Abstract
Background:
Dilated cardiomyopathy (DCM) in children is an important cause of severe heart failure and carries a poor prognosis. Adults with heart failure are at increased risk of anxiety and depression and such symptoms predict adverse clinical outcomes such as mortality. In children with DCM, studies examining these associations are scarce.
Aims:
We studied whether in children with DCM: (1) the level of emotional and behavioral problems was increased as compared to normative data, and (2) depressive and anxiety problems were associated with the combined risk of death or cardiac transplantation.
Methods:
To assess emotional and behavioral problems in children with DCM, parents of 68 children, aged 1.5–18 years (6.9±5.7 years), completed the Child Behavior Checklist.
Results:
Compared to normative data, more young children (1.5–5 years) with DCM had somatic complaints (24.3% vs. 8.0%; p < .001), but fewer had externalizing problems (5.4% vs. 17.0%; p = .049). Overall internalizing problems did not reach significance. Compared to normative data, more older children (6–18 years) showed internalizing problems (38.7% vs. 17.0%; p = .001), including depressive (29.0% vs. 8.0%; p < .001) and anxiety problems (19.4% vs. 8.0%; p = .023), and somatic complaints (29.0% vs. 8.0%; p < .001). Anxiety and depressive problems, corrected for heart failure severity, did not predict the risk of death or cardiac transplantation.
Conclusion:
Children of 6 years and older showed more depressive and anxiety problems than the normative population. Moreover, in both age groups, somatic problems were common. No association with outcome could be demonstrated.
Keywords: Dilated cardiomyopathy, pediatrics, heart failure, emotional and behavioral problems, psychosocial support
Introduction
Cardiomyopathies are disorders characterized by structural and functional abnormalities of the heart. The most common subtype in children is dilated cardiomyopathy (DCM), accounting for approximately 60% of pediatric cardiomyopathies.1,2 DCM, which is characterized by impaired systolic function and dilation of the left ventricle,3 is estimated to affect 0.57 per 100,000 children annually.4 Though disease presentation can vary greatly, 80% of patients show symptoms related to heart failure, such as fatigue, orthopnea, edema, and excessive sweating. Symptoms can also include circulatory collapse, arrhythmias, thromboembolic events, and sudden death.5 Although some children recover,6 the prognosis of DCM generally is poor: within 2 years after diagnosis, approximately 40% of children die or undergo cardiac transplantation,4,6–8 making DCM the leading indication for cardiac transplantation worldwide.9–11 Considering the symptoms and prognosis of DCM, substantial effects on psychosocial wellbeing can be expected.12
Compelling evidence from two meta-analyses shows that adults with heart failure are at increased risk of anxiety and depression.13,14 Few studies have examined the psychosocial wellbeing of children with DCM, but, indeed, it has been found that children with DCM have a lower health-related quality of life (HRQoL) than healthy children.12,15–18 However, studies examining emotional and behavioral problems in children with DCM are scarce. Moreover, the currently available studies have small sample sizes (n ⩽ 15) and show contradictory results. In a cross-sectional study, half (n = 6 out of 12) of children with cardiomyopathy listed for cardiac transplantation showed clinically significant overall emotional and behavioral problems.19 In contrast, a study examining depressive symptoms in children with DCM (n = 15) did not find higher rates of symptoms compared with healthy children.20 However, it should be noted that these studies used different questionnaires.
Regarding the impact of impaired HRQoL on cardiac outcomes, two studies have shown that children’s physical HRQoL (reported by parents) predicts mortality and cardiac transplantation, independent from heart failure severity.15,17 Also, a meta-analysis and reviews have consistently found that depressive and anxiety symptoms in adults with heart failure predict mortality and other adverse clinical outcomes,13,21,22 such as hospitalization and arrhythmias. However, to the best of our knowledge, the predictive value of depressive and anxiety symptoms in children with DCM has not been previously studied. Information on the predictive value of depressive and anxiety symptoms may be valuable for clinical management strategies. Depressive and anxiety problems may lead to poorer self-care and, in turn, to disease progression.22
The aim of the present study was twofold: firstly, we evaluated the level of parent-reported emotional and behavioral problems in children with DCM compared with the general population. Secondly, we exploratively examined whether the level of parent-reported anxiety and depressive problems predicted the combined risk of death and cardiac transplantation whilst controlling for heart failure severity. Based on the aforementioned adult studies, we hypothesized that children with DCM would show more anxiety and depressive problems than children in the general population. Moreover, we hypothesized that anxiety and depressive problems would predict mortality in children with DCM independent from heart failure severity.
Material and methods
All data used in this observational, cross-sectional study were derived from a larger multicenter longitudinal study in children with heart failure secondary to cardiomyopathy.15 The study protocol was approved by the Medical Ethics Committee of the Erasmus Medical Center (protocol number NL45663.078) and by the institutional review boards of all participating centers. The study performed conformed to the ethical guidelines of the Declaration of Helsinki,23 and reported following the STROBE statement. Before participation, written informed consent was obtained from all patients’ parents or legal guardians and from all patients aged 12 years or above.
Participants
Participants were recruited from October 1, 2010 to November 1, 2015 through seven tertiary centers for pediatric cardiology in the Netherlands. The database was closed on July 1, 2017. Children were eligible to participate if they had heart failure secondary to DCM. DCM was defined as fractional shortening ⩽25% and left ventricular end-diastolic dimension z-score > 2 for body surface area. DCM could be idiopathic or secondary to other causes. Exclusion criteria were known mental retardation, congenital heart disease, neuromuscular disease, and insufficient mastery of the Dutch language by parents. In the current study, we only included 1.5–18-year-old children due to age restrictions of the used questionnaire. Since age and gender-matched normative data on emotional and behavioral problems is available, we did not recruit a healthy control group.24
Procedure
Children were either included at DCM diagnosis or were included at an outpatient appointment for a previously diagnosed DCM in one of the participating tertiary pediatric cardiology centers. Demographic variables were obtained at inclusion. Socioeconomic status was based on the highest of both parents’ occupations and categorized into low, low to middle, middle, or high according to the international classification system.25 Parents were asked to complete a questionnaire assessing their child’s emotional and behavioral problems during an outpatient clinic visit. During the same visit, a pediatric cardiologist completed the New York University Pediatric Heart Failure Index (NYU PHFI).26 This validated index assesses heart failure severity based on symptoms and medication use. Scores range from 0 to 30. A higher score indicates more severe heart failure.
Emotional and behavioral problems
One of each participant’s parents completed the problem section of the Child Behavior Checklist (CBCL).27 Depending on the child’s age, the CBCL 1½–5 (100 items; children aged 1.5–5 years) or the CBCL/6–18 (120 items; children aged 6–18 years) was completed. For both versions, response categories range from 0 (not true) to 2 (very true or often true). The CBCL assesses overall emotional and behavioral problems and specific aspects of mental health and problem behavior. In addition to an overall total problem score, broadband scale scores can be calculated for externalizing problems (i.e. externally directed problems affecting the environment, such as aggression and delinquency) and internalizing problems (i.e. internally directed problems such as depression, anxiety, and somatic complaints). Furthermore, the CBCL 1½–5 consists of five scales based on the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5; i.e. depressive problems, anxiety problems, attention deficit/hyperactivity problems, oppositional defiant problems, and autism spectrum problems). In addition, seven empirical scales can be calculated (i.e. anxious/depressed, somatic complaints, attention problems, aggressive behavior, emotionally reactive, withdrawn, and sleep problems). The CBCL/6–18 consists of six DSM-5 based scales (i.e. depressive symptoms, anxiety problems, somatic problems, attention deficit/hyperactivity problems, oppositional defiant problems, and conduct problems) and eight empirical scales (i.e. anxious/depressed, withdrawn/depressed, somatic complaints, social problems, thought problems, attention problems, rule-breaking behavior, and aggressive behavior). On all scales, higher scores indicate more problems. For each scale, scores can be interpreted as falling in the normal, borderline, or clinical range by comparing scale scores with norm data. Scores in the borderline or clinical range indicate psychopathological problems with a need for clinical follow-up and/or intervention. The CBCL has adequate psychometric properties and normative data from the Dutch general population are available.24
Endpoint
We used a combined endpoint of death and cardiac transplantation. Information on mortality and cardiac transplantation was retrieved from patient records. Follow-up was censored at July 1, 2017.
Statistical analyses
Firstly, we examined whether the proportion of children scoring in the borderline or clinical range of emotional and behavioral problems was larger in our DCM study population than in the general population. All raw scale scores were converted to percentiles using the Achenbach System of Empirically Based Assessment Standard norm data, which is based on data of Dutch children from the general population and accounts for age and gender.27 Conforming with the CBCL manual,27 for the total problems scale, internalizing problems scale, and externalizing problems scale, percentile scores of 83 or lower were defined as non-clinical and percentile scores of 84 or higher were defined as borderline/clinical. For the DSM scales and empirical scales, percentile scores of 92 or lower were defined as non-clinical and percentile scores of 93 or higher were defined as borderline/clinical. One sample binomial tests were conducted for each scale of the CBCL 1½–5 and the CBCL/6–18 to test whether the proportion of children with DCM scoring in the borderline/clinical range was higher than the proportion in the norm group.
Secondly, we conducted a Cox regression analysis to examine whether anxiety and depressive problems predicted the combined endpoint of death and cardiac transplantation whilst controlling for heart failure severity. The covariates entered into the model were the CBCL scale scores for anxiety problems and depressive problems and the NYU PHFI. We used t-scores to account for differences between the two versions of the CBCL (i.e. CBCL 1½–5 and CBCL/6-18) anxiety problems and depressive problems scale scores. The NYU PHFI was added to the model because heart failure severity is a known predictor of mortality.15 All analyses were performed using SPSS Statistics version 24.0.28
Results
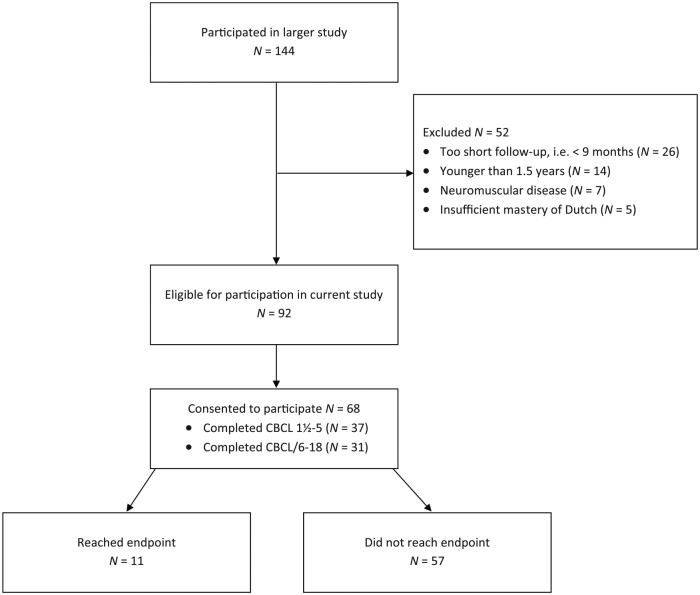
In total, 144 children with DCM participated in the larger multicenter longitudinal study in children with heart failure secondary to cardiomyopathy from which data for the current study was derived. Of this group, 52 children were excluded from participation in the current study (N = 26 had a too short follow-up period to fill out the CBCL, N = 14 were younger than 1.5 years, N = 7 had a neuromuscular disease, N = 5 did not master the Dutch language sufficiently). Therefore, 92 children met the eligibility criteria for the current study, 68 of whom consented to participate in the current study (see Figure 1). Participant characteristics are presented in Table 1.
Figure 1.
Participation flowchart.
Table 1.
Participant characteristics.
| Characteristic | Overall group (N = 68) |
Did not reach endpoint (N = 57) |
Reached endpoint (N = 11) |
|||
|---|---|---|---|---|---|---|
| 1.5–5 years (N = 37) | 6–18 years (N = 31) | 1.5–5 years (N = 31) | 6–18 years (N = 26) | 1.5–5 years(N = 6) | 6–18 years (N = 5) | |
| Male gender, N (%) | 20 (54.1%) | 17 (54.8%) | 18 (58.1%) | 16 (61.5%) | 2 (33.3%) | 1 (20.0%) |
| Age in years, M (SD) | 2.2 (1.3) | 12.4 (3.5) | 2.0 (0.5) | 12.5 (3.6) | 3.3 (1.2) | 11.8 (3.4) |
| Time since DCM diagnosis in months, median (IQR) | 19.0 (12.0–36.0) | 57.0 (24.0–107.0) | 18.0 (12.0–24.0) | 58.5 (27.0–110.0) | 40.5 (24.5–59.0) | 24.0 (17.0–73.5) |
| NYU PHFI, M (SD) | 6.3 (4.8) | 7.2 (3.8) | 4.8 (3.2) | 5.5 (3.1) | 14.2 (3.9) | 12.6 (2.1) |
| Socioeconomic statusa, N (%) | ||||||
| Low | 1 (2.7%) | 2 (6.5%) | 1 (3.2%) | 2 (7.7%) | 0 (0.0%) | 0 (0.0%) |
| Low to middle | 10 (27.0%) | 10 (32.3%) | 8 (25.8%) | 10 (38.5%) | 2 (33.3%) | 0 (0.0%) |
| Middle | 3 (8.1%) | 6 (19.4%) | 3 (9.7%) | 6 (23.1%) | 0 (0.0%) | 0 (0.0%) |
| High | 16 (43.2%) | 10 (32.3%) | 13 (41.9%) | 5 (19.2%) | 3 (50.0%) | 5 (100.0%) |
| Missing | 7 (18.9%) | 3 (9.7%) | 6 (19.4%) | 3 (11.5%) | 1 (16.7%) | 0 (0.0%) |
IQR = Interquartile range; NYU PHFI = New York University Pediatric Heart Failure Index.
Socioeconomic status was determined by parents’ occupation level.25
Emotional and behvaioral problems compared with the general population
1.5- to 5-year-old children (CBCL 1½–5)
The proportion of parent-reported emotional and behavioral problems in 1.5- to 5-year-old children with DCM and children in the norm group is shown in Table 2. The CBCL 1½–5 was completed for 37 participants (by N = 9 fathers, N = 25 mothers, N = 3 parents together) at a median time of 19.0 months after DCM diagnosis (range 10.0–65.0 months; see Table 1). Compared with the normative data of same-aged children (8.0% borderline or clinical score), a significantly larger proportion of children with DCM (24.0% borderline or clinical score) showed somatic complaints in the borderline or clinical range, p < .001. In contrast, the proportion of children showing a borderline or clinical level of externalizing problems was significantly smaller in the DCM study group (5.4% clinical or borderline score) than in the general population (17.0% borderline or clinical score), p = .049.
Table 2.
Distribution of non-clinical versus borderline/clinical emotional and behavioral problems reported by parents of 1.5- to 5-year-old children (CBCL 1½–5).
| CBCL 1½–5 scale | DCM patients (N = 37)a |
General population |
p-value | ||
|---|---|---|---|---|---|
| Non-clinical, N (%) | Borderline/clinical, N (%) | Non-clinical % | Borderline/clinical % | ||
| Broadband scales | |||||
| Internalizing problems | 29 (78.4%) | 8 (21.6%) | 83% | 17% | .298 |
| Externalizing problems | 35 (94.6%) | 2 (5.4%) | 83% | 17% | .049 |
| Total problems | 31 (83.8%) | 6 (16.2%) | 83% | 17% | .500 |
| Syndrome scales | |||||
| Anxious/depressed | 36 (97.3%) | 1 (2.7%) | 92% | 8% | .188 |
| Somatic complaints | 28 (75.7%) | 9 (24.3%) | 92% | 8% | < .001 |
| Attention problems | 36 (97.3%) | 1 (2.7%) | 92% | 8% | .188 |
| Aggressive behavior | 35 (94.6%) | 2 (5.4%) | 92% | 8% | .390 |
| Emotionally reactive | 31 (83.8%) | 6 (16.2%) | 92% | 8% | .062 |
| Withdrawn | 33 (89.2%) | 4 (10.8%) | 92% | 8% | .372 |
| Sleep problems | 34 (91.9%) | 3 (8.1%) | 92% | 8% | .500 |
| DSM-oriented scales | |||||
| Depressive problems | 31 (83.8%) | 6 (16.2%) | 92% | 8% | .062 |
| Anxiety problems | 32 (86.5%) | 5 (13.5%) | 92% | 8% | .175 |
| Attention deficit/hyperactivity problems | 37 (100%) | 0 (0%) | 92% | 8% | .068 |
| Oppositional defiant problems | 36 (97.3%) | 1 (2.7%) | 92% | 8% | .188 |
| Autism spectrum problems | 34 (91.9%) | 3 (8.1%) | 92% | 8% | .500 |
Reported by fathers (N = 9), mothers (N = 25), or both parents together (N = 3).
For the other scales, the proportions of borderline and clinical problems in children with DCM and children from the general population did not significantly differ. However, trends towards significance were found for more emotionally reactive (p = .062) and depressive problems (p = .062), and less attention deficit/hyperactivity problems (p = .068).
Six- to 18-year-old children (CBCL/6–18)
The distribution of parent-reported emotional and behavioral problems in 6- to 18-year-old children with DCM and children from the general population is shown in Table 3. The CBCL/6–18 was completed for 31 children (by N = 5 fathers, N = 23 mothers, N = 3 parents together) at a median time of 39.0 months after DCM diagnosis (range 12.0–177.0 months; see Table 1). Compared with normative data of same-aged peers, significantly larger proportions of children with DCM showed problems in the borderline or clinical range on the following scales: internalizing problems (p = .001; 17.0% vs. 38.7%), anxious/depressed problems (p = .023; 8.0% vs. 19.4%), somatic complaints (p < .001; 8.0% vs. 29.0%), depressive problems (p < .001; 8.0% vs. 29.0%), anxiety problems (p = .023; 8.0% vs. 19.4%), and somatic problems (p < .001; 8.0% vs. 25.8%). For the other scales, the proportion of borderline and clinical problems did not significantly differ between children with DCM and children from the general population.
Table 3.
Distribution of non-clinical versus borderline/clinical emotional and behavioral problems reported by parents of 6- to 18-year-old children (CBCL/6–18).
| CBCL/6–18 scale | DCM patients (N = 31)a |
General population |
p-value | ||
|---|---|---|---|---|---|
| Non-clinical, N (%) | Borderline/clinical, N (%) | Non-clinical % | Borderline/clinical % | ||
| Broadband scales | |||||
| Internalizing problems | 19 (61.3%) | 12 (38.7%) | 83% | 17% | .001 |
| Externalizing problems | 28 (90.3%) | 3 (9.7%) | 83% | 17% | .199 |
| Total problems | 26 (83.8%) | 5 (16.1%) | 83% | 17% | .500 |
| Syndrome scales | |||||
| Anxious/depressed | 25 (80.6%) | 6 (19.4%) | 92% | 8% | .023 |
| Withdrawn/depressed | 28 (90.3%) | 3 (9.7%) | 92% | 8% | .495 |
| Somatic complaints | 22 (71.0%) | 9 (29.0%) | 92% | 8% | < .001 |
| Social problems | 28 (90.3%) | 3 (9.7%) | 92% | 8% | .495 |
| Thought problems | 26 (83.9%) | 5 (16.1%) | 92% | 8% | .091 |
| Attention problems | 27 (87.1%) | 4 (12.9%) | 92% | 8% | .250 |
| Rule breaking behavior | 31 (100%) | 0 (0%) | 92% | 8% | .095 |
| Aggressive behavior | 29 (93.5%) | 2 (6.5%) | 92% | 8% | .500 |
| DSM-oriented scales | |||||
| Depressive problems | 22 (71.0%) | 9 (29.0%) | 92% | 8% | < .001 |
| Anxiety problems | 25 (80.6%) | 6 (19.4%) | 92% | 8% | .023 |
| Somatic problems | 23 (74.2%) | 8 (25.8%) | 92% | 8% | < .001 |
| Attention deficit/hyperactivity problems | 28 (90.3%) | 3 (9.7%) | 92% | 8% | .495 |
| Oppositional defiant problems | 28 (90.3%) | 3 (9.7%) | 92% | 8% | .495 |
| Conduct problems | 29 (93.5%) | 2 (6.5%) | 92% | 8% | .500 |
Reported by fathers (N = 5), mothers (N = 23), or both parents together (N = 3).
Predictive value of anxiety and depressive problems on endpoint
We examined whether anxiety and depressive symptoms predicted the combined risk of death or cardiac transplantation whilst controlling for NYU PHFI. The proportional hazard assumptions were not violated. Before July 1, 2017, 11 participants (16.2%) had reached an endpoint. One had died and 10 had undergone cardiac transplantation. The results of the Cox regression analysis are presented in Table 4. Anxiety problems and depressive problems did not significantly predict death or cardiac transplantation. However, the NYU PHFI did significantly predict the risk of death or cardiac transplantation, p < .001. A one unit increase in the NYU PHFI resulted in a 42% higher risk of death or cardiac transplantation (hazard ratio 1.42, 95% confidence interval 1.19–1.69).
Table 4.
Results of Cox regression analysis.
| Variable | HR | 95% CI |
p-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Anxiety Problems (t-score) | 0.98 | 0.89 | 1.09 | .72 |
| Depressive Problems (t-score) | 0.98 | 0.88 | 1.08 | .64 |
| NYU PHFI (per unit) | 1.42 | 1.19 | 1.69 | < .001 |
CI = confidence interval; HR = hazard ratio; NYU PHFI = New York University Pediatric Heart Failure Index.
Discussion
The current study is the first to investigate emotional and behavioral problems in a substantial cohort of children with DCM. Some results are in line with our expectations. Importantly, we found that, compared with normative data of same-aged peers, larger percentages of older children (6–18 years old) with DCM showed overall internalizing problems, anxiety problems, and depressive problems. Also, we found trends towards significance suggesting that, compared with normative data of same-aged peers, larger percentages of younger children (1.5–5 years old) with DCM showed emotionally reactive problems and depressive problems. These results are in line with meta-analyses in adult heart failure populations, which demonstrate an increased risk of anxiety and depression.13,14
Until now, only two studies have examined emotional and behavioral problems in children with DCM. The first study was conducted by Wray and Radley-Smith,19 who found that 50% of the children with cardiomyopathy in their study (N = 19, age 3½–17 years) showed a clinical level of overall emotional and behavioral problems on the CBCL questionnaire. In our study, this percentage was markedly lower (i.e. 10.8% in younger children and 16.1% in older children). This difference may be due to the fact that all children with cardiomyopathy in Wray and Radley-Smith’s study were listed for cardiac transplantation, whereas in our study this was not the case.
In the second study, Menteer et al. compared the level of depressive symptoms in children (aged 7–21 years) with DCM (N = 15), children who had successfully undergone cardiac transplantation for heart failure (N = 23), and healthy children (N = 24).20 In contrast to our results, they found similar levels of depressive symptoms in all groups. That is, the level of depressive symptoms in children with DCM did not significantly differ from the level of depressive symptoms in healthy children and children who had undergone cardiac transplantation. However, it should be noted that Menteer and colleagues used small sample sizes, which limits the statistical power to detect differences between groups. Moreover, this discordance in results may be explained by the fact that we assessed depressive problems through the CBCL questionnaire whereas Menteer et al. used the Children’s Depression Inventory (CDI).29 Although both instruments assess depressive symptoms, previously, moderate correlations between CDI total scores and CBCL depressive problems scores have been found.30
Depressive and anxiety problems in children with DCM may be caused by factors directly or indirectly related to the illness. For example, in other chronic illnesses, it has been shown that the symptoms of the illness itself,31,32 and side effects of medical treatments,33 can provoke anxiety and depressive symptoms. More indirectly, illness uncertainty (i.e. uncertainty regarding prognosis, disease course, and treatment) can increase symptoms of depression and anxiety.31,34 This can be explained by the cognitive coping theory,35 which states that children interpret situations based on previous knowledge and experiences. When such information is lacking, a situation may be interpreted as a threat, which consequently increases symptoms of depression and anxiety.31,36–39 Similarly, medical treatments such as injections may be experienced as distressing and threatening, thereby increasing children’s anxiety levels.31 Furthermore, it is known that parental overprotectiveness can promote anxiety and depressive symptoms in children with a chronic illness.31,34,40 Depressive and anxiety problems may also have a biological cause. In adult and pediatric heart failure populations,41–43 reduced brain tissue volumes have been found in brain areas which regulate mood. Future research is needed to draw definite conclusions as to biological causes of mood problems in DCM.
Besides increased anxiety and depressive problems, we demonstrated that, compared with normative data, a larger percentage of young and older children with DCM showed a borderline or clinical level of somatic problems. This is not surprising considering all children had heart failure problems secondary to DCM. Furthermore, previous studies have reported reduced levels of physical health related quality of life in this population.15
Other results of the current study were unexpected or contrary to our hypotheses. Firstly, we found that, compared with normative data, a smaller percentage of young children with DCM showed a borderline or clinical level of externalizing problems. In line with this result, we found a trend towards significance suggesting that, compared with normative data, a smaller percentage of young children with DCM showed attention deficit/hyperactivity problems. This might be explained by increased levels of fatigue reported in DCM,5 which may contribute to children showing less hyperactive behavior.
Secondly, contrary to our hypothesis, we found that anxiety and depressive problems in children with DCM did not predict the risk of death and cardiac transplantation whilst controlling for heart failure severity. However, in line with the results of a previous DCM study, heart failure severity (NYU PHFI) did predict the risk of death and cardiac transplantation.15 In contrast with our findings, in adult heart failure populations, a multitude of studies have shown that depressive problems predict mortality and other adverse clinical outcomes.13,21,44–48 Furthermore, increasing evidence shows that anxiety problems predict mortality in adult heart failure.22 An explanation for our different findings is that, in adults, depressive and anxiety problems can lead to poorer self-care.49 In children, however, parents may compensate for children’s poorer self-care behaviors, which subsequently diminishes the impact of depressive and anxiety problems on their physical health. Also, it should be noted that statistical power to detect associations was limited.
This study has several strengths. Studies exclusively examining pediatric cardiomyopathy patients are scarce.12 As stated, the current study is the first to examine emotional and behavioral problems in a relatively large cohort of children with DCM. We recruited children with DCM through seven tertiary centers for pediatric cardiology. Also, we investigated problems in a broad age range (1.5–18 years), using an internationally well-validated questionnaire (CBCL) to assess a wide range of emotional and behavioral problems. The multicenter recruitment and the inclusion of a broad age range improve the generalizability of our results in the pediatric DCM population. Moreover, we examined the predictive value of depressive and anxiety problems on mortality and cardiac transplantation whilst controlling for heart failure severity, which is a known predictor of adverse outcomes. Furthermore, results from our study population were compared to representative normative data matched on age and gender.
The results of this study must also be interpreted in light of a few limitations. Firstly, although the study sample is relatively large considering the prevalence of DCM, the number of events in the study was 11, which limits the statistical power of the prediction analyses. Secondly, we only used proxy-reports completed by parents because most participating children were too young to complete the self-report version of the CBCL.50 Of the children who were old enough to complete the self-report version of the CBCL an insufficient number to analyze completed the questionnaire. The use of proxy-reports has been frequently debated. Studies have found that parent proxy-reports of quality of life in pediatric cardiac populations may differ from child self-reports.51,52 In another pediatric cardiac population, Patel and colleagues found that parent–child agreement was stronger for more readily observable variables such as physical functioning and externalizing behavior and lower for variables which tend to be less visible,53 such as anxiety, emotional functioning, and internalizing behavior. In contrast, in a pediatric cardiac population, Marino and colleagues found that parent-proxy reports and child self-reports on quality of life did not differ.54 Moreover, Wilmot and colleagues reported moderate parent–child agreement on quality of life of children with cardiomyopathy.16 Considering the scarcity of research into emotional and behavioral problems in children with DCM, further research is needed using well-attuned self-reports as well. Thirdly, we combined both father and mother reports in our analyses. Although this may induce bias,55 it should be noted that the majority of questionnaires were completed by mothers and previous research has found moderately high inter-parent agreement on the CBCL.56 Fourthly, considering the relatively long period of time between DCM diagnosis and participation in the current study (see Table 1), it should be noted that the current cohort represents children with chronic heart failure. Children who reached an endpoint or recovered shortly after diagnosis are likely underrepresented.
In conclusion, this first study specifically examining emotional and behavioral problems of children with DCM showed, compared with normative data, significantly more borderline or clinical levels of anxiety, depressive problems, and somatic problems in 6–18-year-olds and significantly more borderline or clinical somatic problems and less externalizing problems in 1.5–5-year-olds. These findings demonstrate the importance of including routine screening for internalizing problems to the clinical management of children with DCM,10 and of providing psychosocial support attuned to the needs of these children. Considering the previously mentioned influence of parental behavior on anxiety and depressive symptoms in pediatric chronic illness, such psychosocial support should not only focus on the children themselves but also include their parents. Future research should focus on evidence-based psychosocial programs to treat and prevent internalizing problems in pediatric cardiomyopathy. As the available literature on emotional and behavioral wellbeing in pediatric cardiomyopathy is limited, many aspects remain to be studied. Considering previous adult studies and our findings, future research should focus on anxiety and depression in pediatric DCM. Moreover, as the results of our study show that emotional and behavioral problems in DCM seem to differ per age group, it would be useful to examine this in more age groups. Furthermore, a previous study found that HRQoL in children with DCM was more impaired at diagnosis than more than 1 year after diagnosis.15 Whether this is also the case for emotional and behavioral problems remains to be studied. Also, since little is known about the psychosocial wellbeing of children with cardiomyopathy, future qualitative studies would be valuable.
Footnotes
Declaration of Conflicting Interests: The authors declare that there are no conflicts of interest.
Funding: This work was supported by the Netherlands Heart Foundation/“Stichting Hartedroom” (grant number 2013T087).
Implications for Practice
- Six- to 18-year-olds with dilated cardiomyopathy showed more anxiety problems.
- Six- to 18-year-olds with dilated cardiomyopathy showed more depressive problems.
- Children with dilated cardiomyopathy should be screened for internalizing problems.
- Psychosocial support should be offered to children with dilated cardiomyopathy.
References
- 1. Lipshultz SE, Sleeper LA, Towbin JA, et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med 2003; 348: 1647-1655. [DOI] [PubMed] [Google Scholar]
- 2. Nugent AW, Daubeney PE, Chondros P, et al. The epidemiology of childhood cardiomyopathy in Australia. N Engl J Med 2003; 348: 1639-1646. [DOI] [PubMed] [Google Scholar]
- 3. Jefferies JL, Towbin JA. Dilated cardiomyopathy. Lancet 2010; 375: 752-762. [DOI] [PubMed] [Google Scholar]
- 4. Towbin JA, Lowe AM, Colan SD, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA 2006; 296: 1867-1876. [DOI] [PubMed] [Google Scholar]
- 5. Weintraub RG, Semsarian C, Macdonald P. Dilated cardiomyopathy. Lancet 2017; 390: 400-414. [DOI] [PubMed] [Google Scholar]
- 6. Everitt MD, Sleeper LA, Lu M, et al. Recovery of echocardiographic function in children with idiopathic dilated cardiomyopathy: results from the pediatric cardiomyopathy registry. J Am Coll Cardiol 2014; 63: 1405-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alexander PM, Daubeney PE, Nugent AW, et al. Long-term outcomes of dilated cardiomyopathy diagnosed during childhood: results from a national population-based study of childhood cardiomyopathy. Circulation 2013; 128: 2039-2046. [DOI] [PubMed] [Google Scholar]
- 8. Den Boer SL, van Osch-Gevers M, van Ingen G, et al. Management of children with dilated cardiomyopathy in The Netherlands: implications of a low early transplantation rate. J Heart Lung Transplant 2015; 34: 963-969. [DOI] [PubMed] [Google Scholar]
- 9. Boucek MM, Waltz DA, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation: ninth official pediatric heart transplantation report – 2006. J Heart Lung Transplant 2006; 25: 893-903. [DOI] [PubMed] [Google Scholar]
- 10. Kirk R, Dipchand AI, Rosenthal DN, et al. The International Society for Heart and Lung Transplantation guidelines for the management of pediatric heart failure: executive summary. J Heart Lung Transplant 2014; 33: 888-909. [DOI] [PubMed] [Google Scholar]
- 11. Rossano JW, Cherikh WS, Chambers DC, et al. The Registry of the International Society for Heart and Lung Transplantation: twentieth pediatric heart transplantation report-2017; focus theme: allograft ischemic time. J Heart Lung Transplant 2017; 36: 1060-1069. [DOI] [PubMed] [Google Scholar]
- 12. Glotzbach K, May L, Wray J. Health related quality of life and functional outcomes in pediatric cardiomyopathy. Progress in Pediatric Cardiology 2018; 48: 26-35. [Google Scholar]
- 13. Rutledge T, Reis VA, Linke SE, et al. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol 2006; 48: 1527-1537. [DOI] [PubMed] [Google Scholar]
- 14. Fan H, Yu W, Zhang Q, et al. Depression after heart failure and risk of cardiovascular and all-cause mortality: a meta-analysis. Prev Med 2014; 63: 36-42. [DOI] [PubMed] [Google Scholar]
- 15. Den Boer SL, Baart SJ, van der Meulen MH, et al. Parent reports of health-related quality of life and heart failure severity score independently predict outcome in children with dilated cardiomyopathy. Cardiol Young 2017; 27: 1194-1202. [DOI] [PubMed] [Google Scholar]
- 16. Wilmot I, Cephus CE, Cassedy A, et al. Health-related quality of life in children with heart failure as perceived by children and parents. Cardiol Young 2016; 26: 885-893. [DOI] [PubMed] [Google Scholar]
- 17. Sleeper LA, Towbin JA, Colan SD, et al. Health-related quality of life and functional status are associated with cardiac status and clinical outcome in children with cardiomyopathy. J Pediatr 2016; 170: 173-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hollander SA, Callus E. Cognitive and psycholologic considerations in pediatric heart failure. J Card Fail 2014; 20: 782-785. [DOI] [PubMed] [Google Scholar]
- 19. Wray J, Radley-Smith R. Cognitive and behavioral functioning of children listed for heart and/or lung transplantation. Am J Transplant 2010; 10: 2527-2535. [DOI] [PubMed] [Google Scholar]
- 20. Menteer J, Beas VN, Chang JC, et al. Mood and health-related quality of life among pediatric patients with heart failure. Pediatr Cardiol 2013; 34: 431-437. [DOI] [PubMed] [Google Scholar]
- 21. Celano CM, Villegas AC, Albanese AM, et al. Depression and anxiety in heart failure: a review. Harv Rev Psychiatry 2018; 26: 175-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vongmany J, Hickman LD, Lewis J, et al. Anxiety in chronic heart failure and the risk of increased hospitalisations and mortality: a systematic review. Eur J Cardiovasc Nurs 2016; 15: 478-485. [DOI] [PubMed] [Google Scholar]
- 23. Rickham PP. Human experimentation. Code of ethics of the World Medical Association. Declaration of Helsinki. Br Med J 1964; 2: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Achenbach TM, Becker A, Dopfner M, et al. Multicultural assessment of child and adolescent psychopathology with ASEBA and SDQ instruments: research findings, applications, and future directions. J Child Psychol Psychiatry 2008; 49: 251-275. [DOI] [PubMed] [Google Scholar]
- 25. International Labour Organization. International standard classification of occupations: ISCO-08. Geneva: International Labour Organization, 2012. [Google Scholar]
- 26. Connolly D, Rutkowski M, Auslender M, et al. The New York University Pediatric Heart Failure Index: a new method of quantifying chronic heart failure severity in children. J Pediatr 2001; 138: 644-658. [DOI] [PubMed] [Google Scholar]
- 27. Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms & profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families, 2001. [Google Scholar]
- 28. IBM Corp. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp, 2016. [Google Scholar]
- 29. Kovacs M. Rating scales to assess depression in school-aged children. Acta Paedopsychiatr 1981; 46: 305-315. [PubMed] [Google Scholar]
- 30. Nakamura BJ, Ebesutani C, Bernstein A, et al. A psychometric analysis of the Child Behavior Checklist DSM-oriented scales. J Psychopathol Behav Assess 2009; 31: 178-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pinquart M, Shen Y. Anxiety in children and adolescents with chronic physical illnesses: a meta-analysis. Acta Paediatr 2011; 100: 1069-1076. [DOI] [PubMed] [Google Scholar]
- 32. Hommel KA, Chaney JM, Wagner JL, et al. Anxiety and depression in older adolescents with long-standing asthma: the role of illness uncertainty. Children's Health Care 2003; 32: 51-63. [Google Scholar]
- 33. Miller JM, Kustra RP, Vuong A, et al. Depressive symptoms in epilepsy: prevalence, impact, aetiology, biological correlates and effect of treatment with antiepileptic drugs. Drugs 2008; 68: 1493-1509. [DOI] [PubMed] [Google Scholar]
- 34. Pinquart M, Shen Y. Depressive symptoms in children and adolescents with chronic physical illness: an updated meta-analysis. J Pediatr Psychol 2011; 36: 375-384. [DOI] [PubMed] [Google Scholar]
- 35. Mishel MH. The measurement of uncertainty in illness. Nurs Res 1981; 30: 258-263. [PubMed] [Google Scholar]
- 36. Steele RG, Aylward BS, Jensen CD, et al. Parent-and youth-reported illness uncertainty: Associations with distress and psychosocial functioning among recipients of liver and kidney transplantations. Children's Health Care 2009; 38: 185-199. [Google Scholar]
- 37. Fortier MA, Batista ML, Wahi A, et al. Illness uncertainty and quality of life in children with cancer. J Pediatr Hematol Oncol 2013; 35: 366-370. [DOI] [PubMed] [Google Scholar]
- 38. Stewart JL, Mishel MH. Uncertainty in childhood illness: a synthesis of the parent and child literature. Sch Inq Nurs Pract 2000; 14: 299-319. [PubMed] [Google Scholar]
- 39. Pao M, Bosk A. Anxiety in medically ill children/adolescents. Depress Anxiety 2011; 28: 40-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pinquart M. Do the parent–child relationship and parenting behaviors differ between families with a child with and without chronic illness? A meta-analysis. J Pediatr Psychol 2013; 38: 708-721. [DOI] [PubMed] [Google Scholar]
- 41. Woo MA, Macey PM, Fonarow GC, et al. Regional brain gray matter loss in heart failure. J Appl Physiol 2003; 95: 677-684. [DOI] [PubMed] [Google Scholar]
- 42. Woo MA, Macey PM, Keens PT, et al. Functional abnormalities in brain areas that mediate autonomic nervous system control in advanced heart failure. J Card Fail 2005; 11: 437-446. [DOI] [PubMed] [Google Scholar]
- 43. Menteer J, Macey PM, Woo MA, et al. Central nervous system changes in pediatric heart failure: a volumetric study. Pediatr Cardiol 2010; 31: 969-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rumsfeld JS, Havranek E, Masoudi FA, et al. Depressive symptoms are the strongest predictors of short-term declines in health status in patients with heart failure. J Am Coll Cardiol 2003; 42: 1811-1817. [DOI] [PubMed] [Google Scholar]
- 45. Junger J, Schellberg D, Muller-Tasch T, et al. Depression increasingly predicts mortality in the course of congestive heart failure. Eur J Heart Fail 2005; 7: 261-267. [DOI] [PubMed] [Google Scholar]
- 46. Sherwood A, Blumenthal JA, Hinderliter AL, et al. Worsening depressive symptoms are associated with adverse clinical outcomes in patients with heart failure. J Am Coll Cardiol 2011; 57: 418-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sokoreli I, Pauws SC, Steyerberg EW, et al. Prognostic value of psychosocial factors for first and recurrent hospitalizations and mortality in heart failure patients: insights from the OPERA-HF study. Eur J Heart Fail 2018; 20: 689-696. [DOI] [PubMed] [Google Scholar]
- 48. Angermann CE, Ertl G. Depression, anxiety, and cognitive impairment: comorbid mental health disorders in heart failure. Curr Heart Fail Rep 2018; 15: 398-410. [DOI] [PubMed] [Google Scholar]
- 49. Luyster FS, Hughes JW, Gunstad J. Depression and anxiety symptoms are associated with reduced dietary adherence in heart failure patients treated with an implantable cardioverter defibrillator. J Cardiovasc Nurs 2009; 24: 10-17. [DOI] [PubMed] [Google Scholar]
- 50. Achenbach TM. Manual for the youth self-report and 1991 profile. Burlington, VT: University of Vermont Department of Psychiatry, 1991. [Google Scholar]
- 51. Berkes A, Varni JW, Pataki I, et al. Measuring health-related quality of life in Hungarian children attending a cardiology clinic with the Pediatric Quality of Life Inventory. Eur J Pediatr 2010; 169: 333-347. [DOI] [PubMed] [Google Scholar]
- 52. Uzark K, Jones K, Slusher J, et al. Quality of life in children with heart disease as perceived by children and parents. Pediatrics 2008; 121: e1060-e1067. [DOI] [PubMed] [Google Scholar]
- 53. Patel BJ, Lai L, Goldfield G, et al. Psychosocial health and quality of life among children with cardiac diagnoses: agreement and discrepancies between parent and child reports. Cardiol Young 2017; 27: 713-721. [DOI] [PubMed] [Google Scholar]
- 54. Marino BS, Shera D, Wernovsky G, et al. The development of the pediatric cardiac quality of life inventory: a quality of life measure for children and adolescents with heart disease. Qual Life Res 2008; 17: 613-626. [DOI] [PubMed] [Google Scholar]
- 55. Janse AJ, Sinnema G, Uiterwaal CSPM, et al. Quality of life in chronic illness: children, parents and paediatricians have different, but stable perceptions. Acta Paediatrica 2008; 97: 1118-1124. [DOI] [PubMed] [Google Scholar]
- 56. Schroeder JF, Hood MM, Hughes HM. Inter-parent agreement on the syndrome scales of the Child Behavior Checklist (CBCL): correspondence and discrepancies. J Child Fam Stud 2010; 19: 646-653. [Google Scholar]