Abstract
Parkinson’s disease (PD) is a debilitating and common neurodegenerative disease. New insights implicating c-Abl activation as a driving force in PD have opened a new drug development avenue for PD treatment beyond the symptomatic relief by L-DOPA. BCR-Abl inhibitors, which include nilotinib and ponatinib, have been found to inhibit this process, and nilotinib has shown improvement in outcomes in a 12-patient, nonrandomized trial. However, nilotinib is a potent inhibitor of hERG, a cardiac K+ channel whose inhibition increases risk of sudden death. We used our machine learning approach to predict novel molecules that would inhibit c-Abl while also having minimal liability against hERG. Of our six novel compounds tested, we identified two that had c-Abl potencies comparable to nilotinib, but with significantly improved profiles regarding the hERG channel. Our best compound exhibited a hERG IC50 of 12.1 μM (compared to nilotinib with an IC50 of 0.45 μM and ponatinib with IC50 of 0.767 μM). This work is a step forward for a machine learning enabled, multiparameter optimization of a chemical space and represents a significant advance in the development of novel Parkinson’s therapies.
Keywords: Parkinson’s disease, c-Abl inhibitors, hERG, QT prolongation, machine learning
Neurodegenerative disease, a category which includes Parkinson’s disease (PD) and Alzheimer’s disease, represents a significant healthcare burden worldwide that is expected to increase as lifespans are prolonged.1,2 A 2015 United Nations report on world population aging anticipates a doubling of the number of people aged 60 years or older over the next 35 years, and this demographic is the most at risk for developing neurodegenerative disease.3 PD is the second most common neurodegenerative disease, affecting 2–3% of those over 65 years of age.4 The classic motor symptoms include bradykinesia, postural instability, resting tremor, and muscle stiffness. Additionally, there is significant disability for the patient in that dementia, depression, dysphagia, insomnia, and loss of smell frequently develop as PD progresses.5 These nonmotor symptoms are key determinants of the quality of life in Parkinson’s patients and are gaining recognition as important aspects to consider in therapeutic development.5,6
The underlying pathogenesis of PD is complex. A key component of the disease process is neurodegeneration due to selective and progressive loss of dopaminergic neurons in the substantia nigra pars compacta.7 The intracellular accumulation of α-synuclein and the subsequent formation of Lewy bodies in the brainstem and olfactory system is an additional histopathological feature of PD, but neither Lewy body formation nor dopaminergic neuron loss of the substantia nigra alone define Parkinson’s.8 Instead, it is the combination of these two histopathological features that results in the disease.8,9
Current therapy for PD is entirely focused on symptomatic relief, and there are severe side effects associated with most of the first line agents in clinical use.10 For example, dyskinesias, exacerbation of insomnia, movement freezing, and dose failure are common consequences of L-DOPA/carbidopa therapy.11 However, new insights into the pathological mechanisms have inspired a novel strategy for PD drug development. Work in both animal models of PD and in post-mortem analysis of human tissue affected by Parkinson’s has revealed the role of oxidative stress in dopaminergic neurons as a key pathogenic step in PD.2,12 One of the key links between oxidative stress and neurodegeneration is c-Abl, a kinase historically associated with the BCR-Abl fusion gene in certain leukemias.13 Recent work has linked aberrations in c-Abl activity resulting from oxidative stress to neurodegeneration in both Alzheimer’s and Parkinson’s diseases.14−18 c-Abl activation and its subsequent phosphorylation of parkin result in inhibition of the ubiquitin ligase activity of parkin, leading to the accumulation of toxic parkin substrates, including parkin interacting substrate (PARIS) and aminoacyl tRNA synthetase complex-interacting multifunctional protein 2 (AIMP2).19,20 The findings that both PARIS and AIMP2 accumulate in familial PD caused by parkin mutations and in MPTP toxin mouse models of PD strongly implicates c-Abl in the pathogenesis of the disease.19,20
Based on these findings, several groups have explored the use of the known BCR-Abl inhibitors that are used clinically for treating chronic myeloid leukemia to also treat PD. This class of drugs, which includes imatinib, nilotinib, and ponatinib among others (Figure 1), has exhibited activity against untranslocated c-Abl.21 Specifically, imatinib was found to restore parkin ubiquitin ligase activity and reduce PARIS and AIMP2 levels in the MPTP mouse model.16,17 Similarly, administration of nilotinib protected against MPTP-induced dopaminergic deficits and reversed the loss of dopamine neurons, a result known to improve motor behavior.22,23 Additionally, a 12-patient, nonrandomized phase I trial conducted at Georgetown University demonstrated that nilotinib treatment improved motor and cognitive outcomes.24 A follow-up study evaluating the pharmacokinetics and pharmacodynamics of nilotinib in PD patients found that a 200 mg single dose of nilotinib was capable of increasing cerebrospinal fluid concentrations of dopamine metabolites.25 Currently, a phase II trial evaluating nilotinib in PD is underway (NCT02954978).
Figure 1.
Selection of approved c-Abl inhibitors.
There are, however, significant side effects associated with these drugs that may adversely impact their use in treating Parkinson’s disease. Nilotinib is known to be a potent inhibitor of the human Ether-à-go-go-Related Gene (hERG), a potassium channel that generates the repolarization current in the cardiac action potential. Inhibition of this repolarization process leads to prolongation of the QT interval, which can progress to torsades de points and cardiac arrest. Nilotinib thus carries a black box warning for QT prolongation and sudden death (2.1% of patients experience QT increases of >60 ms, while <1% experience QT increases of >500 ms; sudden deaths reported in 0.6% of patients).26,27 In addition, nilotinib is a potent inhibitor of CYP3A4, which could preclude its use in patients who are also taking drugs that are metabolized by this enzyme. Similarly, ponatinib has a black box warning for vascular occlusion, heart failure, and hepatotoxicity.28−30
We were intrigued by the possibility of designing new chemical entities that exhibit both good activity against c-Abl and minimal off-target liabilities against hERG. Given our previous successes in using machine learning to optimize the properties of known drugs,34,35 we sought to design novel c-Abl inhibitors with these features.
Results and Discussion
Our initial investigation began with the design of two machine learning workflows. The first employed a single Naïve Bayes Network (NBN) exclusively designed to predict the potency of novel small molecules against c-Abl (the compounds ranked from this workflow will be referred to as single-NBN compounds). The second used two NBNs working in concert; one designed for potency against c-Abl and the other to predict molecules with IC50 values greater than 1 μM against hERG (compounds ranked with this method will be referred to as dual-NBN compounds). To this end, we acquired and curated c-Abl and hERG data sets from ChEMBL and enhanced them with additional information from the patent literature to train the desired NBNs.31−33 In addition, by prospectively testing both workflows, we could experimentally determine if there was a potency reduction resulting from the additional filtering step in the dual-NBN workflow. An overview of our machine learning approaches, as well as the general workflows employed, is briefly discussed in our previous publications.34,35
In selecting the core scaffolds that would be evaluated by our algorithms, we considered the medicinal chemistry profile of nilotinib, since it had shown disease modification in animal studies, as well as ponatinib, which has not yet been evaluated in Parkinson’s disease. From our perspective, analogs of ponatinib provide two key advantages over nilotinib analogs: the ponatinib scaffold is straightforward to prepare, and the parent compound offers a significantly more potent starting point for new analog creation. As a consequence, losses in c-Abl potency that may result from the inclusion of a hERG NBN might still provide us with the opportunity to obtain low nanomolar inhibitors.
Workflows were constructed in Pipeline Pilot36 and used to determine the chemical space most amenable to address the c-Abl/hERG selectivity problem. Using this approach, we were able to explore millions of synthetic candidates not previously reported in the scientific or patent literature. The rankings of the top candidates produced by single-NBN (c-Abl only) and dual-NBN (c-Abl/hERG) workflows are shown in Figure 2. The reader will note that several highly prioritized candidates were not prepared because of the synthetic challenges they presented.
Figure 2.
Synthetic targets generated for the single-NBN and the dual-NBN workflows.
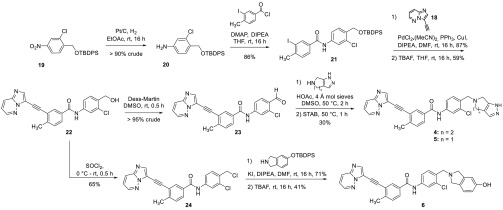
Two synthetic approaches were used to prepare the candidate compounds, a linear synthesis for the single-NBN compounds and a divergent synthesis for the dual-NBN series (Schemes 1 and 2). The linear synthesis began with alkylation of the TBDPS and methyl ether piperazinyl alcohols (9a and 9b) by 7 or 8 to afford 10a, 10b, and 11, respectively (details are included in the accompanying Supporting Information). Suzuki coupling with cyclopropyl boronic acid and subsequent hydrogenation produced 13a and 13b in >76% yield over two steps. Direct hydrogenation of 11 afforded 14 in >95% yield. Compounds 13a, 13b, and 14 were each allowed to react with the indicated acyl chloride to afford amides 15–17 in 70%, 77%, and 85% yield, respectively. Sonogashira coupling of 17 with 18 afforded target compound 3 in 31% yield, and the TBDPS protected 15 and 16 were similarly coupled with 18 in 72% and 33% yield, respectively. Subsequent desilylation of these two compounds gave target compounds 1 and 2 in 91% and 73%, respectively.
Scheme 1. Linear Synthesis of Single-NBN Compounds (c-Abl Alone).
Scheme 2. Divergent Synthesis of the Dual-NBN Compounds (cAbl/hERG).
The divergent synthesis for the dual-NBN compounds began with reduction and acylation of 19 to give the desired amide 21 in greater than >77% yield over two steps (Scheme 2). Sonogashira coupling with alkyne 18 afforded key intermediate 22 in 51% yield over two steps. From 22, target compounds 4 and 5 were accessed via Dess Martin oxidation of the benzylic alcohol, followed by reductive amination with the requisite bicyclic amine in >30% yield over three steps. Additionally, target compound 6 was prepared by chlorination of 22 and subsequent alkylation of the resulting benzylic chloride with the indicated isoindoline followed by deprotection. This strategy was used for 6 as the reductive amination conditions which were successful for 4 and 5 ultimately failed to provide 6. These compounds were then evaluated for their biological activities against c-Abl, hERG, and CYP3A4.
Table 1 shows the biological activities of our compounds against the indicated targets. Compounds 1–3 are single-NBN compounds evaluated solely by the c-Abl NBN and, as predicted, exhibited picomolar IC50’s against c-Abl. However, like their parent structure, they also potently inhibited hERG, rendering them unsuitable for further clinical development. Alternatively, compounds 4 and 5, designed with the dual-NBN, were highly active against c-Abl (<10 nM) and exhibited significantly improved IC50 values against hERG (i.e., 5.02 and 12.1 μM, respectively). Additionally, these compounds had IC50 values greater than 5 μM against CYP3A4. Moreover, compounds 4 and 5 were found to have substantially greater human liver microsome half-lives than nilotinib, although they were not as good as ponatinib (Table 1). Compounds 4 and 5 exhibit negligible toxicity in THLE-3 and HEK293 cell lines. Taken collectively, these data indicate that4and5possess an improved profile relative to both nilotinib and ponatinib. Importantly, while the submicromolar hERG inhibition of ponatinib makes it unsuitable for use in patient populations that have cardiovascular issues, it is unlikely that compounds 4 and 5 could ever achieve the double digit micromolar in vivo concentrations required to significantly inhibit hERG.37
Table 1. cAbl, hERG, and CYP3A4 Activities as Well as Metabolic Stability and CC50 Values for Target Compounds.
| cAbl IC50 (nM) | hERG IC50 (μM) | CYP3A4 IC50 (μM) | Mouse Liver Microsomes t1/2 (min) | Human Liver Microsomes t1/2 (min) | THLE-3 CC50 (μM) | HEK293 CC50 (μM) | |
|---|---|---|---|---|---|---|---|
| 1 | 0.30 | 0.34 | 11.7 | ||||
| 2 | 0.39 | 0.10 | 13.4 | ||||
| 3 | 0.65 | 0.08 | 13.5 | ||||
| 4 | 9.96 | 5.02 | 5.58 | 10.95 | 26.87 | 17.0 | 14.5 |
| 5 | 8.31 | 12.1 | 7.28 | 16.78 | 39.84 | >50.0 | >50.0 |
| 6 | >30 | 0.78 | 4.61 | 26.45 | 157.53 | ||
| Nilotinib | 5.56 | 0.45 | 0.58 | 7.89 | 7.89 | ||
| Ponatinib | 0.31 | 0.77 | 11.4 | 31.94 | 182.4 |
Because of the high structural homology of the ATP-binding pockets of kinases, all c-Abl inhibitors run the risk of inhibiting other kinases.38 However, inhibition of multiple kinases may not necessarily be deleterious, as Fowler et al. have demonstrated that inhibition of the Discoidin Domain Receptors (DDR1/2), the platelet-derived growth factor receptor α and β (PDGFRs), and the tyrosine kinase, Src, potentiate the effect of c-Abl inhibition.39 To evaluate the kinase inhibition profile of 4 and 5, we had them screened at 10 μM concentrations against the panel of >300 kinases maintained by the University of Dundee.40−42 This screen revealed that at 10 μM nilotinib inhibited 9 kinases at >50%. Similarly, ponatinib inhibited 73 kinases, while compounds 4 and 5 inhibited 31 and 43 kinases, respectively (see Supporting Information). Table 2 shows the percent activity remaining of selected kinases screened at 10 μM against nilotinib, ponatinib, 4, and 5, as well as the IC50 values for 4 and 5. Compound 4 was very potent against PDGFRα, modestly inhibited Src, but did not inhibit DDR2. Conversely, compound 5 inhibited PDGRFα, Src, and DDR2. Since our algorithm was not trained to predict kinase selectivity, we consider the inhibition of kinases known to potentiate c-Abl by compounds 4 and 5 to be largely fortuitous. However, since the structure of each compound encodes its intrinsic response in a given measurement, the structural similarities of our compounds to nilotinib and ponatinib undoubtedly bias them to have somewhat comparable profiles. More importantly, compounds 4 and 5 represent an excellent starting point for designing next generation c-Abl inhibitors with more desirable properties.
Table 2. % Activity Remaining and IC50 Values of Nilotinib, Ponatinib, 4, and 5 for Selected Kinases.
| % Activity Remaining |
% Activity Remaining and [IC50 (μM)] |
|||
|---|---|---|---|---|
| kinase | Nilotinib | Ponatinib | 4 | 5 |
| ABL | 2 | 1 | 8 [0.0031] | 11 [0.0013] |
| DDR2 | 4 | 3 | 70 [>20] | 14 [0.2862] |
| PDGFRa | 56 | 25 | 10 [0.0021] | 16 [0.0020] |
| Src | 74 | 11 | 23 [0.7097] | 15 [0.4313] |
| EPHB2 | 25 | 2 | 2 [0.0932] | 1 [0.0708] |
| EPHB3 | 52 | 1 | 6 [0.0505] | 5 [0.0195] |
| Lck | 37 | 29 | 22 [0.0908] | 17 [0.0261] |
| EPHA4 | 23 | 4 | 6 [0.1381] | 2 [0.0846] |
| NEK6 | 97 | 52 | 45 [3.3060] | 9 [5.3500] |
| VEGFR | 75 | 28 | 14 [0.0336] | 13 [0.0448] |
| MAP4K3 | 107 | 2 | 62 [>20] | 14 [0.2404] |
Conclusions
We utilized a machine learning paradigm to identify a new series of compounds that were simultaneously optimized for on-target potency (c-Abl inhibition) and off-target safety (hERG). We employed a single NBN to predict novel, highly potent ponatinib analogs. By using two NBNs in concert, one optimized for c-Abl potency and the other for minimizing hERG liability, we identified novel compounds, 4 and 5, with single digit nanomolar potencies and only modest inhibition of hERG. Although less potent than ponatinib, our best compounds compare favorably to nilotinib, the current agent in clinical trials for Parkinson’s disease. Moreover, our best compounds exhibited a much improved CYP3A4 profile, as well as improved metabolic stability as compared to nilotinib. Initial development revealed our compounds to be nontoxic against THLE-3 cells and HEK293 cells. Also, compound 4 potently inhibited PDGFRα and Src, while compound 5 inhibited PDGFRα, Src, and DDR2, kinase profiles that recent data suggests will improve efficacy in treating PD. These unexpected kinase profiles were not anticipated at the time of machine learning design and serve to highlight the important role serendipity often plays in drug development. However, key biological experiments remain that will demonstrate the utility of compounds 4 and 5. In particular, the cerebrospinal fluid concentration that can be achieved by 4 and 5 will likely be a key predictor of their efficacy in treating PD, and this is a key data point which will be acquired in future animal studies. Most importantly, however, by using machine learning, we were able to significantly accelerate the hit-to-lead optimization of these kinase inhibitors, as well as simultaneously address multiple defects in the properties of clinical agents. The work reported herein bodes well for the potential utility of the compounds designed in this study to treat Parkinson’s disease.
Acknowledgments
The authors wish to thank Sameshnee Pelly for her excellent assistance in preparing this manuscript.
Glossary
Abbreviations
- PD
Parkinson’s Disease
- L-DOPA
L-3,4-dihydroxy-phenylalanine
- BCR
breakpoint cluster region
- PARIS
parkin interacting substrate
- AIMP2
aminoacyl tRNA synthetase complex-interacting multifunctional protein 2
- NBN
Naïve Bayes Network.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.9b00612.
Complete description of the synthesis and characterization of all of the compounds described in the manuscript. Complete details of the biological assessments. (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Elbaz A.; Carcaillon L.; Kab S.; Moisan F. Epidemiology of Parkinson’s disease. Rev. Neurol. 2016, 172, 14–16. 10.1016/j.neurol.2015.09.012. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T. Ageing, neurodegeneration and brain rejuvenation. Nature 2016, 539, 180–186. 10.1038/nature20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Population Ageing 2015 (ST/ESA/SER.A/390); United Nations: New York, NY, 2015. [Google Scholar]
- Poewe W.; Seppi K.; Tanner C. M.; Halliday G. M.; Brundin P.; Volkmann J.; Schrag A.-E.; Lang A. E. Parkinson disease. Nature Reviews Disease Primers 2017, 3, 17013. 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- Chaudhuri K. R.; Schapira A. H. V. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009, 8, 464–474. 10.1016/S1474-4422(09)70068-7. [DOI] [PubMed] [Google Scholar]
- Parkinson’s Disease: National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care; Royal College of Physicians: London, UK, 2006; Vol. 35. [PubMed] [Google Scholar]
- Benarroch E. E.; Cutsforth-Gregory J. K.; Flemming K. D.. Mayo Clinic Medical Neurosciences: Organized by Neurologic System and Level, 6th ed.; Oxford University Press: New York, NY, 2018. [Google Scholar]
- Dickson D. W.; Braak H.; Duda J. E.; Duyckaerts C.; Gasser T.; Halliday G. M.; Hardy J.; Leverenz J. B.; Tredici K. D.; Wszolek Z. K.; Litvan I. Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol. 2009, 8, 1150–1157. 10.1016/S1474-4422(09)70238-8. [DOI] [PubMed] [Google Scholar]
- Braak H.; Tredici K. D.; Rüba U.; Vos R. A. I. d.; Steur E. N. H. J.; Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. 10.1016/S0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Poewe W.; Mahlknecht P.; Jankovic J. Emerging therapies for Parkinson’s disease. Curr. Opin. Neurol. 2012, 25, 448–459. 10.1097/WCO.0b013e3283542fde. [DOI] [PubMed] [Google Scholar]
- Jankovic J.; Poewe W. Therapies in Parkinson’s disease. Curr. Opin. Neurol. 2012, 25, 433–447. 10.1097/WCO.0b013e3283542fc2. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Dawson V. L.; Dawson T. M. Oxidative Stress and Genetics in the Pathogenesis of Parkinson’s Disease. Neurobiol. Dis. 2000, 7, 240–250. 10.1006/nbdi.2000.0319. [DOI] [PubMed] [Google Scholar]
- Druker B. J.; Lydon N. B. Lessons learned from the development of an Abl tyrosine kinase inhibitor for chronic myelogenous leukemia. J. Clin. Invest. 2000, 105, 3–7. 10.1172/JCI9083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B. c-Abl in oxidative stress, aging and cancer. Cell Cycle 2005, 4, 201–203. 10.4161/cc.4.2.1490. [DOI] [PubMed] [Google Scholar]
- Schlatterer S. D.; Acker C. M.; Davies P. c-Abl in Neurodegenerative Disease. J. Mol. Neurosci. 2011, 45, 445–452. 10.1007/s12031-011-9588-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam S. Z.; Zhou Q.; Yamamoto A.; Valente A. J.; Ali S. F.; Bains M.; Roberts J. L.; Kahle P. J.; Clark R. A.; Li S. Novel Regulation of Parkin Function through c-Abl-Mediated Tyrosine Phosphorylation: Implications for Parkinson’s Disease. J. Neurosci. 2011, 31, 157–163. 10.1523/JNEUROSCI.1833-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H. S.; Lee Y.; Shin J.-H.; Karuppagounder S. S.; Gadad B. S.; Koleske A. J.; Pletnikova O.; Troncoso J. C.; Dawson V. L.; Dawson T. M. Phosphorylation by the c-Abl protein tyrosine kinase inhibits parkin’s ubiquitination and protective function. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 16691–16696. 10.1073/pnas.1006083107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahul-Mellier A.-L.; Fauvet B.; Gysbers A.; Dikiy I.; Oueslati A.; Georgeon S.; Lamontanara A. J.; Bisquertt A.; Eliezer D.; Masliah E.; Halliday G.; Hantsche O.; Lashuel H. A. c-Abl phosphorylates α-synuclein and regulates its degradation: implication for α-synuclein clearance and contribution to the pathogenesis of Parkinson’s disease. Hum. Mol. Genet. 2014, 23, 2858–2879. 10.1093/hmg/ddt674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J.-H.; Ko H. S.; Kang H.; Lee Y.; Lee Y.-I.; Pletinkova O.; Troconso J. C.; Dawson V. L.; Dawson T. M. PARIS (ZNF746) Repression of PGC-1α Contributes to Neurodegeneration in Parkinson’s Disease. Cell 2011, 144, 689–702. 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.; Karuppagounder S. S.; Shin J.-H.; Lee Y.-I.; Ko H. S.; Swing D.; Jiang H.; Kang S.-U.; Lee B. D.; Kang H. C.; Kim D.; Tessarollo L.; Dawson V. L.; Dawson T. M. Parthanatos mediates AIMP2-activated age-dependent dopaminergic neuronal loss. Nat. Neurosci. 2013, 16, 1392–1400. 10.1038/nn.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossari F.; Minutolo F.; Orciuolo E. Past, present, and future of Bcr-Abl inhibitors: from chemical development to clinical efficacy. J. Hematol. Oncol. 2018, 11, 84. 10.1186/s13045-018-0624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karuppagounder S. S.; Brahmachari S.; Lee Y.; Dawson V. L.; Dawson T. M.; Ko H. S. The c-Abl inhibitor, Nilotinib, protects dopaminergic neurons in a preclinical animal model of Parkinson’s disease. Sci. Rep. 2015, 4, 4874. 10.1038/srep04874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebron M. L.; Lonskaya I.; Moussa C. E.-H. Nilotinib reverses loss of dopamine neurons and improves motor behavior via autophagic degradation of α-synuclein in Parkinson’s disease models. Hum. Mol. Genet. 2013, 22, 3315–3328. 10.1093/hmg/ddt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagan F.; Hebron M.; Valadez E. H.; Torres-Yaghi Y.; Huang X.; Mills R. R.; Wilmarth B. M.; Howard H.; Dunn C.; Carlson A.; Lawler A.; Rogers S. L.; Falconer R. A.; Ahn J.; Li Z.; Moussa C. Nilotinib Effects in Parkinson’s Disease and Dementia with Lewy Bodies. J. Parkinson's Dis. 2016, 6, 503–517. 10.3233/JPD-160867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagan F. L.; Hebron M. L.; Wilmarth B.; Torres-Yaghi Y.; Lawler A.; Mundel E. E.; Yusuf N.; Starr N. J.; Arellano J.; Howard H. H.; Peyton M.; Matar S.; Liu X.; Fowler A. J.; Schwartz S. L.; Ahn J.; Moussa C.. Pharmacokinetics and pharmacodynamics of a single dose Nilotinib in individuals with Parkinson’s disease; Pharmacology Research & Perspectives, 2019, e00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty K. R.; Wappel R. L.; Talbert D. R.; Trusk P. B.; Moran D. M.; Kramer J. W.; Brown A. M.; Shell S. A.; Bacus S. Multi-parameter in vitro toxicity testing of crizotinib, sunitinib, erlotinib, and nilotinib in human cardiomyocytes. Toxicol. Appl. Pharmacol. 2013, 272, 245–255. 10.1016/j.taap.2013.04.027. [DOI] [PubMed] [Google Scholar]
- Goldenberg M. M. Pharmaceutical Approval Update. Pharmacy and Therapeutics 2008, 33, 54–57. [PMC free article] [PubMed] [Google Scholar]
- Rivera V. M.; Pritchard J. R.; Gonzalvez F.; Baker T.; Gozgit J. M.; Hodgson G. Comparative TKI Profiling Analyses to Explore Potential Mechanisms of Ponatinib-Associated Arterial Thrombotic Events. Blood 2014, 124, 1783. 10.1182/blood.V124.21.1783.1783. [DOI] [Google Scholar]
- Latifi Y.; Moccetti F.; Wu M.; Xie A.; Packwood W.; Qi Y.; Ozawa K.; Shentu W.; Brown E.; Shirai T.; McCarty O. J.; Ruggeri Z.; Moslehi J.; Chen J.; Druker B. J.; López J. A.; Lindner J. R. Thrombotic microangipathy as a cause for cardiovascular toxicity from the BCR-ABL1 tyrosine kinase inhibitor ponatinib. Blood 2019, 133, 1597–1606. 10.1182/blood-2018-10-881557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy S. M. Ponatinib: a review of its use in adults with chronic myeloid leukaemia or Philadelphia chromosome-positive acute lymphoblastic leukaemia. Drugs 2014, 74, 793–806. 10.1007/s40265-014-0216-6. [DOI] [PubMed] [Google Scholar]
- Bento A. P.; Gaulton A.; Hersey A.; Bellis L. J.; Chambers J.; Davies M.; Kruger F. A.; Light Y.; Mak L.; McGlinchey S.; Nowotka M.; Papadatos G.; Santos R.; Overington J. P. The ChEMBL bioactivity database: an update. Nucleic Acids Res. 2014, 42, 1083–1090. 10.1093/nar/gkt1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadatos G.; Gaulton A.; Hersey A.; Overington J. P. Activity, assay and target data curation and quality in the ChEMBL database. J. Comput.-Aided Mol. Des. 2015, 29, 885–896. 10.1007/s10822-015-9860-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaulton A.; Bellis L. J.; Bento A. P.; Chambers J.; Davies M.; Hersey A.; Light Y.; McGlinchey S.; Michalovich D.; Al-Lazikani B.; Overington J. P. ChEMBL: a large-scale bioactivity database for drug discovery. Nucleic Acids Res. 2012, 40 (D1), D1100–D1107. 10.1093/nar/gkr777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser T. M.; Burger P. B.; Butch C. J.; Pelly S. C.; Liotta D. C. A Machine Learning Approach for Predicting HIV Reverse Transcriptase Mutation Susceptibility of Biologically Active Compounds. J. Chem. Inf. Model. 2018, 58, 1544–1552. 10.1021/acs.jcim.7b00475. [DOI] [PubMed] [Google Scholar]
- Shi Q.; Kaiser T. M.; Dentmon Z. W.; Ceruso M.; Vullo D.; Supuran C. T.; Snyder J. P. Design and Validation of FRESH, A drug Discovery Paradigm Resting on Robust Chemical Synthesis. ACS Med. Chem. Lett. 2015, 6, 518–522. 10.1021/acsmedchemlett.5b00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIOVIA Pipeline Pilot. https://www.3dsbiovia.com/products/collaborative-science/biovia-pipeline-pilot/ (accessed 2020-02-16).
- Compound 6 failed to meet our targeted profile (c-Abl IC50 value > 30 nM; hERG IC50 = 780 nM) indicating that our algorithms need further refinement. .
- Robinson D. D.; Sherman W.; Farid R. Understanding Kinase Selectivity Through Energetic Analysis of Binding Site Waters. ChemMedChem 2010, 5, 618–627. 10.1002/cmdc.200900501. [DOI] [PubMed] [Google Scholar]
- Fowler A. J.; Hebron M.; Missner A. A.; Wang R.; Gao X.; Kurd-Misto B. T.; Liu X.; Moussa C. E. H. Multikinase Abl/DDR/Src Inhibition Produces Optimal Effects for Tyrosine Kinase Inhibition in Neurodegeneration. Drugs R&D 2019, 19, 149–166. 10.1007/s40268-019-0266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa D.; Yokota K.; Gouda M.; Narumi Y.; Ohmoto H.; Nishiwaki E.; Akita K.; Kirii Y. Activity-based kinase profiling of approved tyrosine kinase inhibitors. Genes to Cells 2013, 18, 110–122. 10.1111/gtc.12022. [DOI] [PubMed] [Google Scholar]
- Hastie C. J.; McLauchlan H. J.; Cohen P. Assay of protein kinases using radiolabeled ATP: a protocol. Nat. Protoc. 2006, 1 (2), 968–71. 10.1038/nprot.2006.149. [DOI] [PubMed] [Google Scholar]
- Bain J.; Plater L.; Elliott M.; Shpiro N.; Hastie C. J.; McLauchlan H.; Klevernic I.; Arthur J. S.; Alessi D. R.; Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem. J. 2007, 408 (3), 297–315. 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.