Short abstract
This case report describes a novel procedure for opening the lumen of a completely obstructed anastomosis when open surgery is not an option. Two patients underwent ileocecal or colorectal resection and one-stage anastomosis reconstruction without diverging ileostomy. The patients developed post-surgical abdominal distension and nausea. Emergency imaging indicated complete anastomotic obstruction and distal intestinal anastomosis emptiness. Colonoscopy revealed an anastomosis that was completely discontinued by a membranous structure. Considering that open surgery was not a viable treatment option, a minimally invasive endoscopic approach was adopted to repair the obstruction. A needle knife was used to puncture the linear white scar and contrast agent was injected under endoscopy and fluoroscopic guidance. Fluoroscopically, the proximal bowel was identified and a dual knife-mediated membrane puncture was performed. A guidewire was then passed through the incision into the proximal bowel and progressive pneumatic dilatation was performed successively with a controlled radial expansion balloon dilator until a 1.8 cm diameter dilation was achieved. After conventional balloon dilatation, the endoscope easily passed through the anastomosis without any patient discomfort. There were no postoperative signs of immediate or delayed complications. Overall, endoscopic incision and dilatation was a safe and effective treatment for acute anastomotic obstruction after colorectal surgery.
Keywords: Anastomosis stenosis, endoscopic, colorectal resection, anastomotic obstruction, anastomosis complication, ileostomy
Introduction
Anastomotic stricture is a common complication after colorectal surgery, with an incidence ranging from 3–30% after colorectal cancer resection.1,2 Risk factors for anastomotic strictures are still poorly understood, but the aetiology of anastomotic stricture might correlate with factors including general nutritive status, anastomotic technique, anastomotic tension, anastomotic manner, bilateral blood circulation, digestive tract obstruction and infection.3 Strictures occur more frequently with stapled anastomoses than with handsewn anastomoses, possibly because stapler use results in an overactive inflammatory response and healing by secondary intention.4–6 Severe anastomotic stricture requires invasive interventions and treatment options may include endoscopic dilation or surgical revision of the anastomosis.7 Endoscopic intervention, including endoscopic electrocision, balloon dilatation and self-expanding metal stents, is the first treatment option.8 According to previous research, endoscopic balloon dilatation is the best approach for patients with anastomotic stenosis after colorectal surgery.9 Indeed, short-segment strictures presenting as stenosis around the anastomosis usually respond well to endoscopic dilation.9
This current report describes two cases of anastomosis stricture that are unique because of their early onset and the completeness of the stenosis. Both were successfully treated by endoscopic puncture and dilation.
Case reports
Case 1
A 23-year-old male patient underwent a colostomy of the sigmoid colon due a volvulus 6 months prior to presentation to the Department of Gastrointestinal Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei Province, China in April 2014. The reversion of the colostomy was accomplished by an end-to-side anastomosis from the proximal to the distal end using a circular stapler (Ethicon Circular Stapler CDH29A; Ethicon, Somerville, NJ, USA). One week after the reversion, the patient could consume a soft diet and had a bowel movement. He was discharged on postoperative day (POD) 7. On POD 9, he had no additional bowel movements or flatus and was readmitted to the Department of Gastrointestinal Surgery on POD 11. Emergency computed tomography (CT) indicated anastomotic obstruction. Emergent colonoscopy was performed on POD 19, showing a blind end and a white linear scar around the supposed site of anastomosis. Liquid dung leaking through the scar was not observed. On POD 20, another colonoscopy (Video Colonoscope CF-H260AI; Olympus Optical, Tokyo, Japan) was attempted and open surgery was selected as a backup procedure. Upon fluorography, no air travelled upward through the closed anastomosis. A needle knife (KD-10Q-1; Olympus Optical) was used to puncture the anastomosis scar and liquid dung leaked out (Figure 1). A guidewire (Dreamwire ST Guidewire M00556141; Boston Scientific, Marlborough, MA, USA) was then successfully inserted through the small channel established by the needle knife. The colon was visualized with contrast to verify the guidewire position and the stenosis was dilated to 10 mm in diameter with a balloon (M00558430; Boston Scientific). A fully covered metal stent (Cook Medical, Bloomington, IN, USA) with a fully expanded diameter of 22 mm was placed within the anastomosis. The patient recovered quickly and the stent was removed 4 days later. The patient remained symptomless and barium enema did not reveal the presence of further stricture. Colonoscopies were performed 1 and 5 years postoperatively and showed anastomosis patency.
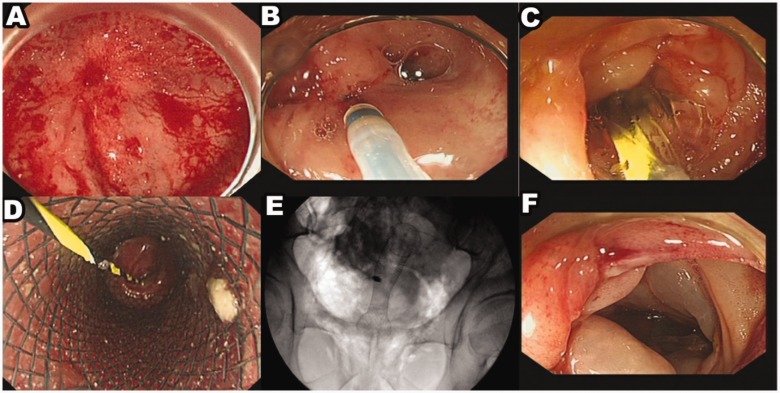
Figure 1.
Representative colonoscopy and fluoroscopy images of a 23-year-old male patient that underwent a colostomy of the sigmoid colon due a volvulus 6 months prior to presentation. (a) The anastomosis was completely discontinued by a membranous structure. (b) A needle knife was used to puncture the anastomosis scar. (c) The stenosis was dilated with a balloon. (d) A fully covered metal stent was placed within the anastomosis. (e) The stent position under fluoroscopy was well placed. (f) After 1 year of follow-up, there was no anastomotic stenosis. The colour version of this figure is available at: http://imr.sagepub.com.
Case 2
A 67-year-old female patient presented to at a local hospital in September 2016 with an obstruction of the ascending colon due to primary gastrointestinal lymphoma (diffused B cell type non-Hodgkin’s lymphoma). She underwent right side semi-colectomy and ileum end to transverse colon side anastomosis via a circular stapler (Ethicon Circular Stapler CDH29A; Ethicon). She had flatus in the first week, which then stopped, and had no bowel movement after the operation. The patient was treated with gastric tube decompression, somatostatin and conservative care. On POD 38, she was referred to the Department of Gastrointestinal Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei Province, China in October 2016. CT scan showed an obstruction at the anastomosis site as indicated by a ring of stapler nails. Emergent colonoscopy (Video Colonoscope CF-H260AI; Olympus Optical) was performed with emergency open surgery determined as a backup option (Figure 2). A tapered blind end was encountered 50 cm superior to the anus. A 1 cm long linear white scar was found near the end. An attempt was made to insert a guidewire (Dreamwire ST Guidewire M00556141; Boston Scientific) with the assistance of a sphincterotome (KD-28Q-1; Olympus Optical) but this failed. A needle knife (KD-10Q-1; Olympus Optical) was advanced to puncture the scar and contrast fluorography showed the colon lumen. A guidewire followed by a dilation balloon (M00558430; Boston Scientific) and diameter of 15–18 mm progressive dilating balloons were inserted until a 20 mm diameter dilation was achieved. Thereafter, the endoscope was easily inserted through the anastomosis. Immediately following the procedure, the patient had bowel movements and the abdominal distension was alleviated. She began a liquid diet 2 days later and ascites was observed on CT. Percutaneous drainage was performed and the ascites was verified to be chylous leakage due to the lymphoma. An iodine contrast enema showed anastomosis patency 2 weeks after the endoscopic procedure and chemotherapy was initiated. Colonoscopy was performed 1 year after surgery and showed anastomosis patency.
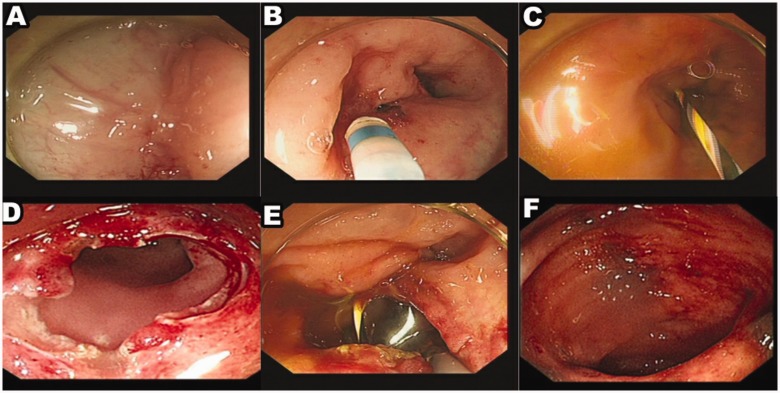
Figure 2.
Representative colonoscopy images of a 67-year-old female patient that presented with an obstruction of the ascending colon due to primary gastrointestinal lymphoma (diffused B cell type non-Hodgkin’s lymphoma). (a) The anastomosis was completely discontinued by a membranous structure. (b) A needle knife was used to puncture the anastomosis scar. (c) The guide wire was used to pass through the stenosis and was located in the upper lumen. (d) Complete incision of the stenosis; (e) The stenosis was dilated with a balloon. (f) After 1 year of follow-up, there was no anastomotic stenosis. The colour version of this figure is available at: http://imr.sagepub.com.
Informed consent was obtained from both patients prior to participation in the study. The study was approved by the Ethics Committee Examination and Approval Committee of Medical Ethics of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei Province, China, (no. 07/11/2018; IORG no: IORG0003571). The medical interventions administered to the human participants complied with the principles set out in the Declaration of Helsinki.
Discussion
An anastomotic stricture does not always involve a long segment around the anastomosis. In this type of stricture, there is no narrowing of the lumen away from the anastomosis, but annular stenosis or closure of the anastomosis ring is present. In this case, the intestinal wall near the anastomosis site remains soft and the mucosal layer also remains normal. The membrane-like stricture, easily observed via endoscopy, might make diagnosis during CT or barium enema challenging unless the stricture is severe enough to cause signs of obstruction. Thus, the incidence of stenotic anastomoses might be underestimated. The two cases of anastomosis stricture that are presented in this case report were verified to be of this type of anastomosis.
Risk factors related to anastomosis stricture may include tension between both sides of the anastomosis, ischaemia, dehiscence, radiation therapy and anastomosis method.3 As previously mentioned, stricture is more frequently reported following staple use than following hand sewing, possibly because staples generate an overactive inflammatory response during healing.4 The diverting stoma has also been associated with stricture formation because the absence of faeces may induce atrophy of the muscle cells and subsequent stricture.10 Table 1 summarizes all previous reports on complete closure of the anastomosis canal that have been cured by endoscopic approaches.11–20 In these studies, all patients underwent endoscopic approaches including balloon dilation and endoscopic cutting by electrocautery with a hook or papillotomy knife, and the strictures were diagnosed when the patients came back for stoma reversal.11–20 The two cases in this current report involved complete anastomosis closure in the colon, a region wherein anastomosis tension or ischaemia is seldomly encountered.
Table 1.
| Case | Reference | Year | Country | Sex | Age, years | Primary surgery | Level of anastomosis | Diagnosis | Treatment | Previous diverting stomas |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Yuan11 | 2019 | China | M | 67 | Low anterior resection and single barrel ileostomy | Anastomosis at 8 cm from the anal verge | COS | Incision was made by a needle knife and sequentially dilated by using a wire-guided balloon dilator. | Yes |
| 2 | Nunes12 | 2019 | Portugal | M | 57 | Colorectal anastomosis with protective ileostomy was performed | Low anastomosis | COS | Endoscopic ultrasound-guided recanalization of complete colorectal anastomotic stenosis and using a lumen-apposing metal stent | Yes |
| 3 | Bong13 | 2019 | South Korea | M | 49 | Low anterior resection | Low anastomosis | COS | Transanal minimally invasive surgery | Yes |
| 4 | Moyer14 | 2017 | USA | F | 30 | Partial transverse colectomy | Transverse colocecal anastomosis | COS | Using the combined antegrade-retrograde dilation procedure | Yes |
| 5 | Gandhi15 | 2015 | New Zealand | F | 38 | Anterior resection | Low anastomosis | COS | Retrograde endoscopic treatment of completely obstructed anastomotic | Yes |
| 6 | Gornals16 | 2015 | Spain | M | 66 | Low anterior resection | Low anastomosis | COS | Endoscopic ultrasound-guided and using a lumen-apposing metal stent | Yes |
| 7 | Yazawa17 | 2014 | Japan | M | 79 | Redo rectal resection | Low anastomosis | COS | Endoscopy: blunt penetration technique | Yes |
| 8 | Curcio18 | 2010 | Italy | M | 70 | Low anterior resection | Low anastomosis | COS | Non-electrosurgical endoscopic approach before balloon dilatation | Yes |
| 9 | De Lusong19 | 2008 | USA | F | 40 | Sigmoidectomy | Colorectal anastomosis | COS | Using a prototype forward-array echoendoscope and facilitated by SpyGlass | Yes |
| 10 | Chen20 | 2018 | China | M | 66 | End-to-end anastomosis and ileocecal stoma | Colorectal anastomosis | COS | Endoscopic incision | Yes |
Interestingly, the current cases involved a unique type of stenosis that developed without affecting the proximal side of the anastomosis. The complete closure caused intestinal obstruction and was therefore easy to detect. Although there are few reports on the timing of anastomosis stricture development, the symptoms reflecting complete obstruction within 10 days of the operation were surprising; a second unusual feature of the current cases.
In these current cases, the short-segment anastomosis stricture responded well to balloon dilation and intermediate cutting in the radial direction on the stenosis ring. The technique is usually successful provided there is a recognizable opening at the anastomosis stricture. The dilating balloon is easily inserted and advanced to a suitable site, and the cutting procedure is easily initiated on the anastomosis ring margin.
In cases of complete stenosis, the endoscopic procedure can be complicated if the stricture characteristics are not understood and a guidewire cannot be readily placed. The different anastomotic methods used have a considerable influence on the endoscopic operation. In all cases reported in the current literature, the anastomosis was made on the end-to-end.11–20 Conversely, in the two current cases, the anastomosis was made on the end-to-side. Therefore, there would be two liner scars inside the lumen of the intestine distal to the anastomosis, namely the anastomosis stenosis and the blind end. The appearance of these two scars might be similar, but the anastomosis was located on the side and not the end. Accurate determination of membrane structure or intestinal wall to avoid iatrogenic perforation is crucial. Based on our research, in our opinion this technique should be performed by experienced endoscopic surgeons. For the closure of the lumen caused by this membrane structure, the instrument should be operated under fluoroscopy to slowly penetrate the structure and a small amount of contrast agent should be injected. Under fluoroscopy, it should be observed whether the contrast agent is in the lumen or slightly inflated into the lumen to determine whether it is in the lumen. When identifying the non-intestinal wall of the membrane structure, slow incision or dilation was performed to observe whether the intestinal mucosa could be seen at the proximal end of the stenosis, so as to achieve surgical safety and avoid iatrogenic perforation. Based on intestinal anatomy, the upper lumen on the other side of the scar was more often in a right-angled direction. Caution should be maintained as the intestine proximal to the anastomosis might not run in a horizontal plane with the intestine distal to the anastomosis.
Computed tomography surveillance or endoscopic ultrasound-guided recanalization is preferred over surgery for a complete stricture. However, in this current report, neither was performed as emergency endoscopy showed stenosis and a short-segment stricture. Additionally, the injection needle had safely allowed visualization of the lumen on the upper side of the anastomosis, and even established a small opening on the scar in case 1, which healed 2 weeks after the complete obstruction. In case 2, a small opening was made by cautiously cutting with a dual knife in the area of the scar while monitoring by endoscopy and X-ray surveillance.
In conclusion, emergent endoscopy can provide important information about the stricture in an anastomotic obstruction after colorectal surgery. Moreover, for early-onset anastomosis stenosis where complete obstruction is possible, or an end-to-side reconstruction, endoscopic recanalization using X-ray surveillance electrocautery with a small anastomosis margin followed by dilation is feasible.
Acknowledgements
The authors sincerely thank the entire staff of our department for offering their assistance with the medical service and manuscript writing processes.
Author contributions
S.D., Y.C. and J.G. contributed equally to this work. S.D., J.L. and K.W. contributed to the study design and literature search. S.D., Y.C. and J.G. contributed to the literature search and the writing of the manuscript. J.W. and K.C. contributed to the review and revise of the manuscript.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Shenghe Deng https://orcid.org/0000-0001-6184-1389
References
- 1.Zuloaga J, Arias-Diaz J, Fernandez-Diez S, et al. Late ischemic stricture following anterior rectal resection. Rev Esp Enferm Dig 2008; 100: 49–52. [DOI] [PubMed] [Google Scholar]
- 2.Hiranyakas A, Da Silva G, Denoya P, et al. Colorectal anastomotic stricture: is it associated with inadequate colonic mobilization. Tech Coloproctol 2013; 17: 371–375. [DOI] [PubMed] [Google Scholar]
- 3.Rizk NP, Bach PB, Schrag D, et al. The impact of complications on outcomes after resection for esophageal and gastroesophageal junction carcinoma. J Am Coll Surg 2004; 198: 42–50. [DOI] [PubMed] [Google Scholar]
- 4.Bannura GC, Cumsille MA, Barrera AE, et al. Predictive factors of stenosis after stapled colorectal anastomosis: prospective analysis of 179 consecutive patients. World J Surg 2004; 28: 921–925. [DOI] [PubMed] [Google Scholar]
- 5.Neutzling CB, Lustosa SA, Proenca IM, et al. Stapled versus handsewn methods for colorectal anastomosis surgery. Cochrane Database Syst Rev 2012: CD003144. Doi: 10.1002/14651858.CD003144.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korontzi MI, Kontovounisios C, Armoutidis V, et al. Dehiscence, stenosis and local recurrence after anterior resection in relation to the level of rectal cancer. Hellenic J Surg 2011; 83: 274–283. [Google Scholar]
- 7.Davis B, Rivadeneira DE. Complications of colorectal anastomoses: leaks, strictures, and bleeding. Surg Clin North Am 2013; 93: 61–87. [DOI] [PubMed] [Google Scholar]
- 8.Biraima M, Adamina M, Jost R, et al. Long-term results of endoscopic balloon dilation for treatment of colorectal anastomotic stenosis. Surg Endosc 2016; 30: 4432–4437. [DOI] [PubMed] [Google Scholar]
- 9.Kim PH, Song HY, Park JH, et al. Safe and effective treatment of colorectal anastomotic stricture using a well-defined balloon dilation protocol. J Vasc Interv Radiol 2012; 23: 675–680. [DOI] [PubMed] [Google Scholar]
- 10.Forshaw MJ, Maphosa G, Sankararajah D, et al. Endoscopic alternatives in managing anastomotic strictures of the colon and rectum. Tech Coloproctol 2006; 10: 21–27. [DOI] [PubMed] [Google Scholar]
- 11.Yuan X, Liu W, Ye L, et al. Combination of endoscopic incision and balloon dilation for treatment of a completely obstructed anastomotic stenosis following colorectal resection: a case report. Medicine (Baltimore) 2019; 98: e16292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nunes G, Marques PP, Patita M, et al. EUS-guided recanalization of complete colorectal anastomotic stenosis using a lumen-apposing metal stent. Endosc Ultrasound 2019; 8: 211–212. Doi: 10.4103/eus.eus_62_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bong JW, Lim SB. Transanal minimally invasive surgery as a treatment option for a completely occluded anastomosis after low anterior resection: a new approach to severe anastomotic stenosis. Asian J Endosc Surg 2019; 12: 175–177. [DOI] [PubMed] [Google Scholar]
- 14.Moyer MT, Mathew A, Chintanaboina J, et al. Restoration of colonic patency of a completely obstructed Crohn’s stricture using the combined antegrade-retrograde dilation procedure. VideoGIE 2017; 2: 359–360. Doi: 10.1016/j.vgie.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gandhi J, Avery N, Keating JP. Retrograde endoscopic treatment of completely obstructed anastomotic stricture after anterior resection. Int Arch Med 2015; 8. Doi: 10.3823/1689. [Google Scholar]
- 16.Gornals JB, Albines G, Trenti L, et al. EUS-guided recanalization of a complete rectal anastomotic stenosis by use of a lumen-apposing metal stent. Gastrointest Endosc 2015; 82: 752. [DOI] [PubMed] [Google Scholar]
- 17.Yazawa K, Morioka D, Matsumoto C, et al. Blunt penetration technique for treatment of a completely obstructed anastomosis after rectal resection: a case report. J Med Case Rep 2014; 8: 236. Published 2014 Jun 27. Doi: 10.1186/1752-1947-8-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curcio G, Spada M, di Francesco F, et al. , Completely obstructed colorectal anastomosis: a new non-electrosurgical endoscopic approach before balloon dilatation. World J Gastroenterol 2010; 16: 4751–4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Lusong MA, Shah JN, Soetikno R, et al. Treatment of a completely obstructed colonic anastomotic stricture by using a prototype forward-array echoendoscope and facilitated by SpyGlass (with videos). Gastrointest Endosc 2008; 68: 988–992. [DOI] [PubMed] [Google Scholar]
- 20.Chen H, Liu W, Jiang S, et al . Completely occluded colorectal anastomotic stenosis treated using an endoscopic incision method. Am J Gastroenterol 2018; 113: 174. [DOI] [PubMed] [Google Scholar]