Short abstract
Background
Obesity is a continuing national epidemic, and the condition can have a physical, psychological, as well as social impact on one’s well-being. Consequently, it is critical to diagnose and document obesity accurately in the patient’s electronic medical record (EMR), so that the information can be used and shared to improve clinical decision making and health communication and, in turn, the patient’s prognosis. It is therefore worthwhile identifying the various factors that play a role in documenting obesity diagnosis and the methods to improve current documentation practices.
Method
We used a retrospective cross-sectional design to analyze outpatient EMRs of patients at an academic outpatient clinic. Obese patients were identified using the measured body mass index (BMI; ≥30 kg/m2) entry in the EMR, recorded at each visit, and checked for documentation of obesity in the EMR problem list. Patients were categorized into two groups (diagnosed or undiagnosed) based on a documented diagnosis (or omission) of obesity in the EMR problem list and compared.
Results
A total of 10,208 unique patient records of obese patients were included for analysis, of which 4119 (40%) did not have any documentation of obesity in their problem list. Chi-square analysis between the diagnosed and undiagnosed groups revealed significant associations between documentation of obesity in the EMR and patient characteristics.
Conclusion
EMR designers and developers must consider employing automated decision support techniques to populate and update problem lists based on the existing recorded BMI in the EMR in order to prevent omissions occurring from manual entry.
Keywords: Obesity, body mass index, electronic medical record, medical records, weight management, clinical decision support
Introduction
Obesity continues to be a major epidemic in the USA, affecting individuals across diverse demographics and spectrums.1 The continued growth of the obesity epidemic is particularly concerning because obesity can have a physical, psychological, as well as social impact on one’s well-being.2 Obesity is also known to be associated with several significant health issues, such as hypertension,3 sleep apnea,4 type II diabetes,5 cardiovascular disease,6 and even mortality.7 Consequently, it is critical to diagnose and document obesity accurately in the patient’s electronic medical record (EMR), so that the information can be used and shared to improve clinical decision making, such as screening, prescribing, and identifying targeted therapies for treatment, and, in turn, to improve the patient’s prognosis. Furthermore, proper documentation of diagnoses in an EMR8 also allows for this information to be shared with the patient and other clinicians, thus encouraging collaborative practice.9
Obesity is generally classified on the basis of the body mass index (BMI), calculated as the total body weight (in kilograms) divided by the height (in meters squared). According to the Centers for Disease Control, obesity is defined as a BMI ≥30 kg/m2, while a BMI of ≥40 kg/m2 is defined as class 3 obesity or morbid obesity.
The Centers for Medicare and Medicaid Services (CMS) Electronic Health Record Incentive Programs10 allow health-care providers to become eligible for payments as an incentive for using an EMR system, or face penalty upon failure to adopt such a system. An integral component of the incentive program, Meaningful Use Stage 1, mandated the recording of height, weight, blood pressure, and BMI as core measures of vital signs in the EMR at each patient visit.11
Besides documentation of individual body weight and height at each visit, most modern EMR systems also allow clinicians to maintain individual patients’ problem lists, utilizing standardized terminologies such as the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM).12 These problem lists serve as critical records to identify and remind clinicians quickly of the presenting patient’s health issues. While they are part of modern EMR systems, these problem lists still require clinicians to update them manually by adding any new and current health problems while removing (or archiving) prior resolved health conditions.
Acknowledgment and subsequent diagnosis are the first steps toward addressing any health condition. Prior evidence reveals that increased use of the EMR is associated with the likelihood of the documentation of obesity in the patient’s medical record.13 Moreover, obese patients with documentation of obesity in their problem list are more likely to receive obesity treatment and management from their physicians.14 Yet, obesity remains largely underdiagnosed15 for several reasons, including the use of appearance rather than BMI for diagnosis,16 lack of preventive care, weight stigma, and implicit anti-fat bias among clinicians.17,18
Broader adoption of EMRs has given rise to the rapid collection of vast amounts of health data. This big data can be further harnessed by performing analytics to find patterns in the data and develop novel machine learning algorithms for use in clinical decision support. Prior evidence demonstrates the value attributed to the secondary use of these existing data to improve health quality and patient outcomes by the implementation of these models in clinical decision support.19–21
Therefore, identifying the various factors that play a role in documenting obesity diagnosis and methods to improve current documentation practices deserves further investigation. This paper examines the extent of obesity-related problem list omissions at an academic outpatient clinic and identifies patient-specific factors associated with documentation of obesity in the EMR. We also discuss potential solutions and techniques to aid the maintenance of accurate and updated problem lists.
Method
Study sample and inclusion criteria
Institutional Review Board (IRB) approval (#UMCIRB 15-000907) was obtained prior to commencing any study activities. We utilized a retrospective cross-sectional design to analyze the outpatient EMRs of patients at East Carolina University (ECU Physicians). ECU Physicians is the medical practice of the Brody School of Medicine, spread across 26 locations in Eastern North Carolina. Office visit summary data were extracted from the EMR for all adult patients (aged ≥18 years) who completed at least one office visit at any of the outpatient clinic locations at least once between January 1, 2017, and December 31, 2017, and had a corresponding BMI measure for the visit. Obese patients were identified using the BMI (≥30 kg/m2) which was recorded in the EMR for each office visit. For patients with more than one visit during the time period, the BMI from only the most recent visit was included for analysis in order to reflect their most recent BMI, consistent with their diagnosis state.
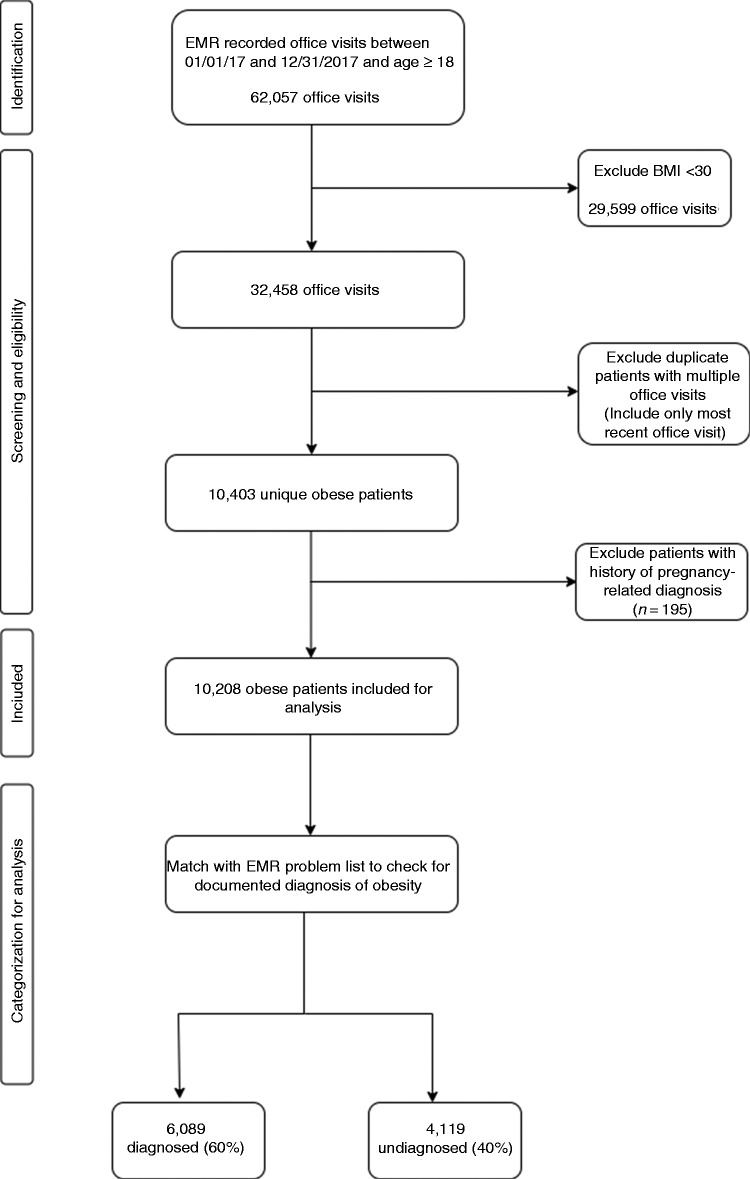
We subsequently extracted problem lists for the obese patients identified above to check for documentation of an obesity diagnosis in the EMR. Documentation of obesity in the problem list was identified using the corresponding ICD-10 category code (E66 and below). Patients with any history of any pregnancy-related diagnosis were identified using corresponding ICD-10 category codes O00 through O9A and excluded in order to avoid confounding the results. Finally, all the identified obese patients were categorized into two groups (diagnosed or undiagnosed) based on the presence of a documented diagnosis for obesity or its omission in the EMR problem list. Figure 1 illustrates the steps associated with the identification of the study sample and the gathering of the associated data.
Figure 1.
Steps for identification of study sample and categorization into study groups.
The two groups were compared based on demographics (sex, age group, race and ethnicity, and insurance status) to assess which specific patient characteristics were associated with the documentation of obesity in the EMR.
We also compared the two groups based on the patients’ use of the online patient portal. Prior evidence indicates that patients who have access to their medical records typically have more complete and accurate health information in the EMR, which is attributed to patient cross-checking and giving feedback on their health records.22–24 Therefore, within our outpatient practice, we wanted to assess if obese patients who were patient portal users may have a more updated and accurate problem list than nonusers of the patient portal. A patient portal user was defined as any patient who had activated their patient portal account and had logged in to the patient portal at least once six months beyond the activation date.
Similarly, we compared the documentation of obesity among two groups based on their diagnosis of other associated comorbidities (coronary heart disease, type II diabetes, hyperlipidemia, hypertension, osteoarthritis, and sleep apnea) to assess whether they played a role in the documentation of obesity in the EMR. We selected these chronic health conditions because there is abundant empirical evidence indicating their association with obesity.17,25–28
Statistical analysis
We conducted nonparametric chi-square tests to assess the differences in the distribution of the sample between the two groups based on their demographics, patient portal use, and diagnosed comorbidities. We used logistic regression analysis to predict the likelihood of the presence or omission of a documented diagnosis for all obese patients. An α of 0.05 was used as the threshold level of significance. All statistical analyses were conducted using IBM SPSS Statistics for Windows v25.0. (IBM Corp., Armonk, NY).
Results
EMRs for 10,208 unique patients with an obesity BMI were included for analysis, of which 4119 (40%) did not have a corresponding diagnosis of obesity in the EMR problem list. Table 1 lists the distribution of the patients across the diagnosed and the undiagnosed groups by patient characteristics (obesity group, sex, age, race, patient portal use, and insurance provider). Chi-square analyses revealed statistically significant differences between the two groups for each of the patient characteristics assessed (80% for morbidly obese vs. 52% for obese patients; 62% for females vs. 55% for males; 66% for patients aged ≤65 years vs. 34% for patients aged ≥66 years; 65% for black patients vs. 55%, 54%, and 53% for Hispanic, other, and white patients, respectively; 62% for patient portal users vs. 59% for nonusers; and 61% of publicly insured vs. 59% and 56% for privately insured and uninsured).
Table 1.
Presence of obesity diagnosis in EMR problem list across various patient characteristics for obese patients (BMI ≥30 kg/m2).
| Patient characteristic | Obesity diagnosis present in EMR problem list (%) | Obesity diagnosis absent in EMR problem list (%) | p |
|---|---|---|---|
| Obesity group | <0.0001 | ||
| Obese (BMI 30–39 kg/m2) | 3947 (52%) | 3581 (48%) | |
| Morbidly obese (BMI ≥40 kg/m2) | 2142 (80%) | 538 (20%) | |
| Sex | <0.0001 | ||
| Female | 4259 (62%) | 2631 (38%) | |
| Male | 1830 (55%) | 1488 (45%) | |
| Age (years) | <0.0001 | ||
| 18–35 | 1269 (64%) | 713 (36%) | |
| 36–45 | 1177 (67%) | 591 (33%) | |
| 46–55 | 1467 (67%) | 723 (33%) | |
| 56–65 | 1463 (68%) | 692 (32%) | |
| 66–75 | 545 (37%) | 943 (63%) | |
| 76+ | 168 (27%) | 457 (73%) | |
| Race | <0.0001 | ||
| Black | 3854 (65%) | 2114 (35%) | |
| Hispanic | 77 (55%) | 63 (45%) | |
| Other | 78 (54%) | 67 (46%) | |
| White | 2080 (53%) | 1875 (47%) | |
| Patient portal use | <0.0133 | ||
| Users | 1787 (62%) | 1116 (38%) | |
| Nonusers | 4302 (59%) | 3003 (41%) | |
| Insurance provider | 0.01805 | ||
| Private | 3255 (59%) | 2296 (41%) | |
| Public | 2653 (61%) | 1683 (39%) | |
| Uninsured | 181 (56%) | 140 (44%) | |
| Total | 6089 (60%) | 4119 (40%) |
Statistically significant values are shown in bold.
EMR: electronic medical record; BMI: body mass index.
Results of logistic regression analyses indicate that the patient characteristics assessed were predictive of the presence of an obesity diagnosis in the EMR problem list for all obese patients (inferred from BMI ≥30 kg/m2). As indicated by odds ratios (OR), obese patients who were female (OR=1.3), black (OR=1.6), and aged ≤65 years (OR=3.9) had higher odds of a presence of an obesity diagnosis in the EMR problem list compared to obese patients who were male, white, and aged ≥66 years. Additionally, morbidly obese patients (BMI ≥40 kg/m2) were far more likely to be diagnosed with obesity (OR=3.6) compared to other obese patients (BMI 30–39 kg/m2).
Figure 2 depicts the receiver-operating characteristic (ROC) curve, displaying the collective strength of the patient characteristics assessed in being able to predict the presence of an obesity diagnosis in the EMR for obese patients. The c-statistic for the ROC, which describes the area under the curve, was 0.713, indicating that patient characteristics can effectively discriminate29 between the two study groups to predict whether an obese patient would have an obesity diagnosis documented in the EMR problem list.
Figure 2.
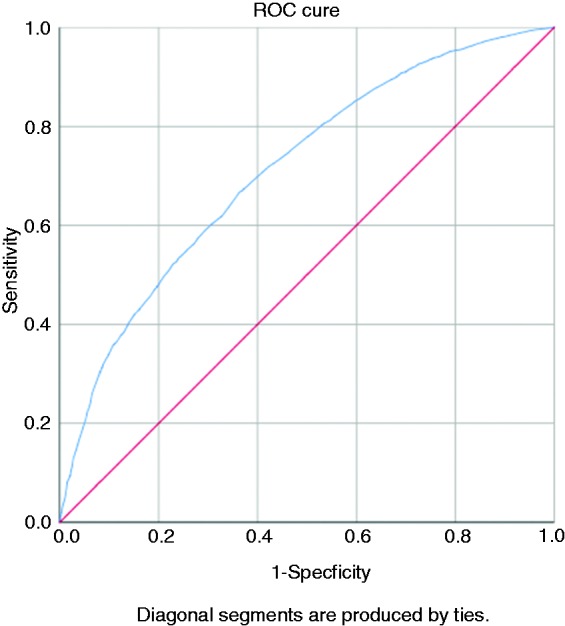
Receiver-operating characteristic curve based on patient characteristics as variables in predicting the presence of an obesity diagnosis in the electronic medical record problem list for obese patients (body mass index ≥30 kg/m2).
We also assessed the presence of an obesity diagnosis in the EMR problem list for the two study groups based on the presence of diagnoses for other obesity-related comorbidities (coronary heart disease, type II diabetes, hyperlipidemia, hypertension, osteoarthritis, and sleep apnea; Table 2). The results of a chi-square test indicated statistically significant differences among the two study groups based on the presence of some comorbidities (coronary heart disease, type II diabetes, hypertension, and sleep apnea). We found no significant differences for obese patients with hyperlipidemia or osteoarthritis. Interestingly, obese or morbidly obese patients without coronary heart disease were more likely to have obesity documented in the EMR problem list than obese or morbidly obese patients with coronary heart disease were.
Table 2.
Presence of obesity diagnosis in EMR problem list by comorbidities for obese patients (BMI ≥30 kg/m2).
| Comorbidity | Obesity diagnosis present in EMR problem list | Obesity diagnosis absent in EMR problem list | p |
|---|---|---|---|
| Coronary heart disease | <0.0001 | ||
| Yes | 410 (52%) | 372 (48%) | |
| No | 5679 (60%) | 3747 (40%) | |
| Type II diabetes | <0.0001 | ||
| Yes | 1878 (64%) | 1046 (36%) | |
| No | 4211 (60%) | 3073 (42%) | |
| Hyperlipidemia | 0.29 | ||
| Yes | 1573 (59%) | 1102 (41%) | |
| No | 4518 (60%) | 3015 (40%) | |
| Hypertension | 0.0004 | ||
| Yes | 3814 (61%) | 2436 (39%) | |
| No | 2273 (58%) | 1682 (42%) | |
| Osteoarthritis | 0.2 | ||
| Yes | 1084 (58%) | 773 (42%) | |
| No | 5005 (60%) | 3346 (40%) | |
| Sleep apnea | <0.0001 | ||
| Yes | 366 (73%) | 133 (27%) | |
| No | 5723 (59%) | 3986 (41%) | |
Statistically significant values are shown in bold.
Discussion
The results of our assessment of EMRs of obese patients at our outpatient practice reveal several interesting findings. Underdiagnosis of obesity was clear, represented by 40% of all obese patients included. There was a clear demographic divide among our obese patient population in terms of receiving a diagnosis for obesity or not. Females, blacks, and younger patients were more likely to receive a diagnosis of obesity compared to obese males, whites, and older obese patients.
We found that patients with morbid obesity (class 3 obesity) were more likely to be diagnosed. These results indicate that providers may often wait until the health condition gets severe enough to warrant documentation in the EMR. Prevention, health education, and early treatment are all effective strategies for weight management, highlighting the need to recognize and address obesity at the early stage.30–32
Early treatment is especially crucial in order to prevent the onset of several related comorbidities, such as coronary heart disease, type II diabetes, hypertension, hyperlipidemia, osteoarthritis, and sleep apnea. Long-term treatment of these associated chronic health conditions not only has a negative impact on the patient’s physical health and quality of life, but also contributes to increased health-care costs for the patient.
In terms of associated comorbidities, some of the results are difficult to explain, particularly how a coronary heart disease diagnosis had an inverse association with the documentation of an obesity diagnosis, while obese patients with other conditions such as type II diabetes, hypertension, and sleep apnea were more likely to have obesity documented. It is possible that physicians associate these conditions more closely with obesity compared to coronary heart disease or hyperlipidemia.
It is also interesting to note that obese patients who were active users of the patient portal had more accurate and complete EMR problem lists, which was attributed to them being able to access their medical records routinely and cross-check for any inaccuracies. Our results are consistent with prior findings that highlight the relationship between patient access to their medical records and the accuracy and completeness of EMR problem lists.22–24
Limitations
There is still no consensus between experts regarding how to define and measure obesity properly.33 While BMI is the accepted standard adopted by various national and international health organizations, it has been shown to suffer from various limitations, especially its use in population-wide studies of obesity, which may include individuals who are athletes or individuals with heights well below or above the population average.
Our results are representative of the diagnostic practices of physicians across outpatient clinics located primarily in a mid-size city and surrounding rural communities for an academic health system. Therefore, our findings may not be generalizable across other diverse health systems.
Finally, our study examines patient characteristics associated with obesity documentation in the EMR. To attain a better understanding of the reasons behind obesity underdiagnosis, it is also imperative to study physician-related factors that are motivators or barriers to documenting obesity in the EMR problem list.
Conclusion
Despite the Meaningful Use Stage 1 mandate for the recording and documentation of height, weight, blood pressure, and BMI as core measures of vital signs,34 it is evident that this information is not being fully harnessed and applied in the delivery of health care. Automated techniques such as machine learning and natural language processing have been shown to be helpful for the automated inference of patient problems from structured EMR data.35 EMR designers and developers must also consider the use of automated decision support techniques to populate and update problem lists by utilizing structured EMR data. Furthermore, larger-scale studies to analyze how EMR data can be used to predict patient populations that are more likely to be diagnosed with obesity may lead to new knowledge that could be incorporated in clinical decision support systems.
Further studies are recommended to investigate underlying causes of lower rates of obesity documentation for certain socio-demographic groups, and to study the impact of automated documentation. Similarly, further studies on physician-specific factors that play a role in the documentation of obesity in the EMR are recommended in order to provide a holistic view of the underdiagnosis problem.
Health-care providers must also continue patient engagement efforts and encourage patient portal use as a means of improving the quality of existing problem lists.
Contributorship
A.K. conceived the study and researched literature. A.K., J.K., and X.Z. were involved in protocol development and gaining ethical approval. A.G. was involved in data acquisition. A.K. conducted data analysis and wrote the first draft of the manuscript. S.H. provided expert guidance on medical coding and assisted in manuscript preparation. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
The IRB of East Carolina University approved this study (#UMCIRB 15-000907).
Guarantor
A.K.
ORCID iD
Akshat Kapoor https://orcid.org/0000-0002-0895-6440
Peer review
This manuscript was reviewed by reviewers who have chosen to remain anonymous.
References
- 1.Hales CM, Fryar CD, Carroll MD, et al. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007–2008 to 2015–2016. JAMA 2018; 319: 1723–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sullivan MB, Sullivan LG, Kral JG. Quality of life assessment in obesity: physical, psychological, and social function. Gastroenterol Clin North Am 1987; 16: 433–442. [PubMed] [Google Scholar]
- 3.Hall JE. Pathophysiology of obesity hypertension. Curr Hypertens Rep 2000; 2: 139–147. [DOI] [PubMed] [Google Scholar]
- 4.Vgontzas AN, Tan TL, Bixler EO, et al. Sleep apnea and sleep disruption in obese patients. Arch Intern Med 1994; 154: 1705–1711. [PubMed] [Google Scholar]
- 5.Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature 2001; 409: 307–312. [DOI] [PubMed] [Google Scholar]
- 6.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature 2006; 444: 875–880. [DOI] [PubMed] [Google Scholar]
- 7.Abdelaal M, Le Roux CW, Docherty NG. Morbidity and mortality associated with obesity. Ann Transl Med 2017; 5: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans JA. Electronic medical records system. US5924074A, https://patents.google.com/patent/US5924074A/en (accessed 14 November 2019).
- 9.Bergeron BP. Can electronic medical records really achieve information sharing? Postgrad Med 1998; 104: 25–27. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Medicare and Medicaid Services (CMS), HHS. Medicare and Medicaid programs; electronic health record incentive program. Final rule. Fed Regist 2010; 75: 44313–44588. [PubMed] [Google Scholar]
- 11.Marcotte L, Seidman J, Trudel K, et al. Achieving meaningful use of health information technology: a guide for physicians to the EHR incentive programs. Arch Intern Med 2012; 172: 731–736. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. ICD-10: international statistical classification of diseases and related health problems: tenth revision, https://apps.who.int/iris/handle/10665/42980 (accessed 19 November 2019).
- 13.Bordowitz R, Morland K, Reich D. The use of an electronic medical record to improve documentation and treatment of obesity. Fam Med 2007; 39: 274–279. [PubMed] [Google Scholar]
- 14.Ruser CB, Sanders L, Brescia GR, et al. Identification and management of overweight and obesity by internal medicine residents. J Gen Intern Med 2005; 20: 1139–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bardia A, Holtan SG, Slezak JM, et al. Diagnosis of obesity by primary care physicians and impact on obesity management. Mayo Clin Proc 2007; 82: 927–932. [DOI] [PubMed] [Google Scholar]
- 16.Lemay CA, Cashman S, Savageau J, et al. Underdiagnosis of obesity at a community health center. J Am Board Fam Pract 2003; 16: 14–21. [DOI] [PubMed] [Google Scholar]
- 17.Ma J, Xiao L, Stafford RS. Underdiagnosis of obesity in adults in US outpatient settings. Arch Intern Med 2009; 169: 312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teachman BA, Brownell KD. Implicit anti-fat bias among health professionals: is anyone immune? Int J Obes 2001; 25: 1525–1531. [DOI] [PubMed] [Google Scholar]
- 19.Kohn MS, Sun J, Knoop S, et al. IBM’s health analytics and clinical decision support. Yearb Med Inform 2014; 9: 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baig MM, Hosseini HG, Lindén M. Machine learning-based clinical decision support system for early diagnosis from real-time physiological data. In: 2016 IEEE Region 10 Conference (TENCON), Singapore, 2016, pp.2943–2946.
- 21.Castaneda C, Nalley K, Mannion C, et al. Clinical decision support systems for improving diagnostic accuracy and achieving precision medicine. J Clin Bioinform 2015; 5: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dullabh PM, Sondheimer NK, Katsh E, et al. How patients can improve the accuracy of their medical records. EGEMS (Wash DC) 2014; 2: 1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staroselsky M, Volk LA, Tsurikova R, et al. Improving electronic health record (EHR) accuracy and increasing compliance with health maintenance clinical guidelines through patient access and input. Int J Med Inform 2006; 75: 693–700. [DOI] [PubMed] [Google Scholar]
- 24.Staroselsky M, Volk LA, Tsurikova R, et al. An effort to improve electronic health record medication list accuracy between visits: patients’ and physicians’ response. Int J Med Inform 2008; 77: 153–160. [DOI] [PubMed] [Google Scholar]
- 25.Alexander JK. Obesity and coronary heart disease. Am J Med Sci 2001; 321: 215–224. [DOI] [PubMed] [Google Scholar]
- 26.Felber J-P, Golay A. Pathways from obesity to diabetes. Int J Obes 2002; 26: S39–45. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan PW, Ghushchyan VH, Ben-Joseph R. The impact of obesity on diabetes, hyperlipidemia and hypertension in the United States. Qual Life Res 2008; 17: 1063. [DOI] [PubMed] [Google Scholar]
- 28.Wolk R, Shamsuzzaman ASM, Somers VK. Obesity, sleep apnea, and hypertension. Hypertension 2003; 42: 1067–1074. [DOI] [PubMed] [Google Scholar]
- 29.Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol 2010; 5: 1315–1316. [DOI] [PubMed] [Google Scholar]
- 30.Kumanyika S, Jeffery RW, Morabia A, et al. Obesity prevention: the case for action. Int J Obes 2002; 26: 425–436. [DOI] [PubMed] [Google Scholar]
- 31.Müller MJ, Asbeck I, Mast M, et al. Prevention of obesity – more than an intention. Concept and first results of the Kiel Obesity Prevention Study (KOPS). Int J Obes 2001; 25: S66–74. [DOI] [PubMed] [Google Scholar]
- 32.Swinburn B, Gill T, Kumanyika S. Obesity prevention: a proposed framework for translating evidence into action. Obes Rev 2005; 6: 23–33. [DOI] [PubMed] [Google Scholar]
- 33.Rothman KJ. BMI-related errors in the measurement of obesity. Int J Obes 2008; 32: S56–59. [DOI] [PubMed] [Google Scholar]
- 34.Eligible Professional Meaningful Use Core Measures Measure 8 of 14, https://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/Downloads/2013DefinitionEP_8_Record_Vital_Signs.pdf (accessed 4 November 2019).
- 35.Wright A, Pang J, Feblowitz JC, et al. A method and knowledge base for automated inference of patient problems from structured data in an electronic medical record. J Am Med Inform Assoc 2011; 18: 859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]