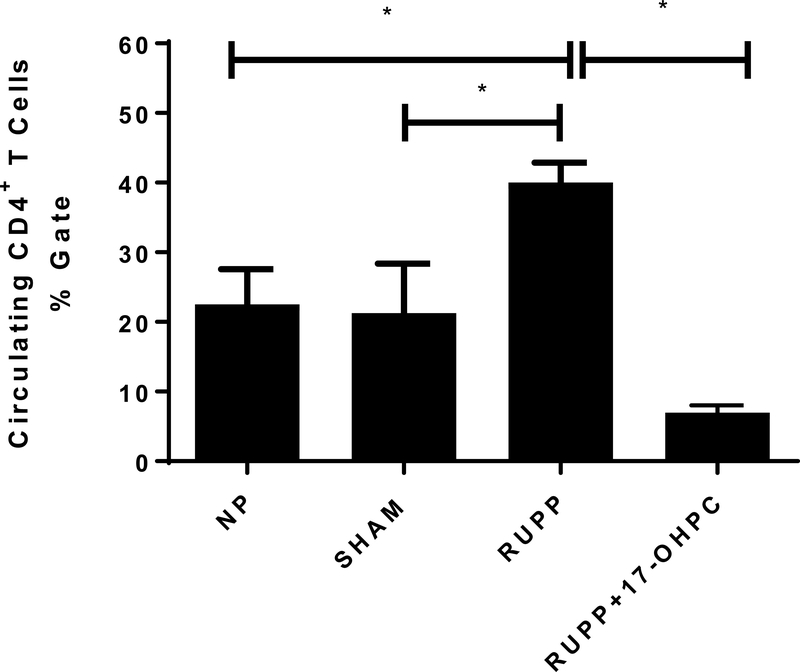
Figure 3.
Early administration of 17-OHPC decreases CD4+ T Cells. One-way ANOVA with Bonferroni multiple comparisons test as post hoc analysis was performed to generate p values (*p <0.05).
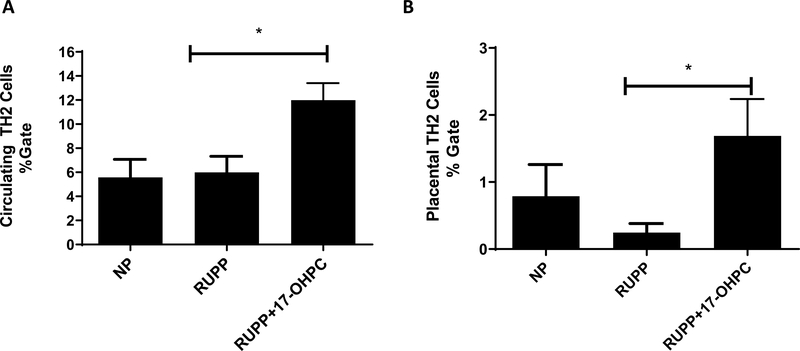
Figure 4.
Early administration of 17-OHPC increases circulating and placental TH2 cells in RUPP rats. Student’s t test was performed to generate p values (*p <0.05).
3. RESULTS
Administration of 17-OHPC blunted hypertension in RUPP rats
Mean arterial pressure (MAP) was not different between NP and SHAM operated controls. MAP was elevated in response to placental ischemia in RUPP rats and it was significantly increased compared to either NP rats or SHAM operated controls (p<0.05). Administration of 17-OHPC attenuated hypertension in response to placental ischemia. MAP in NP rats (n=9) was 100 ± 2, 104±6 in Sham rats (n=8), 128 ± 2 in RUPP (n=11) and 115±3 mmHg in RUPP+17-OHPC (n=10, p<0.05, Figure 1A). We have recently published that 17-OHPC given to NP rats did not show any difference in pregnancy outcomes when compared with untreated NP rats. (45)
Administration of 17-OHPC improved Pup Weight in RUPP rats
Pup weight from RUPP rats (1.9 ± 0.2 g, n=11) was significantly lower than that of NP or SHAM rats (2.4±0.1 g, n=9, 2.3±0.1, n=8, p<0.05). Importantly, 17-OHPC supplementation of RUPP rats improved pup weight to 2.3 ± 0.1g (n=10, p<0.05, Figure 1B) compared to RUPP rats.
Administration of 17-OHPC reduced Uterine Artery Resistance Index (UARI) in RUPP rats
UARI was increased in response to placental ischemia during pregnancy and improved with administration of 17-OHPC. UARI was 0.55±0.02 in NP (n=7), 0.74±0.03 in RUPP rats (n=6), which improved to 0.60±0.02 in RUPP+17-OHPC (n=4, p<0.05, Figure 2).
Administration of 17-OHPC lowered circulating CD4+ T cells in RUPP rats
CD4+ T cells were not different between NP and SHAM operated controls. CD4+T cells were increased in RUPP rats compared to NP or SHAM controls and were reduced after 17-OHPC administration to RUPP rats. CD4+ T cells were 22±5 in NP rats (n=9), 22±7 in Sham rats (n=5), which were elevated to 40±3 in RUPP rats (n=7) and were significantly decreased to 7±1 RUPP+17-OHPC rats (p<0.05, n=3, Figure 3).
Administration of 17-OHPC increased the circulating number of TH2 cells in RUPP rats
We have recently shown that circulating and placental TH2 are decreased in RUPPs compared to NP rats (49). Circulating TH2 cells were 16 ±4 in NP (n=5), 6.0 ± 1% in RUPP rats (n=5), which increased to 11±1 % gate in RUPP+17-OHPC (n=4, p<0.05, Figure 4A).Placental TH2 cells were 1±0.5 in NP, 0.3±0.1 in RUPP rats and 2±0.5 in RUPP+17-OHPC (n=4, p<0.05 Figure 4B).
Administration of 17-OHPC decreased the total number of placental and cytolytic NK cells in RUPP rats
Placental NK cells were not different between NP and SHAM operated controls. Total Placental NK Cells (ANK61) were 21 ± 3% gate in NP rats (n=9) and 14± 5 % gated in Sham rats. Total placental NK cells increased to 38 ± 5% gate in RUPP rats (n=12) but was significantly reduced to 1.61 ± 0.54% gated (n=5) in RUPP + 17- OHPC rats (p<0.05, Figure 5A). In addition placental cytolytic NK cells (ANK44) were 2 ± 1% gate in NP and 1±0.2 in Sham rats which increased to 12 + 2 % gate in RUPP rats (n=6). 17 OHPC significantly reduced cytolytic NK cells to 0.32 ± 0.20% gate (n=5) in RUPP + 17- OHPC (p<0.05, figure 5B)
Administration of 17-OHPC decreased Perforin-Granzyme B in RUPP placentas
Granzyme B levels were reduced in RUPP+17-OHPC to 0.8 ± 0.1 pg/mg/mL (n=6) compared to 4.2 ± 1.2pg/mg/mL (n=4) in RUPP rats (p<0.05, Figure 5C). Perforin levels was decreased in RUPP+17-OHPC to 21.9 ± 3 pg/mg/mL (n=6, p<0.05, Figure 5D) compared to 41.8 ± 10 pg/mg/mL (n=4) in RUPP rats.
4. Discussion
Many factors play a pivotal role in the maintenance of a successful and healthy pregnancy. Disruption of this harmony can lead to many outcomes. Growing evidence indicates that altered immune mechanisms play an important role in the pathophysiology of PE and result in a state of chronic inflammation. Increased CD4+ T cells, inflammatory mediators such as TNF-α, IL-6, and decreased regulatory mechanisms such as regulatory T cells (Tregs) and IL-10 have been noted in PE. (17, 31, 50–52) These physiologic alterations from normal results in the chronic inflammatory state that characteristic of PE. (8, 10, 53, 54)
Over the years our laboratory has performed a series of studies demonstrating the importance of the alteration in these immune factors in causing the symptoms of PE in the RUPP rat model of PE. The importance of the T cell paradigm has been established through a series of such papers by our lab demonstrating the importance of T regs,TH 1,TH17 cells.(34–36) Wallace et al. demonstrated that adoptive transfer of CD4+ T cells from placental ischemic RUPP rat model of PE elicits many characteristics of PE in the normal pregnant rat.(34) More recent data illustrates the importance of TH17 cells to cause fetal demise and hypertension during pregnancy.(35) Moreover, Cornelius, et al. published that adoptive transfer of TRegs from NP rats into RUPP rats decreased blood pressure and vasoactive factors.(36) Importantly, we recently published that NK cell depletion proves beneficial to lowering blood pressure and improving fetal weight in RUPP pregnant rats. (48) Although adoptive transfer and other mechanisms of direct cellular manipulations among various animal models will lend insight into mechanisms and pathways that could be important to manage hypertension during pregnancy, such manipulations cannot be performed in the clinical setting.
Importantly, in the current study, we found that 17-OHPC given in early gestation decreases both total and cytolytic placental NK cells and the placental perforin grazyme B levels in response to placental ischemia. In addition, administration of 17-OHPC lowered circulating CD4+ T cells in RUPP rats while improving both circulating and placental TH2 cells. Importantly, 17-OHPC improves hypertension and fetal weight in response to placental ischemia. Although still in its infancy, 17-OHPC and other therapies that target cytolytic NK cells could eventually be viable treatment options for patients with or at risk for developing PE.
An increase in the cytolytic NK cell population in patients with PE versus healthy control patients has been demonstrated in prior clinical research. (28, 29). In the presence of cell stress and disease, cytolytic NK cells activate pathways that initiate a chain of cellular events which results in the secretion of the pore-forming molecule perforin and the “lytic granules” granzymes A and B which form an interface between the NK cell and the target cell. (55–57) Moreover NK cells are increased in patients suffering from miscarriage .(58) This leads us to believe that this association between the increase in the cytolytic NK cells with the pathophysiology and adverse outcomes seen in PE and miscarriage may be in part due to the increase in cytolytic NK cells.
17-OHPC is safely and widely used to lessen the risk of recurrent preterm labor. (18) It is believed that this agent has both anti-inflammatory and vasodilatory properties. It is well known that progesterone has great importance for pregnancy well-being. Its potential utility for the amelioration or prevention of PE is unclear despite prior studies showing lower serum progesterone levels in patients with PE compared to normal pregnant women. (15–17, 44)
A complete understanding of the exact mechanism of action of 17-OHPC remains elusive. However, it is believed to interact with the progesterone receptors to facilitate an increase in nitric oxide production and thereby promote uterine relaxation. Prior data showed that 17-OHPC administration on day 18 of gestation (late gestation) in the RUPP rat model improved hypertension in RUPP rats which was associated with improved inflammation by attenuating CD4+ T cells and other pro-inflammatory cytokines. Improvements were also noted in renal and placental ET-1, UARI, litter size, vascular eNOS expression and NO bioavailability. (22, 44, 59) Due to lack of changes in fetal weight we began a series of studies administering 17OHPC at an earlier time frame, gestation day 15, to RUPP rats. Consistent with prior work reported in the literature, we were able to demonstrate improvements in blood pressure and inflammation with administration of 17-OHPC in early gestation (day 15) in response to placental ischemia in the RUPP rats. (17, 22, 60) Reduction in fetal weight significantly improve when 17-OHPC is administered on day 15 which was associated with improved UARI. This current study recapitulates these findings in that, importantly, hypertension, fetal weight and UARI are all improved in RUPP rats when 17 OHPC is administered on day 15 of pregnancy.
As mentioned above, the NK cell population is altered in the RUPP rats. Progesterone is thought to exert a vital role in the control of differentiation, migration from the periphery and recruitment of NK cells. While the exact mechanism by which this occurs is still an area of ongoing research and debate, it is thought to act either by direct or indirect mechanisms. In the periphery, progesterone binds directly to NK cells via progesterone receptors and decreases cell number, activation, and cytotoxicity (38) whereas indirectly it promotes release of TH2 cytokines and progesterone-induced blocking factor by T cells which in turn decreases NK cell cytolytic activity. In the endometrium progesterone promotes uterine NK cell proliferation and differentiation, thus supporting placental and trophoblast development.(38–41, 61)
The Cochrane Collaboration review described that there is limited data to support the hypothesis that either progesterone or 17-OHPC may be used for preventing PE .(62) Previous clinical trials using progestogens for PE prevention were inconclusive regarding the role of progesterone in this pregnancy disorder. (63)Moreover, most recent published data from the PROLONG study have concluded that 17-OHPC did not decrease recurrent preterm label and/or increase fetal death.(64) To date, further studies are needed to investigate the role of 17-OHPC for preterm delivery on risks for PE.
Importantly, our data demonstrate that 17-OHPC increases circulating and placental TH2 cells which are reduced in both PE patients and in the RUPP rat. Moreover we demonstrate significant improvements in NK cells when 17 OHPC is administered. Collectively these results suggests that 17-OHPC exerts direct binding effects and indirect signaling to significantly suppress inflammation that occurs in response to placental ischemia during pregnancy. This reduced inflammation is strongly associated with improved UARI, fetal weight and hypertension, the three defining clinical characteristic indicative of preeclampsia. Therefore this study reiterates the positive effects 17 OHPC could have on pregnancies stricken with high levels of NK cells, placental ischemia and IUGR and that it should be further considered for addition to the clinical management of this disease in order to improve the pregnancy outcomes for both mothers and babies
Acknowledgments
Funding:
This work was also supported by UMMC Office of Research and National Institutes of Health grants R01HD067541 HL105324, HL124715, HL78147, HL51971 and MS CEPR P20GM121, AHA 19CDA34670055. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.American College of O, Gynecologists, Task Force on Hypertension in P. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–31. [DOI] [PubMed] [Google Scholar]
- 2.Creasy RK, Resnik R, Greene MF, Iams JD, Lockwood CJ. Creasy and Resnik’s maternal-fetal medicine : principles and practice. Available from: http://www.clinicalkey.com/dura/browse/bookChapter/3-s2.0-C20110040644.
- 3.World Health Organization. The world health report : report of the Director-General. Geneva: World Health Organization; 1995. p. volumes. [Google Scholar]
- 4.Duley L. Maternal mortality associated with hypertensive disorders of pregnancy in Africa, Asia, Latin America and the Caribbean. Br J Obstet Gynaecol. 1992;99(7):547–53. [DOI] [PubMed] [Google Scholar]
- 5.Committee on Obstetric P. Committee Opinion No. 623: Emergent therapy for acute-onset, severe hypertension during pregnancy and the postpartum period. Obstet Gynecol. 2015;125(2):521–5. [DOI] [PubMed] [Google Scholar]
- 6.Stevens TA, Swaim LS, Clark SL. The Role of Obstetrics/Gynecology Hospitalists in Reducing Maternal Mortality. Obstet Gynecol Clin North Am. 2015;42(3):463–75. [DOI] [PubMed] [Google Scholar]
- 7.Cantwell R, Clutton-Brock T, Cooper G, Dawson A, Drife J, Garrod D, et al. Saving Mothers’ Lives: Reviewing maternal deaths to make motherhood safer: 2006–2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG. 2011;118 Suppl 1:1–203. [DOI] [PubMed] [Google Scholar]
- 8.Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. American journal of reproductive immunology. 1997;37(3):240–9. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. American journal of physiology Heart and circulatory physiology. 2008;294(2):H541–50. [DOI] [PubMed] [Google Scholar]
- 10.Lamarca B. The role of immune activation in contributing to vascular dysfunction and the pathophysiology of hypertension during preeclampsia. Minerva ginecologica. 2010;62(2):105–20. [PMC free article] [PubMed] [Google Scholar]
- 11.Matsubara K, Matsubara Y, Hyodo S, Katayama T, Ito M. Role of nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. The journal of obstetrics and gynaecology research. 2010;36(2):239–47. [DOI] [PubMed] [Google Scholar]
- 12.Noris M, Perico N, Remuzzi G. Mechanisms of disease: Pre-eclampsia. Nature clinical practice Nephrology. 2005;1(2):98–114; quiz 20. [DOI] [PubMed] [Google Scholar]
- 13.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308(5728):1592–4. [DOI] [PubMed] [Google Scholar]
- 14.Sandrim VC, Montenegro MF, Palei AC, Metzger IF, Sertorio JT, Cavalli RC, et al. Increased circulating cell-free hemoglobin levels reduce nitric oxide bioavailability in preeclampsia. Free radical biology & medicine. 2010;49(3):493–500. [DOI] [PubMed] [Google Scholar]
- 15.Selles J, Polini N, Alvarez C, Massheimer V. Progesterone and 17 beta-estradiol acutely stimulate nitric oxide synthase activity in rat aorta and inhibit platelet aggregation. Life sciences. 2001;69(7):815–27. [DOI] [PubMed] [Google Scholar]
- 16.Simoncini T, Fu XD, Caruso A, Garibaldi S, Baldacci C, Giretti MS, et al. Drospirenone increases endothelial nitric oxide synthesis via a combined action on progesterone and mineralocorticoid receptors. Human reproduction. 2007;22(8):2325–34. [DOI] [PubMed] [Google Scholar]
- 17.Amaral LM, Kiprono L, Cornelius DC, Shoemaker C, Wallace K, Moseley J, et al. Progesterone supplementation attenuates hypertension and the autoantibody to the angiotensin II type I receptor in response to elevated interleukin-6 during pregnancy. American journal of obstetrics and gynecology. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meis PJ. The role of 17 alpha-hydroxyprogesterone caproate in the prevention of preterm birth. Women’s health. 2006;2(6):819–24. [DOI] [PubMed] [Google Scholar]
- 19.Merlob P, Stahl B, Klinger G. 17alpha Hydroxyprogesterone caproate for prevention of recurrent spontaneous preterm birth. Reproductive toxicology. 2012;33(1):15–9. [DOI] [PubMed] [Google Scholar]
- 20.Sfakianaki AK, Norwitz ER. Mechanisms of progesterone action in inhibiting prematurity. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2006;19(12):763–72. [DOI] [PubMed] [Google Scholar]
- 21.Dodd JM, Jones L, Flenady V, Cincotta R, Crowther CA. Prenatal administration of progesterone for preventing preterm birth in women considered to be at risk of preterm birth. The Cochrane database of systematic reviews. 2013;7:CD004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amaral LM, Cornelius DC, Harmon A, Moseley J, Martin JN Jr., LaMarca B. 17-hydroxyprogesterone caproate significantly improves clinical characteristics of preeclampsia in the reduced uterine perfusion pressure rat model. Hypertension. 2015;65(1):225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Committee on Practice Bulletins-Obstetrics TACoO, Gynecologists. Practice bulletin no. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120(4):964–73. [DOI] [PubMed] [Google Scholar]
- 24.Saito S, Sakai M, Sasaki Y, Tanebe K, Tsuda H, Michimata T. Quantitative analysis of peripheral blood Th0, Th1, Th2 and the Th1:Th2 cell ratio during normal human pregnancy and preeclampsia. Clinical and experimental immunology. 1999;117(3):550–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. American journal of reproductive immunology. 2010;63(6):601–10. [DOI] [PubMed] [Google Scholar]
- 26.Veenstra van Nieuwenhoven AL, Heineman MJ, Faas MM. The immunology of successful pregnancy. Human reproduction update. 2003;9(4):347–57. [DOI] [PubMed] [Google Scholar]
- 27.Raghupathy R. Th1-type immunity is incompatible with successful pregnancy. Immunology today. 1997;18(10):478–82. [DOI] [PubMed] [Google Scholar]
- 28.Fukui A, Funamizu A, Yokota M, Yamada K, Nakamua R, Fukuhara R, et al. Uterine and circulating natural killer cells and their roles in women with recurrent pregnancy loss, implantation failure and preeclampsia. Journal of reproductive immunology. 2011;90(1):105–10. [DOI] [PubMed] [Google Scholar]
- 29.Fukui A, Yokota M, Funamizu A, Nakamua R, Fukuhara R, Yamada K, et al. Changes of NK cells in preeclampsia. American journal of reproductive immunology. 2012;67(4):278–86. [DOI] [PubMed] [Google Scholar]
- 30.Sargent IL, Borzychowski AM, Redman CW. NK cells and human pregnancy--an inflammatory view. Trends in immunology. 2006;27(9):399–404. [DOI] [PubMed] [Google Scholar]
- 31.Toldi G, Svec P, Vasarhelyi B, Meszaros G, Rigo J, Tulassay T, et al. Decreased number of FoxP3+ regulatory T cells in preeclampsia. Acta obstetricia et gynecologica Scandinavica. 2008;87(11):1229–33. [DOI] [PubMed] [Google Scholar]
- 32.Darmochwal-Kolarz D, Kludka-Sternik M, Tabarkiewicz J, Kolarz B, Rolinski J, Leszczynska-Gorzelak B, et al. The predominance of Th17 lymphocytes and decreased number and function of Treg cells in preeclampsia. Journal of reproductive immunology. 2012;93(2):75–81. [DOI] [PubMed] [Google Scholar]
- 33.Santner-Nanan B, Peek MJ, Khanam R, Richarts L, Zhu E, Fazekas de St Groth B, et al. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. Journal of immunology. 2009;183(11):7023–30. [DOI] [PubMed] [Google Scholar]
- 34.Wallace K, Richards S, Dhillon P, Weimer A, Edholm ES, Bengten E, et al. CD4+ T-helper cells stimulated in response to placental ischemia mediate hypertension during pregnancy. Hypertension. 2011;57(5):949–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cornelius DC, Amaral LM, Wallace K, Campbell N, Thomas AJ, Scott J, et al. Reduced uterine perfusion pressure T-helper 17 cells cause pathophysiology associated with preeclampsia during pregnancy. American journal of physiology Regulatory, integrative and comparative physiology. 2016;311(6):R1192–R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cornelius DC, Amaral LM, Harmon A, Wallace K, Thomas AJ, Campbell N, et al. An increased population of regulatory T cells improves the pathophysiology of placental ischemia in a rat model of preeclampsia. American journal of physiology Regulatory, integrative and comparative physiology. 2015;309(8):R884–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2(9):656–63. [DOI] [PubMed] [Google Scholar]
- 38.Dosiou C, Giudice LC. Natural killer cells in pregnancy and recurrent pregnancy loss: endocrine and immunologic perspectives. Endocrine reviews. 2005;26(1):44–62. [DOI] [PubMed] [Google Scholar]
- 39.Schumacher A, Costa SD, Zenclussen AC. Endocrine factors modulating immune responses in pregnancy. Front Immunol. 2014;5:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borzychowski AM, Chantakru S, Minhas K, Paffaro VA, Yamada AT, He H, et al. Functional analysis of murine uterine natural killer cells genetically devoid of oestrogen receptors. Placenta. 2003;24(4):403–11. [DOI] [PubMed] [Google Scholar]
- 41.van den Heuvel MJ, Xie X, Tayade C, Peralta C, Fang Y, Leonard S, et al. A review of trafficking and activation of uterine natural killer cells. American journal of reproductive immunology. 2005;54(6):322–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Granger JP, LaMarca BB, Cockrell K, Sedeek M, Balzi C, Chandler D, et al. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods in molecular medicine. 2006;122:383–92. [DOI] [PubMed] [Google Scholar]
- 43.LaMarca B, Wallace K, Herse F, Wallukat G, Martin JN Jr., Weimer A, et al. Hypertension in response to placental ischemia during pregnancy: role of B lymphocytes. Hypertension. 2011;57(4):865–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiprono LV, Wallace K, Moseley J, Martin J Jr., Lamarca B. Progesterone blunts vascular endothelial cell secretion of endothelin-1 in response to placental ischemia. American journal of obstetrics and gynecology. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amaral LM, Faulkner JL, Elfarra J, Cornelius DC, Cunningham MW, Ibrahim T, et al. Continued Investigation Into 17-OHPC: Results From the Preclinical RUPP Rat Model of Preeclampsia. Hypertension. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tam Tam KB1 GE, Cockrell K, Arany M, Speed J, Martin JN Jr, Lamarca B, Granger JP. Endothelin type A receptor antagonist attenuates placental ischemia-induced hypertension and uterine vascular resistance. American journal of obstetrics and gynecology. 2011; April;204(4):330.e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giezeman-Smits KM, Jonges LE, Chambers WH, Brisette-Storkus CS, Van Vlierberghe RL, Van Eendenburg JD, et al. Novel monoclonal antibodies against membrane structures that are preferentially expressed on IL-2-activated rat NK cells. J Leukoc Biol. 1998;63(2):209–15. [DOI] [PubMed] [Google Scholar]
- 48.Elfarra J, Amaral LM, McCalmon M, Scott JD, Cunningham MW Jr., Gnam A, et al. Natural Killer Cells Mediate Pathophysiology in Response to Reduced Uterine Perfusion Pressure. Clinical science. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cottrell JN, Amaral LM, Harmon AC, Cornelius DC, Cunningham MW Jr., Vaka VR, et al. Interleukin-4 supplementation improves the pathophysiology of hypertension in response to placental ischemia in RUPP rats. American journal of physiology Regulatory, integrative and comparative physiology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prins JR, Boelens HM, Heimweg J, Van der Heide S, Dubois AE, Van Oosterhout AJ, et al. Preeclampsia is associated with lower percentages of regulatory T cells in maternal blood. Hypertension in pregnancy : official journal of the International Society for the Study of Hypertension in Pregnancy. 2009;28(3):300–11. [DOI] [PubMed] [Google Scholar]
- 51.Toldi G, Saito S, Shima T, Halmos A, Veresh Z, Vasarhelyi B, et al. The frequency of peripheral blood CD4+ CD25high FoxP3+ and CD4+ CD25- FoxP3+ regulatory T cells in normal pregnancy and pre-eclampsia. American journal of reproductive immunology. 2012;68(2):175–80. [DOI] [PubMed] [Google Scholar]
- 52.LaMarca BD, Ryan MJ, Gilbert JS, Murphy SR, Granger JP. Inflammatory cytokines in the pathophysiology of hypertension during preeclampsia. Current hypertension reports. 2007;9(6):480–5. [DOI] [PubMed] [Google Scholar]
- 53.Granger JP. Inflammatory cytokines, vascular function, and hypertension. American journal of physiology Regulatory, integrative and comparative physiology. 2004;286(6):R989–90. [DOI] [PubMed] [Google Scholar]
- 54.Lamarca B, Speed J, Ray LF, Cockrell K, Wallukat G, Dechend R, et al. Hypertension in response to IL-6 during pregnancy: role of AT1-receptor activation. International journal of interferon, cytokine and mediator research : IJIM. 2011;2011(3):65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hsu HT, Mace EM, Carisey AF, Viswanath DI, Christakou AE, Wiklund M, et al. NK cells converge lytic granules to promote cytotoxicity and prevent bystander killing. J Cell Biol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kerdiles Y, Ugolini S, Vivier E. T cell regulation of natural killer cells. The Journal of experimental medicine. 2013;210(6):1065–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katsumoto T, Kimura M, Yamashita M, Hosokawa H, Hashimoto K, Hasegawa A, et al. STAT6-dependent differentiation and production of IL-5 and IL-13 in murine NK2 cells. Journal of immunology. 2004;173(8):4967–75. [DOI] [PubMed] [Google Scholar]
- 58.Ebina Y, Nishino Y, Deguchi M, Maesawa Y, Nakashima Y, Yamada H. Natural killer cell activity in women with recurrent miscarriage: Etiology and pregnancy outcome. Journal of reproductive immunology. 2017;120:42–7. [DOI] [PubMed] [Google Scholar]
- 59.Keiser SD, Veillon EW, Parrish MR, Bennett W, Cockrell K, Fournier L, et al. Effects of 17-hydroxyprogesterone on tumor necrosis factor-alpha-induced hypertension during pregnancy. American journal of hypertension. 2009;22(10):1120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harmon AC, Cornelius DC, Amaral LM, Faulkner JL, Cunningham MW Jr., Wallace K, et al. The role of inflammation in the pathology of preeclampsia. Clinical science. 2016;130(6):409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333(24):1581–7. [DOI] [PubMed] [Google Scholar]
- 62.Meher S, Duley L. Progesterone for preventing pre-eclampsia and its complications. The Cochrane database of systematic reviews. 2006(4):CD006175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Norman JE, Mackenzie F, Owen P, Mactier H, Hanretty K, Cooper S, et al. Progesterone for the prevention of preterm birth in twin pregnancy (STOPPIT): a randomised, double-blind, placebo-controlled study and meta-analysis. Lancet. 2009;373(9680):2034–40. [DOI] [PubMed] [Google Scholar]
- 64.Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr., Chauhan SP, Hughes BL, Louis JM, et al. 17-OHPC to Prevent Recurrent Preterm Birth in Singleton Gestations (PROLONG Study): A Multicenter, International, Randomized Double-Blind Trial. Am J Perinatol. 2019. [DOI] [PubMed] [Google Scholar]