Abstract
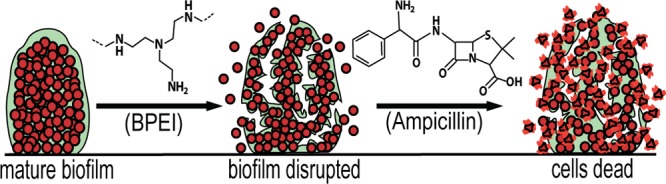
Methicillin-resistant Staphylococcus aureus (MRSA) infections pose a serious threat worldwide. MRSA is the predominant species isolated from medical-device-related biofilm infections and chronic wounds. Its ability to form biofilms grants it resistance to almost all antibiotics on the market. Answering the call for alternative treatments, our lab has been investigating the efficacy of 600 Da branched polyethylenimine (BPEI) as a β-lactam potentiator against bacterial biofilms. Our previous study showed promise against methicillin-resistant Staphylococcus epidermidis biofilms. This study extends our previous findings to eradicate a more virulent pathogen: MRSA biofilms. Microtiter minimum biofilm eradication concentration models, crystal violet assays, and electron microscopy images show synergistic effects between BPEI and ampicillin as a two-step mechanism: step one is the removal of the extracellular polymeric substances (EPS) to expose individual bacteria targets, and step two involves electrostatic interaction of BPEI with anionic teichoic acid in the cell wall to potentiate the antibiotic.
Keywords: MRSA biofilm, MBEC, BPEI, Antibiotic potentiator, Antibiotic resistance
The threat posed by antimicrobial resistance (AMR) on human health is well-known. We recently reported that 600 Da BPEI eliminates β-lactam resistance in methicillin-resistance Staphylococcus aureus (MRSA) by preventing the essential localization of PBP4 enzymes.1 However, the sinister nature of AMR infections is amplified when the pathogens are sequestered in biofilms that shield them from effective antimicrobials and/or the innate immune system. According to a systematic review and meta-analysis,2 the prevalence of biofilms in chronic wounds is almost 80%. Many of the predominant species found in chronic wounds are from the genus Staphylococcus (∼60%).3 In addition to compromising wound healing,4Staphylococcus aureus contributes a high percentage to biomedical device infections.5 Bacterial biofilms are resilient because their self-produced matrix of extracellular polymetric substances (EPS) grants them protection against host defenses and antibiotics.6−8 The EPS matrix contains hydrated carbohydrate polymers, proteins, and extracellular DNA (eDNA) in a complex architecture to provide nutrients, promote the transfer of genetic material, and protect the biofilm against harsh conditions. Only the outermost layers of cells in a biofilm are metabolically active, while the persistent inner-layer cells remain dormant, thereby evading antibiotics.7 First-line β-lactam antibiotics, such as ampicillin, are the most commonly prescribed drugs for bacterial infections. In many developing countries, these antibiotics are sold over the counter, and their use in livestock is poorly regulated. Lack of regulation can lead to overexposure, thereby encouraging acquired antimicrobial resistance. As the most common agricultural pathogens in developing countries, AMR has a convenient means of spreading to humans.9 According to the Centers for Disease Control and Prevention (CDC), MRSA infections pose a grave threat to the society and economy.10 One out of seven severe cases of MRSA results in death.11 Its resistance has been documented within all available antibiotic classes, including the last-resort antibiotics.12 With a dwindling collection of new antibiotics and in the absence of antibiofilm drugs on the market, alternative treatments that combine existing drugs with potentiators have become a central line of research. Here, we demonstrate the ability of 600 Da branched polyethylenimine (BPEI) to eradicate MRSA biofilms. Our previous studies have shown that this low-molecular-weight BPEI exhibits low in vitro cytotoxicity on human cells13 and strong potentiation with β-lactam antibiotics against planktonic MRSA cells.13,14 Strong synergy was also found against methicillin-resistant Staphylococcus epidermidis (MRSE) and its biofilms.15,16 Thus, we hypothesize that BPEI would potentiate ampicillin against MRSA biofilms using similar biochemical mechanisms.
Gram-positive bacteria, such as S. aureus and S. epidermidis, have a thick peptidoglycan layer in their cell walls. For each division cycle, penicillin-binding proteins (PBPs) are responsible for one of the last stages of cell wall synthesis: cross-linking the subunits of the peptidoglycan. β-Lactam antibiotics irreversibly bind to PBPs, preventing them from performing this vital function. Consequently, the bacteria are unable to divide and eventually burst from excessive cytoplasmic pressure. However, in MRSA/MRSE, the enzymes PBP2a and PBP4 with low binding affinity to β-lactams allow the bacteria to withstand the antibiotic attack. An important regulator of PBP2a/4 is wall teichoic acid (WTA) that is decorated with N-acetylglucosamine, d-alanine, and hydroxyl on a phosphodiester backbone.17,18 In Gram-positive bacteria, WTA polymer can be divided in two main components: a disaccharide linkage unit and a repeating unit. The disaccharide linkage unit is highly conserved across Gram-positive bacteria. The repeating unit exhibits structural diversity and can be divided into four different classes: polyol phosphate, glycosylpolyol phosphate, glycosyl phosphate polyol phosphate, and polyol phosphate-glycosylpolyol phosphate. d-Alanine content is variable and can be tailored on the repeating unit hydroxyls, depending on environmental conditions. Despite their diversity, all WTAs share a total of negatively charge due to their anionic phosphate backbone.19 The phosphates impart strong anionic properties to WTA and consequently WTA attracts essential metal cations to the cell wall environment.20−24 However, we have shown that the anionic nature of WTA can be exploited to circumvent the PBP2a/4 enzymes responsible for β-lactam resistance in MRSA. The 600 Da BPEI, a small cationic polymer, electrostatically binds to anionic WTA in the bacterial cell wall, thus prohibiting WTA from properly localizing PBP2a/4 enzymes. This process effectively potentiates β-lactams against planktonic MRSA1,13,14 and MRSE.15,16 As described below, we extend the investigation of 600 Da BPEI potentiators to MRSA biofilms and demonstrate strong efficacy against two biofilm-forming MRSA clinical isolates (MRSA OU6 and OU11) that are strongly resistant to antibiotics (clinical data in the Supporting Information).
Methods (described in the Supporting Information) were adapted from previous work.16 Minimum biofilm eradication concentration (MBEC) assays were utilized on the two clinical isolates of MRSA (OU6 and OU11) and a lab strain MRSA ATCC 43300. The MRSA bacteria were used to inoculate a 96-well inoculation plate, where MRSA biofilms were grown on prongs protruding from the plate lid, known as the MBEC inoculator lid and based on the Calgary biofilm device. The inoculator lid was washed to remove unattached MRSA cells and transferred into a separate 96-well base for treatment with BPEI and ampicillin combinations arranged in a checkerboard assay pattern, the so-called challenge plate. The final step is moving the treated inoculation lid to a third plate (the recovery plate) containing growth-media only and using sonication to dislodge the biofilm and recover cells remaining in the biofilm. In this manner, we are able to evaluate the synergy of BPEI and ampicillin against MRSA biofilms. Standard CLSI (Clinical & Laboratory Standards Institute) guidelines describe a standard MIC assay using 96-well plates inoculated with a standard cell density, usually ∼106 CFU/mL. However, the MIC data reported here is nonstandard because, rather than inoculation via micropipet transfer from an overnight culture, inoculation of the challenge plate occurs from the biofilm-coated inoculation lid where treatment challenge disrupts the protective biofilm EPS matrix. MRSA cells are dislodged and dispersed into the challenge plate media. These cells in the challenge plate media are susceptible to killing by the 600 Da BPEI, ampicillin, or their combinations, and a minimum inhibitory concentration can be determined. We refer to this value as MICCP to differentiate it from MIC measurements made with standard methods. The MBEC is determined from cell growth in the recovery plate and reflects the ability of 600 Da BPEI, ampicillin, or its combinations to kill the biofilm remaining attached to the prongs of the inoculation lid. The MICCP and MBEC data are shown for comparison (Table 1).
Table 1. Synergistic Effects between BPEI and Ampicillin against MRSA Biofilms.
| BPEI (μg/mL) |
ampicillin (μg/mL) |
||||||
|---|---|---|---|---|---|---|---|
| strain | MICCP | MBEC | MICCP | MBEC | MBEC + 64 μg/mL BPEI | FICI | synergy? |
| MRSA 43300 | 64 | >256 | 128 | >256 | 2 | 0.13 | yes |
| MRSA OU6 | >256 | >256 | 256 | >256 | 64 | 0.25 | yes |
| MRSA OU11 | >256 | >256 | 128 | >256 | 32 | 0.19 | yes |
As shown in Table 1, MRSA 43300s BPEI MBEC (>256 μg/mL) is much larger than its MICCP (64 μg/mL). Similarly, the ampicillin MBEC (>256 μg/mL) is higher than the corresponding MICCP (128 μg/mL). The MBECs for BPEI and ampicillin against the two clinical isolates, MRSA OU6 and OU11, are greater than the highest amount tested, 256 μg/mL. Although the MBECs exceeded the tested concentrations, strong synergy (FICI < 0.5) was found between BPEI and ampicillin against the biofilms of MRSA 43300, OU11, and OU6 with an FICI of 0.13, 0.25, and 0.19, respectively. For example, when combined with 64 μg/mL of BPEI, the ampicillin MBECs for MRSA 43300, OU6, and OU11 were reduced to 2, 64, and 32 μg/mL, respectively. For these strains, the MICCP is higher than previously reported values for planktonic MRSA cells evaluated with CLSI methods,1 which showed that 600 Da BPEI lowers the MIC for the planktonic cells and renders them susceptible to oxacillin. As described above, the disparity arises from different methods of inoculation and the cell density in the challenge plate media is unknown and likely varies between wells. Nevertheless, the MICCP can be used to show that BPEI and ampicillin combinations can be used to kill antibiotic-resistant cells dislodged from the inoculation lid.
Heat maps of the average checkerboard results are shown in Figure 1. Data used to determine MICCP in the challenge plate containing MRSA planktonic data are shown on the left (Figure 1Ai, Bi, and Ci), and the corresponding biofilm data are on the right (Figure 1Aii, Bii, and Cii). As expected, the MBECs are larger than the respective MICCP values. This demonstrates the intrinsic protective nature of biofilms against antimicrobial agents. The staircase pattern found in the heat maps indicates that multiple combinations of BPEI and ampicillin are effective against both planktonic and biofilm forms of MRSA 43300, OU6, and OU11 strains. As BPEI concentration increases, the required MICCP and MBEC values of ampicillin decrease to achieve high inhibition percentage, highlighting the potentiating ability of BPEI against pathogenic biofilms.
Figure 1.
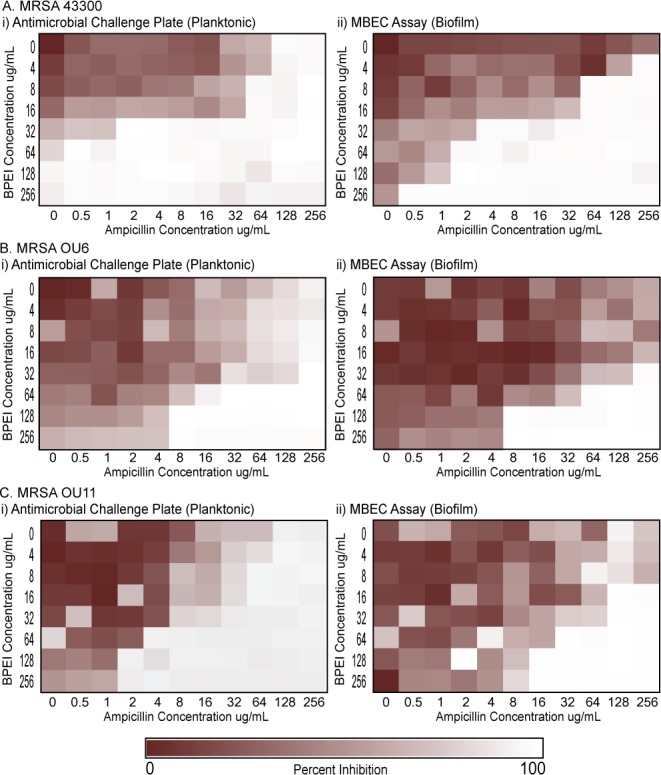
Synergy between BPEI and ampicillin against MRSA 43300 (A), MRSA OU6 (B), and MRSA OU11 (C). Checkerboard assay data on planktonic bacteria are shown on the left (Ai, Bi, and Ci), and corresponding biofilm data are shown on the right (Aii, Bii, and Cii).
To better elucidate the antibiofilm activity of BPEI, biofilm disruption assays were conducted along with a comparison study using the common cationic antibiotic polymyxin B. Briefly, MRSA OU6 biofilms were grown on the bottom of a 96-well plate for 24 h. After repeated washing, the biofilms were stained with crystal violet for semiquantitative analysis. The biofilms were then treated to investigate the ability of BPEI or polymyxin-B to disrupt the biofilm. As shown in Figure 2, the negative control of water only had no impact on disrupting the MRSA biofilms because the biofilm layer remained intact in the bottom (top-down photographic image in Figure 2A). On the other hand, 600 Da BPEI (64 and 128 μg/mL) completely dispersed the MRSA biofilms into its solution in a manner similar to that of the positive control, acetic acid. However, exposure to polymyxin B, a U.S. Food and Drug Administration (FDA)-approved cationic polypeptide antibiotic, resulted in a slight dissolution in biomass, although 128 μg/mL was more effective than 64 μg/mL. The biofilm-disrupting properties are quantitatively reported as OD550 measurements of the amount of biofilm dislodged (Figure 2B). This demonstrates BPEI’s ability to eradicate MRSA biofilms by forcing them to detach and disperse its bacterial cells into planktonic culture, where they transition from a persistent quiescent state into a metabolically active realm and thus become vulnerable to antibiotics.
Figure 2.
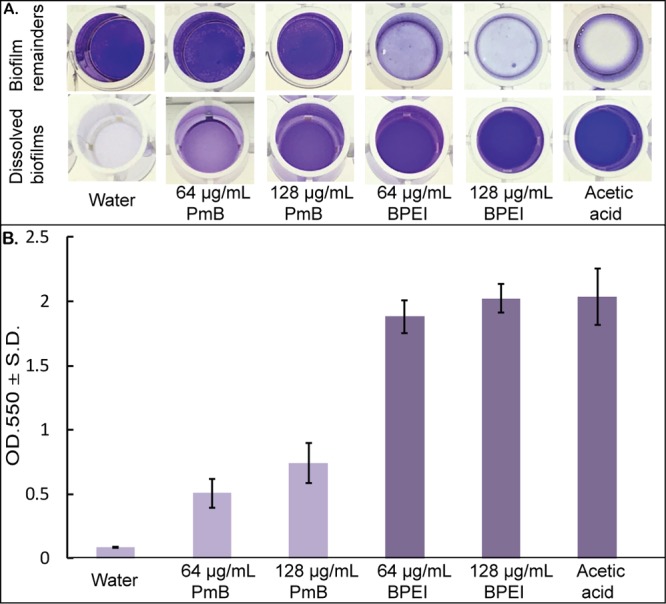
Established MRSA OU6 biofilms stained with crystal violet were treated with polymyxin B (PmB) and 600 Da BPEI for 20 h as well as the negative control (water only) and positive control (30% acetic acid). The dissolved biofilm solutions were transferred to a new plate, and the biofilm remainders are shown in a top-down view (A). The mean OD550 of the dissolved biofilm solution was measured (B). Error bars denote standard deviation (n = 10).
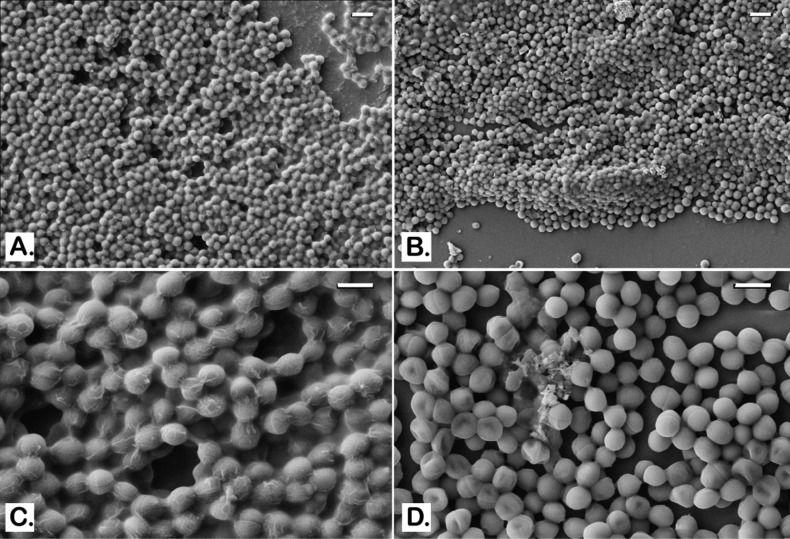
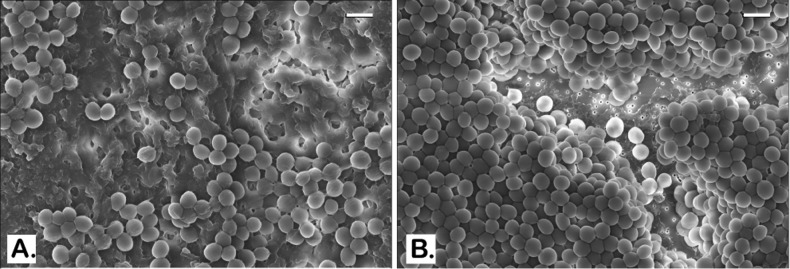
To better characterize the effect of BPEI on MRSA biofilms, morphological analysis was performed using scanning electron microscopy (SEM). Twenty-four hr-established MRSA biofilms on glass coverslips were treated with 128 μg/mL of BPEI. An untreated control and the BPEI-treated samples were then fixed and imaged with SEM. As shown in Figure 3A and 3C, the untreated control MRSA biofilm is enclosed in a thick coat of EPS. Like all biofilm-forming bacteria, the EPS is their self-made protection against harsh environments and antibiotics. With BPEI treatment, the preformed MRSA biofilm lost most of its EPS coat (Figure 3B). At higher magnification (Figure 3D), the lack of EPS in the treated sample rendered the inner layers of the bacteria, which were hidden in the untreated control, visible. To mimic a wound environment, MRSA biofilms were grown on polycarbonate (PC) membrane filters (0.1 μm pore size) placed directly on tryptic soy agar. The membrane pores allow for nutrient absorption and we found that these biofilms are more robust than those grown on glass slides. In the untreated control sample (Figure 4A), the EPS is so thick that the SEM scan cannot locate the bottom of the PC membrane filter. In BPEI-treated sample (Figure 4B), many areas are exposed from the absence of EPS, including the bottom surface of the membrane filter whose nanosize pores (tiny white dots through the crack in Figure 4B) are clearly visible.
Figure 3.
SEM images of MRSA OU11 biofilms on glass coverslips. Untreated control biofilms are shown to be covered and wrapped around in the matrix of EPS (A and C). BPEI-treated samples have much less EPS with many cells being exposed (B and D). Scale bars in (A) and (B) = 2 μm. Scale bars in (C) and (D) = 1 μm.
Figure 4.
SEM images of established MRSA OU6 biofilms on PC membranes. Very thick coat of the EPS matrix is present in the untreated control biofilm on the PC membrane which also blocks the bacterial cells from being captured in the microscope (A). BPEI-treated sample has a much clearer view as the EPS removed and even the membrane surface is exposed as many nanosize pores are seen at the bottom (B). Scale bars in (A) and (B) = 1 μm.
The biofilm EPS of S. aureus contains a high fraction of polysaccharide intracellular adhesin (PIA) and anionic species that are prime targets for 600 Da BPEI binding, such as eDNA and extracellular teichoic acid (TA). The latter is a key component in the biofilm EPS matrix of S. epidermidis(25) and S. aureus.7,26 It enhances bacterial adhesion to biotic and artificial surfaces, which is the first step of biofilm formation. TA has a negative net charge at neutral pH because it contains more negatively charged phosphates than positively charged d-alanine residues.26 Using nuclear magnetic resonance spectroscopy, we found that 600 Da BPEI electrostatically binds wall teichoic acid, which indirectly hinders the resistance factor PBP2a/4.14 Similarly, BPEI most likely binds extracellular TA in the EPS matrix, and also eDNA, to disrupt biofilm structural integrity, as seen in Figures 3 and 4. The exposure of individual bacteria could enhance their contact with various drugs and components of the immune system.
Skin or soft-tissue infections (SSTIs) arise from abrasions, nonsurgical wounds, burns, or chronic health problems.27 For chronic wound infections associated with MRSA and its biofilm, treatment options are scarce. Patients afflicted with these chronic wounds suffer from physical pain and disabilities in addition to psychological and emotional stresses and poor quality of life. Current inpatient treatments include cleansing, debridement, maintaining a moist tissue environment, and, when possible, eliminating the underlying pathology or factors that contribute to poor wound healing.28 In advanced cases, amputation may become necessary. Death, especially in elderly patients, may result from sepsis that can be associated with chronic wounds. Antibiotics can be used effectively against susceptible infections. For drug-resistant infections, the best-practices for effective inpatient intervention are strict sanitary guidelines and antibiotics, such as intravenous vancomycin plus piperacillin/tazobactam or IV treatment with new antibiotics of last resort.28 Nevertheless, biofilms and antimicrobial resistance create substantial technological barriers to treating chronic wound infections. This presents a significant and critical need for a way to counteract biofilms and antimicrobial resistance. The 600 Da BPEI is a dual-function potentiator because it disrupts biofilms that are otherwise impenetrable to antibiotics, and also it counteracts β-lactam resistance mechanisms in MRSA. However, success requires that 600 Da BPEI have low toxicity. In dermal applications, low-molecular-weight BPEI was shown to have high biocompatibility and low genotoxic potential.29 We also confirmed the noncytotoxicity of 600 Da BPEI toward human kidney, colon, and HeLa cells with IC50’s of 1090 and 690 μg/mL on human HeLa cells and HEK293, respectively. Additionally, lactate dehydrogenase (LDH) assays showed that 600 Da BPEI gave the lowest nephrotoxicity of 3.5% at 63 μg/mL (even lower than Polymyxin E/Colistin which was >20% nephrotoxicity at the same concentration tested).13,14 Additional experiments are planned to determine 600 Da BPEI’s toxicity levels in dermal and subcutaneous layers. With bacterial evolution outpacing the discovery of antimicrobial agents, it is imperative to seek alternative treatment options, such as coupling existing drugs with potentiators. With a dual-function mechanism that eliminates antibiotic efficacy barriers in both planktonic and biofilm-encased bacteria, 600 Da BPEI has promise as a therapeutic agent for improving wound care and combating medical device infections. Potency of first-line antibiotics such as ampicillin can now be restored by the addition of BPEI against drug-resistant MRSA, as seen by their strong synergistic effects. Combinations of BPEI and antibiotics could be administered to diagnosed or suspected staph-biofilm infections, which would improve the efficacy of treatment of resistant, biofilm-forming pathogens.
Acknowledgments
This work was possible due to the kindness and contributions of Robert Cichewicz, PhD, Preston Larson, PhD, and Robert Brennan, PhD. We also want to thank Cindy McCloskey, M.D. for the clinical isolates.
Glossary
Abbreviations
- MRSA
methicillin-resistant Staphylococcus aureus
- MRSE
methicillin-resistant Staphylococcus epidermidis
- AMR
antimicrobial resistance
- BPEI
branched polyethylenimine
- Da
Dalton
- SEM
scanning electron microscopy
- CDC
Centers for Disease Control and Prevention
- WTA
wall teichoic acid
- PBP
penicillin-binding protein
- MIC
minimum inhibitory concentration
- MBEC
minimum biofilm eradication concentration
- OD600
optical density at 600 nm
- OD550
optical density at 550 nm
- PC
polycarbonate.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.9b00595.
Experimental methods and clinical antibiotic resistance data (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Funding was provided by the National Institutes of Health (C.V.R., R03AI142420-01), Oklahoma Center of Advancement of Science and Technology (C.V.R., HR16-084-3), and The University of Oklahoma.
The authors declare no competing financial interest.
Supplementary Material
References
- Hill M. A.; Lam A. K.; Reed P.; Harney M. C.; Wilson B. A.; Moen E. L.; Wright S. N.; Pinho M. G.; Rice C. V. BPEI-Induced Delocalization of PBP4 Potentiates β-Lactams against MRSA. Biochemistry 2019, 58, 3813–3822. 10.1021/acs.biochem.9b00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone M.; Bjarnsholt T.; McBain A. J.; James G. A.; Stoodley P.; Leaper D.; Tachi M.; Schultz G.; Swanson T.; Wolcott R. D. The prevalence of biofilms in chronic wounds: a systematic review and meta-analysis of published data. Journal of Wound Care 2017, 26, 20–25. 10.12968/jowc.2017.26.1.20. [DOI] [PubMed] [Google Scholar]
- James G. A.; Swogger E.; Wolcott R.; Pulcini E.; Secor P.; Sestrich J.; Costerton J. W.; Stewart P. S. Biofilms in chronic wounds. Wound Repair Regen 2008, 16, 37–44. 10.1111/j.1524-475X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- Roy S.; Santra S.; Das A.; Dixith S.; Sinha M.; Ghatak S.; Ghosh N.; Banerjee P.; Khanna S.; Mathew-Steiner S.; Ghatak P. D.; Blackstone B. N.; Powell H. M.; Bergdall V. K.; Wozniak D. J.; Sen C. K. Staphylococcus aureus Biofilm Infection Compromises Wound Healing by Causing Deficiencies in Granulation Tissue Collagen. Ann. Surg. 2020, 10.1097/SLA.0000000000003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arciola C. R.; Campoccia D.; Speziale P.; Montanaro L.; Costerton J. W. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials 2012, 33, 5967–5982. 10.1016/j.biomaterials.2012.05.031. [DOI] [PubMed] [Google Scholar]
- Donlan R. M. Biofilms: microbial life on surfaces. Emerging Infect. Dis. 2002, 8, 881–890. 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laverty G.; Gorman S. P.; Gilmore B. F. Biomolecular mechanisms of staphylococcal biofilm formation. Future Microbiol. 2013, 8, 509–524. 10.2217/fmb.13.7. [DOI] [PubMed] [Google Scholar]
- Romling U.; Balsalobre C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med. 2012, 272, 541–561. 10.1111/joim.12004. [DOI] [PubMed] [Google Scholar]
- Grace D.Review of evidence on antimicrobial resistance and animal agriculture in developing countries; Evidence on Demand, 2015; 10.12774/eod_cr.june2015.graced. [DOI]
- Malani P. N. National burden of invasive methicillin-resistant Staphylococcus aureus infection. JAMA 2014, 311, 1438–1439. 10.1001/jama.2014.1666. [DOI] [PubMed] [Google Scholar]
- Dantes R.; Mu Y.; Belflower R.; Aragon D.; Dumyati G.; Harrison L. H.; Lessa F. C.; Lynfield R.; Nadle J.; Petit S. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA internal medicine 2013, 173, 1970–1978. 10.1001/jamainternmed.2013.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard M.; Frees D.; Ingmer H.. Antibiotic Resistance and the MRSA Problem. In Gram-Positive Pathogens; Fischetti V. A., Novick R. P., Ferretti J. J., Portnoy D.A., Braunstein M., Rood J. I., Eds.; American Society for Microbiology, 2019; 10.1128/9781683670131.ch47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxley M. A.; Wright S. N.; Lam A. K.; Friedline A. W.; Strange S. J.; Xiao M. T.; Moen E. L.; Rice C. V. Targeting Wall Teichoic Acid in Situ with Branched Polyethylenimine Potentiates beta-Lactam Efficacy against MRSA. ACS Med. Chem. Lett. 2017, 8, 1083–1088. 10.1021/acsmedchemlett.7b00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxley M. A.; Friedline A. W.; Jensen J. M.; Nimmo S. L.; Scull E. M.; King J. B.; Strange S.; Xiao M. T.; Smith B. E.; Thomas K. J. Iii; Glatzhofer D. T.; Cichewicz R. H.; Rice C. V. Efficacy of ampicillin against methicillin-resistant Staphylococcus aureus restored through synergy with branched poly(ethylenimine). J. Antibiot. 2016, 69, 871–878. 10.1038/ja.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam A. K.; Hill M. A.; Moen E. L.; Pusavat J.; Wouters C. L.; Rice C. V. Cationic Branched Polyethylenimine (BPEI) Disables Antibiotic Resistance in Methicillin-Resistant Staphylococcus epidermidis (MRSE). ChemMedChem 2018, 13, 2240–2248. 10.1002/cmdc.201800433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam A. K.; Wouters C. L.; Moen E. L.; Pusavat J.; Rice C. V. Antibiofilm Synergy of beta-Lactams and Branched Polyethylenimine against Methicillin-Resistant Staphylococcus epidermidis. Biomacromolecules 2019, 20, 3778–3785. 10.1021/acs.biomac.9b00849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farha M. A.; Leung A.; Sewell E. W.; D’Elia M. A.; Allison S. E.; Ejim L.; Pereira P. M.; Pinho M. G.; Wright G. D.; Brown E. D. Inhibition of WTA synthesis blocks the cooperative action of PBPs and sensitizes MRSA to beta-lactams. ACS Chem. Biol. 2013, 8, 226–233. 10.1021/cb300413m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atilano M. L.; Pereira P. M.; Yates J.; Reed P.; Veiga H.; Pinho M. G.; Filipe S. R. Teichoic acids are temporal and spatial regulators of peptidoglycan cross-linking in Staphylococcus aureus. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 18991–18996. 10.1073/pnas.1004304107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S.; Santa Maria J. P. Jr.; Walker S. Wall teichoic acids of gram-positive bacteria. Annu. Rev. Microbiol. 2013, 67, 313–336. 10.1146/annurev-micro-092412-155620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garimella R.; Halye J. L.; Harrison W.; Klebba P. E.; Rice C. V. Conformation of the Phosphate D-Alanine Zwitterion in Bacterial Teichoic Acid from Nuclear Magnetic Resonance Spectroscopy. Biochemistry 2009, 48, 9242–9249. 10.1021/bi900503k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halye J. L.; Rice C. V. Cadmium Chelation by Bacterial Teichoic Acid from Solid-State Nuclear Magnetic Resonance Spectroscopy. Biomacromolecules 2010, 11, 333–340. 10.1021/bm9010479. [DOI] [PubMed] [Google Scholar]
- Thomas K. J.; Rice C. V. Revised model of calcium and magnesium binding to the bacterial cell wall. BioMetals 2014, 27, 1361–1370. 10.1007/s10534-014-9797-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. J.; Rice C. V. Equilibrium binding behavior of magnesium to wall teichoic acid. Biochim. Biophys. Acta, Biomembr. 2015, 1848, 1981–1987. 10.1016/j.bbamem.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham J. R.; Halye J. L.; Kashtanov S.; Khandogin J.; Rice C. V. Revisiting Magnesium Chelation by Teichoic Acid with Phosphorus Solid-State NMR and Theoretical Calculations. J. Phys. Chem. B 2009, 113, 2177–2183. 10.1021/jp809313j. [DOI] [PubMed] [Google Scholar]
- Wagstaff J. L.; Sadovskaya I.; Vinogradov E.; Jabbouri S.; Howard M. J. Poly-N-acetylglucosamine and poly(glycerol phosphate) teichoic acid identification from staphylococcal biofilm extracts using excitation sculptured TOCSY NMR. Mol. BioSyst. 2008, 4, 170–174. 10.1039/B715242F. [DOI] [PubMed] [Google Scholar]
- Gross M.; Cramton S. E.; Gotz F.; Peschel A. Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infect. Immun. 2001, 69, 3423–3426. 10.1128/IAI.69.5.3423-3426.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H. W.; Collins S. A. B.; Resneck J. S. Jr.; Bolognia J. L.; Hodge J. A.; Rohrer T. A.; Van Beek M. J.; Margolis D. J.; Sober A. J.; Weinstock M. A.; Nerenz D. R.; Smith Begolka W.; Moyano J. V. The burden of skin disease in the United States. J. Am. Acad. Dermatol. 2017, 76, 958–972.e2. 10.1016/j.jaad.2016.12.043. [DOI] [PubMed] [Google Scholar]
- Stevens D. L.; Bisno A. L.; Chambers H. F.; Dellinger E. P.; Goldstein E. J.; Gorbach S. L.; Hirschmann J. V.; Kaplan S. L.; Montoya J. G.; Wade J. C. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin. Infect. Dis. 2014, 59, 147–159. 10.1093/cid/ciu444. [DOI] [PubMed] [Google Scholar]
- Wiegand C.; Bauer M.; Hipler U.-C.; Fischer D. Poly (ethyleneimines) in dermal applications: biocompatibility and antimicrobial effects. Int. J. Pharm. 2013, 456, 165–174. 10.1016/j.ijpharm.2013.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.