Abstract
Boron neutron capture therapy (BNCT) is a binary therapeutic approach. Nonradioactive boron-10 atoms accumulated in tumor cells combining with the neutron beams produce two highly energetic particles that could eradicate the cell that takes it and the neighboring cells. Small molecules that carry boron atom, e.g. 5- and 6-boronated and 2,7-diboronated tryptophans, were assessed for their boron accumulation in U87-MG, LN229, and 3T3 for BNCT. TriBoc tryptophan, TB-6-BT, shows boron-10 at 300 ppm in both types of tumor cells with a tumor to normal ratio (T/N) of 5.19–5.25 (4 h). TB-5-BT and DBA-5-BT show boron-10 at 300 ppm (2 h) in U87-MG cells. TB-5-BT exerts a T/N of >9.66 (1 h) in LN229 compared with the current clinical boronophenyl alanine with a highest T/N of 2.3 (1 h) and accumulation concentration of <50 ppm. TB-5-BT and TB-6-BT warrant further animal study.
Keywords: BNCT, tryptophan, boronodehalogenation, tumor to normal, U87-MG, protecting group
As it is the most common type of primary brain tumor in adults, glioblastoma multiforme (GBM) is classified as a World Health Organization grade IV astrocytoma. Its estimated incidence in North America is 3.0 per 100,000 people according to age-adjusted statistics. Due to its aggressive malignancy, GBM has an estimated 2-year survival rate of 8.7%. Even after maximum treatment, the median duration of survival is 12–18 months.1 Whereas the tumor pathogenesis has been well deciphered, the median overall survival of patients has increased by only 3.3 months (from 11.3 months to 14.6 months) over the past 25 years. This disappointing prognosis is mostly associated with the prevalent recurrence of tumors after initial treatment with the maximum safe surgical resection, radiotherapy, and chemotherapy.1 Moreover, the cause of death of glioma patients is not a distant metastasis but the failure of local control of the tumor. When the tumor repeatedly happens, additional systemic and local therapies as well as repeat surgery are commonly considered. While each of these therapies has potential benefits, each also carries associated risks, particularly surgery.2
The invasive nature of glioma often prevents total surgical excision, hence adjuvant therapy for the residual tumor is essential. However, conventional chemotherapy, immunotherapy, and radiotherapy have been proven to be of limited value.3 The highly proliferating malignant tumor cells may require significant substrates such as sugars and amino acids. If a specific upregulation of the amino acid transport system in malignant tumor cells does exist, it could be a molecular target for therapy.3
The leucine-type amino acid transporter (LAT1) is a Na-independent neutral amino acid transport agent and essential for the transport of large neutral amino acids through the plasma membrane.4,5 LAT1 exhibits high affinity for several nutritionally essential amino acids such as leucine, isoleucine, valine, phenylalanine, tryptophan and methionine.3 Thus, one such amino acid analog of phenylalanine bearing a boron-10 atom can be triggered to release energy upon irradiation with neutron beams.
Boron neutron capture therapy (BNCT) is a targeted radiation therapy that significantly increases the therapeutic ratio relative to conventional radiotherapeutic modalities (Figure 1). BNCT is a binary approach: a boron-10 (10B)-labeled compound is administered that delivers high concentrations of 10B to the target tumor relative to surrounding normal tissues. This is followed by irradiation with thermal neutrons or epithermal neutrons which become thermalized at a depth in tissues. The short-range (5–9 μm) of the α and 7Li particles released from the 10B(n, α)7Li neutron capture reaction makes the microdistribution of 10B of critical importance in therapy.6
Figure 1.
Illustration of the basic principles of boron neutron capture therapy (BNCT).
One of the advantages of a binary system is the potential that each component can be manipulated independently. Only when the timing is correct for achieving the maximal cytotoxic effect upon malignant cells, together with the maximum tolerated dose to contiguous normal cells, are the two components juxtaposed. If this approach is to succeed, it is essential that at least one of the components be confined rather specifically to tumor cells, while the second component is exposed to all cells in a particular area.7
The potential for developing NCT rests upon the fact that the various normal elemental constituents of tissue have very low capture cross-sectional values for thermal neutrons compared with boron-10 (3838 barns). The ideal boron agents should have the following features: (1) low intrinsic toxicity; (2) high tumor uptake (∼20–50 μg 10B) and low normal tissue uptake, ideally with tumor to normal tissue and tumor to blood boron concentration ratios of >3:1; and (3) relatively rapid clearance from the blood and normal tissues along with persistence in the tumor for at least several hours during neutron irradiation.8 The present boron-bearing compounds used for BNCT are briefly divided into two types: small molecules, e.g. boronophenylalanine (BPA), and cage-like boron clusters, e.g. sodium borocaptate (Na210B12H11SH, BSH).8 The clinically studied amino acid analog BPA exhibits acceptable in vivo tumor targeting and has proven to be sufficient in the treatment of brain tumors in humans.9 Although BPA and BSH remain unsatisfactory according to the above criteria, these two agents are the clinically used drugs. Other amino acid derivatives of tryptophan such as α-11C-methyl-L-tryptophan (11C-AMT) are amino acid PET tracers that can measure tryptophan metabolism via the immunomodulatory kynurenine pathway.10 It has been shown that 11C-AMT accumulates because of both transport and metabolism in both untreated and recurrent World Health Organization (WHO) grade II–IV gliomas. A standardized uptake value (SUV) of 2.68 is distributed over the brain tumor along with a lesion to cortex ratio of 2.14 from a clinical study for 21 adults.
Although liposomal and nanoparticle compounds have been intensively developed as boron carriers, the accumulated boron in brain tumors remains low, i.e. < 0.4% injection dose/g.11,12 Satisfactorily high concentrations of 50 ppm 10B have been achieved recently.13 However, the preparation is relatively uncommon to organic chemists, i.e. via grinding of a micron-sized 10B-enriched rock-hard powder to give the particle size of 85 nm. Hence, we are encouraged to prepare single-boron carried small molecule via the common covalent-bond formation with the hope of improving tumoral accumulation. With respect to the similar features to those of BPA such as the transportation mechanism via LAT-1, essential nutrition source, and insignificant cytotoxicity, we intend to prepare the amino acid-like boron derivatives of tryptophan (Figure 2). The pseudotryptophan structure can be regarded as a prodrug that can diffuse through the BBB layer via LAT-1 mediated transportation.14 This is similar to the concept of a pseudonutrient that has been applied by DOPA, a clinically used prodrug of dopamine.15 Hence, the protecting groups are optionally attached to examine their effects on assisting the penetration across the cell membrane for accumulation. The present study is aimed to prepare boronopinacol tryptophan derivatives with protecting groups for assessing their potencies as BNCT agents. The relevant in vitro assay includes the accumulation, cytotoxicity, and the eradication effects through neutron irradiation.
Figure 2.
Boronopinacoltryptophan derivatives described in this work.
Electrophilic substitution on the indole-based tryptophan is apt to take place at the pyrrole ring or the 5- or 6-position of the benzene ring. However, borylation on 5- and 6-positions via regioselective control over a common intermediate remains difficult.16 In general, the reactivity of the two fused rings has been speculated (Figure 3). The intermediates formed from 4- or 7-substitution lead to the entire loss of aromaticity of both rings. Experimental data17 and theoretical calculation18 predicts that the reactivity under the catalysis of Pd(0) is in an order of C-4 > C-6 > C-5 > C-7. Furthermore, Pd(0)-catalyzed oxidative addition for the boronopinacol group at the six-membered ring moiety suffers from other disadvantages. For example, Pd(0) is deactivated by electron donors, such as −NH2 through its coordination to Pd, thereby rendering the metal insertion into R-X inefficient. The bulky electron-withdrawing Boc as the protecting group for −NH2 not only blocks its capacity for coordination but also weakens the electron delocalization to the halogenated carbon, thereby retaining the polarizability of the C–Br bond (Scheme 1(A)).
Figure 3.
Electrophilic substitution prefers the route via a cation intermediate (a) over that of a cation (b).
Scheme 1. (A) Deactivating Pd(0) through Electron Delocalization. (B) TriBoc Protected 5-Boronopinacol Tryptophan Analog TB-5-BT (1) and Its Deprotection Leading to 5-BT (4).
Reaction conditions: (a) SOCl2, CH3OH; Boc2O, DMAP, CH3CN, 50% over two steps. (b) Diborono pinacol, Ferocene2PdCl2, DMSO, KOAc, 100 °C, 2 h, 90%. (c) As listed in Table 1.
Results and Discussion
Chemical Synthesis
Starting from a d,l-mixture of the commercial 5-bromotryptophan, the subsequent Miyamura borylation proceeded smoothly when an appropriate solvent was chosen, e.g., DMSO (Scheme 1(A)). After several batches of optimization, the boronopinacol tryptophan TB-5-BT (1)19 was obtained in a greater than 90% yield.
Whereas t-Boc in peptide synthesis is typically removed by an acid such as trifluoroacetic acid (pKa = 0.30),20,21 only trimethylsilyl iodide (TMSI)22,23 has been reported for the deprotection of boronopinacol tryptophan derivatives. The inherent instability of boron groups associated with the indole ring prevents the use of acidic conditions. In our hands, a milder acid H3PO4 (pKa = 2.15) also failed. Only under the modified conditions of TMSI at 0 °C can the three Boc groups be removed (Table 1). Whereas the in situ generation of TMSI (entry 1 and 2) can eliminate the moisture disturbance and should be capable of increasing the yield, the mudlike mixture [CH3CN+Si(CH3)3]I– was difficult to quantitatively assess for equivalence control. On the other hand, the commercial solution of TMSI was straightforward but required the reaction to be terminated in time to obtain an acceptable yield.
Table 1. Conditions for the Removal of triBoc Moieties of TB-5-BT (1) to Give 5-BT (4).
| Entry | Reagent | Temp | Solvent | TMSI (eq) | Reaction time (h) | Yield |
|---|---|---|---|---|---|---|
| Lit. | TMSI | rt | CHCl3 | 8 | 2 | 81%22 |
| 1 | TMSCl/NaI | rt | CN3CN | 8 | 2 | 16% |
| 2 | TMSCl/NaI | rt | CHCl3 | 4 | 1 | 27% |
| 3 | TMSI | rt | CHCl3 | 4 | 0.5 | 42% |
| 4 | TMSI | 0 °C | CHCl3 | 4 + 2 | 1 | 68% |
For expanding the structural variation and pursuing an inexpensive 5-bromo tryptophan source, an attempt was made to assemble 5-bromoindole and an in situ generated compound 8(24) (Scheme 2). However, we were unable to reproduce the results and only found oligomerized byproducts. Compound 8 is typically obtained through the condensation between acetamide and pyruvic acid using the Dean–Stark setup.25−28 In our hands, Soxhlet extraction also proceeded smoothly. Compound 10 obtained from Friedel–Crafts alkylation was identified as a d,l-mixture in a ratio of 3:1 from HPLC analysis. The final borylation afforded a similar yield of products DBA-5-BT (2)29 and NA-5-BT (3) from the full-Boc-protected bromo tryptophan 11 and partial protected compound 10, respectively. As discussed above, the partial protected 10 can deactivate the Pd(0) catalysis thereby rendering the reaction incomplete. The mixture of 10 and NA-5-BT (3) is inseparable through chromatography but can be completely transformed to NA-5-BT (3) by repeating the reaction. Although, as expected, 5-bromoindole is capable of producing 5-boronopinacol indole,30 the subsequent Friedel–Crafts coupling with compound 9(31,32) failed to give any desired product. Thus, the pathway of Scheme 2 is adopted throughout this study.
Scheme 2. 5-Boronopinacol Tryptophan Analog DBA-5-BT (2) and NA-5-BT (3) Prepared from an Indole Analog.
Reaction conditions: (a) toluene, reflux, Soxhlet, 55%. (b) CH3I, K2CO3, acetone, 60-65 °C, 93%. (c) EtAlCl3, CH2Cl2, 0 °C, 85%. (d) Boc2O, DMAP, CH3CN, 89%. (e) diborono pinacol, Pd(fer)2Cl2, DMSO, KOAc, 100 °C, 2 h, 64% for DBA-5-BT (2); 65% for NA-5-BT (3) from a consecutive repeated reaction.
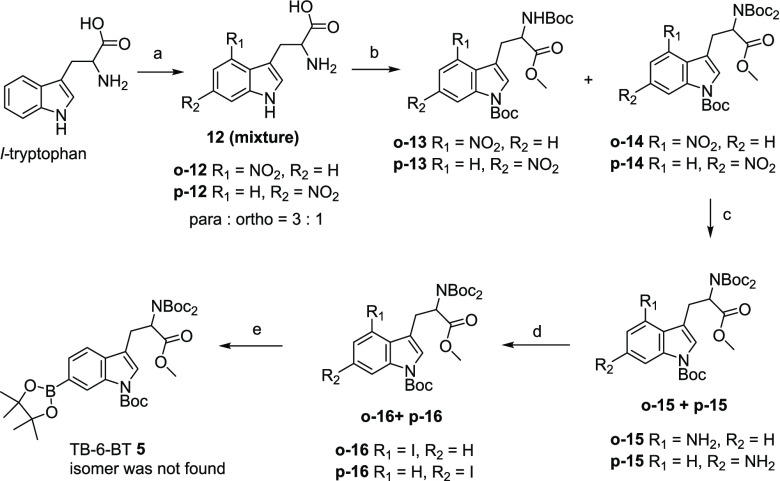
Another synthetic route to prepare a constitutional isomer of the above TB-5-BT (1), i.e., TB-6-BT (5), is illustrated in Scheme 3. Starting from the nitration reaction of the tryptophan,33 the desired nitro tryptophan was obtained as two constitutional isomers with a ratio of p-12:o-12 = 3:1. Due to the significant yield advantage by tri-Boc protected iodotryptophan, i.e. 70–90% vs 30–60% yield (triBoc vs diBoc), the tri-Boc tryptophan precursor 14 was chosen for the next preparation.22,34,35
Scheme 3. TriBoc Protected 6-Boronopinacol Tryptophan Analog TB-6-BT (5) Prepared from Tryptophan.
Reaction conditions: (a) HNO3, AcOH, 41%. (b) SOCl2, CH3OH; Boc2O, DMAP; 70% for 13 and 30% for 14 from individual preparation. (c) Zn, AcOH, 92%. (d) NaNO2, HCl (aq), KI, I2, THF, 51%. (e) Diborono pinacol, Pd(fer)2Cl2, DMSO, KOAc, 100 °C, 1 h, 63%.
Introduction of the three Bocs did not proceed smoothly. Partially protected 13 and fully protected 14 were obtained in a ratio of 1:3.36−38 Incomplete protection was due to the disturbance of the cocrystallized salt of nitrotryptophan·H2O·HNO3 from the previous nitration step. Hence, neutralization with Na2CO3 is a prerequisite before esterification. The reduction reaction using Zn powder generated the amino derivative 15 in a quantitative yield. However, in some experimental batches, the reaction was retarded due to the incomplete removal of the residual base from the previous chromatography. An additional one equivalent of Zn powder and optional solvent can drive the reaction toward completeness. Followed by a subsequent Sandmeyer reaction,39 the desired iodo tryptophan analog 16 was obtained in a 51% yield which contained an unseparated deiodinated byproduct (∼10%) even after HPLC purification. After borylation, the 6-isomer TB-6-BT (5) was obtained as the sole product from column chromatography.
The third target compound A,Z-2,7-DBT (6) with two boron atoms is thought to be more useful than the above single boron tryptophan derivatives (Scheme 4). Ir-assisted direct borylation on tryptophan has been thoroughly documented in several reports.40−42 However, intrinsic instability is associated with the diboronopinacol moieties, especially when oxidizing using H2O2.41 Whereas Ir-involved borylation did not produce a satisfactory yield of the diboron product AZ-2,7-DBT (6), the quantity was enough for the in vitro biological analysis.
Scheme 4. 2,7-Diboronopinacol Tryptophan Analog AZ-2,7-DBT 6 Prepared from Tryptophan.
Reaction conditions: (a) SOCl2, CH3OH. (b) TfN3, CuSO4, K2CO3, CH3OH, rt, over 2 steps, 69%. (c) [Ir(COD)OMe]2, (CH3)4phen, B2pin2, THF, 80 °C, 11% for AZ-2,7-DBT (6) and 18, respectively.
Cytotoxicity
The cytotoxicities of these tryptophan derivatives were assessed in three cell lines including two tumor cell lines, U87-MG and LN-229 from human, and one control cell line, 3T3 fibroblast cell from mice. The reduction potential of mitochondrial was evaluated by the formation of a dark yellow precipitate when treating WST-1 reagents with aliquots of compounds in a series of dilutions from 1 nM to 100 μM. The cell viability was thus obtained as shown in Supporting Information Figure 22 and Table 2. To prepare the samples as a solution phase, compounds were first dissolved in 100% DMSO to give 10 mM as a stock for the following dilutions. The most concentrated aliquots (100 μM) contain 1% DMSO. Except for the control 3T3 cells, both tumor cells tolerate the presence of 1% DMSO well. Furthermore, the Boc-protected derivatives, e.g., TB-6-BT (5), TB-5-BT (1), and DBA-5-BT 2, are slightly toxic against 3T3 cells but insignificant when compared with the control compound BPA. BPA was generally regarded as nontoxic.43−48
Table 2. Compound Toxicity (100 μM) against Tumor (U87-MG and LN-229) and Normal (3T3) Cellsa.
| Cell
viability (%) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Cell lines | TB-6-BT | TB-5-BT | DBA-5-BT | NA-5-BT | 5-BT | AZ-2,7-DBT | BPA | DMSO (1%) |
| U87 | 60.1 ± 1.9 | 67.4 ± 1.6 | 70.2 ± 0.1 | 66.2 ± 3.2 | 65.8 ± 3.3 | 61.3 ± 5.0 | 67.0 ± 2.0 | 97 |
| LN229 | 53.2 ± 2.0 | 60.7 ± 5.2 | 70.8 ± 1.4 | 69.9 ± 2.9 | 67.3 ± 1.9 | 57.2 ± 6.2 | 79.6 ± 3.6 | 91 |
| 3T3 | 63.6 ± 0.9 | 60.2 ± 2.3 | 66.6 ± 1.8 | 42.9 ± 0.7 | 47.6 ± 1.5 | 109.9 ± 1.1 | 53.0 ± 1.6 | 57 |
Results were tabulated from the most concentrated (100 μM) aliquots in 1% DMSO after 24-h treatment. Data were obtained in quadruplicate and were expressed as mean ± SD. GraphPad Prism 5 software was used.
In-Vitro Accumulation
The cellular accumulation of boron tryptophan derivatives was evaluated by determining the boron concentration using induced coupled mass spectrometry (ICP-MS). The three tryptophan derivatives with Boc groups including TB-5-BT (1), DBA-5-BT (2), and TB-6-BT (5) showed better accumulation than the partial protected derivatives 5-BT (4) and NA-5-BT (3) (Figure 4). Both the triBoc-protected TB-5-BT (1) and TB-6-BT (5) accumulated more than 100 ppm of 10B after 1 h incubation in LN229 cell. Moreover, TB-5-BT (1) and DBA-5-BT (2) reach a high level of 300 ppm after 2 h incubation in U87-MG cells, and TB-5-BT (1) exhibited an even higher accumulation level of 450 ppm after 1 h of incubation in LN229 cell. By comparison with the 6-boronopinacol derivative, 5-boronopinacol derivatives accumulated preferentially in U87-MG cells. Setting the 3T3 cells as a control, accumulation selectivity indices defined by the tumor vs normal (T/N) ratio of TB-6-BT 6 are >5 (T/N) 4 h after addition in both types of tumor cells (Figure 5). TB-5-BT (1) showed an average T/N > 2 and an impressive T/N > 9 in LN229 cells before 1 h. Thus, the most highly accumulated and selective TB-5-BT (1) warrants further study. Furthermore, to serve as a clinically useful BNCT agent, plenty of dosages (>1g/kg) are commonly given to patients over 2 h prior to neutron irradiation. Based on this rationale, the 6-boronopinacol derivative TB-6-BT (5) is potent because of its increasing accumulation trend. Due to the slight toxicity of 100 μM of some tryptophan derivatives (Table 2), half concentration (50 μM) was employed for neutron irradiation experiments. The results of the BNCT were assessed using WST-1 assay. After neutron irradiation, more cells treated with the most accumulated TB-5-BT (1), DBA-5-BT (2), and TB-6-BT (5) were rendered nonviable than those treated with BPA (Figure 6). Unlike TB-6-BT (1) and DBA-5-BT (2), with both showing enhanced cancerous eradication, TB-5-BT (5) in U87-MG cell is somewhat inferior probably due to the bias as indicated by the internal standard of DMSO with increasing survival rate of greater than 100%.
Figure 4.
Schematic comparison of boron accumulation by boronotryptophan derivatives in tumor (U87-MG and LN229) and normal brain cells (3T3).
Figure 5.
Schematic comparison of boron accumulation ratio (T/N) in tumor (U87-MG and LN229) vs normal brain cells (3T3).
Figure 6.
Comparison of the cell survival ratio from the treatment of boron agents (50 μM) with neutron irradiation (A and continued part B). Data were collected from quadruplicate of three sets and two sets of experiments for cancer cells (LN229 and U87-MG) and normal cell 3T3, respectively.
LAT-1 Expression Assay
It is necessary to examine whether these tryptophan derivatives can affect the expression of LAT-1. Although no direct binding assay was performed, the protein expression by Western blot can generate a qualitative comparison (Figure 7). In general, no significant inhibition of LAT-1 was observed over the 2-h time course except 3T3 cell. Especially, the two more T/N selective TB-5-BT (1) and TB-6-BT (5) can be due to the differential expression of LAT-1. In addition, the preferential accumulation of these tryptophan derivatives in tumor cells can be accounted for by two reasons. First, the hydrophobic characteristics of these compounds can facilitate diffusion across the cell membrane. Second, tumor cells tend to ingest more tryptophan derivatives as nutrition sources than normal cells do.
Figure 7.

Western blot of the LAT-1 expression level of the three cell lines after 2 h incubation of the selected tryptophan derivatives. Primary antibody LAT-1 (1:2500, Cell Signaling Tech) and α-tubulin (1:5000, GeneTex).
Conclusion
The three Boc-protected tryptophan derivatives TB-5-BT (1), DBA-5-BT (2), and TB-6-BT (5) are not soluble in aqueous solution, and hence, the question now rests on the formulation optimization for injection in an animal study (Figure 8). In brief, TB-5-BT (1) and DBA-5-BT (2) as well as TB-6-BT (5) showed better tumor accumulation and selectivity, and their in vivo application should occur in due course.
Figure 8.

Aqueous solubility test for the tryptophan derivatives (1 mM, 20% EtOH in saline).
Acknowledgments
We are grateful to the Ministry of Science and Technology of Taiwan and the Chang-Gung Medical Research Project for providing financial support via the following grants: MOST-107-2113-M-007-025-, MOST-106-2113-M-007-012-, MOST-108-2113-M-007-023-, MOST-106-2314B-182A-058-MY2, NMRPG3G612, and CMRPG3F1511.
Glossary
Abbreviations
- BNCT
boron neutron capture therapy
- LAT1
leucine-type amino acid transporter 1
- T/N
tumor to normal accumulation ratio
- BPA
boronophenyl alanine.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.0c00064.
Experimental procedures for the preparation of both described tryptophan derivatives and biological data including the cell survival assay, cellular accumulation assay, neutron irradiation, and LAT-1 blotting. Figures 1–21, 1H NMR, 13C-DEPT-135 NMR, LC-ESI-HRMS, and chromatogram; Figure 22, Cytotoxicity of boronopincaol tryptophan derivatives in various concentrations ranging from 1 nM to 100 μM. (PDF)
Author Contributions
∥ C.-M.C. and Y.-C.H. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Easaw J. C.; Mason W. P.; Perry J.; Laperriere N.; Eisenstat D. D.; Del Maestro R.; Belanger K.; Fulton D.; Macdonald D.; Glioblastoma C. Canadian recommendations for the treatment of recurrent or progressive glioblastoma multiforme. Current Oncology 2011, 18 (3), E126–E136. 10.3747/co.v18i3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. K.; Hodges T.; Arko L.; Shen M.; Dello Iacono D.; McNabb A.; Bailey N. O.; Kreisl T. N.; Iwamoto F. M.; Sul J.; Auh S.; Park G. E.; Fine H. A.; Black P. M. Scale to Predict Survival After Surgery for Recurrent Glioblastoma Multiforme. J. Clin. Oncol. 2010, 28 (24), 3838–3843. 10.1200/JCO.2010.30.0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawashiro H.; Otani N.; Shinomiya N.; Fukui S.; Ooigawa H.; Shima K.; Matsuo H.; Kanai Y.; Endou H. L-type amino acid transporter 1 as a potential molecular target in human astrocytic tumors. Int. J. Cancer 2006, 119 (3), 484–92. 10.1002/ijc.21866. [DOI] [PubMed] [Google Scholar]
- Habermeier A.; Graf J.; Sandhofer B. F.; Boissel J. P.; Roesch F.; Closs E. I. System L amino acid transporter LAT1 accumulates O-(2-fluoroethyl)-L-tyrosine (FET). Amino Acids 2015, 47 (2), 335–344. 10.1007/s00726-014-1863-3. [DOI] [PubMed] [Google Scholar]
- Fuchs B. C.; Bode B. P. Amino acid transporters ASCT2 and LAT1 in cancer: partners in crime?. Semin. Cancer Biol. 2005, 15 (4), 254–66. 10.1016/j.semcancer.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Coderre J. A.; Morris G. M. The radiation biology of boron neutron capture therapy. Radiat. Res. 1999, 151 (1), 1–18. 10.2307/3579742. [DOI] [PubMed] [Google Scholar]
- Soloway A. H.; Tjarks W.; Barnum B. A.; Rong F. G.; Barth R. F.; Codogni I. M.; Wilson J. G. The chemistry of neutron capture therapy (vol 98, pg 1515, 1998). Chem. Rev. 1998, 98 (6), 2389–2389. 10.1021/cr980493e. [DOI] [PubMed] [Google Scholar]
- Barth R. F.; Mi P.; Yang W. L. Boron delivery agents for neutron capture therapy of cancer. Cancer Communications 2018, 38, 15. 10.1186/s40880-018-0299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth R. F.; Coderre J. A.; Vicente M. G. H.; Blue T. E. Boron neutron capture therapy of cancer: Current status and future prospects. Clin. Cancer Res. 2005, 11 (11), 3987–4002. 10.1158/1078-0432.CCR-05-0035. [DOI] [PubMed] [Google Scholar]
- Alkonyi B.; Barger G. R.; Mittal S.; Muzik O.; Chugani D. C.; Bahl G.; Robinette N. L.; Kupsky W. J.; Chakraborty P. K.; Juhasz C. Accurate Differentiation of Recurrent Gliomas from Radiation Injury by Kinetic Analysis of alpha-C-11-Methyl-L-Tryptophan PET. J. Nucl. Med. 2012, 53 (7), 1058–1064. 10.2967/jnumed.111.097881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peura L.; Malmioja K.; Huttunen K.; Leppanen J.; Hamalainen M.; Forsberg M. M.; Rautio J.; Laine K. Design, Synthesis and Brain Uptake of LAT1-Targeted Amino Acid Prodrugs of Dopamine. Pharm. Res. 2013, 30 (10), 2523–2537. 10.1007/s11095-012-0966-3. [DOI] [PubMed] [Google Scholar]
- Gomes P.; Soares-da-Silva P. L-DOPA transport properties in an immortalised cell line of rat capillary cerebral endothelial cells, RBE 4. Brain Res. 1999, 829 (1–2), 143–150. 10.1016/S0006-8993(99)01387-6. [DOI] [PubMed] [Google Scholar]
- Fan C. H.; Wang T. W.; Hsieh Y. K.; Wang C. F.; Gao Z. Y.; Kim A.; Nagasaki Y.; Yeh C. K. Enhancing Boron Uptake in Brain Glioma by a Boron-Polymer/Microbubble Complex with Focused Ultrasound. ACS Appl. Mater. Interfaces 2019, 11 (12), 11144–11156. 10.1021/acsami.8b22468. [DOI] [PubMed] [Google Scholar]
- Shi Y. X.; Li J. Y.; Zhang Z. Z.; Duan D. B.; Zhang Z. C.; Liu H.; Liu T.; Liu Z. B. Tracing Boron with Fluorescence and Positron Emission Tomography Imaging of Boronated Porphyrin Nanocomplex for Imaging-Guided Boron Neutron Capture Therapy. ACS Appl. Mater. Interfaces 2018, 10 (50), 43387–43395. 10.1021/acsami.8b14682. [DOI] [PubMed] [Google Scholar]
- Kuthala N.; Vankayala R.; Li Y. N.; Chiang C. S.; Hwang K. C. Engineering Novel Targeted Boron-10-Enriched Theranostic Nanomedicine to Combat against Murine Brain Tumors via MR Imaging-Guided Boron Neutron Capture Therapy. Adv. Mater. 2017, 29 (31), 10. 10.1002/adma.201700850. [DOI] [PubMed] [Google Scholar]
- Huang Z.; Kwon O.; Huang H. Y.; Fadli A.; Marat X.; Moreau M.; Lumb J. P. A Bioinspired Synthesis of Polyfunctional Indoles. Angew. Chem., Int. Ed. 2018, 57 (37), 11963–11967. 10.1002/anie.201806490. [DOI] [PubMed] [Google Scholar]
- Katritzky A. R.; Ramsden C. A.; Joule J. A.; Zhdankin V. V.. Handbook of heterocyclic chemistry, 3rd ed.; Elsevier: Amsterdam, 2010. [Google Scholar]
- Leitch J. A.; Bhonoah Y.; Frost C. G. Beyond C2 and C3: Transition-Metal-Catalyzed C-H Functionalization of Indole. ACS Catal. 2017, 7 (9), 5618–5627. 10.1021/acscatal.7b01785. [DOI] [Google Scholar]
- Giglio B. C.; Fei H. Y.; Wang M. Z.; Wang H.; He L.; Feng H. J.; Wu Z. H.; Lu H. J.; Li Z. B. Synthesis of 5-[F-18]Fluoro-alpha-methyl Tryptophan: New Trp Based PET Agents. Theranostics 2017, 7 (6), 1524–1530. 10.7150/thno.19371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isidro-Llobet A.; Alvarez M.; Albericio F. Amino Acid-Protecting Groups. Chem. Rev. 2009, 109 (6), 2455–2504. 10.1021/cr800323s. [DOI] [PubMed] [Google Scholar]
- Jarowicki K.; Kocienski P. Protecting groups. Journal of the Chemical Society-Perkin Transactions 2001, 1 (18), 2109–2135. 10.1039/b103282h. [DOI] [Google Scholar]
- Jia Y. X.; Bois-Choussy M.; Zhu J. P. Synthesis of DEFG ring of complestatin and chloropeptin I: Highly atropdiastereoselective macrocyclization by intramolecular Suzuki-Miyaura reaction. Org. Lett. 2007, 9 (12), 2401–2404. 10.1021/ol070889p. [DOI] [PubMed] [Google Scholar]
- Shinohara T.; Deng H. B.; Snapper M. L.; Hoveyda A. H. Isocomplestatin: Total synthesis and stereochemical revision. J. Am. Chem. Soc. 2005, 127 (20), 7334–7336. 10.1021/ja051790l. [DOI] [PubMed] [Google Scholar]
- Yokoyama Y.; Hikawa H.; Mitsuhashi M.; Uyama A.; Hiroki Y.; Murakami Y. Total synthesis without protection: Three-step synthesis of optically active clavicipitic acids by a biomimetic route. Eur. J. Org. Chem. 2004, 6, 1244–1253. 10.1002/ejoc.200300603. [DOI] [Google Scholar]
- Kadayifcioglu N.; Acar H. Y. Synthesis of poly(2-acetamidoacrylic acid) and its PEGMA block copolymer via ATRP in water. Eur. Polym. J. 2013, 49 (10), 3366–3376. 10.1016/j.eurpolymj.2013.07.014. [DOI] [Google Scholar]
- Dedeoglu B.; Ugur I.; Degirmenci I.; Aviyente V.; Barcin B.; Cayli G.; Acar H. Y. First RAFT polymerization of captodative 2-acetamidoacrylic acid (AAA) monomer: An experimental and theoretical study. Polymer 2013, 54 (19), 5122–5132. 10.1016/j.polymer.2013.07.028. [DOI] [Google Scholar]
- Ha E. J.; Kim B. S.; Park C. H.; Lee J. O.; Paik H. J. Electroactive hydrogel comprising poly(methyl 2-acetamido acrylate) for an artificial actuator. J. Appl. Phys. 2013, 114 (5), 054701. 10.1063/1.4815932. [DOI] [Google Scholar]
- Rothstein E. Experiments in the Synthesis of Derivatives of Alpha-Aminoacrylic Acid from Serine and N-Substituted Serines. J. Chem. Soc. 1949, 0 (Aug), 1968–1972. 10.1039/JR9490001968. [DOI] [Google Scholar]
- Xie Y.-T.Preparation and Biological Assessment of 6-Boronopinacol Tryptophan Analogs and 5-Boronfenbufen for Boron Neutron Capture Therapy; National Tsing Hua University, 2018. [Google Scholar]
- Bartolucci S.; Bartoccini F.; Righi M.; Piersanti G. Direct, Regioselective, and Chemoselective Preparation of Novel Boronated Tryptophans by Friedel-Crafts Alkylation. Org. Lett. 2012, 14 (2), 600–603. 10.1021/ol203216h. [DOI] [PubMed] [Google Scholar]
- Simons C.; Hanefeld U.; Arends I. W. C. E.; Sheldon R. A.; Maschmeyer T. Noncovalent anchoring of asymmetric hydrogenation catalysts on a new mesoporous aluminosilicate: Application and solvent effects. Chem. - Eur. J. 2004, 10 (22), 5829–5835. 10.1002/chem.200400528. [DOI] [PubMed] [Google Scholar]
- Gladiali S.; Pinna L. Asymmetric Hydroformylation of N-Acyl 1-Aminoacrylic Acid-Derivatives by Rhodium/Chiral Diphosphine Catalysts. Tetrahedron: Asymmetry 1991, 2 (7), 623–632. 10.1016/S0957-4166(00)86114-5. [DOI] [Google Scholar]
- Tomon Moriya K. H. A Facile Synthesis of 6-Chloro-D-tryptophan. Bull. Chem. Soc. Jpn. 1975, 48, 2217–2218. 10.1246/bcsj.48.2217. [DOI] [Google Scholar]
- Michaux J.; Retailleau P.; Campagne J. M. Synthesis of the central tryptophan-leucine residue of celogentin C. Synlett 2008, 10, 1532–1536. 10.1055/s-2008-1078411. [DOI] [Google Scholar]
- Zlatopolskiy B. D.; Zischler J.; Schafer D.; Urusova E. A.; Guliyev M.; Bannykh O.; Endepols H.; Neumaier B. Discovery of 7-[F-18]Fluorotryptophan as a Novel Positron Emission Tomography (PET) Probe for the Visualization of Tryptophan Metabolism in Vivo. J. Med. Chem. 2018, 61 (1), 189–206. 10.1021/acs.jmedchem.7b01245. [DOI] [PubMed] [Google Scholar]
- Li L.; Hu W. M.; Jia Y. X. Synthetic studies of cyclic peptides stephanotic acid methyl ester, celogentin C, and moroidin. Tetrahedron 2014, 70 (42), 7753–7762. 10.1016/j.tet.2014.05.082. [DOI] [Google Scholar]
- Hu W. M.; Zhang F. Y.; Xu Z. R.; Liu Q.; Cui Y. X.; Jia Y. X. Stereocontrolled and Efficient Total Synthesis of (−)-Stephanotic Acid Methyl Ester and (−)-Celogentin C. Org. Lett. 2010, 12 (5), 956–959. 10.1021/ol902944f. [DOI] [PubMed] [Google Scholar]
- Li B. T. Y.; White J. M.; Hutton C. A. Synthesis of the Leu-Trp Component of the Celogentin Family of Cyclic Peptides Through a C-H Activation-Cross-Coupling Strategy. Aust. J. Chem. 2010, 63 (3), 438–444. 10.1071/CH10033. [DOI] [Google Scholar]
- Hodgson H. H. The Sandmeyer Reaction. Chem. Rev. 1947, 40 (2), 251–277. 10.1021/cr60126a003. [DOI] [PubMed] [Google Scholar]
- Mkhalid I. A. I.; Barnard J. H.; Marder T. B.; Murphy J. M.; Hartwig J. F. C-H Activation for the Construction of C-B Bonds. Chem. Rev. 2010, 110 (2), 890–931. 10.1021/cr900206p. [DOI] [PubMed] [Google Scholar]
- Homer J. A.; Sperry J. A short synthesis of the endogenous plant metabolite 7-hydroxyoxindole-3-acetic acid (7-OH-OxIAA) using simultaneous C-H borylations. Tetrahedron Lett. 2014, 55 (42), 5798–5800. 10.1016/j.tetlet.2014.08.104. [DOI] [Google Scholar]
- Loach R. P.; Fenton O. S.; Amaike K.; Siegel D. S.; Ozkal E.; Movassaghi M. C7-Derivatization of C3-Alkylindoles Including Tryptophans and Tryptamines. J. Org. Chem. 2014, 79 (22), 11254–11263. 10.1021/jo502062z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simone U.; Manzo L.; Ferrari C.; Bakeine J.; Locatelli C.; Coccini T. Short and long-term exposure of CNS cell lines to BPA-f a radiosensitizer for Boron Neutron Capture Therapy: safety dose evaluation by a battery of cytotoxicity tests. NeuroToxicology 2013, 35, 84–90. 10.1016/j.neuro.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Imperio D.; Del Grosso E.; Fallarini S.; Lombardi G.; Panza L. Anomeric sugar boronic acid analogues as potential agents for boron neutron capture therapy. Beilstein J. Org. Chem. 2019, 15, 1355–1359. 10.3762/bjoc.15.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperio D.; Del Grosso E.; Fallarini S.; Lombardi G.; Panza L. Synthesis of Sugar-Boronic Acid Derivatives: A Class of Potential Agents for Boron Neutron Capture Therapy. Org. Lett. 2017, 19 (7), 1678–1681. 10.1021/acs.orglett.7b00382. [DOI] [PubMed] [Google Scholar]
- Luderer M. J.; Muz B.; de la Puente P.; Chavalmane S.; Kapoor V.; Marcelo R.; Biswas P.; Thotala D.; Rogers B.; Azab A. K. A Hypoxia-Targeted Boron Neutron Capture Therapy Agent for the Treatment of Glioma. Pharm. Res. 2016, 33 (10), 2530–2539. 10.1007/s11095-016-1977-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y.; Asano T.; Niki Y.; Kondoh H.; Kirihata M.; Yamaguchi Y.; Wakamiya T. Study on the compounds containing F-19 and B-10 atoms in a single molecule for the application to MRI and BNCT. Bioorg. Med. Chem. 2006, 14 (10), 3258–3262. 10.1016/j.bmc.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Hattori Y.; Kurihara K.; Kondoh H.; Asano T.; Kirihata M.; Yamaguchi Y.; Wakamiya T. Biological evaluation of fluorinated p-boronophenylalanine derivatives as a boron carrier. Protein Pept. Lett. 2007, 14 (3), 269–272. 10.2174/092986607780090856. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.