Abstract
Studies reported that Serenoa repens was effective in relieving lower urinary tract symptoms (LUTS). This article carried out a systematic review and meta-analysis to compare Serenoa repens with tamsulosin in the treatment of benign prostatic hyperplasia (BPH) after at least 6-month treatment cycle. Four studies involving 1,080 patients (543 in the Serenoa repens group and 537 in the tamsulosin group) were included in the meta-analysis. The results were as follows: compared with tamsulosin, Serenoa repens had a same effect in treating BPH in terms of International Prostate Symptom Score (IPSS) (mean difference [MD] 0.63, 95% confidence interval [CI] [−0.33, 1.59], p = 0.20), quality of life (QoL) (MD 1.51, 95% CI [−1.51, 4.52], p = 0.33), maximum flow rate (Qmax) (MD 0.27, 95% CI [−0.15, 0.68], p = 0.21), postvoid residual volume (PVR) (MD −4.23, 95% CI [−22.97, 14.44], p = 0.65), prostate-specific antigen (PSA) (MD 0.46, 95% CI [−0.06, 0.97], p = 0.08) with the exception of prostate volume (PV) (MD −0.29, 95% CI [−0.41, −0.17], p < 0.00001). For side effects, Serenoa repens was well tolerated compared with tamsulosin especially in ejaculation disorders (odds ratio [OR] = 12.56, 95% CI [3.83, 41.18], p < 0.0001) and decreased libido (OR = 5.40; 95% CI [1.17, 24.87]; p = 0.03). This study indicated that Serenoa repens had the same effect in treating BPH compared with tamsulosin in terms of IPSS, QoL, and PVR after at least 6-month treatment cycle, however, the latter had a greater improvement in PV compared with the former. And Serenoa repens did not increase the risk of adverse events especially with respect to ejaculation disorders and libido decrease.
Keywords: benign prostatic hyperplasia, tamsulosin, Serenoa repens, randomized controlled trials, meta-analysis
Benign prostatic hyperplasia (BPH) is one of the most common diseases in lower urinary tract symptoms (LUTS), which can cause urinary dysfunction in middle-aged and elderly men and may affect the normal life of patients (Buck, 2015; Gray & Allensworth, 1990; Holtgrewe, 1998; Lowe & Fagelman, 1999; Oesterling, 1995; Wilt et al., 1998). Drug therapy has become a major treatment model for BPH, mainly including alpha-blockers, 5α-reductase inhibitors, and phytotherapeutics (Boyle et al., 1996; Di Salle et al., 1994; Salle et al., 1994). Different types of drugs have different side effects. Alpha-1 blockers can be associated with orthostatic hypotension and 5α-reductase inhibitors are associated with sexual dysfunction (Clifford & Farmer, 2000). Increasing attention has been focused on the use of phytotherapeutic agents to alleviate the LUTS.
Serenoa repens (also known as the saw palmetto) has been widely used in Europe for many years and Americans have recognized its help in prostate health in the past decade, which has been assessed in numerous studies (Debruyne et al., 2002; Gerber et al., 2001; Lowe, 2001; Pytel et al., 2002; Sinescu et al., 2011). In vitro, Serenoa repens extract has demonstrated anti-inflammatory, antiandrogenic, and estrogenic effects along with a decrease in sexual hormone-binding globulin; inhibition of 5α-reductase, muscarinic cholinoceptors, dihydropyridine receptors, and vanilloid receptors; and neutralization of free radicals (Ficarra et al., 2014; Habib, 2009).
Many studies have found that Serenoa repens played an important role in the treatment of BPH, however, there were few retrospective articles comparing Serenoa repens with tamsulosin in the treatment of BPH. To assess the efficacy and safety of tamsulosin (0.4 mg) compared with Serenoa repens (320 mg) for the treatment of LUTS/BPH, this study performed a systematic review and meta-analysis of randomized controlled trials (RCTs).
Materials and Methods
Search Strategy
The study searched MEDLINE, Embase, and Cochrane Controlled Trials Register databases for RCTs published before May 2019, using the following search criteria: BPH, RCT, tamsulosin, and Serenoa repens. The analysis confined our search to published studies in English only, and obtained certain essential information directly from the authors. Some relevant references were also screened in this study.
Inclusion Criteria
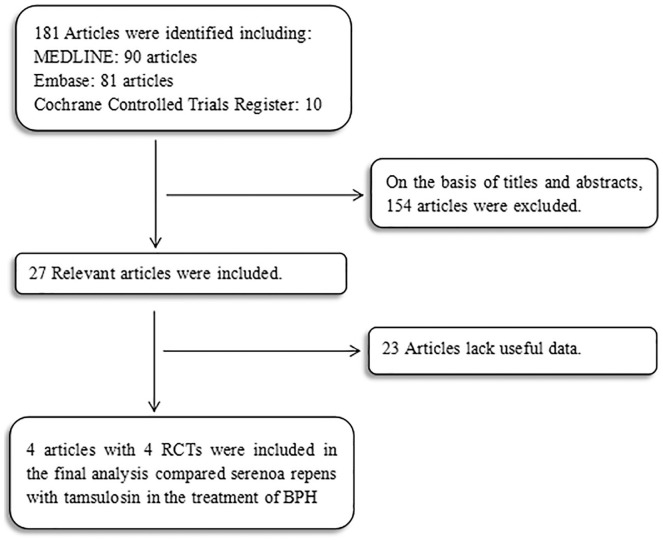
The study should meet the following characteristics: (a) Serenoa repens and tamsulosin for the treatment of BPH/LUTS; (b) available full text; and (c) provided accurate data for analysis, including the total number of subjects and the values of each indicator. The most recently published study was included in the meta-analysis if an identical study was published in distinct journals or at a different time point. When the same group of researchers investigated a certain subject group in multiple experiments, each study was included. As presented in Figure 1, the meta-analysis used a flowchart to show the selection process.
Figure 1.
Flowchart of the study selection process. RCTs = randomized controlled trials.
Quality Assessment
Jadad and Rennie’s (1998) scale was used to determine the quality of the retrieved RCTs (Jadad & Rennie, 1998). This meta-analysis did not consider the quality score and used all of the identified RCTs. The methodological quality of each study was assessed based on how patients were allocated to the aims of the study, the concealment of distribution procedures, blinding, and data lost due to attrition. According to the guidelines published in the Cochrane Handbook for Systematic Reviews of Interventions v.5.1.0, the studies were then classified qualitatively. Each article was evaluated and assigned according to three quality classification criteria: (a) if the study has all quality criteria and it would have a low risk of bias; (b) the study was considered to have a moderate risk of bias, when one or more quality criteria were merely partially met or were ambiguous; or (c) the study was considered to have a high risk of bias when one or more of these criteria were rarely met or not involved. All authors participated in the RCTs’ quality assessment and resolved the differences through discussion.
Data Extraction
The following information from the studies was recorded: (a) regimen patients received; (b) design of study and size of sample; (c) name of the RCT; (d) the area of study; (e) changes in the following parameters, such as International Prostate Symptom Score (IPSS), quality of life (QoL), maximum flow rate (Qmax), postvoid residual volume (PVR), prostate volume (PV), prostate-specific antigen (PSA), ejaculation disorders, libido decrease, rhinitis, fatigue, dizziness, postural hypotension, dry mouth, and headache.
Statistical Analysis and Meta-Analysis
RevMan v.5.1.0 (Cochrane Collaboration, Oxford, UK) was used to perform this meta-analysis (Higgins & Green, 2008). The difference of study between the entry and endpoint was evaluated according to changes in the IPSS, QoL, Qmax, PVR, PV, PSA, ejaculation disorders, libido decrease, rhinitis, fatigue, dizziness, postural hypotension, dry mouth, and headache. The mean difference (MD) was used to evaluate continuous data, and the odds ratio (OR) with 95% confidence interval (CI) was used to evaluate dichotomous data. A fixed-effects model was suitable for studies with p > .05, which was recognized as homogeneous. Inconsistent results were analyzed using the I2 statistic, which represents the proportion of heterogeneity across trials (Thompson & Thompson, 2005). The study used a random-effects model for studies with p < .05 and where I2 > 50%. Meanwhile, if p < .05, the result was considered statistically significant.
Results
Characteristics of the Individual Studies
One hundred and eighty-one studies were identified in all databases. According to the inclusion and exclusion criteria described above, reviewers removed 154 studies after reviewing the titles and abstracts of the articles. Twenty-three studies were excluded for lack of useful data. Thus four articles reporting data from four RCTs (Argirovic & Argirovic, 2013; Debruyne et al., 2002; Kaplan, 2016; Morgia et al., 2015) that compared Serenoa repens with tamsulosin over 24-week treatment cycle were included in the analysis (Figure 1). Table 1 presents the baseline characteristics of studies.
Table 1.
Study and Patient Characteristics.
| Sample size | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Country | Therapy in experimental group | Therapy in control group | Experimental | Control | Method | Time of therapy (weeks) | Dosage (mg/mg) | Main inclusion criteria |
| Argirovic and Argirovic (2013) | Serbia | T | SR | 98 | 107 | Oral | 24 | 0.4 mg once-daily/320 mg daily | PV < 50 ml, IPSS = 7–18, QoLs > 3, Qmax of 5–15 ml/s, with PVR < 150; PSA < 4 ng/ml |
| Debruyne et al. (2002) | Europe | T | SR | 340 | 345 | Oral | 48 | 0.4 mg once-daily/320 mg daily | IPSS ≥ 10, Qmax of 5–15 ml/s with a PVR ≤ 150 ml, PV ≥ 25 cc, and PSA ≤ 4 ng/ml |
| Hızlı (2007) | Turkey | T | SR | 20 | 20 | Oral | 24 | 0.4 mg once-daily/320 mg daily | IPSS ≥ 10, Qmax of 5–15 ml/s with a PVR ≤ 150 ml, PV ≥ 25 cc, and PSA ≤ 4 ng/ml |
| Morgia (2014) | Italy | T | SR+Ly+Se | 79 | 71 | Oral | 24 | 0.4 mg once-daily/320 mg daily | Age between 55 and 80 years old, PSA ≤ 4 ng/ml, IPSS ≥ 12, PV ≤ 60 cc, Qmax ≤ 15 ml/s, PVR urine < 150 ml |
Note. T = tamsulosin; SR = Serenoa repens; Ly = lycopene; Se = selenium; PV = prostate volume; IPSS = International Prostate Symptom Score, PSA = prostate-specific antigen; QoLs = Quality of Life score; Qmax = maximal urinary flow rate; PVR = postvoid residual volume.
Quality of the Individual Studies
All four studies were RCTs and double-blind. At the same time, their randomization process has been elaborated in all the papers. All of the included studies calculated the efficiency and determined the best sample size, and all included studies conducted power calculation to determine the best sample size (Table 2). Table 2 presents the quality of each study included. The funnel plot shows the results of the qualitative estimation of publication bias in various studies, showing no bias evidence (Figure 2).
Table 2.
Quality Assessment of Individual Study.
| Study | Allocation sequence generation | Allocation concealment | Blinding | Lost to follow-up | Calculation of sample size | Statistical analysis | ITT analysis | Level of quality |
|---|---|---|---|---|---|---|---|---|
| Argirovic and Argirovic (2013) | A | A | A | 3 | YES | ANCOVA | NO | A |
| Debruyne et al. (2002) | A | A | A | 0 | YES | ANCOVA | NO | A |
| Hızlı (2007) | A | A | A | 7 | YES | ANCOVA | NO | A |
| Morgia (2014) | A | A | A | 0 | YES | ANCOVA | NO | B |
Note. A = all quality criteria met (adequate): low risk of bias; B = one or more of the quality criteria only partly met (unclear): moderate risk of bias; C = one or more criteria not met (inadequate or not used): high risk of bias; ITT = intention-to-treat analysis; ANCOVA = analysis of covariance.
Figure 2.

Funnel plot of the studies included in our meta-analysis. MD = mean difference; SE = standard error.
Efficacy
International Prostate Symptom Score
Four studies involving 1,080 patients (543 in the Serenoa repens group and 537 in the tamsulosin group) contained meaningful data. A random-effects model was used to evaluate changes between the two groups, which showed an MD of 0.63, 95% CI [−0.33, 1.59], p = 0.20. The result demonstrated that patients who received treatment of Serenoa repens had the same effect in IPSS compared with the tamsulosin group (Figure 3).
Figure 3.
Forest plots showing changes between two groups in (a) International Prostate Symptom Score (IPSS), (b) quality of life (QoL), (c) maximum flow rate (Qmax), (d) postvoid residual volume (PVR), (e) prostate volume (PV), (f) prostate-specific antigen (PSA). SD = standard deviation; IV = inverse variance; CI = confidence interval; df = degrees of freedom.
Quality of Life
Three studies involving 395 patients (198 in the Serenoa repens group and 197 in the tamsulosin group) contained meaningful data. A random-effects model was used to evaluate changes between the two groups, which showed an MD of 1.15, 95% CI [−1.51, 4.52], p = 0.33. The result demonstrated that the Serenoa repens group had the same effect in QoL compared with the control group (Figure 3).
Maximal Urinary Flow Rate
Four studies involving 1,080 patients (543 in the Serenoa repens group and 537 in the tamsulosin group) were included. A fixed-effects model was used to evaluate changes between the two groups, which showed an MD of 0.27, 95% CI [−0.15, 0.68], p = 0.21. The result demonstrated that Serenoa repens had the same effect in Qmax compared with the tamsulosin group (Figure 3).
Postvoid Residual Volume
Three studies involving 395 patients (198 in the Serenoa repens group and 197 in the tamsulosin group) were included. A random-effects model was used to evaluate changes between the two groups, which showed an MD of −4.27, 95% CI [−22.97, 14.44], p = 0.65. The result reported that Serenoa repens had the same effect in PVR compared with the tamsulosin group (Figure 3).
Prostate Volume
Three studies including 930 patients (472 in the Serenoa repens group and 458 in the tamsulosin group) contained meaningful data. A random-effects model was used to evaluate changes between the two groups, which showed an MD of −0.29, 95% CI [−0.41, −0.17], p < 0.00001. The result reported that the tamsulosin group had a greater improvement in PV compared with the tamsulosin group (Figure 3).
Prostate-Specific Antigen
Four studies involving 1,080 patients (543 in the Serenoa repens group and 537 in the tamsulosin group) contained meaningful data. A random-effects model was used to evaluate changes between the two groups, which showed an MD of 0.46, 95% CI [−0.06, 0.97], p = 0.08. The result reported that patients who received treatment of Serenoa repens had the same effect in PSA compared with the tamsulosin group (Figure 3).
Safety
Side Effect
Three RCTs including 930 participants (472 in the Serenoa repens group and 458 in the tamsulosin group) were involved in the research for side effect (OR = 11.80; 95% CI [0.27, 515.58]; p = 0.20). These results indicated that there was no significant difference between the two groups in terms of side effect (Figure 5).
Figure 5.
Forest plots showing changes between two groups in (a) side effect, (b) rhinitis, (c) dizziness, (d) fatigue, (e) postural hypotension, (f) dry mouth, and (g) headache. CI = confidence interval; df = degrees of freedom; M-H = Mantel-Haenszel.
Ejaculation Disorders and Libido Decrease
Three RCTs including 930 participants (472 in the Serenoa repens group and 458 in the tamsulosin group) were involved in the research for ejaculation disorders (OR = 12.56; 95% CI [3.83, 41.18]; p < 0.0001). The data about libido decrease in three RCTs (OR = 5.40; 95% CI [1.17, 24.87]; p = 0.03). The result indicated that the tamsulosin group had a higher incidence than the Serenoa repens group with respect to ejaculation disorders and libido decrease (Figure 4).
Figure 4.
Forest plots showing changes between two groups in (a) ejaculation disorders and (b) libido decrease. CI = confidence interval; df = degrees of freedom; M-H = Mantel-Haenszel.
Rhinitis, Dizziness, Fatigue, Postural Hypotension, Dry Mouth, and Headache
Three RCTs including 930 participants (472 in the Serenoa repens group and 458 in the tamsulosin group) were involved in the research for rhinitis (OR = 1.62; 95% CI [1.00, 2.61]; p = 0.05), dizziness (OR = 1.32; 95% CI [0.49, 3.57]; p = 0.59), fatigue (OR = 0.93; 95% CI [0.39, 2.20]; p = 0.86), postural hypotension (OR = 1.65; 95% CI [0.54, 5.08]; p = 0.38), and dry mouth (OR = 2.35; 95% CI [0.72, 7.62]; p = 0.16). Two RCTs including 890 participants (438 in the tamsulosin group and 452 in the Serenoa repens group) were involved in the research for headache (OR = 1.03, 95% CI [0.44, 2.44]; p = 0.94). These results indicated that there was no significant difference between the two groups in terms of rhinitis, dizziness, fatigue, postural hypotension, dry mouth, and headache (Figure 5).
Discussion
BPH is a noncancerous hyperplasia of the prostate. The occurrence of this disease is related to androgen and there is no obvious symptom in the early stage (Neal, 1997). It is currently recognized that advanced age and functional tests are two important factors in the pathogenesis of BPH, both of which are indispensable (Oelke et al., 2013; Welch et al., 2002). The use of Serenoa repens to treat BPH has become more and more popular, especially in some developed countries. In vitro studies have shown that Serenoa repens is a non-competitive inhibitor of type I 5-alpha-reductase and non-competitively inhibits the type II isozyme. (Boyle et al., 2015; Iehlé et al., 1995; Lowe, 2001; Willetts et al., 2015).
This systematic review and quantitative meta-analysis summarized the evidence from RCTs regarding the efficacy and safety of Serenoa repens comparing with tamsulosin for BPH treatment. In this meta-analysis, the inclusion criteria were men aged between 55 and 80 years old, PSA ≤ 4 ng/ml, IPSS ≥ 7, PV = 25–60 cc, Qmax = 5–15 ml/s, and PVR < 150 ml. There was no difference in baseline characteristics between the Serenoa repens group and tamsulosin group. Based on the result, compared with tamsulosin, Serenoa repens had a same effect in treating BPH in terms of IPSS (p = 0.20), QoL (p = 0.33), Qmax (p = 0.21), PVR (p = 0.65), and PSA (p = 0.08) with the exception of PV (p < 0.00001). This analysis found that phytotherapy with Serenoa repens was an effective pharmacotherapy in management of men with LUTS/BPH. However, Novara et al. (2016) and Vela-Navarrete et al. (2018) reported that Serenoa repens had an efficacy for relieving LUTS similar to that of tamsulosin. What is certain is that urologist should be aware and informed about phytotherapy as it inevitably becomes part of the standard medical therapy for men with LUTS/BPH.
Tamsulosin is a selective a1-adrenalin receptor blocker, it can selectively block the a1-adrenalin receptor in the prostate gland, relax the prostate smooth muscle, thereby improving the symptoms of dysuria caused by BPH. Serenoa repens would act by inhibiting the 5α-reductase and the binding between the dihydrotestosterone and the androgen receptor, antagonizing the a1-adrenergic receptor, and inhibiting cell proliferation and the production of COX-2 and 5-leukotrienes (Minutoli et al., 2013). Meanwhile, Serenoa repens can improve patients’ LUTS by changing the PV (p < 0.00001), in which Serenoa repens is different from tamsulosin. In addition, Morgia et al. (2015) reported that Serenoa repens combined with other compounds (such as Selenium [Se] and the carotenoid lycopene [Ly]) would act through some selenoproteins promoting an optimal balance between oxidants/antioxidants, with significant beneficial effects on BPH. Some studies have also reported that taking Serenoa repens for 3 months can improve patients’ LUTS (Morgia et al., 2015).
The incidence of adverse reactions was similar between Serenoa repens and tamsulosin, such as rhinitis, fatigue, dizziness, postural hypotension, dry mouth, and headache. These results demonstrate the safety of Serenoa repens in treating BPH. Additionally, Serenoa repens has triple mechanisms, namely antiandrogenic, antiproliferative, and anti-inflammatory, are enhanced with the severity of LUTS, which allows the drug to reduce both obstructive and irritative symptoms (Gerber, 2000; Kaplan, 2002). Besides, in terms of ejaculation disorders (p < 0.0001) and decreased libido (p = 0.03), Serenoa repens has less impact on patients’ erectile ability compared with tamsulosin, which is conducive to the promotion of this drug (Gacci et al., 2011; Lowe, 2015; Rosen et al., 2003). Moreover, Novara et al. (2016) reported that Serenoa repens had a favorable safety profile with a very limited impact on sexual function, which is significantly affected by all other available drugs for LUTS/BPH.
Serenoa repens is recognized by more and more people in the following aspects: (a) people are dissatisfied with traditional treatment methods (Cherkin, 1998; Furnham & Forey, 1994; Vincent & Furnham, 2011). (b) The study found that the treatment of Serenoa repens had no negative effects on male sexual function, especially in ejaculation. At the same time, the drug is extracted from berries without any obvious toxicity. (c) In many countries, people can use without a prescription, which greatly increases the awareness rate of this drug. It has become the first-line therapy in many countries (Barry et al., 2011; Mcvary, 2006).
All in all, this meta-analysis included four RCTs and had advantages compared with previous studies. First, the result of the meta-analysis was derived from randomized, double-blind, controlled trials. According to the quality assessment scale, quality of each study in the meta-analysis was met. Second, the data came from the latest data, with accuracy. Therefore, the results of this analysis are of great value both from a scientific perspective and from a daily clinical perspective. However, there are certain limitations to the research. Serenoa repens is the most commonly found natural compound whose quality may vary depending on the growing environment of the plant or the technique of extraction, which may affect the test result. At the same time, different ethnic groups have different tolerance to drugs, which will affect the test results to some extent. Finally, more appropriate high-quality randomized trial is needed to improve the accuracy of results.
Conclusions
This study indicated that Serenoa repens had the same effect in treating BPH compared with tamsulosin in terms of IPSS, QoL, and PVR after at least 6-month treatment cycle, however, the latter had a greater improvement in PV compared with the former. And Serenoa repens did not increase the risk of adverse events especially with respect to ejaculation disorders and libido decrease.
Supplemental Material
Supplemental material, authorship_change_request for Comparison of Serenoa repens With Tamsulosin in the Treatment of Benign Prostatic Hyperplasia: A Systematic Review and Meta-Analysis by Tong Cai, Yuanshan Cui, Shaoxia Yu, Qian Li, Zhongbao Zhou and Zhenli Gao in American Journal of Men's Health
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Nature Science Foundation of China (81801429).
ORCID iDs: Tong Cai  https://orcid.org/0000-0001-9416-5508
https://orcid.org/0000-0001-9416-5508
Yuanshan Cui  https://orcid.org/0000-0002-9810-8145
https://orcid.org/0000-0002-9810-8145
References
- Argirovic A., Argirovic D. (2013). Does the addition of Serenoa repens to tamsulosin improve its therapeutical efficacy in benign prostatic hyperplasia? Vojnosanitetski Pregled, 70(12), 1091–1096. [DOI] [PubMed] [Google Scholar]
- Barry M. J., Meleth S., Lee J. Y., Kreder K. J., Avins A. L., Nickel J. C., Roehrborn C. G., Crawford E. D., Foster H. E., Jr, Kaplan S. A., McCullough A., Andriole G. L., Naslund M. J., Williams O. D., Kusek J. W., Meyers C. M., Betz J. M., Cantor A., McVary K. T., & Complementary and Alternative Medicine for Urological Symptoms (CAMUS) Study Group. (2011). Effect of increasing doses of saw palmetto extract on lower urinary tract symptoms: A randomized trial. JAMA, 306(12), 1344–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle P., Gould A. L., Roehrborn C. G. (1996). Prostate volume predicts outcome of treatment of benign prostatic hyperplasia with finasteride: Meta-analysis of randomized clinical trials. Urology, 48(3), 398–405. [DOI] [PubMed] [Google Scholar]
- Boyle P., Robertson C. F., Roehrborn C. (2015). Updated meta-analysis of clinical trials of Serenoa repens extract in the treatment of symptomatic benign prostatic hyperplasia. BJU International, 93(6), 751–756. [DOI] [PubMed] [Google Scholar]
- Buck A. C. (2015). Phytotherapy for the prostate. British Journal of Urology, 78(3), 325–336. [DOI] [PubMed] [Google Scholar]
- Cherkin D. (1998). Why patients use alternative medicine. Journal of the American Medical Association, 279(19), 1548–1553.9605899 [Google Scholar]
- Clifford G. M., Farmer R. D. (2000). Medical therapy for benign prostatic hyperplasia: A review of the literature. European Urology, 38(1), 2–19. [DOI] [PubMed] [Google Scholar]
- Debruyne F., Koch G., Boyle P., Da Silva F. C., Gillenwater J. G., Hamdy F.C., Perrin P., Teillac P., Vela-Navarrete R., Raynaud J.-P. (2002). Comparison of a phytotherapeutic agent (Permixon) with an α-blocker (Tamsulosin) in the treatment of benign prostatic hyperplasia: A 1-year randomized international study; European Urology, 1(1), 108. [PubMed] [Google Scholar]
- Di Salle E., Briatico G., Giudici D., Ornati G., Zaccheo T., Buzzetti F., Nesi M., Panzeri A. (1994). Novel aromatase and 5 alpha-reductase inhibitors. The Journal of Steroid Biochemistry and Molecular Biology, 49(4–6), 289–294. [DOI] [PubMed] [Google Scholar]
- Ficarra V., Rossanese M., Zazzara M., Giannarini G., Abbinante M., Bartoletti R., Mirone V., Scaglione F. (2014). The role of inflammation in lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia (BPH) and its potential impact on medical therapy. Current Urology Reports, 15(12), 463. [DOI] [PubMed] [Google Scholar]
- Furnham A., Forey J. (1994). The attitudes, behaviors and beliefs of patients of conventional vs. complementary (alternative) medicine. Journal of Clinical Psychology, 50(3), 458–469. [DOI] [PubMed] [Google Scholar]
- Gacci M., Eardley I., Giuliano F., Hatzichristou D., Kaplan S. A., Maggi M., McVary K. T., Mirone V., Porst H., Roehrborn C. G. (2011). Critical analysis of the relationship between sexual dysfunctions and lower urinary tract symptoms due to benign prostatic hyperplasia. European Urology, 60(4), 809–825. [DOI] [PubMed] [Google Scholar]
- Gerber G. S. (2000). Saw palmetto for the treatment of men with lower urinary tract symptoms. Journal of Urology, 163(5), 1408–1412. [PubMed] [Google Scholar]
- Gerber G. S., Kuznetsov D., Johnson B. C., Burstein J. D. (2001). Randomized, double-blind, placebo-controlled trial of saw palmetto in men with lower urinary tract symptoms. Urology, 58(6), 960–963. [DOI] [PubMed] [Google Scholar]
- Gray M., Allensworth D. (1990). Medical management of benign prostatic hyperplasia. Urology, 36(1), 5–12. [DOI] [PubMed] [Google Scholar]
- Habib F. K. (2009). Serenoa repens: The scientific basis for the treatment of benign prostatic hyperplasia. European Urology Supplements, 8(13), 887–893. [Google Scholar]
- Higgins J. P., Green S. (2008). Cochrane handbook for systematic reviews of interventions. Cochrane Book Series. [Google Scholar]
- Holtgrewe H. L. (1998). Current trends in management of men with lower urinary tract symptoms and benign prostatic hyperplasia. Urology, 51(4A Suppl), 1–7. [DOI] [PubMed] [Google Scholar]
- Hızlı F., Uygur M. C. (2007). A prospective study of the efficacy of serenoa repens, tamsulosin, and serenoa repens plus tamsulosin treatment for patients with benign prostate hyperplasia. International Urology & Nephrology, 39(3), 879–886. [DOI] [PubMed] [Google Scholar]
- Iehlé C., Délos S., Guirou O., Tate R., Raynaud J. P., Martin P. M. (1995). Human prostatic steroid 5α-reductase isoforms—a comparative study of selective inhibitors. The Journal of Steroid Biochemistry and Molecular Biology, 54(5–6), 273–279. [DOI] [PubMed] [Google Scholar]
- Jadad A. R., Rennie D. (1998). The randomized controlled trial gets a middle-aged checkup. Journal of the American Medical Association, 279(4), 319–320. [DOI] [PubMed] [Google Scholar]
- Kaplan S. A. (2002). Saw palmetto alters nuclear measurements reflecting DNA content in men with symptomatic BPH: Evidence for a possible molecular mechanism. Urology, 60(4), 617–622. [DOI] [PubMed] [Google Scholar]
- Kaplan S. A. (2016). Re: Comparison of tamsulosin plus Serenoa repens with tamsulosin in the treatment of benign prostatic hyperplasia in Korean men: 1-year randomized open label study. Journal of Urology, 196(2), 503–503. [DOI] [PubMed] [Google Scholar]
- Lowe F. C. (2001). Phytotherapy in the management of benign prostatic hyperplasia. Urology, 58(6), 71–76. [DOI] [PubMed] [Google Scholar]
- Lowe F. C. (2015). Treatment of lower urinary tract symptoms suggestive of benign prostatic hyperplasia: Sexual function. BJU International, 95(4 Suppl), 12–18. [DOI] [PubMed] [Google Scholar]
- Lowe F. C., Fagelman E. (1999). Phytotherapy in the treatment of benign prostatic hyperplasia. Urology, 53(4), 671–678. [DOI] [PubMed] [Google Scholar]
- Mcvary K. T. (2006). Saw palmetto for benign prostatic hyperplasia. Focus on Alternative & Complementary Therapies, 11(3), 1950–1951. [Google Scholar]
- Minutoli L., Bitto A., Squadrito F., Marini H., Irrera N., Morgia G., Passantino A., Altavilla D. (2013). Serenoa repens, lycopene and selenium: A triple therapeutic approach to manage benign prostatic hyperplasia. Current Medicinal Chemistry, 20(10), 1306–1312. [DOI] [PubMed] [Google Scholar]
- Morgia G., Russo G. I., Voce S., Palmieri F., Gentile M., Giannantoni A., Blefari F., Carini M., Minervini A., Ginepri A., Salvia G., Vespasiani G., Santelli G., Cimino S., Allegro R., Collura Z., Fragalà E., Arnone S., Pareo R. M. (2015). Serenoa repens, lycopene and selenium versus tamsulosin for the treatment of LUTS/BPH. An Italian multicenter double-blinded randomized study between single or combination therapy (PROCOMB trial). Prostate, 74(15), 1471–1480. [DOI] [PubMed] [Google Scholar]
- Neal D. E. (1997). Watchful waiting or drug therapy for benign prostatic hyperplasia? Lancet, 350(9074), 305–306. [DOI] [PubMed] [Google Scholar]
- Novara G., Giannarini G., Alcaraz A., Cózar-Olmo J.-M., Descazeaud A., Montorsi F., Vincenzo F. (2016). Efficacy and safety of hexanic lipidosterolic extract of Serenoa repens (Permixon) in the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia: Systematic review and meta-analysis of randomized controlled trials; European Urology Focus, 2(5), 553–561. [DOI] [PubMed] [Google Scholar]
- Oelke M., Bachmann A., Descazeaud A., Emberton M., Gravas S., Michel M. C., N'dow J., Nordling J., de la Rosette J. J. (2013). EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. European Urology, 64(1), 118–140. [DOI] [PubMed] [Google Scholar]
- Oesterling J. E. (1995). Benign prostatic hyperplasia. Medical and minimally invasive treatment options. New England Journal of Medicine, 332(2), 99–109. [DOI] [PubMed] [Google Scholar]
- Pytel Y. A., Vinarov A., Lopatkin N., Sivkov A., Gorilovsky L., Raynaud J. P. (2002). Long-term clinical and biologic effects of the lipidosterolic extract of Serenoa repens in patients with symptomatic benign prostatic hyperplasia. Advances in Therapy, 19(6), 297–306. [DOI] [PubMed] [Google Scholar]
- Rosen R., Altwein J., Boyle P., Kirby R. S., Lukacs B., Meuleman E., O’Leary M. P., Puppo P., Robertson C., Giuliano F. (2003). Lower urinary tract symptoms and male sexual dysfunction: The multinational survey of the aging male (MSAM-7). European Urology, 44(6), 637–649. [DOI] [PubMed] [Google Scholar]
- Salle E. D., Briatico G., Giudici D., Ornati G., Panzeri A. (1994). Endocrine properties of the testosterone 5α-reductase inhibitor turosteride (FCE 26073). Journal of Steroid Biochemistry & Molecular Biology, 48(2–3), 241–248. [DOI] [PubMed] [Google Scholar]
- Sinescu I., Geavlete P., Multescu R., Gangu C., Miclea F., Coman I., Ioiart I., Ambert V., Constantin T., Petrut B., Feciche B. (2011). Long-term efficacy of Serenoa repens treatment in patients with mild and moderate symptomatic benign prostatic hyperplasia. Urologia Internationalis, 86(3), 284–289. [DOI] [PubMed] [Google Scholar]
- Thompson S. G., Thompson S. G. (2005). Meta-analysis of clinical trials. Biostatistis Decoded. [Google Scholar]
- Vela-Navarrete R., Alcaraz A., Rodríguez-Antolín A., López B. M., Fernández-Gómez J. M., Angulo J. C., Díaz D. C., Romero-Otero J., Brenes F. J., Carballido J., Molero García J. M., Fernández-Pro Ledesma A., Olmos J. M. C., Dalmau J, M., Cachinero I. S., Herdman M., Ficarra V. (2018). Efficacy and safety of a hexanic extract of Serenoa repens (Permixon®) for the treatment of lower urinary tract symptoms associated with benign prostatic hyperplasia (LUTS/BPH): Systematic review and meta-analysis of randomised controlled trials and observational studies. BJU International, 122(6), 1049–1065. [DOI] [PubMed] [Google Scholar]
- Vincent C., Furnham A. (2011). Why do patients turn to complementary medicine? An empirical study. British Journal of Clinical Psychology, 35(1), 37–48. [DOI] [PubMed] [Google Scholar]
- Welch G., Weinger K., Barry M. J. (2002). Quality-of-life impact of lower urinary tract symptom severity: Results from the health professionals follow-up study. Urology, 59(2), 245–250. [DOI] [PubMed] [Google Scholar]
- Willetts K. E., Clements M. S., Ehsman S., Eden J. A. (2015). Serenoa repens extract for benign prostate hyperplasia: A randomized controlled trial; British Journal of Urology International, 92(3), 267–270. [DOI] [PubMed] [Google Scholar]
- Wilt T. J., Ishani A., Stark G., Macdonald R., Lau J., Mulrow C. (1998). Saw palmetto extracts for treatment of benign prostatic hyperplasia: A systematic review. Complementary Therapies in Medicine, 7(18), 1604–1609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, authorship_change_request for Comparison of Serenoa repens With Tamsulosin in the Treatment of Benign Prostatic Hyperplasia: A Systematic Review and Meta-Analysis by Tong Cai, Yuanshan Cui, Shaoxia Yu, Qian Li, Zhongbao Zhou and Zhenli Gao in American Journal of Men's Health