Short abstract
Myocardial dysfunction is a prime cause of death in sepsis. This study is to delve into the function of lncRNA KCNQ1OT1 in myocardial injury induced by sepsis. Sepsis-induced myocardial injury model in rat was initiated by intraperitoneally injecting of LPS (10 mg/kg) in vivo, and cardiomyocyte H9c2 was treated with LPS to mimic sepsis in vitro. KCNQ1OT1 and miR-192-5p expressions were detected by qRT-PCR. The cell viability was probed with CCK-8 experiment and the apoptosis of the cardiomyocytes was tested using flow cytometry analysis. Western blot was operated to determine apoptosis-related proteins expressions. ELISA was used to evaluate the levels of TNF-α, IL-6, and IL-1β. Bioinformatics analysis, RT-PCR, dual luciferase reporter assay, and RNA immunoprecipitation experiment were utilized to detect the interrelation of genes. Herein, we proved that KCNQ1OT1 was considerably down-regulated, whereas miR-192-5p was markedly increased in myocardial tissues of septic rats. KCNQ1OT1 interrelated with miR-192-5p, and negatively modulated its expression levels. Overexpression of KCNQ1OT1 or the transfection of miR-192-5p inhibitors greatly facilitated the viability and impeded the apoptosis of H9c2 cardiomyocytes. miR-192-5p paired with the 3ʹUTR of XIAP, and repressed its protein expression, and XIAP was modulated positively by KCNQ1OT1. In conclusion, our work indicates that down-regulation of KCNQ1OT1 advances cardiac injury through regulating miR-192-5p/XIAP axis during sepsis.
Impact statement
Sepsis-induced cardiomyopathy remains to be a major challenge to health care systems around the globe. There are no known therapies currently available that can cure the disease. This study provides convincing evidence that KCNQ1OT1 could attenuate sepsis-mediated myocardial injury. We further demonstrate that the beneficial function of KCNQ1OT1 was achieved by regulating the miR-192-5p/XIAP axis. We therefore found a new mechanism of cardioprotective effect of KCNQ1OT1, one which also offers a novel theoretical basis for the therapy of sepsis-induced cardiomyopathy.
Keywords: lncRNA KCNQ1OT1, miR-192-5p, sepsis, myocardial injury
Introduction
Sepsis is a comprehensive systemic disease characterized by life-threatening organ dysfunction in the body’s uncontrolled infection response.1 Sepsis-induced cardiomyopathy (SIC) is a common dysfunction of the heart owing to sepsis, and its pathophysiological process is reversible. According to epidemiological investigations, SIC occurs in at least 40% of patients with sepsis, and if SIC is not controlled in time and effectively, the patient mortality rate will increase significantly.2 From the pathogenesis, sepsis-mediated myocardial injury develops with abnormal expression and dysfunction of genes, RNA, and proteins. Effective modulation of this abnormal molecular expression may alleviate SIC. Therefore, investigating the detailed mechanisms of SIC is at play in exploring novel treatment options.
LncRNAs are RNA molecules with over 200 nucleotides in length and without the ability of encoding proteins. LncRNA is involved in multiple biological processes, X chromosome imprinting, chromatin remodeling, alternative splicing and decay of RNA, cell differentiation, cell fate control, cell migration, and so on included.3,4 Research reports often offer inferences that lncRNA exerts a meaningful part in the pathophysiological responses of bacterial inflammatory reactions such as pneumonia and nephritis.5,6 Ascending evidence denoted that lncRNAs are enrolled in sepsis.7,8 Exploring the mechanism of lncRNAs in the pathophysiological process of sepsis may be helpful in finding effective therapeutic targets. KCNQ1 opposite strand/antisense transcript 1 (KCNQ1OT1) is abnormally expressed in several diseases, and is associated with inflammatory responses and cell apoptosis.9,10 Nevertheless, the mechanism of action of KCNQ1OT1 in sepsis remains unclear.
MicroRNAs (MiRs), which also belong to ncRNAs, are highly conserved ncRNA molecules of 21–25 nt in length. MiRs are of great importance in cell differentiation, metabolism, proliferation, and apoptosis.11 Reports have denoted that miRNAs participate in the progression of sepsis.12,13 Researches have suggested that miR-192-5p was diminished in some tumors such as lung cancer, bladder carcinoma, and so on.14,15 Instead, miR-192-5p function in the pathophysiological process of sepsis needs further exploration.
X-linked inhibitor of apoptosis protein (XIAP) is a crucial caspase inhibitor during apoptosis. XIAP functions in the inhibition of apoptosis driven by endogenous or exogenous apoptosis through a diversity of signaling pathways.16,17 Interestingly, studies have shown that in sepsis, miR-23b exerted immunosuppressive effects in the late periods of sepsis via impeding the NF-κB signaling pathway and elevating pro-apoptotic signaling pathways influenced by NIK, TRAF1, and XIAP.18 This study suggests that XIAP is involved in sepsis and inflammatory responses and can be targeted by miRNAs.
Herein, we manifested that KCNQ1OT1 expression was remarkably decreased in myocardial tissue of a SIC rat model. We further validated that the regulation of the expression of KCNQ1OT1 affected cardiac injury. Ultimately, we made clear that KCNQ1OT1 controlled the cell proliferation and apoptosis through modulating miR-192-5p/XIAP axis. This study found that KCNQ1OT1 regulated the proliferation and apoptosis of cardiomyocytes and paved the way for the therapy of SIC.
Materials and methods
Establishment of rat model of SIC
The Experimental Animal Center of Fudan University was the provider of male Sprague-Dawley rats (220–250 g). They were classified into different groups (20 in a group) at random. There existed no conspicuous dissimilarities in age and weight among the groups. To establish SIC models, each group was intraperitoneally injected with lipopolysaccharide (LPS). After 12 h, the heart function of each group was examined by ultrasound. Then 100 μL purified adenovirus solution (1 × 1011 PFU) carrying vector plasmid or KCNQ1OT1 overexpression plasmid (Hongtuo Biotechnology Co., Ltd, Hangzhou, China) was injected into the rats via caudal vein. Two weeks after injection, rats were treated with LPS to establish sepsis model.
Echocardiography detection
Echocardiography of each group of rats was carried out by a Mylab 30 cv ultrasound system (Esoate, S.P.A, Genoa, Italy) and a 10 mhz linear ultrasound sensor. After shaving in the anterior thoracic region and anesthetizing of the rats, the rats were placed on a plate at 37°C with the left side facing up. Test indicators included ejection fraction (%), fractional shortening (%), and heart rate.
Hematoxylin-eosin staining
Fresh heart tissue of rats was obtained and settled in 10% paraformaldehyde, dehydrated by ethanol and xylene, embedded in paraffin, and sliced into section. After dewaxing, hematoxylin eosin staining and neutral resin sealing were carried out, and the histological changes of myocardial tissue were determined using light microscope.
Cell culture and transfection
The Chinese Academy of Sciences was the provider of human cardiomyocyte H9c2 cells. Cells, placed in a humidified and 5% CO2 incubator at 37°C, were cultured in DMEM (Invitrogen, Carlsbad, CA, US) supplemented with L-glutamine (Gibco, New York, CA, US), 100 U/mL penicillin and 100 mg/mL streptomycin (Hyclone, New York, CA, US), and 10% fetal bovine serum (FBS; Gibco, New York, CA, US). H9c2 cardiomyocytes were treated with lipopolysaccharide (LPS; Sigma, St. Louis, MO, USA) to construct the LPS-induced sepsis model in vitro. RiboBio Co., Ltd. (Guangzhou, China) was the provider of miR-192-5p mimics, miR-192-5p inhibitors, KCNQ1OT1 overexpression plasmid, and KCNQ1OT1 shRNA. H9c2 cells were transfected with Lipofectamine™ 2000 (Invitrogen, Shanghai, China) in compliance with protocols.
Quantitative real-time polymerase chain reaction
TRIzol (Invitrogen, Shanghai, China) was used to extract the total RNA from myocardial tissue and H9c2 cells. Reverse transcription was conducted by MMLV transcriptase (Invitrogen, Shanghai, China) to get cDNA. qRT-PCR was done with SYBR premix EX TAQ II kit (TaKaRa, Dalian, China). GAPDH functioned as the internal reference for KCNQ1OT1 and XIAP. U6 was that of miR-192-5p. 2−ΔΔCt method was used to quantify the expression levels of genes. The information of primer sequences employed is provided in Table 1.
Table 1.
qRT-PCR primer sequences.
| Name | Primer sequences |
|---|---|
| KCNQ1OT1 | Forward:5′-CCCAGAAATCCACACCTCGG-3′ |
| Reverse:5′-TCCTCAGTGAGCAGATGGAGA-3′ | |
| GAPDH | Forward:5′-TATGATGATATCAAGAGGGTAGT-3′ |
| Reverse:5′-TGTATCCAAACTCATTGTCATAC-3′ | |
| miR-192-5p | Forward:5′-GGACTTTCTTCATTC ACACCG-3′ |
| Reverse:5′-GACCACTGAGGTTAGAGCCA-3′ | |
| XIAP | Forward:5′-CCGTGCGGTGCTTTAGTTGT-3′ |
| Reverse:5′-TTCCTCGGGTATATGGTGTCTGAT-3′ | |
| U6 | Forward:5′-GCTTCGGCAGCACATATACTAAAAT-3′ |
| Reverse:5′-CGCTTCACGAATTTGCGTGTCAT-3′ |
Cell counting kit-8 assay
The viability of cardiomyocytes was examined by CCK-8 assay. H9c2 cells transfected for 24 h were inoculated in 96-well plates with a density of 5 × 103/mL, and cultured for four consecutive days. On each day, 10 μL CCK-8 solution (Dojindo, Kumamoto, Japan) was loaded, and the cells were incubated in the incubator for additional 1 h, and the absorbance value was monitored at 450 nm. The curves were drawn with time as abscissa and OD 450 nm as ordinate.
Apoptosis assay
H9c2 cells at log growth phase were trypsinized to make a single cell suspension. Afterwards, cardiomyocytes were centrifuged at 1500 r/min for 5 min, and rinsed twice. Then the centrifugation was conducted again. After that, 400 μL of 1 × Binding Buffer was adopted to resuspend the cells, and 4 μL of Annexin V-FITC apoptosis detection kit (Abcam, Shanghai, China) with 4 μL of propidium iodide solution were dripped into the mix. After incubation in dark for 30 min, flow cytometry analysis was done to monitor the apoptosis.
RNA immunoprecipitation assay
Magna RIP™ RNA-Binding Protein Immunoprecipitation Kit (Millipore, Billerica, MA, USA) was utilized to conduct RIP in accord to the protocols; 100 μL H9c2 cell lysate was prepared using 100 μL RIP buffer, 0.25 μL RNase inhibitors, and 0.5 μL protease inhibitors. After high-speed centrifugation, the supernatant was cultured with protein-A/G-Sepharose beads to obtain RNA binding protein complex. KCNQ1OT1 enrichment and miR-192-5p enrichment in the complex were probed by RT-PCR.
Western blot
Cells were cleaned three times with PBS buffer, and total protein extraction was performed via loading RIPA lysate (Beyotime, Shanghai, China) with PMSF, and then denatured in boiling water for 5 min. Then protein samples were dissociated by 10% SDS-PAGE and transferred to PVDF membrane. Then the proteins were blocked with 5% skim milk for 30 min, and then anti-XIAP antibody (ab21278, abcam, 1:1000), anti-Caspase-3 antibody (ab13847, abcam, 1:1000), anti-Bax antibody (ab53154, abcam, 1:1000) were loaded, and the membrane was incubated at 4°C overnight and rinsed, and then secondary antibody was loaded and incubated for 60 min. Finally, immunoreactivity was determined with enhanced chemiluminescence kit (Amersham, Pittsburgh, PA, USA).
Dual luciferase reporter assay
Targeting sequences of wild-type (wt) KCNQ1OT1 and mutated (mut) KCNQ1OT1 were established and inserted into pGL3 vector (Promega, Madison, WI, USA), formulating pGL3-KCNQ1OT1-wild type (KCNQ1OT1-wt) and pGL3-KCNQ1OT1-mutant (KCNQ1OT1-mut) reporter vector. The cells were then cultivated in 24-well plates at 105 cells per well. Then the miR-192-5p mimics or the negative control was co-transfected into H9c2 cardiomyocytes with wild type or mutant reporter vectors, respectively. After 48 h, the activity of luciferase was determined.
Enzyme-linked immunosorbent assay
ELISA was used to probe the expression levels of pro-inflammatory cytokines. Myocardial tissues were lysed with RIPA lysis buffer (Beyotime, Shanghai, China), and then the lysis was centrifuged at 14,000 × g for 5 min. Then the cytokines were detected by ELISA in accordance with the manufacturer’s protocols.
Statistical analysis
The analysis was operated with SPSS 21.0 (IBM, Armonk, NY, USA). Data were stated as mean ± standard deviation. Student’s t test or one-way ANOVA was used to evaluate the differences among two or more groups. P < 0.05 implied significant.
Results
Down-regulation of KCNQ1OT1 in myocardial tissue of rats treated with LPS
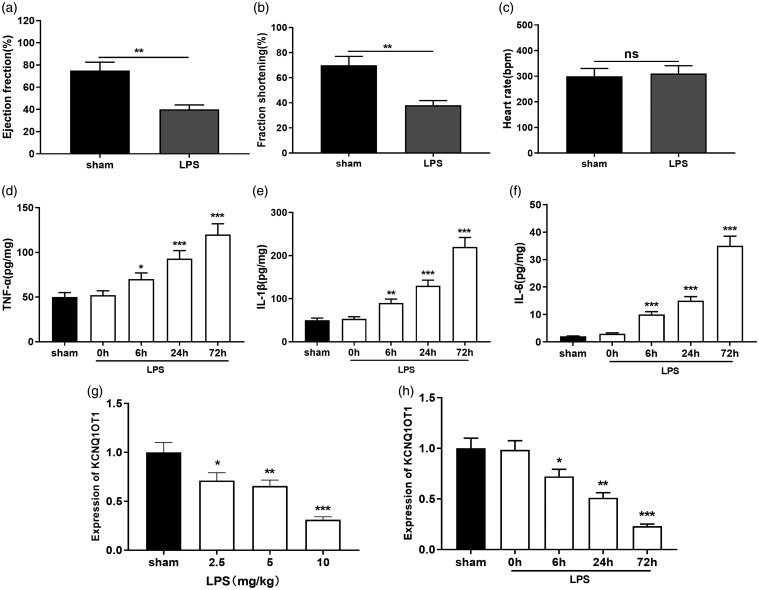
To initially investigate the function of KCNQ1OT1 in SIC, we first created a rat model of sepsis-induced myocardial injury. HE staining proved that as opposed to the sham group, vacuolar degeneration, swelling of myocardium, and interstitial edema were only observed in septic rats, suggesting myocardial injury in SIC group was successfully induced (Supplemental Figure 1). We particularly monitored the cardiac function of the rats. Ultrasound findings indicated that in the LPS group, EF (%) and FS (%) were dramatically dwindled than the sham group (Figure 1(a) and (b)). There was no marked difference in heart rate (Figure 1(c)), which was consistent with previous report.19 Further, ELISA was applied in order to detect the inflammatory factors in myocardial tissue. It was showed that TNF-α, IL-1β, and IL-6 were greatly accumulated in the LPS group as against the sham group (Figure 1(d) and (f)). We subsequently tested KCNQ1OT1 expression in rat myocardial tissue via qRT-PCR. This data indicated that KCNQ1OT1 expression was inhibited by LPS induction and significantly down-regulated in rat myocardial tissue in both time and dose-dependent manners (Figure 1(g) and (h)).
Figure 1.
KCNQ1OT1 was down-regulated in rat SIC model. (a–c) Ultrasound system was used to detect EF (%), FS (%), and heart rate of sham group and LPS-treated rats. (d–f) TNF-α, IL-1β, and IL-6 expressions in myocardial tissue were evaluated by ELISA. (g–h) qRT-PCR was employed to detect KCNQ1OT1 expression in the myocardial tissue of rats with different LPS concentrations and time of treatment. *, **, *** indicated P < 0.05, P < 0.01, P < 0.001, respectively.
Up-regulation of the expression of KCNQ1OT1 protected H9c2 cells from LPS-induced injury
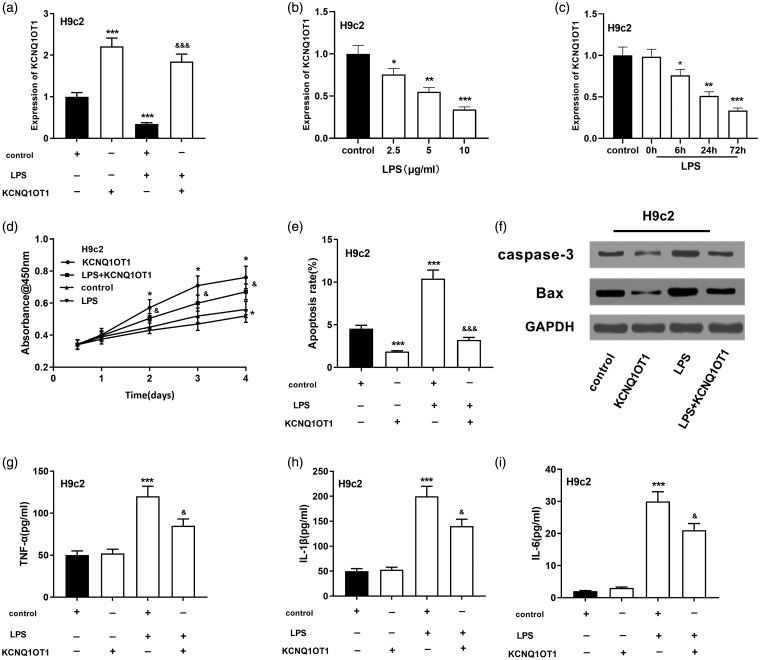
To inquire into the function of KCNQ1OT1 in sepsis-mediated myocardial injury, we employed LPS to treat H9c2 cells to build a myocardial injury model in vitro.20 We learned that LPS dramatically repressed KCNQ1OT1 expression of H9c2 cells in time- and dose-dependent manners (Figure 2(a) to (c)). We also successfully constructed the H9c2 cell line with KCNQ1OT1 overexpressed (Figure 2(a)). Further experiments indicated that ectogenic overexpression of KCNQ1OT1 greatly accelerated the proliferation of H9c2 cells (Figure 2(d)) and inhibited the expression level of apoptosis-related proteins caspase3 and Bax (Figure 2(e) and (f)). In addition, after overexpression of KCNQ1OT1, TNF-α, IL-1β, and IL-6 expressions in the supernatant of H9c2 cells were significantly down-regulated (Figure 2(g) to (i)).
Figure 2.
The functions of KCNQ1OT1 on the viability and apoptosis of LPS-induced H9c2 cardiomyocytes. (a) qRT-PCR validated the overexpression of KCNQ1OT1 and its change when treated with LPS in H9c2 cells. (b) qRT-PCR detected KCNQ1OT1 expressions in H9c2 cells at different LPS concentrations. (c) qRT-PCR was adopted to detect KCNQ1OT1 expressions in H9c2 cells at different treatment times. (d) CCK-8 assay was employed to evaluate the viability of LPS-induced H9c2 cardiomyocytes after KCNQ1OT overexpression. (e) The apoptosis of LPS-induced H9c2 cells after KCNQ1OT overexpression was detected. (f) Western blot was conducted to detect caspase-3 and Bax after KCNQ1OT overexpression in H9c2 cells. ( g–i) ELISA was used to detect TNF-α, IL-1β and IL-6 expressions in the supernatant of cells. v.s. control group: *, **, *** indicated P < 0.05, P < 0.01, P < 0.001, respectively. v.s LPS group: &, &&, &&& indicated P < 0.05, P < 0.01, P < 0.001, respectively.
MiR-192-5p was a target of KCNQ1OT1
qRT-PCR denoted that as opposed to normal rat tissues, miR-192-5p expression was greatly elevated in rat myocardial tissue along with growing LPS concentration (Figure 3(a)). With longer treatment time, miR-192-5p expression in myocardial tissue of the LPS group was also up-regulated gradually (Figure 3(b)). We then conducted a bioinformatics analysis through the StarBase database, which signified that KCNQ1OT1 carried a conserved combining site for miR-192-5p (Figure 3(c)). To attest the correlation between KCNQ1OT1 and miR-192-5p, we conducted the dual luciferase reporter assay, which denoted that the miR-192-5p mimics impeded the luciferase activity of the wild type KCNQ1OT1 vector, but exerted no notable effect on that of the KCNQ1OT1-mut vector (Figure 3(d)). Furthermore, miR-192-5p expression was remarkably down-regulated after up-regulation of KCNQ1OT1 in LPS-induced H9c2 cardiomyocytes (Figure 3(e)). In contrast, after down-regulating it, miR-192-5p expression was considerably elevated (Figure 3(f)). To make sure their binding between miR-192-5p and KCNQ1OT1, we performed an anti-Ago2 RIP experiment. The results revealed that the Anti-Ago2 antibody was enriched with more KCNQ1OT1 compared to the control Anti-IgG (Figure 3(g)). The results of this experiment implied that there were binding relationships between miR-192-5p and KCNQ1OT1 in sepsis-induced myocardial injury, and miR-192-5p was inhibited by KCNQ1OT1.
Figure 3.
The expression characteristics of miR-192-5p in LPS-treated rat tissues and cells, and its interaction with KCNQ1OT1. (a) qRT-PCR was employed to examine miR-192-5p expressions in myocardial tissue of sham and LPS rats at different LPS concentrations. (b) qRT-PCR was applied to figure out miR-192-5p expressions in myocardial tissue of sham group and LPS rats at different LPS treatment times. (c) Predicted binding site between KCNQ1OT1 sequence and miR-192-5p. (d) The binding between KCNQ1OT1 and miR-192-5p was validated by double luciferase report assay. (e–f) The effects of KCNQ1OT1 and LPS treatment on miR-192-5p expressions in H9c2 cells were observed by qRT-PCR. (g) The interaction between KCNQ1OT1 and miR-192-5p was validated by RIP experiment. *, **, *** indicated P < 0.05, P < 0.01, P < 0.001, respectively. (A color version of this figure is available in the online journal.)
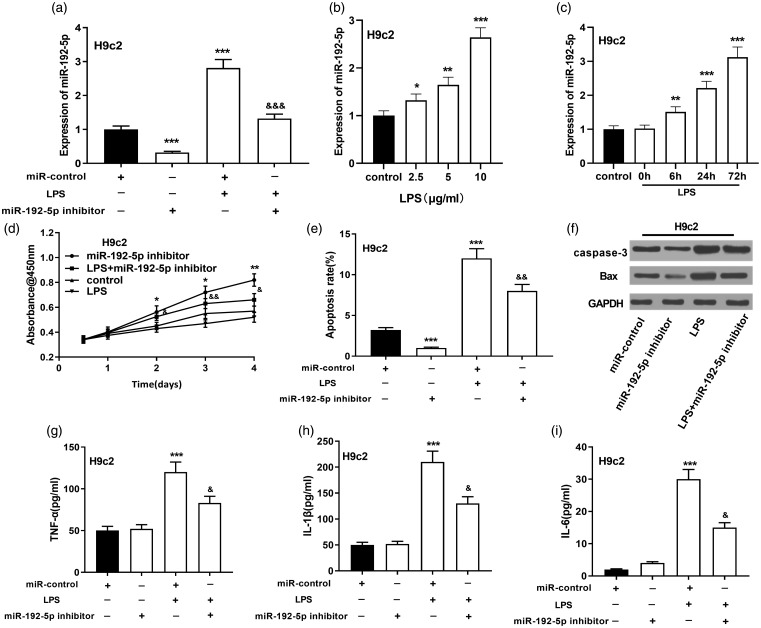
MiR-192-5p inhibition prompted proliferation and restrained apoptosis of H9c2 cells
We later proved that LPS considerably elevated miR-192-5p expressions in H9c2 cells with increasing LPS concentration and treatment time (Figure 4(a) to (c)). Further studies revealed that inhibition of miR-192-5p dramatically facilitated the viability and impeded the apoptosis of H9c2 cardiomyocytes, with decreased expressions of caspase3 and Bax (Figure 4(d) to (f)). Also, after inhibition of miR-192-5p, TNF-α, IL-1β, and IL-6 expressions in culture supernatant of H9c2 cardiomyocytes were considerably reduced (Figure 4(g) to (i)). These data implied that miR-192-5p was an injurious factor in SIC.
Figure 4.
Effect of miR-192-5p inhibition on the viability and apoptosis of H9c2 cardiomyocytes. (a) qRT-PCR was utilized to measure miR-192-5p in LPS-induced H9c2 cardiomyocytes. (b) qRT-PCR was adopted to work out miR-192-5p in H9c2 cells at different LPS concentrations. (c) MiR-192-5p in H9c2 cells were detected by qRT-PCR at different treatment times. (d) CCK-8 assay was conducted to delve into the viability of LPS-induced H9c2 cardiomyocytes after inhibiting miR-192-5p. (e) Flow cytometry analysis was operated to detect the apoptosis of H9c2 cardiomyocytes induced by LPS after miR-192-5p inhibition. (f) Western blot was conducted to detect caspase-3 and Bax after inhibition of miR-192-5p in H9c2 cardiomyocytes. (g–i) ELISA was carried out to quantify TNF-α, IL-1β and IL-6 expressions in the cell supernatant. vs. miR-control: *, **, *** indicated P < 0.05, P < 0.01, P < 0.001, respectively. vs. LPS group: &, &&, &&& indicated P < 0.05, P < 0.01, P < 0.001, respectively.
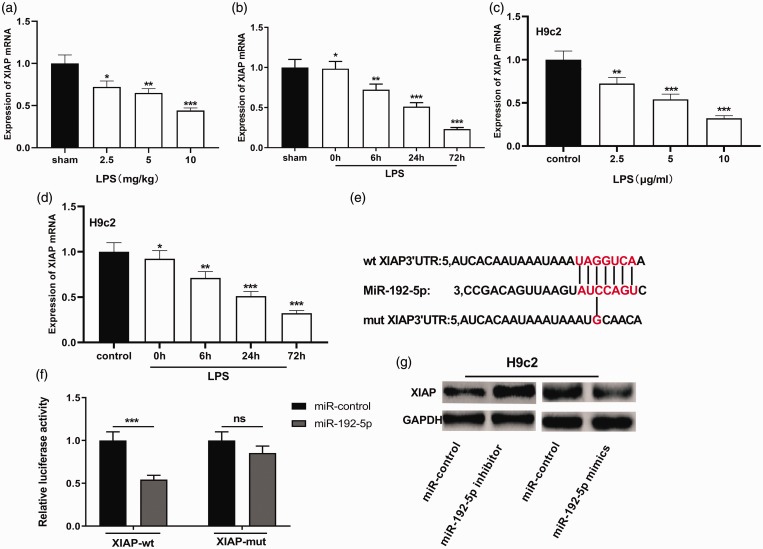
MiR-192-5p directly targets XIAP
To figure out mechanisms of miR-192-5p in mediating sepsis myocardial injury and the role of XIAP in this process, we examined whether the XIAP was abnormally expressed by qRT-PCR. Results denoted that XIAP mRNA expression was significantly declined in rat myocardial tissue along with both increasing LPS concentration and treatment time (Figure 5(a) and (b)). XIAP mRNA expression was remarkably down-regulated in H9c2 cells treated with LPS (Figure 5(c) and (d)). We also searched the StarBase database and learned that XIAP was a hidden target of miR-192-5p (Figure 5(e)). Dual luciferase reporter assay was adopted to confirm the linking relation between miR-192-5p and XIAP. MiR-192-5p mimics can impede the luciferase activity of the XIAP-wt reporter, and there was no obvious influence on the luciferase activity of the XIAP-mut vector (Figure 5(f)). Western blot also manifested that XIAP expression was dramatically down-regulated by miR-192-5p mimics, and was greatly elevated by of miR-192-5p inhibitor (Figure 5(g)).
Figure 5.
XIAP was down-regulated in SIC models and targeted by miR-192-5p. (a) qRT-PCR was operated to figure out XIAP mRNA expressions in myocardial tissue of sham and SIC rats at different LPS concentrations. (b) qRT-PCR was used to delve into XIAP mRNA expressions in myocardial tissue of sham group and SIC rats at different LPS treatment times. (c) qRT-PCR was operated to figure out XIAP mRNA expressions in H9c2 cells at different LPS concentrations. (d) qRT-PCR was adopted to delve into XIAP mRNA expressions in H9c2 cells at different LPS treatment times. (e) Base complementary pairing relationship between the sequence of XIAP 3ʹUTR and miR-192-5p. (f) Dual luciferase reporter assay was utilized to verify the binding between miR-192-5p and XIAP. (g) The effects of miR-192-5p overexpression and inhibition on XIAP expression in H9c2 cardiomyocytes were examined by Western blot. *, **, *** indicated P < 0.05, P < 0.01, P < 0.001, respectively. (A color version of this figure is available in the online journal.)
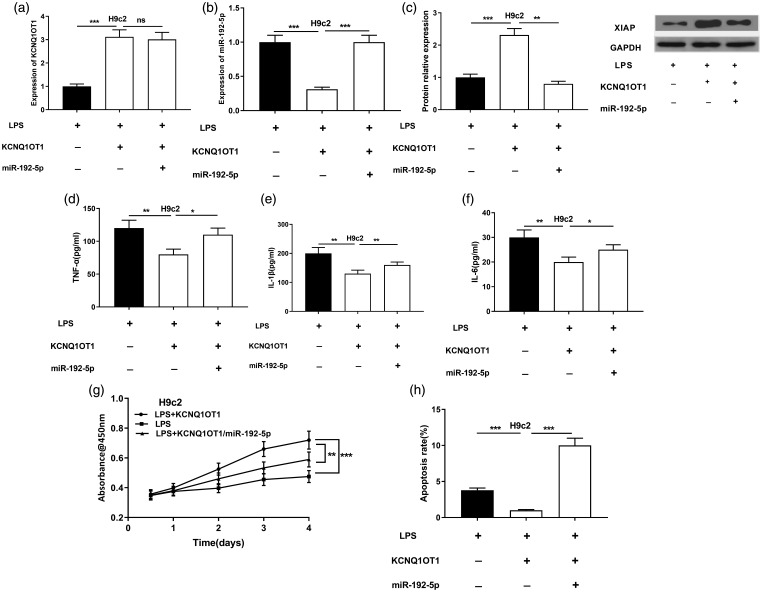
MiR-192-5p reversed the effects mediated by KCNQ1OT1 on H9c2 cells
To further verify that KCNQ1OT1 can function in regulating miR-192-5p in SIC, miR-192-5p mimics was transfected into KCNQ1OT1 overexpressed cells, and qRT-PCR demonstrated that miR-192-5p mimics had no evident influence on KCNQ1OT1 expressions in LPS-induced H9c2 cells (Figure 6(a) and (b)). The expression level of XIAP was greatly ascended by KCNQ1OT1 overexpression but this effect was reversed by miR-192-5p mimics (Figure 6(c)). TNF-α, IL-1β, and IL-6 expressions in culture supernatant of H9c2 cells were remarkably up-regulated after transfection of miR-192-5p mimics (Figure 6(d) to (f)). Additionally, cell proliferation and apoptosis experiments revealed that against as the LPS group, the cell proliferation of the overexpression group of KCNQ1OT1 was considerably enhanced, and the apoptosis was greatly down-regulated, but these phenotypes were partly reversed by co-transfection of miR-192-5p (Figure 6(g) and (h)). Collectively, it implied that miR-192-5p and XIAP were crucial downstream effectors of KCNQ1OT1 during the process of SIC.
Figure 6.
miR-192-5p reversed the effects mediated by KCNQ1OT1 on H9c2 cardiomyocytes. (a) qRT-PCR was operated to delve into KCNQ1OT1 expressions in H9c2 cells. (b) qRT-PCR detected miR-192-5p expressions in H9c2 cells. (c) Western blot was performed to evaluate the changes of XIAP in H9c2 cells. (d–f) ELISA was employed to detect TNF-α, IL-1β and IL-6 expressions. (g) The proliferation of H9c2 cells treated with LPS was detected by CCK-8 method. (h) Flow cytometry analysis was adopted to examine the apoptotic rates of H9c2 cardiomyocyte induced by LPS. *, **, *** indicated P < 0.05, P < 0.01, P < 0.001, respectively.
KCNQ1OT1 affects the progression of SIC through miR-192-5p/XIAP axis in vivo
Vector plasmids (100 μL) or KCNQ1OT1 overexpression plasmids were injected into rats via the tail vein. After the rats were treated with LPS, as against the control group, qRT-PCR results showed that the SIC + KCNQ1OT1 group had a marked increase in EF (%) and FS (%) (Figure 7(a) and (b)). There was no marked change in heart rate (Figure 7(c)). MiR-192-5p expression was also markedly declined in myocardial tissue, and KCNQ1OT1 and XIAP were dramatically up-regulated (Figure 7(d) to (f)). Additionally, TNF-α, IL-1β, and IL-6 expressions in the SIC+KCNQ1OT1 group were significantly down-regulated (Figure 7(g)). These in vivo data further suggested KCNQ1OT1 ameliorated the progression of SIC through the miR-192-5p/XIAP axis.
Figure 7.
KCNQ1OT1 attenuated septic myocardial injury of rats via miR-192-5p/XIAP axis. (a–c) Ultrasound system was used to detect the [EF (%)], [FS (%)] and heart rate in the myocardial tissues of rats. (d) qRT-PCR was conducted to observe KCNQ1OT1 expressions in the myocardial tissues of rats. (e) MiR-192-5p expressions in the myocardial tissue of rats were detected with qRT-PCR. (f) XIAP mRNA expressions in the myocardial tissues of rats were detected with qRT-PCR. (g) TNF-α, IL-1β, and IL-6 expressions in the myocardial tissues of rats were detected by ELISA. *, **, *** indicated P < 0.05, P < 0.01, P < 0.001, respectively.
Discussion
Sepsis and SIC are important causes of death in ICU patients, and seriously threaten the health and safety of patients. We observed the function of KCNQ1OT1 in the pathophysiological mechanism of SIC by means of in vivo and in vitro experiments. We found that KCNQ1OT1 was significantly down-regulated in sepsis-mediated myocardial tissue of rats and LPS-induced cardiomyocytes, and the overexpression of KCNQ1OT1 can strikingly facilitate the proliferation and attenuate the apoptosis of cardiomyocytes, suggesting that KCNQ1OT1 can attenuate the sepsis-mediated myocardial injury caused by sepsis.
The pathogenesis of SIC is complex, including changes in hemodynamics, cardiomyocyte metabolism, cardiomyocyte apoptosis, and so on.21,22 Pro-inflammatory cytokines exert a leading effect in the pathogenesis of sepsis.23 TNF-α and IL-1β undergo an autocrine and paracrine inflammatory cascade that activates macrophage secretion of other pro-inflammatory cytokines (such as IL-6, IL-8, and MIF), lipid mediators, reactive oxygen species, and nitrogen, etc.,24 which has a negative impact on myocardial tissue, leading to myocardial sepsis, myocardial injury, and eventually cardiac dysfunction. In addition, inflammatory factors and oxidative stress also promote apoptosis, thus aggravating sepsis-mediated myocardial damage. In this work, we found that TNF-α, IL-1β, and IL-6 expressions were dramatically up-regulated after down-regulating KCNQ1OT1 in H9c2 cells.
Increasing studies have connoted that lncRNA features more prominently in the progress of sepsis. LncRNA regulates the inflammatory response by effectively controlling related proteins. For example, during urogenic sepsis, lncRNA TapSAKI enhances kidney injury.25 There are also reports that high lncRNA NEAT1 expression is linked with high expression of pro-inflammatory cytokines and poor prognosis in patients with sepsis.26 Moreover, lncRNA also includes critical modulators in the progression of SIC. For example, LncRNA HOTAIR facilitates LPS-induced TNF-α expression in rat cardiomyocytes by initiating the NF-κB cascade, thereby facilitating myocardial injury.27 Recently, accumulating researches have denoted that KCNQIOT1 features in the progression of multiple human diseases. For instance, KCNQ1OT1 is up-regulated in acute myeloid leukemia, which is significantly related with poor prognosis of patients.28 Interestingly, investigations have shown that KCNQ1OT1 was up-regulated in diabetes-induced cardiomyopathy, which can significantly accelerate apoptosis in diabetic-induced cardiomyopathy cells.29 In the present work, we uncovered that KCNQ1OT1 was significantly decreased in the SIC model, and the gain-and loss-assay confirmed that KCNQ1OT1 inhibited the progression of sepsis-induced myocardial injury.
It has been found that microRNAs are also crucial regulators in the progression of sepsis. For instance, in spleen macrophages, miR-146a attenuates the organ injury engendered by inflammation and sepsis.30 Magnolol reduces sepsis-induced acute kidney injury by regulating miR-218-5p.20 In the sepsis induced myocardial injury, miRNA is also involved. For example, miR-23b attenuates inflammatory responses by inhibiting TRAF6, and ameliorates sepsis-induced cardiomyopathy by inhibiting apoptosis and preventing NF-κB activation.31 In recent years, miR-192-5p has been linked with many human diseases. Specifically, miR-192-5p is considerably down-regulated in renal biopsy specimens from patients with hypertension.32 Additionally, miR-192-5p expression is greatly declined in oxidative stress-mediated apoptosis, which can aggravate liver injury.33 The current research studied the characteristics of miR-192-5p in the pathophysiological mechanism of SIC. We first validated that miR-192-5p was greatly elevated in septic myocardial tissue and LPS-induced cardiomyocytes. Inhibiting miR-192-5p significantly advanced the survival and attenuated the apoptosis of cardiomyocytes, suggesting that miR-192-5p can aggravate sepsis-mediated myocardial injury caused by LPS.
Recently, it has been reported that lncRNAs interact with microRNAs and inhibit the effect of microRNAs via a “sponge” mechanism. For example, in sepsis, lncRNA NEAT1 can enhance the function of immunosuppression of septic mice by targeting miR-125 to increase the expression of MCEMP1.34 Furthermore, LncRNA HOTAIR can inhibit renal cell apoptosis by modulating the miR-34a/Bcl-2 axis, thereby attenuating acute kidney injury in septic rats.35 Additionally, lncRNA MALAT1 exacerbates inflammatory response and cardiac dysfunction in sepsis through sponging miR-125b to modulate the p38MAPK/NFκB signaling.36 Additionally, it is reported that KCNQ1OT1 accelerates the development of diabetic retinopathy by targeting miR-1470.37 We supposed that KCNQ1OT1 might also be a ceRNA, so we worked out the potential interaction of KCNQ1OT1 with miR-192-5p. Our data denoted that KCNQ1OT1 can bind to miR-192-5p, and functional experiments demonstrated that KCNQ1OT1 was negatively interrelated with miR-192-5p expressions. Transfection of miR-192-5p mimics could reverse the role of KCNQ1OT1 on promoting proliferation and inhibition of apoptosis of H9c2 cells. These data demonstrate that KCNQ1OT1 may be a miR-192-5p sponge in SIC.
XIAP is a modulator in cell viability, apoptosis, and inflammatory responses. For example, studies have demonstrated that CCN1 promoted FasL-induced apoptosis of H9c2 cardiomyocytes by disrupting XIAP.38 Interestingly, it is reported that Urocortin-1 prevented the heart from ischemia and reperfusion injury by increasing cell survival and stimulating the apoptosis-related genes CD40lg, XIAP, and BAD.39 Recently, studies have shown that the interaction of miRNAs and their target genes can affect the progression of human diseases. For example, miR-146a prohibits against myocardial ischaemia reperfusion injury via targeting Med1.40 Moreover, in ethanol-induced cardiomyocytes, miR-186-5p is highly expressed and modulates apoptosis through targeting XIAP.41 Herein, our data indicated that miR-192-5p targeted XIAP. It was also manifested that XIAP expression was dramatically declined after transfection of miR-192-5p mimics, and XIAP was greatly up-regulated after transfection of its inhibitors. Eventually, we constructed a SIC model of rats with KCNQ1OT1 overexpressed, and validated that KCNQ1OT1 regulated cardiomyocyte viability, apoptosis, and inflammatory responses via the miR-192-5p/XIAP axis.
In conclusion, our study proved that KCNQ1OT1 was down-regulated in SIC tissues and cells. Functional experiments confirmed that KCNQ1OT1 was a valuable and promising therapeutic target to attenuate SIC by modulating miR-192-5p and XIAP.
Supplemental Material
Supplemental material, EBM908041 Supplemental Material for LncRNA KCNQ1OT1 attenuates sepsis-induced myocardial injury via regulating miR-192-5p/XIAP axis by Fangyuan Sun, Weifang Yuan, Hao Wu, Gang Chen, Yuxia Sun, Lin Yuan, Wei Zhang and Ming Lei in Experimental Biology and Medicine
Authors’ contributions
FYS and ML designed the experiments; WY, HW, WQW, GC, YXS, LY, WZ, and YLW performed the experiments; WY did the statistical analysis; WY and ML wrote the paper. The final manuscript has the approval of all authors.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Data availability statement
Data supporting the results of the survey can be obtained from the appropriate authors on request.
Ethics statement
Our study was given the green light by the ethics review board of our hospital.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Budget Project of Shanghai University of Traditional Chinese Medicine(18LK068), the Outstanding Clinical Discipline Project of Shanghai Pudong (PWYgy2018-01), the Science and Technology Development Fund of Shanghai Pudong (PKJ2019-Y16), the Talents Training Program of Seventh People’s Hospital of Shanghai University of Traditional Chinese Medicine (XX2018-02).
ORCID iD
SUPPLEMENTAL MATERIAL
Supplemental material for this article is available online.
References
- 1.Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, Angus DC, Rubenfeld GD, Singer M, Sepsis Definitions Task Force. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 315:775–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hochstadt A, Meroz Y, Landesberg G. Myocardial dysfunction in severe sepsis and septic shock: more questions than answers? J Cardiothorac Vasc Anesth 2011; 25:526–35 [DOI] [PubMed] [Google Scholar]
- 3.Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell 2013; 152:1298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell 2009; 136:629–41 [DOI] [PubMed] [Google Scholar]
- 5.Zhou Z, Zhu Y, Gao G, Zhang Y. Long noncoding RNA SNHG16 targets miR-146a-5p/CCL5 to regulate LPS-induced WI-38 cell apoptosis and inflammation in acute pneumonia. Life Sci 2019; 228:189–97 [DOI] [PubMed] [Google Scholar]
- 6.Gao JR, Qin XJ, Jiang H, Gao YC, Guo MF, Jiang NN. Potential role of lncRNAs in contributing to pathogenesis of chronic glomerulonephritis based on microarray data. Gene 2018; 643:46–54 [DOI] [PubMed] [Google Scholar]
- 7.Jia Y, Li Z, Cai W, Xiao D, Han S, Han F, Bai X, Wang K, Liu Y, Li X, Guan H, Hu D. SIRT1 regulates inflammation response of macrophages in sepsis mediated by long noncoding RNA. Biochim Biophys Acta Mol Basis Dis 2018; 1864:784–92 [DOI] [PubMed] [Google Scholar]
- 8.Dai Y, Liang Z, Li Y, Li C, Chen L. Circulating long noncoding RNAs as potential biomarkers of sepsis: a preliminary study. Genet Test Mol Biomarkers 2017; 21:649–57 [DOI] [PubMed] [Google Scholar]
- 9.Gu H, Li Z, Lv XF, Zhao AB, Zhu MY, Zhang Y. KCNQ1OT1 delayed fracture healing through the Wnt/β-catenin pathway. Eur Rev Med Pharmacol Sci 2019; 23:4575–83 [DOI] [PubMed] [Google Scholar]
- 10.Li X, Dai Y, Yan S, Shi Y, Han B, Li J, Cha L, Mu J. Down-regulation of KCNQ1OT1 protects against myocardial ischemia/reperfusion injury following acute myocardial infarction. Biochem Biophys Res Commun 2017; 491:1026–33 [DOI] [PubMed] [Google Scholar]
- 11.Sayed D, Abdellatif M. MicroRNAs in development and disease. Physiol Rev 2011; 91:827–87 [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Zhang P, Chen W, Feng D, Jia Y, Xie L. Serum microRNA signatures identified by solexa sequencing predict sepsis patients’ mortality: a prospective observational study. PLoS One 2012; 7:e38885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasilescu C, Dragomir M, Tanase M, Giza D, Purnichescu-Purtan R, Chen M, Yeung SJ, Calin GA. Circulating miRNAs in sepsis-A network under attack: an in-silico prediction of the potential existence of miRNA sponges in sepsis. PLoS One 2017; 12:e0183334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye M, Zhang J, Zhang J, Miao Q, Yao L, Zhang J. Curcumin promotes apoptosis by activating the p53-miR-192-5p/215-XIAP pathway in non-small cell lung cancer. Cancer Lett 2015; 357:196–205 [DOI] [PubMed] [Google Scholar]
- 15.Ji D, Jiang L, Li Y. MiR-192-5p suppresses the growth of bladder cancer cells via targeting yin yang 1. Hum Cell 2018; 31:210–9 [DOI] [PubMed] [Google Scholar]
- 16.Srinivasula SM, Hegde R, Saleh A, Datta P, Shiozaki E, Chai J, Lee RA, Robbins PD, Fernandes-Alnemri T, Shi Y, Alnemri ES. A conserved XIAP-interaction motif in caspase-9 and SMAC/DIABLO regulates caspase activity and apoptosis. Nature 2001; 410:112–6 [DOI] [PubMed] [Google Scholar]
- 17.Rijal D, Ariana A, Wight A, Kim K, Alturki NA, Aamir Z, Ametepe ES, Korneluk RG, Tiedje C, Menon MB, Gaestel M, McComb S, Sad S. Differentiated macrophages acquire a pro-inflammatory and cell death-resistant phenotype due to increasing XIAP and p38-mediated inhibition of RipK1. J Biol Chem 2018; 293:11913–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H, Li H, Shaikh A, Caudle Y, Yao B, Yin D. Inhibition of MicroRNA-23b attenuates immunosuppression during late sepsis through NIK, TRAF1, and XIAP. J Infect Dis 2018; 218:300–11 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Wang SM, Liu GQ, Xian HB, Si JL, Qi SX, Yu YP. LncRNA NEAT1 alleviates sepsis-induced myocardial injury by regulating the TLR2/NF-κB signaling pathway. Eur Rev Med Pharmacol Sci 2019; 23:4898–907 [DOI] [PubMed] [Google Scholar]
- 20.Zhang T, Xiang L. Honokiol alleviates sepsis-induced acute kidney injury in mice by targeting the miR-218-5p/heme oxygenase-1 signaling pathway. Cell Mol Biol Lett 2019; 24:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y-C, Yu M-M, Shou S-T, Chai Y-F. Sepsis-Induced cardiomyopathy: mechanisms and treatments. Front Immunol 2017; 8:1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Datta D, Grahamslaw J, Gray AJ, Graham C, Walker CA. Lactate-arterial and venous agreement in sepsis: a prospective observational study. Eur J Emerg Med 2018; 25:85–91 [DOI] [PubMed] [Google Scholar]
- 23.Kakihana Y, Ito T, Nakahara M, Yamaguchi K, Yasuda T. Sepsis-induced myocardial dysfunction: pathophysiology and management. J Intensive Care 2016; 4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis DH, Chan DL, Pinheiro D, Armitage-Chan E, Garden OA. The immunopathology of sepsis: pathogen recognition, systemic inflammation, the compensatory anti-inflammatory response, and regulatory T cells. J Vet Intern Med 2012; 26:457–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen J, Liu L, Zhang F, Gu J, Pan G. LncRNA TapSAKI promotes inflammation injury in HK-2 cells and urine derived sepsis-induced kidney injury. J Pharm Pharmacol 2019; 71:839–48 [DOI] [PubMed] [Google Scholar]
- 26.Huang Q, Huang C, Luo Y, He F, Zhang R. Circulating lncRNA NEAT1 correlates with increased risk, elevated severity and unfavorable prognosis in sepsis patients. Am J Emerg Med 2018; 36:1659–63 [DOI] [PubMed] [Google Scholar]
- 27.Wu H, Liu J, Li W, Liu G, Li Z. LncRNA-HOTAIR promotes TNF-α production in cardiomyocytes of LPS-induced sepsis mice by activating NF-κB pathway. Biochem Biophys Res Commun 2016; 471:240–6 [DOI] [PubMed] [Google Scholar]
- 28.Jia ZW, Li Y, Cui GR, Zhao HB, Li PY, Luo JM. Expression and clinical significance of KCNQ1OT1 in patients with acute myeloid leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2018; 26:653–7 [DOI] [PubMed] [Google Scholar]
- 29.Yang F, Qin Y, Wang Y, Li A, Lv J, Sun X, Che H, Han T, Meng S, Bai Y, Wang L. KCNQ1OT1 mediates pyroptosis in diabetic cardiomyopathy. Cell Physiol Biochem 2018; 50:1230–44 [DOI] [PubMed] [Google Scholar]
- 30.Funahashi Y, Kato N, Masuda T, Nishio F, Kitai H, Ishimoto T, Tsuboi N, Matsuda N, Maruyama S, Kadomatsu K. miR-146a targeted to splenic macrophages prevents sepsis-induced multiple organ injury. Lab Invest 2019; 99:1130–42 [DOI] [PubMed] [Google Scholar]
- 31.Cao C, Zhang Y, Chai Y, Wang L, Yin C, Shou S, Jin H. Attenuation of Sepsis-Induced cardiomyopathy by regulation of MicroRNA-23b is mediated through targeting of MyD88-Mediated NF-κB activation. Inflammation 2019; 42:973–86 [DOI] [PubMed] [Google Scholar]
- 32.Baker MA, Wang F, Liu Y, Kriegel AJ, Geurts AM, Usa K, Xue H, Wang D, Kong Y, Liang M. MiR-192-5p in the kidney protects against the development of hypertension. Hypertension 2019; 73:399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roy S, Benz F, Alder J, Bantel H, Janssen J, Vucur M, Gautheron J, Schneider A, Schüller F, Loosen S, Luedde M, Koch A, Tacke F, Luedde T, Trautwein C, Roderburg C. Down-regulation of miR-192-5p protects from oxidative stress-induced acute liver injury. Clin Sci 2016; 130:1197–207 [DOI] [PubMed] [Google Scholar]
- 34.Chen JX, Xu X, Zhang S. Silence of long noncoding RNA NEAT1 exerts suppressive effects on immunity during sepsis by promoting microRNA-125-dependent MCEMP1 downregulation. IUBMB Life 2019; 71:956–68 [DOI] [PubMed] [Google Scholar]
- 35.Jiang ZJ, Zhang MY, Fan ZW, Sun WL, Tang Y. Influence of lncRNA HOTAIR on acute kidney injury in sepsis rats through regulating miR-34a/bcl-2 pathway. Eur Rev Med Pharmacol Sci 2019; 23:3512–9 [DOI] [PubMed] [Google Scholar]
- 36.Chen H, Wang X, Yan X, Cheng X, He X, Zheng W. LncRNA MALAT1 regulates sepsis-induced cardiac inflammation and dysfunction via interaction with miR-125b and p38 MAPK/NFκB. Int Immunopharmacol 2018; 55:69–76 [DOI] [PubMed] [Google Scholar]
- 37.Shao J, Pan X, Yin X, Fan G, Tan C, Yao Y, Xin Y, Sun C. KCNQ1OT1 affects the progression of diabetic retinopathy by regulating miR-1470 and epidermal growth factor receptor. J Cell Physiol 2019; 234:17269–79 [DOI] [PubMed] [Google Scholar]
- 38.Chen C, Liu TS, Zhao SC, Yang WZ, Chen ZP, Yan Y. XIAP impairs mitochondrial function during apoptosis by regulating the bcl-2 family in renal cell carcinoma. Exp Ther Med 2018; 15:4587–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calderón-Sánchez E, Díaz I, Ordóñez A, Smani T. Urocortin-1 mediated cardioprotection involves XIAP and CD40-Ligand recovery: role of EPAC2 and ERK1/2. PLoS One 2016; 11:e0147375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Huang R, Zhou W, Zhao Q, Lü Z. miR-192-5p mediates hypoxia/reoxygenation-induced apoptosis in H9c2 cardiomyocytes via targeting of FABP3. J Biochem Mol Toxicol 2017;31:e21873 [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Yu B. MicroRNA‑186‑5p is expressed highly in ethanol‑induced cardiomyocytes and regulates apoptosis via the target gene XIAP. Mol Med Rep 2019; 19:3179–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, EBM908041 Supplemental Material for LncRNA KCNQ1OT1 attenuates sepsis-induced myocardial injury via regulating miR-192-5p/XIAP axis by Fangyuan Sun, Weifang Yuan, Hao Wu, Gang Chen, Yuxia Sun, Lin Yuan, Wei Zhang and Ming Lei in Experimental Biology and Medicine
Data Availability Statement
Data supporting the results of the survey can be obtained from the appropriate authors on request.