Abstract
Pyxinol, the main metabolite of 20S-protopanaxadiol in human liver, was chosen as a novel skeleton for the development of anti-inflammatory agents. Pyxinol derivatives modified at C-3, C-12, or C-25 and selected stereoisomers were designed, prepared, and investigated for in vitro anti-inflammatory activities. Structure–activity relationship (SAR), focused on skeleton, was analyzed based on their ability to inhibit lipopolysaccharide (LPS)-induced nitric oxide (NO) synthesis. The preliminary SAR results signified that the biological activity of the pyxinol derivatives is largely dependent on the R/S stereochemistry of pyxinol skeleton and the hydroxy at C-3 is a modifiable position. Among the tested compounds, the 3-oximinopyxinol (4a) exhibited the most potent NO-inhibitory activity and was even comparable to the steroid drug. Furthermore, compound 4a also significantly decreased LPS-induced TNF-α and IL-6 synthesis and iNOS and COX-2 expressions via the NF-κB pathway. This study proves that pyxinol is an interesting skeleton for anti-inflammatory drug discovery.
Keywords: Pyxinol, ginsenoside, anti-inflammatory activity, structure−activity relationship, macrophages
Inflammation is a primary defense response of the immune system and protects against infection and injury.1 Macrophages are the key cells that produce inflammatory mediators, including nitric oxide (NO), prostaglandins (PGs), and proinflammatory cytokines, including tumor necrosis factor α (TNF-α) and interleukin-6 (IL-6). However, prolonged inflammation can lead to immune over-reactions that cause tissue damage and lead to chronic diseases, such as Alzheimer’s disease and diabetes.2,3 Steroid drugs, such as hydrocortisone, are powerful at reducing inflammation, but they have many serious side effects, such as water and sodium retention and osteoporosis.4 Other drugs are also limited by side effects.5 Therefore, the development of new anti-inflammatory agents that are safer and more effective is ongoing. Natural products are a key source and play an important role in discovering next generation medicines.6
Ginseng, the roots of Panax ginseng Meyer, is well-known to extend life, enhance vitality and play roles against kinds of conditions, such as tumors, depression, and inflammation.7,8 Ginsenosides, the widely orally administered components of ginseng, are recognized as its major active pharmacological components with various activities.7 Their anti-inflammatory activities are well-known because of the structural similarity with cortisone, a steroid hormone with powerful anti-inflammatory activity. Some of them have been applied to clinical treatment. Previous studies and reviews have summarized that the protopanoxadiol type ginsenosides (Rb1, Rb2, Rd, and Rh2) exert anti-inflammatory activities by decreasing the generation of inflammatory mediators and proinflammatory cytokines and regulating the inflammatory signaling pathways.8,9 20S-Protopanoxadiol (PPD), the principal intestinal metabolite of ginsenosides, was recognized as the pharmacophore of ginsenosides and showed increased absorption in the gastrointestinal tract.10,11 PPD also showed anti-inflammatory activity by inactivation of nuclear factor κB (NF-κB) and inducing heme oxygenase 1 expression.12 These results suggested that the metabolites of ginsenosides could be promising candidates for inflammation treatment.
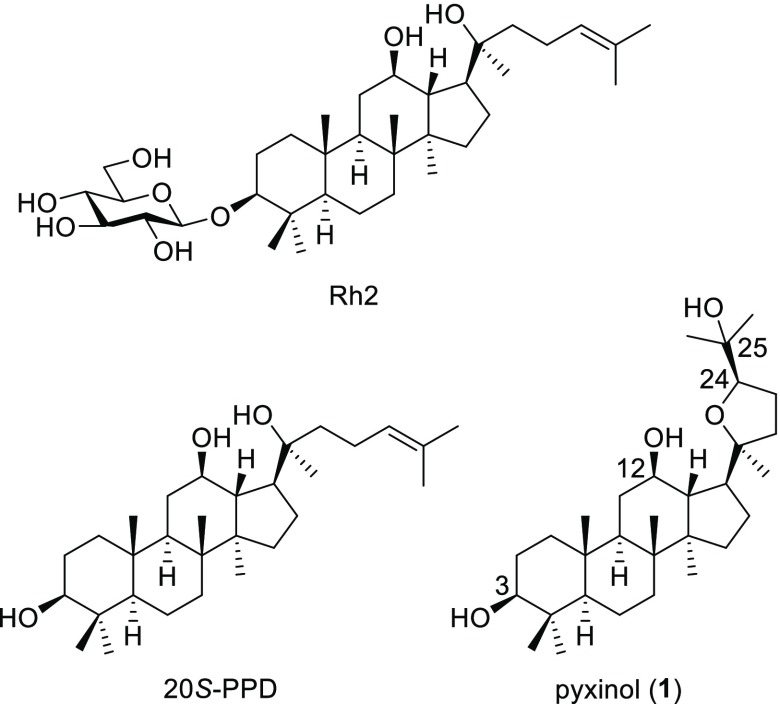
Pyxinol, an ocotillol-type triterpene shown in Figure 1, was first isolated from lichen in 1972 and recently was identified as the main metabolite of PPD in human liver.13−15 Pyxinol was found to have better oral bioavailability and a lower metabolism burden in organisms compared with the parent compounds PPD and protopanoxadiol-type ginsenosides, suggesting its medicinal development value.14,16 Therefore, pyxinol and its derivatives have been extensively studied for drug development against a variety of conditions, such as cardiovascular diseases,17−19 tumors, and bacterial infection.20−24 Nevertheless, there are few reports of pyxinol or pyxinol analogs with anti-inflammatory activity.
Figure 1.
Chemical structures of ginsenosides.
On the basis of above view for development of novel anti-inflammatory agents, pyxinol was first used as a lead compound in this study and different modifications were carried out at the C-3, C-12, or C-25 of pyxinol to yield 18 compounds including several stereoisomers. Their anti-inflammatory activities were assessed by measuring NO-inhibitory activity in lipopolysaccharide (LPS)-induced macrophages. The anti-inflammatory effects of the most potent derivative 4a with an oxime moiety at C-3 were further confirmed by its inhibition of proinflammatory cytokines that were comparable to the clinical steroid drug hydrocortisone sodium succinate.
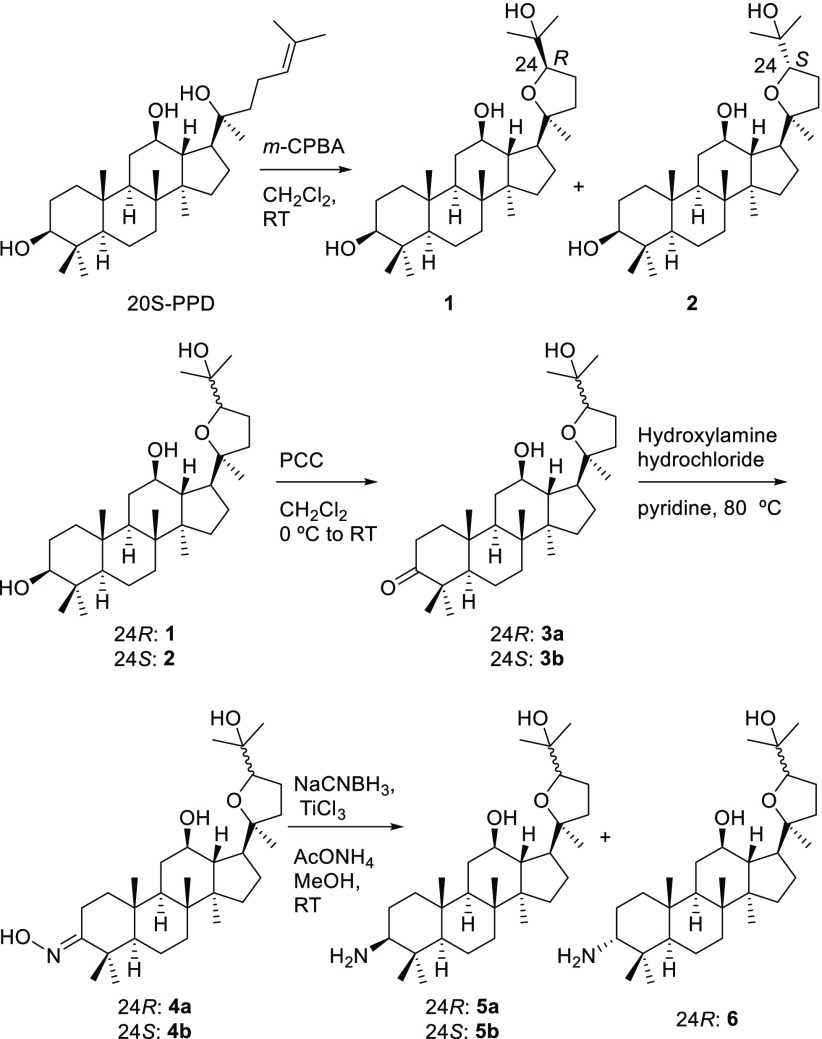
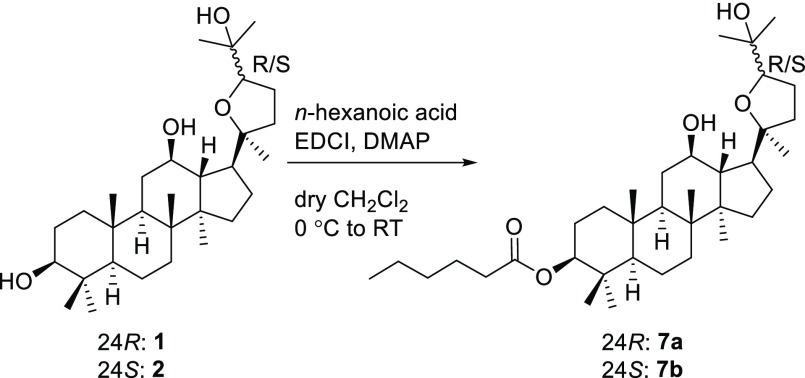
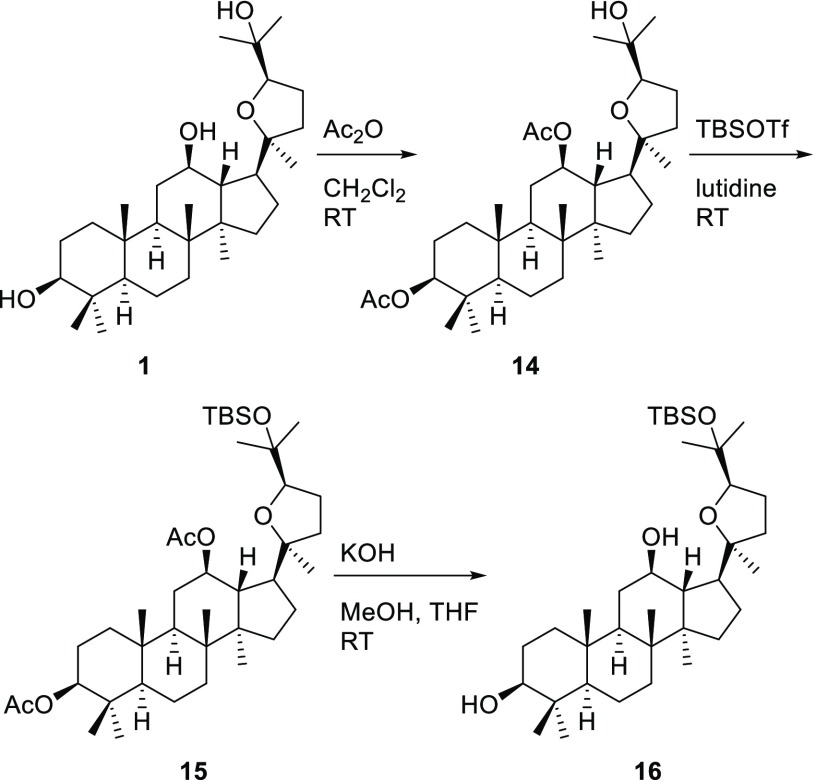
To explore the effects of hydroxy groups and stereochemistry of pyxinol on its anti-inflammatory activities, a series of pyxinol derivatives and selected stereoisomers were designed in which the hydroxy group at C-3, C-12, or C-25 was modified. Pyxinol (1) and its epimer 24S-pyxinol (2) were synthesized by epoxidization of the commercially available PPD and following nucleophilic addition in situ as previously described.25 The modification of the hydroxy group at the C-3 of pyxinol gave the derivatives 3–6 as shown in Scheme 1 following our previously reported procedures.20 The C-3 ester derivatives (7a and 7b) were synthesized by selective esterification as shown in Scheme 2.
Scheme 1. Synthesis of Pyxinol Derivatives 1–6.
Scheme 2. Synthesis of Pyxinol Derivatives 7a,b.
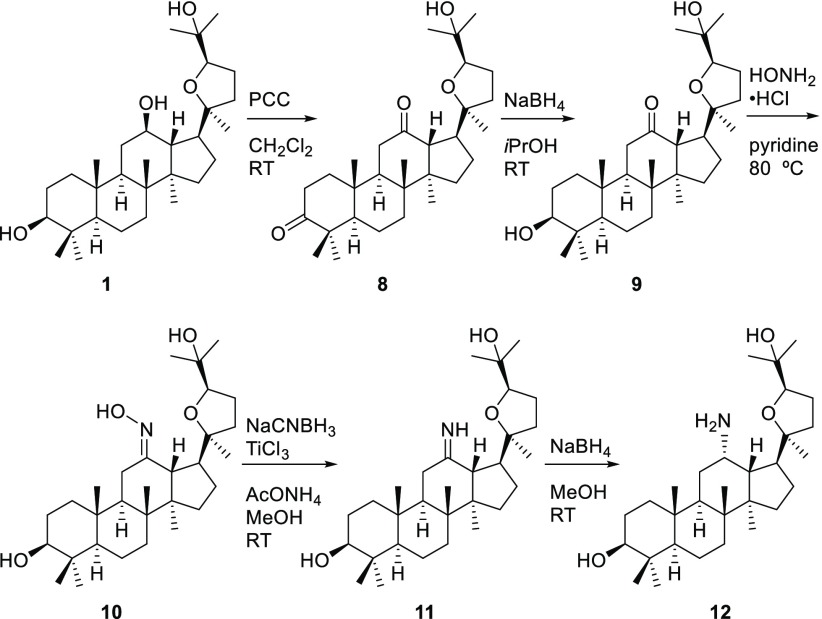
The modification of the hydroxy group at the C-12 of pyxinol is shown in Scheme 3. The oxidation of pyxinol with an excess of pyridinium chlorochromate (PCC) gave 3,12-diketopyxinol (8), subsequent selective reduction by NaBH4 in isopropanol gave 12-ketopyxinol (9), and further oximation gave 12-oximinopyxinol (10). An unexpected stable imine (11) was attained by the reduction of oxime with NaCNBH3 in the presence of AcONH4 and titanium(III) chloride in methanol. Then reduction of the imine with NaBH4 in methanol afforded the 12-aminopyxinol (12).
Scheme 3. Synthesis of Pyxinol Derivatives 8–12.
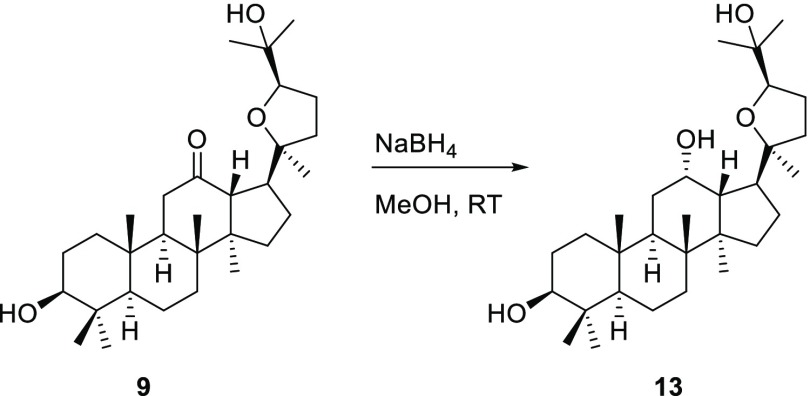
Another stereoisomer of pyxinol (13) was attained by reduction of 9 with NaBH4 in methanol as shown in Scheme 4. The pyxinol derivative 16 modified at C-25 was synthesized as depicted in Scheme 5.
Scheme 4. Synthesis of Isomer 13.
Scheme 5. Synthesis of Pyxinol Derivative 16.
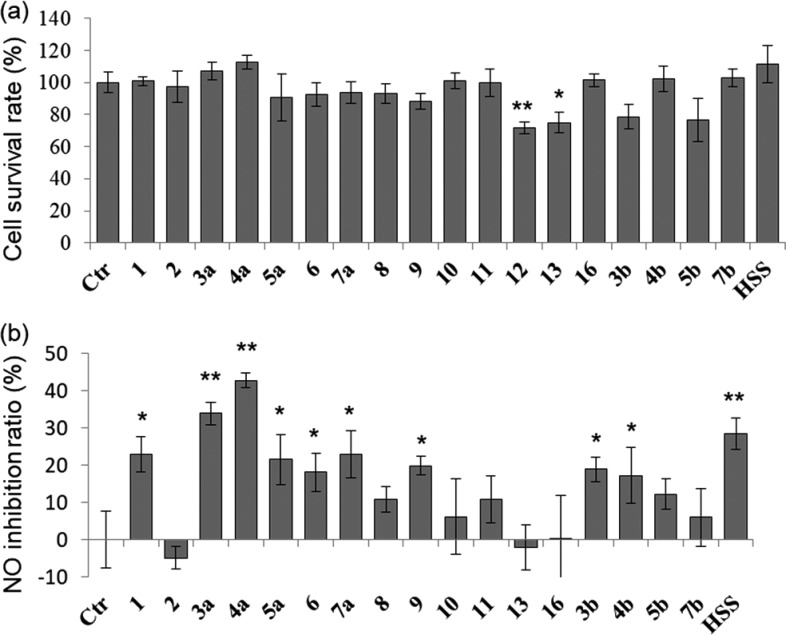
The NO-inhibitory activity of a compound is always related to its anti-inflammatory activity.26−28 In order to avoid the cytotoxic interference of the synthesized compounds on NO-inhibitory effect, the cytotoxicity was evaluated. As shown in Figure 2a, compounds 1–11 and 16 at the test concentrations (20 μM) had no obvious effects on cytotoxicity in RAW264.7 cells, but compound 12 showed a notably significant cytotoxicity (p < 0.01). Therefore, compounds 1–11, 13, and 16 were further tested for their anti-inflammatory activities. The cells were co-treated with the pyxinol derivatives and LPS for 24 h. After that, the LPS-induced NO production was determined by the Griess assay. Hydrocortisone sodium succinate (20 μM) was the positive control (Figure S1). As shown in Figure 2b, relative to the control group, pyxinol (1) significantly inhibited the LPS-mediated NO release (p < 0.05) to a similar extent as that of the positive control. However, compound 2 had no obvious NO-inhibitory activity. Pyxinol and 2, a pair of stereoisomers at C-24, were known as the main metabolites of PPD in human liver microsomes.14,15 These results suggested that pyxinol may be the major active form of PPD in vivo on anti-inflammation and the stereochemistry at C-24 of pyxinol influences the NO-inhibitory capacity with an R-configuration favored.
Figure 2.
Inhibitory effects of pyxinol derivatives on LPS-induced NO generation in RAW264.7 cells: (a) effect of pyxinol derivatives and LPS on the cell survival by MTT assays; (b) inhibitory effect of pyxinol derivatives on LPS-induced NO generation. The cells were co-treated with pyxinol derivatives (20 μM) and LPS (1 μg/mL) for 24 h. Data were denoted as the mean ± SD (n = 3): (∗) p < 0.05, (∗∗) p < 0.01, relative to control group; Ctr, control; HSS, hydrocortisone sodium succinate.
Furthermore, pyxinol derivatives 3a, 4a, 5a, 6, 7a, and 9 also exhibited significant NO-inhibitory activity (p < 0.05) (Figure 2b). The stereoisomer of pyxinol (13) had no obvious NO-inhibitory activity. In addition to 9, pyxinol derivatives 10, 11, and 16, in which the hydroxy groups at C-12 or C-25 were modified, also had no significant NO-inhibitory activity. Interestingly, 3-oximinopyxinol (4a) exhibited the most potent NO-inhibitory activity that was even stronger than that of the positive control, hydrocortisone sodium succinate, at the same concentration. Fernández-Herrera et al. have reported similar result that incorporating oxime functionality has improved the biological activities of the steroids.29 These results indicated that pyxinol is an interesting skeleton for anti-inflammatory drug development and its derivative 4a is a promising candidate and worthy of more detailed studies.
As shown in Figure 2, in the absence of any obvious cytotoxicity, derivatives 3a, 4a, 5a, 6, and 7a modified at the C-3 of pyxinol showed significant NO-inhibitory activity similar to pyxinol, while derivatives 10, 11, and 16 modified at C-12 or C-25 had no obvious NO-inhibitory activity. This suggested that the hydroxy moieties at C-12 and C-25 of pyxinol are important for its anti-inflammatory activity and that the C-3 position is amenable to modification. Stereoisomers 1, 2, and 13 displayed dramatically dissimilar NO-inhibitory activities, which suggested that the stereochemistry of the hydroxy moiety at C-12 and/or the isopropanol moiety at C-24 may considerably influence the binding of the bioactive compounds to their target in macrophages. This was further confirmed by the comparison that the NO-inhibitory activity of derivatives 3b, 4b, 5b, and 7b with 24S-configuration was lower than that of corresponding derivatives 3a, 4a, 5a, and 7a with 24R-configuration. Stereoisomers 5a and 6 with epimerization at C-3 displayed similar NO-inhibitory activities, which further suggested that the C-3 of pyxinol is a modifiable position for the discovery of anti-inflammatory drugs.
On the basis of the NO-inhibitory activity of pyxinol derivatives, a probable structure–activity relationship (SAR) could be predicted as shown in Figure 3. The hydroxy group at C-3 is a modifiable position, and the anti-inflammatory activity of a pyxinol derivative incorporating oxime functionality at C-3 was improved. Hydroxy moieties at C-12 and C-25 are crucial for the activity. Furthermore, the stereochemistry at C-12 and C-24 considerably influences the NO-inhibitory activity with an R-configuration preferred.
Figure 3.
Preliminary structure–activity relationships of pyxinol derivatives.
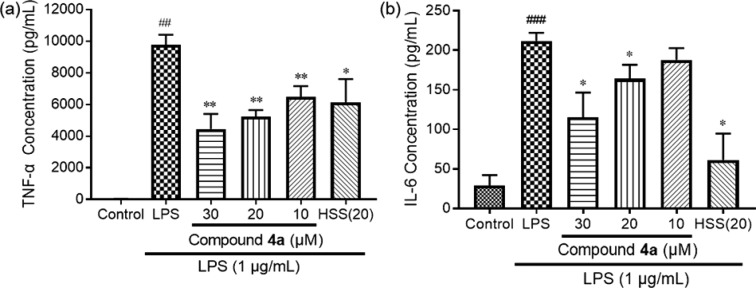
In LPS-activated macrophages, overproduction of proinflammatory cytokines such as TNF-α and interleukins and the resulting tissue damage can be inhibited by anti-inflammatory agents.30 To confirm the anti-inflammatory capacity of 4a, its inhibition effects on 1 μg/mL of LPS-induced proinflammatory cytokines (TNF-α, IL-6) were evaluated in RAW264.7 cells. Hydrocortisone sodium succinate (20 μM) was the positive control. Compared with the basal level in macrophages, the LPS-stimulation considerably increased the TNF-α and IL-6 productions as expected (Figure 4). In the absence of any obvious cytotoxicity, the LPS-stimulated increase of TNF-α and IL-6 was suppressed concentration-dependently through pretreatment with 4a. In particular, the inhibition effect of 4a on LPS-stimulated TNF-α increase was better than that of hydrocortisone sodium succinate, a clinical steroid drug. These results indicated that 4a can alleviate an over-immune-reaction by inhibiting the LPS-stimulated increase of proinflammatory cytokines.
Figure 4.
Inhibitory effect of 4a on LPS-mediated (a) TNF-α and (b) IL-6 synthesis in RAW264.7 cells, which were pretreated with 4a (10, 20, 30 μM) or HSS (20 μM) for 2 h before the LPS stimulation (1 μg/mL) for 4 h. The data are expressed as the mean ± SD (n = 3): (##) p < 0.01, (###) p < 0.001, relative to the control group; (∗) p < 0.05, (∗∗) p < 0.01, relative to LPS-stimulated group; HSS, hydrocortisone sodium succinate.
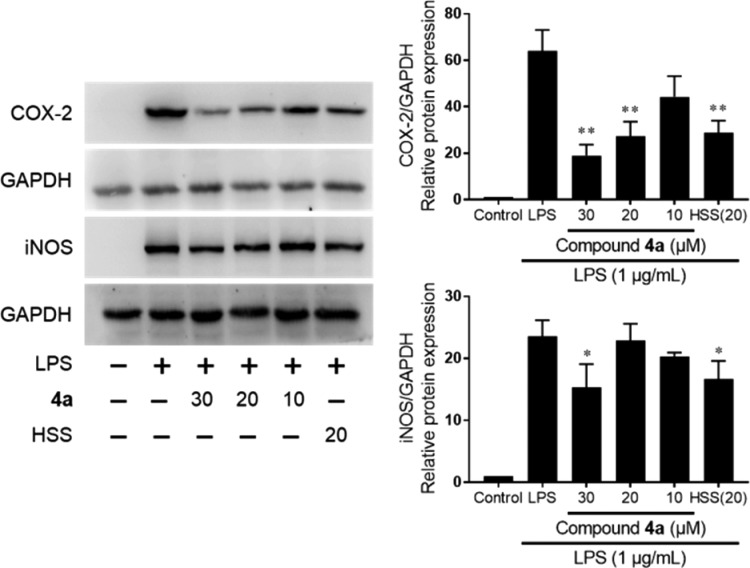
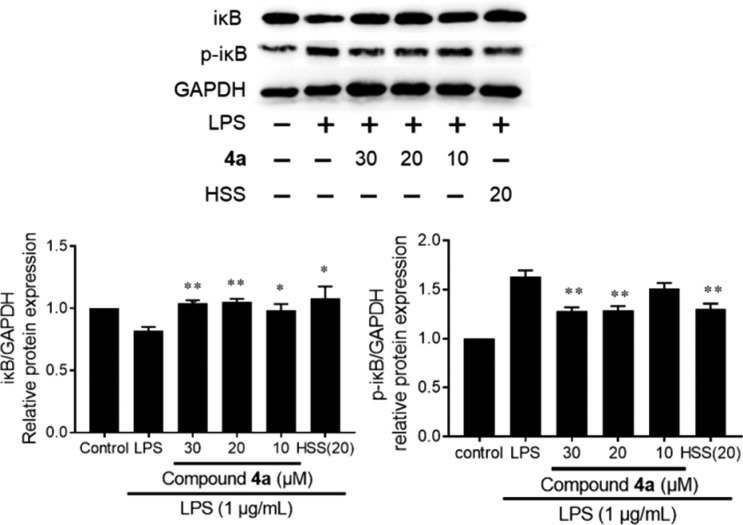
Inflammatory mediators, such as PGs and NO, play pivotal roles in inflammatory diseases and are closely related to the expression of cyclooxygenase 2 (COX-2) and inducible NO synthase (iNOS).31 The levels of COX-2 and iNOS were investigated in LPS-induced RAW264.7 cells by Western blotting. Hydrocortisone sodium succinate (20 μM) was the positive control. As shown in Figure 5, the LPS-stimulation obviously increased protein levels of COX-2 and iNOS as expected. Similar to the positive control, co-treatment with 4a significantly decreased LPS-stimulated COX-2 and iNOS expressions, respectively. These inhibitions were in a dose-dependent manner. COX-2 and iNOS are positively modulated by the NF-κB pathway in inflammation.32 The LPS-activated NF-κB pathway involves phosphorylation and degradation of iκB. Compound 4a could suppress the LPS-induced iκB degradation, as well as its phosphorylation, similar to the positive control (hydrocortisone sodium succinate) (Figure 6). However, unlike that of hydrocortisone sodium succinate, the anti-inflammatory activities of 4a could not be blocked by the RU-486 (the glucocorticoid receptor antagonist), even though their core structures were similar (data not shown). It suggested that the anti-inflammatory activities of 4a are not associated with glucocorticoid receptor. Ahn et al. reported that the ginsenosides have valid binding affinity with NF-κB to exert their anti-inflammatory activity by molecular docking study.33 It is interesting to further study the target of pyxinol analogs on anti-inflammatory activities. Anyway, our results revealed that 4a could modulate the expression of proinflammatory proteins by inhibiting the LPS-activated NF-κB pathway in macrophages.
Figure 5.
Effect of 4a on LPS-stimulated COX-2 and iNOS protein expressions in RAW264.7 cells co-treated with LPS (1 μg/mL) and 4a (10, 20, 30 μM) or HSS (20 μM) for 24 h. The data are expressed as the mean ± SD (n = 3) of three independent experiments: (∗) p < 0.05, (∗∗) p < 0.01, relative to LPS-stimulated group; HSS, hydrocortisone sodium succinate.
Figure 6.
Effect of 4a on LPS-stimulated NF-κB signaling pathway in RAW264.7 cells co-treated with LPS (1 μg/mL) and 4a (10, 20, 30 μM) or HSS (20 μM) for 2 h. The data are expressed as the mean ± SD (n = 3): (∗) p < 0.05, (∗∗) p < 0.01, relative to LPS-stimulated group; HSS, hydrocortisone sodium succinate.
Inflammatory mediators, such as PGs and NO, are the pivotal signal transduction molecules of inflammation and are regulated by endogenous enzymes. PGs are mainly synthesized via mediation of COX-2 in LPS-stimulated macrophages.34 Therefore, blocking protein expression of COX-2 is a valid approach to alleviate the inflammation by inhibiting PGs production. Additionally, NO is synthesized endogenously through a series of biochemical reactions by NO synthases. In inflammatory responses, NO is primarily regulated by iNOS.35 The increased protein expression of iNOS can also cause chronic inflammation.36 Therefore, blocking the protein expression of iNOS is one of the effective ways to treat inflammatory diseases. Compound 4a could block both COX-2 and iNOS expression (Figure 5) to inhibit inflammatory mediators (Figure 2) in LPS-activated macrophages. These results all demonstrated that 4a is a promising anti-inflammatory agent.
Pyxinol, the primary in vivo metabolite of PPD which acted as the pharmacophore of ginsenosides, was first chosen as the lead compound for the development of novel anti-inflammatory agents. Its derivatives were designed and synthesized by structural modification at the C-3, C-12, or C-25 of pyxinol based on the structure characteristics. The inhibitory activities of the pyxinol derivatives toward LPS-induced NO production were evaluated. The preliminary SAR results of these pyxinol derivatives indicated that pyxinol is responsible for the inhibitory activity of the LPS-induced NO release, the hydroxy at C-3 is a modifiable position, and the activity is improved when the C-3 is an oxime. Additionally, hydroxy groups at C-12 and C-25 are essential for the activity, and the stereochemistries at C-12 and C-24 significantly affect the NO-inhibitory activity with an R-configuration preferred. Among the tested compounds, 3-oximinopyxinol (4a) displayed the strongest inhibitory activity on LPS-induced NO synthesis and was even comparable to the clinical steroid drug. Furthermore, the results indicated that compound 4a can reduce LPS-induced production of TNF-α and IL-6 and the protein expressions of COX-2 and iNOS via NF-κB pathway. Further studies on the mechanism responsible for the anti-inflammatory action and structural optimization are ongoing.
Acknowledgments
The authors are grateful to National Demonstration Center for Experimental Pharmacy Education (Yantai University) for the support.
Glossary
Abbreviations
- COX-2
cyclooxygenase 2
- IL-6
interleukin-6
- iNOS
inducible NO synthase
- LPS
lipopolysaccharide
- NF-κB
nuclear factor κB
- NO
nitric oxide
- PCC
pyridinium chlorochromate
- PG
prostaglandin
- PPD
20S-protopanoxadiol
- SAR
structure–activity relationship
- TNF-α
tumor necrosis factor α
- TLC
thin layer chromatography
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.9b00562.
Figure S1, experimental protocols, NMR spectra, and mass spectra (PDF)
Author Contributions
§ Y.S. and X.F. contributed equally. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This study was supported by Key Research Project of Yantai City (Grant 2018ZHGY085), Key Research Project of Shandong Province (Grant 2019GSF108241), and National Natural Science Foundation of China (Grant 21502164).
The authors declare no competing financial interest.
Supplementary Material
References
- Medzhitov R. Origin and physiological roles of inflammation. Nature 2008, 454 (7203), 428–35. 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Kokiko-Cochran O. N.; Godbout J. P. The Inflammatory Continuum of Traumatic Brain Injury and Alzheimer’s Disease. Front. Immunol. 2018, 9, 672–672. 10.3389/fimmu.2018.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donath M. Y.; Shoelson S. E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11 (2), 98–107. 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- Song Y. Q.; Zhao F.; Zhang L. M.; Du Y.; Wang T.; Fu F. H. Ginsenoside Rg1 exerts synergistic anti-inflammatory effects with low doses of glucocorticoids in vitro. Fitoterapia 2013, 91, 173–179. 10.1016/j.fitote.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Pereira-Leite C.; Nunes C.; Jamal S. K.; Cuccovia I. M.; Reis S. Nonsteroidal Anti-Inflammatory Therapy: A Journey Toward Safety. Med. Res. Rev. 2017, 37 (4), 802–859. 10.1002/med.21424. [DOI] [PubMed] [Google Scholar]
- Newman D. J.; Cragg G. M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79 (3), 629–61. 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- Helliwell R. M.; ShioukHuey C. O.; Dhuna K.; Molero J. C.; Ye J. M.; Xue C. C.; Stokes L. Selected ginsenosides of the protopanaxdiol series are novel positive allosteric modulators of P2X7 receptors. Br. J. Pharmacol. 2015, 172 (13), 3326–3340. 10.1111/bph.13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H.; Yi Y. S.; Kim M. Y.; Cho J. Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2017, 41 (4), 435–443. 10.1016/j.jgr.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.; Lee J.; Rhee M. H.; Yu T.; Baek K. S.; Sung N. Y.; Kim Y.; Yoon K.; Kim J. H.; Kwak Y. S.; Hong S.; Kim J.-H.; Cho J. Y. Molecular mechanism of protopanaxadiol saponin fraction-mediated anti-inflammatory actions. J. Ginseng Res. 2015, 39 (1), 61–68. 10.1016/j.jgr.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H.-c.; Sun J.-g.; Wang G.-j.; A J.-y.; Xie H.-t.; Zha W.-b.; Yan B.; Sun F.-z.; Hao H.-p.; Gu S.-h.; Sheng L.-s.; Shao F.; Shi J.; Zhou F. Sensitive determination of 20(S)-protopanaxadiol in rat plasma using HPLC–APCI-MS: Application of pharmacokinetic study in rats. J. Pharm. Biomed. Anal. 2008, 48 (5), 1476–1480. 10.1016/j.jpba.2008.09.045. [DOI] [PubMed] [Google Scholar]
- Kim D.-H. Gut microbiota-mediated pharmacokinetics of ginseng saponins. J. Ginseng Res. 2018, 42 (3), 255–263. 10.1016/j.jgr.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H.; Seo G. S.; Ko G.; Kim J. B.; Sohn D. H. Anti-inflammatory activity of 20(S)-protopanaxadiol: enhanced heme oxygenase 1 expression in RAW 264.7 cells. Planta Med. 2005, 71 (12), 1167–70. 10.1055/s-2005-873147. [DOI] [PubMed] [Google Scholar]
- Yosioka I.; Yamauchi H.; Kitagawa I. Lichen Triterpenoids. V. On the Neutral Triterpenoids of Pyxine endochrysina NYL. Chem. Pharm. Bull. 1972, 20 (3), 502–513. 10.1248/cpb.20.502. [DOI] [Google Scholar]
- Li L.; Chen X.; Li D.; Zhong D. Identification of 20(S)-protopanaxadiol metabolites in human liver microsomes and human hepatocytes. Drug Metab. Dispos. 2011, 39 (3), 472–83. 10.1124/dmd.110.036723. [DOI] [PubMed] [Google Scholar]
- Wang W.; Wang L.; Wu X.; Xu L.; Meng Q.; Liu W. Stereoselective formation and metabolism of 20(s)-protopanaxadiol ocotillol type epimers in vivo and in vitro. Chirality 2015, 27 (2), 170–6. 10.1002/chir.22407. [DOI] [PubMed] [Google Scholar]
- Wang W.; Wu X.; Wang L.; Meng Q.; Liu W. Stereoselective Property of 20 (S)-Protopanaxadiol Ocotillol Type Epimers Affects Its Absorption and Also the Inhibition of P-Glycoprotein. PLoS One 2014, 9 (6), e98887 10.1371/journal.pone.0098887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T.; Meng Q.; Zhang J.; Bi Y.; Jiang N. Study on the structure-function relationship of 20(S)-panaxadiol and its epimeric derivatives in myocardial injury induced by isoproterenol. Fitoterapia 2010, 81 (7), 783–7. 10.1016/j.fitote.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Bi Y.; Wang T.; Meng Q.; Zhang J.; Wang L.; Li Q.; Zhao F.; Sun H. Synthesis and Myocardial Ischemia Protective Effect of Ocotillol-Type Derivatives. Rec. Nat. Prod. 2012, 6 (3), 242–254. [Google Scholar]
- Yang G. Q.; Yang Y. T.; Yang Q.; Li Y.; Jiang Y. T.; Fu F. H.; Wang H. B. Novel Fluorescent Pyxinol-Based Probes: Design, Synthesis and Biological Evaluation. Chin. J. Org. Chem. 2017, 37 (8), 2109–2114. 10.6023/cjoc201705039. [DOI] [Google Scholar]
- Ren Q.; Yang G.; Guo M.; Guo J.; Li Y.; Lu J.; Yang Q.; Tang H.; Li Y.; Fang X.; Sun Y.; Qi J. G.; Tian J.; Wang H. Design, synthesis, and discovery of ocotillol-type amide derivatives as orally available modulators of P-glycoprotein-mediated multidrug resistance. Eur. J. Med. Chem. 2019, 161, 118–130. 10.1016/j.ejmech.2018.10.038. [DOI] [PubMed] [Google Scholar]
- Liu J.; Xu Y. R.; Yang J. J.; Wang W. Z.; Zhang J. Q.; Zhang R. M.; Meng Q. G. Discovery, semisynthesis, biological activities, and metabolism of ocotillol-type saponins. J. Ginseng Res. 2017, 41 (3), 373–378. 10.1016/j.jgr.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Y.; Yang J.; Ma C.; Liu Z. Y.; Zhang T. T.; Zhang X. C.; Lu J.; Meng Q. G. Design, synthesis and in vitro NO-releasing activities of ocotillol-type furoxans. Pharmazie 2015, 70 (4), 213–218. [PubMed] [Google Scholar]
- Zhou Z.; Ma C.; Zhang H.; Bi Y.; Chen X.; Tian H.; Xie X.; Meng Q.; Lewis P. J.; Xu J. Synthesis and biological evaluation of novel ocotillol-type triterpenoid derivatives as antibacterial agents. Eur. J. Med. Chem. 2013, 68, 444–453. 10.1016/j.ejmech.2013.07.041. [DOI] [PubMed] [Google Scholar]
- Bi Y.; Yang X.; Zhang T.; Liu Z.; Zhang X.; Lu J.; Cheng K.; Xu J.; Wang H.; Lv G.; Lewis P. J.; Meng Q.; Ma C. Design, synthesis, nitric oxide release and antibacterial evaluation of novel nitrated ocotillol-type derivatives. Eur. J. Med. Chem. 2015, 101, 71–80. 10.1016/j.ejmech.2015.06.021. [DOI] [PubMed] [Google Scholar]
- Yang G. Q.; Li Y.; Yang Q.; Yue X.; Yao L.; Jiang Y. T. Simple and Efficient Synthesis of Pseudoginsenoside HQ. Chin. J. Org. Chem. 2017, 37 (6), 1530–1536. 10.6023/cjoc201703006. [DOI] [Google Scholar]
- Li B.; Cai S.; Yang Y. A.; Chen S. C.; Chen R.; Shi J. B.; Liu X. H.; Tang W. J. Novel unsaturated glycyrrhetic acids derivatives: Design, synthesis and anti-inflammatory activity. Eur. J. Med. Chem. 2017, 139, 337–348. 10.1016/j.ejmech.2017.08.002. [DOI] [PubMed] [Google Scholar]
- Zhao F.; Gao Z. T.; Jiao W. H.; Chen L. P.; Chen L.; Yao X. S. In vitro Anti-Inflammatory Effects of Beta-Carboline Alkaloids, Isolated from Picrasma quassioides, through Inhibition of the iNOS Pathway. Planta Med. 2012, 78 (18), 1906–1911. 10.1055/s-0032-1327883. [DOI] [PubMed] [Google Scholar]
- Sun C. P.; Qiu C. Y.; Zhao F.; Kang N.; Chen L. X.; Qiu F. Physalins V-IX, 16,24-cyclo-13,14-seco withanolides from Physalis angulata and their antiproliferative and anti-inflammatory activities. Sci. Rep. 2017, 7, 4057. 10.1038/s41598-017-03849-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeferino-Díaz R.; Olivera-Castillo L.; Dávalos A.; Grant G.; Kantún-Moreno N.; Rodriguez-Canul R.; Bernès S.; Sandoval-Ramírez J.; Fernández-Herrera M. A. 22-Oxocholestane oximes as potential anti-inflammatory drug candidates. Eur. J. Med. Chem. 2019, 168, 78–86. 10.1016/j.ejmech.2019.02.035. [DOI] [PubMed] [Google Scholar]
- Xia Q.; Bao X.; Sun C.; Wu D.; Rong X.; Liu Z.; Gu Y.; Zhou J.; Liang G. Design, synthesis and biological evaluation of novel 2-sulfonylindoles as potential anti-inflammatory therapeutic agents for treatment of acute lung injury. Eur. J. Med. Chem. 2018, 160, 120–132. 10.1016/j.ejmech.2018.10.014. [DOI] [PubMed] [Google Scholar]
- Tsatsanis C.; Androulidaki A.; Venihaki M.; Margioris A. N. Signalling networks regulating cyclooxygenase-2. Int. J. Biochem. Cell Biol. 2006, 38 (10), 1654–61. 10.1016/j.biocel.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Lee S. M.; Kim N.-H.; Lee S.; Kim Y. N.; Heo J. D.; Jeong E. J.; Rho J.-R. Deacetylphylloketal, a New Phylloketal Derivative from a Marine Sponge, Genus Phyllospongia, with Potent Anti-Inflammatory Activity in In Vitro Co-Culture Model of Intestine. Mar. Drugs 2019, 17, 634. 10.3390/md17110634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S.; Siddiqi M. H.; Noh H.-Y.; Kim Y.-J.; Kim Y.-J.; Jin C.-G.; Yang D.-C. Anti-inflammatory activity of ginsenosides in LPS-stimulated RAW 264.7 cells. Sci. Bull. 2015, 60 (8), 773–784. 10.1007/s11434-015-0773-4. [DOI] [Google Scholar]
- Tsatsanis C.; Androulidaki A.; Dermitzaki E.; Gravanis A.; Margioris A. N. Corticotropin releasing factor receptor 1 (CRF1) and CRF2 agonists exert an anti-inflammatory effect during the early phase of inflammation suppressing LPS-induced TNF-alpha release from macrophages via induction of COX-2 and PGE2. J. Cell. Physiol. 2007, 210 (3), 774–83. 10.1002/jcp.20900. [DOI] [PubMed] [Google Scholar]
- Kong F. H.; Lee B. H.; Wei K. 5-Hydroxymethylfurfural Mitigates Lipopolysaccharide-Stimulated Inflammation via Suppression of MAPK, NF-B and mTOR Activation in RAW 264.7 Cells. Molecules 2019, 24 (2), 275. 10.3390/molecules24020275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suschek C. V.; Schnorr O.; Kolb-Bachofen V. The role of iNOS in chronic inflammatory processes in vivo: is it damage-promoting, protective, or active at all?. Curr. Mol. Med. 2004, 4 (7), 763–75. 10.2174/1566524043359908. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.