Abstract
Mucopolysaccharidoses (MPSs) are a group of rare lysosomal storage diseases with multisystem manifestations, including carpal tunnel syndrome (CTS). This study comprised a systematic review of literature and hospital guidelines addressing the method and frequency of screening for carpal tunnel syndrome in mucopolysaccharidosis patients and a review of carpal tunnel syndrome in patients seen in the multidisciplinary mucopolysaccharidosis clinic of a pediatric hospital, in order to develop screening recommendations. The literature reported the importance of routine carpal tunnel syndrome screening from early childhood in patients with mucopolysaccharidosis I, II, IV, and VI. Screening methods included physical examination, nerve conduction studies, electromyography, and ultrasonography. Ten of 20 mucopolysaccharidosis patients in our series underwent carpal tunnel syndrome surgery. Given the high incidence of carpal tunnel syndrome at a young age in mucopolysaccharidosis, the authors recommend performing physical examination and obtaining patient and caregiver history for carpal tunnel syndrome every 6 months from the time of mucopolysaccharidosis diagnosis, supplemented by annual nerve conduction studies in cases with poor history or equivocal examination.
Keywords: mucopolysaccharidoses, carpal tunnel syndrome, pediatrics, decompression surgery, inborn genetic diseases
Mucopolysaccharidoses are a group of inherited metabolic disorders that result in progressive lysosomal accumulation of glycosaminoglycans in cells and tissues. There are 7 distinct subtypes with some overlap in signs, symptoms, and variable severity of phenotype. New treatment modalities such as enzyme replacement therapy and hematopoietic stem cell transplantation can significantly improve cardiorespiratory function, cognitive ability, and life expectancy in certain patients.1 Further evidence is required regarding the effects of systemic therapies on musculoskeletal complications and effective screening must be employed for those manifestations where clear benefit of early intervention has been demonstrated.2,3
Carpal tunnel syndrome is among one of the most common musculoskeletal manifestations of mucopolysaccharidosis, reported in mucopolysaccharidosis types I, II, IV, and VI.4,5 The early symptoms and signs of carpal tunnel syndrome in mucopolysaccharidosis patients may be minimal and may not follow the classical clinical manifestations of the disease in adults.6 Carpal tunnel syndrome in mucopolysaccharidosis is commonly diagnosed clinically only when thenar wasting and loss of hand function occurs.7 The early nonspecific symptoms, compounded with communication barriers due to age and intellectual disability, lead to delayed diagnosis and potential permanent loss of hand function.8
Most centers that treat mucopolysaccharidosis patients perform screening for the presence of carpal tunnel syndrome.9 Typical screening strategies include a combination of regular physical examination and neurophysiological tests such as nerve conduction studies, nerve ultrasonography, or electromyography.9–11 These methods differ in sensitivity, specificity, and practicality. There is a lack of consensus in current literature regarding the timing, nature, and frequency of screening for carpal tunnel syndrome in mucopolysaccharidosis patients. This study comprised a systematic review of studies of methods and frequency of screening for carpal tunnel syndrome in mucopolysaccharidosis patients, as well as a review of carpal tunnel syndrome in patients seen in the multidisciplinary mucopolysaccharidosis clinic of a pediatric hospital.
Methods
Literature Review
A database search pertaining to diagnosis and screening of carpal tunnel syndrome in mucopolysaccharidosis patients was performed for the following dates, 1960–August 21, 2018 (OVID MEDLINE), 1974–August 21, 2018 (Embase), and 1960–August 21, 2018 (CINAHL), in accordance with published guidelines from the Joanna Briggs Institute of Evidence Based Medicine.12 The search strategy was developed with the assistance of a medical librarian skilled in systematic review using relevant keywords and medical subject headings. Articles were initially assessed using title and abstract, followed by full-text review for relevant articles. Two reviewers (PP and NW) independently determined article inclusion and discussed any discrepancies. Protocols and guidelines from recognized hospitals and clinicians that regularly treat mucopolysaccharidosis patients with carpal tunnel syndrome were also assessed. Hospitals and clinicians were identified through the National MPS Society website13 and screening protocols were sourced from hospital websites and/or email contact with practitioners.
All mucopolysaccharidosis patients diagnosed with carpal tunnel syndrome regardless of sex, age, or current treatment were included. Only articles in English were reviewed.
Data extraction was performed using a Microsoft Excel spreadsheet with predetermined data fields including patient age at carpal tunnel syndrome diagnosis, mucopolysaccharidosis type, diagnostic method, and details regarding screening protocol recommendations. Additionally, comments regarding limitations within each recommendation were noted.
Case Series
Ethics committee approval was acquired for a retrospective medical record review (audit no. 644A) for all patients with a diagnosis of mucopolysaccharidosis seen by the metabolic service at the Women’s and Children’s Hospital, Adelaide, Australia. The following information was extracted from the medical record: gender, mucopolysaccharidosis type, mucopolysaccharidosis treatment (enzyme replacement therapy and/or hematopoietic stem cell transplantation), age at carpal tunnel syndrome diagnosis, carpal tunnel syndrome signs and symptoms, investigations, treatment, and findings at follow-up.
Results
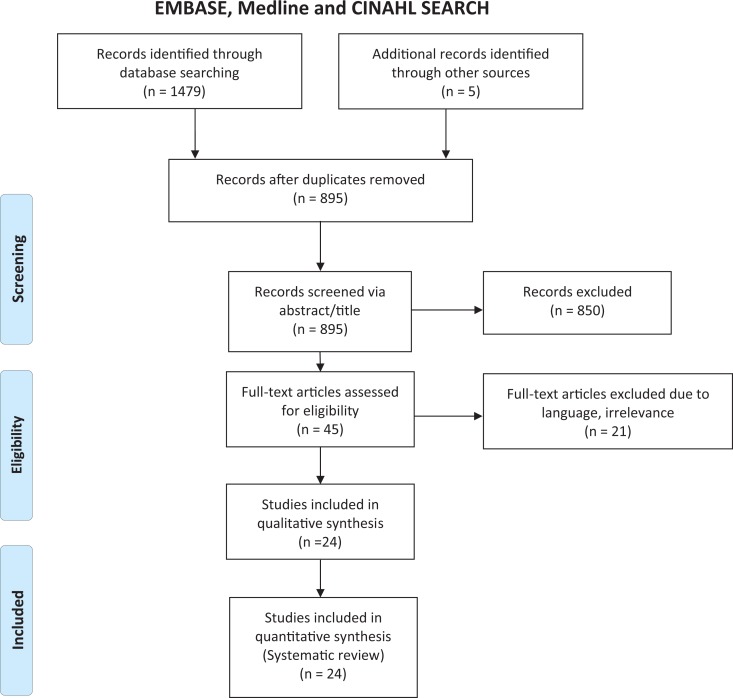
The search strategy identified 1479 published articles (Figure 1). Following title and abstract screening, 45 studies underwent full-text assessment, of which 24 made the inclusion criteria. Reasons for exclusion were lack of relevance, insufficient information regarding details of carpal tunnel syndrome diagnosis, and/or diagnostic method or absence of English translation. Clinicians and hospitals from 11 different countries were contacted with information regarding guidelines received from clinicians in England,14 Japan (T. Akiyuma, personal communication, January 16, 2019), Ireland (E. Crushell, personal communication, January 22, 2019), Switzerland (A. Wiesbauer, personal communication, January 16, 2019), Germany (C. Lampe, January 21, 2019), and Austria (L. Florian, personal communication, February 16, 2019). Protocols were provided by clinicians in 4 of these countries, with the remainder reporting that no screening protocol currently existed.
Figure 1.
PRISMA flow diagram showing the results of a combined Medline, EMBASE, and CINAHL search and subsequent article review.
Demographics and Diagnostic Methods
From the literature, 462 cases of carpal tunnel syndrome were reported in patients with mucopolysaccharidosis (mucopolysaccharidosis I: 417 patients, mucopolysaccharidosis II: 22 patients, mucopolysaccharidosis IV: 4 patients, mucopolysaccharidosis VI: 5 patients, mucopolysaccharidosis type unspecified: 14 patients; Table 1). All cases of carpal tunnel syndrome were diagnosed with a combination of physical examination and neurophysiology testing. No cases were diagnosed with clinical examination alone. Two hundred fourteen patients were diagnosed with nerve conduction studies. In 21 cases, nerve conduction studies were combined with electromyography, and in 20 cases, nerve conduction studies were combined with nerve ultrasonography. Eleven patients had electromyography alone. For 237 patients, there was no specification of any clinical or neurophysiological method of diagnosis. For the papers that reported mean age of carpal tunnel syndrome diagnosis, the overall mean varied between mucopolysaccharidosis subtypes (mucopolysaccharidosis 1: 5.3 years, mucopolysaccharidosis II: 5.3 years, mucopolysaccharidosis IV: 9.5 years, mucopolysaccharidosis VI: 7.4 years).
Table 1.
Demographics of MPS Patients With CTS in the Literature and Current Case Series.
| Study (country) | MPS type | No. of patients | Age at CTS diagnosisa: median (range) |
|---|---|---|---|
| Authors’ Case Series (Australia) | I | 6 | 9.2 y (5.6-13.3) |
| II | 1 | 5.6 y | |
| VI | 3 | 7.3 y (6.5-8.0) | |
| Wyffels et al 201715 (USA) | IH | 35 | 5.2 y (reported as mean) |
| Viskochil et al 201710 (USA) | IH | 153 | 5.2 y (0.8-16.1) |
| IHS/S | 138 | 11.9 y (1.7-44.1) | |
| Argenta et al 201711 (USA) | II | 1 | 11 y |
| Bäumer et al 20169 (Germany) | I | 6 | 6.5 y (2.1-14.6) |
| II | 2 | 4.1 y (1.7-6.6) | |
| Jadhav et al 20155 (Australia) | I | 2 | 7.5 y (5.0-10.0) |
| II | 5 | 8.0 y (5.0-12.0) | |
| IV | 2 | 13.5 y (12.0-15.0) | |
| Davis et al 201416 | IH | 1 | 3.0 y |
| Del Toro et al 2013 (Spain)17 | I, II, IV | 9 | 9.9 (4.0-15.0) |
| Meyer-Marcotty et al 2012 (Germany)18 | IH | 11 | 6.9 y (3.6-11.9) |
| Bahadir et al 200919 (Turkey) | IS | 2 | 25.5 (21.0-30.0) |
| Khanna et al 200720 (USA) | IH | 23 | 4.8 (2.5-10.9) |
| Van Meir et al 2003 (Belgium)21 | I | 2 | 3.8 (2.5-6.0) |
| Van Heest et al 1998 (USA)8 | IH | 11 | 6.5 (3.9-10.3) |
| IHS | 1 | 10.0 | |
| IS | 1 | 9.2 | |
| II | 3 | 4.1 (3.8-4.3) | |
| Cruz et al 199822 (Spain) | IV | 1 | 11.0 |
| Haddad et al 19976 (England) | I | 23 | – |
| II | 7 | – | |
| VI | 5 | 3.7 (2.5-15.0) | |
| Bona et al 199423 (France) | IH | 2 | 4.5 (4.0-5.0) |
| II | 2 | 4.5 (4.0-5.0) |
Abbreviations: CTS, carpal tunnel syndrome; MPS, mucopolysaccharidosis.
aAge of diagnosis defined as either date of confirmation of CTS by nerve conduction studies or date of carpal tunnel release surgery.
Screening Protocol Recommendations
Eleven papers and hospital guidelines provided recommendations for screening for carpal tunnel syndrome in mucopolysaccharidosis that included at least 1 of the following 3 key parameters: diagnostic method, age of screening commencement, and frequency of screening (Table 2).
Table 2.
Summary of Published Recommendations and Hospital Guidelines for Screening of Carpal Tunnel Syndrome in the Mucopolysaccharidoses.
| Study (country) | MPS type | Starting age of screening | Screening intervals | ||
|---|---|---|---|---|---|
| Clinical examination | NCS | Clinical examination | NCS | ||
| Viskochil et al 201710 (USA) | IH | – | 3 y | ||
| IHS/S | – | 5-7 y | |||
| Argenta et al 201711 (USA) | II | 3 y | 3 y | ||
| Jadhav et al 20155 (Australia) | I, II, IV | 4 y | |||
| White et al 20107 (USA) | unspecified | At diagnosis | At diagnosis | 1 y | 1-3 y |
| Muenzer et al 200924 (USA) | II | At diagnosis | 4-5 y | 1 y | 1-2 y |
| Khanna et al 200720 (USA) | IH | 1 y after HSCT | 1 y | ||
| Vellodi et al 201114 (England) | I, II, VI | 3-mo | 1 y | ||
| Crushell 2019 (Ireland)a | IH | At diagnosis | 4-5 y | 1.5-2 y (if initial NCS normal) ½-1 y (if initial NCS mildly abnormal) |
|
| Lampe 2019 (Germany)a | I, II, VI, VII | At diagnosis | At diagnosis | 1-2 y | 1-2 y |
| Lagler 2019a (Austria)a | unspecified | At diagnosis | At diagnosis | ½ y | 1 y |
Abbreviations: CTS, carpal tunnel syndrome; HSCT, hematopoietic stem cell transplantation; MPS, mucopolysaccharidosis; NCS, nerve conduction studies.
aPersonal email correspondence.
Clinical examination screening for carpal tunnel syndrome in mucopolysaccharidosis
Clinical examination was recommended by all papers and hospital guidelines (11/11)—however, never as the only method of diagnosis.
Method of clinical examination for carpal tunnel syndrome: clinical examination had several reported limitations. Across 2 studies, only approximately one-third (13/38) of mucopolysaccharidosis patients with nerve conduction studies suggestive of carpal tunnel syndrome demonstrated any positive clinical signs.8,9 Limitations included difficulty assessing thenar atrophy and muscle strength due to associated hand pathology, ineffectiveness of sensory tests due to communication barriers, and the poor sensitivity of clinical examination.7,9 Bäumer et al9 defined diagnostic criteria for carpal tunnel syndrome as the presence of any one of the following features: thenar atrophy, disturbances of nail growth in digits I, II, and III, pain/sensory symptoms in the first 3 fingers, or a positive Tinel sign at the wrist.
The importance of assessing subtle signs of carpal tunnel syndrome in children has been emphasized.7 These include manual clumsiness, gnawing of hands, difficulty with fine motor tasks, and alterations in playing pattern. Heightened awareness of these indirect signs is needed in order to adequately educate carers.11
Timing of clinical examination for carpal tunnel syndrome screening
Five of the 6 papers and hospital guidelines covering mucopolysaccharidosis types I, II, and VI recommended carpal tunnel syndrome screening at the time of initial mucopolysaccharidosis diagnosis.7,24 Argenta et al11 suggested routine clinical screening for mucopolysaccharidosis II from 3 years of age.
Three of the 5 papers and hospital guidelines recommended clinical assessments once every 1 to 2 years.7,10 Austrian and English mucopolysaccharidosis clinicians suggested more rigorous screening with checks once every 3 to 6 months.
Neurophysiological Testing for carpal tunnel syndrome Screening in mucopolysaccharidosis
Method of neurophysiological testing for carpal tunnel syndrome screening
All papers or guidelines (11/11) recommended nerve conduction studies as their choice of neurophysiology testing in carpal tunnel syndrome screening. Electromyography was not recommended by any sources in screening for carpal tunnel syndrome because of the intolerability of electromyography in the unsedated child.24
Two papers stated similar criteria for evaluating nerve conduction studies in children with mucopolysaccharidosis.6,9 Sensory studies were graded based on the amplitude of the sensory nerve action potential (SNAP) from the ring finger, whereas motor studies involved assessment of the distal motor latency and compound muscle action potential from the abductor pollicis brevis. During interpretation of nerve conduction studies, there was difficulty in obtaining comparative normative data because of the bilateral nature of the disease in mucopolysaccharidosis children.7,15 As a result, the ipsilateral ulnar nerve over the equivalent length segment was used for comparison.
Timing of neurophysiological testing for carpal tunnel syndrome screening
Recommendations for age at nerve conduction studies screening commencement were at diagnosis (2/6 papers) or 4 to 5 years of age (4/6 papers).5,7,24 Furthermore, there was a lack of consensus regarding the frequency of screening. Five of 7 sources that discussed frequency of screening suggested intervals of once every 1 to 2 years, with the remainder suggesting every 3 to 7 years. Papers addressing more attenuated mucopolysaccharidosis subtypes such as Scheie (1S) or Hurler-Scheie (1HS) suggested less frequent screening intervals (5-7 years).10 Clinicians from the Irish National Centre of Inherited Metabolic Disorders alter screening intervals based on whether previous nerve conduction studies were interpreted as normal or mildly abnormal. The poor tolerance of nerve conduction studies, especially in children with cognitive impairment, was a notable limitation in the literature.7,9
Median nerve ultrasonography for carpal tunnel syndrome screening in mucopolysaccharidosis
One study recommended using nerve ultrasonography alongside nerve conduction studies in carpal tunnel syndrome diagnosis.9 Using ultrasonography, carpal tunnel pathology was classified as absent, moderate, or prominent through assessment of median nerve echogenicity at either the site proximal to the retinaculum flexorum or the site of greatest nerve enlargement, as well as the wrist-to-forearm ratio of the proximal and distal cross-sectional area measurement of the median nerve. Moderate signs included either hypoechogenicity or a wrist-to-forearm ratio >1.5-2, whereas prominent signs included both hypoechogenicity and a wrist-to-forearm ratio >1.5 being present. The study reports superior sensitivity of ultrasonography compared with nerve conduction studies alone, especially in cases of mild carpal tunnel syndrome. Seven of 43 patients in that study showed no significant changes on nerve conduction studies, despite having carpal tunnel pathology on nerve ultrasonography.
Case Series
Patients
Of 20 patients with mucopolysaccharidosis seen in our clinic, 10 had undergone surgery for carpal tunnel syndrome (mucopolysaccharidosis I 6/7 patients, mucopolysaccharidosis II 1/1 patient, mucopolysaccharidosis III 0/6, mucopolysaccharidosis IV 0/2, and mucopolysaccharidosis VI 3/4) (Table 1). Enzyme replacement therapy or hematopoietic stem cell transplantation treatment was not used in 2 cases of mucopolysaccharidosis VI, with the remaining 8 mucopolysaccharidosis patients with carpal tunnel syndrome previously receiving hematopoietic stem cell transplantation, enzyme replacement therapy, or a combination. Neurophysiologic testing was conducted based on clinical suspicion, with no formal protocol for carpal tunnel syndrome screening in mucopolysaccharidosis patients in place during the time of this case series.
Initial signs and symptoms varied between each patient. Eight of 10 patients reported a combination of motor weakness and subsequent limitations in functional capabilities. Reported functional deficits included clumsiness of the hands, reduced hand usage, and difficulty with grabbing objects, twisting tops, and writing. One patient and carer reported no symptoms despite significant objective motor weakness on clinical examination. In 5 patients, clinical examination demonstrated weakness of at least 1 of abductor pollicis brevis, grip strength, and/or pincer grip. Three of 10 patients reported paraesthesia in the median nerve distribution. Three of 10 patients had thenar muscle wasting with clawing of their hands, and 2 of 10 cases described hand gnawing as one of their initial symptoms. Only 1 patient reported pain as his initial symptom, localized to the index and middle finger bilaterally.
All 10 patients underwent nerve conduction studies to confirm carpal tunnel syndrome, with 8 of 10 reports available. The median age at first nerve conduction study was 8.7 years (range 5.8-13.3). One patient with clinical suspicion of carpal tunnel syndrome but normal nerve conduction study results was subsequently suggested to have nerve ultrasonography. This, however, was not conducted, and carpal tunnel decompression was performed with successful postoperative relief of symptoms.
Bilateral carpal tunnel release surgery was performed in all 10 patients with no complications. Median age at surgery was 9.0 years (range 6.5-13.3). The majority of operative reports described thickened flexor retinaculum and/or median nerve constriction. Four operations involved supplementary flexor synovectomy. All cases reported positive outcomes postoperatively, with subjective reports of improvement in symptoms and observed improvement in hand function.
Discussion
Modern mucopolysaccharidosis treatment (hematopoietic stem cell transplantation and/or enzyme replacement therapy and multidisciplinary specialist care) has improved life expectancy by reducing complications from fatal cardiac and neurologic pathology. Its effect on musculoskeletal complications is still not proven.10,11 Routine monitoring of musculoskeletal complications is therefore, important, regardless of systemic treatment.25
Mucopolysaccharidosis patients were reportedly diagnosed with carpal tunnel syndrome in a late, irreversible stage.9,20 This could be due to a combination of factors, including other more urgent life-threatening pathology, difficulty with communication of symptoms, and the lack of routine clinical and neurophysiological screening.10,11,24
Typical early carpal tunnel syndrome symptoms in nonmucopolysaccharidosis adults such as paraesthesia, nocturnal pain, numbness, and positive Tinel and Phalen signs may be absent in children.6,7,25,26 The mucopolysaccharidoses have more subtle presentations such as hand gnawing, manual clumsiness, and difficulties with fine motor tasks.7 The nontypical nature of this presentation may contribute to underdiagnosis. Our own case series demonstrated that a majority of patients with carpal tunnel syndrome presented with functional deficits rather than typical sensory manifestations. Carpal tunnel syndrome symptoms may be underreported by mucopolysaccharidosis patients. Haddad et al6 described a majority of patients claiming to be asymptomatic despite severe functional and neurophysiological deficits. A recent study demonstrated that only 10/24 mucopolysaccharidosis patients with carpal tunnel syndrome were able to reliably report clinical symptoms.9 One patient in our case series was asymptomatic with both carer and patient denying symptoms despite clinical examination showing functional deficits and weakness of the abductor pollicis brevis. However, the majority of our patients (9/10) reported symptoms prior to confirmatory nerve conduction studies. This is in contrast with the majority of reviewed literature, which report the unreliability of clinical examination in diagnosing carpal tunnel syndrome in mucopolysaccharidosis.15,26 This may be due to the relatively healthy cognitive state of most patients seen at our center. This raises the possibility of the underestimated value of clinical examination in a cognitively intact child with mucopolysaccharidosis and an argument against mandatory nerve conduction studies before carpal tunnel decompression (CTD) in patients with suggestive symptoms and/or positive clinical examination findings. CTD is a relatively low-risk operation of short duration, can be timed with other common mucopolysaccharidosis operations requiring anesthetic such as ear or dental procedures, and typically has very good postoperative outcomes.27 One patient (mucopolysaccharidosis I) in our series had postoperative relief of clinical symptoms and signs following CTD despite equivocal preoperative nerve conduction study findings. The value of clinical examination should not be ignored by clinicians. Both regular clinical screening and education of the subtle clinical profile of carpal tunnel syndrome to clinicians and carers seems imperative for early diagnosis and positive outcomes.
Neurophysiologic testing is frequently used to confirm carpal tunnel syndrome in the general adult population because of its high sensitivity and specificity in this population. The sensitivity and specificity in the pediatric and mucopolysaccharidosis population is unknown. Carpal tunnel syndrome is a rare diagnosis in nonmucopolysaccharidosis children, and nerve conduction studies can be poorly tolerated in children with and without mucopolysaccharidosis.9
The lack of a contralateral unaffected median nerve for comparison makes interpretation of nerve conduction study findings difficult. Using the ulnar nerve for comparison has limitations considering Guyon’s canal pathology, and ulnar neuropathy has been reported in mucopolysaccharidosis patients.28 Furthermore, small hands and the confounding effects of proximal nerve and spinal cord pathology must also be considered when interpreting nerve conduction study findings.7 Nerve conduction studies remain the most useful investigation available for screening for carpal tunnel syndrome in mucopolysaccharidosis, particularly in patients with difficult clinical examination and history. But further research is required to define pathologic values, and improvements could be made in terms of enhancing the patient experience and achieving appropriate interpretation.
Ultrasonography of the median nerve and carpal tunnel is an appealing alternative or adjunct to nerve conduction studies as a screening modality for carpal tunnel syndrome in mucopolysaccharidosis patients. It is very tolerable in children, with reported sensitivity of up to 95% in the nonmucopolysaccharidosis population where it has a use for diagnosis of mild cases of carpal tunnel syndrome.29 Nerve ultrasonography, however, is user dependent and can be difficult to interpret, with no current consensus regarding cut-off values for diagnosis.30
The timing of carpal tunnel syndrome screening in mucopolysaccharidosis varied in the literature. The most frequently used protocol was carpal tunnel syndrome screening once every 1 to 2 years from age of mucopolysaccharidosis diagnosis. Carpal tunnel syndrome can deteriorate both clinically and neurophysiologically within 6 months, and more rigorous screening protocols may be considered.5 Carpal tunnel syndrome is diagnosed in mucopolysaccharidosis relatively early in childhood, providing justification for screening from the time of diagnosis. Mean age at diagnosis of carpal tunnel syndrome in current literature is 5.5 years; however, there was no evidence that these patients underwent regular neurophysiological screening for carpal tunnel syndrome.5,6,8,9,11,15,16,19,22,23 Considering the typical late reporting of symptoms and signs, it is possible that with use of a neurophysiological screening protocol, the age of carpal tunnel syndrome diagnosis in mucopolysaccharidosis may be lower than the current literature suggests. Early diagnosis and treatment of carpal tunnel syndrome in the mucopolysaccharidoses has been linked to better neurophysiological and functional outcomes.6,8
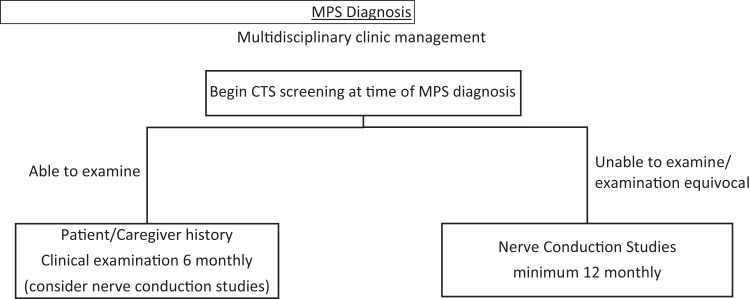
Development of a screening protocol for manifestations of rare genetic diseases such as mucopolysaccharidosis is difficult. With current literature lacking consensus in carpal tunnel syndrome screening protocols for any mucopolysaccharidosis type, a broad screening protocol can be used for the subtypes most commonly associated with carpal tunnel syndrome (mucopolysaccharidosis I, II, IV, and VI).26,31 We particularly stress its use in Hurler (IH), Hunter (II), and Maroteaux-Lamy (VI), all of which have high prevalence of carpal tunnel syndrome.15,31 The small number of mucopolysaccharidosis cases makes it difficult to generate screening protocols for individual subtypes. Further long-term studies are required to develop these protocols. We recommend commencing carpal tunnel syndrome screening at the time of mucopolysaccharidosis diagnosis with clinical examination every 6 months and history from the patient or caregiver including reduced manual dexterity and gnawing of thumb, index, and middle finger. Based on our experience, the underreporting of clinical findings is due to poor patient communication of symptoms and signs. Thus, history and clinical examination of mucopolysaccharidosis patients who can reliably communicate findings with the clinician can still lead to early intervention and positive outcomes. In situations where history and examination is difficult to interpret or equivocal, we recommend nerve conduction studies at least once every 12 months (Figure 2).
Figure 2.
Authors’ suggested screening protocol for carpal tunnel syndrome (CTS) in mucopolysaccharidosis (MPS) patients.
A limitation of this study includes the small sample size of the literature. Carpal tunnel release surgery has been reported in mucopolysaccharidosis III patients but was not seen in our study.32 This raises the possibility that it has been underdiagnosed. Additionally, with gene therapy trials and evolving systemic treatment, the presentation of carpal tunnel syndrome may change over time.33
Acknowledgment
The authors would like to thank Natalie Dempster for her assistance with literature search strategy and article identification.
Footnotes
Author Contributions: PP conducted the literature review and completed first draft and revisions of the manuscript. GA provided guidance with data extraction and interpretation and manuscript review. DC reviewed and edited manuscript drafts with particular attention to the clinical neurology aspects. DK contributed data to the case series and reviewed and edited mansucript drafts with particular attention to metabolic aspects. NW conceived the idea for the manuscript, reviewed all identified articles against the review inclusion criteria and supervised manuscript preparation. All authors have reviewed and approved the final manuscript.
Declaration of Conflicting Interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: In the past 5 years, NW has received reimbursement for attending symposia, speaker’s fees, and institutional research funds from BioMarin Pharmaceutical, Inc.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded and made available for Open Access by BioMarin Pharmaceutical Inc. [Grant Number 106285]. The authors confirm independence from the sponsors; the content of the article has not been reviewed or influenced by the sponsors.
Ethical Approval: Institutional ethics committee approval was obtained for retrospective medical record review (Audit No 644A).
References
- 1. Van der Linden MH, Kruyt MC, Sakkers RJ, De Koning TJ, Oner FC, Castelein RM. Orthopaedic management of Hurler’s disease after hematopoietic stem cell transplantation: a systematic review. J Inherit Metab Dis. 2011;34(3):657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Souillet G, Guffon N, Maire I, et al. Outcome of 27 patients with Hurler’s syndrome transplanted from either related or unrelated haematopoietic stem cell sources. Bone Marrow Transplant. 2003;31(12):1105–1117. [DOI] [PubMed] [Google Scholar]
- 3. Vellodi A, Young EP, Cooper A, et al. Bone marrow transplantation for mucopolysaccharidosis type I: experience of two British centres. Arch Dis Child. 1997;76(2):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nørmark MB, Kjaer N, Lund AM. Prevalence of mucopolysaccharidosis types I, II, and VI in the pediatric and adult population with carpal tunnel syndrome (CTS). Retrospective and prospective analysis of patients treated for CTS. JIMD Rep. 2017;36:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jadhav TM, Kornberg AJ, Peters H, Lee J, Ryan MM. Carpal tunnel syndrome in pediatric mucopolysaccharidoses. J Int Child Neurol Assoc. 2015;15:101. [Google Scholar]
- 6. Haddad FS, Jones DH, Vellodi A, Kane N, Pitt MC. Carpal tunnel syndrome in the mucopolysaccharidoses and mucolipidoses. J Bone Joint Surg Br. 1997;79(4):576–582. [DOI] [PubMed] [Google Scholar]
- 7. White K, Kim T, Neufeld J. Clinical assessment and treatment of carpal tunnel syndrome in the mucopolysaccharidoses. J Pediatr Rehabil Med. 2010;3(1):57–62. [DOI] [PubMed] [Google Scholar]
- 8. Van Heest AE, House J, Krivit W, Walker K. Surgical treatment of carpal tunnel syndrome and trigger digits in children with mucopolysaccharide storage disorders. J Hand Surg Am. 1998;23(2):236–243. [DOI] [PubMed] [Google Scholar]
- 9. Bäumer T, Bühring N, Schelle T, Münchau A, Muschol N. Nerve ultrasound in clinical management of carpal tunnel syndrome in mucopolysaccharidosis. Dev Med Child Neurol. 2016;58(11):1172–1179. [DOI] [PubMed] [Google Scholar]
- 10. Viskochil D, Muenzer J, Guffon N, et al. Carpal tunnel syndrome in mucopolysaccharidosis I: a registry-based cohort study. Dev Med Child Neurol. 2017;59(12):1269–1275. [DOI] [PubMed] [Google Scholar]
- 11. Argenta AE, Davit A. Carpal tunnel syndrome in the setting of mucopolysaccharidosis II (Hunter syndrome). Plast Reconstr Surg Glob Open. 2017;5(8):1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. The Joanna Briggs Institute. “Joanna Briggs Institute Reviewer’s Manual.” The Joanna Briggs Institute for Systematic Review Research. https://wiki.joannabriggs.org/display/manual. Published 2018 Accessed June 30, 2018
- 13. MPS Society. Resources https://mpssociety.org/support/resources. Published 2019. Accessed June 30, 2018.
- 14. Vellodi A, Wraith JE, Cleary MA. Guidelines for the investigation and management of mucopolysaccharidosis type II. Department of Health. http://docplayer.net/14342011-Guidelines-for-the-investigation-and-management-of-mucopolysaccharidosis-type-ii.html. Published 2011. Accessed February 20, 2019.
- 15. Wyffels ML, Orchard PJ, Shanley RM, Miller WP. The frequency of carpal tunnel syndrome in Hurler syndrome after peritransplant enzyme replacement therapy: a retrospective comparison. J Hand Surg Am. 2017;42(7):573.e1-573.e8. [DOI] [PubMed] [Google Scholar]
- 16. Davis L, Vedanarayanan VV. Carpal tunnel syndrome in children. Pediatr Neurol. 2014;50(1):57–59. [DOI] [PubMed] [Google Scholar]
- 17. Del Toro M, Ortiz S, Garcia-Fontecha CG, et al. Carpal tunnel syndrome in young patients with mucopolysaccharidosis. J Inherit Metab Dis. 2013;36(2):291. [Google Scholar]
- 18. Meyer-Marcotty MV, Kollewe K, Dengler R, et al. Carpal tunnel syndrome in children with mucopolysaccharidosis type 1H: diagnosis and therapy in an interdisciplinary centre. Handchir Mikrochir Plast Chir. 2012;44(1):23–28. [DOI] [PubMed] [Google Scholar]
- 19. Bahadir C, Kurtulus D, Cihandide E. Mucopolysaccharidosis type-IS presenting with onset of carpal tunnel syndrome at adolescence. J Clin Rheumatol. 2009;15(8):402–404. [DOI] [PubMed] [Google Scholar]
- 20. Khanna G, Van Heest AE, Agel J, et al. Analysis of factors affecting development of carpal tunnel syndrome in patients with Hurler syndrome after hematopoietic cell transplantation. Bone Marrow Transplant. 2007;39(6):331–334. [DOI] [PubMed] [Google Scholar]
- 21. Van Meir N, De Smet L. Carpal tunnel syndrome in children. Acta Orthop Belg. 2003;69(5):387–395. [PubMed] [Google Scholar]
- 22. Cruz Martínez A, Arpa J. Carpal tunnel syndrome in childhood: study of 6 cases. Electroencephalogr Clin Neurophysiol. 1998;109(4):304–308. [DOI] [PubMed] [Google Scholar]
- 23. Bona I, Vial C, Brunet P, et al. Carpal tunnel syndrome in mucopolysaccharidoses. A report of four cases in child. Electromyogr Clin Neurophysiol. 1994;34(8):471–475. [PubMed] [Google Scholar]
- 24. Muenzer J, Beck M, Eng CM, et al. Multidisciplinary management of Hunter syndrome. Pediatrics. 2009;124(6):1228–1239. [DOI] [PubMed] [Google Scholar]
- 25. Wraith JE, Alani SM. Carpal tunnel syndrome in the mucopolysaccharidoses and related disorders. Arch Dis Child. 1990;65(9):962–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yuen A, Dowling G, Johnstone B, Kornberg A, Coombs C. Carpal tunnel syndrome in children with mucopolysaccharidoses. J Child Neurol. 2007;22(3):260–263. [DOI] [PubMed] [Google Scholar]
- 27. Karl JW, Gancarczyk SM, Strauch RJ. Complications of carpal tunnel release. Orthop Clin North Am. 2016;47(2):425–433. [DOI] [PubMed] [Google Scholar]
- 28. Swift TR, McDonald TF. Peripheral nerve involvement in Hunter syndrome (mucopolysaccharidosis II). Arch Neurol. 1976;33(12):845–846. [DOI] [PubMed] [Google Scholar]
- 29. Fowler JR, Gaughan JP, Ilyas AM. The sensitivity and specificity of ultrasound for the diagnosis of carpal tunnel syndrome: a meta-analysis. Clin Orthop Relat Res. 2011;469(4):1089–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Descatha A, Huard L, Aubert F, Barbato B, Gorand O, Chastang JF. Meta-analysis on the performance of sonography for the diagnosis of carpal tunnel syndrome. Semin Arthritis Rheum. 2012;41(6):914–922. [DOI] [PubMed] [Google Scholar]
- 31. Kwon JY, Ko K, Sohn YB, et al. High prevalence of carpal tunnel syndrome in children with mucopolysaccharidosis type II (Hunter syndrome). Am J Med Genet A. 2011;155(6):1329–1335. [DOI] [PubMed] [Google Scholar]
- 32. White KK, Karol LA, White DR, Hale S. Musculoskeletal manifestations of Sanfilippo syndrome (mucopolysaccharidosis type III). J Pediatr Orthop. 2011;31(5):594–598. [DOI] [PubMed] [Google Scholar]
- 33. Di Domenico C, Villani GR, Di napoli D, et al. Gene therapy for a mucopolysaccharidosis type I murine model with lentiviral-IDUA vector. Hum Gene Ther. 2005;16(1):81–90. [DOI] [PubMed] [Google Scholar]