Abstract
In order to improve the antitumor potency of the natural product evodiamine, novel boron-containing evodiamine derivatives were designed by incorporating boronic acid and boronate as trigger units. Boronate derivative 13a could be triggered by reactive oxygen species (ROS) in the HCT116 colon cancer cell line and showed excellent antitumor activity in vitro and in vivo. It induced apoptosis in HCT116 cancer cells in a dose-dependent manner and cell growth arrest at the G2 phase.
Keywords: Evodiamine, borate unit, ROS, antitumor, Top1, Top2
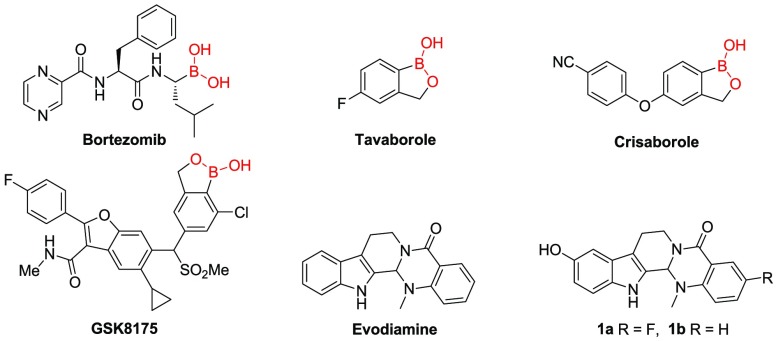
Boron is an essential element of diverse cells and is ubiquitous in nature.1 It has an empty p-orbital with an electrophilic property, which can form covalent bonds with biological nucleophiles including amine and hydroxyl groups in nucleic acids. Moreover, the sp2 boron center can be easily changed to sp3 hybridization under certain physiological conditions.2−6 Given the special characteristics of the boron atom, boron-containing compounds (BCCs) have attracted great interests in medicinal chemistry, and several of them have been approved (e.g., bortezomib, tavaborole, Figure 1)7,8 or under clinical trials (e.g., crisaborole, GSK8175, Figure 1).9,10
Figure 1.
Chemical structures of bortezomib, tavaborole, crisaborole, GSK8175, evodiamine, and evodiamine derivatives.
Reactive oxygen species (ROS), including hydrogen peroxide (H2O2), which act as a pivotal part in cell signaling and homeostasis, are natural byproducts of normal oxygen metabolism.11 In particular, cancer cells exhibit an exclusive feature of increased ROS (mainly H2O2),12−14 which can be used to design drugs for targeted cancer therapy.15,16 Moreover, borates, including boronate and boronic acid, are ROS responsive functional groups.11,17−22 Borates attached to carbon are easily cleavable by ROS.11,17−22 Therefore, it is advantageous to attach borates as a “trigger unit” to improve the pharmacological activity and druggability of antitumor agents.17−22
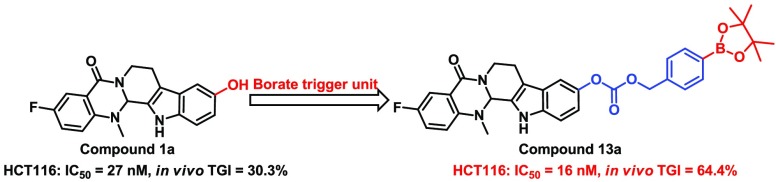
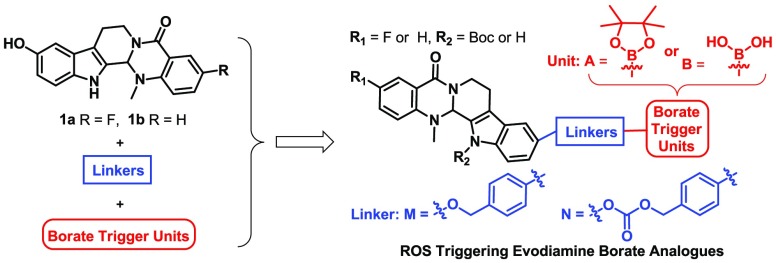
In recent years, evodiamine (Figure 1) has been investigated as an antitumor lead compound that possesses multitargeting profiles.23−26 Previously, systemic structural optimizations of evodiamine were performed by our group, and several highly active evodiamine derivatives (1a and 1b, Figure 1) were identified.27−29 Unfortunately, further development of evodiamine derivatives was hindered by the unsatisfied in vivo antitumor potency. In light of the success of borate in drug discovery,17−22 herein, a series of novel boron-containing evodiamine analogues were reported by incorporating borates as trigger units at the C10 position of compounds 1a and 1b through various cleavable linkers with an aim to improve their in vivo antitumor efficacy (Figure 2).
Figure 2.
Design of ROS triggering evodiamine borate analogues.
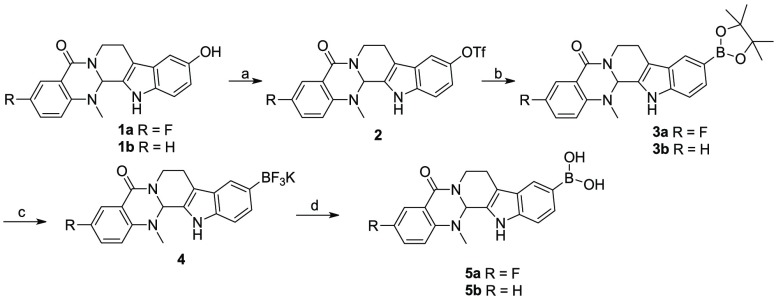
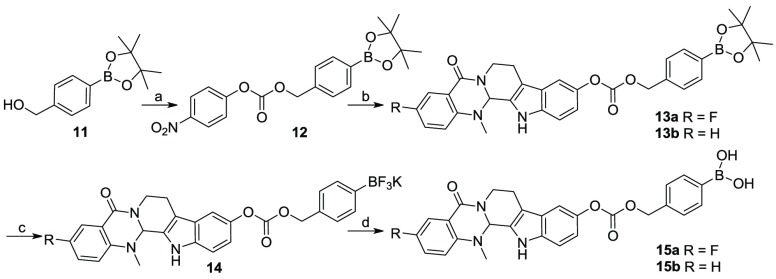
The syntheses of evodiamine borates were depicted in Schemes 1–3. First, starting from evodiamine derivatives 1, triflates 2 were prepared according to the literature.22 Then, the Miyaura reaction was performed with 2 to afford boronoates 3. Deprotection of 3 in the presence of potassium difluorohydride (KHF2) and methanol at room temperature gave potassium trifluoroborates 4, followed by hydrolysis under acid conditions to yield target compound 5 (Scheme 1).
Scheme 1. Chemical Synthesis of Compounds 3 and 5.
Reagents and conditions: (a) Tf2O, pyridine, DCM, 0 °C, 3 h, yield 87%; (b) bis(pinacolato)diboron, Pd(dppf)Cl2, KOAc, 1,4-dioxane, 100 °C, 8 h, yield 90–93%; (c) KHF2, MeOH, rt, 30 min, yield 63–68%; (d) TMSCl, H2O, MeCN, 2 h, yield 95–98%.
Scheme 3. Chemical Synthesis of Compounds 13 and 15.
Reagents and conditions: (a) 4-nitrophenyl chloroformate, TEA, THF, rt, 6 h, yield 58%; (b) 1, K2CO3, THF, 70 °C, 10 h, yield 76–80%; (c) KHF2, MeOH, rt, 30 min, yield 74–76%; (d) TMSCl, H2O, MeCN, 2 h, yield 59–62%.
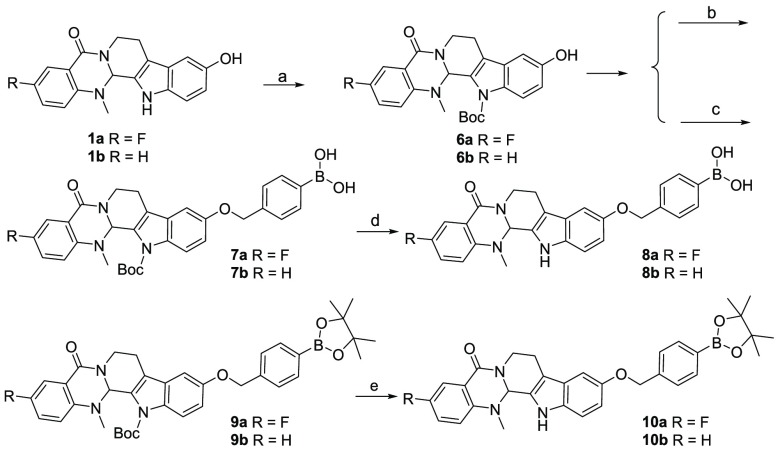
Target compounds 7–10 were synthesized as shown in Scheme 2. The NH groups of compounds 1 were protected with the Boc group via three steps to afford intermediates 6. In the presence of NaH and 4-bromomethylphenylboronic acid or 4-bromomethylphenylboronic acid pinacol ester, phenylboronic acids 7 and phenylboronates 9 were obtained, respectively. Finally, Boc deprotection was conducted to give target compounds 8 and 10.
Scheme 2. Chemical Synthesis of Compounds 7–10.
Reagents and conditions: (a) (1) DMAP, (Boc)2O, THF, 2 h; (2) K2CO3, MeOH, 4 h; (3) AcOH, 1 h, yield 62–65%; (b) NaH, 4-bromomethylphenylboronic acid, DMF, 0 °C, 8 h, 1 N HCl, pH = 2, yield 80–83%; (c) NaH, 4-bromomethylphenylboronic acid pinacol ester, DMF, 0 °C, 8 h, yield 82–83%; (d) TFA, DCM, rt, 2 h, yield 63–66%; (e) TFA, DCM, rt, 2 h, yield 45–48%.
As depicted in Scheme 3, evodiamine boronate derivatives with carbonate linker (13 and 15) were prepared. Boronate pinacol ester 11 was reacted with 4-nitrophenyl chloroformate and trimethylamine (TFA) in tetrahydrofuran (THF) to afford intermediate 12, which was reacted with compounds 1 to give target compounds 13 with high yields. Then, target compounds 15 were prepared using similar synthetic methods as described for derivatives 5.
The in vitro antitumor activities of evodiamine borates (Table 1) were assayed against HCT116, MCF-7,and A549 cell lines using the CCK8 assay.30 Evodiamine derivatives 1a and 1b and camptothecin (CPT) were selected as positive controls. As illustrated in Table 1, boronic acids derivatives (5a, 5b, 8a, 8b, and 15b) generally showed better antitumor activity than the corresponding boronates (3a, 3b, 10a, 10b, and 13b). Most N13-Boc protected intermediates (7a, 7b, 9a, and 9b) showed decreased antitumor activity against the three cell lines. Interestingly, excellent antitumor activity was retained for evodiamine borates with a carbonate linker (13 and 15). Most boronic acid derivatives showed comparable antitumor activity to lead compounds 1a and 1b. In particular, compound 13a exhibited excellent antiproliferative activity (IC50 range: 16–37 nM), whose efficacy against HCT116 cells (IC50 = 16 nM) was superior to that of lead compound 1a (IC50 = 27 nM).
Table 1. In Vitro Antitumor Activity of Borate Evodiamines (IC50, μM).
| Compds | HCT116 | MCF-7 | A549 |
|---|---|---|---|
| 3a | 0.17 ± 0.004 | 0.96 ± 0.2 | 0.11 ± 0.14 |
| 3b | 0.22 ± 0.03 | 0.39 ± 0.075 | 0.17 ± 0.022 |
| 5a | 0.063 ± 0.003 | 0.094 ± 0.006 | 0.040 ± 0.006 |
| 5b | 0.069 ± 0.005 | 0.16 ± 0.031 | 0.10 ± 0.008 |
| 7a | 4.91 ± 0.97 | 2.39 ± 0.48 | 2.13 ± 0.15 |
| 7b | 0.52 ± 0.36 | 1.58 ± 0.32 | 7.20 ± 1.4 |
| 8a | 0.083 ± 0.001 | 0.059 ± 0.010 | 0.073 ± 0.003 |
| 8b | 0.12 ± 0.04 | 0.11 ± 0.021 | 0.074 ± 0.014 |
| 9a | 2.17 ± 0.47 | 7.85 ± 0.92 | 2.23 ± 0.43 |
| 9b | 4.18 ± 0.79 | 4.13 ± 0.83 | 3.44 ± 0.65 |
| 10a | 0.15 ± 0.014 | 0.097 ± 0.016 | 0.12 ± 0.001 |
| 10b | 0.10 ± 0.06 | 0.16 ± 0.031 | 0.39 ± 0.075 |
| 13a | 0.016 ± 0.003 | 0.033 ± 0.008 | 0.037 ± 0.002 |
| 13b | 0.065 ± 0.022 | 0.086 ± 0.017 | 0.10 ± 0.006 |
| 15a | 0.041 ± 0.005 | 0.032 ± 0.006 | 0.044 ± 0.002 |
| 15b | 0.052 ± 0.007 | 0.14 ± 0.028 | 0.13 ± 0.05 |
| 1a | 0.027 ± 0.006 | 0.031 ± 0.008 | 0.029 ± 0.004 |
| 1b | 0.055 ± 0.005 | 0.27 ± 0.006 | 0.084 ± 0.013 |
| CPT | 0.009 ± 0.003 | 0.084 ± 0.005 | 0.039 ± 0.008 |
Previously, topoisomerase I (Top1) and topoisomerase II (Top2) were identified as targets of compounds 1a and 1b. Herein, evodiamine borates with good cytotoxicity were selected to investigate their Top inhibitory activities. As depicted in Figure 3A, all the compounds showed strong inhibitory effect against Top1 at 500 μM. At a lower concentration of 125 μM, six compounds (8a, 8b, 13a, 13b, 15a, and 15b) were still effective. Particularly, the activity of compounds 13a and 15a were active at 83.3 μM (Figure 3B). Moreover, five compounds, namely, 8b, 13a, 13b, 15a, and 15b, retained Top2 inhibitory potency at 50 μM (Figure 3C,D), and three of them (8a, 13a, 15a) exhibited comparable potency to the reference drug etoposide (ETO). Given that 3-chloro-10-hydroxy thio-evodiamine was identified as a Top1/Top2/tubulin inhibitor, the tubulin inhibitory activities of compounds 13a, 13b, 15a, and 15b were further determined.28 However, only compound 15b was active with an IC50 value of 25.7 μM (Table S3). On the basis of the above results, compounds 8b, 13a, 13b, and 15a were proven to be Top1/Top2 dual inhibitors, indicating that these borate compounds might act by a similar antitumor mechanism to that of lead compounds 1a and 1b.29
Figure 3.
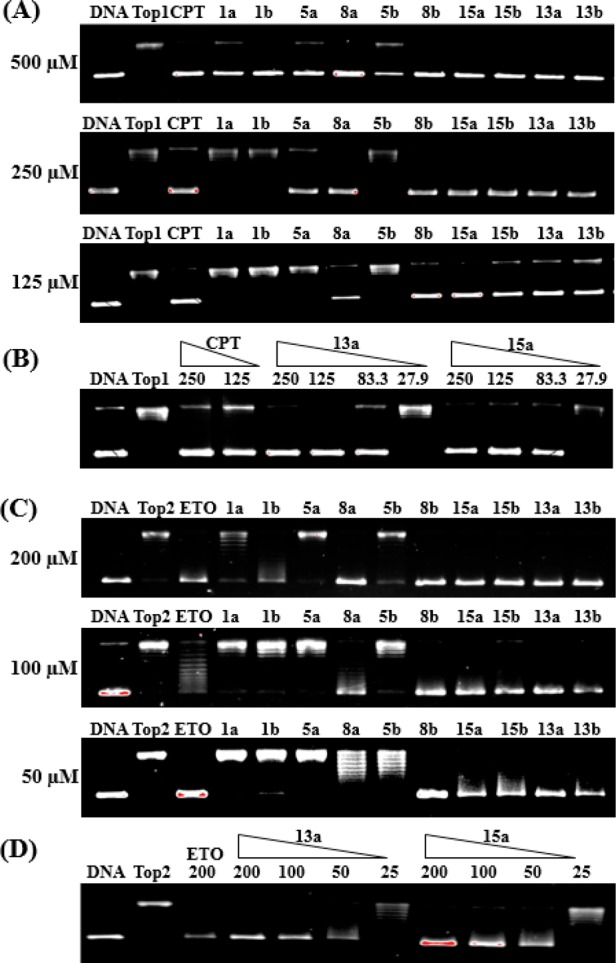
(A) Top1 inhibitory activity assay of evodiamine borate derivatives at 500, 250, and 125 μM, respectively. (B) Top1 inhibitory activity assay of compounds 13a and 15a ranging from 27.9 to 250 μM. (C) Top2 inhibitory activity assay of evodiamine borate derivatives at 200, 100, and 50 μM, respectively. (D) Top2 inhibitory activity assay of compounds 13a and 15a ranging from 25 to 200 μM.
To better illuminate the binding mode with Top1 and Top2, compounds 1a, 1b, 13a, and 15a were subjected to molecule docking. Similar to compounds 1a and 1b (Figure S1A,B), the A-ring of compound 13a formed π–π interactions with the TGP11 base pair, enhancing the base stacking at the active site of Top1 (Figure 4A). The carbonyl group and carbonate carbonyl group formed hydrogen bonding interactions with Try426 and Asn722, respectively. The boric acid group of compound 15a additionally formed a hydrogen bond with DA11 and DG12 (Figure 4B). Additional hydrogen bonds were also found for compounds 13a and 15a in the active site of Top1. Moreover, the four compounds fitted well into the ATPase of Top2α. For compounds 1, hydrogen bonds between the hydroxy group and Top2α were the predominant interactions (1a, DC8 and His758; 1b, DC8 and Ser763, Figure S1C,D). However, compounds 13a and 15a bound to the active domain of Top2 through different ways. For compound 13a, the D-ring carbonyl group participated in the formation of a hydrogen bond with Ser464 (Figure 4C). For compound 15a, the D-ring carbonyl group formed a hydrogen bond with Ser763 and the boric acid group formed hydrogen bonds with His758 and DC8, respectively (Figure 4D).
Figure 4.
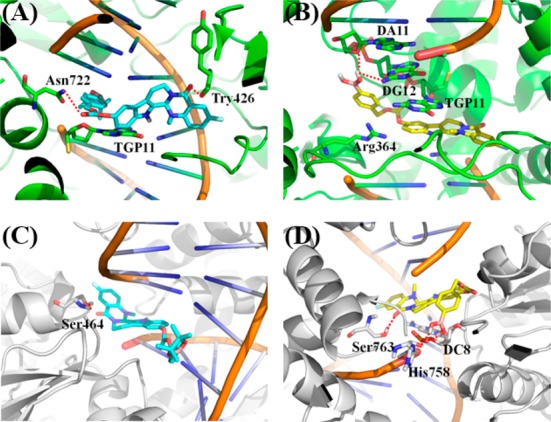
Binding modes of compounds 13a (A, C) and 15a (B, D) with the Top1–DNA complex (PDB code: 1T8I) and ATPase domain of Top2α (PDB code: 5GWK).
On the basis of in vitro antitumor activities, two different types of evodiamine borates 13a and 15a were selected to test the ability to induce apoptosis and cell-cycle arrest in the HCT116 cell line. As shown in Figure 5A, compounds 13a and 15a demonstrated significant apoptosis-inducing activity in a dose-dependent manner in HCT116 cells. The percentages of apoptotic cells were increased significantly (13a: from 42.28% to 61.73%; 15a: from 44.40% to 64.47%) as compared to the control population (6.25%). The cell-cycle arrest of compounds 13a and 15a in HCT116 cells was evaluated by the flow cytometric method (Figure 5B). After exposure to compound 13a at 0.05, 0.2, and 0.4 μM, the percentages of cells in the G2 fraction were 26.88%, 45.49%, and 56.25%, respectively. Similarly, the percentages of cells exposed to compound 15a at the G2 phase were changed dramatically (29.47%, 43.45%, and 51.23%, respectively). As compared with the ratio (10.8%) of the untreated cells at the G2 phase, compounds 13a and 15a were confirmed to induce cell-cycle arrest in HCT116 cells at the G2 phase, which was consistent with that of lead compound 1a.29
Figure 5.
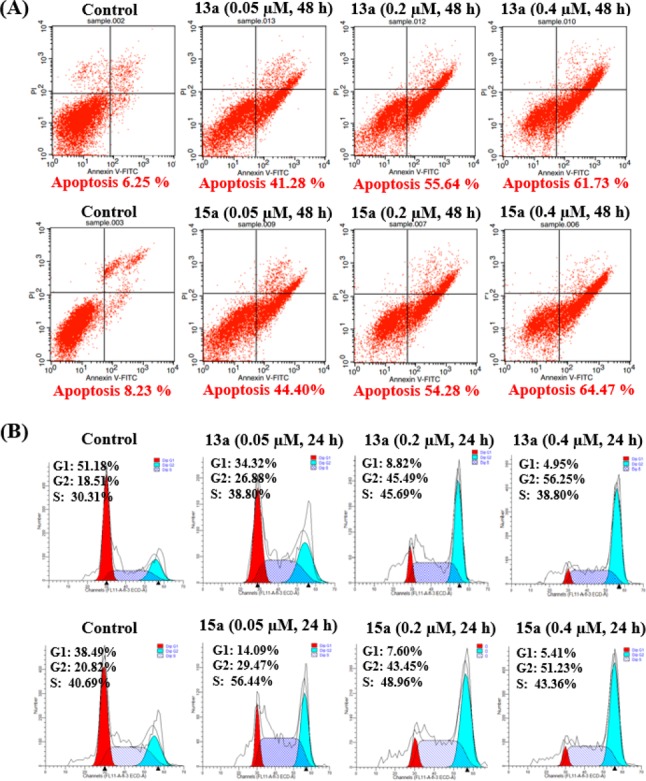
Apoptosis and cell-cycle arrest induced by compounds 13a and 15a. (A) HCT116 cells were incubated with 0.05, 0.2, and 0.4 μM of compounds 13a or 15a for 48 h. Apoptosis was assayed by flow cytometry (n = 3). (B) Cell-cycle effect of compounds 13a and 15a. HCT116 cells were incubated with 0.05, 0.2, and 0.4 μM of compounds 13a or 15a for 24 h. At least three independent experiments were done for each condition.
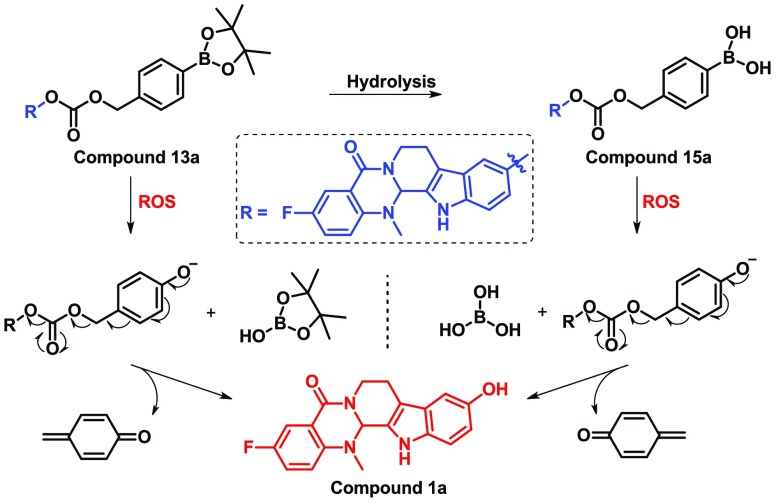
Compounds 13a and 15a were selected to investigate the ability to release compound 1a by treating different concentrations of H2O2 in PBS (pH = 7.4) using high performance liquid chromatography (HPLC). At 0.05 mM H2O2, compound 1a could be detected with relative drug release rates for compounds 13a and 15a of 11.6% and 20.8%, respectively. Compound 1a could be released in a concentration-dependent manner, indicating that it can be activated by H2O2 (Figures S1 and S2). Furthermore, ultraperformance liquid chromatography quadrupole time-of-flight mass spectrometry (UPLC-QTOF/MS) was used to assess the cleavage of borate derivatives undergoing intracellular ROS in HCT116 cancer cells. The concentrations of compounds 13a, 15a, and 1a in cell culture media were analyzed after incubation with HCT116 cancer cells for 8, 48, and 72 h. Relative drug release rates were measured by relative peak areas. Compound 15a (retention time: 8.6 min) was directly converted into compound 1a (retention time: 7.3 min) with the relative drug release rates of 18.5%, 24.5%, and 35.5%, respectively (Figures S4–S6). In contrast, compound 13a (retention time: 11.4 min) was first converted to compound 15a (78.6%, 43.7%, and 36.3%, respectively) and then transformed to compound 1a (13.6%, 39.9%, and 53.1%, respectively, Figures S4–S6). Thus, compounds 13a and 15a could be triggered by ROS in HCT116 cells (Scheme 4). The relative drug release rates were increased in a time-dependent manner. Reactive quinone methide (QM) precursor 4-(chloromethyl)phenyl acetate was also used to test its antiproliferative effect for cancer cells, and it was almost inactive against cancer cells (Table S1), suggesting the released QM has little effect on cancer cells. Furthermore, in vitro metabolic stabilities of compounds 13a, 15a, and 1a were evaluated through the liver microsome assay. Compound 13a had a terminal half-life (t1/2) of 8.36 min, which was much longer than that of compounds 15a (t1/2 = 0.74 min) and 1a (t1/2 = 3.69 min, Table S2). The cLogP values of compounds 13, 15, and 1 were also predicted (Table S4). Compounds 13 had better in vitro antitumor potency, possibly due to their tumor penetration with increased lipophilicity of the boronate units.
Scheme 4. Proposed Reaction Mechanism of Evodiamine Borate Analogues with ROS.
Finally, compound 13a was administrated intraperitoneally (IP) at 10 mg/kg to evaluate its in vivo antitumor activity in a HCT116 xenograft model in mice. After 21 consecutive days, compound 13a achieved the tumor growth inhibition (TGI) of 64.4% (Figure 6A), which was superior to compound 1a (TGI = 30.3% at 2 mg/kg,29 highly toxic at 10 mg/kg, Figure S7) and comparable to the control TPT (TGI = 68.8%). Meanwhile, compound 13a had no significant effects on the decrease of the body weight (P > 0.05, Figure 6B). In contrast, TPT had significant toxicity on the treated mice (P < 0.01), suggesting that the toxicity of compound 13a might be lower than that of TPT.
Figure 6.
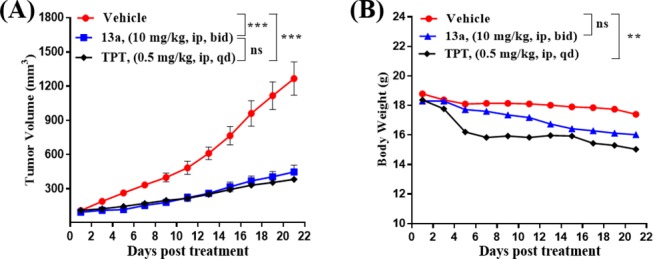
Antitumor activity of compound 13a and TPT in a HCT116 tumor xenograft model. (A) Tumor growth inhibition. (B) The change of body weight. Data are presented as the mean ± SEM: *P < 0.05, **P < 0.01, and ***P < 0.001 vs vehicle group, determined with Student’s t test.
In summary, novel boron-containing evodiamine analogues were reported by incorporating boronic acid and boronate as trigger units. In particular, compound 13a showed excellent antitumor activity in a HCT116 xenograft model in mice, which induced apoptosis in a dose-dependent manner and cell growth arrest at the G2 phase. Compound 13a acted as a ROS triggering evodiamine borate analogues and showed improved in vivo antitumor potency compared to parent evodiamine derivative 1a. Taken together, this study demonstrated a good example of using borates as trigger units to improve the in vivo antitumor potency of a natural product. Compound 13a represents a promising antitumor lead compound, and further structural optimization and antitumor mechanism studies are currently in progress.
Glossary
Abbreviations
- BCCs
boron-containing compounds
- ROS
reactive oxygen species
- CPT
camptothecin
- ETO
etoposide
- Top1
topoisomerase I
- Top2
topoisomerase II
- HPLC
high performance liquid chromatography
- UPLC-QTOF/MS
ultra performance liquid chromatography quadrupole time-of-flight mass spectrometry
- IP
intraperitoneally
- TGI
tumor growth inhibition.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.9b00513.
Chemical synthesis and structural characterization of the target compounds; protocols of the biological assays; relative drug release; NMR spectra; HPLC purity of representative compounds; certificate of STR analysis (PDF)
Author Contributions
∇ X.L., S.W., and G.D. contributed equally to this work. D.L. and C.S. designed the experiments and revised the manuscript. X.L., S.W., and G.D. synthesized evodiamine borate derivatives and carried out the biological experiments. S.C. and Z.M. assisted with the biological experiments and in the preparation of the manuscript. All authors discussed the results and commented on the manuscript. All authors read and approved the final manuscript.
This work was supported by National Natural Science Foundation of China (grants 21807113 to S.W., 81872742 to G.D., and 81725020 to C.S.), the National Key R&D Program of China (grant 2017YFA0506000 to C.S.), Shanghai Sailing Program (18YF1429100 to S.W.), and the Innovation Program of Shanghai Municipal Education Commission (Grant 2019-01-07-00-07-E00073 to C.S.).
The authors declare no competing financial interest.
Supplementary Material
References
- Farfan-Garcia E. D.; Castillo-Mendieta N. T.; Cipres-Flores F. J.; Padilla-Martinez I. I.; Trujillo-Ferrara J. G.; Soriano-Ursua M. A. Current data regarding the structure-toxicity relationship of boron-containing compounds. Toxicol. Lett. 2016, 258, 115–125. 10.1016/j.toxlet.2016.06.018. [DOI] [PubMed] [Google Scholar]
- Yang F.; Zhu M.; Zhang J.; Zhou H. Synthesis of biologically active boron-containing compounds. MedChemComm 2018, 9, 201–211. 10.1039/C7MD00552K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban H. S.; Nakamura H. Boron-based drug design. Chem. Rec. 2015, 15, 616–635. 10.1002/tcr.201402100. [DOI] [PubMed] [Google Scholar]
- Adamczyk-Wozniak A.; Borys K. M.; Sporzynski A. Recent developments in the chemistry and biological applications of benzoxaboroles. Chem. Rev. 2015, 115, 5224–5247. 10.1021/cr500642d. [DOI] [PubMed] [Google Scholar]
- Liu C. T.; Tomsho J. W.; Benkovic S. J. The unique chemistry of benzoxaboroles: current and emerging applications in biotechnology and therapeutic treatments. Bioorg. Med. Chem. 2014, 22, 4462–4473. 10.1016/j.bmc.2014.04.065. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Zhu M.; Lin Y.; Zhou H. The synthesis of benzoxaboroles and their applications in medicinal chemistry. Sci. China: Chem. 2013, 56, 1372–1381. 10.1007/s11426-013-4981-y. [DOI] [Google Scholar]
- Zhang J.; Zhang J.; Hao G.; Xin W.; Yang F.; Zhu M.; Zhou H. Design, synthesis, and structure-activity relationship of 7-propanamide benzoxaboroles as potent anticancer agents. J. Med. Chem. 2019, 62, 6765–6784. 10.1021/acs.jmedchem.9b00736. [DOI] [PubMed] [Google Scholar]
- Akama T.; Baker S. J.; Zhang Y. K.; Hernandez V.; Zhou H.; Sanders V.; Freund Y.; Kimura R.; Maples K. R.; Plattner J. J. Discovery and structure-activity study of a novel benzoxaborole anti-inflammatory agent (AN2728) for the potential topical treatment of psoriasis and atopic dermatitis. Bioorg. Med. Chem. Lett. 2009, 19, 2129–2132. 10.1016/j.bmcl.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Nocentini A.; Supuran C. T.; Winum J. Y. Benzoxaborole compounds for therapeutic uses: a patent review (2010–2018). Expert Opin. Ther. Pat. 2018, 28, 493–504. 10.1080/13543776.2018.1473379. [DOI] [PubMed] [Google Scholar]
- Chong P. Y.; Shotwell J. B.; Miller J.; Price D. J.; Maynard A.; Voitenleitner C.; Mathis A.; Williams S.; Pouliot J. J.; Creech K.; Wang F.; Fang J.; Zhang H.; Tai V. W.; Turner E.; Kahler K. M.; Crosby R.; Peat A. J. Design of N-benzoxaborole benzofuran GSK8175-optimization of human pharmacokinetics inspired by metabolites of a failed clinical HCV inhibitor. J. Med. Chem. 2019, 62, 3254–3267. 10.1021/acs.jmedchem.8b01719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubelius A.; Lee S.; Almutairi A. The chemistry of boronic acids in nanomaterials for drug delivery. Acc. Chem. Res. 2019, 52, 3108. 10.1021/acs.accounts.9b00292. [DOI] [PubMed] [Google Scholar]
- Szatrowski T. P.; Nathan C. F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991, 51, 794–798. [PubMed] [Google Scholar]
- Kawanishi S.; Hiraku Y.; Pinlaor S.; Ma N. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol. Chem. 2006, 387, 365–372. 10.1515/BC.2006.049. [DOI] [PubMed] [Google Scholar]
- Toyokuni S.; Okamoto K.; Yodoi J.; Hiai H. Persistent oxidative stress in cancer. FEBS Lett. 1995, 358, 1–3. 10.1016/0014-5793(94)01368-B. [DOI] [PubMed] [Google Scholar]
- Lopez-Lazaro M. Dual role of hydrogen peroxide in cancer: possible relevance to cancer chemoprevention and therapy. Cancer Lett. 2007, 252, 1–8. 10.1016/j.canlet.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Trachootham D.; Alexandre J.; Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach?. Nat. Rev. Drug Discovery 2009, 8, 579–591. 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- Fernandes G. F. S.; Denny W. A.; Dos Santos J. L. Boron in drug design: Recent advances in the development of new therapeutic agents. Eur. J. Med. Chem. 2019, 179, 791–804. 10.1016/j.ejmech.2019.06.092. [DOI] [PubMed] [Google Scholar]
- Kim E. J.; Bhuniya S.; Lee H.; Kim H. M.; Cheong C.; Maiti S.; Hong K. S.; Kim J. S. An activatable prodrug for the treatment of metastatic tumors. J. Am. Chem. Soc. 2014, 136, 13888–13894. 10.1021/ja5077684. [DOI] [PubMed] [Google Scholar]
- Li J.; Huang J.; Lyu Y.; Huang J.; Jiang Y.; Xie C.; Pu K. Photoactivatable organic semiconducting pro-nanoenzymes. J. Am. Chem. Soc. 2019, 141, 4073–4079. 10.1021/jacs.8b13507. [DOI] [PubMed] [Google Scholar]
- Yang W.; Gao X.; Wang B. Boronic acid compounds as potential pharmaceutical agents. Med. Res. Rev. 2003, 23, 346–368. 10.1002/med.10043. [DOI] [PubMed] [Google Scholar]
- Liao Y.; Xu L.; Ou S.; Edwards H.; Luedtke D.; Ge Y.; Qin Z. H2O2/Peroxynitrite-activated hydroxamic acid HDAC inhibitor prodrugs show antileukemic activities against AML cells. ACS Med. Chem. Lett. 2018, 9, 635–640. 10.1021/acsmedchemlett.8b00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.; Xie S.; Ma L.; Chen Y.; Lu W. 10-Boronic acid substituted camptothecin as prodrug of SN-38. Eur. J. Med. Chem. 2016, 116, 84–89. 10.1016/j.ejmech.2016.03.063. [DOI] [PubMed] [Google Scholar]
- Yu H.; Jin H.; Gong W.; Wang Z.; Liang H. Pharmacological actions of multi-target-directed evodiamine. Molecules 2013, 18, 1826–1843. 10.3390/molecules18021826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X.; Li D.; Chu C.; Li X.; Wang X.; Jia Y.; Hua H.; Xu F. Antiproliferative effects of alkaloid evodiamine and its derivatives. Int. J. Mol. Sci. 2018, 19, 3403–3434. 10.3390/ijms19113403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.; Dong G.; Wu S.; Liu N.; Zhang W.; Sheng C. Novel fluorescent probes of 10-hydroxyevodiamine: autophagy and apoptosis-inducing anticancer mechanisms. Acta Pharm. Sin. B 2019, 9, 144–156. 10.1016/j.apsb.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J.; Hu C. Evodiamine: a novel anti-cancer alkaloid from Evodia rutaecarpa. Molecules 2009, 14, 1852–1859. 10.3390/molecules14051852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G.; Sheng C.; Wang S.; Miao Z.; Yao J.; Zhang W. Selection of evodiamine as a novel topoisomerase I inhibitor by structure-based virtual screening and hit optimization of evodiamine derivatives as antitumor agents. J. Med. Chem. 2010, 53, 7521–7531. 10.1021/jm100387d. [DOI] [PubMed] [Google Scholar]
- Wang S.; Fang K.; Dong G.; Chen S.; Liu N.; Miao Z.; Yao J.; Li J.; Zhang W.; Sheng C. Scaffold diversity inspired by the natural product evodiamine: discovery of highly potent and multitargeting antitumor agents. J. Med. Chem. 2015, 58, 6678–6696. 10.1021/acs.jmedchem.5b00910. [DOI] [PubMed] [Google Scholar]
- Dong G.; Wang S.; Miao Z.; Yao J.; Zhang Y.; Guo Z.; Zhang W.; Sheng C. New tricks for an old natural product: discovery of highly potent evodiamine derivatives as novel antitumor agents by systemic structure-activity relationship analysis and biological evaluations. J. Med. Chem. 2012, 55, 7593–7613. 10.1021/jm300605m. [DOI] [PubMed] [Google Scholar]
- Liu W.; Chen S.; Zhang F.; He S.; Wang S.; Sheng C. Design, synthesis and biological evaluation of novel antitumor spirodihydrothiopyran-oxindole derivatives. Bioorg. Med. Chem. Lett. 2019, 29, 1636–1642. 10.1016/j.bmcl.2019.04.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.