Abstract
Background
Reverse shoulder arthroplasty provides predictable pain relief and improvements in function, but concerns remain regarding complication rates and there is little long-term outcome data. The aim of this study was to review the clinical and radiographic outcomes of the Delta Xtend reverse shoulder arthroplasty at a minimum of five years.
Methods
Ninety-six Delta Xtend reverse shoulder arthroplasty procedures were performed in 93 patients. There were 41 males and 52 females with an average age of 74.9 years. All available patients returned for clinical and radiographic analysis, including completion of patient reported outcome measures.
Results
The complication rate was 9.4%. There were three revisions (3.1%) and two other reoperations (2.1%). Fifty-nine shoulders were available for review at an average of 81 months. Average forward flexion was 142°. Average American Shoulder and Elbow Assessment Score improved from 27.6 to 78.5 (p<0.001). Radiolucent lines and/or proximal bone resorption was seen in 35.4%. Scapula notching was observed in 69.1%, with Grade III or IV notching in 20%. These findings had no effect on patient reported outcome measures.
Discussion
This study confirms the clinical benefits of reverse shoulder arthroplasty, with improvements maintained out to 10 years. The high rate of scapula notching remains a concern. Further study is needed to fully understand the clinical significance of notching, as well as the potential benefits of newer implant designs.
Keywords: shoulder joint replacement, reverse shoulder arthroplasty, complication, scapula notching, patient reported outcome measures
Introduction
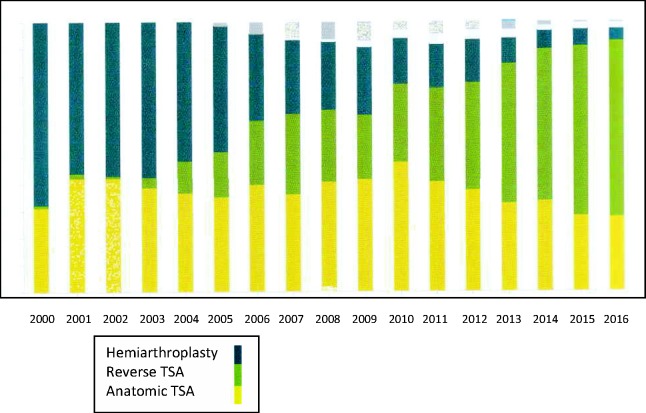
Reverse shoulder arthroplasty (RSA) is now a well-established treatment method to relieve pain and improve function for a variety of shoulder conditions that have in common loss or dysfunction of the rotator cuff (RC). Although initially developed for the treatment of RC tear arthropathy, as surgeons have gained more experience the indications for reverse TSA have expanded. They now include revision arthroplasty,1 inflammatory arthropathy,2,3 osteoarthritis,4,5 painful and irreparable RC tears without arthritis,6 acute proximal humeral fractures,7,8 fracture sequelae,9,10 and tumour.11 As a result, significantly more of these procedures are now being undertaken, including in younger more active patients, with National Joint Registries reporting that use of RSA now outnumbers anatomical joint replacement (Figure 1).
Figure 1.
Graphical representation of the increasing use of RSA in New Zealand (data obtained from The New Zealand Joint Registry 18-year report) www.nzoa.org.nz/nz-joint-registry. TSA: total shoulder arthroplasty.
Since 1985, there have been numerous modifications of the original Delta reverse shoulder prosthesis introduced by Grammont et al.12 and Grammont and Baulot13 and currently there are many different design options available on the market. Many of these have shown good to excellent clinical outcomes at short-term follow-up. However, many controversies and challenges in the use of RSA continue to be refined,14 and concerns remain regarding complication and failure rates and the incidence of scapula notching. In addition, long-term outcome data on the use of RSA for newer indications and in younger patients are still lacking.15,16
The DePuy Delta Xtend RSA System (DePuy Orthopaedics, Inc., 700 Orthopaedic Drive, Warsaw, IN 46581-0988, USA) is a prosthesis based on the original Grammont design and was introduced into New Zealand in June 2007. It offers a fluted modular humeral stem design intended for uncemented use, along with eccentric epiphysis options to better preserve bone in the proximal humerus whilst retaining the Grammont style 155° neck-shaft angle. The system also offers a curved back and smaller diameter metaglene design with locking screw options, in addition to a newer glenosphere design with eccentric options. The aim of this study was to review the clinical and radiographic outcomes of the Delta Xtend RSA performed by a single surgeon for all indications at a minimum follow-up of five years.
Materials and methods
Ninety-six consecutive shoulders in 93 patients underwent RSA utilising the Delta Xtend System between June 2007 and June 2012. All surgery was performed by the senior author (CMB) using a standardised surgical technique. In all but one patient the surgery was performed through a deltopectoral approach, with patients under general anaesthesia supplemented with an interscalene block.
Uncemented hydroxyapatite-coated modular stems were used in 72 patients and cemented monobloc stems in 24 patients. The indications for cementation of the stem included acute fracture, fracture sequelae, and revision surgery. All humeral stems were implanted with a retroversion between 10° and 20°. The hydroxyapatite-coated metaglene baseplate was implanted in a neutral position as inferiorly as possible, with the aim to achieve inferior glenosphere overhang as described by Nyffeler et al.17 The largest size glenosphere to best obtain soft tissue tension and stability was selected, which meant in most males a glenosphere size of 42 mm was used; in most females 38 mm. Only four eccentric glenospheres were used. In 52% of cases the humeral liner size was +6 mm; in 36% of cases the humeral liner size was +3 mm. In only three cases was a high mobility liner used.
There were 42 males and 54 female shoulders with an average age of 74.9 years at the time of surgery (range 51–89 years). There were 42 left shoulders and 54 right shoulders. The indications for surgery included RC tear arthropathy (Hamada IV, V) in 22 patients, massive irreparable RC tear with or without pseudoparalysis (Hamada I–III) in 38 patients,18 osteoarthritis with or without RC tear in 13 patients, acute proximal humerus fracture in 11 patients, post fracture sequelae in 6 patients, and revision to RSA in 6 patients (Table 1). In two cases, supplemental bone grafting of the glenoid was undertaken using autograft from the humeral head.19
Table 1.
Indications for performing reverse TSA.
| Indication for surgery | Number |
|---|---|
| RC tear arthropathy | 22 |
| Massive irreparable RC tear | 38 |
| Osteoarthritis | 13 |
| Acute proximal humerus fracture | 11 |
| Post-fracture sequelae | 6 |
| Revision to reverse TSA | 6 |
RC: rotator cuff; TSA: total shoulder arthroplasty.
All patients had undergone a standardised pre-operative clinical and radiologic assessment and had completed the American Shoulder and Elbow Assessment Score (ASES).20 All available patients were invited back for an independent post-operative assessment including clinical examination, standardised radiograph analysis, and completion of various patient reported outcome measures (PROMs), including the ASES, the Simple Shoulder Score (SST),21 the Oxford Score,22 and the Disabilities of the Shoulder and Hand Score (DASH).23 Written informed consent was obtained from all patients for their anonymised information to be published in this article.
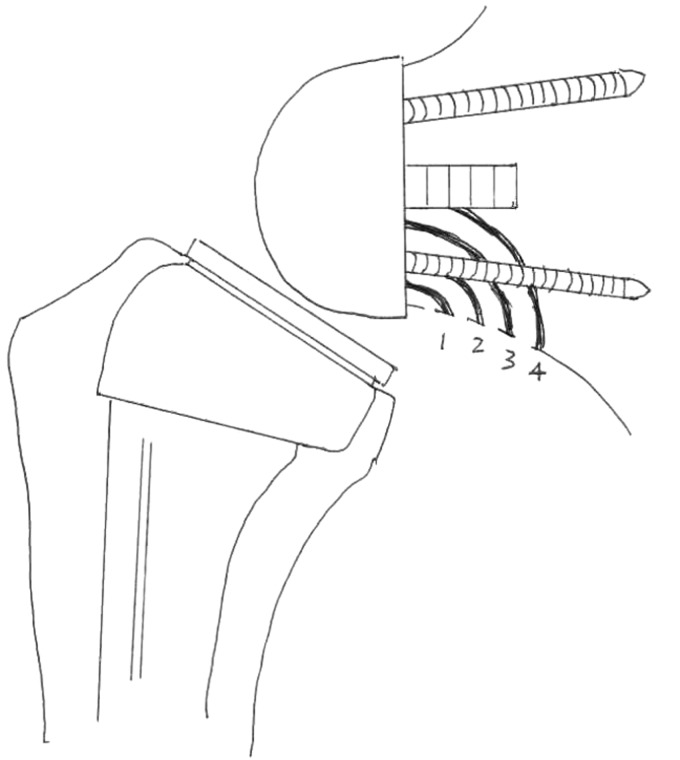
Anteroposterior, lateral, and axillary radiographs were evaluated for the presence of component malposition, radiolucent lines, loosening, osteolysis, scapula notching, heterotopic ossification, and evidence of wear or prosthesis failure. Radiolucent lines were assessed according to the criteria of Sperling et al.24 Stress shielding was defined as thinning of either the medial or lateral cortical bone with osteopenia as classified by Melis et al.25 Scapula notching was graded according to the classification of Sirveaux et al.26 (Figure 2).
Figure 2.
Radiographic grades of scapula notching.
The age and gender for those with specific indications for reverse TSA were compared using independent t-tests and chi-square or Fisher's exact tests. PROMs were compared between the indication groups using independent t-tests. The exploratory nature of these hypotheses with this observational cohort meant an unadjusted two-tailed p-value ≤ 0.05 was used to indicate statistical significance.
Results
Fifty-nine shoulders in 57 patients were available for review (61.5%) at an average follow-up of 81 months (range 60–115 months). There were 29 males and 30 female shoulders with an average age at the time of follow-up of 79.1 years. Thirty-seven shoulders in 37 patients were not available for review. Of these, 24 patients were deceased, 5 had severe dementia and were not able to participate, 7 were unable to be contacted or had moved overseas, and 1 declined to be involved. A review of the New Zealand Shoulder Arthroplasty Registry revealed that none of these 37 patients had undergone a reoperation or revision procedure of their prosthesis.
The overall complication rate was 9.4%. These complications included an intraoperative fracture of the calcar in one patient, an axillary nerve palsy in one patient, humeral stem loosening in one patient, a traumatic peri-prosthetic fracture of the metaglene in one patient, and acromial stress fractures in five patients (5.2%). There were no prosthetic dislocations and no cases of infection. A further 16.7% of patients experienced a minor transient neuropraxia of the thumb and index finger that resolved completely within 3–6 months and had no effect on clinical outcome.
There were three revisions in the 96 shoulders for an overall revision rate of 3.1%. There was one metaglene revision and liner exchange for a peri-prosthetic fracture around the metaglene that occurred following a fall four years after a successful primary procedure. This patient required allograft bone grafting to the glenoid at the time of the revision. There was one cemented stem revision for symptomatic stem loosening three years after the primary procedure for fracture sequelae. The index procedure required a long, cemented stem because of proximal humerus bone loss. There was one revision of the glenosphere with liner exchange for Grade IV notching in a patient with pain and mechanical symptoms. There were two other reoperations (overall reoperation rate 2.1%), both for traumatic acromial fractures, of which neither were successful in obtaining union.
In the 59 shoulders available for review, the average post-operative range of forward flexion was 142°; the average range of post-operative active external rotation with the elbow at the side was 19.8°. The average pre-operative ASES score was 27.6. This improved to an average post-operative ASES score of 78.5. This improvement was highly statistically significant (p<0.001). There was no difference between male and female patients (p = 0.85).
The average SST score at final review was 67.6 (0 = worst; 100 = best function). The average DASH score was 25.3 (0 = best; 100 = worst). The average Oxford Shoulder Score was 40.1 (0 = worst; 48 = best), of which there was no difference between male and female patients (p = 0.75). The average post-operative patient satisfaction was 8.5 out of 10.
There was a significant correlation between final range of forward flexion and better ASES and OSS scores (r = 0.62, p<0.001 and r = 0.60, p<0.001, respectively). There was no correlation between final range of motion or any of the PROMs and whether the patient had undergone prior RC surgery to the shoulder. The worst PROMs were seen in those patients whose indication for surgery was acute fracture, sequelae of fracture, and revision surgery. The average age at the time of surgery for the acute fracture group was 81.1 years (p = 0.004).
In terms of complications and their effect on outcome, all complications did result in worse PROMs and overall range of motion. In addition, all patients requiring a revision or reoperation had worse PROMs and overall range of motion. These findings were statistically significant (p<0.001).
There were 55 radiographs available for review. On the humeral side, this included 7 cemented stems and 48 uncemented stems. Of the seven cemented stems, one stem required revision for symptomatic loosening three years after the index procedure. This procedure had been undertaken for fracture sequelae with proximal humeral bone loss, which may have contributed to the stem loosening by not having sufficient proximal bone support for the implant27 (Figure 3).
Figure 3.
Long cemented stem implanted for fracture sequelae and proximal humeral bone loss.
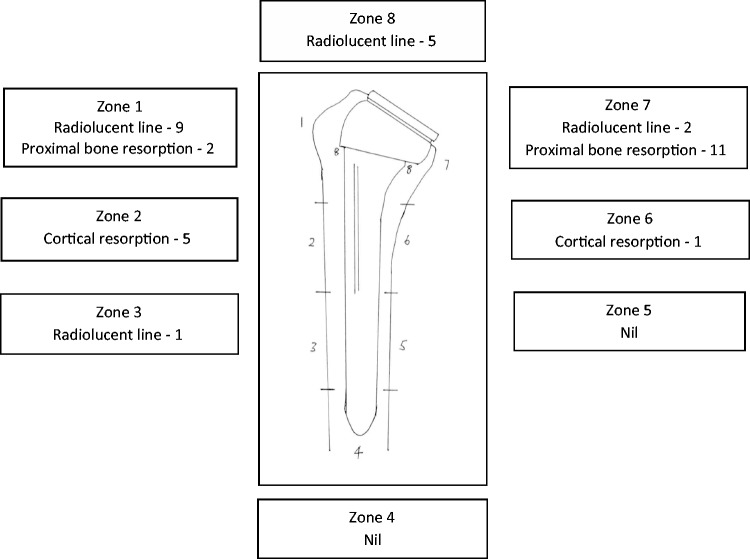
Of the 48 uncemented stems, 3 had evidence of slight subsidence (1 mm), which had no effect on any outcome measure. There were 17 humeral radiolucent lines (nine zone 1, five zone 8, two zone 7, one zone 3), but all were less than 1 mm. There were 6 radiographs with minor cortical resorption (five zone 2, one zone 6), and there were 14 radiographs with proximal bone resorption confined to the metaphysis (11 medial, 2 lateral, 1 both). The overall incidence of radiolucent lines was 35.4% (Figure 4). There were no cases of uncemented stem loosening or stem at risk. Proximal bone loss on the medial side of the humerus (22.9%) was seen more commonly in patients with higher grades of notching. None of the bony changes were observed to have any influence on overall range of motion or PROMs.
Figure 4.
Analysis of bony adaptions around the 48 uncemented humeral stems.
On the glenoid side 36 patients had a scapula neck angle <105° and 19 had a scapula neck angle >105°. The scapula neck angle has been used previously by authors to characterise glenoid morpholgy.28 In 41 patients, a satisfactory amount of glenosphere overhang was observed (>2 mm); in the remaining 14 patients there was minimal or no overhang. In four of the radiographs there was minimal lucency around the superior screw of uncertain significance. Otherwise none of the radiographs showed evidence of baseplate or screw loosening. Seventeen radiographs showed some triceps ossification (31%). This observation was more common in men but had no effect on PROMs or overall range of motion. There were no glenoid baseplate failures or glenosphere dissociations.
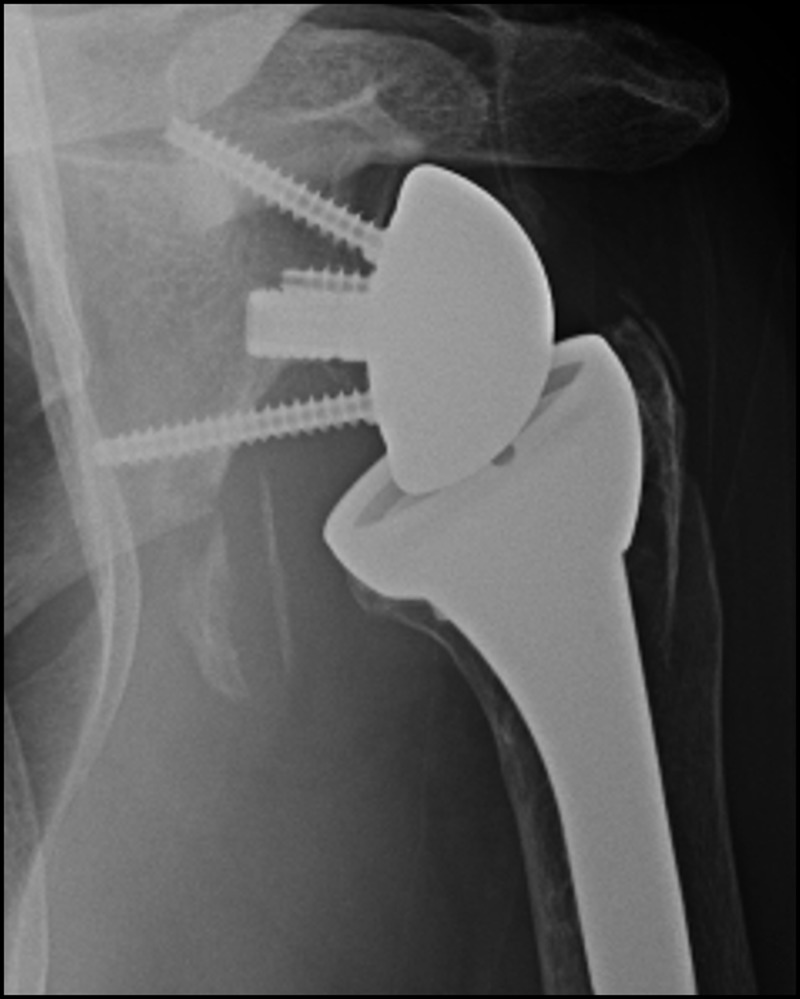
Scapula notching was observed in 38 of the 55 radiographs (69.1%), including 11 patients with either Grade III or IV notching (20%). The presence or grade of notching had no measurable effect on any PROM. With the numbers available, scapula neck angle, glenosphere size, patient gender, or the amount of glenosphere overhang did not appear to have any influence on notching grade. However, there was a correlation between higher grade of notching and longer follow-up. One patient with Grade IV notching underwent glenosphere and liner exchange because of pain and mechanical symptoms thought related to the degree of notching (Figure 5). At the time of revision significant medial wear of the polyethylene liner was noted, but the baseplate was not loose, and his symptoms improved after liner exchange and implantation of a larger glenosphere.
Figure 5.
Grade IV notching in a patient with pain and mechanical symptoms.
Discussion
The present study shows that the Delta Xtend RSA can provide significant improvements in pain and function, even when used for different indications. There were significant gains in both range of motion and PROMs, and these clinical benefits were maintained out to 10 years' post-surgery. Previous studies have reported a gradual deterioration in range of motion and functional scores with longer follow-up.29,30 It has been hypothesised that this may be the result of over-tensioning of the deltoid muscle and periscapular muscle fatigue.31–33 However, in our elderly population the results with the Delta Xtend RSA were maintained, with no significant difference between male and female patients.34
Traditionally the best results following RSA have been reported in patients with RC tear arthropathy.29,35–37 Use of the Delta Xtend RSA in this series also resulted in predictable pain relief and improvements in function for patients with massive irreparable RC tears without arthrosis, and in patients with osteoarthritis. Outcomes after RSA for massive irreparable RC tears without arthritis have not been well defined in the literature.6 However, a recent systematic review confirmed that RSA in these patients can result in significant improvement of the patient's functional status and relief of pain and can be recommended.38
For patients with osteoarthritis who are at risk of poor outcomes and early failure after anatomic TSA (e.g. in patients with significant glenoid bone loss and/or humeral head subluxation, and in very elderly patients with an intact but compromised RC), the use of RSA is an attractive option.4 A recent matched cohort study reported that patients with glenohumeral osteoarthritis have similar functional improvements whether they undergo anatomic or reverse TSA and suggested that in certain instances reverse TSA provided a reasonable alternative.5
Consistent with previous studies,36,39,40 our worst results were observed in patients undergoing RSA for acute fracture and fracture sequelae, and when RSA is used in the revision setting. However, significant improvements in pain relief and PROMs are still observed in these patients, and they have very high satisfaction scores. It appears to be primarily the range of motion recovery in these patients that can be unpredictable when compared to other indications for RSA.
Our complication and revision rates are consistent with previous studies.29,41 Zumstein et al.41 reviewed 21 studies and found complication, reoperation, and revision rates of 24, 3.5, and 10%, respectively. The most common and serious complication reported following RSA is dislocation, with an incidence of up to 31%.41–44 Although Mole et al.45 found lower instability rates with the anterosuperior approach, in our series we used the deltopectoral approach almost exclusively and had no dislocations. We also routinely repaired the subscapularis tendon when there was tendon tissue remaining.46
Infection has been reported as the second most common cause for revision, and deep infection rates of 1–10% after RSA have been reported.42,47–50 All operations in this series were performed in a standard operating room with positive-pressure ventilation, and without the use of ultraviolet lights or body exhaust suits. We observed no patients with deep infection but accept that the incidence of infection following RSA is probably underestimated.
Most of the current literature on RSA involves patients where a cemented humeral stem was used.36,51–54 This originally arose from concerns about possible higher rates of loosening due to the semi-constrained nature of the RSA articulation. However, a recent study reported that uncemented stems have at least equivalent clinical and radiographic outcomes compared with cemented stems.55 In addition, as with anatomic TSA, humeral component loosening has not been reported with high incidence, and a shift towards the use of press-fit uncemented humeral systems has recently been seen, even in the very elderly.
Few studies have assessed the radiographic changes seen with uncemented stems in RSA.25,56–58 Our loosening rate of 0.0% in our uncemented stems is consistent with that reported for modern press-fit humeral stems. However, we did see evidence of radiolucent lines in 35.4% of the cases. This system achieves fixation primarily in the metadiaphyseal region, whereas other long-stem humeral systems achieve fixation in the diaphysis alone. Our finding may therefore be secondary to the location of fixation achieved, given that systems with primarily diaphyseal fixation report a very low rate of radiolucent lines.57,59
In contrast, stress shielding, defined as an adaptation of the bone to changes in stress distribution according to Wolff's law,60 is seen more commonly in diaphyseal press-fit humeral stems,57 and is inversely proportional to the diameter of the stem.61 Our incidence of stress shielding and proximal bone resorption was 22.9%, and in addition there were six radiographs with minor cortical resorption (12.5%). This is consistent with previous reports,25,58 although not as high as a recent study by Harmsen and Norris57 who observed a rate of what they termed internal proximal stress shielding of 97.4%.
Most cases of proximal bone resorption occurred on the medial side of the proximal humerus, and this was associated with higher grades of scapula notching. This is in keeping with the research of Sirveaux et al.26 and Al-Hadithy et al.,29 who found that three of their four patients with proximal humeral bone resorption had associated scapula notching. They suggested that this may be due to direct impingement on the scapula neck, causing both scapula notching and proximal humeral resorption. Proximal bone loss in this region may therefore not be solely the result of stress shielding, but rather a response to mechanical impingement.
The long-term clinical significance of stress shielding and other bony changes seen in RSA is unknown. Studies that have evaluated stress shielding in anatomic TSA suggest that the adaptive changes seen are not associated with a negative effect on functional outcomes.62,63 In our series, we observed no influence of these changes on range of motion or PROMs. Similarly, we observed no influence of the presence of heterotopic ossification (observed in 31% cases) on any outcome measure.64
Our scapula notching rate of 69.1% is consistent with previous studies, with rates ranging from 44% to as high as 100%.26,65–67 Although early reports suggested that the development of scapula notching could negatively influence functional results and long-term survival of the glenoid component,66 this has not been confirmed by all authors.32,39,54 We found no relationship between scapula notching and range of motion, function or pain at latest follow-up. However, the high percentage of Grade III and IV notching in our series is concerning (20%), particularly considering that there was a positive correlation between length of follow-up and the severity of scapula notching. This suggests that in some patients the development of scapula notching is a progressive phenomenon and is invariably associated with damage to the polyethylene liner.66,68 Whilst progressive notching may not become a major issue when managing elderly patients as in our series, the concern is that in the younger more active patient progressive scapula notching could become a clinical problem.69–72
Although numerous solutions have been proposed to minimise the risk of scapula notching,28,53,73–75 even in the most optimal conditions notching can still occur.65 Previous studies have suggested that increased prosthetic overhang and larger glenosphere size can both reduce the incidence of scapula notching.65,73 However, a recent study by Muller et al.76 reported no difference regarding the extent of inferior scapular notching or shoulder function in 68 patients with two different glenosphere sizes (36 and 44 mm). Our study also showed no relationship between glenosphere size and overhang, or scapula neck angle, on the development of scapula notching. In addition, these variables also had no influence on range of motion or PROMs.
It is likely that although the 155° neck-shaft angle design of the Delta Xtend System is inherently more stable, when coupled with a medialised centre of rotation (COR) and medialised humeral offset this may be the cause of our high rate of scapula notching. Such designs also result in greater humeral lengthening, which whilst important for optimising deltoid tensioning may contribute to an increased incidence of nerve complications and acromial fractures. Although we did not formally measure humeral lengthening, our incidence of these complications is in line with what has been previously reported,77,78 but is still worryingly high. Neurologic complications following RSA are not uncommon, but usually do not have a significant impact on patient outcome.77 However, post-operative scapula fractures invariably do lead to inferior clinical results.78,79
With improved understanding of RSA biomechanics and the complications that can occur, there is renewed interest in lateralisation of the COR, changes in neck-shaft angle, and lateralisation of humeral offset. The aim of these design changes is to maximise ROM (especially rotation) whilst minimising scapula notching, instability, and the complications that can occur because of excessive humeral lengthening. Currently it remains very difficult to select the optimal implant configuration for each patient,80 but with further study of different implants designs one day we may ultimately achieve patient-specific implant planning. However, whether such prosthetic changes ultimately affect implant longevity and long-term patient outcome remains to be seen.
To our knowledge, this is the first study in the English language literature to report on a consecutive series of Delta Xtend RSAs at a minimum follow-up of five years using standardised outcome measures.81 However, this study has several limitations that need to be considered when interpreting our results. This is a retrospective series, and the small number of patients limits the ability to perform extensive subgroup analysis and examine all potential confounding variables. A significant number of patients were also not available for review, mostly because of poor health and mortality. This is to be expected given the age group of the patients involved in the study and the length of follow-up post-surgery. However, we did account for all available follow-up times in the final analysis to provide a better overall assessment, and of the 37 patients unavailable for review none had undergone a reoperation or revision procedure of their prosthesis according to our National Joint Registry.
The patients were all treated by a single surgeon, which might limit the ability to broadly extrapolate the results but does limit the number of confounding variables that can arise from multi-surgeon studies. The senior surgeon is shoulder fellowship trained and had significant experience in shoulder arthroplasty before undertaking this study. Because this was a consecutive series of patients, multiple indications for reverse TSA were included in the study. This meant that the number of patients in some of the treatments groups was small, which could affect conclusions when comparing such variables as outcomes and complication rates. Therefore, our results should be interpreted with caution.
Conclusion
Our experience with use of the Delta Xtend RSA has been encouraging, with significant improvements in pain, range of motion, and PROMs observed in patients regardless of surgical indication. These results were maintained out to 10 years' follow-up and were not influenced by patient gender, glenosphere size and placement, or the presence of scapula notching. Our complication rate was acceptable and in line with previous reports. However, the high rate of scapula notching remains a concern, especially when considering the increasing use of these implants in younger more active patients. Further study is needed to fully understand the clinical significance of this frequent finding, as well as the influence of newer implant designs on its occurrence.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Outside funding was received from DePuy Orthopaedics, Inc. (700 Orthopaedic Drive, Warsaw, IN 46581-0988, USA) to assist in data collection (obtaining patient radiographic analysis) of this study.
Ethical Review and Patient Consent
This study was deemed exempt from requiring Ethical Committee Approval by the New Zealand Health and Disability Ethics Committee. Written informed consent was obtained from all patients for their involvement in this study and for their anonymised information to be published in this article.
References
- 1.Walker M, Willis MP, Brooks JP, et al. The use of the reverse shoulder arthroplasty for treatment of failed total shoulder arthroplasty. J Shoulder Elbow Surg 2012; 21: 514–522. [DOI] [PubMed] [Google Scholar]
- 2.Hattrup SJ, Sanchez-Sotelo J, Sperling JW, et al. Reverse shoulder replacement for patients with inflammatory arthritis. J Hand Surg Am 2012; 37: 1888–1894. [DOI] [PubMed] [Google Scholar]
- 3.Young AA, Smith MM, Bacle G, et al. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am 2011; 93: 1915–1923. [DOI] [PubMed] [Google Scholar]
- 4.Mizuno N, Denard PJ, Raiss P, et al. Reverse total shoulder arthroplasty for primary glenohumeral osteoarthritis in patients with a biconcave glenoid. J Bone Joint Surg Am 2013; 95: 1297–1304. [DOI] [PubMed] [Google Scholar]
- 5.Steen BM, Cabezas AF, Santoni BG, et al. Outcome and value of reverse shoulder arthroplasty for treatment of glenohumeral osteoarthritis: a matched cohort. J Shoulder Elbow Surg 2015; 24: 1433–1441. [DOI] [PubMed] [Google Scholar]
- 6.Mulieri P, Dunning P, Klein S, et al. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am 2010; 92: 2544–2556. [DOI] [PubMed] [Google Scholar]
- 7.Bufquin T, Hersan A, Hubert L, et al. Reverse shoulder arthroplasty for the treatment of three- and four-part fractures of the proximal humerus in the elderly: a prospective review of 43 cases with a short-term follow-up. J Bone Joint Surg Br 2007; 89: 516–520. [DOI] [PubMed] [Google Scholar]
- 8.Ross M, Hope B, Stokes A, et al. Reverse shoulder arthroplasty for the treatment of three-part and four-part proximal humeral fractures in the elderly. J Shoulder Elbow Surg 2015; 24: 215–222. [DOI] [PubMed] [Google Scholar]
- 9.Hattrup SJ, Waldrop R, Sanchez-Sotelo J. Reverse total shoulder arthroplasty for posttraumatic sequelae. J Orthop Trauma 2016; 30: e41–e47. [DOI] [PubMed] [Google Scholar]
- 10.Willis M, Min W, Brooks JP, et al. Proximal humeral malunion treated with reverse shoulder arthroplasty. J Shoulder Elbow Surg 2012; 21: 507–513. [DOI] [PubMed] [Google Scholar]
- 11.DeWilde L, Sys G, Julien Y, Van Ovost E, et al. The reversed Delta shoulder prosthesis in reconstruction of the proximal humerus after tumour resection. Acta Orthop Belg 2003; 69: 495–500. [PubMed] [Google Scholar]
- 12.Grammont P, Trouilloud P, Laffay J, et al. Concept study and realisation of a new total shoulder prosthesis. Rheumatologie 1987; 39: 407–418. [Google Scholar]
- 13.Grammont P, Baulot E. Delta shoulder prosthesis for rotator cuff rupture. Orthopedics 1993; 16: 65–68. [DOI] [PubMed] [Google Scholar]
- 14.Churchill JL, Garrigues GE. Current controversies in reverse total shoulder arthroplasty. J Bone Joint Surg Rev 2016; 4: 1–7. [DOI] [PubMed] [Google Scholar]
- 15.Ernstbrunner L, Suter A, Catanzaro S, et al. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears before the age of 60 years: long-term results. J Bone Joint Surg Am 2017; 99: 1721–1729. [DOI] [PubMed] [Google Scholar]
- 16.Otto RJ, Clark RE, Frankle MA. Reverse shoulder arthroplasty in patients younger than 55 years: 2 to 12 year follow-up. J Shoulder Elbow Surg 2017; 26: 792–797. [DOI] [PubMed] [Google Scholar]
- 17.Nyffeler RW, Werner CM, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg 2005; 14: 524–528. [DOI] [PubMed] [Google Scholar]
- 18.Hamada K, Yamanaka K, Uchiyama Y, et al. A radiographic classification of massive rotator cuff tear arthritis. Clin Orthop Relat Res 2011; 469: 2452–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ernstbrunner L, Werthel JD, Wagner E, et al. Glenoid bone grafting in primary reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2017; 26: 1441–1447. [DOI] [PubMed] [Google Scholar]
- 20.Richards RR, An AK, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg 1994; 3: 347–352. [DOI] [PubMed] [Google Scholar]
- 21.Lippitt SB, Harryman DT II and Matsen FA III. A practical tool for evaluation function: the simple shoulder test. In: Matsen FA III, Fu FH and Hawkins RJ (eds) The Shoulder: A Balance of Mobility and Stability. Rosemont, IL: American Academy of Orthopaedic Surgeons, 1993, pp.501–551.
- 22.Dawson J, Fitzpatrick R, Carr A. Questionnaire on the perception of patients about shoulder surgery. J Bone Joint Surg Br 1996; 78: 593–600. [PubMed] [Google Scholar]
- 23.Gummesson C, Atroshi I, Ekdahl C. The disabilities of the arm, shoulder and hand (DASH) outcome questionnaire: longitudinal construct validity and measuring self-rated health change after surgery. BMC Musculoskelet Disord 2003; 4: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sperling JW, Cofield RH, O'Driscoll SW, et al. Radiographic assessment of ingrowth total shoulder arthroplasty. J Shoulder Elbow Surg 2000; 9: 507–513. [DOI] [PubMed] [Google Scholar]
- 25.Melis B, DeFranco M, Ladermann A, et al. An evaluation of the radiological changes around the Grammont reverse geometry shoulder arthroplasty after eight to 12 years. J Bone Joint Surg Br 2011; 93: 1240–1246. [DOI] [PubMed] [Google Scholar]
- 26.Sirveaux F, Favard L, Oudet D, et al. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. J Bone Joint Surg 2004; 86: 388–395. [DOI] [PubMed] [Google Scholar]
- 27.Cuff D, Levy JC, Gutierrez S, et al. Torsional stability of modular and non-modular reverse shoulder humeral components in a proximal humeral bone loss model. J Shoulder Elbow Surg 2011; 20: 646–651. [DOI] [PubMed] [Google Scholar]
- 28.Berhouet J, Garaud P, Slimane M, et al. Effect of scapular pillar anatomy on scapular impingement in adduction and rotation after reverse shoulder arthroplasty. Orthop Traumatol Surg Res 2014; 100: 495–502. [DOI] [PubMed] [Google Scholar]
- 29.Al-Hadithy N, Domos P, Sewell MD, et al. Reverse shoulder arthroplasty in 41 patients with cuff tear arthropathy with a mean follow-up period of 5 years. J Shoulder Elbow Surg 2014; 23: 1662–1668. [DOI] [PubMed] [Google Scholar]
- 30.Favard L, Levigne C, Nerot C, et al. Reverse prostheses in arthropathies with cuff tear: are survivorship and function maintained over time?. Clin Orthop Relat Res 2011; 469: 2469–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim MS, Lim KY, Lee DH, et al. How does scapula motion change after reverse total shoulder arthroplasty? A preliminary report. BMC Musculoskelet Disord 2012; 13: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsen FA III, Boileau P, Walch G, et al. The reverse total shoulder arthroplasty. J Bone Joint Surg Am 2007; 89: 660–667. . [DOI] [PubMed] [Google Scholar]
- 33.Mollon B, Mahure SA, Roche CP, et al. Impact of scapula notching on clinical outcomes after reverse total shoulder arthroplasty: an analysis of 476 shoulders. J Shoulder Elbow Surg 2017; 26: 1253–1261. [DOI] [PubMed] [Google Scholar]
- 34.Wong SE, Pitcher AA, Ding DY, et al. The effect of patient gender on outcomes after reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2017; 26: 1889–1896. [DOI] [PubMed] [Google Scholar]
- 35.Nolan BM, Ankerson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop Relat Res 2011; 469: 2476–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wall B, Nove-Josserand L, O'Connor DP, et al. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am 2007; 89: 1476–1485. [DOI] [PubMed] [Google Scholar]
- 37.Wellmann M, Struck M, Pastor MF, et al. Short and midterm results of reverse shoulder arthroplasty according to the preoperative etiology. Arch Orthop Trauma Surg 2013; 133: 463–471. [DOI] [PubMed] [Google Scholar]
- 38.Sevivas N, Ferreira N, Andrade R, et al. Reverse shoulder arthroplasty for irreparable massive rotator cuff tears: a systematic review with meta-analysis and meta-regression. J Shoulder Elbow Surg 2017; 26: e265–e277. [DOI] [PubMed] [Google Scholar]
- 39.Boileau P, Watkinson D, Hatzidakis AM, et al. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg 2006; 15: 527–540. [DOI] [PubMed] [Google Scholar]
- 40.Kelly JD, 2nd, Zhao JX, Hobgood ER, et al. Clinical results of revision shoulder arthroplasty using the reverse prosthesis. J Shoulder Elbow Surg 2012; 21: 1516–1525. [DOI] [PubMed] [Google Scholar]
- 41.Zumstein MA, Pinedo M, Old J, et al. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 2011; 20: 146–157. [DOI] [PubMed] [Google Scholar]
- 42.Boileau P. Complications and revision of reverse total shoulder arthroplasty. Orthop Traumatol Surg Res 2016; 102: S33–S43. [DOI] [PubMed] [Google Scholar]
- 43.Chalmers PN, Rahman Z, Romeo AA, et al. Early dislocation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2014; 23: 737–744. [DOI] [PubMed] [Google Scholar]
- 44.Gallo RA, Gamradt SC, Mattern CJ, et al. Instability after reverse total shoulder replacement. J Shoulder Elbow Surg 2011; 20: 584–590. [DOI] [PubMed] [Google Scholar]
- 45.Mole D, Wein F, Dezaly C, et al. Surgical technique: the anterosuperior approach for reverse shoulder arthroplasty. Clin Orthop Relat Res 2011; 469: 2461–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edwards TB, Williams MD, Labriola JE, et al. Subscapularis insufficiency and the risk of shoulder dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg 2009; 18: 892–896. [DOI] [PubMed] [Google Scholar]
- 47.Cuff D, Clark R, Pupello D, et al. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency: a concise follow-up, at a minimum of five years, of a previous report. J Bone Joint Surg Am 2012; 94: 1996–2000. [DOI] [PubMed] [Google Scholar]
- 48.Jacquot A, Sirveaux F, Roche O, et al. Surgical management of the infected reversed shoulder arthroplasty: a French multicenter study of reoperation in 32 patients. J Shoulder Elbow Surg 2015; 24: 1713–1722. [DOI] [PubMed] [Google Scholar]
- 49.Morris BJ, O'Connor DP, Torres D, et al. Risk factors for periprosthetic infection after reverse shoulder arthroplasty. J Shoulder Elbow Surg 2015; 24: 161–166. [DOI] [PubMed] [Google Scholar]
- 50.Saltzman BM, Chalmers PN, Gupta AK, et al. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2014; 23: 1647–1654. [DOI] [PubMed] [Google Scholar]
- 51.Boileau P, Gonzalez JF, Chuinard C, et al. Reverse total shoulder arthroplasty after failed rotator cuff surgery. J Shoulder Elbow Surg 2009; 18: 600–606. [DOI] [PubMed] [Google Scholar]
- 52.Cuff D, Pupello D, Virani N, et al. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Jt Surg Am 2008; 90: 1244–1251. [DOI] [PubMed] [Google Scholar]
- 53.Frankle M, Siegal S, Pupello D, et al. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am 2005; 87: 1697–1705. [DOI] [PubMed] [Google Scholar]
- 54.Werner CM, Steinmann PA, Gilbart M, et al. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am 2005; 87: 1476–1486. [DOI] [PubMed] [Google Scholar]
- 55.Phadnis J, Huang T, Watts A, et al. Cemented or cementless humeral fixation in reverse total shoulder arthroplasty? A systematic review. Bone Joint J 2016; 98-B: 65–74. [DOI] [PubMed] [Google Scholar]
- 56.Gilot G, Alvarez-Pinzon AM, Wright TW, et al. The incidence of radiographic aseptic loosening of the humeral component in reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2015; 24: 1555–1559. [DOI] [PubMed] [Google Scholar]
- 57.Harmsen SM, Norris TR. Radiographic changes and clinical outcomes associated with an adjustable diaphyseal press-fit humeral stem in primary reverse shoulder arthroplasty. J Shoulder Elbow Surg 2017; 26: 1589–1597. [DOI] [PubMed] [Google Scholar]
- 58.Wiater JM, Moravek JE, Jr, Budge MD, et al. Clinical and radiographic results of cementless reverse total shoulder arthroplasty: a comparative study with 2 to 5 years of follow-up. J Shoulder Elbow Surg 2014; 23: 1208–1214. [DOI] [PubMed] [Google Scholar]
- 59.Youn SM, Deo S, Poon PC. Functional and radiologic outcomes of uncemented reverse shoulder arthroplasty in proximal humeral fractures: cementing the humeral component is not necessary. J Shoulder Elbow Surg 2016; 25: e83–e89. [DOI] [PubMed] [Google Scholar]
- 60.Wolffe J. The law of bone remodelling, Heidelberg: Springer-Verlag, 1986. [Google Scholar]
- 61.Nagels J, Stokdijk M, Rozing PM. Stress shielding and bone resorption in shoulder arthroplasty. J Shoulder Elbow Surg 2003; 12: 35–39. [DOI] [PubMed] [Google Scholar]
- 62.Raiss P, Edwards TB, Deutsch A, et al. Radiographic changes around humeral components in shoulder arthroplasty. J Bone Joint Surg Am 2014; 96: e54. [DOI] [PubMed] [Google Scholar]
- 63.Schnetzke M, Coda S, Raiss P, et al. Radiologic bone adaptations on a cementless short-stem shoulder prosthesis. J Shoulder Elbow Surg 2016; 25: 650–657. [DOI] [PubMed] [Google Scholar]
- 64.Ko JK, Tompson JD, Sholder DS, et al. Heterotopic ossification of the long head of the triceps after reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2016; 25: 1810–1815. [DOI] [PubMed] [Google Scholar]
- 65.Bigorre N, Lancigu R, Bizot P, et al. Predictive factors of scapular notching in patients with reverse shoulder arthroplasty. Orthop Traumatol Surg Res 2014; 100: 711–714. [DOI] [PubMed] [Google Scholar]
- 66.Levigne C, Garret J, Boileau P, et al. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how?. Clin Orthop Relat Res 2011; 469: 2512–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Simovitch RW, Zumstein MA, Lohri E, et al. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg Am 2007; 89: 588–600. [DOI] [PubMed] [Google Scholar]
- 68.Wiater BP, Baker EA, Salisbury MR, et al. Elucidating trends in revision reverse total shoulder arthroplasty procedures: a retrieval study evaluating clinical, radiographic, and functional outcomes data. J Shoulder Elbow Surg 2015; 24: 1915–1925. [DOI] [PubMed] [Google Scholar]
- 69.Ek ET, Neukom L, Catanzaro S, et al. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg 2013; 22: 1199–1208. [DOI] [PubMed] [Google Scholar]
- 70.Muh SJ, Streit JJ, Wanner JP, et al. Early follow-up of reverse total shoulder arthroplasty in patients sixty years of age or younger. J Bone Joint Surg Am 2013; 95: 1877–1883. [DOI] [PubMed] [Google Scholar]
- 71.Sershon RA, Van Thiel GS, Lin EC, et al. Clinical outcomes of reverse total shoulder arthroplasty in patients aged younger than 60 years. J Shoulder Elbow Surg 2014; 23: 395–400. [DOI] [PubMed] [Google Scholar]
- 72.Samuelsen BT, Wagner ER, Houdek MT, et al. Primary reverse shoulder arthroplasty in patients aged 65 years or younger. J Shoulder Elbow Surg 2017; 26: e13–e17. [DOI] [PubMed] [Google Scholar]
- 73.Berhouet J, Garaud P, Favard L. Evaluation of the role of glenosphere design and humeral component retroversion in avoiding scapular notching during reverse shoulder arthroplasty. J Shoulder Elbow Surg 2014; 23: 151–158. [DOI] [PubMed] [Google Scholar]
- 74.Boileau P, Moineau G, Roussanne Y, et al. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res 2011; 469: 2558–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Wilde LF, Poncet D, Middernacht B, et al. Prosthetic overhang is the most effective way to prevent scapular conflict in a reverse total shoulder prosthesis. Acta Orthop 2010; 81: 719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Muller AM, Born M, Jung C, et al. Glenosphere size in reverse shoulder arthroplasty: is larger better for external rotation and abduction strength? J Shoulder Elbow Surg 2018; 27: 44–52. [DOI] [PubMed]
- 77.Ball CM. Neurologic complications of shoulder joint replacement. J Shoulder Elbow Surg 2017; 26: 2125–2132. [DOI] [PubMed] [Google Scholar]
- 78.Hattrup SJ. The influence of postoperative acromial and scapular spine fractures on the results of reverse shoulder arthroplasty. Orthopedics 2010, pp. 33. [DOI] [PubMed] [Google Scholar]
- 79.Teusink MJ, Otto RJ, Cottrell BJ, et al. What is the effect of postoperative scapular fracture on outcomes of reverse shoulder arthroplasty?. J Shoulder Elbow Surg 2014; 23: 782–790. [DOI] [PubMed] [Google Scholar]
- 80.Walker DR, Kinney AL, Wright TW, et al. How sensitive is the deltoid moment arm to humeral offset changes with reverse total shoulder arthroplasty?. J Shoulder Elbow Surg 2016; 25: 998–1004. [DOI] [PubMed] [Google Scholar]
- 81.Gruber S, Schoch C, Geyer M. [The reverse shoulder arthroplasty Delta Xtend: mid-term results]. Orthopade 2017; 46: 222–226. [DOI] [PubMed] [Google Scholar]