Abstract
Psilocybin, an active component in “magic mushroom”, may have the potential to meet the therapeutic needs for a number of indications without the addictiveness and overdose risk of other mind-altering drugs, such as cocaine, heroin, alcohol, methamphetamine, and so forth. The need for new therapies is urgent because addiction, overdose, and suicide deaths have risen throughout the United States and around the world. Anecdotal and contemporary pharmacological reports have provided some indication about the therapeutic use of psilocybin for the treatment of mental health disorders such as major depressive disorder and addiction disorders. In this Viewpoint, I summarize the current state of psilocybin therapeutic research and attempt to provide some insight into future directions on which the scientific community may wish to focus.
Keywords: Psilocybin, psilocin, depression, psychedelic, serotonin, ketanserin
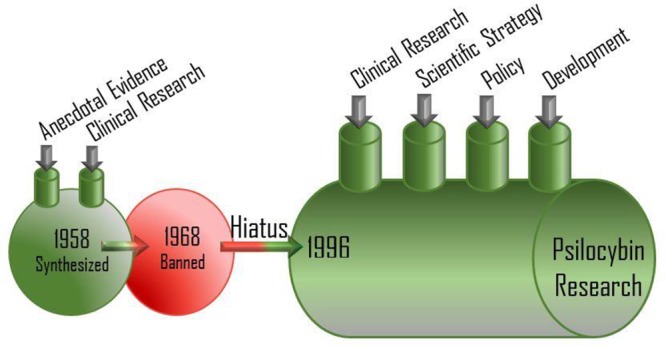
Recorded use of psilocybin by humans has been demonstrated for over a century.1 There are reports suggesting that the molecule has potent effects on behavior, cognition, thought, spirituality, and retrospection. In the 1950s, scientists began to realize the potential of psychedelic compounds in neuroscience, mood disorders, behaviors, and psychiatry. A notable quality of these compounds was they showed little propensity for habit formation, overdoses, or addiction.1 In contrast to psilocybin, opioid use and addiction has spread within the U.S. population irrespective of profession or age. In 2016, drug overdose deaths exceeded 59,000, which is the largest annual jump ever recorded in the U.S.2 Equally disturbing, the rate of suicide among Americans aged 35 to 64 increased nearly 30% from 1999 to 2010.3 This troubling trend is considered underreported due to the stigma associated with suicide. Attempts at combating suicide include collaborative efforts between different agencies involved in substance abuse, mental health services, preventive services, and public education. However, there is little sign that these efforts have had a dramatic effect in slowing down the trend of suicide, overdoses, and depression. In 2017, the U.S deaths from alcohol, drugs, and suicide hit their highest level since record-keeping began.4 The problem is exacerbated by the lack of significant advances in psychiatric drug development, as current treatments are plagued with limited efficacy, significant side effects, and dependency on long time use, which may lead some patients to develop treatment-resistance. Academic research along with anecdotal reports suggest that psychedelics have promising therapeutic potential, and the United States National Institutes of Health has issued a mandate that funded research should include participants from diverse population.5
This renewed interest in psilocybin research is beleaguered by several years of scientific dormancy, caused in part by governmental regulation and societal taboo; this has left many unanswered questions regarding the pharmacology and toxicology of psilocybin. The classification of psilocybin as a Schedule I drug in the United States and Class A drug in the United Kingdom has resulted in a relative dearth of scientific inquiry into the biology of psilocybin until recently. The therapeutic potential of psychedelics is demonstrated, especially in psychiatry over the past decade with more than ten completed clinical trials. A meta-analysis of LSD-assisted treatment of alcoholism in the 1960s demonstrated robust clinically and meaningful effects, which persisted up to 6 months after a single high-dose LSD session.6 In a related study conducted in 2012, participants treated with psilocybin for alcohol use disorder (AUD) and cigarette addiction demonstrated impressive improvements. Furthermore, psilocybin-assisted psychotherapy has been effective in the treatment of depression and anxiety in cancer patients and also in the treatment of resistant depression.7
Studies exploring psilocybin in the treatment of AUD have reported a wide range of psychological experiences and processes in participants that are considered to be critical for positive changes with respect to alcohol consumption habits and addiction. Many of these experiences are not adequately captured by measures used in clinical trials. Some recent studies have supported the hypothesis that mystical experience is a mediator of change in participants, experiences that are high in internal unity/oneness and involve the attractiveness to one’s surroundings, knowledge of the ultimate reality, insightfulness, and spiritual or religious blessedness.8 The definition and correlative findings for mystical experience upon high dose psilocybin administration are a positive development in quantifying the psilocybin-induced psychophysiological experience as other metrics, such as peak-psychedelic experience, are subjective and may be characterized primarily by the experience of unity or loss of the sense of a separate self.
Effects of Psilocybin
The effects of psilocybin administration may provide therapeutic potential in conjunction with modern scientific approaches. Considering anecdotal reports and recent clinical studies, psilocybin’s effect can be generally classified into four areas: perceptual, cognitive, emotional, and ego dissolution.9
The perceptual changes are dose-dependent and could range from mental imagery, distortion, perceptual intensification, illusion, elementary hallucinations, and complex hallucinations. Sense of time, location, and causal sequence can lose their usual linear cause–effect relationship. Cognitive effects of psilocybin can be paradoxical and enigmatic. Certain cognitive traits associated with creativity can increase, such as divergent thinking, unlikely word associations or language patterns, expansion of semantic activation, and attribution of meaning to musical stimuli. The emotional effects are characterized by intensification of feelings, a broadening in the overall range of emotions felt over the duration of psilocybin exposure, and increased access to emotions such as unique states of euphoria characterized by involuntary grinning, uncontrollable laughter, forgiveness, connectedness, silliness, giddiness, playfulness, exuberance, or negative emotions. The majority of emotional effects of psilocybin in supportive contexts are experienced as positive or bias emotion toward constructive responses to social and environmental stimuli. There can be subtle to drastic ego dissolution experiences in which a sense of self- and ego loss occur, which are also exhibited in a dose-dependent fashion. The notion of self- and identity dissolution, sense of connection with the universe or environment, “mystical-type” experiences may reliably occur at higher doses and can be modulated by external stimuli such as music. This experience is more likely to influence long-term changes in life outlook and personality traits.9
Obviously, psilocybin effects are not one size fits all scenarios; however, the harm potential of psilocybin compared to other mind-altering drugs is relatively low.10 Psilocybin administration may result in effects such as silliness, laughter, playfulness, and so forth, which may raise “eye brows”; however, they could be modulated to address an individual’s desired clinical outcome. For example, for an individual who may be undergoing a severe state of depression, the ability of a psilocybin therapy to enable controllable/uncontrollable laughter or playfulness could be enigmatic and therapeutic, which will be a far cry from the occasioned suicidal tendencies associate with major depressive disorder (MDD). This points to the need for psilocybin to be considered like any other medication. Illicit use of psilocybin is ill-advised and is tantamount to consuming a prescription drug outside of medical care.
Neuroscience and Pharmacology
The pharmacology of psilocybin has been described in several models/hypotheses such as psychoses, filtration, and psychoanalytical hypothesis in which psilocybin’s effects share descriptive elements, a wide range of subjective contents, and elements of psychoses, respectively. These hypotheses or models strongly correlate the delicate balance between neuronal activity, including critical integrative mechanisms, processing across sensory stimuli, neural excitation, and functional structures within the brain. The pioneering work of Rick Strassman and co-workers, ushered psychedelics into modern medicine in the early 1990s, when they reported on the biological and behavioral effects of N,N-dimethyltryptamine (DMT).11 In 1998, Franz Vollenweider, who is one of the pioneers of psychedelic neuroscience, demonstrated that psychedelics such as LSD and psilocybin work on the human brain by activating serotonin 5-HT2A receptors.9
David Nichols has discussed the psychedelic drug molecule impacts on the neuron by binding to and altering the conformation of receptors on the surface of the neuron. The receptor interaction most implicated in producing classic psychedelic drug effects is the agonist or partial agonist activity at serotonin (5-hydroxytrptamine) type 2A (5-HT2A) receptors (Figure 1).9 Classic serotonergic psychedelics like psilocybin bind to serotonin receptors and generally exhibit agonist properties. Unlike hallucinogens associated with dopaminergic “psychotic” experiences, 5-HT2A serotonin receptor activation tends to produce “dream-like” effects with awareness/insight as the basis for the hallucinatory experience.
Figure 1.
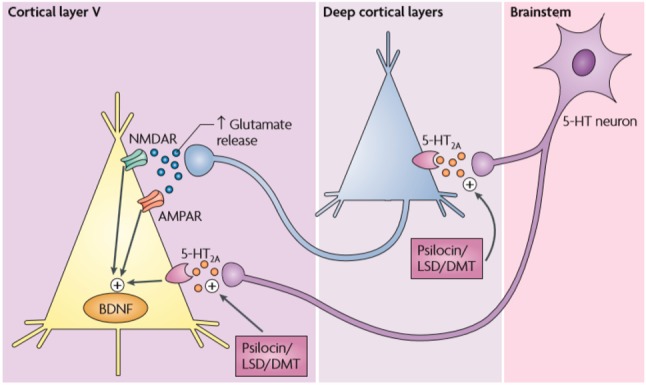
Glutamatergic synaptic activity is modulated by specific 5-HT2A antagonists such as AMPA (α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionic acid), positive allosteric modulators of metabotropic glutamate receptor 2 (mGluR2) and selective antagonists of the NR2b subunit of NMDA receptors. (Reprinted with permission from Springer Nature: Nature Reviews Neuroscience, 2010).9
Another idea that was introduced in 2014 by Carhart-Harris et al. involved the entropic brain theory (EBT), which introduced and links the phenomenology and neurophysiology of psychedelic effects by characterizing in terms of both quantitative notions of entropy and uncertainty.12 The EBT proposes that the conscious state depends on the system’s entropy measured via key parameters of brain function, which undergo perceptual destabilization, ego dissolution, and cognitive flexibility that can be mapped directly onto elevated levels of entropy/uncertainty measured in brain activity.9 Recently, Carhart-Harris proposed the relaxed beliefs under psychedelics (REBUS), a model that explains a broad range of phenomena associated with the psychedelic experience as it relates to the potential therapeutic use. The model integrates the free-energy principle and entropic brain hypothesis, where psychedelics relax the high-level priors or beliefs for example and liberate information in a bottom-up manner from the limbic system.12
Future Direction
The future of therapeutic psychedelics research in general and psilocybin in particular holds enormous potential to save lives and meet unmet medical needs through the world. Sales of the top 12 selling psychiatry drugs in the United States (June 2013 – June 2014) were estimated by the research firm IMS to be $23 billion, despite the numerous liabilities and underwhelming efficacy of these drugs.13 The promise of psilocybin therapeutic has drawn the attention of the FDA and EMA. For example, the FDA recently designated psilocybin and MDMA as “Breakthrough Therapies” for treatment-resistant depression and post-traumatic stress disorder, respectively, a status reserved for drugs that display advantages over current options for serious or life-threatening conditions.
Molecular Biology Infrastructure
Psychedelic research in general lacks high-throughput screening techniques, which are needed to test for drug interactions and effects in cell-based biological systems. In the current psilocybin pharmaceutical research, there is a lack of studies that use biomarkers for genotypes and expression levels of 5-HT2A receptors in parallel with clinical end points. Such studies could shed light on the therapeutic mechanism of 5-HT2A receptors in the use of psilocybin and also facilitate the development of personalized medicines in the treatment of various diseases such as anxiety and stress-related disorders.
There is insufficient modern literature on psilocybin’s structure–activity relationships as it relates to dose–response, lack of large sample size on potential abuse or side effects, the combination of psilocybin with an SSRI, and the influence of psychotherapeutic approaches. Furthermore, clinical outcomes that distinguish the relationship between drug-induced experience and its integration into the psychotherapeutic process are needed, to enable better understanding of the behavioral changes and the unique effect of the drug on neuroplasticity.
Clinical Study Design
Recent psychedelic clinical trials implement extensive exclusion criteria and each expansion of trial size may introduce new potential variables. This could pose significant complexities as the treatment reaches a wider population. Based on studies to date, therapeutic outcomes in response to psychedelic treatment may be uniquely coupled to the subjective cognitive effects of the participants during their dosing sessions. Consequently, any variable that may influence the subject’s cognitive experience (e.g., mindset, physical setting, guide’s influence) needs to be considered as a potentially confounding variable to the study. On like a regular drug discovery program, obtaining a control or placebo group is challenging because it is difficult, if not impossible, to develop a credible placebo that blinds the psychedelic experience. As a result, the execution of clinical studies requires a high degree of blinding and separation of functional roles such that outcome raters and investigators remain unaware of the subject’s experience and/or outward signs of psychedelic activity.
Funding Gap
A recent Tufts study finds the cost of bringing a new drug to market in the US to be roughly $3 billion and could be more than 12 years.14 The potential disruptive nature of psilocybin treatment to the SSRIs market is expected to be significant. Psilocybin treatment requires only a small dose, which is nonaddictive, and may be a once in a lifetime treatment. Because these drugs are naturally occurring, patentability is minimal and manufacturers seeking FDA-approval are likely to only received 5-year exclusivity afforded to new drug approvals (against generics). Gaps in funding have been filled by nonprofit organizations such as the Multidisciplinary Association for Psychedelic Studies (MAPS), Usona Institute, and The Heffter Research Institute. Psychedelic research has potential therapeutic benefit to society but will require large investment and given the lingering historical stigmatization, it will be imperative for funding and advocacy to go beyond nonprofit organizations. The situation has resemblance to the orphan drug market, which received incentives from the FDA since it addresses neglected diseases by the pharmaceutical industry. The number of orphan drugs approved and under development has soared since these incentives were enacted. Psychedelic therapy is in a similar situation but lacks such incentive. It will be a crucial aspect in helping to remove regulatory barriers involved in basic and clinical research and stigmatization of these medications. Societal understanding/acceptance of psilocybin therapeutic research is vague, and researchers are carefully reintroducing psychedelic research into the modern-day scientific paradigm.
Conclusion
Psychedelic research has been traditionally neglected by the pharmaceutical industry. A combination of anecdotal and recent clinical studies has demonstrated potential disease-modifying therapeutics of psychedelics. Psilocybin-assisted psychotherapy has been effective in the treatment of depression, anxiety in cancer patients, and treatment resistant depression. The renewed interest in psilocybin research is beleaguered by several years of scientific dormancy, which have left many unanswered questions regarding the mechanism of action and pharmacology. The significant infrastructure, funding, and regulatory requirements for any drug discovery program warrant that psychedelic and psilocybin research in particular involves governmental, pharmaceutical, and nonprofit organizations, for the therapeutic benefit to reach the general populace.
Acknowledgments
The author thanks Poncho Meisenheimer, Alex Sherwood, Robert Barrow, Charles Raison, David E. Nichols, Tura G. Patterson, Joel Walker, Bill Linton, and Katchen Anderson for suggestions and revisions.
Views expressed in this editorial are those of the author and not necessarily the views of the ACS nor the Usona Institute to which the author is affiliated.
References
- Nutt D.; King L. A.; Saulsbury W.; Blakemore C. Developing of a rational scale to assess the harm of drugs of potential misuse. Lancet 2007, 369, 1047. 10.1016/S0140-6736(07)60464-4. [DOI] [PubMed] [Google Scholar]
- Katz J.Drug Deaths in America Are Rising Faster Than Ever; New York Times, published online June 5, 2017; https://nyti.ms/2rI5lBB (accessed 2019-08-10). [Google Scholar]
- Parker-Pope T.Suicide Rates Rise Sharply. New York times, published online May 03, 2013; https://www.nytimes.com/2013/05/03/health/suicide-rate-rises-sharply-in-us.html?_r=0 (accessed 2019-03-16). [Google Scholar]
- https://amp.usatoday.com/amp/3033124002 (accessed 2019-03-16).
- Michaels T. I.; Purdon J.; Collins A.; Williams M. T. Inclusion of people of color in psychedelic-assisted psychotherapy: a review of the literature. BMC Psychiatry 2018, 18, 245. 10.1186/s12888-018-1824-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs T. S.; Johansen P. O. Lysergic acid diethylamide (LSD) for alcoholism: meta-analysis of randomized controlled trials. J. Psychopharmacol. 2012, 26, 994. 10.1177/0269881112439253. [DOI] [PubMed] [Google Scholar]
- Griffiths R. R.; Johnson M. W.; Carducci M. A.; Umbricht A.; Richards W. A.; Richards B. D.; et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J. Psychopharmacol. 2016, 30, 1181. 10.1177/0269881116675513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett F. S.; Johnson M. W.; Griffiths R. R. Validation of the revised mystical experience questionnaire in experimental sessions with psilocybin. J. Psychopharmacol. 2015, 29, 1182. 10.1177/0269881115609019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider F. X.; Kometer M. The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat. Rev. Neurosci. 2010, 11, 642. 10.1038/nrn2884. [DOI] [PubMed] [Google Scholar]
- Nutt D. J.; King L. A.; Phillips L. D. Drug harms in the UK: a multicriteria decision analysis. Lancet 2010, 376, 1558. 10.1016/S0140-6736(10)61462-6. [DOI] [PubMed] [Google Scholar]
- Strassman R. J.; Qualls C. R.; Uhlenhuth E. H.; Kellner R. Dose-Response Study of N,N-Diemthyltryptamine in Humans: II. Subjective Effects and Preliminary Results of a New Rating Scale. Arch. Gen. Psychiatry 1994, 51, 98. 10.1001/archpsyc.1994.03950020022002. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris R. L.; Friston K. J. REBUS and the Anarchic Brain: Toward a Unified Model of the Brain Action of Psychedelics. Pharmacol. Pharmacol. Rev. 2019, 71, 316. 10.1124/pr.118.017160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mental Health Daily. Top 12 Selling Psychiatric Drugs: United States (July 2013 – June 2014) https://mentalhealthdaily.com/2014/08/12/top-selling-psychiatric-drugs-united-states-july-2013-june-2014/ (accessed 2019-09-14).
- For example, See:Mullin R.Tufts study finds big rise in cost of drug development. Chem. Eng. News [online], November 20, 2014https://cen.acs.org/articles/92/web/2014/11/Tufts-Study-Finds-Big-Rise.html (accessed 2019-11-09).


