Abstract
Alkaloids are a large cluster of molecules found in Mother Nature all over the world. They are all secondary compounds and collection of miscellaneous elements and biomolecules, derived from amino acids or from transamination. This diverse chemical group is categorized, based on the amino acids that deliver their nitrogen atom and part of their skeleton. Alkaloids from a similar origin or having the same basic nucleus may have dissimilar biosynthetic pathways and different biological activity. They are derived from l-phenylalanine, l-tyrosine, anthranilic acid or acetate, l-histidine, l-ornithine, nicotinic acid, and l-lysine. Apart from other types of alkaloids, indole, tropane, and isoquinoline alkaloids are very important. People from all over the world are using them in their everyday life. Alkaloids can also have an animal origin, which may be endogenous or exogenous. This chapter highlights the phytochemistry of the main representatives among the diverse group of alkaloids. This chapter also focuses on their extraction, purification, fractionation, identification, and quantification procedures. A fundamental understanding of the biological activity of important alkaloids is also highlighted in this chapter. With the potential of revealing new compounds and diverse pharmacological properties, alkaloids still embrace a great potential for the future of drug discovery.
Keywords: Indole alkaloid, Tropane alkaloid, Isoquinoline alkaloid, Phytochemistry, Biological activity
15.1. Introduction
Plants are the renowned cradle of traditional medicine system that assuages human diseases and promotes health for thousands of years (Rupani and Chavez, 2018; Sadia et al., 2018). Plants are a rich reservoir of a vast array of active constituents that have significant therapeutic applications like antiviral, anticancer, analgesic, antitubercular (Ishtiyak and Hussain, 2017; Uniyal et al., 2006). Among them, alkaloids are the important secondary metabolites that were initially discovered and used as early as 4000 years ago and are well recognized for their rich therapeutic potential (Amirkia and Heinrich, 2014). Based on their heterocyclic ring system and biosynthetic precursor, alkaloids are classified into diverse categories, viz. indole, purine, quinoline, isoquinoline, tropane, imidazole, among others (Kaur and Arora, 2015; Roy, 2017). Alkaloids have antiproliferative, antibacterial, antioxidant potential, which can be used for the development of drugs (Qiu et al., 2014). This therapeutic potential of alkaloids grows up their industrial application. Numerous research have been carried out in pharmaceutical properties of different alkaloids extracted from plants. In this chapter, we will systematically summarize the chemistry, isolation and identification techniques, biological activities along with potential applications in a single platform.
Alkaloids are an assembly of naturally occurring chemical composites, which typically comprise basic nitrogen atoms. They may also contain some neutral or weakly acidic compounds (Manske and Holmes, 2014; McNaught and McNaught, 1997). Few synthetic compounds are also considered as alkaloids too (Lewis, 1998). Apart from carbon, nitrogen, or hydrogen, alkaloids may comprise sulfur and rarely bromine, phosphorus, or chlorine (Knunyants and Zefirov, 1988).
These secondary metabolites are formed by a large variety of entities, including plants, animals, fungi, and bacteria. Because of their vast array of pharmacological actions (anticancer, antimalarial, anesthetic, stimulant), they are purified from the crude extract by acid-base extraction (Ziegler and Facchini, 2008).
The word “alkaloid” was first coined by the German chemist Carl F. W. Meissner in 1819, derived from the Arabic name al-qali, which is associated to the plant from which soda was first sequestered (Croteau et al., 2000). Alkaloids are low-molecular-weight structures and form approximately 20% of plant-based secondary metabolites (Kaur and Arora, 2015). So far, approximately 12,000 alkaloids are isolated from various genera of the plant kingdom (Kaur and Arora, 2015).
Alkaloids are mostly solids and are known to occur in higher plants. They are prevalent in the plants belonging to the following botanical families: Apocynaceae, Annonaceae, Amaryllidaceae, Berberidaceae, Boraginaceae, Gnetaceae, Liliaceae, Leguminoceae, Lauraceae, Loganiaceae, Magnoliaceae, Menispermaceae, Papaveraceae, Piperaceae, Rutaceae, Rubiaceae, Ranunculaceae, Solanaceae, etc. (Boit, 1961).
15.2. Phytochemistry and classification of alkaloids
15.2.1. Classification established upon the biogenesis
Alkaloids illustrate large diversity not only in their botanical and biochemical origin but also in structure and pharmacological action. In this connection, various systems of classification are possible. From a structural perception, alkaloids can be classified, based on their molecular precursor, structures, and origins or on the biological pathways used to obtain the molecule.
There are three central types of alkaloids: (1) true alkaloids, (2) protoalkaloids, and (3) pseudoalkaloids. True alkaloids and protoalkaloids are produced from amino acids, whereas pseudoalkaloids are not derived from these compounds.
15.2.1.1. True alkaloids
This type of alkaloids are obtained from amino acids and they share a nitrogen-containing heterocyclic ring. They are highly reactive in nature and have potent biological activity. They form water-soluble salts, and many of them are crystalline in nature, which conjugates with acid and forms a salt. Almost all true alkaloids are bitter in taste and solid, except nicotine, which is a brown liquid (Aniszewski, 2007).
Their occurrence in plants occurs in three forms: (a) in Free-state, (b) as N-oxide, or (c) as salts. Various amino acids like l-phenylalanine/l-tyrosine, l-ornithine, l-histidine, l-lysine are the main sources of true alkaloids (Table 15.1 ) (Dewick, 2002; Pelletier, 1999). Cocaine, morphine, quinine are the common true alkaloids found in nature.
Table 15.1.
Amino acid and their involvement in alkaloid synthesis (Aniszewski, 1994, Aniszewski, 2007; Bentley, 2006; Chini et al., 1992; Fusco and Giacovazzo, 1997; Hartmann, 1999; Ihara and Fukumoto, 1996; Leonard, 1999).
| Alkaloid type | Major group of alkaloid | Chemical group of alkaloid | Amino acid precursor |
|---|---|---|---|
| Tryptophan-derived alkaloids | True alkaloid | Ergot alkaloids Pyrroloindole alkaloids Indole alkaloids Aspidosperma alkaloids Quinoline alkaloids |
l-Threonine l-Proline l-Tryptophan l-Serine |
| Protoalkaloids | Terpenoid indole alkaloids | ||
| Arginine-derived alkaloids | True alkaloid | Marine alkaloids |
l-Asparagine l-Alanine l-Aspartic acid l-arginine |
| Ornithine-derived alkaloids | True alkaloid | Pyrrolizidine alkaloids Tropane alkaloids Pyrrolidine alkaloids |
l-Ornithine |
| Histidine-derived alkaloids | True alkaloid | Manzamine alkaloids Imidazole alkaloids |
l-Histidine |
| Nicotinic acid-derived alkaloids | True alkaloid | Sesquiterpene pyridine alkaloids Pyridine alkaloids |
Nicotinic acid |
| Lysine-derived alkaloids | True alkaloid | Indolizidine alkaloids Quinolizidine alkaloids Piperidine alkaloids |
l-Lysine l-Leucine l-Isoleucine |
| Anthranilic acid-derived alkaloids | True alkaloid | Acridine alkaloids Quinoline alkaloids Quinazoline alkaloids |
Anthranilic acid |
| Tryptophan-derived alkaloids | Protoalkaloids | Terpenoid indole alkaloids |
l-Threonine l-Proline l-Tryptophan l-Serine |
| Tyrosine-derived alkaloids | Protoalkaloids | Phenylethylamine alkaloids | l-Tyrosine |
15.2.1.2. Protoalkaloids
This type of alkaloids contains a nitrogen atom, which is derived from an amino acid but is not part of the heterocyclic ring system. l-Tryptophan and l-tyrosine are the main precursors of this type of alkaloids. This minor group is structurally composed of simple alkaloids. Yohimbine, mescaline, and hordenine are the main alkaloids of this type. They are used in various health disorders, including mental illness, pain, and neuralgia (Chini et al., 1992).
15.2.1.3. Pseudoalkaloids
The basic carbon skeleton of pseudoalkaloids is not directly derived from amino acids; instead, they are connected with amino acid pathways where they are derived from by amination or transamination reaction from forerunners or postcursors of amino acid (Dewick, 2002; Jakubke and Jeschkeit, 1994). Nonamino-acid precursors can also produce pseudoalkaloids. They can be phenylalanine or acetate derived. Capsaicin, caffeine, ephedrine are very common examples of pseudoalkaloids (Table 15.2 ).
Table 15.2.
Involvement of parent compound in pseudoalkaloids synthesis (Aniszewski, 2007).
| Parent compounds | Precursor compound | Chemical group of alkaloids | Examples |
|---|---|---|---|
| Terpenoid | Geraniol | Terpenoid Alkaloids |
Gentianine Aconitine β-Skytanthine Actinidine |
| Sesquiterpene | Acetate | Sesquiterpene Alkaloids |
Evonoline Cassinine Evorine Celapanin |
| Phenyl | Ferulic acid | Aromatic Alkaloids |
Capsaicin |
| Piperidine | Acetate | Piperidine Alkaloids |
Pinidine Coniceine Coniine |
| Purine | Adenine/Guanine | Purine alkaloids | Theophylline Theobromine Caffeine |
15.2.2. Classification established upon the ring structure
This is the most comprehensively established classification, based on the presence of a basic heterocyclic nucleus in their structure.
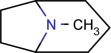
15.2.2.1. Tropane alkaloid (Biastoff and Dräger, 2007; Gossauer, 2003; Hemscheidt, 2000)
This category of alkaloids has tropane (C4N skeleton) nucleus. They are abundantly found in the Solanaceae family. They are derived from ornithine and acetoacetate. Structurally, pyrrolines are the precursor of these type of alkaloids. Maximum of them are esters of mono, di, trihydroxytropane, having a wide range of hydroxylation arrangements. Cocaine, atropine, scopolamine, and their derivatives are widely studied since the 19th century because of their enormous pharmacological actions (Fig. 15.1 ).
Fig. 15.1.
Basic structure of the tropane nucleus.
15.2.2.2. Pyrrolizidine alkaloids (Hartmann and Ober, 2000; Liddell, 2002; Rizk, 1990; Robins, 1982; Schardl et al., 2007; Wrobel, 1985)
The pyrrolizidine nucleus is distinctive of this group of alkaloids. They occur in the plants from Asteraceae and Fabaceae family. Majority of pyrrolizidine alkaloids occur in the plants as N-oxides, whose role being lost during the isolation process. They have extensively reviewed alkaloids because of their toxic effects, especially liver damage. These alkaloids enter into the food chain and become antifeedants for the animals who eat them. Senecionine is the popular alkaloid of this type (Fig. 15.2 ).
Fig. 15.2.
Basic structure of the Pyrrolizidine nucleus.
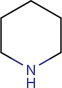
15.2.2.3. Piperidine alkaloids (Strunz and Findlay, 1985)
Piperidine nucleus is the basic ring system of this group of alkaloids. Monocycle compounds with the C5N nucleus is the important feature of true piperidine alkaloids. Presence of odor is the common feature of piperidine alkaloids. They exert chronic neurotoxicity. Many of them are originated from plants. Although piperidine itself is a lysine-derived alkaloid, some of the piperidine alkaloids also derived from acetate, acetoacetate, in an analogous fashion to the simple pyrrolidine alkaloids. Lobeline is one of the important alkaloids in this group (Fig. 15.3 ).
Fig. 15.3.
Basic structure of the piperidine nucleus.
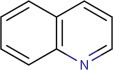
15.2.2.4. Quinolines alkaloid (Michael, 2008; Soares et al., 2007)
This type of quinolone-nucleus-containing alkaloid is achieved exclusively from the bark of the Cinchona plant. But a variety of simple heteroaromatic quinolines are also isolated from various marine sources (4,8-quinolinediol from cephalopod ink and 2-heptyl-4-hydroxyquinoline from a marine pseudomonad). The major alkaloid of this specific group is cinchonine, cinchonidine, quinine, and quinidine (Fig. 15.4 ).
Fig. 15.4.
Basic structure of the Quinoline nucleus.
15.2.2.5. Isoquinoline alkaloids (Baker, 1996; Bentley, 2006; Hu et al., 2003a; Schiff Jr, 1987; Thomas, 2004)
Isoquinoline alkaloids are an extremely large group of alkaloids mostly occurring in higher plants, but few groups are also isoquinolinoid marine alkaloids. Isoquinoline nucleus is the basic structural feature. These groups of alkaloids have huge types of medicinal properties like antiviral, antifungal, anticancer, antioxidant, antispasmodic, and an enzyme inhibitor. Morphine and codeine are the major and widely studied isoquinoline alkaloids. They are derived from tyrosine or phenylalanine. They are made from a predecessor of dopamine (3,4-dihydroxytryptamine) associated with a ketone or aldehyde. This group of alkaloids is further classified as follows: Simple isoquinoline alkaloids (e.g., salsoline, mimosamycin), benzylisoquinoline alkaloids (e.g., reticuline, imbricatine), bisbenzylisoquinoline alkaloids (e.g., fumaricine), manzamine alkaloids (e.g., manzamine a), pseudobenzylisoquinoline alkaloids (e.g., polycarpine, ledecorine), secobisbenzylisoquinoline alkaloids (e.g., baluchistanamine), bisbenzylisoquinoline alkaloids containing one ether link (e.g., dauricine), bisbenzylisoquinoline alkaloids containing two ether links (e.g., berbamine), bisbenzylisoquinoline alkaloids containing aryl links only (e.g., pisopowetine), bisbenzylisoquinoline alkaloids containing one aromatic link and one or two ether links (e.g., rodiasine) (Fig. 15.5 ).
Fig. 15.5.
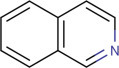
Basic structure of the isoquinoline nucleus.
15.2.2.6. Indole alkaloids (Fresneda and Molina, 2004; Kam and Choo, 2006; Kawasaki and Higuchi, 2005; Knölker, 2008; Mukherjee and Menge, 2000; Schardl et al., 2006; Somei and Yamada, 2004; Somei et al., 2000)
This is the largest and most interesting alkaloid group derived from tryptophan. The important alkaloids from this group include simple tryptamine derivatives, carbazoles (where the ethanamine chain has been lost), a diversity of alkaloids where one or more prenyl residues are combined with tryptamine, and others where integration of regular monoterpenoid or diterpenoid units occurred. Although structural diversity varies according to the terrestrial and marine source, classical research studies have been carried out on alkaloids from both origins and the fungal source. Polyhalogenation is a common feature of these alkaloids. They are further classified as follows: simple indole alkaloids (e.g., Aplysinopsin, Gramine), bisindoles (e.g., Indirubin, 6,6′-Dibromoindigotin), simple tryptamine alkaloids (e.g., Tryptamine), cyclotryptamine alkaloids (e.g., Physostigmine), quinazolinocarbazole alkaloids (e.g., Rutaecarpine), β-carboline alkaloids (e.g., Harman), carbazole alkaloids (e.g., Ekeberginine), indolonaphthyridine alkaloids (e.g., Canthin-6-one), ergot alkaloids (e.g., ergotamine) (Fig. 15.6 ).
Fig. 15.6.
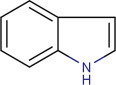
Basic structure of the indole nucleus.
15.2.2.7. Steroidal alkaloids (Ata and Andersh, 2008; Dey et al., 2018; Habermehl, 1967; Keeler, 1986; Ripperger, 1998; Tomko and Votický, 1973; Zhao et al., 2015)
1,2-Cyclopentane phenanthrene ring system is the characteristic of this type of alkaloids. They are typically originated from higher plants, which belong to Liliaceae, Solanaceae, Apocynaceae, Buxaceae families, but some are also isolated from amphibians too. These alkaloids are divided into various other subtypes, among them various types of aminopregnanes are the simplest type. The others types of steroidal alkaloids are Salamandra type (e.g., cycloneosamandione), jerveratrum type (e.g., jervine), spirosolane type (e.g., soladulcidine), solanidine type (e.g., rubijervine), cerveratrum type (e.g., 3,6-cevanediol), conanine type (e.g., didymeline), Buxus type (e.g., cyclobuxine), pregnane type (e.g., 20α-dimethylamino-3β-senecioylamino-16β-hydroxy-pregn-5-ene), cephalostatins/ritterazines (e.g., ritterazines a), miscellaneous steroidal alkaloids (e.g., bufotoxin) (Fig. 15.7 ).
Fig. 15.7.
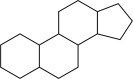
Basic structure of the steroidal alkaloid nucleus.
15.2.2.8. Imidazole alkaloid (Jin, 2006; Maat and Beyerman, 1984)
The imidazole ring structure is the characteristic of this type of alkaloid. The imidazole ring of these alkaloids is previously made at the stage of the precursor, so they are an exemption in the transformation procedure of structures. This type of alkaloids contains numerous structurally different examples, particularly among marine and microbial alkaloids. They display a wide array of biological activities and significant pharmaceutical potential. Pilocarpine is the most pharmaceutically significant imidazole alkaloid (Fig. 15.8 ).
Fig. 15.8.
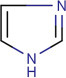
Basic structure of the imidazole nucleus.
15.2.2.9. Purine alkaloids (Ashihara et al., 2013; Lean et al., 2011; Rosemeyer, 2004)
Purine is the nitrogenous base of nucleotide (building block of DNA and RNA), which consist of purine ring and pentose sugar along with another base pyrimidine. Caffeine, Theophylline and Theobromine are typical examples of purine alkaloids. They are popular as plant alkaloids, but they can be also originated in marine organisms with substituted purines (e.g., Phidolopin) and a variety of terpenoid-purine alkaloids, such as the age lines and others (Fig. 15.9 ).
Fig. 15.9.
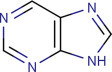
Basic structure of the purine nucleus.
15.2.2.10. Pyrrolidine alkaloids (Cheeke, 1988; Robertson and Stevens, 2014)
Pyrrolidine (C4N skeleton) nucleus constitutes the basic nucleus of pyrrolidine alkaloids. Many pyrrolidine alkaloids are known from plants. Hygrine (biosynthesized from ornithine), ficine (where the pyrrolidine ring is involved to a flavone nucleus), and brevicolline (wherein it is attached to a β-carboline unit) are some examples of this type of alkaloids (Fig. 15.10 ).
Fig. 15.10.
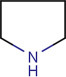
Basic structure of the pyrrolidine nucleus.
15.3. The biological activity of alkaloids
Plant secondary metabolites are a diverse range of biologically active molecules having multiple pharmacological actions like antimicrobial, stimulant, analgesic, anthelmintic, anticoagulant, antiacne, and antioxidant, among others (Debnath et al., 2012; Dey and Bhakta, 2012, Dey and Bhakta, 2013; Dey et al., 2012b, Dey et al., 2013, Dey et al., 2014; Karuna et al., 2018a, Karuna et al., 2018b; Kundu et al., 2019). Different synthetic compounds show potential biological activity in vitro as well as in vivo (Jeon et al., 2017; Jeong et al., 2017a, Jeong et al., 2017b; Kim et al., 2018; Lee et al., 2018, 2019; Richa et al., 2019; Tae et al., 2018), and various formulations of existing drugs are also developed to increase the safety profile (Gorain et al., 2014; Karmakar et al., 2015; Maity et al., 2016). In spite of this technological development, humans from almost every culture are efficiently employing the plant-derived compounds for the anticipation and management of multiple health disorders for many centuries (Dey et al., 2012a, Dey et al., 2012c).
15.3.1. The biological activity of indole alkaloids
Among all indole alkaloids, reserpine (antihypertensive agent) from Rauvolfia serpentine (Sagi et al., 2016), vinblastine and vincristine (antitumor lead) from Catharanthus roseus (El-Sayed and Verpoorte, 2007) are the most significant. Other indole alkaloids also possess essential and potent pharmacological activities such as antimicrobial, antifungal, CNS stimulant, antiviral. Marine-derived indole alkaloids are very promising and an active group of molecules. They possess various biological activities like antiparasitic, cytotoxic, serotonin and antagonistic realms, antiinflammatory, and antiviral (Gul and Hamann, 2005).
This exclusive type of phytochemicals possesses miscellaneous therapeutic and pharmacological activities which will be discussed in this section.
15.3.1.1. Antimicrobial/antiparasitic activity (Grellier et al., 1999)
Vincristine and vinblastine were isolated from the periwinkle plant C. roseus, also known as V. rosea belongs to the family Apocynaceae and discovered by Robert Noble and Charles Beer of Canada in the 1950s (Anitha, 2016). Trypanosomiasis is an insect-borne disease, which is caused by Trypanosoma cruzi effects in human and other animals. They showed differential effects on the cell division of T. cruzi epimastigote forms in a dose-dependent manner. At a concentration of 50 μM (vincristine) and 15 μM (vinblastine), they avoid both nuclear division and cytokinesis, and also affect cell shape. Whereas at 10 μM (vincristine) and 3 μM (vinblastine) concentration, cytokinesis was repressed without effect on cell-cycle progression. Variations of interactions between microtubules and associated proteins by vincristine and vinblastine may be primarily responsible for the suppression of cytokinesis, rather than from inhibition of microtubule dynamics, which is usually anticipated for these indole alkaloids.
Caboxine A (isolated from air-dried and powdered leaves and bark of Aspidosperma rigidum) had noteworthy antiparasitic activity at a dose of 100 μg/mL and was toxic against Leishmania infantum than T. cruzi with an 82.13% and 69.92% mortality, respectively. Caboxine B was active against T. cruzi with 68.92% mortality of the parasite, while carapanaubine was inactive. None of these compounds were toxic against mammalian CHO cells (Reina et al., 2011).
Reserpine showed potential antimycobacterial activity against Mycobacterium tuberculosis, strain H37Rv, and antioxidant activities. Reserpine displays 55% of growth inhibition of M. tuberculosis H37Rv (ATCC 27294) at 6.25 μg/mL concentration (Begum et al., 2012).
Halocyamine A inhibited the growth of several Gram-positive bacteria, including Bacillus subtilis, Bacillus megaterium, Bacillus cereus, and also the yeast Candida neoformans with a MIC 150, 50, 100, and 100 μg/mL, respectively (Azumi et al., 1990).
Nortopsentins A, B, and C displayed reasonable antifungal activity against Candida albicans (Sakemi and Sun, 1991).
Eudistomin E inhibits the growth of Escherichia coli and Penicillium atrovenetum (Gul and Hamann, 2005).
Dragmacidin D introverted the growth of several gram-positive and gram-negative microbes, including E. coli, B. subtilis with MIC values of 15.6 and 3.1 μg/mL, respectively. It also inhibits the growth of several opportunistic yeasts like Candida aeruginosa, C. albicans, and C. neoformans at a MIC of 62.5, 15.6, and 3.9 μg/mL, correspondingly (Wright et al., 1992).
Styelin D exhibited potent antimicrobial activity against methicillin-resistant and susceptible strain of Staphylococcus aureus. It has also an effect on the outer and inner membranes on E. coli. The penetration into outer membrane by Nitrocefin (a β-lactamase substrate) and inner membrane by o-nitrophenyl β-d-galactopyranoside (a β-galactosidase substrate) is possible after the treatment of Styelin D (Taylor et al., 2000).
Chelonin A exhibited antibacterial activity against the gram-positive bacteria B. subtilis at a concentration of 100 μg/disk (Bobzin and John Faulkner, 1991).
Pibocin B showed potential growth inhibition against B. subtilis, C. albicans, and S. aureus (Makarieva et al., 2001).
Caulerpin inhibits the growth of M. tuberculosis strain, H37Rv at an IC50 of 0.24 μM (Chay et al., 2014).
Vincamine appeared as the most active antibacterial agent against E. coli, Pseudomonas aeruginosa, S. aureus, B. subtilis with MIC values between 2 and 8 μg/mL. This compound also having anti-Candida activity at 4 μg/mL (Özçelik et al., 2011).
15.3.1.2. Anticancer activity (Ferguson et al., 1984)
The vinca alkaloids, vincristine, and vinblastine have mostly been used as a chemotherapeutic agent in the treatment of cancer. They potentially inhibit the growth of multiple cancer cell lines, like mouse neuroblastoma cells, human leukemia HL-60 cells, HeLa cells, mouse lymphoma S49 cells, mouse leukemia L1210 cells with an IC50 of 33 and 15 nM, 4.1 and 5.3 nM, 1.4 and 2.6 nM, 5 and 3.5 nM, 4.4 and 4.0 nM, respectively. The cytotoxic activity of vinca alkaloids (vincristine and vinblastine) is mainly attributed to the interruption of mitotic spindle assembly via the interaction with tubulin in the microtubules, which comprise the mitotic spindles, and ultimately triggering the metaphase arrest (Gupta et al., 2016; Mohapatra and Mittra, 2016; Seleim et al., 2014; Singh and Prasad, 2014; Sinha, 2014; Abduyeva-Ismayilova, 2016; Thivya et al., 2014). Other biochemical pathways, which are triggered by these alkaloids, may or may not be involved in their influence on microtubules.
In particular, vinca alkaloids bind precisely to the receptor sites on β- tubulin (vincristine binds strongly and intermediate level of binding by vinblastine) and detach it from the colchicine, guanosine-5′-triphosphate, taxanes, and podophyllotoxin. At the end of microtubule, there exist 16–17 high-affinity binding sites and in every mole of tubulin dimer, two vinca alkaloid-binding sites are present. Binding of the vinca alkaloids to the binding sites blocks its capability to polymerize with β-tubulin into microtubules. This enables vinca alkaloids in inhibiting in cell-cycle progression via mitosis and kill actively dividing cells (Creamer and Baucom, 2013; Dang, 2013; Dewir et al., 2014; Pillai and Jayaraj, 2015; Nada et al., 2017; Nomura and Shiina, 2016; Abduyeva-Ismayilova, 2016; Vaidya et al., 2014).
Valparicine, the pentacyclic indole alkaloid of the pericine type, was isolated from a Malayan Kopsia (Lim et al., 2006, Lim et al., 2007). Valparicine was found to exhibit strong cytotoxicity activity toward drug-sensitive and drug-resistant KB cells (KB/VJ300) as well as Jurkat cells with an IC50 of 13.0, 2.72 and 0.91 μM, respectively.
Vallesiachotamine (isolated from the leaves of Palicourea rigida) possesses significant anticancer activity against human (SK-MEL-37) melanoma cells with a possible mechanism of apoptosis. The inhibitory concentration (IC50) against SK-MEL-37 cells was 14.7 ± 1.2 μM for 24 h of drug treatment. Flow cytometry analysis revealed that vallesiachotamine induced cell-cycle arrest in G0/G1 phase and increased the percentage of sub-G1 hypodiploid cells at 11 and 22 μM concentration, and the outcome was not reliant on the time of incubation. Vallesiachotamine at a concentration of 50 μM for 24 h caused extensive cytotoxicity and necrosis (Soares et al., 2012).
The brominated tris-indole alkaloid gelliusine A (isolated from a deep-water new Caledonian sponge). The crude extract of the sponge possesses weak cytotoxic activity against KB, P388, P388/dox, HT29 and NSCLCN6 cell lines (IC50 is 10–20 μg/mL for each test system) (Christopherson, 1985).
The in vitro IC50 of Dragmacidin (isolated from a deep-water marine sponge Dragmacidin sp.) against P-388 cell lines was 15 and 1–10 μg/mL against A-549 (human lung cancer cell lines), HCT-8 (human colon cancer cell lines), and MDA-MB (human mammary cancer cell lines) (Kohmoto et al., 1988; Gul and Hamann, 2005).
A potent antitumor antibiotic Staurosporine [isolated from Streptomyces staurosporeus Awaya (AM-2282)] (Omura et al., 1977). and other actinomycetes, including Streptomyces actuosus and Streptomyces strain M-193) (Oka et al., 1986). possesses high cytotoxic activity against KB and P-388 cancer cell lines with an ED50 value of 0.0024 and < 0.08 μg/mL, respectively (Meksuriyen and Cordell, 1988). It did not hinder the tubulin polymerization or disrupt microtubular function (Gul and Hamann, 2005). Staurosporine also acts as a cytotoxic agent against HT-29 (human colon cancer), MDA-MB-231 (human breast cancer), and A549 (human lung cancer) at GI50 values of 10.9, 7.1, and 2.4 nM, respectively (Reyes et al., 2008).
Eudistomin K (isolated from Caribbean ascidian Eudistoma olivaceum) is an indole alkaloid, which contains a novel oxathiazepine ring, and is found to be an antitumor lead against L-1210, A-549, HCT-8, and P-388 cell lines. The in vitro IC50 value of this compound was 0.01 μg/mL against P-388 cell line (Lake et al., 1989; Gul and Hamann, 2005).
Grossularine 1 and 2 (isolated from isolated from the tunicate Dendrodoa grossularia) (Moquin-Pattey and Guyot, 1989) possess potent cytotoxic activity against L-1210 cancer cell line with IC50 values of 6 and 4 μg/mL, respectively, and also of IC50 values of < 0.01 μg/mL against WiDr (colon cancer cell line) and MCF7 (breast cancer cell line) (Moquin-Pattey and Guyot, 1989). Grossularine 1 and 2 act as a monointercalating agent of DNA and also accumulate the cell in G1 phase at a concentration of 10 and 1.5 μg/mL, respectively (Gul and Hamann, 2005).
Halocyamine A (isolated from the solitary ascidian Halocynthia roretzi) (Azumi et al., 1990; Gul and Hamann, 2005) was found cytotoxic at a concentration of 160 μM for 24 h against neuroblastoma N-18 cells. Halocyamine A also causes deterioration of the neurite at a concentration of 100 μM against neuronal cells cultured from rat fetal brain. The cytotoxic activity of halocyamine A was also proved against HepG2 cancer cell line at a concentration of 200 μM for 24 h.
Topsentin (isolated from the Caribbean deep-sea sponge Spongosorites ruetzleri) possesses in vitro activity cytotoxic activity against P-388 with an IC50 3.0 and 20 μg/mL for human tumor cells (HCT-8, A-549, and T47D). The compound also shows in vivo antitumor activity against P-388 (T/C 137%) and B16 melanoma (T/C 144%) at a concentration of 150 mg/kg and 37.5 mg/kg, respectively (Tsujii et al., 1988; Gul and Hamann, 2005).
Hyrtiosins A and B (isolated from the Okinawan sponge Hyrtios erecta) exhibited cytotoxic activities higher than 5-hydroxyindole-3-aldehyde, whose IC50 was reported to be 4.3 μg/mL against in vitro against human epidermoid carcinoma KB cells (Kobayashi et al., 1990; Gul and Hamann, 2005).
Nortopsentins A, B, and C (isolated from the Caribbean deep-sea sponge S. ruetzleri) act as cytotoxic compounds against P-388 cells with IC50 values of 7.6, 7.8, and 1.7 μg/mL, respectively (Sakemi and Sun, 1991; Gul and Hamann, 2005).
Eudistomin E and Eudistalbin A (extracted from the marine tunicate Eudistoma album) possess in vitro cytotoxic activities with ED50 < 0.005 and 3.2 μg/mL, respectively, against KB cells (Gul and Hamann, 2005).
Dragmacidin D, a bis (indole)-derived sponge metabolite, (isolated from the sponge Spongosorites sp. Dragmacidin D) exhibits in vitro anticancer activity against P-388 and A-549 with IC50 values of 1.4 and 4.4 μg/mL, respectively (Gul and Hamann, 2005; Wright et al., 1992).
Makaluvamine G (isolated from the Indonesian sponge Histodermella sp.) displayed cytotoxic activity with IC50 values of 0.5, 0.5, 0.5, 0.5, and 0.4 μg/mL against P-388, A-549, HT-29, MCF-7 and KB, respectively (Carney et al., 1993; Gul and Hamann, 2005).
Gelliusines A and B (isolated from a deep-water New Caledonian sponge Gellius or Orina sp.) (Bifulco et al., 1994) showed anticancer activity against KB, P-388, P-388/dox, HT-29, and NSCLCN-6 cell lines with IC50 values of between 10 and 20 μg/mL (Bifulco et al., 1994; Gul and Hamann, 2005).
Konbamidin is an indole alkaloid (isolated from the Okinawan marine sponge Ircinia sp.) displayed in vitro cytotoxicity against Hela cells with an IC50 value of 5.4 μg/mL (Shinonaga et al., 1994; Gul and Hamann, 2005).
A peptide, Kapakahine B (isolated from the marine sponge Cribrochalina olemda) (Nakao et al., 1995), showed reasonable anticancer activity against P-388 murine leukemia cells with an IC50 value of 5.0 μg/mL (Gul and Hamann, 2005).
Another unique peptide Styelin D (isolated from the hemocytes of the solitary ascidian Styela clava) demonstrated anticancer activity against HCT-116 and human ME-180 cervical epithelial cells with an IC50 value of 10.1 μg/mL and ED50 value of 50 μg/mL, respectively (Gul and Hamann, 2005).
Topsentin B1 and B2 (isolated from the Mediterranean marine sponge Rhaphisia lacazei) inhibited NSCLC-N6 (human bronchopulmonary cancer cells) with IC50 values of 12.0 and 6.3 μg/mL, respectively (Casapullo et al., 2000; Gul and Hamann, 2005).
Pibocin B (isolated from the ascidian Eudistoma sp.) showed cytotoxic activity against mouse Ehrlich carcinoma cells with an ED50 value of 25 μg/mL (Makarieva et al., 2001; Gul and Hamann, 2005).
Convolutamydine A (isolated from marine bryozoan Amathia convolute) showed significant anticancer activity against HL-60 (human promyelocytic leukemia cells). At a concentration of 0.1–25 μg/mL, this indole alkaloid brings a change in culture plate adhesion, induces growth arrest, and phagocytosis (Gul and Hamann, 2005; Kamano et al., 1995).
Manzamine B, E, and F (collected from various sponges, including an Amphimedon sp.) have been presented with cytotoxic activity against P-388 murine leukemia cells with an IC50 values of 6.0, 5.0, and 5.0 μg/mL, respectively (Hu et al., 2003b; Gul and Hamann, 2005).
Echinosulfonic acid D (isolated from the New-Caledonian sponge Psammoclemma sp.) (Ovenden and Capon, 1999) was found to be cytotoxic at an IC50 of 2 mg/mL to KB cells (Rubnov et al., 2005).
15.3.1.3. Antiangiogenesis effect
At concentrations of 0.1–1.0 pmol/L, vinblastine blocks endothelial proliferation, chemotaxis, and spreading on fibronectin without affecting other fibroblasts and lymphoid tumors (Amalraj et al., 2013; Freire et al., 2013; Malafaia et al., 2013; Mishra and Prasad, 2016; Reggia et al., 2016; Srivastava and Sharma, 2014; Shi et al., 2013; Sudhir Kumar et al., 2016; Kumar et al., 2013b). A combination of low doses of vinblastine and antibodies against VGFR (vascular endothelial growth factor) upturns the antitumor effect of the drug even in resistant tumors also (Anitha, 2016).
15.3.1.4. Antioxidant activities (Begum et al., 2012)
In the DPPH radical-scavenging assay, it was found that reserpine possesses 42% inhibition of DPPH radical.
Lind et al. performed two different biochemical assays, FRAP (Ferric-Reducing Antioxidant Power) and ORAC (Oxygen Radical Absorbance Capacity), to find the antioxidant activity of barettin. According to their experiment, barettin showed a potential antioxidant profile in a dose-dependent manner. At a concentration of 30 μg/mL barettin had FRAP and ORAC values of 77 and 5.5 μM Trolox equivalents (TE), respectively (Lind et al., 2013).
15.3.1.5. Antihypertensive activities
Reserpine is highly used as a first-line therapeutic agent in reducing blood pressure in primary hypertension. A dose of reserpine 0.5 mg/day or greater attained the statistically significant SBP (systolic blood pressure) effects (Shamon and Perez, 2016). The underlying mechanism of the antihypertensive action of reserpine is, it reduces the catecholamines concentration from the peripheral sympathetic nerve endings. Reserpine has the higher affinity for VMAT2 (vesicular monoamine transporter), irreversibly binds to their receptors, and irrevocably blocks the VMAT2 (Schuldiner et al., 1993). VMATS is responsible for the transportation of released and free nor-adrenaline or nor-epinephrine, dopamine, and serotonin (5-HT) from the cytoplasm of the presynaptic nerve terminal into storage vesicles for further release into the synaptic cleft (Blackman et al., 1959; Nammi et al., 2005). Due to uptake obstruction, these unshielded and susceptible neurotransmitters never reach the synapse to exert their actions as they are metabolized by COMT (catecholamine-o-methyl transferase) and MAO (monoamine oxidase) in the cytoplasm. Reserpine has the higher affinity for VMAT2 and binds irreversibly to their receptors. It is a potent antihypertensive agent and tranquilizer, but its sustained usage stimulates the release of prolactin and causes breast cancer (Meenakshi and Priyanka, 2010).
15.3.1.6. Treatment for antidyskinesia syndromes (Shen, 2008) and others (Singh, 2017a)
Formerly reserpine was used to treat indications of dyskinesia in patients suffering from Huntington’s disease. Reserpine is also used in various psychiatric diseases by acting as a tranquilizing agent.
15.3.1.7. Settlement inhibition of barnacle larvae or antifouling agents
Barettin (cyclo[(6-bromo-8-entryptophan)arginine]) and 8,9-dihydrobarettin (cyclo[(6-bromotryptophan)arginine]) are brominated cyclodipeptides, which has been isolated from a marine sponge Geodia barrette family Geodiidae. These two compounds, barettin and 8,9-dihydrobarettin, intensely prevent the settlement of the barnacle larva of Balanus improvisus and the blue mussel Mytilus edulis in a dose-dependent manner in concentrations ranging from 0.5 to 25 μM with EC50 values of 0.9 and 7.9 μM for barettin and 8,9-dihydrobarettin, respectively (Sjögren et al., 2004a, Sjögren et al., 2004b).
15.3.1.8. Interaction with human serotonin receptors
Barettin especially interacted with the three different serotonin receptors 5-HT2A, 5-HT2C, and 5-HT4 at approximately similar concentrations that of endogenous serotonin, with the K i values 1.93, 0.34, and 1.91 μM, respectively. This is possible because of the structural resemblance between tryptophan residue and the endogenous serotonin (Erik et al., 2006). On the other hand, 8,9-dihydrobarettin interacted with only 5-HT2C receptor with a K i value of 4.63 μM (Erik et al., 2006).
Gelliusine A was acting as a restricted agonist for serotonin receptor(s). At lower concentration, this compound blocks the serotoninergic receptor(s) by acting as a serotonin antagonist, which was confirmed by experimenting on male guinea pig ileum; however, at higher concentration (5–70 μg/mL), it was responsible for serotonin-like and methysergide-sensitive contraction. This result recommends the possible role of gelliusine A as a serotoninergic system modulator (Bifulco et al., 1994; Christopherson, 1985).
15.3.1.9. Antinociceptive and antiinflammatory activities
Barettin was also able to inhibit the secretion of the inflammatory cytokines IL-1β and TNFα from LPS-stimulated human acute monocytic leukemia cell line THP-1 cells (Lind et al., 2013).
Dragmacidin has shown to have antiinflammatory activity by inactivating the bee venom PLA2 (in vitro assay) and mouse ear edema test (in vivo assays) (Jiang et al., 1994).
Chelonin A (isolated from the sponge Chelonaplysilla sp.) displayed potential antiinflammatory activity by inhibiting 60% PMA-induced inflammation in a mouse ear model at a concentration of 50 μg/ear (Bobzin and John Faulkner, 1991; Gul and Hamann, 2005).
Caulerpin (isolated and purified from the lipid extract of Caulerpa racemose) (Lunagariya et al., 2017) possesses comparable analgesic activity (0.0945 μmol) in contrast to dypirone (0.0426 μmol) by reducing acetic-acid-induced nociception in the abdominal constriction test. In the hot plate test also, caulerpin inhibits the nociception in vivo at a dose of 100 μmol/kg, p.o., which proves caulerpin shows a central activity, without altering the motor action. Caulerpin, at a dose of 100 μmol/kg, p.o. inhibits the inflammation by 55.8% in capsaicin-induced ear edema model. The promising antiinflammatory activity was also detected after the reduction of the number of recruit cells by 48.3% significantly at a dose of 100 μmol/kg, p.o. in the carrageenan-induced peritonitis model (de Souza et al., 2009).
15.3.1.10. Immunomodulatory effects (Lind et al., 2015)
Lind et al. showed that barettin obstructs two inflammatory protein kinases, receptor-interacting serine/threonine kinase 2 (RIPK2) and calcium/calmodulin-dependent protein kinase 1α (CAMK1α). According to them, barettin possibly inhibits CAMK1α, which ultimately results in inhibition of antiinflammatory cytokine interleukin-10 (IL-10) production in a dose- and time-dependent manner. It also diminishes the excretion of monocyte chemotactic protein-1 (MCP-1) from immune cells. All these highlight the atheroprotective effect of barettin.
15.3.1.11. Anticoagulant activities
Tissue factor (TF), the transmembrane glycoprotein, is a major stimulator of blood coagulation, which is synthesized by monocyte (Osterud and Bjorklid, 2012). It binds with Factor VII and forms a complex TF-FVII complex, which in turn activates Factors IX and X, and subsequently generates thrombin, as well as activates platelet and fibrin deposition (Mann et al., 2006). Apart from the role of coagulation, this TF-factor VII complex is also involved in other pathophysiological roles like atherosclerosis, tumor metastasis, inflammation, and angiogenesis (Åberg and Siegbahn, 2013; Schaffner et al., 2012). Barettin acts in this pathway and acts as a potent anticoagulant (Lind, 2015).
15.3.1.12. Antiviral activity
Eudistomin K inhibits the growth of HSV-1 (Herpes simplex virus) at a concentration of 0.25 and 0.10 μg/disk, respectively (Rinehart Jr et al., 1987).
Topsentin exhibited in vitro antiviral activity against HSV-1, VSV (Vesicular stomatitis virus), and the corona virus A-59 (Gul and Hamann, 2005).
Eudistomin E possesses antiviral activity against both DNA (e.g., HSV-1, 2 and vaccinia virus) and RNA viruses (such as Coxsackievirus A-21 and equine rhinovirus) (Gul and Hamann, 2005).
Dragmacidin D showed in vitro antiviral activity by repressing the replication of feline leukemia virus (FeLV) at an IC50 of 6.25 μg/mL (Wright et al., 1992).
The alpha and beta phases are very essential where factors responsible for the regulation of viral replication, antigenic presentation, and genome replication are synthesized. Caulerpin inhibits the alpha and beta phases of the replication cycle. Hence, it can be used as a substitute to acyclovir as an anti-HSV-1 drug (Macedo et al., 2012).
15.3.1.13. Enzyme inhibitor
Dragmacidin D inhibits serine-threonine protein phosphatases. It also inhibits the activity of neural nitric oxide synthase (βNOS), which may corroborate to help in Huntington’s, Parkinson’s, and Alzheimer’s disease treatments (Yang et al., 2002).
15.3.1.14. Antitopoisomerase-I activity
Makaluvamine G possesses moderate inhibitory activity against topoisomerase-I (IC50 3.0 μM). It also inhibits the activity of DNA, and protein at an IC50 of 15, 15, and 21 μM (Carney et al., 1993).
15.3.1.15. Calmodulin-antagonistic activity
Eudistomidin A was isolated from the Okinawan tunicate Eudistoma glaucus (Kobayashi et al., 1986). This alkaloid was the first calmodulin antagonist reported from a marine source. The IC50 (2 × 10− 5 M) of eudistomidin A was 15 times more potent than W-7 (3 × 10− 4 M), a well-known calmodulin antagonist, used against calmodulin-activated brain phosphodiesterase (Gul and Hamann, 2005).
15.3.1.16. Plant growth regulator
Caulerpin is the first plant growth regulator isolated from marine origin. This bis-indole alkaloid possesses excellent root growth and development promotion activity equivalent to indole-3-pyruvic acid and indole-3-acrylic acid (Raub et al., 1987).
15.3.1.17. Antidiabetic and antiobesity activity (Mao et al., 2006)
Type II diabetes and obesity have emerged as a worldwide health issue. Human protein tyrosine phosphatase 1B (hPTP1B) is one of the important enzymes, which hydrolyze phosphotyrosines on the insulin receptor, hence deactivating it. So, hPTP1B is considered a strategic target to tackle these two diseases. Caulerpin displayed potent hPTP1B inhibitory activity at an IC50 value of 3.77 μM.
15.3.1.18. Spasmolytic effect (Cavalcante-Silva et al., 2013)
Caulerpin exhibits the spasmolytic effect on guinea pig ileum. This bis-indole alkaloid inhibited phasic contractions induced by carbachol, histamine, and serotonin in a nonselective manner. Caulerpin marginally repressed the CaCl2-encouraged contractions in depolarizing medium without Ca2 +, and hence shifting the curves to the right and with E max reduction. So, this attributes the mechanism of this spasmolytic effect of caulerpin as it inhibits the Ca2 + influx via voltage-gated calcium channels (Cav).
15.3.1.19. Effect on central nervous system and brain (Borzeix and Cahn, 1984)
Vincamine, the common nitrogen-based secondary metabolites, comprise one or more indole/indoline moieties. This was first isolated from Vinca Minor L (Periwinkle) and belongs to the apocynaceae family. In male Wistar rats, vincamine ketoglutarate, vincamine teproside, and vincamine hydrochloride prohibited the manifestation of cerebral edema and inhibited the neurological insufficiency caused by the administration of triethyltin hydrochloride.
15.3.1.20. Other imperative medicinal uses of indole alkaloids (Dhayalan et al., 2015; Jordan and Wilson, 2004)
The cotreatment of vinblastine along with bleomycin and methotrexate in VBM chemotherapy for Stage IA or IIA Hodgkin lymphomas was found markedly effective. Likewise, this alkaloid was also used for the subsequent treatments like mycosis fungicides, testicular cancer, Letterer-Siwe disease, Hodgkin’s sickness, non-Hodgkin’s lymphomas, Kaposi’s sarcoma related to acquired immune deficiency syndrome (AIDS), etc.
Vincristine is administered through intravenous infusion for several categories of a therapeutic regime. As a part of the chemotherapeutic regime, this alkaloid is used against non-Hodgkin’s lymphoma. Vincristine is sporadically used as an immunosuppressant, for example, in case of treating thrombotic thrombocytopenic purpura or chronic idiopathic thrombocytopenic purpura. For the treatment of childhood leukemia, vincristine is used in combination with prednisone. This alkaloid is also used for the therapeutic application of many diseases like idiopathic thrombocytopenia purpura, bladder cancer, cervical cancer, nonsmall-cell lung cancer, autoimmune hemolytic anemia, neck cancer, and head cancer (Dhayalan et al., 2015; Qweider et al., 2007).
15.3.2. The biological activity of tropane alkaloid
15.3.2.1. Effect on asthma
Atropine is isolated from dried leaves and flowering tops of Datura metal of Solanaceae family.
It has an action against Nocturnal Asthma. Nocturnal asthma can be effectively treated by atropine methyl nitrate (Sur et al., 1990), atropine sulfate (Owens and George, 1991), and atropine in blended with metaproterenol (Young and Freitas, 1991) and albuterol, correspondingly. In humans, lung mucociliary function can be improved by inhalation of atropine (Groth et al., 1991).
15.3.2.2. Protection against organophosphorus compounds
Toxic effect of organophosphate can be neutralized by atropine only and with cholinesterase reactivator (Ligtenstein and Moes, 1991; Romano et al., 1991). A combination of clonidine (Wu-Fu, 1991) and diazepam (Tsung-Ming, 1991) was also found active with atropine sulfate.
Scopolamine exerts a protective effect against Organophosphate Exposure (Solana et al., 1991). In vivo muscarinic cholinergic receptor imaging, besides through positron emission tomography, can be done by using C-scopolamine (Frey et al., 1992).
15.3.2.3. Activity against hyperglycemia and parkinsonism
Atropine suppresses neostigmine-induced hyperglycemia (Iguchi et al., 1991). Atropine influences the vagal tone of diabetic patients (Julu et al., 1991). Tremor in parkinsonism of a monkey model can be reposed by atropine (Gomez-Mancilla et al., 1991).
Hyoscine antagonizes the cholinergic activity at the muscarinic receptors in the striatum and increases DA activity, hence used to treat PD (Houghton and Howes, 2008).
15.3.2.4. Effect on ANS (Fodor and Dharanipragada, 1994)
Atropine acts as a competitive antagonist of the activities of acetylcholine and other muscarinic agonists. It also contests for a mutual binding site on all muscarinic receptors. Atropine blocks the muscarinic receptors in smooth and ganglia and intramural neurons.
15.3.2.5. Effect on CVS (Fodor and Dharanipragada, 1994)
Atropine blocks the muscarinic receptors in cardiac muscle. It also used in cardiac dysfunction.
15.3.2.6. DNA protectant and mitotic inhibitor
Colchicine is a strong spindle fiber poison and inhibits the polymerization of tubulin (Foye, 1995).
15.3.2.7. Anticancer and antiinflammatory activity
At toxic or nearly toxic dosage, colchicine is effective against chronic myelocytic leukemia and gout (Wedge and Camper, 1999).
15.3.2.8. Use against nausea, drooling, and Alzheimer disease
Scopolamine is applied to get rid of nausea and drooling in Alzheimer and Parkinson diseases. Postoperative nausea and vomiting of morphine can be minimized by transdermal application of scopolamine (Horimoto et al., 1991). Transdermal scopolamine was used as nausea prophylaxis (Harris et al., 1991) and against drooling (Dreyfuss et al., 1991; Siegel and Klingbeil, 1991). Scopolamine has an impact on environmentally induced pain reactivity (Grau et al., 1991).
15.3.2.9. For treating motion sickness
The complication of motion sickness is overcome by the use of transdermal scopolamine. When applied behind the ear 5 h erstwhile to sailing, this drug is found effective in inhibiting seasickness (Attias et al., 1987).
15.3.2.10. Antispasmodic and antiallergic effects
Hyoscyamine and atropine contribute antispasmodic and antiallergic effects by acting as a competitive antagonist at the muscarinic acetylcholine receptor site (Kinghorn, 2001; Sneader, 2005).
15.3.2.11. Hyperactivity: Effect on slowing
In mice, scopolamine-activated hyperactivity is shared by the cholinergic and dopaminergic representatives (Shannon and Peters, 1990). Riekkinen and Valjakka et al. mentioned the effects of tetrahydroaminoacridine (THA, an acetylcholinesterase inhibitor) on scopolamine-induced neocortical spectral electroencephalography (EEG) slowing (Riekkinen Jr et al., 1991; Valjakka et al., 1991). Acetylcholine synthesis in the brain was marked affected by scopolamine, oxotremorine, and physostigmine (Bertrand and Beley, 1990).
15.3.2.12. Central sedative, antiemetic, and amnestic effects (Renner et al., 2005)
Scopolamine is a nonselective muscarinic antagonist. The mechanism of action of the anticholinergic drug scopolamine includes the competitive antagonism of acetylcholine at the muscarinic receptors (M1). This blockage of acetylcholine inhibits the acetylcholine-mediated nerve impulse from traveling throughout the body. This antimuscarinic property allows scopolamine to produce the central sedative, antiemetic, and amnestic effects.
15.3.2.13. Miscellaneous
Atropine has an influence on the pelvic pouch and anal sphincter functions (Hallgren et al., 1991). Atropine impacts radial arm maze enactment of the rat when centrally administered (Sala et al., 1991).
15.3.3. The biological activity of isoquinoline alkaloids
15.3.3.1. Effect against neurodegenerative diseases
Berberine is one of the important isoquinoline alkaloids. It is present in medicinal plants, especially in the genus Berberis like Berberis vulgaris. Berberine is potentially effective against multineurodegenerative diseases such as Alzheimer, Parkinson, and Huntington diseases (Ahamad et al., 1969).
15.3.3.2. Antibacterial activity (Peng et al., 2015)
At minimum inhibition concentration (MIC) of 78 μg/mL, berberine shows antibacterial activity by severely damaging bacterial cell membrane structure by inhibiting cellular proteins, which were confirmed by TEM and SDS-PAGE. This compound altered bacterial DNA synthesis. It also hinders the biofilm formation of methicillin-resistant S. aureus (MRSA) in a concentration-dependent manner ranging from 1 to 64 μg/mL via inhibiting phenol-soluble modulins (PSMs) aggregation into amyloid fibrils (Chu et al., 2016).
15.3.3.3. Anticancer activity
Berberine inhibits the cancer cells keratinocytes at an IC50 of 30 μmol/L (Müller et al., 1995). It also reduces the entwined sarcoma in an animal by 0%, 53%, and 33 % at the doses of 5, 2.5, and 0.5, mg/kg, respectively (Anis et al., 2001). In vitro antiproliferative activity of berberine is also achieved against HeLa cells, Dalton lymphoma of ascitic cancer cells, and L929 cells at an IC50 of 7.2, > 1000, and 40 mg/L, respectively (Anis et al., 1999).
Emetine shows cytotoxicity by apoptotic mechanism in various cancer cell lines: at EC50 of 0.05 μM against CCRF-CEM (Human T-cell lymphoblast-like cell line) (Meijerman et al., 1999), at LD50 of 55 μg/mL against A549-S (lung adenocarcinoma) (Watanabe et al., 2002), at EC50 of 2 μM against CEM/ADR5000 (leukemia cell line) (Möller et al., 2006), at EC50 of 0.17 μM against Jurkat T cells (T-cell leukemia) (Möller et al., 2006). Emetine inhibits the protein synthesis and interacts with DNA and exerts its cytotoxic activity (Grollman, 1968). In PC3 (prostate cancer cell line), MCF-7 (breast cancer cell line) emetine significantly decreases the ratio of BCL-xS/BCL-xL (Boon-Unge et al., 2007). Treatment with emetine in Jurkat cells results in downregulation of EGFR, BCL2, TNF and upregulation of the TNFRS11B, AKT1, TNFSF13, Caspase 8, TNFRSF6, BAK1, caspase 9, MST1, GZMB, DAXX (Möller et al., 2007).
15.3.3.4. Antidiabetic activity
Berberine (methanolic extract) shows its antidiabetic potential at the dose of 500 mg/kg. Not only antidiabetic activity, but also Berberis aristata (methanolic extract) show its potential effect on carbohydrate metabolism as well as HDL and cholesterol levels (Saravanan and Pari, 2003; Yadav et al., 2005).
15.3.3.5. Antiosteoporosis activity
Berberine shows mild laxative as well as hypocholesterolemic activity (Chauhan, 1999). Berberine and its methanolic extract displays significant antiosteoporosis activity and substantiates the ethnic use in the treatment of postmenopausal osteoporosis (Potdar et al., 2012; Yogesh et al., 2011).
15.3.3.6. Treatment in cardiovascular diseases
In cardiovascular diseases, berberine compound is used as an antihyperlipidemic agent. After the administration of berberine, LDL cholesterol, triglycerides, and cholesterol content were reduced by 25%, 35%, and 29% within three months, respectively (Kong et al., 2004; Shenoy and Yoganarasimhan, 2009).
15.3.3.7. Antioxidant and antiinflammatory activity (Li et al., 2014)
Berberine inhibits the oxidative stress and inflammation in various tissues, including liver, kidney, pancreas, and adipose tissue by targeting various signaling pathways and cellular kinases like mitogen-activated protein kinases (MAPKs), nuclear factor-κB (NF-κB) pathway, AMPactivated protein kinase (AMPK), and nuclear factor erythroid-2-related factor-2 (Nrf2) pathway.
15.3.3.8. MAO inhibitor and others
Berberine moderately inhibits the monoamine oxidase (MAO) enzyme, K i = 110 μmol/L, IC50 = 126 μmol/L (Kong et al., 2001). Berberine also has antidiarrheal, antifungal, and antiprotozoal activity. Currently, research indicates that berberine shows its protective mechanism against atherosclerosis. Tincture of the root of berberine is better than quinine and cinchona in the treatment of intermittent fever because it does not produce cardiac depression (Chatterjee and Pakrashi, 1991).
15.3.3.9. Antiviral activity
Psychotria ipecacuanha Stokes (Rubiaceae) is one of the major sources of Emetine. This isoquinoline alkaloid mainly occurs in three plant families comprising Icacinaceae, Rubiaceae, and Alangiaceae. The main active constituent of ipecac root is emetine (Wiegrebe et al., 1984). Possessing vital biological functions to make emetine is a major constituent to molecular biologists and pharmacologists.
Emetine shows antipoxviral activity by inhibiting vaccinia virus replication at noncytotoxic doses (inhibition of plaque at 0.25 μM; IC99: 0.1 μM for 48 h) (Deng et al., 2007). Antiviral activity against four serotypes of dengue virus (DENV) at an early stage has also been reported in a dose-dependent manner at a noncytotoxic dose by affecting viral replication cycle either by disturbing viral protein translation pathway or damaging viral RNA synthesis pathway (Yin Low et al., 2009). At a dose of 0.1 μM, DENV II was repressed by 33%, while 90% inhibition was observed at 0.5 μM to 10 μM dose (Yin Low et al., 2009).
15.3.3.10. Contraceptive activity
Emetine is used as a contraceptive agent in the rabbit uterus and it shows an antiimplantation effect in a concentration-dependent manner (Moyer et al., 1977). Another research study on five rodent species: hamster, rat, mouse, guinea pig, and rabbit via oral and intravaginal routes demonstrated the prospective use of emetine ditartrate as an alternative contraceptive agent (Mehrotra et al., 2004). The main site of action of emetine ditartrate includes uterus and primary embryos nearby implantation, probably the trophoblast and endometrial cells at the addition site.
15.3.3.11. Antiparasitic activity and others
Emetine inhibits the growth of Entamoeba histolytica responsible for amoebiasis and amebic dysentery (Lambert, 1918; Thompson, 1913; Vedder, 1912). Hence, it is effective in the treatment of perianal skin amoebiasis and amoebic liver (Hughes and Petri Jr, 2000; Ruiz-Moreno, 1967). Emetine induces programmed cell death in this same parasite and induces nuclear condensation, DNA fragmentation, and inhibits the maintenance of cell membrane integrity (Villalba-Magdaleno et al., 2007).
Emetine is also effective against leishmaniasis by inhibiting Leishmania donavani (Muhammad et al., 2003). Emetine also inhibits the protein biosynthesis and induces DNA fragmentation in Trypanosoma b. brucei (Rosenkranz and Wink, 2008). It also possesses anthelmintic activity in infected goat and sheep at a dose of 1 mg/kg against Protostrongylus rufescens (Akinboye and Bakare, 2011; Shahlapour et al., 1970). Emetine interferes with the function of alcohol dehydrogenase and therefore alters the pathological function of alcohol addiction (Nikolaenko, 2001).
15.3.3.12. Miscellaneous
The mechanistic approach of codeine includes K+ conductance (opening of IC channels) with neurons responding via hyperpolarization. A reduction of the Ca2 + entry into nerve termini (closing of voltage-gated Ca2 + channels) consequently prevents the secretion of excitatory neurotransmitters and synaptic activity. Such types of inhibitory effects on synaptic cleft may produce either a depressant or an excitant effect. Opioid alkaloids, especially morphine and other narcotic agents, have produced their pharmacological effect through mu (μ), kappa (κ), and delta (δ) receptors (Fries, 1995; Gutstein and Akil, 2001; Lüllmann et al., 2000).
The primary effect of these opium alkaloids (morphine and codeine) and morphine-like narcotic antagonists in CNS involves analgesia, drowsiness, euphoria, a sense of detachment, respiratory depression, depressed cough reflex (partially via direct action on the medullary cough center), and hypothermia. These types of alkaloids also act on the parasympathetic nervous system, and the specially oculomotor nucleus is responsible for pupillary miosis.
The other effects of these opium alkaloids (morphine and codeine) include reduced gastrointestinal motility, increased resting tone and spasm, and increased anal sphincter tone. The major therapeutic action of morphine is relief from mild or severe pain. It is also used as an anesthetic intraoperative cases. Sometimes, morphine is applied for acute pulmonary edema due to the presence of the hemodynamic property (Fries, 1995; Gutstein and Akil, 2001).
Thebaine has a stimulatory effect on the central nervous system. It produces hyperirritability and motor activity on animal model, especially in mouse, rabbit, cat, and dog, at doses around 2–10 mg/kg s.c. or i.m. Many published reports suggest that thebaine upsurges gastrointestinal tone and intestinal activity.
Papaverine has smooth-muscle relaxant property. The smooth musculature of the larger blood vessels is relaxed, including the coronary, systemic peripheral, and pulmonary arteries. The vasodilation effect of papaverine has been credited to inhibition of cyclic nucleotide phosphodiesterases, with resulting rises in intracellular levels of the cyclic AMP and cyclic GMP accompanied by declines in Ca2 +. This alkaloid produces prolonged myocardial refractory period by decreasing the conduction rate (Cocolas, 1982; Lindner, 1985; Schmeller and Wink, 1998). This alkaloid is often used as a topical gel in sexual dysfunction in order to obtain an erection in patients with spinal cord injuries. Due to the presence of the above property, this alkaloid got Orphan Drug status.
Noscapine possesses analgesic properties. It also has antitussive activity equal to that of codeine. That is why the name was changed to noscapine. Besides noscapine with small doses also showed a bronchodilatory effect. A large dose of noscapine produces bronchoconstriction and transient hypotension due to secretion of histamine (Abd-Rabbo, 1969; Grollman, 1968; Schmeller and Wink, 1998).
The application of curare is very less except as a source of alkaloids. The commercial product such as Tubocurarine chloride is used as a muscle relaxant in the surgical case and neurological disorder (Dewick, 2002; Willette, 1998).
15.4. Techniques of extraction, purification
Generally, extraction is the first step to separate the compounds from the mixture of solid or liquids by a suitable solvent (Fabricant and Farnsworth, 2001; Huie, 2002b). Moreover, extraction is also the preliminary step in the drug discovery process and developments in the indole, isoquinoline, and tropane groups of plants. Many procedures have been established to obtain extracts showing a range of polarities and augmented widespread secondary metabolites such as alkaloids. There is a wide range of well-established techniques to extract alkaloids such as maceration, infusion, decoction and boiling under reflux, microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE), supercritical fluid extraction (SFE), and pressurized liquid extraction (PLE) (Table 15.3 ).
Table 15.3.
Different types of extraction processes.
| Type | Sample size; solvent | Temperature | Pressure/time | Investment | Inference |
|---|---|---|---|---|---|
| Soxhlet | 1–2000 g; 4–8000 mL | Depends upon the solvent | Atmospheric/6–8 h | Very low | Efficient for polar and nonpolar BAC |
| SFE | 25–100 g; continuous flow | – | 25–45 MPa; 1–2 h | High | Efficient for nonpolar BAC |
| PLE | 1–30 g; 10–100 mL | 80–200°C | 1–10 MPa; 10–30 min | High | Efficient for polar and nonpolar BAC, Safety |
| MAE | 1–20 g; 10–50 mL | 80–150°C | Variable/10–30 min | Moderate | Use of solvents is risky |
| Hydro distillation | 10–100 g | 40–60°C | Atmospheric/6–8 h | Low | Residual hydrotropic |
Fig. 15.11 describe the general flowchart of alkaloid extraction and purification.
Fig. 15.11.
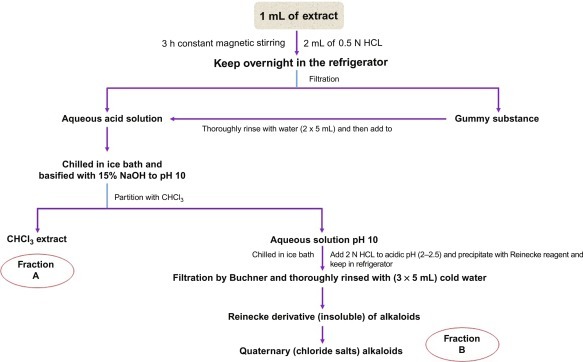
General techniques of alkaloid extraction.
15.4.1. Mechanical methods
Mortar and pestle, grinding mill, and mixer grinder are generally used for grounding plant tissues before extraction. For instance, lignified plant material was frozen and pulverized with liquid nitrogen in a mortar and pestle (Kirakosyan et al., 2016). Sonicator was also used for extraction of plant products grown by cell suspension culture methods (Bhargavi et al., 2018).
15.4.2. Chemical degradation
Various types of potential chemicals are used for breaking down of cell walls. The insoluble matrix, as well as the molecular structure of the cell wall, can be disrupted by detergents. For example, Triton X-100, octyl glycoside, and Tween-20 are some of the nonionic detergents. Ionic detergents such as sodium dodecyl sulfate (SDS) are used for the aforementioned purposes (Bhargavi et al., 2018; Fabricant and Farnsworth, 2001; Huie, 2002b).
15.4.3. Precautions to be taken during extraction processes
Compounds may be destroyed by the presence of excessive heat, certain chemicals, enzymatic reaction inside the cell. In the case of water-soluble materials, one needs to be aware of the buffer system. Due to methodical error in the extraction technique as well as isolation process, polyphenols may be deactivated by insoluble polyvinyl polypyrrolidine (PVPP), soluble polyvinyl pyrrolidine (PVP). A chelating agent such as EDTA may be added to eliminate divalent cations from the extracts. Foams are suppressed by using suitable antifoaming agents such as DC-544 (Dow Corning) or SAG-30 (Union Carbide) (Bhargavi et al., 2018; Fabricant and Farnsworth, 2001; Huie, 2002b).
15.4.4. Extraction with solvents (liquid-liquid extraction or solid-liquid extraction)
The homogenized cell is kept in the extracting solvent for a certain time so that all parts of the cells are easily penetrated by the solvent. The main primary key of the extraction process is to find out the suitable solvent and effect of temperature. An ideal extraction method should avoid some important phenomena associated with physical incompatibility. These important phenomena include decomposition, isomerization, or polymerization. Another important parameter is compound stability. The stability of the compound depends on light, heat, and solvent polarity. Extraction is a separation technique of the desired compound from a matrix as in the case of soxhlet extraction and countercurrent extraction (Bhargavi et al., 2018; Fabricant and Farnsworth, 2001; Huie, 2002b; Pandey and Tripathi, 2014).
Fig. 15.12 describes the isolation techniques of opium alkaloids (isoquinoline).
Fig. 15.12.
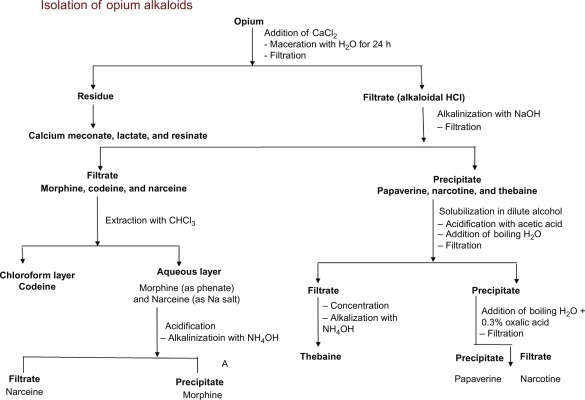
Extraction and purification techniques of opium alkaloids.
An ideal solvent dissolves the desired compound, leaving the other constituents.
Polarity index is a golden standard of measurement of the intensity of interaction of the solvent with various polar solutes. For extraction of the nonpolar compounds, nonpolar solvents are applied. Hexane is used for extraction of fixed oil, chlorophylls, steroids, and terpenoids. Highly polar compounds such as glycosides, sugars, amino acids, proteins, and polysaccharides can be extracted with polar solvents. Less polar compounds such as isoflavones, flavanones, methylated flavones, and flavonols are extracted with low polar solvents such as chloroform, dichloromethane, diethyl ether, or ethyl acetate; and the polar flavonoids and flavonoid glycosides are extracted with alcohols or aqueous alcohol mixtures (Barnes, 1999; Pandey and Tripathi, 2014; Patil et al., 2012).
Volatility is a major drawback of a compound during the extraction process. Such type of problem may be overcome by using solvents with a low boiling point without denaturation at high temperature.
The solvent selection is necessary for better extraction technique. By modification, the solvent becomes more acidic and more basic in certain extraction processes. Acidified solvents are mainly used for alkaloid extraction, whereas the basic nature of a certain solvent is applied for phenolic compounds. Generally, 5% HCl is applied for the alkaloids extraction, amines, and pyrazines, 5% NaOH or 5% KOH preferentially extracts phenolics compounds. For extraction of catechins, methanol is used as an ideal solvent. For procyanidins, 70% acetone is used. In the case of anthocyanins extraction, a slight acidic solvent is selected and thus 0.1% HCl in methanol is used. 7% acetic acid or 3% trifluoroacetic acid has been also used for the extraction of anthocyanins. Acyl groups can be affected by using high concentrated mineral acid or raised temperature due to the hydrolysis reaction (Barnes, 1999; Magistretti, 1980; Pandey and Tripathi, 2014) (Table 15.4 ).
Table 15.4.
Physical properties of common solvents used in phytochemistry (Barnes, 1999; Pandey and Tripathi, 2014).
| Solvent | Polarity index | Refractive index | B.P. | Specific gravity (20°C) |
|---|---|---|---|---|
| n-Pentane | 0.0 | 1.358 | 36 | |
| n-Hexane | 0.0 | 1.375 | 69 | 0.659 |
| Petroleum ether (low boiling) | 0.0 | – | 40–60 | |
| Petroleum ether (high boiling) | 0.0 | – | 60–80 | |
| Heptane | 0.0 | 1.387 | 98 | |
| Cyclohexane | 0.2 | 1.426 | 81 | 0.779 |
| Carbon tetrachloride | 1.6 | 1.466 | 77 | 1.594 |
| Toluene | 2.4 | 1.496 | 111 | 0.867 |
| Xylene | 2.5 | 1.500 | 139 | 0.860 |
| Benzene | 2.7 | 1.501 | 80 | 0.879 |
| Diethyl ether | 2.8 | 1.353 | 35 | 0.714 |
| Dichloromethane (methylene chloride) | 3.1 | 1.424 | 41 | 1.325 |
| 1,2-Dichloroethane (ethylene chloride) | 3.5 | 1.445 | 84 | |
| Isopropanol (2-propanol) | 3.9 | 1.380 | 82 | 0.785 |
| n-Propanol | 4 | 1.380 | 97 | 0.804 |
| Tetrahydrofuran | 4.0 | 1.407 | 65 | 0.887 |
| n-Butanol | 3.9 | 1.399 | 125 | 0.810 |
| Chloroform | 4.1 | 1.443 | 61 | 1.486 |
| Ethyl acetate | 4.4 | 1.370 | 77 | 0.901 |
| Acetone | 5.1 | 1.359 | 56 | 0.791 |
| Methanol | 5.1 | 1.329 | 65 | 0.792 |
| Ethanol | 5.2 | 1.361 | 78 | |
| Pyridine | 5.3 | 1.510 | 0.982 | |
| Acetonitrile | 5.8 | 1.344 | 82 | 0.782 |
| Acetic acid | 6.2 | 1.372 | 118 | 1.049 |
| Dimethyl sulfoxide | 7.2 | 1.477 | 189 | 1.101 |
| Water | 9.0 | 1.330 | 100 |
15.4.5. Types of extraction processes
Classification of extraction can be based on heat. These are hot and cold extractions. Depending upon the physical state, extraction processes are categorized into the solid-liquid extraction and liquid extraction (Cos et al., 2006; Culture and Health, 1996; Pandey and Tripathi, 2014). Different methods of extraction techniques (Barnes, 1999) are summarized in Fig. 15.13 .
Fig. 15.13.
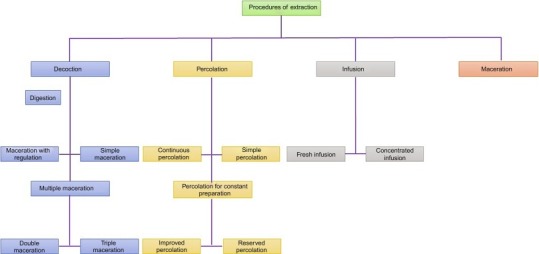
Different methods of extraction.
15.4.6. Cold and hot extraction
Cold extraction is performed at room temperature. Several types of cold extraction technique are available in small- as well as large-scale purposes like percolation, maceration, and superfluid extraction. Thermolabile components are mainly extracted by such types of techniques (Harborne, 1984).
The use of hot extraction depends on some key factors like prolonged time and higher temperature. Hot extraction processes include digestion, reflexion, and steam distillation. The major drawback of the hot extraction method is excess temperature and volatility of compounds. Due to these disadvantages, polymerization and decomposition of protein take place (Azwanida, 2015; Barnes, 1999).
Fig. 15.14 describes the isolation techniques of indole alkaloids.
Fig. 15.14.
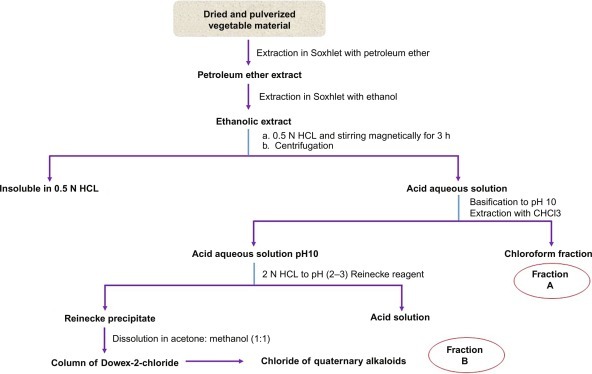
Extraction and purification techniques of indole alkaloids.
15.4.6.1. Liquid-liquid and solid-liquid extractions
If the precursor is liquid in nature, the partition method is applied, where the distribution coefficient between the liquid form and solvent is appreciable. This is an example of liquid-liquid extraction.
In the case of extraction from solids, there are several subtypes of solid-liquid extraction.
15.4.6.2. Maceration
In this method powder or bulk, plant compound is transferred in a stoppered container and covered with a solvent for a certain time until the solubilized part is dissolved in the solvent. It is an example of the cold extraction process (Cunha et al., 2004; Majors, 1996; Pandey and Tripathi, 2014; Phrompittayarat et al., 2007; Poole and Poole, 1996; Ronald, 1999; Sasidharan et al., 2008; Valérie, 2001; Woisky and Salantino, 1998).
15.4.6.3. Percolation
Indole-, isoquinoline-, and tropane-derived plant products are transferred in a percolation tube plugged with cotton with a filter. For maceration, the solvent is put in the plant material. The total experiment is carried on at room temperature. The extract along with extracted solvent is collected by a stopper at bellow. The process is continued until the proper evaporation for the last residue of the solvent from the percolator (Saberi, 2015).
Fig. 15.15 describes the isolation techniques of datura alkaloids (tropane alkaloids).
Fig. 15.15.
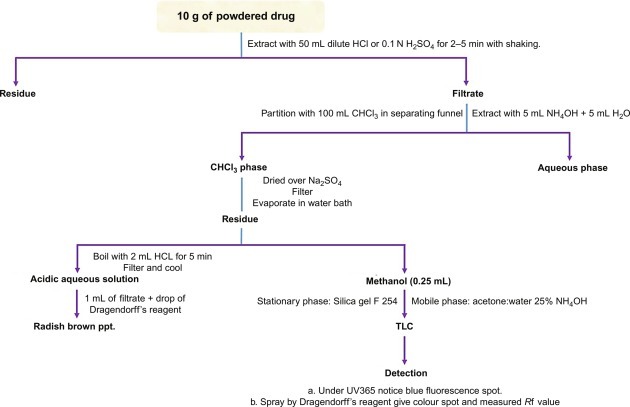
Extraction and purification techniques of datura alkaloids.
15.4.6.4. Digestion
In this step, temperature about (40–60°C) is applied at the time of extraction. The process is suitable for thermostable plant materials. Modification of method is done by mixing the plants products using magnetic stirrer and mechanical stirrer. The extract is filtered after 8–12 h, and then the fresh solvent is added. The method is repeated until the extraction of desire solutes (Komárek et al., 2006; Pandey and Tripathi, 2014).
15.4.6.5. Infusion
In this step, the plant material is extracted by cold water or boiling water for a brief time (Pandey and Tripathi, 2014).
15.4.6.6. Decoction
This process is appropriate for extracting for thermostable and water-soluble plant materials. Firstly the plant material is boiled in water, cooled, and strained (Pandey and Tripathi, 2014).
15.4.6.7. Extraction with boiling solvents (Reflexion)
In this step, the plant material is kept with hot water. The solvent vapor is condensed by condenser fitted on top of the container and recycled (Johansen et al., 1996).
Tincture
Plant material extracted in presence of alcohol. Generally, ethyl alcohol is used at the ratio 1:5. Due to alcohol content, the tinctures are stored in the closed system to avoid decomposition.
15.4.7. Pressurized liquid extraction (PLE)
Another name of this extraction method is an accelerated solvent extraction (ASE) system or enhanced solvent extraction (ESE) system. In this technique, temperature and pressure gradually increase. This elevated temperature promotes the extraction process by increasing the diffusivity of the solvent, whereas increased pressure can promote the penetration process through matrix pore without altering the liquid state of organic solvent. The instrument provides better working efficiency under an inert atmosphere and with protection from light. The advantages of this extraction process are a requirement of less solvent and less time (Björn et al., 1999; Liu et al., 2013; Richter et al., 1996; Skalicka-Wozniak and Glowniak, 2012).
15.4.8. Soxhlet extraction
Soxhlet extraction is the best technique for the continuous extraction of a solid by a hot solvent.
Soxhlet apparatus is constructed by unique glass materials used for organic solvent extractions purpose. During the extraction procedure, the solvent is boiled gently inside round bottomed (RB) flask, the vapor passes through the side tube, is condensed by condenser, and falls drop by drop into thimble-containing material. By this away, Soxhlet is filled up gradually, and when it reaches at the top the tube it siphons over into the flask. The removal portion of the materials, which it has extracted and the process repeats (Huie, 2002a; Zygmunt and Namieśnik, 2003).
15.4.9. Steam distillation
The essential oil is isolated from natural product via a steam distillation process. This process is very simple where vapors are made by steam, passing through the compounds. The steam volatile oil is recovered by condensation, where oil detached from the water.
15.4.10. Hydrodistillation
Hydrodistillation process is a most popular technique for isolation of essential oil. Plant products are soaked in water and boiled using a heating mantle. The essential oil is separated out from the oil gland in the plant materials and transfer with the steam. Clevenger apparatus are used for condensing steam oil mixture and detaching the oil part from the aqueous portion (Farhat et al., 2009; Özek et al., 2010; Sahraoui et al., 2008).
15.4.11. Enfleurage
This process is mainly applied to a delicate fragrance. The flower petals are extended over a layer of refined fat, which picks up the odor of the flowers and the saturated fat is treated with a solvent. Mainly alcohol is used for soluble of the fragrant component. The residual fat is separated by cooling the alcoholic extract at 20°C. After separation of fatty substances, volatile component is collected from alcohol by rotary evaporator (Mahajan et al., 2015).
15.4.12. Supercritical fluid extraction
The manufacture of the supercritical fluid is performed by heating at an above critical temperature and compressing over the critical pressure. A supercritical fluid is a liquid as well as that of gas in nature. The penetration of supercritical fluid is like gas under pressure and can be handled as a liquid. CO2 is the most popular supercritical fluid. Another example like ethylene, ethane, propylene, propane, and nitrous oxide can also be used. CO2 has a comparatively low critical temperature (31.1°C) and pressure (73.8 bar). The extraction is performed at a temparature of 35°C and 36°C in the presence of CO2. The method has been in use in large scale. Sometimes methods are modified by the addition of polar compound for efficient extraction. Delicious flavor, perfume chemicals are manufactured by this sophisticated technique. The benefits of this technique are mild conditions, which is suitable for thermolabile compounds (Lutfun and Sarker, 2012; Patil and Shettigar, 2010; Zougagh et al., 2004).
15.4.13. Ultrasonic extraction
In this technique, high-frequency sound is applied to liberating the phytochemicals from the plant tissue. Ultrasound effect promotes the extraction used with mixtures of immiscible solvents. The major drawback of this technique is generating heat, which is harmful to thermolabile products. To avoid such types of problem, extraction is carried on under an ice bath to reduce the temperature. This process is not suitable for the isolation of large molecules like proteins or DNA (Vinatoru, 2001).
15.4.14. Microwave-assisted extraction
The electromagnetic radiations with a frequency range of 0.3–300 GHz are used in MAE. A microwave frequency of about 2.45 GHz is used as general domestic extraction purpose (Delazar et al., 2012). Microwave energy is passed through the solvent, with brief periods of cooling time as the process generates much heat. The electric field is responsible for the heating of substrates through the dipolar rotation and ionic conduction. Temperature is increased gradually with higher dielectric constant. In terms of yield value of an extract, microwaves extraction needs less time compared with Soxhlet extraction. Due to heat in microwaves technique, weak hydrogen bonds are broken down. So extraction of thermolabile compounds with low dielectric constant needs cold environment. To avoid this drawback, exhaust fans and solvent vapor detectors are not allowed in laboratory premises.
15.4.15. Solid-phase extraction
This type of extraction technique needs cartridges and disks with a variety of sorbents, where the solute molecules are especially attached over the stationary phase surfaces. Normal phase, reverse phase, and ion exchange solid-phase extraction (SPE) units are available. For example, polar compounds are eluted by using “Sep-Pak C18” cartridges (reverse phase), whereas the less polar compounds can be removed later (Tekel and Hatrík, 1996).
15.4.16. Solid-phase microextraction
This extraction was done without solvent and depended on adsorbent fibers. Solid-phase microextraction (SPME) can be attached to GC or high-performance liquid chromatography (HPLC). Volatiles compounds are extracted by SPME technique. The device of SPME apparatus is very simple. It appears like a modified syringe containing a fiber holder and a fiber assembly, the latter containing a 1–2 cm-long retractable SPME fiber. The SPME fiber itself is a thin fused-silica optical fiber, coated with a thin polymer film [such as polydimethylsiloxane (PDMS)]. The fiber is exposed and the analytes are eluted by the mobile phase. The common nonpolar liquid adsorbent phases are polydimethylsiloxane (PDMS), divinyl benzene (DB), while polyacrylate (PA) and carbowax (CW) are polar adsorbents (Buchholz and Pawliszyn, 1994; Zini et al., 2001).
15.4.17. Headspace-solid-phase microextraction
HS-SPME is a most popular method for volatile compounds. This technique has a highly significant role in chemical ecology and fragrance industries. The benefits of the method over the existing processes such as hydrodistillation are shorter sampling time, field analysis, repeated and cost-effective analysis (Vankar, 2004).
15.4.18. Sequential and selective extraction
In sequential extraction, the extraction is carried out on the same plant material successively in the order of polarity of the solvent. It is also known as successive extraction. In selective extraction, a particular solvent is used for the extraction and once the extraction is over, the fresh plant material is used for further extraction with other solvents.
15.4.19. The concentration of the extract
The heating bath temperature is recommended to be around 20–30°C higher than the boiling point of the solvent. Usually, the extract is concentrated on a water bath if the solvent has boiling point below 80°C, whereas a heating mantle, sand bath, or hot plate is used for higher boiling solvents.
15.4.20. Rotary evaporator
A rotary evaporator is mainly used for a thermolabile product where the solvent is removed by using a vacuum evaporator. It follows the PV = nRT equation, a reduction of pressure lowers temperature. The working mechanism is just opposite to a pressure cooker. The liquid becomes boiled, when the vapor pressure of the liquid is equaled to atmospheric pressure and thus at a reduced pressure only less vapor pressure is needed for boiling and hence solvents boil at reduced temperature. At a vacuum of 72 mbar, water boils at 40°C, ethanol boils at 40°C at a vacuum of 240 mbar, and butanol boils at 40°C at 25 mbar. Molecular weight, density, vapor are the important parameters of boiling of any types of liquid. Proper heat transfer with reduction of the bumping phenomenon of solvents is improved by the rotation.
A layer is formed inside the flask and increases the surface area, which ultimately increases the rate of evaporation. For simple distillation, rotavapor with diagonal condensers is used for high boiling (> 100oC) solvents. In case of low boiling solvents, acetone and dry ice are used as coolants. Methanol, ethanol, or isopropanol is used as antifreeze agents when very low temperature is required (Barnes, 1999; Kahol et al., 1998; Pandey and Tripathi, 2014; Wang and Weller, 2006).
Fig. 15.16, Fig. 15.17 describe the isolation techniques of atropine (tropane alkaloid) and ergotamine alkaloid (indole alkaloids).
Fig. 15.16.
Extraction and purification techniques of atropine alkaloid.
Fig. 15.17.

Extraction and purification techniques of ergotamine alkaloid.
15.4.21. Evaporator centrifuge
It is applied for evaporation of solvents by vacuum. Bubbling of solvent is reduced with the help of centrifugal force. Temperature stability is controlled by evaporation of the solvent.
15.4.22. Freeze drying (lyophilization)
Freeze drying is mainly used for concentrating the thermolabile substances such as proteins, antibiotics, and enzymes. The principle of lyophilization is that the aqueous solution is frozen and the ice is sublimed off to leave a dry residue. Initially, the material is cooled below its triple point. At this time, temperature generally should remain at the range − 50°C to − 80°C. About 95% of the water in the compound is sublimated at the time of primary drying stage. The sublimed water vapor becomes condensed on a cold outward (− 50°C). In the secondary drying stage, residual water molecules are removed from the frozen material. After removing water from the compound, nearby atmospheric temperature becomes equal. Finally, the residual water content reaches around 0.5% (Barnes, 1999; Pandey and Tripathi, 2014; Wang and Weller, 2006).
15.4.23. Preservation of the extract
Moisture, temperature, light, presence of oxygen and microorganisms are the important key factor for proper storage of the indole-, isoquinoline-, and tropane-derived plant extracts. To avoid water contamination, a suitable drying agent such as anhydrous sodium sulfate, calcium chloride, P2O5, CuSO4, K2CO3, Sodium, CaO, MgSO4 is used at the time of storage. Toluene is used as an antifungal agent in the aqueous extract. The extract should be kept in dark for the protection of oxidation reaction. The extract should be transferred in N2 atmosphere to prevent decomposition. For prevention of decomposition, a reducing substance such as cysteine (1%, pH 7) is used and stored at cold temperature in the refrigerator or a deep freezer, and sealed hermetically (Barnes, 1999; Pandey and Tripathi, 2014; Regnier et al., 2012).
The schematic process of isolation of major indole, tropane, and isoquinoline alkaloids is represented in figure.
15.5. Techniques of identification and quantification
There are wide ranges of bioactive compounds present in indole-, isoquinoline-, and tropane-derived plant extracts. Due to a wide range of polarity index, separation techniques, as well as the identification and characterization of bioactive compounds, are an important issue. For the isolation of active components, various analytical techniques have been applied such as TLC, HPTLC, paper chromatography, column chromatography (CC), gas chromatography (GC), optimum performance laminar chromatography (OPLC), and HPLC, After that, the isolated compounds are used for structure elucidation as well as biological activity (Sasidharan et al., 2011).
15.5.1. Chromatographic techniques
The principle of this technique is separation. The separation technique of active components is based on their size, shape, and charge (Coskun, 2016). At the time of analysis with chromatography, analytes are present in a solution and transferred through a solid phase that acts as a sieving material. In this regard, paper and thin-layer chromatography are the ideal techniques to provide qualitative as well as quantitative data.
15.5.2. Adsorption chromatography
The principle of adsorption chromatography is an interaction between the solute and stationary phase. The active site of the stationary phase is detached by noncovalent bonds, nonpolar interactions, Van der Waals forces, and hydrophobic interactions. The loosely bound components will be eluted out firstly by the mobile phase.
15.5.3. Partition chromatography
In this method, molecules are separated on the basis of the interaction between two immiscible liquid phases. Another name of this chromatography is liquid/liquid chromatography.
15.5.4. Ion-exchange chromatography
The active principle of this method is a separation of ions and polar components based on electrical properties of the components (Rathore and Gupta, 2012).
15.5.5. Affinity chromatography
In this method, separations depend on interactions between interacting pairs of substances such as macromolecules and its substrates, cofactors, allosteric effectors, or inhibitors. At the time of chromatography, a mixture of components passed through the column with the help of the mobile phase. Substance those have more affinity toward ligand are passed slowly and those that have less affinity are eluted fast due to less binding with a legend. A buffer having wide ranges of pH or an increased ionic strength is used to elute the analyte out.
15.5.6. Size exclusion chromatography
In this type of chromatography, separation of molecules is based on size without any chemical interactions. It is also labeled as gel permeation chromatography and molecular sieve chromatography.
15.5.7. Paper chromatography
In this technique, the separation was carried out on a simple filter paper. Another advantage of this technique is the considerable reproducibility of Rf (retention factor) values determined on paper. In this chromatography, filter paper is used as a stationary phase (Singh, 2017b). The sample is applied to the bottom portion of filter paper. This filter paper is kept in a chromatographic chamber with solvent. The solvent along with soluble molecules is run by capillary action. The capacity of movement of the solvent depends on the porosity of paper.
15.5.8. Thin-layer chromatography
Thin-layer chromatography (TLC) is a more sensitive, flexible, and rapid technique than paper chromatography (Bele and Khale, 2011). The active principle of this technique adsorption. That is why it is called adsorption chromatography. In this case, the sample is separated, based on adsorption on the surface of the solid adsorbent attached to the plate. The separation is mainly based on low-molecular-weight compounds. Several types of adsorbents are used for this chromatography (Fried, 2005).
15.5.9. Column chromatography
The active principle of CC is adsorption. The materials are diluted with mobile phase and pass through the stationary phase. Compounds that have more affinity toward the stationary phase move slowly and fewer affinity molecules elute first. Fractions of elute materials can be more concentrated than the original solution applied to the column.
15.5.10. Gas chromatography
This chromatography is suitable for only volatile compounds. Gas and liquid phase is used for this purpose. The gas phase is used as a mobile phase and a liquid acts as a stationary phase (Eiceman, 2002). The rate of migration depends on the distribution of chemical species in the gas phase. If the distribution is 100% into liquid stationary phase, it will not migrate at all. If the distribution of species is partly in both phases, it will migrate at an intermediate rate. Samples are initially vaporized and injected on the chromatographic column and then pass through the column by the flow of inert, gaseous mobile phase. Stationary phase remains fixed inside the column (Pravallika, 2016).
15.5.11. High-performance liquid chromatography
HPLC is the most widely used analytical technique for separation as well as the determination of organic and inorganic solutes in any sample, especially biological, pharmaceutical, food, environmental, industrial, etc. (Thammana, 2016). The active principle of this type of chromatography is adsorption. Separation of the compound depends on the interaction between polar and nonpolar compounds tightly packed inside the column and running phase. In the pharmaceutical industry and food industry, stationary phase remains nonpolar in nature and mobile phase-polar in nature. To elute the molecules from the column, high pressure up to 400 bars is required before they pass through a diode array detector (DAD). Vaporized compounds cannot be considered for the HPLC analysis. Even thermolabile compounds cannot allow for the HPLC analysis, and it provides a good complement to GC for detection of indole, isoquinoline, and tropane derivatives. Various types of an analytical column like C18, C8 column are used for HPLC analysis. Many types of detectors like UV, DAD, FID are used (Swartz, 2010).
Fig. 15.18 describes the isolation techniques of strychnine and brucine (indole alkaloid).
Fig. 15.18.
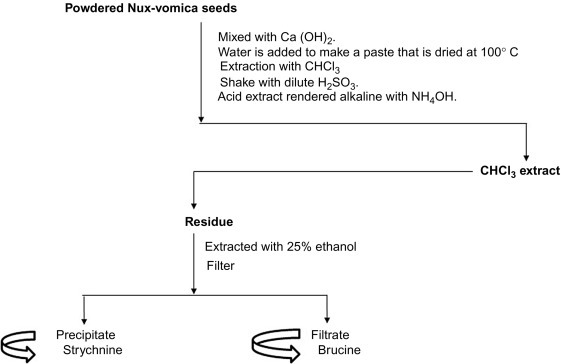
Extraction and purification techniques of strychnine and brucine alkaloid.
15.5.12. High-performance thin-layer chromatography (Attimarad et al., 2011; Modi et al., 2016)
The active principle of high-performance thin-layer chromatography (HPTLC) is a separation of molecules based on high-performance layers with detection and data acquisition. In this chromatography, a precoated plate is used with particle size 5–7 μm and a layer thickness of 150–200 μm. Better efficacy of separation depends on the reduction of particle size. Small particle size increases the plating efficiency.
15.5.13. Optimum performance laminar chromatography (Bryson and Papillard, 2004; Ingle et al., 2017)
This technique is associated with TLC and HPTLC. It is used as analytical as well as a preparative tool in research-cum-analytical field. This is the more efficient separation technique based on user-friendly interface and resolution of HPLC with the capacity of flash chromatography and multidimensionality of TLC.
Like other chromatographic techniques, high pressure is applied on liquid mobile phase through a stationary phase, such as silica or a bonded phase medium (C8, C18, amino, cyano, diol, and ion exchange). The structure of the column is the flat planar type. The pressure of the column is used up to 50 bars with a constant linear velocity of the mobile phase by the pump.
Phytochemicals are nothing but a large number of secondary indole-, isoquinoline-, and tropane-derived metabolic compounds found in plants. The different chemical compounds, which are present in plant extracts, have been identified through different methods of detection, which are discussed as follows and these are important tools in bioactive compound analyses.
15.5.14. Fourier-transform infrared spectroscopy
Fourier-transform infrared spectroscopy (FTIR) is an important instrument for the elucidation of functional groups present in plant extract. It also helps in the structure determination of an active constituent of indole-, isoquinoline-, and tropane-derived extract (Hazra et al., 2007; Eberhardt et al., 2007). The sample preparation technique of the FTIR is different. Liquid samples about one drop are placed between two plates of sodium chloride. This one drop produces a thin film.
Potassium bromide (KBr) is used for solid sample analysis. Sometimes, methylene chloride is also used for dissolving the solid samples and the little amount of solution is then transferred onto a single high attenuated total reflectance (HATR) plates and percentage transmittance was recorded with the help of spectra.
15.5.15. Nuclear magnetic resonance spectroscopy (Exarchou et al., 2001; Heyman and Meyer, 2012; Schripsema, 2010)
Nuclear magnetic resonance (NMR) is one of the valuable tools for measurement of physical, chemical as well as biological properties of indole-, isoquinoline-, and tropane-derived plant extracts. There are two types of NMR analytical technique used for the plant’s structure elucidation. Plant molecules with a complicated structure could be achieved through two-dimensional NMR techniques. Otherwise, in the general case, the one-dimensional technique is routinely used. For elucidation of the molecular structure of a solid, solid-state NMR spectroscopy is used. C-NMR is used to identify the types of carbon are present in the compound, whereas H-NMR is used for searching the hydrogen nature of any compound.
15.5.16. Mass spectrometry (Altemimi et al., 2017; Kumar, 2017; Rammohan et al., 2016)
Mass spectrometry (MS) is the most popular and powerful technique for qualitative as well as quantitative analysis of indole, isoquinoline, and tropane derivatives. The application of this technique is structure elucidation as well as identify the physical and chemical characteristics of indole, isoquinoline, and tropane derivatives. Molecular weight is also determined with the help of mass spectrometry. The tool is widely used for the structural elucidation of organic compounds, peptide or oligonucleotide sequencing.
15.5.17. Levels found in foods/plants
Presence of phytoconstituents in foods or plants depends upon their geographical location. For example, reserpine usually occurs at about 0.10%–0.16% of natural extracts (Varchi et al., 2005). Apart from hyoscyamine and scopolamine, which are major tropane alkaloids found in the green parts of Datura species, various other tropane alkaloids are also found at subordinate concentrations. The percentage of alkaloids varies among species. Approximately 30 different tropane alkaloids have been found in all tissues (especially roots) of the plant, except the capsules and the woody portions of the roots and stems of some Datura species (like, D. stramonium var. stramonium, D. stramonium var. godronii, D. ceratocaula, D. stramonium var. tatula, etc.) (Berkov et al., 2006; Berkova and Zayed, 2004). Stems contain more amounts of tropane alkaloid than leaves, flowers, and seeds (Al-yahya and Evans, 1977; Philipov and Berkov, 2002; Lounasmaa and Tamminen, 1993; Vitale et al., 1995).
Flower and leaves contain a higher amount of two main tropane alkaloids, hyoscyamine and scopolamine than root, and a considerable amount of these alkaloids is found in seeds (Miraldi et al., 2001; Witte et al., 1987). Berkov (2003) and Berkov et al. (2006) reported different tissues contain different amount of tropane alkaloids (Berkov, 2003; Berkov et al., 2006).
Many environmental elements (like climate, plant health, plant growth hormones, fertilization, insects, etc.) can show the positive or negative impact of tropane alkaloid content (Brachet and Cosson, 1983; Demeyer and Dejaegere, 1989; Gupta and Madan, 1975; Karnick and Saxena, 1970; Shonle and Bergelson, 2000; Stecka et al., 1975; van der Velde et al., 1988). The amount of various tropane alkaloids changes with the change of the period of a lifetime also. For instance, in an early age, scopolamine content is higher than the hyoscyamine whose content increases in the time of flowering in D. stramonium (Demeyer and Dejaegere, 1989).
Available analytical data on the tropane alkaloid content in different plant parts of various Datura species are shown in Table 15.5 . A summary of the tropane alkaloid content of some of these
Table 15.5.
Amount or concentration of total tropane alkaloids (TTA), hyoscyamine, and scopolamine content found in various species of datura plant (Alexander, 2008).
| Plant origin | Family | Part | Level found (FW or w/w or mg/kg DW) |
||
|---|---|---|---|---|---|
| TTA | Hyoscyamine | Scopolamine | |||
| Datura ferox | Solanaceae | Seeds | 800–1250 | NA | NA |
| Leaves | 1000–4100 DW | NA | 50–3200DW | ||
| Stem | 200–2800 DW | NA | 200–1500 DW | ||
| D. stramonium var. tatula | Seeds | 1400–1600 FW | 700 DW | 500 DW | |
| Leaves | NA | 1000 DW | 600 DW | ||
| D. stramonium | Seeds | 1600–4200 w/w | 1900 DW | 100–900 DW | |
| Leaves | 1900–3600 w/w | 1550 DW | 150–6500 DW | ||
| D. inoxia | Seeds | 4100 FW | 1400–2900 DW | 700 DW | |
| Leaves | 170–5700 DW; 300 FW | 54 FW | 150–1300 DW; 250 FW | ||
| Stem | 3200 DW; 66 FW | 25 FW | 19 FW | ||
| D. stramonium var. stramonium | Seeds | NA | 700 DW | 1200 DW | |
| Leaves | 100–1800 FW | 700 DW | 650 DW | ||
| D. stramonium | Seeds | 1700 FW | 1400 DW | 1300 DW | |
| var. godronii | Leaves | NA | 400–450 DW | 550–600 DW | |
FW: Fresh weight; w/w: weight by weight; DW: dry weight; NA: not available.
UnlikeTable 15.4where the amount of total tropane alkaloids, scopolamine, and hyoscyamine varies from species to species, this table will explain the same with respect to the geographic distribution.
Datura species is given in Table 15.5, Table 15.6 . One of the difficulties encountered when summarizing the available information is the fact that different investigators have used different analytical methods and different units to express the measured alkaloid concentrations found (see Table 15.6).
Table 15.6.
Level found in various parts of different Datura species in different countries.
| Country | Plant name | Part | Level found (FW or w/w or mg/kg DW) |
Reference | |||
|---|---|---|---|---|---|---|---|
| TTA (mg/kg) | Atropine (mg/kg) | Scopalamine (mg/kg) (% of total) | Hyoscyamine (mg/kg) (% of total) | ||||
| India | D. metal | Stem | 1900–4600* | NA | NA | NA | Karnick and Saxena (1970) |
| Root | 2700–8900* | NA | NA | NA | Karnick and Saxena (1970) | ||
| Leaves | 2500–5800* | NA | NA | NA | Karnick and Saxena (1970) | ||
| Flower | 9390* | 3200* | 5510* | NA | Karnick and Saxena (1970) | ||
| Seed | 900–1900* | NA | NA | NA | Karnick and Saxena (1970) | ||
| Fruit | 600–970* | NA | NA | NA | Karnick and Saxena (1970) | ||
| Paricarps from herbarium | 860* | NA | 480* | 230* | Karnick and Saxena (1970) | ||
| Seed from herbarium | 1910* | NA | 1250* | 650* | Karnick and Saxena (1970) | ||
| D. metal diploid | Fruit | 3300* | NA | NA | NA | Karnick and Saxena (1970) | |
| D. metal tetraploid | Fruit | 4200* | NA | NA | NA | Karnick and Saxena (1970) | |
| D. metal haploid | Fruit | 1500 | NA | NA | NA | Karnick and Saxena (1970) | |
| France | D. inoxia | Leaves | 5700** | NA | NA | NA | Brachet and Cosson (1983) |
| Roots | 6700** | NA | NA | NA | Brachet and Cosson (1983) | ||
| Basal leaves | 1700** | NA | NA | NA | Brachet and Cosson (1983) | ||
| Stem | 6700** | NA | NA | NA | Brachet and Cosson (1983) | ||
| Japan | D. metal var. muricata | Leaves (n = 2) | NA | NA | 810–820** | NA | Hiraoka et al. (1996) |
| D. metal var. metal | Leaves (n = 2) | NA | NA | 2280–2530** | NA | Hiraoka et al. (1996) | |
| D. metal var. fastuosa | Leaves (n = 2) | NA | NA | 690–1200** | NA | Hiraoka et al. (1996) | |
| D. metal var. rubra | Leaves (n = 2) | NA | NA | 460–590** | NA | Hiraoka et al. (1996) | |
| Russia | D. stramonium | Seed | 4200* | NA | 20.5% | 72.5% | Mirzamatov and Lutfullin (1986) |
| Roots during vigorous growth | 1300* | NA | 18.5% | 66.2% | Mirzamatov and Lutfullin (1986) | ||
| Epigeal parts during flowering | 2600* | NA | 17.2% | 69.3% | Mirzamatov and Lutfullin (1986) | ||
| Roots during fruit bearing | 2200* | NA | 15.5% | 55.2% | Mirzamatov and Lutfullin (1986) | ||
| Epigeal parts during fruit bearing | 1900* | NA | 16.5% | 61.2% | Mirzamatov and Lutfullin (1986) | ||
| Roots during flowering | 1900* | NA | 16.3% | 61.1% | Mirzamatov and Lutfullin (1986) | ||
| Epigeal parts during vigorous growth | 3600* | NA | 19.5% | 73.5% | Mirzamatov and Lutfullin (1986) | ||
| Netherlands | D. ceratocaula (from greenhouse) | Seed | NA | NA | 700** | 2500** | Berkov (2003) |
| Stem | NA | NA | 2000** | 4200** | Berkov (2003) | ||
| Flower | NA | NA | 3400** | 4100** | Berkov (2003) | ||
| Roots | NA | NA | 700** | 1600** | Berkov (2003) | ||
| Italy | D. stramonium | Flower | NA | 660–1060** | 2700–2990** | NA | Miraldi et al. (2001) |
| Young plants, root | NA | 1200** | 1210** | NA | Miraldi et al. (2001) | ||
| Seed | NA | 1700–3870** | 120–890** | NA | Miraldi et al. (2001) | ||
| Fully-grown plant, stem | NA | 10** | NA | Miraldi et al. (2001) | |||
| Fully-grown plant, leaves | NA | 1340–1650** | 160–440** | NA | Miraldi et al. (2001) | ||
| Young plants, leaves | NA | 1560–8310** | 350–730** | NA | Miraldi et al. (2001) | ||
| Young plants, stem | NA | 9150** | 1290** | NA | Miraldi et al. (2001) | ||
| Poland | D. metal var. fastuosa | Seed | NA | NA | 773** | 1027** | Mroczek et al. (2006) |
| D. metal | Leaves | NA | NA | 1110** | 904** | Mroczek et al. (2006) | |
| D. stramonium var. tatula | Seed | NA | NA | 522** | 711** | Mroczek et al. (2006) | |
| D. quercifolia | Seed | NA | NA | 387** | 1929** | Mroczek et al. (2006) | |
| D. inoxia | Seed | NA | NA | 672** | 2862** | Mroczek et al. (2006) | |
| D. stramonium var. stramonium | Seed | NA | NA | 1192** | 722** | Mroczek et al. (2006) | |
| Leaves | NA | NA | 713** | 658** | Mroczek et al. (2006) | ||
| D. stramonium var. godronii | Leaves | NA | NA | 571** | 427** | Mroczek et al. (2006) | |
| Seed | NA | NA | 1275** | 1382** | Mroczek et al. (2006) | ||
| USA | D. stramonium | Leaves | NA | NA | 1550** | 1550** | Miraldi et al. (2001) |
| Seed | 3400* | 2900* | 500* | NA | Miraldi et al. (2001) | ||
| Seed | NA | 2270 ± 360* | 530 ± 130* | NA | List et al. (1979) | ||
| Seed | NA | 4000* | NA | NA | List et al. (1979) | ||
| Seed | NA | 2710* | 660* | NA | Klein-Schwartz and Oderda (1984) | ||
| D. inoxia | Seed | NA | 26** | 29** | NA | Galey et al. (1996) | |
| Leaves and stems | NA | 187 ± 103** | 612 ± 278** | NA | Galey et al. (1996) | ||
| Argentian | D. ferox | Seed | NA | NA | 76.3% | NA | Vitale et al. (1995) |
| Leaves | 1000–4100** | NA | 40–3200** | NA | Padula et al. (1976) | ||
| Roots | 800–3600** | NA | 40–120** | NA | Padula et al. (1976) | ||
| Fruits | 1100–2300* | NA | 130–210** | NA | Padula et al. (1976) | ||
| Stem | 200–2800** | NA | 300–1500** | NA | Padula et al. (1976) | ||
| Germany | D. inoxia (from greenhouse) | Flower | 550*** | NA | 520*** | 42*** | Witte et al. (1987) |
| Roots | 1123*** | NA | 100*** | 172*** | Witte et al. (1987) | ||
| Leaves | 314*** | NA | 250*** | 54*** | Witte et al. (1987) | ||
| Stem | 66*** | NA | 19*** | 25*** | Witte et al. (1987) | ||
The subsequent units have been used in this table: *weight by weight, **mg/kg dry weight, and ***mg/kg fresh weight (% of total tropane alkaloids).
Table 15.5 contains tropane alkaloids content in various parts found in different Datura species. But different authors have used different analytical techniques and units to express the measured alkaloid concentrations.
15.5.18. Effect of food processing on phytochemicals
Various vegetables, fruits, grains are good source of phytochemicals, which possess diverse pharmacological activities like anticancer, antiinflammatory, antiaging, antimicrobial, etc. (Liu, 2003, Liu, 2004; Zern et al., 2005). Intake of phytochemical-rich fruits and vegetables or other plant foods can lower the risk of breast, pancreatic, lung, stomach, colon, ovarian, and esophageal cancers (Gladys et al., 1992) and also help to maintain the optimum level of antioxidant in blood serum (Guohua et al., 1998a, Guohua et al., 1998b). However, for better digestion and metabolism, humans process the food items in various ways. Research indicates that nature and extent of phytochemicals alter among and within the plant species and also can be organ dependent within an individual. Quantitative or qualitative medicinal properties are also affected by food-processing techniques.
To maximize decontamination and long-term storage, air drying is the cost-effective technique used in various parts of the globe traditionally (Schweiggert et al., 2007). The dried plant parts have high stability and are easier to handle, standardize, transport, and store. Furthermore, different solid dosage forms, like tablets and capsules, can be manufactured from dried extracts (Couto et al., 2012). But, throughout the drying procedure, phytochemicals are prone to hydrolysis, oxidation, microbial and additional environmental degradation (Kwok et al., 2004). Research indicates that freezing can appropriately preserve the biological property of compounds (Piotr and Lisiewska, 2006; Wollgast and Anklam, 2000; Weiguang and Wetzstein, 2011).
Food-processing techniques like cooking, canning, and drying/dehydration, among others, have significant effect on health-benefiting properties of different phytochemicals such as fruits, vegetables, and grains (Nayak et al., 2015). In this section of the chapter, we will concisely highlight the effect of these thermal and nonthermal food processing techniques on the alkaloids.
15.5.19. Drying
For future use, drying/dehydration is one of the oldest practices for preserving foods items. In this technique, water is removed from the food to minimize bacterial activity. Along with food safety, scientists also give attention to the variations of phytochemicals during food preservation. In some fruits like saskatoon berries, various processing techniques like air-drying, vacuum microwave-drying, and a combination of air-drying and vacuum microwave-drying can reduce the anthocyanin, phenolics content, and hence their antioxidant activity (Kwok et al., 2004). Leusink et al found that ORAC antioxidant activity of dehydrated cranberry products was increased by vacuum microwave-drying (Gwen et al., 2010). Freeze-drying degraded the antioxidant activity of cranberry than vacuum microwave-drying. In the microwave-drying, water molecules inside the food samples absorb more energy and allow it to reach in boiling point than any other drying methods (Majeed et al., 1995). A combinatorial application of both vacuum and microwave drying can facilitate the drying process without an increase in drying temperature, time, and oxygen intake (Oliveira and Franca, 2002). Apart from these two methods, freeze-drying has no severe effect on the phytochemicals of cranberries. Increase in the total antioxidant activity was observed after refractance window and freeze-drying than tray-drying, a combination of microwave and spouted bed drying (Nindoa et al., 2003).
In the oak groats, ferulic acid and vanillin were improved by the application of flaking (100°C, for 20 min) and steaming (100°C, for 1 h), whereas it reduces the caffeic acid, some avenanthramides, and tocotrienols. In the same study, autoclaving degraded avenanthramides, but it increased tocotrienols, p-coumaric acid, tocopherols, and ferulic acid content (Susanne et al., 2002). They also showed that all tools and phenolic compounds were degraded by the application of 8-bar steam pressure (also known as Drum-drying) on rolled oats or wholemeal, whereas avenanthramides are unaffected in this process (Susanne et al., 2002). The microwave-drying process, where the high temperature was applied for a short period, preserves the phytochemical content as compared with conventional drying technique.
15.5.20. Blanching
In order to protect the fruits and vegetables from browning or other possible reactions, inactivation of the enzymatic activity and softening the product was done by blanching. Plant tissue contains numbers of enzymes such as polyphenol oxidase (PPO), peroxidases, and glycosidases, which degraded the phenolic compounds. Anthocyanidins are very unstable and rapidly degraded produced by glycosidases. PPO accelerates the oxidation reaction of o-dihydro phenols to o-quinones that further counter brown polymers. The extent of peroxidases inactivation determines the effectiveness of blanching. Generally, blanching is done by steam or hot water and its time depends on the measurement of the food matrix, like for small food items (e.g., peas) are 1–2 min and for large-sizes food items (e.g., corn on the cob) are 11 min (Irondi et al., 2017). Blanching for a long time can damage the quality of food, whereas a shorter time can reduce the degree of enzyme inactivation and which results in reduced shelf-life, dietetic and efficient value.
As compared to untreated Rome beauty apple peels, blanching them in boiling water for 10–20 seconds increased the total flavonoid and phenolics contents (Wolfe and Liu, 2003). Chu et al. (2000) reported green leaves of sweet potato, with a blanching time of 1 min in boiling water, have been endorsed to preserve antioxidant activity (Chu et al., 2000). Compared to raw purple potato, 8 min of saturated steam blanching preceded drum-drying, upsurging total antioxidant capability (175%) of dry flakes (Nayak et al., 2011a). Blanching may either improve or diminish the antioxidant activity of food items by opening the cell matrix and increasing the polyphenolic yield during extraction. Antioxidant activity of blueberry juice was increased after 3 min steam blanching than the unblanched juice (Margherita et al., 2003). Uyan et al. (2004) reported antioxidant capacity of purple carrots was reduced after blanching in water at 98°C for 2 min (Uyan et al., 2004). Antioxidant and phenolics content of all vegetables except for cabbage and mustard cabbage was reduced after 5–10 min blanching in hot water at 98°C (Badwaik et al., 2015; Ismail and Lee, 2005). The antioxidant activity was increased by 9%, 40%, 4%, and 19% in mustard cabbage, Chinese cabbage, red cabbage, and Chinese white cabbage, respectively, after 15 min of blanching.
15.5.21. Extrusion
Extrusion cooking is a food-processing technique where a combination of mechanical shearing and temperature under pressure is applied, which results in molecular conversion and chemical reactions in processed foods. Phenolic content of cauliflower byproducts (Stojceska et al., 2008) and oats (Zielinski et al., 2001) is increased by extrusion cooking. The antioxidant activity of cauliflower byproducts (Stojceska et al., 2008), fruit powders from raspberries, concord grapes, cranberries, blueberries (Nayak et al., 2011b), and sweet potatoes (Shih et al., 2009) was increased by this method. Phenolic content was reduced in the extruded oat cereals, bean, and oat extrudates by 24%–46%, 19%–21%, 50%, respectively (Jaroslaw et al., 2007; Viscidi et al., 2004; Zadernowskl et al., 1999). The loss of antioxidant activity was attributed to the high temperature during extrusion process, which alters the molecular arrangement of phenolic compounds and diminishes their chemical reactivity and also lessens their extractability because of a certain degree of polymerization (Rubén et al., 2000; Zadernowskl et al., 1999).
15.5.22. Cooking
Cooking, the most common thermal technique, prompts several physical and chemical alteration in phytochemicals. This popular technique may bring two differing spectacles in phytochemicals (1) thermolabile phytochemicals are more prone to thermal degradation, which reduces their amount/concentration, (2) or else, it increases the concentration of phytochemicals with respect to raw material by increasing the extractability of phytochemicals (Palermo et al., 2014). But, supremely, the ultimate effect of cooking on phytochemicals depends on the chemical nature of individual phytochemical, the structure of the food matrix, and also processing factors. Apart from other cooking techniques, steaming is the best technique, which ensures better preservation/extraction yield of various phytochemicals (Palermo et al., 2014). The following factors ensure the same (1) Steamed foods are not in direct contact with water or oil (the major material of cooking of foods), which minimizes the mixing of hydrophilic or hydrophobic compounds in water or oil. (2) Chances of thermal degradation are also minimized.
Cooking of different fruits, vegetables, and grains has exerted diversified action on phytochemicals. Canning of blueberries at 100°C for 22 min and raspberries 100°C for 28 min increases their antioxidant activity and phenolic content by 53% and 50%, respectively (Sablani et al., 2010). Total phenolic content was reduced while processing jam because of heat (Kim and Padilla-Zakour, 2004). The total flavonoid, phenolic content, and antioxidant activity was reduced in cooked green beans at 100–120°C for 10–40 min (Jiratanan and Liu, 2004). Microwave cooking may retain or decrease the phytochemical content in spinach, cauliflower, and peas as compared to boiling (Natella et al., 2010). Antioxidant activity of spinach, garlic, maize, onion, pepper, and Swiss chard was increased after microwave cooking (Jiménez-Monreal et al., 2009). Anthocyanin content of red cabbage was reduced by 29% and 41% after steaming for 10 min and Boiling at 94–96°C for 3 min, respectively (Volden et al., 2008).
15.5.23. Nonthermal processing
Traditional food-processing technique especially the application of heat is a very popular and widely acceptable methodology for preserving foods. But in last decade, different nonthermal technologies such as ultrasound, high hydrostatic pressure, and the pulsed electric field (PEF) have been developed as substitute practices in food processing to advance nutritional qualities of the processed foods (Nayak et al., 2015). Application of ultrasound is responsible for degradation of compounds that control color, and anthocyanins is endorsed to (a) the antioxidant reaction that occurs by the interaction with free radicals produced during sonication; (Portenlänger and Heusinger, 1992) (b) extreme physical conditions (like pressure up to 500 MPa at microscale level and temperature up to 5000 K) produce during sonication (L, 1988). High hydrostatic pressure treatments on vegetable and fruit foodstuffs are reliant on their matrices and different handling parameters like pressure, time, and temperature. A slight modification in the nutritional configuration (vitamins A, E, C, B1, B2, and folic acid), flavonoid content was observed in juices of apple, orange, lemon, and also mixed vegetable soup (Donsi et al., 1996; Quaglia et al., 1996; Sánchez-Moreno et al., 2009).
15.5.24. CO2 treatment
CO2 is a nonflammable and nontoxic gas basically reflected as harmless by the food industries. But, supercritical CO2 has the ability to infiltrate complex configurations and porous food materials due to its zero surface tension and low viscosity (Zhang et al., 2006). When compared with the thermal application (90°C for 1 min), CO2 treatment maintains the antioxidant activity of guava puree (at 35°C temperature, 30.6 MPa pressure, 8% CO2 for 6.8 min) (Nayak et al., 2015). No significant change observed in external anthocyanin content was observed while internal anthocyanin content was decreased significantly after being kept in CO2-treated atmosphere (Gil et al., 1997). This type of opposite behavior is due to a large amount of cyanidin 3-glucoside in peripheral tissue providing higher color stability, whereas pelargonidin glycosides exist in the internal tissue (Nayak et al., 2015).
15.6. Current and potential industrial applications of alkaloids
The production of alkaloids in vitro cultures is a feasible technology pursued by industrial and academic interests. In addition, screening of cell lines along successive subcultures has also enabled the selection of high-yielding cell lines, although they are usually unstable and tend to decrease their production after periodical subcultures (Sayeed et al., 2013).
Although there are only a few reports about the industrial perspectives of alkaloids metabolic engineering, this may be a potent tool for increasing alkaloid production in cell platforms. Usually, enzymes of the biosynthetic pathway are selected as targets for gene cloning and then manipulated by genetic engineering, e.g., to raise metabolic flow rate toward the compound of interest and hence enhance its levels. One of the major limitations of metabolic engineering of these systems is the lack of fully elucidated plant biosynthetic pathways (Dräger, 2008; Sayeed et al., 2013).
15.6.1. Pharmaceutical application
On the basis of the medical approach, alkaloids have led to the production of herbal medicines and their components. The alkaloidal structure is modifying chemically for a better therapeutic response. Generally, after modifications, synthetic drugs show a better response than natural drugs. However, alkaloids have a significant role in phototherapy, homeopathy, and alternative medicine (Aniszewski, 2007; Sayeed et al., 2013).
Indole, isoquinoline, and tropane derivatives have clinical importance. The herbal drugs are modified to medicinal products in the pharmaceutical industry to get a better therapeutic response compared to synthetic drugs. Physicians focus their interest on dispensing the herbal remedies for the treatment of various disorders.
Tropane derivatives like atropine, hyoscine, and hyoscyamine are promoted in large scale and also for clinical purposes. As an example, atropinol contains an active ingredient, atropine sulfate. Buscopan is a derivative of hyoscine. It is used in transdermal plasters. Another compound Bella sanol also encompasses hyoscyamine. There are 50 different products based on alkaloids that have been developed and marketed (Aniszewski, 2007; Dräger, 2008; Grzegorz and Gadzikowska, 2008).
The important alkaloids such as boldine, codeine, narceine, and morphine have a significant role in clinical therapy. Bold oval and Oxyboldine have morphine-like pharmacological action. Codeine is one of the most popular compounds in at least 250 pharmaceutical drugs on the market. Codicaps or Codipront can be applied for the same purposes. Each and every product is modified from opium. Narceine-containing drugs are related to codeine. It is mainly used for cough treatment. Paneraj is an example of a typical trademark (Aniszewski, 2007; Sayeed et al., 2013).
Tubocurarine derivatives like tubarine or jexin have been applied as a muscle relaxant. Morphine-containing drugs such as morphalgin and spasmofen are used in serious cases like surgical operations and postoperation treatments (Aniszewski, 2007).
The indole alkaloids chemical constituents such as ephedrine, ergotamine, ergometrine, and yohimbine are applied in different combination formulations (Kumar et al., 2013a).
The main active ingredient of Dorex or Endrine is ephedrine. It is used for multiple purposes like treating nasal cold symptoms or in bronchial asthma (Aniszewski, 2007).
The main chemical constituent of ergot is ergotamine. Ergotamine is highly available in the market due to its wide applications. Ergostat or Migral are marketed products based on ergotamine. These alkaloids are used for treating migraines. Yohimbine is the main active molecules of aphrodyne or yohimex. At least 20 different compounds are developed, based on this alkaloid. These drugs are applied to impotency-related problems in men (Aniszewski, 2007; Jeffrey et al., 1994; Kumar et al., 2013a; Smeller and Wink, 1998).
The application of alkaloids is very wide. For example, strychnine is used for the treatment of multiple disorders, including eye conditions. The active component of Dysurgal or Pasuma is strychnine use in clinical doses (Aniszewski, 2007; Jeffrey et al., 1994; Smeller and Wink, 1998).
15.6.2. Agricultural application
In food crops, alkaloids are a concern and topic of discussion due to possible health hazards and the fact that they have to be removed from plants by breeding and especially hybridization. Alkaloid-rich (bitter) and alkaloid-poor (sweet) cultivars are developed as a result (Tadeusz, 1992).
The elimination of total alkaloids through breeding is impossible. However, the reduction of alkaloid content is possible by using a suitable application. Alkaloids are also removed from the raw materials by industrial processing. Elimination of total alkaloids can be found in the food industry.
Generally, the clinical doses of functional foods are found much slighter. And alkaloids are potentially used as a developing agent for functional foods and so-called natural plant-based vaccines. This kind of development is noticed in the case of genetically modified plants (GMP), which contain extra genes and generate new proteins (Aniszewski, 2007).
Sometimes alkaloids are applied as biological fertilizers in cultivation. Alkaloids are used in cultivation purposes especially used as cyclical maintenance of a field, garden, or forest ecosystems (Aniszewski, 1995).
Alkaloids are a good source of nitrogen and carbon. However, nitrogen and carbon have an important role in organic cultivation. The balance between macro- and micronutrients is an important part of the carbon- and nitrogen-based soil management systems.
15.6.3. Alkaloids in food
Alkaloidal plants are often used as foods. The low application rate is due to its bioactivity as medicines and drugs. On the other hand, food is screened and restricted for the purposes of keeping alkaloid contaminated food off the market. Contamination of food by alkaloids is considered a health risk (EFSA Panel on Contaminants in the Food Chain, 2017).
15.7. Conclusion
Alkaloids possess a prodigious therapeutic and civic interest as a source of the novel leading compounds for drug development against various deadly diseases. Among all, the indole, tropane, and isoquinoline alkaloids are well acknowledged to exhibit therapeutic potential for treatment of hypertension, cancer, microbial infection, neurological disorders, etc. They are structurally unique bioactive molecules with effective therapeutic ability. Enough scientific evidence is gathered in this chapter regarding various plant-based major types of alkaloids and their phytochemistry, isolation, identification, and biological activities. In conclusion, detailed research on their metabolic transformation, the introduction of a wide range of scientific tools, and cooperative teamwork of professionals among different scientific areas will accelerate the research on this hotspot and will provide new and important healthcare opportunities.
References
- Abd-Rabbo H. Chemotherapy of neoplasia (cancer) with dehydroemetine. J. Trop. Med. Hyg. 1969;72:287–290. [PubMed] [Google Scholar]
- Abduyeva-Ismayilova S.M. Effect of physiological acid salts on swelling, germination and growth processes of seedlings. J. Plant Physiol. Pathol. 2016;4 [Google Scholar]
- Åberg M., Siegbahn A. Tissue factor non-coagulant signaling-molecular mechanisms and biological consequences with a focus on cell migration and apoptosis. J. Thromb. Haemost. 2013;11:817–825. doi: 10.1111/jth.12156. [DOI] [PubMed] [Google Scholar]
- Ahamad J., Mir S.R., Naquvi K.J. Hypoglycemic activity of aqueous extract of Berberis aristata stems bark in STZ-induced rats. J. Res. Indian Med. 1969;4:83–96. [Google Scholar]
- Akinboye E.S., Bakare O. Biological activities of emetine. Open Nat. Prod. J. 2011;4 [Google Scholar]
- Alexander J. Tropane alkaloids (from Datura sp.) as undesirable substances in animal feed. EFSA J. 2008;691:1–55. [Google Scholar]
- Altemimi A., Lakhssassi N., Baharlouei A., Watson D.G., Lightfoot D.A. Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants. 2017;6:42. doi: 10.3390/plants6040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-yahya M.A., Evans W. The alkaloids of Datura quercifolia HBK. Quart. J. Crude Drug Res. 1977;15:131–132. [Google Scholar]
- Amalraj L., Venkateswarlu B., Desai S., Kumar P., Ahmed M., Meenakshi T., Sultana U., Pinisetty S., Narasu L. Effect of polymeric additives, adjuvants, surfactants on survival, stability and plant growth promoting ability of liquid bioinoculants. J. Plant Physiol. Pathol. 2013;1 [Google Scholar]
- Amirkia V., Heinrich M. Alkaloids as drug leads—a predictive structural and biodiversity-based analysis. Phytochem. Lett. 2014;10:xlviii–liii. [Google Scholar]
- Anis K., Kuttan G., Kuttan R. Role of berberine as an adjuvant response modifier during tumour therapy in mice. Pharm. Pharmacol. Commun. 1999;5:697–700. [Google Scholar]
- Anis K., Rajeshkumar N., Kuttan R. Inhibition of chemical carcinogenesis by berberine in rats and mice. J. Pharm. Pharmacol. 2001;53:763–768. doi: 10.1211/0022357011775901. [DOI] [PubMed] [Google Scholar]
- Aniszewski T. The biological basis of quinolizidine alkaloids. Sci. Legum. 1994;1:1–24. [Google Scholar]
- Aniszewski T. The effects of green manure on bulk density and internal balance of garden soil: a one-year experiment. Sci. Legum. 1995;2:149–162. [Google Scholar]
- Aniszewski T. Elsevier; 2007. Alkaloids—Secrets of Life. Alkaloid Chemistry, Biological Significance, Applications and Ecological Role. [Google Scholar]
- Anitha S. Pharmacological activity of vinca alkaloids. Res. Rev. J. Pharmacogn. Phytochem. 2016;4:27–34. [Google Scholar]
- Ashihara H., Yokota T., Crozier A. Springer; 2013. Purine Alkaloids, Cytokinins, and Purine-Like Neurotoxin Alkaloids, Natural Products; pp. 953–975. [Google Scholar]
- Ata A., Andersh B.J. Buxus steroidal alkaloids: chemistry and biology. Alkaloids: Chem. Biol. 2008;66:191–213. doi: 10.1016/s1099-4831(08)00203-4. [DOI] [PubMed] [Google Scholar]
- Attias J., Gordon C., Ribak J., Binah O., Rolnick A. Efficacy of transdermal scopolamine against seasickness: a 3-day study at sea. Aviat. Space Environ. Med. 1987;58:60–62. [PubMed] [Google Scholar]
- Attimarad M., Ahmed K.M., Aldhubaib B.E., Harsha S. High-performance thin layer chromatography: a powerful analytical technique in pharmaceutical drug discovery. Pharm. Methods. 2011;2:71. doi: 10.4103/2229-4708.84436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azumi K., Yokosawa H., Ishii S. Halocyamines: novel antimicrobial tetrapeptide-like substances isolated from the hemocytes of the solitary ascidian Halocynthia roretzi. Biochemistry. 1990;29:159–165. doi: 10.1021/bi00453a021. [DOI] [PubMed] [Google Scholar]
- Azwanida N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med. Aromat. Plants. 2015;4:3–8. [Google Scholar]
- Badwaik L., Gautam G., Deka S. Influence of blanching on antioxidant, nutritional and physical properties of bamboo shoot. J. Agric. Sci. 2015;10:140–150. [Google Scholar]
- Baker B. Alkaloids: Chem. Biol. Perspect. 1996;10:357–407. [Google Scholar]
- Barnes J. Trease and Evans' pharmacognosy. Focus. Altern. Complement. Ther. 1999;4:151–152. [Google Scholar]
- Begum S., Naqvi S.Q.Z., Ahmed A., Tauseef S., Siddiqui B.S. Antimycobacterial and antioxidant activities of reserpine and its derivatives. Nat. Prod. Res. 2012;26:2084–2088. doi: 10.1080/14786419.2011.625502. [DOI] [PubMed] [Google Scholar]
- Bele A.A., Khale A. An overview on thin layer chromatography. IJPSR. 2011;2:256–267. [Google Scholar]
- Bentley K.W. β-Phenylethylamines and the isoquinoline alkaloids. Nat. Prod. Rep. 2006;23:444–463. doi: 10.1039/b509523a. [DOI] [PubMed] [Google Scholar]
- Berkov S. Alkaloids of Datura ceratocaula. Z. Naturforsch. C. 2003;58:455–458. doi: 10.1515/znc-2003-7-801. [DOI] [PubMed] [Google Scholar]
- Berkov S., Zayed R., Doncheva T. Alkaloid patterns in some varieties of Datura stramonium. Fitoterapia. 2006;77:179–182. doi: 10.1016/j.fitote.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Berkova S., Zayed R. Comparison of tropane alkaloid spectra between Datura innoxia grown in Egypt and Bulgaria. Z. Naturforsch. C. 2004;59:184–186. doi: 10.1515/znc-2004-3-409. [DOI] [PubMed] [Google Scholar]
- Bertrand N., Beley A. Effect of oxotremorine, physostigmine, and scopolamine on brain acetylcholine synthesis: a study using HPLC. Neurochem. Res. 1990;15:1097–1100. doi: 10.1007/BF01101710. [DOI] [PubMed] [Google Scholar]
- Bhargavi G., Rao P.N., Renganathan S. IOP Publishing; 2018. Review on the Extraction Methods of Crude Oil From All Generation Biofuels in Last Few Decades, IOP Conference Series: Materials Science and Engineering; p. 012024. [Google Scholar]
- Biastoff S., Dräger B. Calystegines. Alkaloids: Chem. Biol. 2007;64:49–102. doi: 10.1016/s1099-4831(07)64002-4. [DOI] [PubMed] [Google Scholar]
- Bifulco G., Bruno I., Minale L., Riccio R., Calignano A., Debitus C. (±)-Gelliusines A and B, two diastereomeric brominated tris-indole alkaloids from a deep water New Caledonian marine sponge (Gellius or Orina sp.) J. Nat. Prod. 1994;57:1294–1299. doi: 10.1021/np50111a020. [DOI] [PubMed] [Google Scholar]
- Björn B., Danz H., Hamburger M. Pressurized liquid extraction of medicinal plants. J. Chromatogr. A. 1999;837:211–219. doi: 10.1016/s0021-9673(99)00071-0. [DOI] [PubMed] [Google Scholar]
- Blackman J., Campion D., Fastier F. Mechanism of action of reserpine in producing gastric haemorrhage and erosion in the mouse. Br. J. Pharmacol. Chemother. 1959;14:112–116. doi: 10.1111/j.1476-5381.1959.tb00936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobzin S.C., John Faulkner D. Aromatic alkaloids from the marine sponge Chelonaplysilla sp. J. Org. Chem. 1991;56:4403–4407. [Google Scholar]
- Boit H.-G. Akademie-verlag; 1961. Ergebnisse der Alkaloid-chemie bis 1960. [Google Scholar]
- Boon-Unge K., Yu Q., Zou T., Zhou A., Govitrapong P., Zhou J. Emetine regulates the alternative splicing of Bcl-x through a protein phosphatase 1-dependent mechanism. Chem. Biol. 2007;14:1386–1392. doi: 10.1016/j.chembiol.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borzeix M., Cahn J. Cerebral antiedematous effect of Teproside and of some vincamine derivatives. Int. J. Clin. Pharmacol. Res. 1984;4:259–261. [PubMed] [Google Scholar]
- Brachet J., Cosson L. Changes in the total content of Datura inoxia Mill. subjected to salt stress. J. Exp. Bot. 1983;37:650–656. [Google Scholar]
- Bryson N., Papillard D. An introduction to OPLC operation and applications. LC-GC North Am. 2004;22:366–378. [Google Scholar]
- Buchholz K., Pawliszyn J. Optimization of solid-phase microextraction conditions for determination of phenols. Anal. Chem. 1994;66:160–167. [Google Scholar]
- Carney J.R., Scheuer P.J., Kelly-Borges M. Makaluvamine G, a cytotoxic pigment from an an Indonesian sponge Histodermella sp. Tetrahedron. 1993;49:8483–8486. [Google Scholar]
- Casapullo A., Bifulco G., Bruno I., Riccio R. New bisindole alkaloids of the topsentin and hamacanthin classes from the Mediterranean marine sponge Rhaphisia lacazei. J. Nat. Prod. 2000;63:447–451. doi: 10.1021/np9903292. [DOI] [PubMed] [Google Scholar]
- Cavalcante-Silva L., Correia A., Barbosa-Filho J., da Silva B., Santos B., de Lira D., Sousa J., de Miranda G., Cavalcante F., Alexandre-Moreira M. Spasmolytic effect of caulerpine involves blockade of Ca2 + influx on guinea pig ileum. Mar. Drugs. 2013;11:1553–1564. doi: 10.3390/md11051553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee, A., Pakrashi, S.C., 1991. The treatise on Indian medicinal plants: vol. 1. New Delhi: Publications and Information Directorate, CSIR 172p.-illus., col. illus. ISBN 8172360118 En Icones. Includes authentic Sanskrit slokas in both Devnagri and Roman scripts. Plant records. Geog 6.
- Chauhan N.S. Indus Publishing; 1999. Medicinal and Aromatic Plants of Himachal Pradesh. [Google Scholar]
- Chay C., Cansino R., Pinzón C., Torres-Ochoa R., Martínez R. Synthesis and anti-tuberculosis activity of the marine natural product caulerpin and its analogues. Mar. Drug. 2014;12:1757–1772. doi: 10.3390/md12041757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeke P. Toxicity and metabolism of pyrrolizidine alkaloids. J. Anim. Sci. 1988;66:2343–2350. doi: 10.2527/jas1988.6692343x. [DOI] [PubMed] [Google Scholar]
- Chini C., Bilia A.R., Keita A., Morelli I. Protoalkaloids from Boscia angustifolia. Planta Med. 1992;58:476. doi: 10.1055/s-2006-961522. [DOI] [PubMed] [Google Scholar]
- Christopherson C. Academic Press; New York: 1985. The Alkaloids: Chemistry and Pharmacology. [Google Scholar]
- Chu M., Zhang M.-b., Liu Y.-c., Kang J.-r., Chu Z.-y., Yin K.-l., Ding L.-y., Ding R., Xiao R.-x., Yin Y.-n. Role of berberine in the treatment of methicillin-resistant Staphylococcus aureus infections. Sci. Rep. 2016;6 doi: 10.1038/srep24748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y., Chang C., Hsu H. Flavonoid content of several vegetables and their antioxidant activity. J. Sci. Food Agric. 2000;80:561–566. [Google Scholar]
- Cocolas G. Textbook of Organic Medicinal and Pharmaceutical Chemistry. 1982. Cholinergic drugs and related agents; pp. 505–551. [Google Scholar]
- Cos P., Vlietinck A.J., Berghe D.V., Maes L. Anti-infective potential of natural products: how to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006;106:290–302. doi: 10.1016/j.jep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Coskun O. Separation techniques: chromatography. North Clin. Istanb. 2016;3:156–160. doi: 10.14744/nci.2016.32757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto R., Conceição E., Chaul L., Oliveira E., Martins F., Bara M., Rezende K., Alves S., Paula J. Spray-dried rosemary extracts: physicochemical and antioxidant properties. Food Chem. 2012;131:99–105. [Google Scholar]
- Creamer R., Baucom D. Fungal endophytes of locoweeds: a commensal relationship? J. Plant Physiol. Pathol. 2013;1 [Google Scholar]
- Croteau R., Kutchan T.M., Lewis N.G. Natural products (secondary metabolites) Biochem. Mol. Biol. Plants. 2000;24:1250–1319. [Google Scholar]
- Culture and Health . 1996. Orientation Texts-World Decade for Cultural Development 1988–1997. Document CLT/DEC/PRO-1996; p. 129. [Google Scholar]
- Cunha I., Sawaya A., Caetano F., Shimizu M., Marcucci M., Drezza F., Povia G., Carvalho P. Factors that influence the yield and composition of Brazilian propolis extracts. J. Braz. Chem. Soc. 2004;15:964–970. [Google Scholar]
- Dang Q.-L. Improving the quality and reliability of gas exchange measurements. J. Plant Health Technol. 2013;1 [Google Scholar]
- de Souza E., de Lira D., de Queiroz A., da Silva D., de Aquino A., Mella E., Lorenzo V., de Miranda G., de Araújo-Júnior J., Chaves M., Barbosa-Filho J., de Athayde-Filho P., Santos B., Alexandre-Moreira M. The antinociceptive and anti-inflammatory activities of caulerpin, a bisindole alkaloid isolated from seaweeds of the genus Caulerpa. Mar. Drug. 2009;7:689–704. doi: 10.3390/md7040689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath P., Dey P., Chanda A., Bhakta T. vol. 1. Scholars Academic & Scientific Publishers; 2012. A survey on pineapple and its medicinal value; pp. 24–29. [Google Scholar]
- Delazar A., Nahar L., Hamedeyazdan S., Sarker S. Methods Mol. Biol. Humana Press; 2012. Microwave-assisted extraction in natural products isolation; pp. 89–115. [DOI] [PubMed] [Google Scholar]
- Demeyer K., Dejaegere R. Influence of the ion-balance in the growth medium on the yield and alkaloid content of Datura stramonium L. Plant Soil. 1989;114:289–294. [Google Scholar]
- Deng L., Dai P., Ciro A., Smee D.F., Djaballah H., Shuman S. Identification of novel antipoxviral agents: mitoxantrone inhibits vaccinia virus replication by blocking virion assembly. J. Virol. 2007;81:13392–13402. doi: 10.1128/JVI.00770-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewick P.M. John Wiley & Sons; 2002. Medicinal Natural Products: A Biosynthetic Approach. [Google Scholar]
- Dewir Y., El Mahrouk M., Hafez Y., Rihan H., Sáez C., Fuller M. Antioxidative capacity and electrolyte leakage in healthy versus phytoplasma infected tissues of Euphorbia coerulescens and Orbea gigantea. J. Plant Physiol. Pathol. 2014;3 [Google Scholar]
- Dey P., Bardalai D., Kumar N.R., Subramani C., Mukherjee M., Bhakta T. Ethnomedicinal knowledge about various medicinal plants used by the tribes of Tripura. Res. J. Pharmacogn. Phytochem. 2012;4:297. [Google Scholar]
- Dey P., Bhakta T. Evaluation of in vitro anticoagulant activity of Molineria recurpata leaf extract. J. Nat. Prod. Plant Resour. 2012;2:685–688. [Google Scholar]
- Dey P., Bhakta T. Comparative studies on anthelmintic activity of leaf extract of Musa acuminate colla and Cajanus cajan (Linn.) leaf extract. Mintage J. Pharm. Med. Sci. 2013:24–25. [Google Scholar]
- Dey P., Debnath P., Bhakta T. Evaluation of anthelmintic activity of Molineria recurvata leaf extracts. Int. Res. J. Pharm. Appl. Sci. 2012;2:16–20. [Google Scholar]
- Dey P., Debnath P., Bhakta T. Evaluation of anthelmintic activity of pineapple fruit extract using Indian earthworm (Pheritima posthuma) Mintage J. Pharm. Med. Sci. 2013:26–27. [Google Scholar]
- Dey P., Karuna D., Bhakta T. Medicinal plants used as anti-acne agents by tribal and non-tribal people of Tripura, India. AJPCT. 2014;2:556–570. [Google Scholar]
- Dey P., Kundu A., Chakraborty H.J., Kar B., Choi W.S., Lee B.M., Bhakta T., Atanasov A.G., Kim H.S. Therapeutic value of steroidal alkaloids in cancer: current trends and future perspectives. Int. J. Cancer. 2018;145:1731–1744. doi: 10.1002/ijc.31965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey P., Mukherjee M., Bhakta T., Ghosh T. Preliminary phytochemical studies of leaf extracts of Molineria recurvata. J. Chem. Pharm. Res. 2012;4:3727–3730. [Google Scholar]
- Dhayalan M., Jegadeeshwari A., Gandhi N. Biological activity sources from traditionally used tribe and herbal plants material. Asian J. Pharm. Clin. Res. 2015;8:11–23. [Google Scholar]
- Donsi G., Ferrari G., Di Matteo M. High pressure stabilization of orange juice: evaluation of the effects of process conditions. Italian J. Food Sci. 1996;8:99–106. [Google Scholar]
- Dräger B. Biotechnology of Solanaceae alkaloids: a model or an industrial perspective? In: Kayser O., Quax W.J., editors. Medicinal Plant Biotechnology: From Basic Research to Industrial Applications. Wiley-VCH Verlag GmbH & Co. KGaA; 2008. pp. 237–265. [Google Scholar]
- Dreyfuss P., Vogel D., Walsh N. The use of transdermal scopolamine to control drooling. A case report. Am. J. Phys. Med. Rehabil. 1991;70:220–222. doi: 10.1097/00002060-199108000-00012. [DOI] [PubMed] [Google Scholar]
- Eberhardt T.L., Li X., Shupe T.F., Hse C.Y. Chinese tallow tree (Sapium sebiferum) utilization: characterization of extractives and cell-wall chemistry. Wood Fiber Sci. 2007;39:319–324. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain (CONTAM), Knutsen H.K., Alexander J., Barregård L., Bignami M., Brüschweiler B., Ceccatelli S., Cottrill B., Dinovi M., Edler L., Grasl-Kraupp B. Risks for human health related to the presence of pyrrolizidine alkaloids in honey, tea, herbal infusions and food supplements. EFSA J. 2017;15(7):e04908. doi: 10.2903/j.efsa.2017.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiceman G.A. Gas chromatography. Anal. Chem. 2002;74:2771–2780. doi: 10.1021/ac020210p. [DOI] [PubMed] [Google Scholar]
- El-Sayed M., Verpoorte R. Catharanthus terpenoid indole alkaloids: biosynthesis and regulation. Phytochem. Rev. 2007;6:277–305. [Google Scholar]
- Erik H., Martin S., Per-Anders F., Tobias J., Ulf G., Mia D., JPer J., Fred N., Lars B. Brominated cyclodipeptides from the marine sponge Geodia barretti as selective 5-HT ligands. J. Nat. Prod. 2006;69:1421–1424. doi: 10.1021/np0601760. [DOI] [PubMed] [Google Scholar]
- Exarchou V., Troganis A., Gerothanassis I.P., Tsimidou M., Boskou D. Identification and quantification of caffeic and rosmarinic acid in complex plant extracts by the use of variable-temperature two-dimensional nuclear magnetic resonance spectroscopy. J. Agric. Food Chem. 2001;49:2–8. doi: 10.1021/jf990928e. [DOI] [PubMed] [Google Scholar]
- Fabricant D.S., Farnsworth N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001;109:69. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhat A., Ginies C., Romdhane M., Chemat F. Eco-friendly and cleaner process for isolation of essential oil using microwave energy: experimental and theoretical study. J. Chromatogr. A. 2009;1216:5077–5085. doi: 10.1016/j.chroma.2009.04.084. [DOI] [PubMed] [Google Scholar]
- Ferguson P.J., Phillips J.R., Selner M., Cass C.E. Differential activity of vincristine and vinblastine against cultured cells. Cancer Res. 1984;44:3307–3312. [PubMed] [Google Scholar]
- Fodor G., Dharanipragada R. Tropane alkaloids. Nat. Prod. Rep. 1994;11:443–450. doi: 10.1039/np9941100443. [DOI] [PubMed] [Google Scholar]
- Foye W.O. Amer. Chemical Society; 1995. Cancer Chemotherapeutic Agents. [Google Scholar]
- Freire M., de Souza C., Portal T., Machado R., dos Santos P., Mussi-Dias V. Effect of castor bean oil on post harvest fungal pathogen of coconut: Lasiodiplodia theobromae. J. Plant Physiol. Pathol. 2013;1 [Google Scholar]
- Fresneda P.M., Molina P. Application of iminophosphorane-based methodologies for the synthesis of natural products. Synlett. 2004;2004:1–17. [Google Scholar]
- Frey K., Koeppe R., Mulholland G., Jewett D., Hichwa R., Ehrenkaufer R., Carey J., Wieland D., Kuhl D., Agranoff B. In vivo muscarinic cholingeric receptor imaging in human brain with [11C] scopolamine and positron emission tomography. J. Cereb. Blood Flow Metab. 1992;12:147–154. doi: 10.1038/jcbfm.1992.18. [DOI] [PubMed] [Google Scholar]
- Fried J.S.B. Thin layer chromatographic analysis of biological samples. A review. J. Liq. Chromatogr. Relat. Technol. 2005;28:2297–2314. [Google Scholar]
- Fries D. Analgesics. In: Foye W., Lemke T., Williams D., editors. Principles of Medicinal Chemistry. fourth ed. Lippincott Williams & Wilkins, Media; PA: 1995. pp. 247–269. [Google Scholar]
- Fusco B.M., Giacovazzo M. Peppers and pain. Drugs. 1997;53:909–914. doi: 10.2165/00003495-199753060-00001. [DOI] [PubMed] [Google Scholar]
- Galey F.D., Holstege D.M., Francis T., Hyde W., Jack R. Proceedings of the 11th Conference of Racing Analysts and Veterinarians, Queensland, Australia. 1996. Residues of Datura species in horses. pp. 333–337. [Google Scholar]
- Gil M., Holcroft D., Kader A. Changes in strawberry anthocyanins and other polyphenols in response to carbon dioxide treatments. J. Agric. Food Chem. 1997;45:1662–1667. [Google Scholar]
- Gladys B., Patterson B., Subar A. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr. Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- Gomez-Mancilla B., Boucher R., Bedard P.J. Effect of clonidine and atropine on rest tremor in the MPTP monkey model of parkinsonism. Clin. Neuropharmacol. 1991;14:359–366. doi: 10.1097/00002826-199108000-00008. [DOI] [PubMed] [Google Scholar]
- Gorain B., Choudhury H., Kundu A., Sarkar L., Karmakar S., Jaisankar P., Pal T.K. Nanoemulsion strategy for olmesartan medoxomil improves oral absorption and extended antihypertensive activity in hypertensive rats. Colloids Surf. B: Biointerfaces. 2014;115:286–294. doi: 10.1016/j.colsurfb.2013.12.016. [DOI] [PubMed] [Google Scholar]
- Gossauer A. Springer; 2003. Monopyrrolic Natural Compounds Including Tetramic Acid Derivatives, Fortschritte der Chemie organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products; pp. 1–188. [DOI] [PubMed] [Google Scholar]
- Grau J.W., Illich P.A., Chen P.-S., Meagher M.W. Role of cholinergic systems in pain modulation. I. Impact of scopolamine on environmentally induced hypoalgesia and pain reactivity. Behav. Neurosci. 1991;105:62. doi: 10.1037//0735-7044.105.1.62. [DOI] [PubMed] [Google Scholar]
- Grellier P., Sinou V., Garreau-de Loubresse N., Bylen E., Boulard Y., Schrével J. Selective and reversible effects of vinca alkaloids on Trypanosoma cruzi epimastigote forms: blockage of cytokinesis without inhibition of the organelle duplication. Cell Motil. Cytoskeleton. 1999;42:36–47. doi: 10.1002/(SICI)1097-0169(1999)42:1<36::AID-CM4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Grollman A.P. Inhibitors of protein biosynthesis. V. Effects of emetine on protein and nucleic acid biosynthesis in HeLa cells. J. Biol. Chem. 1968;243:4089–4094. [PubMed] [Google Scholar]
- Groth M., Langenback E., Foster W. Influence of inhaled atropine on lung mucociliary function in humans. Am. Rev. Respir. Dis. 1991;144:1042–1047. doi: 10.1164/ajrccm/144.5.1042. [DOI] [PubMed] [Google Scholar]
- Grzegorz G., Gadzikowska M. Tropane alkaloids as medicinally useful natural products and their synthetic derivatives as new drugs. Pharmacol. Rep. 2008;60:439. [PubMed] [Google Scholar]
- Gul W., Hamann M.T. Indole alkaloid marine natural products: an established source of cancer drug leads with considerable promise for the control of parasitic, neurological and other diseases. Life Sci. 2005;78:442–453. doi: 10.1016/j.lfs.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guohua C., Booth S., Sadowski J., Prior R. Increases in human plasma antioxidant capacity after consumption of controlled diets high in fruit and vegetables. Am. J. Clin. Nutr. 1998;68:1081–1087. doi: 10.1093/ajcn/68.5.1081. [DOI] [PubMed] [Google Scholar]
- Guohua C., Russell R., Lischner N., Prior R. Serum antioxidant capacity is increased by consumption of strawberries, spinach, red wine or vitamin C in elderly women. J. Nutr. 1998;128:2383–2390. doi: 10.1093/jn/128.12.2383. [DOI] [PubMed] [Google Scholar]
- Gupta D., Tawussi F., Hamann L., Walther C. Moderate uranium disturbs the nutritional status and induces oxidative stress in Pisum sativum L. J. Plant Physiol. Pathol. 2016;4 [Google Scholar]
- Gupta M.S., Madan C. Effect of growth retardants on growth and alcaloid formation in Datura metel var. fastuosa. Planta Med. 1975;28:193–200. doi: 10.1055/s-0028-1097853. [DOI] [PubMed] [Google Scholar]
- Gutstein H., Akil H. Opioid analgesics. In: Hardman J., Limbird L., Gilman A., editors. The Pharmacological Basis of Therapeutics. 10th ed. McGraw-Hill; New York: 2001. pp. 569–611. [Google Scholar]
- Gwen L., Kitts D., Yaghmaee P., Durance T. Retention of antioxidant capacity of vacuum microwave dried cranberry. J. Food Sci. 2010;75:C311–C316. doi: 10.1111/j.1750-3841.2010.01563.x. [DOI] [PubMed] [Google Scholar]
- Habermehl G. Elsevier; 1967. The Steroid Alkaloids: The Salamandra Group, The Alkaloids: Chemistry and Physiology; pp. 427–439. [Google Scholar]
- Hallgren T., Fasth S., Delbro D., Nordgren S., Öresland T., Hulten L. The effects of atropine or benzilonium on pelvic pouch and anal sphincter functions. Scand. J. Gastroenterol. 1991;26:563–571. doi: 10.3109/00365529108998581. [DOI] [PubMed] [Google Scholar]
- Harborne J. Springer; 1984. Methods of Plant Analysis, Phytochemical Methods; pp. 1–36. [Google Scholar]
- Harris S.N., Sevarino F.B., Sinatra R.S., Preble L., O'connor T.Z., Silverman D.G. Nausea prophylaxis using transdermal scopolamine in the setting of patient-controlled analgesia. Obstet. Gynecol. 1991;78:673–677. [PubMed] [Google Scholar]
- Hartmann T. Chemical ecology of pyrrolizidine alkaloids. Planta. 1999;207:483–495. [Google Scholar]
- Hartmann T., Ober D. Biosynthesis. Springer; 2000. Biosynthesis and metabolism of pyrrolizidine alkaloids in plants and specialized insect herbivores; pp. 207–243. [Google Scholar]
- Hazra K., Roy R., Sen S., Laskar S. Isolation of antibacterial pentahydroxy flavones from the seeds of Mimusops elengi Linn. Afr. J. Biotechnol. 2007;6:1446–1449. [Google Scholar]
- Hemscheidt T. Springer; 2000. Tropane and Related Alkaloids, Biosynthesis; pp. 175–206. [Google Scholar]
- Heyman H.M., Meyer J.J.M. NMR-based metabolomics as a quality control tool for herbal products. S. Afr. J. Bot. 2012;82:21–32. [Google Scholar]
- Hiraoka N., Tashimo K., Kinoshita C., Hiro'oka M. Genotypes and alkaloid contents of Datura metel varieties. Biol. Pharm. Bull. 1996;19:1086–1089. doi: 10.1248/bpb.19.1086. [DOI] [PubMed] [Google Scholar]
- Horimoto Y., Tomie H., Hanzawa K., Nishida Y. Scopolamine patch reduces postoperative emesis in paediatric patients following strabismus surgery. Can. J. Anaesth. 1991;38:441–444. doi: 10.1007/BF03007580. [DOI] [PubMed] [Google Scholar]
- Houghton P.J., Howes M.-J. Selected Topics In The Chemistry Of Natural Products. World Scientific; 2008. Natural products and related compounds of realized and potential use in treating neurodegenerative disease; pp. 377–426. [Google Scholar]
- Hu J.F., Hamann M.T., Hill R., Kelly M. Alkaloids. vol. 60. Academic Press; New York: 2003. The manzamine alkaloids; pp. 207–286. [Google Scholar]
- Hu J.F., Hamann M.T., Hill R., Kelly M. The Alkaloids Chemistry and Biology. Academic Press; New York: 2003. The manzamine alkaloids. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes M.A., Petri W.A., Jr. Amebic liver abscess. Infect. Dis. Clin. N. Am. 2000;14:565–582. doi: 10.1016/s0891-5520(05)70121-5. [DOI] [PubMed] [Google Scholar]
- Huie C. A review of modern sample-preparation techniques for the extraction and analysis of medicinal plants. Anal. Bioanal. Chem. 2002;373:23–30. doi: 10.1007/s00216-002-1265-3. [DOI] [PubMed] [Google Scholar]
- Huie C. A review of modern sample-preparation techniques for the extraction and analysis of medicinal plants. Anal. Bioanal. Chem. 2002;373:23–30. doi: 10.1007/s00216-002-1265-3. [DOI] [PubMed] [Google Scholar]
- Iguchi A., Uemura K., Kunoh Y., Miura H., Ishiguro T., Nonogaki K., Tamagawa T., Gotoh M., Sakamoto N. Hyperglycemia induced by hippocampal administration of neostigmine is suppressed by intrahypothalamic atropine. Neuropharmacology. 1991;30:1129–1131. doi: 10.1016/0028-3908(91)90144-z. [DOI] [PubMed] [Google Scholar]
- Ihara M., Fukumoto K. Recent progress in the chemistry of non-monoterpenoid indole alkaloids. Nat. Prod. Rep. 1996;13:241–261. [Google Scholar]
- Ingle K.P., Deshmukh A.G., Padole D.A., Dudhare M.S., Moharil M.P., Khelurkar V.C. Phytochemicals: Extraction methods, identification and detection of bioactive compounds from plant extracts. J. Pharmacogn. Phytochem. 2017;6:32–36. [Google Scholar]
- Irondi E., Akintunde J., Agboola S., Boligon A., Athayde M. Blanching influences the phenolics composition, antioxidant activity, and inhibitory effect of Adansonia digitata leaves extract on α-amylase, α-glucosidase, and aldose reductase. Food Sci. Nutr. 2017;5:233–242. doi: 10.1002/fsn3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishtiyak P., Hussain S.A. Traditional use of medicinal plants among tribal communities of Bangus Valley, Kashmir Himalaya, India. Stud. Ethno-Med. 2017;11:318–331. [Google Scholar]
- Ismail A., Lee W. Effect of different blanching times on antioxidant properties in selected cruciferous vegetables. J. Sci. Food Agric. 2005;85:2314–2320. [Google Scholar]
- Jakubke H.-D., Jeschkeit H. Trans. Rev. Mary Eagleson.; Berlin, New York, Walter de Gruyter: 1994. Concise Encyclopedia Chemistry. [Google Scholar]
- Jaroslaw K., Gumul D., Czechowska K. Effect of extrusion on the phenolic composition and antioxidant activity of dry beans of Phaseolus vulgaris L. Food Technol. Biotechnol. 2007;45:139–146. [Google Scholar]
- Jeffrey H., Baxter H., Webster F. Phytochemical dictionary: a handbook of bioactive compounds from plants. J. Chem. Ecol. 1994;20:815–818. [Google Scholar]
- Jeon M., Park J., Dey P., Oh Y., Oh H., Han S., Um S.H., Kim H.S., Mishra N.K., Kim I.S. Site-selective Rhodium (III)-catalyzed C-H amination of 7-azaindoles with anthranils: synthesis and anticancer evaluation. Adv. Synth. Catal. 2017;359:3471–3478. [Google Scholar]
- Jeong T., Lee S.H., Mishra N.K., De U., Park J., Dey P., Kwak J.H., Jung Y.H., Kim H.S., Kim I.S. Synthesis and cytotoxic evaluation of N-aroylureas through Rhodium (III)-catalyzed C-H functionalization of indolines with isocyanates. Adv. Synth. Catal. 2017;359:2329–2336. [Google Scholar]
- Jeong T., Mishra N.K., Dey P., Oh H., Han S., Lee S.H., Kim H.S., Park J., Kim I.S. C (sp 3)-H amination of 8-methylquinolines with azodicarboxylates under Rh (iii) catalysis: cytotoxic evaluation of quinolin-8-ylmethanamines. Chem. Commun. 2017;53:11197–11200. doi: 10.1039/c7cc06670h. [DOI] [PubMed] [Google Scholar]
- Jiang B., Smallheer J., Amaral-Ly C., Wuonola M. Total synthesis of (±)-Dragmacidin: a cytotoxic bis(indole)alkaloid of marine origin. J. Org. Chem. 1994;59:6823–6827. [Google Scholar]
- Jiménez-Monreal A.M., García-Diz L., Martínez-Tomé M., Mariscal M., Murcia M.A. Influence of cooking methods on antioxidant activity of vegetables. J. Food Sci. 2009;74:H97–H103. doi: 10.1111/j.1750-3841.2009.01091.x. [DOI] [PubMed] [Google Scholar]
- Jin Z. Imidazole, oxazole and thiazole alkaloids. Nat. Prod. Rep. 2006;23:464–496. doi: 10.1039/b502166a. [DOI] [PubMed] [Google Scholar]
- Jiratanan T., Liu R.H. Antioxidant activity of processed table beets (Beta vulgaris var, conditiva) and green beans (Phaseolus vulgaris L.) J. Agric. Food Chem. 2004;52:2659–2670. doi: 10.1021/jf034861d. [DOI] [PubMed] [Google Scholar]
- Johansen H., Glitsø V., Knudsen K. Influence of extraction solvent and temperature on the quantitative determination of oligosaccharides from plant materials by high-performance liquid chromatography. J. Agric. Food Chem. 1996;44:1470–1474. [Google Scholar]
- Jordan M.A., Wilson L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer. 2004;4:253. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- Julu P., Adler J., Hondo R. Vagal tone in healthy-volunteers given atropine and in diabetic-patients. J. Physiol. Lond. 1991:P33. [Google Scholar]
- Kahol A., Tandon S., Singh K. Developments in the separation technologies for the processing of medicinal and aromatic plants. Chem. Week. Bombay. 1998;44:145–154. [Google Scholar]
- Kam T., Choo Y. Bisindole alkaloids. Alkaloids: Chem. Biol. 2006;63:181–337. doi: 10.1016/s1099-4831(06)63004-6. [DOI] [PubMed] [Google Scholar]
- Kamano Y., Zhang H.-p., Ichihara Y., Kizu H., Komiyama K., Pettit G.R. Convolutamydine A, a novel bioactive hydroxyoxindole alkaloid from marine bryozoan Amathia convoluta. Tetrahedron Lett. 1995;36:2783–2784. [Google Scholar]
- Karmakar S., Biswas S., Bera R., Mondal S., Kundu A., Ali M.A., Sen T. Beverage-induced enhanced bioavailability of carbamazepine and its consequent effect on antiepileptic activity and toxicity. J. Food Drug Anal. 2015;23:327–334. doi: 10.1016/j.jfda.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnick C.R., Saxena M.D. On the variability of alkaloid production in Datura species. Planta Med. 1970;18:266–269. doi: 10.1055/s-0028-1099775. [DOI] [PubMed] [Google Scholar]
- Karuna D., Dey P., Das S., Kundu A., Bhakta T. In vitro antioxidant activities of root extract of Asparagus racemosus Linn. J. Tradit. Complement. Med. 2018;8:60–65. doi: 10.1016/j.jtcme.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karuna D., Dey P., Kundu A., Vishal V., Bhakta T. Evaluation of in vitro antioxidant potential of Aconitum napellus Linn. root extract. Int. J. Pharmacogn. Chinese Med. 2018;2:1–8. [Google Scholar]
- Kaur R., Arora S. Alkaloids-important therapeutic secondary metabolites of plant origin. J. Crit. Rev. 2015;2:1–8. [Google Scholar]
- Kawasaki T., Higuchi K. Simple indole alkaloids and those with a nonrearranged monoterpenoid unit. Nat. Prod. Rep. 2005;22:761–793. doi: 10.1039/b502162f. [DOI] [PubMed] [Google Scholar]
- Keeler R.F. Teratology of steroidal alkaloids. Alkaloids: Chem. Biol. Perspect. 1986;4:389–425. [Google Scholar]
- Kim D.O., Padilla-Zakour O. Jam processing effect on phenolics and antioxidant capacity in anthocyanin-rich fruits: cherry, plum, and raspberry. J. Food Sci. 2004;69:S395–S400. [Google Scholar]
- Kim S., Kundu A., Chun R., Han S.H., Pandey A.K., Yoo S., Park J., Kim H.S., Ku J.-M., Kim I.S. Direct synthesis of 2-acyl acridines using aldimines and anthranils: evaluation of cytotoxicity and anti-inflammatory activity. Asian J. Org. Chem. 2018;7:2069–2075. [Google Scholar]
- Kinghorn A.D. Pharmacognosy in the 21st century. J. Pharm. Pharmacol. 2001;53:135–148. doi: 10.1211/0022357011775334. [DOI] [PubMed] [Google Scholar]
- Kirakosyan A., Noon K.R., McKenzie M., Cseke L.J., Kaufman P.B. CRC Press; 2016. Isolation and Purification of Metabolites, Handbook of Molecular and Cellular Methods in Biology and Medicine; pp. 386–399. [Google Scholar]
- Klein-Schwartz W., Oderda G.M. Jimsonweed intoxication in adolescents and young adults. Am. J. Dis. Child. 1984;138:737–739. doi: 10.1001/archpedi.1984.02140460029010. [DOI] [PubMed] [Google Scholar]
- Knölker H.-J. Synthesis of biologically active carbazole alkaloids using selective transition-metal-catalyzed coupling reactions. Chem. Lett. 2008;38:8–13. [Google Scholar]
- Knunyants I., Zefirov N. Sov. Entsikl.; Moscow: 1988. Chemical Encyclopedia. [Google Scholar]
- Kobayashi J., Murayama T., Ishibashi M., Kosuge S., Takamatsu M., Ohizumi Y., Kobayashi H., Ohta T., Nozoe S., Sasaki T. Hyrtiosins A and B, new indole alkaloids from the Okinawan marine sponge Hyrtios erecta. Tetrahedron. 1990;46:7699–7702. [Google Scholar]
- Kobayashi J., Nakamura H., Ohizumi Y., Hirata Y. Eudistomidin-A, a novel calmodulin antagonist from the Okinawan tunicate Eudistoma glaucus. Tetrahedron Lett. 1986;27:1191–1194. [Google Scholar]
- Kohmoto S., Kashman Y., McConnell O.J., Rinehart K.L., Jr., Wright A., Koehn F. Dragmacidin, a new cytotoxic bis (indole) alkaloid from a deep water marine sponge, Dragmacidon sp. J. Org. Chem. 1988;53:3116–3118. [Google Scholar]
- Komárek M., Chrastný V., Ettler V., Tlustos P. Evaluation of extraction/digestion techniques used to determine lead isotopic composition in forest soils. Anal. Bioanal. Chem. 2006;385:1109–1115. doi: 10.1007/s00216-006-0543-x. [DOI] [PubMed] [Google Scholar]
- Kong L.D., Cheng C.H., Tan R.X. Monoamine oxidase inhibitors from rhizoma of Coptis chinensis. Planta Med. 2001;67:74–76. doi: 10.1055/s-2001-10874. [DOI] [PubMed] [Google Scholar]
- Kong W., Wei J., Abidi P., Lin M., Inaba S., Li C., Wang Y., Wang Z., Si S., Pan H. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat. Med. 2004;10:1344. doi: 10.1038/nm1135. [DOI] [PubMed] [Google Scholar]
- Kumar B.R. Application of HPLC and ESI-MS techniques in the analysis of phenolic acids and flavonoids from green leafy vegetables (GLVs) J. Pharm. Anal. 2017 doi: 10.1016/j.jpha.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar K., Kaushik N., Attri P., Kumar N., Kim C., Verma A., Choi E. Biomedical importance of indoles. Molecules. 2013;18:6620–6662. doi: 10.3390/molecules18066620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Singh A.U., Singh H.S. Initial population density its effect on the pathogenic potential and population growth of Rotylenchulus reniformis on cowpea (Vigna unguiculata L.) J. Plant Physiol. Pathol. 2013;1 [Google Scholar]
- Kundu A., Dey P., Sarkar P., Karmakar S., Tae I.H., Kim K.S., Park J.H., Lee S.H., Lee B.M., Renthlei L., Puia Z., Kim H.S. Protective effects of Croton hookeri on streptozotocin-induced diabetic nephropathy. Food Chem. Toxicol. 2019;7:110873. doi: 10.1016/j.fct.2019.110873. [DOI] [PubMed] [Google Scholar]
- Kwok B., Hu C., Durance T., Kitts D. Dehydration techniques affect phytochemical contents and free radical scavenging activities of Saskatoon berries (Amelanchier alnifolia Nutt.) J. Food Sci. 2004;69:SNQ122–SNQ126. [Google Scholar]
- L K. In: Ultrasound: Its Chemical, Physical, and Biological Effects. Suslick K.S., editor. New York; VCH: 1988. [DOI] [PubMed] [Google Scholar]
- Lake R., Blunt J., Munro M. Eudistomins from the New Zealand ascidian Ritterella sigillinoides. Aust. J. Chem. 1989;42:1201–1206. [Google Scholar]
- Lambert A. The treatment of amoebic dysentery with emetine and bismuth iodide. Br. Med. J. 1918;1:116. doi: 10.1136/bmj.1.2978.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lean M.E., Ashihara H., Clifford M.N., Crozier A. Teas, Cocoa and Coffee: Plant Secondary Metabolites and Health. 2011. Purine alkaloids: a focus on caffeine and related compounds in beverages; pp. 25–44. [Google Scholar]
- Lee S.H., Kundu A., Han S.H., Mishra N.K., Kim K.S., Choi M.H., Pandey A.K., Park J.S., Kim H.S., Kim I.S. Synthesis of TMPA derivatives through sequential Ir (III)-catalyzed C-H alkylation and their antidiabetic evaluation. ACS Omega. 2018;3:2661–2672. doi: 10.1021/acsomega.8b00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Kim K., Jeon Y.U., Kundu A., Dey P., Hwang J.Y., Mishra N.K., Kim H.S., Kim I.S. Lewis acid-mediated cross-coupling reaction of 7-azaindoles and aldehydes: cytotoxic evaluation of C3-linked bis-7-azaindoles. Tetrahedron Lett. 2019;60:1–5. [Google Scholar]
- Leonard J. Recent progress in the chemistry of monoterpenoid indole alkaloids derived from secologanin. Nat. Prod. Rep. 1999;16:319–338. [Google Scholar]
- Lewis R.A. CRC Press; 1998. Lewis’ Dictionary of Toxicology. [Google Scholar]
- Li Z., Geng Y., Jiang J., Kong W. Antioxidant and anti-inflammatory activities of berberine in the treatment of diabetes mellitus. Evid. Based Complement. Alternat. Med. 2014;2014 doi: 10.1155/2014/289264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddell J.R. Pyrrolizidine alkaloids. Nat. Prod. Rep. 2002;19:773–781. doi: 10.1039/b108975g. [DOI] [PubMed] [Google Scholar]
- Ligtenstein D.A., Moes G.W. The synergism of atropine and the cholinesterase reactivator HI-6 in counteracting lethality by organophosphate intoxication in the rat. Toxicol. Appl. Pharmacol. 1991;107:47–53. doi: 10.1016/0041-008x(91)90329-d. [DOI] [PubMed] [Google Scholar]
- Lim K.-H., Hiraku O., Komiyama K., Koyano T., Hayashi M., Kam T.-S. Biologically active indole alkaloids from Kopsia arborea. J. Nat. Prod. 2007;70:1302–1307. doi: 10.1021/np0702234. [DOI] [PubMed] [Google Scholar]
- Lim K.-H., Low Y.-Y., Kam T.-S. Biomimetic oxidative transformations of pericine: partial synthesis of apparicine and valparicine, a new pentacyclic indole alkaloid from Kopsia. Tetrahedron Lett. 2006;47:5037–5039. [Google Scholar]
- Lind K.F. 2015. Bioactivity profile of barettin—with special focus on anti-inflammatory, antioxidant and anticoagulant activities. [Google Scholar]
- Lind K.F., Hansen E., Østerud B., Eilertsen K.-E., Bayer A., Engqvist M., Leszczak K., Jørgensen T.Ø., Andersen J.H. Antioxidant and anti-inflammatory activities of barettin. Mar. Drug. 2013;11:2655–2666. doi: 10.3390/md11072655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind K.F., Østerud B., Hansen E., Jørgensen T.Ø., Andersen J.H. The immunomodulatory effects of barettin and involvement of the kinases CAMK1α and RIPK2. Immunopharmacol. Immunotoxicol. 2015;37:458–464. doi: 10.3109/08923973.2015.1082584. [DOI] [PubMed] [Google Scholar]
- Lindner E. Springer; 1985. Structure Activities and Pharmacological Properties of the Opium Alkaloids, The Chemistry and Biology of Isoquinoline Alkaloids; pp. 38–46. [Google Scholar]
- List G.R., Spencer G.F., Hunt W.H. Toxic weed seed contaminants in soybean processing. J. Am. Oil Chem. Soc. 1979;56:706–710. [Google Scholar]
- Liu A., Chao H., Zhou X., Zhu Z., Huang F., Shen Y. Determination of three capsaicinoids in Capsicum annuum by pressurized liquid extraction combined with LC-MS/MS. J. Sep. Sci. 2013;36:857–862. doi: 10.1002/jssc.201200942. [DOI] [PubMed] [Google Scholar]
- Liu R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003;78:517S–520S. doi: 10.1093/ajcn/78.3.517S. [DOI] [PubMed] [Google Scholar]
- Liu R.H. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J. Nutr. 2004;134:3479S–3485S. doi: 10.1093/jn/134.12.3479S. [DOI] [PubMed] [Google Scholar]
- Lounasmaa M., Tamminen T. The tropane alkaloids: chemistry and biology. In: Cordell G.A., editor. The Alkaloids. Academic Press; New York: 1993. pp. 1–114. [Google Scholar]
- Lüllmann H., Mohr K., Hein L., Bieger D. second ed. Thieme; New York: 2000. Color Atlas of Pharmacology. [Google Scholar]
- Lunagariya J., Bhadja P., Zhong S., Vekariya R., Xu S. Marine natural product bis-indole alkaloid caulerpin: chemistry and biology. Mini Rev. Med. Chem. 2017;17 doi: 10.2174/1389557517666170927154231. [DOI] [PubMed] [Google Scholar]
- Lutfun N., Sarker S. Supercritical fluid extraction in natural products analyses. In: John W., editor. Methods in Molecular Biology. Humana Press; 2012. pp. 43–74. [DOI] [PubMed] [Google Scholar]
- Maat L., Beyerman H. Elsevier; 1984. The Imidazole Alkaloids, The Alkaloids: Chemistry and Pharmacology; pp. 281–333. [Google Scholar]
- Macedo N.R.P.V., Ribeiro M.S., Villaça R.C., Ferreira W., Pinto A.M., Teixeira V.L., Cirne-Santos C., Paixão I.C.N.P., Giongo V. Caulerpin as a potential antiviral drug against herpes simplex virus type 1. Rev. Bras. Farmacogn. 2012;22:861–867. [Google Scholar]
- Magistretti M. Remarks on the pharmacological examination of plant extracts. Fitoterapia. 1980;51:67–79. [Google Scholar]
- Mahajan A., Shelke U., Gadhave P., Bhalerao K. Essential oils: at a glance. World J. Pharm. Pharm. Sci. 2015;4:718–738. [Google Scholar]
- Maity S., Kundu A., Karmakar S., Sa B. In vitro and in vivo correlation of colon-targeted compression-coated tablets. J. Pharm. 2016;2016 doi: 10.1155/2016/5742967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed K., Cooper T., Magee T. Investigation and modelling of combined microwave and air drying. Food Bioprod. Process. 1995;73:0433. [Google Scholar]
- Majors R. Sampler prep perspectives: liquid extraction techniques for sample preparation. LC-GC. 1996;14:88–90. [Google Scholar]
- Makarieva T.N., Dmitrenok A.S., Dmitrenok P.S., Grebnev B.B., Stonik V.A. Pibocin B, the first N-O-methylindole marine alkaloid, a metabolite from the Far-Eastern Ascidian Eudistoma species. J. Nat. Prod. 2001;64:1559–1561. doi: 10.1021/np010161w. [DOI] [PubMed] [Google Scholar]
- Malafaia C., Silva T., do Amaral D., de Almeida C., de Silva M., Correia M., Silva M. Evaluation of the resistance and differential induction of chitinases in tomato in response to inoculation with Fusarium oxysporum f. sp. lycopersici. J. Plant Physiol. Pathol. 2013;1 [Google Scholar]
- Mann K.G., Brummel-Ziedins K., Orfeo T., Butenas S. Models of blood coagulation. Blood Cell Mol. Dis. 2006;36:108–117. doi: 10.1016/j.bcmd.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Manske R.H.F., Holmes H.L. Elsevier; 2014. The Alkaloids: Chemistry and Physiology. [Google Scholar]
- Mao S.-C., Guo Y.-W., Shen X. Two novel aromatic valerenane-type sesquiterpenes from the Chinese green alga Caulerpa taxifolia. Bioorg. Med. Chem. Lett. 2006;16:2947–2950. doi: 10.1016/j.bmcl.2006.02.074. [DOI] [PubMed] [Google Scholar]
- Margherita R., Giussani E., Morelli R., Lo Scalzo R., Nani R.C., Torreggiani D. Effect of fruit blanching on phenolics and radical scavenging activity of highbush blueberry juice. Food Res. Int. 2003;36:999–1005. [Google Scholar]
- McNaught A.D., McNaught A.D. Blackwell Science Oxford; 1997. Compendium of Chemical Terminology. [Google Scholar]
- Meenakshi B., Priyanka M. A novel protocol for micropropagation of Rauvolfia serpentina: in low concentration of growth regulators with sucrose and phenolic acid. Int. J. Plant Sci. (Muzaffarnagar) 2010;5:93–97. [Google Scholar]
- Mehrotra P., Kitchlu S., Dwivedi A., Agnihotri P., Srivastava S., Roy R., Bhaduri A. Emetine ditartrate: a possible lead for emergency contraception. Contraception. 2004;69:379–387. doi: 10.1016/j.contraception.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Meijerman I., Blom W.M., de Bont H.J., Mulder G.J., Nagelkerke J.F. Induction of apoptosis and changes in nuclear G-actin are mediated by different pathways: the effect of inhibitors of protein and RNA synthesis in isolated rat hepatocytes. Toxicol. Appl. Pharmacol. 1999;156:46–55. doi: 10.1006/taap.1998.8616. [DOI] [PubMed] [Google Scholar]
- Meksuriyen D., Cordell G. Biosynthesis of Staurosporine. 1. 1H- and 13C-NMR assignments. J. Nat. Prod. 1988;51:884–892. doi: 10.1021/np50059a012. [DOI] [PubMed] [Google Scholar]
- Michael J.P. Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 2008;25:166–187. doi: 10.1039/b612168n. [DOI] [PubMed] [Google Scholar]
- Miraldi E., Masti A., Ferri S., Barni Comparini I. Distribution of hyoscyamine and scopolamine in Datura stramonium. Fitoterapia. 2001;72:644–648. doi: 10.1016/s0367-326x(01)00291-x. [DOI] [PubMed] [Google Scholar]
- Mirzamatov R.T., Lutfullin K.L. Dynamics of the accumulation of alkaloids in Datura stramonium. Chem. Nat. Compd. 1986;22(3):359. [Google Scholar]
- Mishra P.K., Prasad S.M. Mounting insights over human wellness by utilizing plant’s primed defense against precise/mild oxidative stress. J. Crop Res. 2016;51 [Google Scholar]
- Modi V., Bhatlar S., Prajapati P., Basuri T. A review on various applications of high performance thin layer chromatography (HPTLC) in pharmaceuticals. IJIPSR. 2016;4:384–407. [Google Scholar]
- Mohapatra S., Mittra B. Induction of glutathione, ascorbate and associated enzymes by a low dose CdCl2 pre-treatment alleviate fusarium induced oxidative stress in wheat. J. Plant Physiol. Pathol. 2016;4 doi: 10.1016/j.plaphy.2016.10.014. [DOI] [PubMed] [Google Scholar]
- Möller M., Herzer K., Wenger T., Herr I., Wink M. The alkaloid emetine as a promising agent for the induction and enhancement of drug-induced apoptosis in leukemia cells. Oncol. Rep. 2007;18:737–744. [PubMed] [Google Scholar]
- Möller M., Weiss J., Wink M. Reduction of cytotoxicity of the alkaloid emetine through P-glycoprotein (MDR1/ABCB1) in human Caco-2 cells and leukemia cell lines. Planta Med. 2006;72:1121–1126. doi: 10.1055/s-2006-941546. [DOI] [PubMed] [Google Scholar]
- Moquin-Pattey C., Guyot M. Grossularine-1 and grossularine-2, cytotoxic α-carbolines from the tunicate: Dendrodoa grossularia. Tetrahedron. 1989;45:3445–3450. [Google Scholar]
- Moyer D.L., Thompson R.S., Berger I. Anti-implantation action of a medicated intrauterine delivery system (MIDS) Contraception. 1977;16:39–49. doi: 10.1016/0010-7824(77)90128-7. [DOI] [PubMed] [Google Scholar]
- Mroczek T., Głowniak K., Kowalska J. Solid-liquid extraction and cation-exchange solid-phase extraction using a mixed-mode polymeric sorbent of Datura and related alkaloids. J. Chromatogr. A. 2006;1107:9–18. doi: 10.1016/j.chroma.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Muhammad I., Dunbar D.C., Khan S.I., Tekwani B.L., Bedir E., Takamatsu S., Ferreira D., Walker L.A. Antiparasitic alkaloids from Psychotria klugii. J. Nat. Prod. 2003;66:962–967. doi: 10.1021/np030086k. [DOI] [PubMed] [Google Scholar]
- Mukherjee J., Menge M. New Products and New Areas of Bioprocess Engineering. Springer; 2000. Progress and prospects of ergot alkaloid research; pp. 1–20. [DOI] [PubMed] [Google Scholar]
- Müller K., Ziereis K., Gawlik I. The antipsoriatic Mahonia aquifolium and its active constituents. II. Antiproliferative activity against cell growth of human keratinocytes. Planta Med. 1995;61:74–75. doi: 10.1055/s-2006-958005. [DOI] [PubMed] [Google Scholar]
- Nada B., Vuko E., Ruscic M., Dunkic V. Helichrysum italicum (Roth) G. Don-essential oil composition and activity on tobacco mosaic virus infection. J. Plant Physiol. Pathol. 2017;4:1–12. [Google Scholar]
- Nakao Y., Yeung B.K.S., Yoshida W.Y., Scheuer P.J., Kelly-Borges M. Kapakahine B, a cyclic hexapeptide with an .alpha.-carboline ring system from the marine sponge Cribrochalina olemda. J. Am. Chem. Soc. 1995;117:8271–8272. [Google Scholar]
- Nammi S., Boini K.M., Koppula S., Sreemantula S. Reserpine-induced central effects: pharmacological evidence for the lack of central effects of reserpine methiodide. Can. J. Physiol. Pharmacol. 2005;83:509–515. doi: 10.1139/y05-039. [DOI] [PubMed] [Google Scholar]
- Natella F., Belelli F., Ramberti A., Scaccini C. Microwave and traditional cooking methods: effect of cooking on antioxidant capacity and phenolic compounds content of seven vegetables. J. Food Biochem. 2010;34:796–810. [Google Scholar]
- Nayak B., Berrios J.D.J., Powers J.R., Tang J. Effect of extrusion on the antioxidant capacity and color attributes of expanded extrudates prepared from purple potato and yellow pea flour mixes. J. Food Sci. 2011;76:C874–C883. doi: 10.1111/j.1750-3841.2011.02279.x. [DOI] [PubMed] [Google Scholar]
- Nayak B., Berrios J.D.J., Powers J.R., Tang J., Ji Y. Colored potatoes (Solanum tuberosum L.) dried for antioxidant-rich value-added foods. J. Food Process. Preserv. 2011;35:571–580. [Google Scholar]
- Nayak B., Liu R.H., Tang J. Effect of processing on phenolic antioxidants of fruits, vegetables, and grains—a review. Crit. Rev. Food Sci. Nutr. 2015;55:887–918. doi: 10.1080/10408398.2011.654142. [DOI] [PubMed] [Google Scholar]
- Nikolaenko V. Maintenance of homeostasis of endogenous ethanol as a method for the therapy of alcoholism. Bull. Exp. Biol. Med. 2001;131:231–233. doi: 10.1023/a:1017687012629. [DOI] [PubMed] [Google Scholar]
- Nindoa C.I., T-t S., Wangb S., Tang J., Powers J.R. Evaluation of drying technologies for retention of physical quality and antioxidants in asparagus (Asparagus officinalis, L.) LWT-Food Sci. Technol. 2003;36:507–516. [Google Scholar]
- Nomura H., Shiina T. Plant endosymbiotic organellar calcium signaling under biotic and abiotic stresses. J. Plant Physiol. Pathol. 2016;4 [Google Scholar]
- Oka S., Kodama M., Takeda H., Tomizuka N., Suzuki H. Staurosporine, a potent platelet aggregation inhibitor from a Streptomyces species. Agric. Biol. Chem. 1986;50:2723–2727. [Google Scholar]
- Oliveira M.E.C., Franca A.S. Microwave heating of foodstuffs. J. Food Eng. 2002;53:347–359. [Google Scholar]
- Omura S., Iwai Y., Hirano A., Nakagawa A., Awaya J., Tsuchya H., Takahashi Y., Masuma R. A new alkaloid AM-2282 OF Streptomyces origin. Taxonomy, fermentation, isolation and preliminary characterization. J. Antibiot. (Tokyo) 1977;30:275–282. doi: 10.7164/antibiotics.30.275. [DOI] [PubMed] [Google Scholar]
- Osterud B., Bjorklid E. Tissue factor in blood cells and endothelial cells. Front. Biosci. (Elite Ed.) 2012;4:289–299. doi: 10.2741/e376. [DOI] [PubMed] [Google Scholar]
- Ovenden S., Capon R. Echinosulfonic acids A − C and Echinosulfone A: novel bromoindole sulfonic acids and a sulfone from a Southern Australian marine sponge, Echinodictyum. J. Nat. Prod. 1999;62:1246–1249. doi: 10.1021/np9901027. [DOI] [PubMed] [Google Scholar]
- Owens M.W., George R.B. Nebulized atropine sulfate in the treatment of acute asthma. Chest. 1991;99:1084–1087. doi: 10.1378/chest.99.5.1084. [DOI] [PubMed] [Google Scholar]
- Özçelik B., Kartal M., Orhan I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011;49:396–402. doi: 10.3109/13880209.2010.519390. [DOI] [PubMed] [Google Scholar]
- Özek G., Demirci F., Özek T., Tabanca N., Wedge D., Khan S., Başer K.H.C., Duran A., Hamzaoglu E. Gas chromatographic-mass spectrometric analysis of volatiles obtained by four different techniques from Salvia rosifolia Sm., and evaluation for biological activity. J. Chromatogr. A. 2010;1217:741–748. doi: 10.1016/j.chroma.2009.11.086. [DOI] [PubMed] [Google Scholar]
- Padula L.Z., Bandoni A.L., Rondina R.V., Coussio J.D. Quantitative determination of total alkaloids and scopolamine in Datura ferox growing in Argentina. Planta Med. 1976;29:357–360. doi: 10.1055/s-0028-1097676. [DOI] [PubMed] [Google Scholar]
- Palermo M., Pellegrini N., Fogliano V. The effect of cooking on the phytochemical content of vegetables. J. Sci. Food Agric. 2014;94:1057–1070. doi: 10.1002/jsfa.6478. [DOI] [PubMed] [Google Scholar]
- Pandey A., Tripathi S. Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. J. Pharmacogn. Phytochem. 2014;2 [Google Scholar]
- Patil A., Koli S., Patil D., Phatak A. Evaluation of extraction techniques with various solvents to determine extraction efficiency of selected medicinal plants. Int. J. Pharm. Sci. Res. 2012;3:2607–2612. [Google Scholar]
- Patil P.S., Shettigar R. An advancement of analytical techniques in herbal research. J. Adv. Sci. Res. 2010;1:08–14. [Google Scholar]
- Pelletier S.W. Springer; 1999. Alkaloids: Chemical and Biological Perspectives. [Google Scholar]
- Peng L., Kang S., Yin Z., Jia R., Song X., Li L., Li Z., Zou Y., Liang X., Li L. Antibacterial activity and mechanism of berberine against Streptococcus agalactiae. Int. J. Clin. Exp. Pathol. 2015;8:5217. [PMC free article] [PubMed] [Google Scholar]
- Philipov S., Berkov S. GC-MS investigation of tropane alkaloids in Datura stramonium. Z. Naturforsch. C. 2002;57:559–561. doi: 10.1515/znc-2002-5-627. [DOI] [PubMed] [Google Scholar]
- Phrompittayarat W., Putalun W., Tanaka H., Jetiyanon K., Wittaya-areekul S., Ingkaninan K. Comparison of various extraction methods of Bacopa monnieri. Naresuan Univ. J.: Sci. Technol. (NUJST) 2007;15:29–34. [Google Scholar]
- Pillai T.G., Jayaraj R. Identification of endophytic fungi/oppurtunistic pathogen from the perennial herb of amaranthaceae family. J. Plant Physiol. Pathol. 2015;3 [Google Scholar]
- Piotr G., Lisiewska Z. Comparison of the level of selected antioxidative compounds in frozen broccoli produced using traditional and modified methods. Innovative Food Sci. Emerg. Technol. 2006;7:239–245. [Google Scholar]
- Poole C.F., Poole S.K. Trends in extraction of semivolatile compounds from solids for environmental analysis. Anal. Commun. 1996;33:11H–14H. [Google Scholar]
- Portenlänger G., Heusinger H. Chemical reactions induced by ultrasound and γ-rays in aqueous solutions of l-ascorbic acid. Carbohydr. Res. 1992;232:291–301. [Google Scholar]
- Potdar D., Hirwani R., Dhulap S. Phyto-chemical and pharmacological applications of Berberis aristata. Fitoterapia. 2012;83:817–830. doi: 10.1016/j.fitote.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Pravallika S. Gas chromatography—a mini review. RRJPA. 2016;5:55–62. [Google Scholar]
- Qiu S., Sun H., Zhang A.H., Xu H.Y., Yan G.L., Han Y., Wang X.J. Natural alkaloids: basic aspects, biological roles, and future perspectives. Chin. J. Nat. Med. 2014;12:0401–0406. doi: 10.1016/S1875-5364(14)60063-7. [DOI] [PubMed] [Google Scholar]
- Quaglia G.B., Gravina R., Paperi R., Paoletti f. Effect of high pressure treatments on peroxidase activity, ascorbic acid content and texture in green peas. LWT-Food Sci. Technol. 1996;29:552–555. [Google Scholar]
- Qweider M., Gilsbach J.M., Rohde V. Inadvertent intrathecal vincristine administration: a neurosurgical emergency: case report. J. Neurosurg. Spine. 2007;6:280–283. doi: 10.3171/spi.2007.6.3.280. [DOI] [PubMed] [Google Scholar]
- Rammohan B., Samit K., Chinmoy D., Arup S., Amit K., Ratul S., Sanmoy K., Dipan A., Tuhinadri S. Human cytochrome P450 enzyme modulation by Gymnema sylvestre: a predictive safety evaluation by LC-MS/MS. Pharmacogn. Mag. 2016;12:S389. doi: 10.4103/0973-1296.191441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore K., Gupta P. An overview on ion exchange chromatography. IJARPB. 2012;2:55–64. [Google Scholar]
- Raub M.F., Cardellina J.H., Schwede J.G. The green algal pigment caulerpin as a plant growth regulator. Phytochemistry. 1987;26:619–620. [Google Scholar]
- Reggia L., Hupman K., Johnson G., Keller D., Krill D. Arabidopsis rapid movement response to electrical stimulation. J. Plant Physiol. Pathol. 2016;4 [Google Scholar]
- Regnier T., Combrinck S., Du Plooy W. Essential oils and other plant extracts as food preservatives. In: Rajeev Bhat A.K.A., Paliyath G., editors. Progress in Food Preservation. John Wiley & Sons Ltd; 2012. pp. 539–579. [Google Scholar]
- Reina M., Ruiz-Mesia W., Ruiz-Mesia L., Martínez-Díaz R., González-Coloma A. Indole alkaloids from Aspidosperma rigidum and A. schultesii and their antiparasitic effects. Z. Naturforsch. C. 2011;66:225–234. doi: 10.1515/znc-2011-5-605. [DOI] [PubMed] [Google Scholar]
- Renner U.D., Oertel R., Kirch W. Pharmacokinetics and pharmacodynamics in clinical use of scopolamine. Ther. Drug Monit. 2005;27:655–665. doi: 10.1097/01.ftd.0000168293.48226.57. [DOI] [PubMed] [Google Scholar]
- Reyes F., Fernández R., Rodríguez A., Bueno S., de Eguilior C., Francesch A., Cuevas C. Cytotoxic staurosporines from the Marine Ascidian Cystodytes solitus. J. Nat. Prod. 2008;71:1046–1048. doi: 10.1021/np700748h. [DOI] [PubMed] [Google Scholar]
- Richa S., Dey P., Park C., Yang J., Son J.Y., Park J.H., Lee S.H., Ahn M.Y., Kim I.S., Moon H.R., Kim H.S. A new histone deacetylase inhibitor, MHY4381, induces apoptosis via generation of reactive oxygen species in human prostate cancer cells. Biomol. Ther. 2019;27:1–11. doi: 10.4062/biomolther.2019.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter B., Jones B., Ezzell J., Porter N. Accelerated solvent extraction: a technique for sample preparation. Anal. Chem. 1996;68:1033–1039. [Google Scholar]
- Riekkinen P., Jr., Jäkälä P., Sirviö J., Koivisto E., Miettinen R., Riekkinen P. The effects of THA on scopolamine and nucleus basalis lesion-induced EEG slowing. Brain Res. Bull. 1991;26:633–637. doi: 10.1016/0361-9230(91)90107-u. [DOI] [PubMed] [Google Scholar]
- Rinehart K.L., Jr., Kobayashi J., Harbour G.C., Gilmore J., Mascal M., Holt T.G., Shield L.S., Lafargue F. Eudistomins AQ, beta-carbolines from the antiviral Caribbean tunicate Eudistoma olivaceum. J. Am. Chem. Soc. 1987;109:3378–3387. [Google Scholar]
- Ripperger H. Solanum steroid alkaloids—an update. Alkaloids: Chem. Biol. Perspect. 1998;12:103–185. [Google Scholar]
- Rizk A.-F. CRC Press; 1990. Naturally Occurring Pyrrolizidine Alkaloids. [Google Scholar]
- Robertson J., Stevens K. Pyrrolizidine alkaloids. Nat. Prod. Rep. 2014;31:1721–1788. doi: 10.1039/c4np00055b. [DOI] [PubMed] [Google Scholar]
- Robins D. Springer; 1982. The Pyrrolizidine Alkaloids, Fortschritte der Chemie organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products; pp. 115–202. [DOI] [PubMed] [Google Scholar]
- Romano J.A., Terry M.R., Murrow M.L., Mays M.Z. Protection from lethality and behavioral incapacitation resulting from intoxication by Soman (pinacolyl methylphosphono fluoridate) and treatment with atropine sulfate and 2–PAM chloride in the guinea pig, Cavia porcellus. Drug Chem. Toxicol. 1991;14:21–44. doi: 10.3109/01480549109017867. [DOI] [PubMed] [Google Scholar]
- Ronald M. An overview of sample introduction and sample preparation of volatile organic compounds. LC GC. 1999;17:S7–S13. [Google Scholar]
- Rosemeyer H. The chemodiversity of purine as a constituent of natural products. Chem. Biodivers. 2004;1:361–401. doi: 10.1002/cbdv.200490033. [DOI] [PubMed] [Google Scholar]
- Rosenkranz V., Wink M. Alkaloids induce programmed cell death in bloodstream forms of trypanosomes (Trypanosoma b. brucei) Molecules. 2008;13:2462–2473. doi: 10.3390/molecules13102462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A. A review on the alkaloids an important therapeutic compound from plants. Int. J. Plant Biotechnol. 2017;3:1–9. [Google Scholar]
- Rubén A., Grant G., Dewey P., Marzo F. Nutritional assessment in vitro and in vivo of raw and extruded peas (Pisum s ativum L.) J. Agric. Food Chem. 2000;48:2286–2290. doi: 10.1021/jf000095o. [DOI] [PubMed] [Google Scholar]
- Rubnov S., Chevallier C., Thoison O., Debitus C., Laprevote O., Guénard D., Sévenet T. Echinosulfonic acid D: an ESI MSn evaluation of a new cytotoxic alkaloid from the New-Caledonian sponge Psammoclemma sp. Nat. Prod. Res. 2005;19:75–79. doi: 10.1080/1478641042000199851. [DOI] [PubMed] [Google Scholar]
- Ruiz-Moreno F. Perianal skin amebiases. Dis. Colon Rectum. 1967;10:65–69. doi: 10.1007/BF02617393. [DOI] [PubMed] [Google Scholar]
- Rupani R., Chavez A. Medicinal plants with traditional use: ethnobotany in the Indian subcontinent. Clin. Dermatol. 2018;36:306–309. doi: 10.1016/j.clindermatol.2018.03.005. [DOI] [PubMed] [Google Scholar]
- Saberi A.A. Recent advances in percolation theory and its applications. Phys. Rep. 2015;578:1–32. [Google Scholar]
- Sablani S.S., Andrews P.K., Davies N.M., Walters T., Saez H., Syamaladevi R.M., Mohekar P.R. Effect of thermal treatments on phytochemicals in conventionally and organically grown berries. J. Sci. Food Agric. 2010;90:769–778. doi: 10.1002/jsfa.3882. [DOI] [PubMed] [Google Scholar]
- Sadia S., Tariq A., Shaheen S., Malik K., Khan F., Ahmad M., Qureshi H., Nayyar B.G. Ethnopharmacological profile of anti-arthritic plants of Asia—a systematic review. J. Herb. Med. 2018:1–68. [Google Scholar]
- Sagi S., Avula B., Wang Y.-H., Khan I.A. Quantification and characterization of alkaloids from roots of Rauwolfia serpentina using ultra-high performance liquid chromatography-photo diode array-mass spectrometry. Anal. Bioanal. Chem. 2016;408:177–190. doi: 10.1007/s00216-015-9093-4. [DOI] [PubMed] [Google Scholar]
- Sahraoui N., MA Vian I., Bornard C., Boutekedjiret F.C. Improved microwave steam distillation apparatus for isolation of essential oils: comparison with conventional steam distillation. J. Chromatogr. A. 2008;1210:229–233. doi: 10.1016/j.chroma.2008.09.078. [DOI] [PubMed] [Google Scholar]
- Sakemi S., Sun H.H. Nortopsentins A, B, and C. Cytotoxic and antifungal imidazolediylbis[indoles] from the sponge Spongosorites ruetzleri. J. Org. Chem. 1991;56:4304–4307. [Google Scholar]
- Sala M., Braida D., Calcaterra P., Leone M.P., Comotti F.A., Gianola S., Gori E. Effect of centrally administered atropine and pirenzepine on radial arm maze performance in the rat. Eur. J. Pharmacol. 1991;194:45–49. doi: 10.1016/0014-2999(91)90122-7. [DOI] [PubMed] [Google Scholar]
- Sánchez-Moreno C., de Ancos B., Plaza L., Elez-Martínez P., Cano M.P. Nutritional approaches and health-related properties of plant foods processed by high pressure and pulsed electric fields. Crit. Rev. Food Sci. Nutr. 2009;49:552–576. doi: 10.1080/10408390802145526. [DOI] [PubMed] [Google Scholar]
- Saravanan G., Pari L. Effect of Cogent db, a herbal drug, on serum and tissue lipid metabolism in experimental hyperglycaemic rats. Diabetes. Obes. Metab. 2003;5:156–162. doi: 10.1046/j.1463-1326.2003.00257.x. [DOI] [PubMed] [Google Scholar]
- Sasidharan S., Chen Y., Saravanan D., Sundram K.M., Yoga Latha L. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011;8:1–10. [PMC free article] [PubMed] [Google Scholar]
- Sasidharan S., Darah I., Jain K. In vivo and in vitro. Toxicity study of Gracilaria changii. Pharm. Biol. 2008;46:413–417. [Google Scholar]
- Sayeed A., Garg M., Tamboli E., Abdin M., Ansari S. In vitro production of alkaloids: factors, approaches, challenges and prospects. Pharmacogn. Rev. 2013;7:27. doi: 10.4103/0973-7847.112837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner F., Yokota N., Ruf W. Tissue factor proangiogenic signaling in cancer progression. Thromb. Res. 2012;129:S127–S131. doi: 10.1016/S0049-3848(12)70032-4. [DOI] [PubMed] [Google Scholar]
- Schardl C.L., Grossman R.B., Nagabhyru P., Faulkner J.R., Mallik U.P. Loline alkaloids: currencies of mutualism. Phytochemistry. 2007;68:980–996. doi: 10.1016/j.phytochem.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Schardl C.L., Panaccione D.G., Tudzynski P. Ergot alkaloids—biology and molecular biology. Alkaloids: Chem. Biol. 2006;63:45–86. doi: 10.1016/s1099-4831(06)63002-2. [DOI] [PubMed] [Google Scholar]
- Schiff P.L., Jr. Bisbenzylisoquinoline alkaloids. J. Nat. Prod. 1987;50:529–599. doi: 10.1021/np50052a001. [DOI] [PubMed] [Google Scholar]
- Schmeller T., Wink M. Alkaloids. Springer; 1998. Utilization of alkaloids in modern medicine; pp. 435–459. [Google Scholar]
- Schripsema J. Application of NMR in plant metabolomics: techniques, problems and prospects. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2010;21:14–21. doi: 10.1002/pca.1185. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Liu Y., Edwards R.H. Reserpine binding to a vesicular amine transporter expressed in Chinese hamster ovary fibroblasts. J. Biol. Chem. 1993;268:29–34. [PubMed] [Google Scholar]
- Schweiggert U., Carle R., Schieber A. Conventional and alternative processes for spice production—a review. Trends Food Sci. Technol. 2007;18:260–268. [Google Scholar]
- Seleim M., Abo-Elyousr K., Mohamed A., Al-Marzoky H. Peroxidase and polyphenoloxidase activities as biochemical markers for biocontrol efficacy in the control of tomato bacterial wilt. J. Plant Physiol. Pathol. 2014;2(1):2. [Google Scholar]
- Shahlapour A., Eslami A., Eliazian H. Comparative anthelmintic tests in sheep and goats infected with gastro-intestinal nematodes and lungworms in Iran. Trop. Anim. Health Prod. 1970;2:223–234. [Google Scholar]
- Shamon S.D., Perez M.I. Blood pressure lowering efficacy of reserpine for primary hypertension. Cochrane Database Syst. Rev. 2016;21:1–39. doi: 10.1002/14651858.CD007655.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon H.E., Peters S.C. A comparison of the effects of cholinergic and dopaminergic agents on scopolamine-induced hyperactivity in mice. J. Pharmacol. Exp. Ther. 1990;255:549–553. [PubMed] [Google Scholar]
- Shen H. 2008. Illustrated Pharmacology Memory Cards: PharMnemonics. Minireview. [Google Scholar]
- Shenoy K., Yoganarasimhan S. Evaluation of antibacterial activity of Elanir kujambu—An ayurvedic eye formulation. Indian J. Trad. Knowl. 2009;8:272–274. [Google Scholar]
- Shi C.H., Zheng D., Jin X.L., Shou J.Y., Wu J.G., Zhu J. Analysis of genetic relationships between seed nutrient traits and plant agronomic traits in Indica rice (Oryza sativa L.) J. Plant Physiol. Pathol. 2013;1:1–5. [Google Scholar]
- Shih M.-C., Kuo C.-C., Chiang W. Effects of drying and extrusion on colour, chemical composition, antioxidant activities and mitogenic response of spleen lymphocytes of sweet potatoes. Food Chem. 2009;117:114–121. [Google Scholar]
- Shinonaga H., Shigemori H., Kobayashi J. Konbamidin, a new indole alkaloid from the Okinawan marine sponge Ircinia sp. J. Nat. Prod. 1994;57:1603–1605. doi: 10.1021/np50113a026. [DOI] [PubMed] [Google Scholar]
- Shonle I., Bergelson J. Evolutionary ecology of the tropane alkaloids of Datura stramonium L. (Solanaceae) Evolution. 2000;54:778–788. doi: 10.1111/j.0014-3820.2000.tb00079.x. [DOI] [PubMed] [Google Scholar]
- Siegel L.K., Klingbeil M.A. Control of drooling with transdermal scopolamine in a child with cerebral palsy. Dev. Med. Child Neurol. 1991;33:1013–1014. doi: 10.1111/j.1469-8749.1991.tb14818.x. [DOI] [PubMed] [Google Scholar]
- Singh A.U., Prasad D. Management of plant-parasitic nematodes by the use of botanicals. J. Plant Physiol. Pathol. 2014;2:1–10. [Google Scholar]
- Singh M. Evaluating the therapeutic efficiency and drug targeting ability of alkaloids present in Rauwolfia serpentina. Int. J. Green Pharm. (IJGP) 2017;11 [Google Scholar]
- Singh S. A review on paper chromatography. J. Drug Disc. Therap. 2017;5:23–25. [Google Scholar]
- Sinha P. Modulation of oxidative stress responsive enzymes and non-enzymatic components by excess nickel in C. lanatus lanatus var. fistulosus. J. Plant Physiol. Pathol. 2014;2(1):2. [Google Scholar]
- Sjögren M., Dahlström M., Göransson U., Jonsson P.R., Bohlin L. Recruitment in the field of Balanus improvisus and Mytilus edulis in response to the antifouling cyclopeptides barettin and 8,9-dihydrobarettin from the marine sponge Geodia barretti. Biofouling. 2004;20:291–297. doi: 10.1080/08927010400027027. [DOI] [PubMed] [Google Scholar]
- Sjögren M., Göransson U., Johnson A.-L., Dahlström M., Andersson R., Bergman J., Jonsson P.R., Bohlin L. Antifouling activity of brominated cyclopeptides from the marine sponge Geodia barretti. J. Nat. Prod. 2004;67:368–372. doi: 10.1021/np0302403. [DOI] [PubMed] [Google Scholar]
- Skalicka-Wozniak K., Glowniak K. Pressurized liquid extraction of coumarins from fruits of Heracleum leskowii with application of solvents with different polarity under increasing temperature. Molecules. 2012;17:4133–4141. doi: 10.3390/molecules17044133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeller T., Wink M. Alkaloids in modern medicine. In: Roberts M., Wink M., editors. Alkaloids. Biochemistry, Ecology, and Medicinal Applications. Academic Press; New York, London: 1998. pp. 435–459. [Google Scholar]
- Sneader W. John Wiley & Sons; 2005. Drug Discovery: A History. [Google Scholar]
- Soares M.S., Fernandes J.B., Vieria P.C. Alkyl, aryl, alkylarylquinoline, and related alkaloids. Alkaloids: Chem. Biol. 2007;64:139–214. doi: 10.1016/s1099-4831(07)64004-8. [DOI] [PubMed] [Google Scholar]
- Soares P.R., de Oliveira P.L., de Oliveira C.M., Kato L., Guillo L.A. In vitro antiproliferative effects of the indole alkaloid vallesiachotamine on human melanoma cells. Arch. Pharm. Res. 2012;35:565–571. doi: 10.1007/s12272-012-0320-7. [DOI] [PubMed] [Google Scholar]
- Solana R.P., Gennings C., Carter W.H., Jr., Anderson D., Lennox W.J., Carchman R.A., Harris L.W. Efficacy comparison of two cholinolytics, scopolamine and azaprophen, when used in conjunction with physostigmine and pyridostigmine for protection against organophosphate exposure. J. Am. Coll. Toxicol. 1991;10:215–222. [Google Scholar]
- Somei M., Yamada F. Simple indole alkaloids and those with a nonrearranged monoterpenoid unit. Nat. Prod. Rep. 2004;21:278–311. doi: 10.1039/b212257j. [DOI] [PubMed] [Google Scholar]
- Somei M., Yokoyama Y., Murakami Y., Ninomiya I., Kiguchi T., Naito T. Recent synthetic studies on the ergot alkaloids and related compounds. In: Cordell G.A., editor. The Alkaloids: Chemistry and Biology. vol. 54. Academic Press; San Diego, CA: 2000. p. 191. [Google Scholar]
- Srivastava S., Sharma Y.K. Arsenic induced changes in growth and metabolism of black gram seedlings (Vigna mungo L.) and the role of phosphate as an ameliorating agent. Environ. Process. 2014;1:431–445. [Google Scholar]
- Stecka L., Mruk-Luczkiewicz A., Wilk S. Output and composition of oil from seeds and total alkaloid content in leaves of Datura stramonium L. var. tatula (L.) Torr. from own cultivations. Herba Pol. 1975;21:17–23. [Google Scholar]
- Stojceska V., Ainsworth P., Plunkett A., İbanoğlu E., İbanoğlu Ş. Cauliflower by-products as a new source of dietary fibre, antioxidants and proteins in cereal based ready-to-eat expanded snacks. J. Food Eng. 2008;87:554–563. [Google Scholar]
- Strunz G.M., Findlay J.A. The Alkaloids: Chemistry and Pharmacology. Elsevier; 1985. Pyridine and piperidine alkaloids; pp. 89–183. [Google Scholar]
- Sudhir Kumar I., Srinivasa Rao P., Belum V.S.R., Ravindrababu V., Reddy K.H.P. Heterosis and inbreeding depression in tropical sweet sorghum (Sorghum bicolor (L.) Moench) J. Crop. Res. 2016;51 [Google Scholar]
- Sur S., Mohiuddin A., Vichyanond P., Nelson H. A random double-blind trial of the combination of nebulized atropine methylnitrate and albuterol in nocturnal asthma. Ann. Allergy. 1990;65:384–388. [PubMed] [Google Scholar]
- Susanne B., Dimberg L., Kamal-Eldin A. Effects of commercial processing on levels of antioxidants in oats (Avena sativa L.) J. Agric. Food Chem. 2002;50:1890–1896. doi: 10.1021/jf011222z. [DOI] [PubMed] [Google Scholar]
- Swartz M. HPLC detectors: a brief review. J. Liq. Chromatogr. Relat. Technol. 2010;33:1130–1150. [Google Scholar]
- Tadeusz A. The alkaloid-rich and alkaloid-poor Washington lupine (Lupinus polyphyllus Lindl.) as a potential industrial crop. Ind. Crop. Prod. 1992;1:147–155. [Google Scholar]
- Tae I.H., Park E.Y., Dey P., Son J.Y., Lee S.Y., Jung J.H., Saloni S., Kim M.H., Kim H.S. Novel SIRT1 inhibitor 15-deoxy-Δ12,14-prostaglandin J2 and its derivatives exhibit anticancer activity through apoptotic or autophagic cell death pathways in SKOV3 cells. Int. J. Oncol. 2018;53:2518–2530. doi: 10.3892/ijo.2018.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.W., Craig A.G., Fischer W.H., Park M., Lehrer R.I. Styelin D, an extensively modified antimicrobial peptide from ascidian hemocytes. J. Biol. Chem. 2000;275:38417–38426. doi: 10.1074/jbc.M006762200. [DOI] [PubMed] [Google Scholar]
- Tekel J., Hatrík S. Pesticide residue analyses in plant material by chromatographic methods: clean-up procedures and selective detectors. J. Chromatogr. A. 1996;754:397–410. doi: 10.1016/s0021-9673(96)00489-x. [DOI] [PubMed] [Google Scholar]
- Thammana M. A review on high performance liquid chromatography (HPLC) RRJPA. 2016;5:22–28. [Google Scholar]
- Thivya N., Srilakshmi K., Bhuvaneswari S., Leon Stephan Raj T. Phytoaccumulation of chromium and copper by Mentha spicata L. J. Plant Physiol. Pathol. 2014;2 [Google Scholar]
- Thomas R. Biogenetic speculation and biosynthetic advances. Nat. Prod. Rep. 2004;21:224–248. doi: 10.1039/b311022m. [DOI] [PubMed] [Google Scholar]
- Thompson J.H. The treatment of dysentery by injections of emetine hydrochloride. Dublin J. Med. Sci. (1872–1920) 1913;136:102–109. [Google Scholar]
- Tomko J., Votický Z. Steroid alkaloids: the Veratrum and Buxus groups. Alkaloids: Chem. Physiol. 1973;14:1–79. [Google Scholar]
- Tsujii S., Rinehart K.L., Gunasekera S.P., Kashman Y., Cross S.S., Lui M.S., Pomponi S.A., Diaz M.C. Topsentin, bromotopsentin, and dihydrodeoxybromotopsentin: antiviral and antitumor bis(indolyl)imidazoles from Caribbean deep-sea sponges of the family Halichondriidae. Structural and synthetic studies. J. Org. Chem. 1988;56:5446–5453. [Google Scholar]
- Tsung-Ming S. Cholinergic actions of diazepam and atropine sulfate in soman poisoning. Brain Res. Bull. 1991;26:565–573. doi: 10.1016/0361-9230(91)90097-4. [DOI] [PubMed] [Google Scholar]
- Uniyal S.K., Singh K.N., Jamwal P., Lal B. Traditional use of medicinal plants among the tribal communities of Chhota Bhangal, Western Himalaya. J. Ethnobiol. Ethnomed. 2006;2:1–8. doi: 10.1186/1746-4269-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyan S.E., Baysal T., Yurdagel U., El S.N. Effects of drying process on antioxidant activity of purple carrots. Food/Nahrung. 2004;48:57–60. doi: 10.1002/food.200300373. [DOI] [PubMed] [Google Scholar]
- Vaidya S., Vanaja M., Sathish P., Anitha Y., Jyothi Lakshmi N. Impact of elevated CO2 on growth and physiological parameters of groundnut (Arachis hypogaea L.) genotypes. J. Plant Physiol. Pathol. 2014;3 [Google Scholar]
- Valérie C. Recent extraction techniques for solid matrices—supercritical fluid extraction, pressurized fluid extraction and microwave-assisted extraction: their potential and pitfalls. Analyst. 2001;126:1182–1193. doi: 10.1039/b008243k. [DOI] [PubMed] [Google Scholar]
- Valjakka A., Lukkarinen K., Koivisto E., Riekkinen P., Jr., Miettinen R., Airaksinen M.M., Lammintausta R., Riekkinen P. Modulation of EEG rhythmicity and spike activity in the rat hippocampus by systemically administered tetrahydroaminoacridine, scopolamine and atipamezole. Brain Res. Bull. 1991;26:739–745. doi: 10.1016/0361-9230(91)90169-k. [DOI] [PubMed] [Google Scholar]
- van der Velde H., Demeyer K., Dejaegere R. Influence of IAA and DMAA on hyoscyamine and scopolamine production in Datura stramonium var. tatula. Acta Agron. Hung. 1988;37:55–63. [Google Scholar]
- Vankar P.S. Essential oils and fragrances from natural sources. Resonance. 2004;9:30–41. [Google Scholar]
- Varchi G., Battaglia A., Samorì C., Baldelli E., Danieli B., Fontana G., Guerrini A., Bombardelli E. Synthesis of deserpidine from reserpine. J. Nat. Prod. 2005;68:1629–1631. doi: 10.1021/np050179x. [DOI] [PubMed] [Google Scholar]
- Vedder E. An experimental study of the action of Ipecacuanha on Amoebae. Far Eastern Assoc. Trop. Med. Trans. Second Biennial Congress held at Hongkong; 1912. pp. 87–91. [Google Scholar]
- Villalba-Magdaleno J., Rojas R., Gomez-Garcia M.D.C., Shibayama M., Carrero J.C., Perez-Ishiwara D.G. Emetine produce Entamoeba histolytica death by inducing a programmed cell death. Am. J. Infect. Dis. 2007;3:110–114. [Google Scholar]
- Vinatoru M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001;8:303–313. doi: 10.1016/s1350-4177(01)00071-2. [DOI] [PubMed] [Google Scholar]
- Viscidi K.A., Dougherty M.P., Briggs J., Camire M.E. Complex phenolic compounds reduce lipid oxidation in extruded oat cereals. LWT-Food Sci. Technol. 2004;37:789–796. [Google Scholar]
- Vitale A.A., Acher A., Pomilio A.B. Alkaloids of Datura ferox from Argentina. J. Ethnopharmacol. 1995;49:81–89. doi: 10.1016/0378-8741(95)90035-7. [DOI] [PubMed] [Google Scholar]
- Volden J., Borge G.I.A., Bengtsson G.B., Hansen M., Thygesen I.E., Wicklund T. Effect of thermal treatment on glucosinolates and antioxidant-related parameters in red cabbage (Brassica oleracea L. ssp. capitata f. rubra) Food Chem. 2008;109:595–605. [Google Scholar]
- Wang L., Weller C. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006;17:300–312. [Google Scholar]
- Watanabe N., Iwamoto T., Dickinson D.A., Iles K.E., Forman H.J. Activation of the mitochondrial caspase cascade in the absence of protein synthesis does not require c-Jun N-terminal kinase. Arch. Biochem. Biophys. 2002;405:231–240. doi: 10.1016/s0003-9861(02)00399-5. [DOI] [PubMed] [Google Scholar]
- Wedge D.E., Camper N.D. Biologically Active Natural Products. CRC Press; 1999. Connections between agrochemicals and pharmaceuticals; pp. 13–27. [Google Scholar]
- Weiguang Y., Wetzstein H.Y. Effects of drying and extraction conditions on the biochemical activity of selected herbs. HortScience. 2011;46:70–73. [Google Scholar]
- Wiegrebe W., Kramer W.J., Shamma M. The emetine alkaloids. J. Nat. Prod. 1984;47:397–408. [Google Scholar]
- Willette R.E. Analgesic agents. In: Wilson C.O., Gisvold O., Delgado J.N., Remers W.A., editors. Textbook of Organic Medicinal and Pharmaceutical Chemistry. 10th ed. Lippincott-Raven; Philadelphia, PA: 1998. pp. 687–711. [Google Scholar]
- Witte L., Müller K., Arfmann H.A. Investigation of the alkaloid pattern of Datura innoxia plants by capillary gas-liquid-chromatography-mass spectrometry. Planta Med. 1987;53:192–197. doi: 10.1055/s-2006-962670. [DOI] [PubMed] [Google Scholar]
- Woisky R.G., Salantino A. Analysis of propolis: some parameters and procedures for chemical quality control. J. Apic. Res. 1998;37:99–105. [Google Scholar]
- Wolfe K.L., Liu R.H. Apple peels as a value-added food ingredient. J. Agric. Food Chem. 2003;51:1676–1683. doi: 10.1021/jf025916z. [DOI] [PubMed] [Google Scholar]
- Wollgast J., Anklam E. Review on polyphenols in Theobroma cacao: changes in composition during the manufacture of chocolate and methodology for identification and quantification. Food Res. Int. 2000;33:423–447. [Google Scholar]
- Wright A., Pomponi S., Cross S., McCarthy P. A new bis-(indole) alkaloid from a deep-water marine sponge of the genus Spongosorites. J. Org. Chem. 1992;57:4772–4775. [Google Scholar]
- Wrobel J.T. Pyrrolizidine alkaloids. Alkaloids. 1985;26:327–384. [Google Scholar]
- Wu-Fu L. A symptomatological assessment of organophosphate-induced lethality in mice: comparison of atropine and clonidine protection. Toxicol. Lett. 1991;56:19–32. doi: 10.1016/0378-4274(91)90086-l. [DOI] [PubMed] [Google Scholar]
- Yadav U.C., Moorthy K., Baquer N.Z. Combined treatment of sodium orthovanadate and Momordica charantia fruit extract prevents alterations in lipid profile and lipogenic enzymes in alloxan diabetic rats. Mol. Cell. Biochem. 2005;268:111–120. doi: 10.1007/s11010-005-3703-y. [DOI] [PubMed] [Google Scholar]
- Yang C., Liu G., Jiang B. Preparing functional bis(indole) pyrazine by stepwise cross-coupling reactions: an efficient method to construct the skeleton of Dragmacidin D. J. Org. Chem. 2002;67:9392–9396. doi: 10.1021/jo026450m. [DOI] [PubMed] [Google Scholar]
- Yin Low J., Chen K., Wu K., Ng M.M.-L., Chu J.H. Antiviral activity of emetine dihydrochloride against dengue virus infection. J. Antivir. Antiretrovir. 2009;1 062000. [Google Scholar]
- Yogesh H., Chandrashekhar V., Katti H., Ganapaty S., Raghavendra H., Gowda G.K., Goplakhrishna B. Anti-osteoporotic activity of aqueous-methanol extract of Berberis aristata in ovariectomized rats. J. Ethnopharmacol. 2011;134:334–338. doi: 10.1016/j.jep.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Young G.P., Freitas P. A randomized comparison of atropine and metaproterenol inhalational therapies for refractory status asthmaticus. Ann. Emerg. Med. 1991;20:513–519. doi: 10.1016/s0196-0644(05)81605-1. [DOI] [PubMed] [Google Scholar]
- Zadernowskl R., Nowak-Polakowska H., Rashed A.A. The influence of heat treatment on the activity of lipo- and hydrophilic components of oat grain. J. Food Process. Preserv. 1999;23:177–191. [Google Scholar]
- Zern T.L., Wood R.J., Greene C., West K.L., Liu Y., Aggarwal D., Shachter N.S., Fernandez M.L. Grape polyphenols exert a cardioprotective effect in pre- and postmenopausal women by lowering plasma lipids and reducing oxidative stress. J. Nutr. 2005;135:1911–1917. doi: 10.1093/jn/135.8.1911. [DOI] [PubMed] [Google Scholar]
- Zhang J., Davis T.A., Matthews M.A., Drews M.J., LaBerge M., An Y.H. Sterilization using high-pressure carbon dioxide. J. Supercrit. Fluids. 2006;38:354–372. [Google Scholar]
- Zhao H., Wang X.Y., Li M.K., Hou Z., Zhou Y., Chen Z., Meng J.R., Luo X.x., Tang H.F., Xue X.Y. A novel pregnane-type alkaloid from pachysandra terminalis inhibits methicillin-resistant Staphylococcus aureus in vitro and in vivo. Phytother. Res. 2015;29:373–380. doi: 10.1002/ptr.5261. [DOI] [PubMed] [Google Scholar]
- Ziegler J., Facchini P.J. Alkaloid biosynthesis: metabolism and trafficking. Annu. Rev. Plant Biol. 2008;59:735–769. doi: 10.1146/annurev.arplant.59.032607.092730. [DOI] [PubMed] [Google Scholar]
- Zielinski H., Kozlowska H., Lewczuk B. Bioactive compounds in the cereal grains before and after hydrothermal processing. Innovative Food Sci. Emerg. Technol. 2001;2:159–169. [Google Scholar]
- Zini C.A., Augusto F., Christensen T.E., Smith B.P., Caramão E.B., Pawliszyn J. Monitoring biogenic volatile compounds emitted by Eucalyptus citriodora using SPME. Anal. Chem. 2001;73:4729–4735. doi: 10.1021/ac0103219. [DOI] [PubMed] [Google Scholar]
- Zougagh M., Valcárcel M., Rı´os A. Supercritical fluid extraction: a critical review of its analytical usefulness. Trends Anal. Chem. 2004;23:399–405. [Google Scholar]
- Zygmunt B., Namieśnik J. Preparation of samples of plant material for chromatographic analysis. J. Chromatogr. Sci. 2003;41:109–116. doi: 10.1093/chromsci/41.3.109. [DOI] [PubMed] [Google Scholar]


