Abstract
In amniotes, head movements are encoded by two types of vestibular hair cells (type I and type II) with unique morphology, physiology, and innervation. After hair cell destruction in mature rodents, supporting cells regenerate some type II hair cells, but no type I hair cells are replaced. The transcription factor Atoh1 is required for hair cell development, and Atoh1 is upregulated in supporting cells, the hair cell progenitors, in mature chickens and mice following hair cell damage. We investigated whether Atoh1 is required for type II hair cell regeneration in adult mice after genetic ablation of hair cells. First, we used a knock-in Atoh1 reporter to demonstrate that supporting cells in the utricle, a vestibular organ that detects linear acceleration of the head, upregulate Atoh1 expression by 7 days after hair cell destruction was initiated. Next, we labeled supporting cells prior to damage and fate-mapped them over time to test whether conditional deletion of Atoh1 from supporting cells prevented them from converting into hair cells after damage. In mice with normal Atoh1 expression, fate-mapped supporting cells in the adult utricle gave rise to hundreds of type II hair cells after hair cell destruction, but they did not form new type I hair cells. By contrast, mice with Atoh1 deletion prior to hair cell damage had only 10–20 fate-mapped type II hair cells per utricle at 3 weeks post-damage, and numbers did not change at 12 weeks after hair cell destruction. Supporting cells had normal cell shape and nuclear density up to 12 weeks after Atoh1 deletion. Similar observations were made in two other vestibular organs, the saccule and the lateral ampulla. Our findings demonstrate that Atoh1 is necessary in adult mouse supporting cells for regeneration of type II vestibular hair cells and that deletion of Atoh1 from supporting cells prior to damage does not appear to induce supporting cells to die or to proliferate.
Keywords: Vestibular, hair cell, supporting cell, regeneration, Atoh1
1. Introduction
Vestibular organs in the inner ear sense head movements and convey information to the brain via the vestibulocochlear nerve to regulate critical bodily functions including motor reflexes, cognition, blood pressure, and mood (e.g., reviewed in Hilber et al., 2019; McCall et al., 2017; Smith, 2017; Bill J. Yates et al., 2014). The mammalian inner ear has five vestibular organs, each containing a specialized sensory epithelium (macula or crista) composed of hair cells (the sensory receptors), supporting cells, and afferent and efferent nerve endings (reviewed in Eatock and Songer, 2011). In the two macular organs - utricle and saccule - hair cells encode linear acceleration, and in the cristae of the three semicircular canals (anterior, posterior, and lateral), hair cells encode angular acceleration. Vestibular deficits arise as a result of hair cell degeneration or destruction, which is caused by gene mutations, ototoxic drug exposure, microbial infections, or aging (Gleeson and Felix, 1987; Rauch et al., 2006; Rosenhall and Rubin, 1975; Sedó-Cabezón et al., 2014). Vestibular dysfunction increases the risk of falling (Herdman et al., 2000) and reduces quality of life (Guinand et al., 2012), and it is experienced by an estimated 35% of the U.S. population older than 40 years (Agrawal et al., 2009).
Amniotes have two types of hair cells, type I and type II, which differ with respect to morphology, physiology, molecular markers, and innervation (reviewed in Burns and Stone, 2017; Eatock, 2018; Eatock and Songer, 2011). Adult mammals, including rodents and possibly humans, have a limited capacity to replace damaged or lost vestibular hair cells (Forge et al., 1998, 1993; Golub et al., 2012; Kawamoto et al., 2009; Slowik and Bermingham-Mcdonogh, 2013; Taylor et al., 2018, 2015; Wang et al., 2015; Warchol et al., 1993; Wu et al., 2016). Histological analyses indicate that type II hair cells are regenerated, but type I hair cells are not (Forge et al., 1998; Golub et al., 2012; Kawamoto et al., 2009). Our unpublished data indicate that, in adult mice, ~40% of the type II hair cell population is replaced after near-complete hair cell loss (Stone et al., 2015). Cellular fate-mapping shows that supporting cells give rise to new vestibular hair cells after damage in adult mammals (Bucks et al., 2017; Lin et al., 2011). Because there is little evidence that supporting cells divide prior to hair cell regeneration (e.g., Forge et al., 1993; Golub et al., 2012; Kawamoto et al., 2009; Lin et al., 2011), it is surmised that supporting cells convert into hair cells without dividing. This form of hair cell regeneration - called direct transdifferentiation - also occurs in non-mammalian vertebrates (e.g., Baird et al., 1996; Roberson et al., 2004; Shang et al., 2010).
The molecules that regulate regeneration of vestibular type II hair cells in adult mammals have not been defined. Transcription factors are key regulators of cellular fate during development, normal conditions, and repair from injury. Atoh1 is a basic helix-loop-helix transcription factor required for hair cell development in mice and fish (Bermingham, 1999; Millimaki et al., 2007). In the developing otocyst, Atoh1 is first expressed in prosensory progenitor cells (Bermingham, 1999; Lanford et al., 2000; Matei et al., 2005; Woods et al., 2004; Yang et al., 2010) and then becomes enriched in hair cells as they differentiate (Bermingham, 1999; Chen et al., 2002; Itoh and Chitnis, 2001; Shailam et al., 1999; Woods et al., 2004). Atoh1 activates transcription of a host of hair cell-specific genes, directing hair cell differentiation and maturation (Cai et al., 2015). Accordingly, ectopic expression of Atoh1 is sufficient to induce a hair cell phenotype in non-sensory regions (e.g., Kawamoto et al., 2003; Woods et al., 2004; Zheng and Gao, 2000) or to reprogram supporting cells into hair cells (Gao et al., 2016; Gubbels et al., 2008; Izumikawa et al., 2005; Kelly et al., 2012; Kuo et al., 2015; Liu et al., 2012; Shou et al., 2003; Walters et al., 2017). Atoh1 is also required for survival of developing hair cells, up to a certain point (Cai et al., 2013; Chen et al., 2002; Chonko et al., 2013; Maass et al., 2013; Pan et al., 2012). Once hair cells mature, however, Atoh1 expression is downregulated in supporting cells and hair cells (Driver et al., 2013; Lanford et al., 2000; Yang et al., 2010), except in systems that have continuous hair cell turnover in maturity, such as the vestibular organs of chicken (Cafaro et al., 2007) and mice (Bucks et al., 2017) and the lateral line neuromasts of zebrafish (Ma et al., 2008). In these cases, small numbers of Atoh1-expressing supporting cells and hair cells persist in adulthood.
Atoh1 may be important for regeneration of hair cells because many supporting cells in fish, birds, and mice upregulate Atoh1 after damage and go on to form new hair cells (Atkinson et al., 2018; Cafaro et al., 2007; Cotanche and Kaiser, 2010; Kawamoto et al., 2009; Lewis et al., 2012; Lin et al., 2011; Ma et al., 2008; Wang et al., 2010). However, Atoh1’s requirement for hair cell regeneration has not been tested. In this study, we used CreER-loxP technology to investigate whether Atoh1 is required in adult mouse supporting cells for regeneration of type II vestibular hair cells. We found that Atoh1 deletion from adult supporting cells before hair cell damage prevented them from transdifferentiating into hair cells but did not alter their general shape or nuclear density.
2. Materials and Methods
2.1. Animals
Sox9-CreERT2 (stock #18829, Kopp et al., 2011), Rosa26loxP-stop-loxP-tdTomato (Rosa26tdTomato, also called Ai14, stock #7914, Madisen et al., 2010), Atoh1loxP/loxP (stock #8681; Shroyer et al., 2007), and Atoh1GFP (stock #13593, Rose et al., 2009) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Pou4f3DTR (DTR) mice (Golub et al., 2012; Tong et al., 2015), a gift from Ed Rubel at the University of Washington, were used as heterozygotes. DTR:Atoh1GFP/GFP mice were on a C57BL/6J background. DTR:Sox9-CreERT2:Rosa26tdTomato:Atoh1loxP/loxP (Atoh1 CKO) and DTR:Sox9-CreERT2:Rosa26tdTomato:Atoh1+/+ (Atoh1 WT) were on a C57BL/6J:BALBc:CD1 background. Both genders were used in all studies.
All animal procedures were conducted at the Southern Illinois University School of Medicine (Springfield, IL) using approved protocols from their animal care and use committees. Animal numbers are provided in Table 1. The “n” value represents the number of mice included in each experiment.
TABLE 1.
Hair cell numbers and densities for utricles, saccules, and ampullae
| UTRICLES | Number of hair cells per organ (mean ±SD) | ||||
|---|---|---|---|---|---|
| Group | tdTom+ HCII | tdTom+ HCI | tdTom− HCII | tdTom− HCI | N (mice) |
| WT 3w | 206 ±32 | 0±0 | 83 ±53 | 236 ±153 | 4 |
| WT 12w | 404 ±47 | 0±0 | 74 ±38 | 217 ±23 | 3 |
| CKO 3w | 13 ±6 | 0±1 | 99 ±40 | 259 ±139 | 3 |
| CKO 12w | 20 ±7 | 0±0 | 61 ±13 | 101 ±23 | 3 |
| Effect | Two-way ANOVA p Value | ||||
| Time post-DT | 0.0001 | 0.3018 | 0.1876 | ||
| Atoh1 deletion | <0.0001 | 0.3018 | 0.4692 | ||
| SACCULES | Numbers of hair cells per 10,000 μm^2 (mean ±SD) | ||||
| Group | tdTom+ HCII | tdTom+ HCI | tdTom− HCII | tdTom− HCI | N (mice) |
| WT 3w | 4.82 ±2.01 | 0.0 ±0.0 | 2.78 ±0.90 | 7.26 ±4.39 | 3 |
| CKO 3w | 0.33 ±0.14 | 0.0 ±0.0 | 4.64 ±2.44 | 7.53 ±1.66 | 3 |
| t-test p value | 0.0181 | N/A | 0.2827 | 0.9254 | |
| LAT AMPULLAE | Numbers of hair cells per 10,000 μm^2 (mean ±SD) | ||||
| Group | tdTom+ HCII | tdTom+ HCI | tdTom− HCII | tdTom− HCI | N (mice) |
| WT 3w | 7.05 ±1.36 | 0.0 ±0.0 | 1.39± 1.37 | 8.53 ±6.18 | 3 |
| CKO 3w | 0.07 ±0.13 | 0.0 ±0.0 | 1.53 ±0.07 | 5.99 ±2.27 | 3 |
| t-test p value | 0.0009 | N/A | 0.8626 | 0.5417 | |
2.2. Drug Treatments
Mice aged 6 weeks of age were injected intraperitoneally (IP) with tamoxifen (Sigma-Aldrich, St. Louis, MO) at 9 mg/40 g, given once a day on two consecutive days spaced 20–24 hours apart. Diphtheria toxin (DT, Sigma-Aldrich, St. Louis, MO or List Biological Laboratories, Inc., Campbell, CA) was injected at either 6 or 7 weeks of age at either 25 or 50 ng/g depending on the potency of DT, which varied with each lot and over time during storage at −20°C. DT was delivered intramuscularly (IM) once a day for two days (spaced 44 to 48 hours apart).
2.3. Immunolabeling
Temporal bones were immersion-fixed in electron microscopy grade 4% paraformaldehyde (Polysciences, Inc., Warrington, PA) overnight at room temperature and stored in 10 mM phosphate buffered saline (PBS; Sigma-Aldrich, St. Louis, MO) at 4°C. Whole organs were dissected and immunolabeled in 96-well plates as described in Bucks et al. (2017). The following primary antibodies were used: rabbit anti-myosin VIIa (1:300; Proteus Biosciences Inc., Ramona, CA, cat #25–6790); rabbit anti-calretinin (1:500; EMD Millipore, Burlington, MA, cat# AB5054); rabbit anti-green fluorescent protein (GFP; 1:250, Invitrogen, Carlsbad, CA, cat #A6455); and goat anti-Sox2 (1:500; Santa Cruz Biotechnology, Dallas, TX, cat #sc-17320). Primary antibodies were detected with Alexa Flour-conjugated secondary antibodies (1:400, Invitrogen, Carlsbad, CA). To counter-label nuclei, we used 4’,6’-diamidine-2-phenylindole (DAPI; Sigma-Aldrich, St. Louis, MO) at 1 μg/mL in 10 mM PBS. After immunolabeling, utricles were whole-mounted in Fluoromount-G (Southern Biotech, Birmingham, AL) on slides.
2.4. Tissue Imaging, Criteria for Cell-Typing, Quantitative Analysis, and Statistics
Fluorescent imaging of whole-mount organs was performed using an Olympus FV-1000 microscope (Olympus, Center Valley, PA). For hair cell counts in utricles, we acquired Z-series images of the entire organ using a 60x oil objective starting at the lumenal surface of the epithelium and continuing through the epithelium into the stroma for a few microns. For hair cell counts in ampullae and saccules, we sampled approximately one third of the organ and estimated hair cell counts per organ, including both central/striolar and peripheral/extrastriolar regions.
Olympus files were imported into Fiji (http://fiji.sc/) for qualitative and quantitative analyses and cells were counted using Fiji’s Cell Counter plugin. Hair cells were distinguished from supporting cells by their morphology and immunoreactivity for myosin VIIa. To classify hair cells as type I or II, we used several well established morphological criteria previously described in detail (Bucks et al., 2017; Pujol et al., 2014). Briefly, type II hair cells have heavy myosin VIIa labeling throughout their cytoplasm, which is higher in volume than type I hair cells. Type II hair cells have a thick neck and at least one large cytoplasmic process that is myosin VIIa-positive. The nucleus of each type II hair cell is large and oval and is located closer to the lumen than those of type I hair cells. Type I hair cells have a thin neck, a small round nucleus, and a rounded cell body with relatively little cytoplasmic myosin VIIa. They also lack basolateral processes. In extrastriolar regions, type I hair cell nuclei are located deeper than type II hair cell nuclei. In areas of the epithelium where many continuous supporting cells were tdTomato-positive, we could also determine if a hair cell had a calyx, which presented itself as a non-labeled (black) space between the myosin VIIa-positive hair cell cytoplasm and the tdTomato-positive supporting cell cytoplasm. For some utricles, we used antibodies to calretinin and Sox2 – two type II hair cell markers (Desai et al., 2005; Oesterle et al., 2008; Pujol et al., 2014) to confirm the relative proportions of type I:II hair cells.
For analysis of Atoh1 expression in utricles, we counted GFP-positive supporting cells in 3 DTR:Atoh1GFP/GFP mice at 7, 14, 28, and 60 days post-DT and 3 Atoh1GFP/GFP mice (DTR-negative) at 7–14 days post-DT. For these counts, we generated a z-series images of the entire utricle using a 60x objective with 1x zoom and counted all GFP-positive nuclei per utricle that were located in the supporting cell nuclear layer, checking that such cells were not labeled for the hair cell marker myosin VIIa.
For analysis of the effects of Atoh1 deletion in utricles, we counted hair cells from 3–4 Atoh1 CKO and Atoh1 WT mice. Counts were performed as described for DTR:Atoh1GFP/GFP mice, except we scored every hair cell in the utricle as tdTomato-positive or tdTomato-negative and as type I or type II. For analysis of the effects of Atoh1 deletion in saccules and lateral ampullae, we counted cells in 3 Atoh1 CKO and Atoh1 WT mice. We created z-series using a 60x oil objective and sampled 3–4 regions, which comprised 46% of the saccule (mostly composed of the peripheral zone) and all of the ampulla.
For supporting cell counts in Atoh1 CKO experiments, we generated z-series images of 4 regions of the utricle (2 striolar, 2 lateral extrastriola) using a 60x oil objective (2x zoom), sampling 21% of the macula for each mouse. Supporting cells were identified by the following characteristics: they were myosin VIIa-negative, their cell bodies spanned the depth of the sensory epithelium from the lumen to the basal lamina, and their nuclei were located very close to the basal lamina. We counted supporting cells in utricles from 3–4 Atoh1 CKO and Atoh1 WT mice.
Figures were created using Adobe Photoshop CS4 (San Jose, CA). Statistical analysis was performed with Prism6 (GraphPad Software, San Diego, CA). Differences between groups were considered significant if p ≤ 0.05.
3. Results
3.1. Supporting cells upregulate Atoh1 in adult mouse utricles after hair cell destruction
In mice, the utricle is a disk-shaped organ (Fig. 1A) composed of a sensory epithelium (or macula) overlying loose connective tissue (or stroma). The utricular macula is composed of type I hair cells, type II hair cells, and supporting cells (Fig. 1B, right), and it is subdivided into three regions: the striola, the lateral extrastriola, and the medial extrastriola (Fig. 1B, left).
Figure 1. Supporting cells upregulate Atoh1 prior to regeneration of type II hair cells in damaged adult mouse utricles.
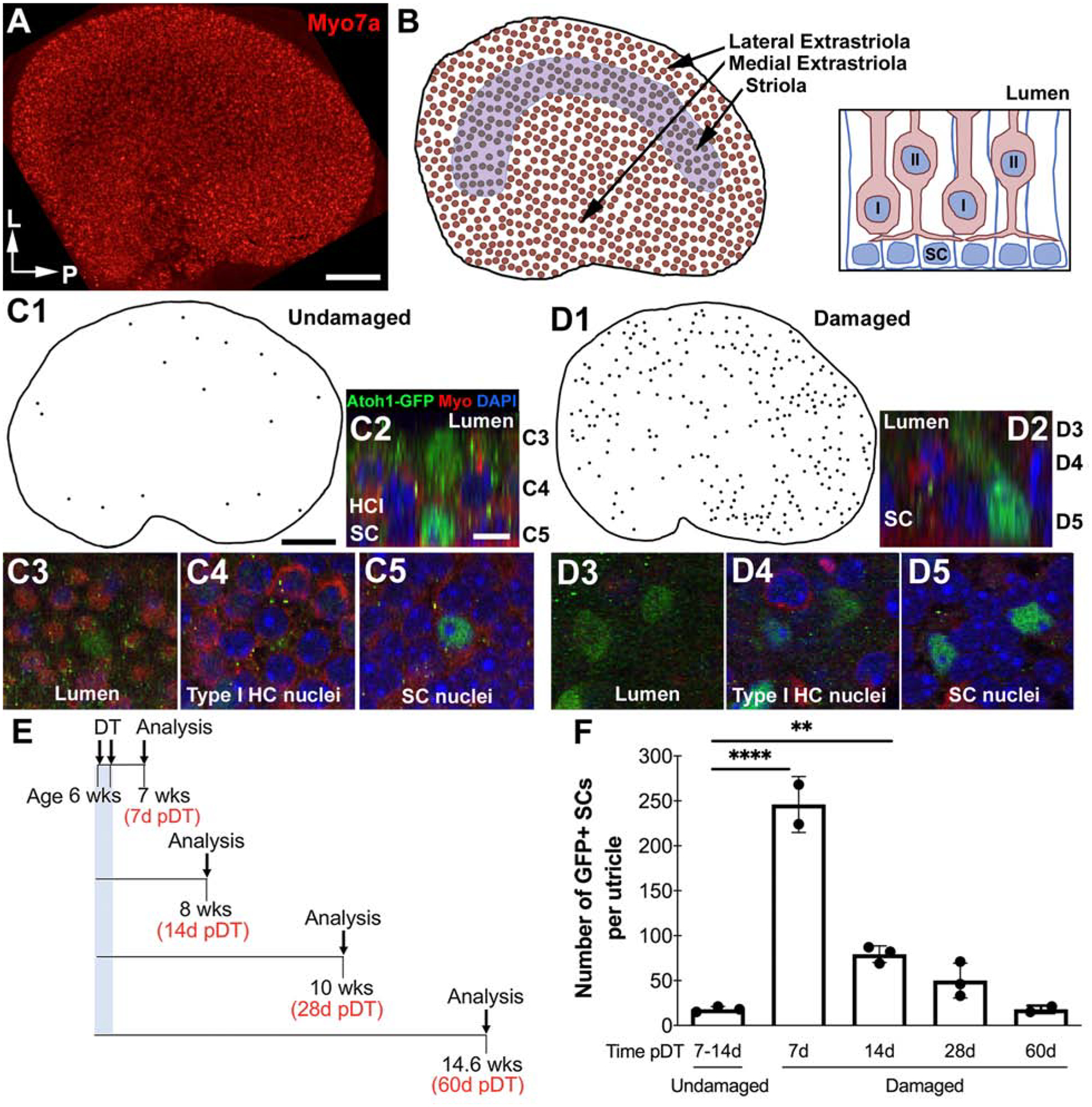
A. Representative confocal horizontal (xy) image of a whole utricle from an adult mouse, labeled with antibodies to the hair cell marker myosin VIIa (myo7a, red). P=posterior, L=lateral. B. Schematic of a top-down view of the utricle (left) and a cross-section through the utricular macula (right). Hair cells are shown in red tint in both drawings. The striola is outlined in purple in the drawing on the left. Cell nuclei in the cross-section are blue. I = type I hair cell; II = type II hair cell; SC = supporting cell. C1–5. Schematic and representative confocal images of an undamaged utricle from an Atoh1GFP/GFP mouse at 7 days post-DT. C1. Map of Atoh1-GFP-positive supporting cells from an exemplary undamaged utricle. C2. Optical section through an Atoh1-GFP-positive supporting cell (green) with hair cells labeled by myosin VIIa (Myo, red) and nuclei labeled with DAPI (blue). C3–5. Series of slices from the same z-stack, showing an Atoh1-GFP-positive supporting cell, focused at the lumen (C3), the type I hair cell (HC) nuclear layer (C4), and the supporting cell nuclear layer (C5). The approximate positions of each slice is shown on the right side of C2. D1–5. Schematic and representative confocal images of a damaged utricle from a DTR:Atoh1GFP/GFP mouse at 7 days post-DT. D1. Map of Atoh1-GFP-positive supporting cells from an exemplary damaged utricle. D2. Optical section through an Atoh1-GFP-positive supporting cell (green) labeled the same as C2. D3–5. Series of slices from the same z-stack, showing an Atoh1-GFP-positive supporting cell, focused at the lumen (C3), the type I hair cell (HC) nuclear layer (D4), and the supporting cell nuclear layer (D5). The approximate positions of each slice is shown on the right side of D2. E. Timeline showing the experimental design for Atoh1 expression analysis in damaged mice. All mice received DT at 6 weeks of age and were examined at 7, 14, 28, or 60 days post-DT (pDT). F. Graph showing the mean number of Atoh1-GFP-positive supporting cells per utricle after DT treatment in undamaged and damaged mice. Horizontal lines over bars and asterisks indicate significant difference from control (one-way ANOVA with Tukey’s multicomparisons test). Error bars = 1 standard deviation from the mean. N = 3 mice per group. Scale bar in A = 150 μm and applies to A, B. Scale bar in C1 = 150 μm and applies to C1 and D1. Scale bar in C2 = 3μm and applies to C2-C5, D2-D5.
To study hair cell regeneration, we employed genetically modified mice (Pou4f3DTR+/−, abbreviated as DTR) in which vestibular hair cells express the human receptor for diphtheria toxin (DT). Following injection of DT, ~94% of vestibular hair cells are destroyed, but other cell types in the sensory organ are spared (Golub et al., 2012). As a first step in exploring Atoh1’s role in vestibular hair cell regeneration, we examined when and where Atoh1 protein levels are upregulated in adult supporting cells (the hair cell progenitors during regeneration) after hair cell destruction. We crossed DTR mice with Atoh1GFP/GFP mice, which express a fusion protein of Atoh1 and green fluorescent protein (GFP) under control of the endogenous Atoh1 promoter (Rose et al., 2009). We injected 6 week-old Atoh1GFP/GFP mice with or without DTR and collected utricles at 7, 14, 28, or 60 days post-DT (Fig. 1E). Utricles were labeled with antibodies to GFP, the hair cell marker myosin VIIa, and the nuclear label DAPI, and examined using confocal microscopy.
In Atoh1GFP/GFP mice lacking DTR, utricular hair cells were present in normal-appearing densities (Fig. 1C3,C4), which is consistent with prior reports indicating hair cells are not damaged by DT in mice that lack the DTR allele (e.g., Golub et al., 2012). In these mice at 7–14 days post-DT, we detected 18 ±3 (mean +SD) Atoh1-GFP-positive supporting cells per utricle (Fig. 1C1–C5,F). These cells were distributed throughout the sensory epithelium, mainly in the periphery (Fig. 1C1). In DTR:Atoh1GFP/GFP mice, there was significant loss of hair cells (Fig. 1D3,4) and a significant increase in Atoh1-GFP-positive supporting cells after DT treatment (Fig. 1D1–D5,F; p=<0.00001, one-way ANOVA for effect of time, where the control is designated as 0d), in conjunction with extensive destruction of vestibular hair cells. At 7 days post-DT, DTR:Atoh1GFP/GFP mice had 227 ±40 Atoh1-GFP-positive supporting cells and at 14 days post-DT, they had 79 ±9 Atoh1-GFP-positive supporting cells. Atoh1-GFP-positive supporting cells were distributed throughout the utricle. At both timepoints, there were significantly fewer labeled supporting cells from undamaged Atoh1GFP/GFP mice (Tukey’s multiple comparisons test; for 7d vs control, p≤0.0001; for 14d vs control, p≤0.01). Numbers of Atoh1-GFP-positive supporting cells in DTR mice at 28 or 60 days post-DT were not significantly different from control (Fig. 1F, Tukey’s multiple comparisons test), presumably paralleling the reduction in new hair cell production that occurs over time (Golub et al., 2012).
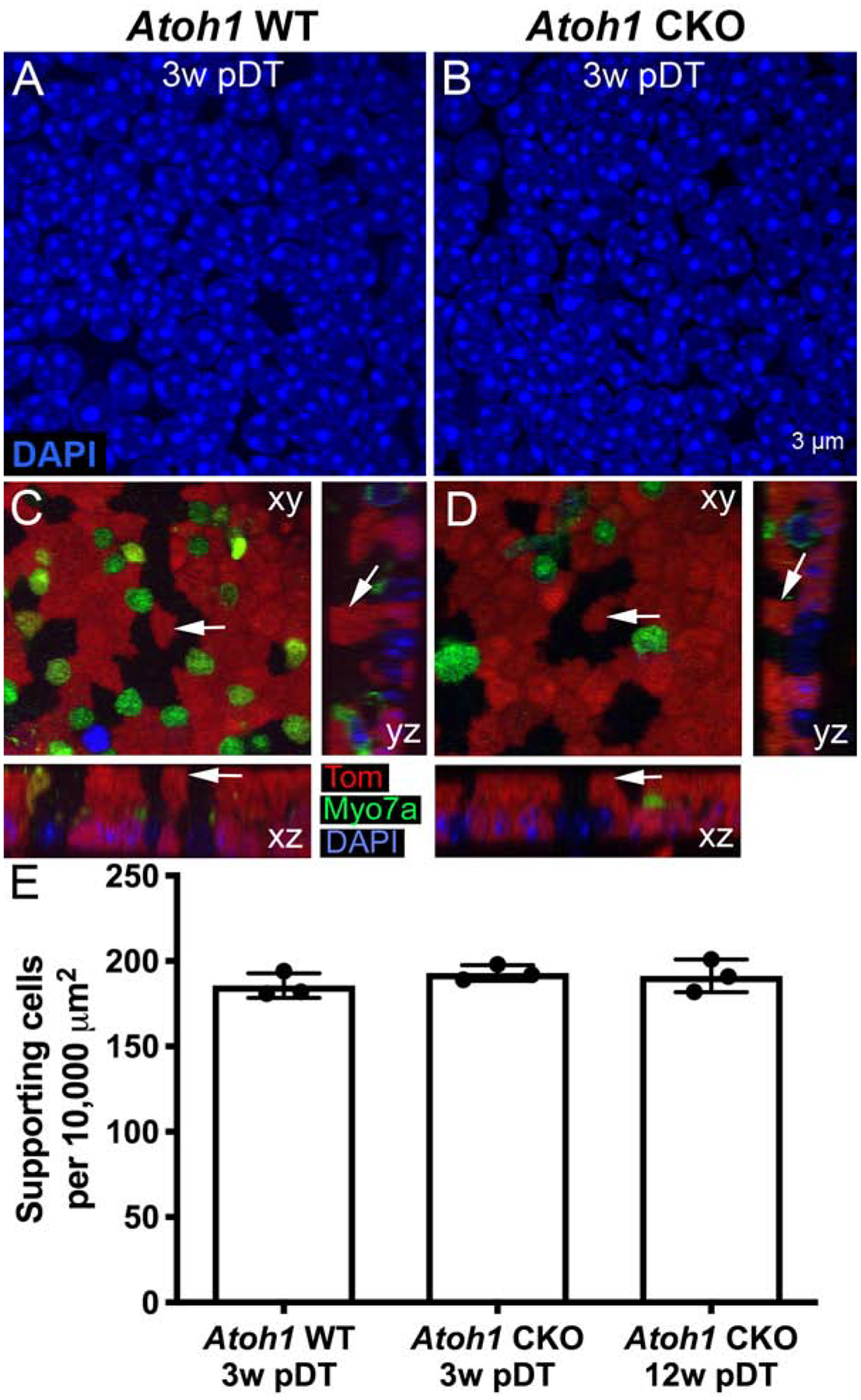
Figure 4. Deletion of Atoh1 from supporting cells does not appear to alter supporting cell nuclear shape or number.
A,B. Representative high-magnification horizontal (xy) confocal slices of the supporting cell nuclear layer from whole-mount utricles from an Atoh1 WT mouse (A) and an Atoh1 CKO mouse (B) at 3 weeks post-DT (pDT) that were labeled with DAPI (blue). C,D. Representative images of the utricular sensory epithelium, showing a top-down view (xy) and two orthogonal slices (yz and xz), to illustrate the shape of supporting cells from Atoh1 WT (C) and Atoh1 CKO (D) mice. The arrows in C and D point to the same cell in each type of view. In the xz slices, supporting cell nuclei are toward the bottom, and in the yz slices, supporting cell nuclei are toward the right. E. Graph showing the number of utricular supporting cell nuclei per 10,000 μm2 from Atoh1 WT and Atoh1 CKO mice. Error bars represent +1 standard deviation. N = 3 mice per group. Scale bar in B applies to A and B.
We also detected Atoh1-GFP-positive hair cells (not shown) at all times post-DT in both undamaged and damaged utricles, as has been described previously for normal and regenerating utricles from adult mice (Bucks et al., 2017; Golub et al., 2012; Lin et al., 2011; Wang et al., 2010).
This expression analysis confirmed that hundreds of supporting cells accumulate Atoh1 protein by 7 days after the start of hair cell death and that higher-than-normal numbers of supporting cells express Atoh1 for at least 2 weeks after damage. These findings suggest that Atoh1 is upregulated in supporting cells before they convert into regenerated type II hair cells.
3.2. Deletion of Atoh1 from utricular supporting cells before hair cell damage prevents regeneration of type II hair cells
To test if Atoh1 expression in supporting cells is required for regeneration of type II hair cells, we used Sox9-CreERT2 mice, which have CreER activity in most supporting cells (~87%) and a small proportion of hair cells (~4%) when tamoxifen is injected at 6 weeks of age (Stone et al., 2018). We crossed DTR:Sox9-CreERT2:Rosa26tdTomato mice with Atoh1loxP/loxP mice, in which the coding region for Atoh1 is flanked by loxP sites (Shroyer et al., 2007). To achieve tdTomato expression and conditional knock-out (CKO) of Atoh1 in supporting cells prior to hair cell damage, we injected experimental mice (DTR:Sox9-CreERT2:Rosa26tdTomato:Atoh1loxP/loxP, hereafter called Atoh1 CKO) with tamoxifen (9 mg/40 g on two consecutive days) at 6 weeks of age. Mice were injected with DT one week later (at 7 weeks of age) to kill hair cells. At 3 and 12 weeks post-DT, mice were euthanized, and utricles were labeled with antibodies to myosin VIIa or with type II-selective markers [the calcium binding protein calretinin (Desai et al., 2005; Pujol et al., 2014) and the transcription factor Sox2 (Oesterle et al., 2008)], to determine if type II hair cell regeneration had occurred. Controls consisted of DTR:Sox9-CreERT2:Rosa26tdTomato:Atoh1+/+ mice (Atoh1 WT, in which the Atoh1 locus is not altered, yet supporting cells still express tdTomato after tamoxifen injection) that were treated exactly the same was as Atoh1 CKO mice.
At 3 weeks post-DT, there was considerable loss of hair cells in both control and experimental mice (compare Fig. 2A,A’ with Fig. 2D,D’ and Fig. 1A). However, hair cells were more abundant in Atoh1 WT mice (Fig. 2A,A’) than in Atoh1 CKO mice (Fig. 1D, D’). Closer inspection of Atoh1 WT mice demonstrated that hundreds of hair cells per utricle were tdTomato-positive (Fig. 2B,B’,F, Table 1), indicating they were derived from supporting cells. All tdTomato-positive hair cells were type II-like; they had heavy myosin VIIa labeling, at least one basolateral process, and were labeled with antibodies to calretinin and Sox2 (Fig. 2C–C””,F). By contrast, Atoh1 CKO mice at 3 weeks post-DT had very few tdTomato-labeled type II hair cells (Fig. 2E,E’,F, Table 1).
Figure 2. Atoh1 is required in supporting cells for regeneration of type II hair cells in the adult utricle.
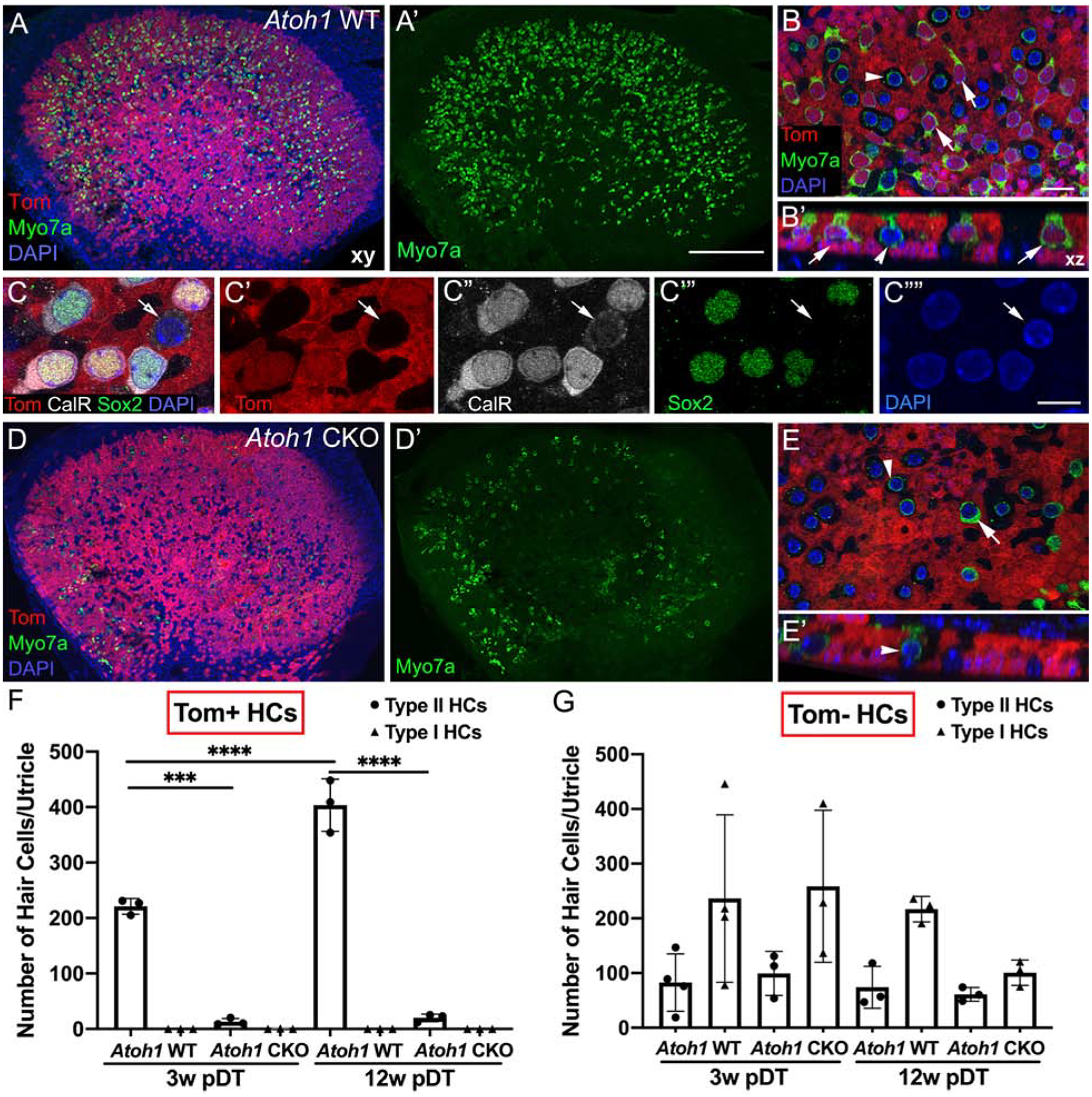
A. Representative low-magnification horizontal (xy) confocal slice of a whole-mount utricle from an Atoh1 WT mouse at 3 weeks post-DT (pDT) and labeled for myosin VIIa (Myo7a, green), tdTomato (red), and DAPI (blue). A’. Same field as A, showing Myo7a labeling only. B. High-magnification xy view of the lateral extrastriolar region of the sensory epithelium from an Atoh1 WT utricle. B’. Optical cross-section from a region taken from B. Arrows point to tdTomato-positive type II hair cells and the arrowhead points to a tdTomato-negative type I hair cell. C-C””. Representative high-magnification xy images of several regenerated type II hair cells, labeled with antibodies to calretinin (CalR, white, labeled nucleus and cytoplasm), Sox2 (green, labeled nucleus), tdTomato (Tom, red), and DAPI (blue, labeled nuclei). Panels C’-C”” show each label individually. Arrows point to the only type I-like hair cell (tdTomato-negative) in the field. D. Representative low-magnification xy confocal slice of a whole-mount utricle from an Atoh1 CKO mouse at 3 weeks pDT and labeled for Myo7a (green), tdTomato (red), and DAPI (blue). D’. Same field as D, showing Myo7a labeling only. E. Representative high-magnification xy view of the lateral extrastriolar region of the sensory epithelium from an Atoh1 CKO utricle. E’. Optical cross-section from a region taken from E. The arrowhead points to a tdTomato-negative type I hair cell, and the arrow points to a tdTomato-negative type II hair cell. F,G. Graphs showing the average number of type I and type II hair cells per utricle that are either tdTomato-positive (Tom+, F) or tdTomato-negative (Tom-, G). Arrows point to groups with average values of 0. Horizontal lines over bars and asterisks indicate significant difference from control (one-way ANOVA with Tukey’s multicomparisons test). Error bars represent +1 standard deviation. N = 3–4 mice per group. Scale bar in A’ = 150 μm and applies to A,A’D,D’. Scale bar in C”” = 6 μm and applies to C-C””. Scale bar in B = 12 μm and applies to B,B’,E,E’.
Atoh1 CKO mice at 3 weeks and at 12 weeks post-DT had significantly fewer tdTomato-labeled type II hair cells than Atoh1 WT mice, indicating that type II hair cell regeneration was likely not delayed, but rather not initiated. Statistical analysis (two-way ANOVA) identified a significant effect of both time post-DT (p =0.0001) and genotype (where Atoh1 WT differed significantly from Atoh1 CKO at both survival times, but there was no significant difference between Atoh1 CKO at 3 weeks or 12 weeks post-DT (Tukey’s multiple comparisons post-hoc test; see Table 1). These findings demonstrate that supporting cells lacking Atoh1 fail to regenerate utricular type II hair cells, even as late as 12 weeks post-DT.
No tdTomato-positive type I hair cells were seen in Atoh1 WT mice at 3 weeks post-DT or in Atoh1 CKO mice at 3 or 12 weeks post-DT (Fig. 2F; Table 1), indicating that Sox9CreER-expressing supporting cells did not regenerate type I hair cells in either condition.
Unlabeled type I and II hair cells (tdTomato-negative) were observed in all utricles examined (Fig. 2B,E,G). Statistical analysis (two-way ANOVA) indicated that numbers of these cells were not significantly different across control and experimental groups (Table 1). It is unlikely that these hair cells were regenerated from the ~13% supporting cells that did not express Sox9-CreERT2, because their numbers did not increase over time. Thus, these unlabeled hair cells likely survived DT treatment since ~6% of vestibular hair cells have been shown to survive DT treatment in DTR mice (Golub et al., 2012).
3.3. Deletion of Atoh1 from supporting cells also prevents hair cell regeneration in saccules and lateral ampullae
Following the same experimental design as described for utricles, we tested the requirement for Atoh1 in supporting cells for hair cell regeneration in two other vestibular organs, the saccule and lateral ampulla (see schematic drawings of the organs at the top of Fig. 3). Tamoxifen given at 6 weeks of age induced tdTomato labeling in ~73% of supporting cells in both organs (Fig. 3A–D). Three weeks after hair cell damage, tdTomato-labeled type II hair cells (regenerated from supporting cells) were common in the saccule (Fig. 3A,A’,E, Table 1) and the lateral ampulla (Fig. 3C, G, Table 1) of Atoh1 WT mice, but they were rarely seen in these organs in Atoh1 CKO mice (Fig. 3B,B’,D,E,G, Table 1). Statistical analysis (two-tailed unpaired Student’s t-test) indicated significant differences in numbers of tdTomato-labeled labeled type II hair cells in Atoh1 WT and Atoh1 CKO mice in saccules (p=0.0181) and lateral ampullae (p=0.0009). There were no statistically significant differences between tdTomato-negative type I and type II hair cells in either organ (Table 1). These findings demonstrate that supporting cells lacking Atoh1 fail to regenerate saccular or ampullary type II hair cells.
Figure 3. Atoh1 is required in supporting cells for regeneration of type II hair cells in the adult saccule and lateral ampulla.

The schematic drawings at the top of the figure depict top-down views of the saccule and the horizontal ampulla, with their different anatomical zones indicated. A,B. Representative high-magnification horizontal (xy) confocal slices of whole-mount saccules from an Atoh1 WT mouse (A) and an Atoh1 CKO mouse (B) at 3 weeks post-DT (pDT) and labeled for myosin VIIa (myo7a, green), tdTomato (red), and DAPI (blue). Arrows point to tdTomato-positive type II hair cells and the arrowhead points to a tdTomato-negative type I hair cell. A’,B’. Optical cross-section from A and B taken at the levels indicated by the yellow arrowhead (bottom left side of A). C,D. Representative high-magnification horizontal (xy) confocal slices of whole-mount lateral cristae from an Atoh1 WT mouse (C) and an Atoh1 CKO mouse (D) at 3 weeks pDT and labeled the same as A,B. Arrows point to tdTomato-labeled type II hair cells, and arrowhead points to a tdTomato-negative type I hair cell. E-H. Each graph shows the numbers of tdTomato-positive (Tom+, E, G) or tdTomato-negative (Tom-, F, H) type I and type II hair cells (HCs) per 10,000 μm2 in the saccules or lateral ampullae from Atoh1 WT and CKO mice at 3 weeks pDT. Arrows point to groups with average values of 0. Horizontal lines over bars indicate significant difference between groups (p ≤ 0.05). Error bars represent +1 standard deviation. N = 3 mice per group. Scale bar in B applies to all images.
3.4. Deletion of Atoh1 from supporting cells does not appear to alter their appearance or survival
A prior study showed that supporting cells do not develop normally when Atoh1 is deleted in the germline (Woods et al., 2004). However, this study did not address whether Atoh1 is required in supporting cells after embryonic development for their maintenance or survival, or if failure to upregulate Atoh1 when hair cells die in adulthood causes supporting cells to undergo apoptosis. We analyzed supporting cells in utricles, saccules, and lateral ampullae at 3 and 12 weeks after DT-mediated hair cell destruction using the same samples in which we quantified hair cells. DAPI labeling suggested that supporting cells in Atoh1 CKO mice maintained their normal nuclear size relative to age-matched Atoh1 WT mice (Fig. 4A,B). Comparison of individual tdTomato-labeled supporting cells in orthogonal views of confocal image stacks indicated that supporting cells in Atoh1 CKO mice had columnar shapes, similar to Atoh1 WT mice (Fig. 4C,D). Quantitative analysis determined that there was no detectable difference in the density of utricular supporting cell nuclei at either 3 or 12 weeks post-DT (Fig. 4E, Table 2).
TABLE 2.
Utricular supporting cell density (mean number of cells per 10,000 μm^2 ±SD)
| Group | Density | N (mice) |
|---|---|---|
| WT 3w | 185 ±7 | 3 |
| CKO 3w | 189 ±2 | 3 |
| CKO 12w | 191 ±9 | 3 |
| 1-way ANOVA p value = 0.4872 |
4. Discussion
Prior morphological studies indicate that adult mammals regenerate some type II hair cells, but no type I hair cells (Bucks et al., 2017; Forge et al., 1998, 1993; Golub et al., 2012; Kawamoto et al., 2009) and only ~40% of all type II hair cells are replaced (Stone et al., 2015). The factors that enable this spontaneous regeneration of vestibular hair cells in adult mammals are not well understood. Here, we tested if the transcription factor Atoh1 is necessary for type II hair cell regeneration in adult mice. We found that Atoh1 expression was increased in supporting cells, the hair cell progenitors, in utricles shortly after hair cell destruction and was reduced a week or so later. Deletion of Atoh1 from supporting cells prior to hair cell damage prevented most supporting cells from transdifferentiating into hair cells in utricles, saccules, and horizontal ampullae. This study demonstrates that Atoh1 is required for type II vestibular hair cell regeneration in adult mice.
4.1. Atoh1 is upregulated in supporting cells throughout the utricle within 7 days after hair cell destruction
Using DTR:Atoh1GFP/GFP reporter mice, we determined that the number of GFP-positive supporting cells was increased over normal levels by 7 days after DT-induced hair cell destruction in vivo. This knock-in mouse line reports Atoh1 protein levels, since Atoh1 and GFP form a fusion protein (Rose et al., 2009). Atoh1 remained elevated in supporting cells at 14 days post-DT and returned to normal levels by 28 days post-DT. Only a subpopulation of supporting cells had elevated Atoh1, and these cells were scattered throughout the utricle. These findings are consistent with prior studies that examined Atoh1 expression in adult mouse utricles after hair cell loss using other methods, including Atoh1 immunolabeling or virally-delivered Atoh1 reporters (Golub et al., 2012; Lin et al., 2011; Wang et al., 2010). The observation that only a subpopulation of vestibular supporting cells accumulate Atoh1 after near-complete hair cell loss may be one reason why only ~40% of type II hair cells are regenerated in adult mice. Notch signaling attenuates Atoh1 accumulation in supporting cells after hair cell damage in mouse utricles (Lin et al., 2011) and in chicken basilar papillae (Lewis et al., 2012). BMP4 also inhibits Atoh1 expression in the chicken basilar papilla (Lewis et al., 2012). Future studies should determine if inhibition of Notch and/or BMP4 signaling can increase the number of regenerated hair cells in adult mouse vestibular epithelia.
4.2. Fate-mapping shows that supporting cells transdifferentiate into type II hair cells, but not type I hair cells after damage
To assess hair cell regeneration, we used Sox9-CreERT2 mice to label ~86% of supporting cells with the fluorescent reporter tdTomato prior to hair cell destruction for fate-mapping. Numerous type II hair cells were tdTomato-positive at 3 weeks after damage, indicating they were derived from supporting cells. However, no labeled type I hair cells were seen. This finding is consistent with prior studies in mature rodents showing morphological evidence for type II, but not type I hair cell regeneration. It is possible, however, that the ~14% of unlabeled supporting cells regenerated some type I hair cells. Yet, additional findings speak against this. Although we found a significant increase in tdTomato-positive type II hair cells between 3 and 12 weeks post-damage, numbers of tdTomato-negative type I hair cells did not change. Thus, it is unlikely that any supporting cells transdifferentiated into type I hair cells during this period. It is unclear why significant numbers of type I hair cells are not replaced in adult mice. However, recent studies showed that only type II hair cells are added to mouse utricles during postnatal development (McInturff et al., 2018; Warchol et al., 2019) and in maturity (Bucks et al., 2017). Therefore, it is possible that the factors needed to drive a type I hair cell fate are not maintained after birth. For example, the downstream targets of Atoh1 needed to drive a type I fate may be epigenetically repressed in adult mouse supporting cells or in hair cell precursors.
4.3. Atoh1 is required for type II hair cell regeneration in adult mice
Atoh1 is necessary for the differentiation and survival of hair cells during development (Bermingham, 1999; Cai et al., 2013; Chen et al., 2002; Chonko et al., 2013; Maass et al., 2013; Pan et al., 2012; Woods et al., 2004). Furthermore, Atoh1 can reprogram non-sensory cells and supporting cells to acquire hair cell-like features (Gao et al., 2016; Gubbels et al., 2008; Izumikawa et al., 2005; Kawamoto et al., 2003; Kelly et al., 2012; Kuo et al., 2015; Liu et al., 2012; Shou et al., 2003; Walters et al., 2017; Woods et al., 2004; Zheng and Gao, 2000). Yet, prior to this study, Atoh1’s requirement for hair cell regeneration had not been tested directly.
We deleted Atoh1 from supporting cells one week before hair cells were killed and used fate-mapping to assess regenerated hair cells. Very little hair cell regeneration occurred in Atoh1 CKO mice, demonstrating that this transcription factor is required for the majority of supporting cells to convert into type II hair cells after damage. However, we detected a small number (10–20) tdTomato-positive type II hair cells per utricle in Atoh1 CKO mice that could be interpreted as evidence that a few hair cells are formed from supporting cells in the absence of Atoh1. In support of this interpretation, an Atoh1-independent pathway for hair cell formation has previously been suggested to occur (Ahmed et al., 2012; Du et al., 2007). However, this phenomenon is poorly understood, and there are other explanations for this finding. For instance, our analysis from Atoh1GFP/GFP mice revealed that each undamaged utricle had, on average, ~18 Atoh1-expressing supporting cells that likely reflect the fact that small numbers of supporting cells form new hair cells in adult mouse utricles under normal physiological conditions (Bucks et al., 2017). Since some type II regenerated hair cells observed in Atoh1 CKO mice expressed tdTomato, they must have also expressed Sox9-CreER and undergone Cre-mediated recombination after tamoxifen injection. However, it is possible that the CreER enzyme failed to delete both copies of Atoh1 in these supporting cells, which would have enabled them to maintain one copy of Atoh1 and to complete the conversion into hair cells. Alternatively, CreER may have deleted both Atoh1 copies in these Atoh1-expressing supporting cells after they had experienced sufficient Atoh1 activity to push them toward the hair cell fate. In that event, they would have continued to differentiate into hair cells, despite Atoh1 deletion and could have escaped DT toxicity if they were immature at the point of DT treatment and had not yet expressed Pou4f3.
Another possible factor that contributed to the presence of some tdTomato-positive hair cells in Atoh1 CKO mice after damage is that some original hair cells may have survived the DT treatment. In a prior study (Stone et al., 2018), we found that 138 hair cells per utricle were tdTomato-positive in adult Sox9-CreERT2:Rosa26tdTomato mice at one week after tamoxifen induction. Although the dose of DT we used kills ~94% of utricular hair cells (Golub et al., 2012), it is possible that some of the original tdTomato-positive hair cells were not killed by DT.
Deletion of Atoh1 from supporting cells prior to hair cell damage prevented them from acquiring overt hair cell properties, including an apically located nucleus, basolateral processes, and expression of the hair cell-specific proteins myosin VIIa and calretinin. Furthermore, in the absence of Atoh1, tdTomato-labeled supporting cells showed no change in cell density or general appearance at 3 or 12 weeks post-DT. Because we only examined a few phenotypic aspects, it is unclear whether supporting cells lacking Atoh1 changed their baseline state after damage. Nonetheless, these findings indicate that supporting cells do not proliferate, degenerate, or die upon hair cell death when they lack Atoh1.
5. Summary
In this study, we characterized the temporal and spatial expression of Atoh1 in the utricular supporting cells of adult mice after hair cell destruction. In the utricle, saccule, and horizontal ampulla, we found that some supporting cells transdifferentiate into type II hair cells, but no new type I hair cells are formed. We showed for the first time that deletion of Atoh1 from vestibular supporting cells prior to damage prevents them from producing new hair cells. Additional studies are needed to understand why only some supporting cells upregulate Atoh1 and convert into hair cells after damage and to identify ways to promote regeneration of type I hair cells in adult mammals.
Highlights.
Expression of Atoh1 is increased in vestibular supporting cells of adult mice after hair cell destruction.
Fate-mapping of supporting cells shows they regenerate type II hair cells but not type I hair cells.
Deletion of Atoh1 from vestibular supporting cells before damage prevents hair cell regeneration.
These findings illustrate that, like development, Atoh1 is required for regeneration of type II vestibular hair cells.
Acknowledgments
We are grateful to Irina Omelchenko, Jialin Shang, Tot Nguyen, Sharada KC, Sophie Seo, Glen MacDonald, and Caitlyn Trullinger-Dwyer for technical assistance at the University of Washington. We are also thankful to Kaley Graves for her help at Southern Illinois University School of Medicine. This work was supported by NIH grant R01DC014441 (BCC), Office of the Assistant Secretary of Defense for Health Affairs Grant W81XWH-15-1-0475 (BCC), R01DC013771 (JSS), and T32DC000018 (KH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
Brandon Cox is a consultant for Turner Scientific, LLC and Otonomy, Inc. Jennifer Stone is serving or has served as a consultant for Decibel Therapeutics and Otonomy, Inc.
References
- Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB, 2009. Disorders of Balance and Vestibular Function in US Adults. Arch. Intern. Med 169, 938 10.1001/archinternmed.2009.66 [DOI] [PubMed] [Google Scholar]
- Ahmed M, Wong EYM, Sun J, Xu J, Wang F, Xu PX, 2012. Eya1-Six1 Interaction Is Sufficient to Induce Hair Cell Fate in the Cochlea by Activating Atoh1 Expression in Cooperation with Sox2. Dev. Cell 22, 377–390. 10.1016/j.devcel.2011.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson PJ, Dong Y, Gu S, Liu W, Najarro EH, Udagawa T, Cheng AG, 2018. Sox2 haploinsufficiency primes regeneration and Wnt responsiveness in the mouse cochlea. J. Clin. Invest 128, 1641–1656. 10.1172/JCI97248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird RA, Steyger PS, Schuff NR, 1996. Mitotic and Nonmitotic hair cell regeneration in the bullforg vestibular otolith organs. Ann N Y Acad SCI 781, 59–70. 10.1111/j.1365-2990.2007.00884.x [DOI] [PubMed] [Google Scholar]
- Bermingham NA, 1999. Math1: An Essential Gene for the Generation of Inner Ear Hair Cells. Science 284, 1837–1841. 10.1126/science.284.5421.1837 [DOI] [PubMed] [Google Scholar]
- Bucks SA, Cox BC, Vlosich BA, Manning JP, Nguyen TB, Stone JS, 2017. Supporting cells remove and replace sensory receptor hair cells in a balance organ of adult mice. Elife 6, e18128 10.7554/eLife.18128.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JC, Stone JS, 2017. Development and regeneration of vestibular hair cells in mammals. Semin. Cell Dev. Biol 65, 96–105. 10.1016/j.semcdb.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafaro J, Lee GS, Stone JB, 2007. Atoh1 expression defines activated progenitors and differentiating hair cells during avian hair cell regeneration. Dev. Dyn 236, 156–170. 10.1002/dvdy.21023 [DOI] [PubMed] [Google Scholar]
- Cai T, Jen H-I, Kang H, Klisch TJ, Zoghbi HY, Groves AK, 2015. Characterization of the transcriptome of nascent hair cells and identification of direct targets of the Atoh1 transcription factor. J. Neurosci 35, 5870–83. 10.1523/JNEUROSCI.5083-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai T, Seymour ML, Zhang H, Pereira FA, Groves AK, 2013. Conditional deletion of Atoh1 reveals distinct critical periods for survival and function of hair cells in the organ of Corti. J. Neurosci 33, 10110–22. 10.1523/JNEUROSCI.5606-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Johnson JE, Zoghbi HY, Segil N, 2002. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development 129, 2495–2505. 10.1007/s00267-008-9138-y [DOI] [PubMed] [Google Scholar]
- Chonko KT, Jahan I, Stone J, Wright MC, Fujiyama T, Hoshino M, Fritzsch B, Maricich SM, 2013. Atoh1 directs hair cell differentiation and survival in the late embryonic mouse inner ear. Dev. Biol 381, 401–410. 10.1016/j.ydbio.2013.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotanche DA, Kaiser CL, 2010. Hair cell fate decisions in cochlear development and regeneration. Hear. Res 266, 18–25. 10.1016/j.heares.2010.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai SS, Zeh C, Lysakowski A, 2005. Comparative Morphology of Rodent Vestibular Periphery. I. Saccular and Utricular Maculae. J. Neurophysiol 93, 251–266. 10.1152/jn.00746.2003 [DOI] [PubMed] [Google Scholar]
- Driver EC, Sillers L, Coate TM, Rose MF, Kelley MW, 2013. The Atoh1-lineage gives rise to hair cells and supporting cells within the mammalian cochlea. Dev. Biol 376, 86–98. 10.1016/j.ydbio.2013.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Jensen P, Goldowitz D, Hamre KM, 2007. Wild-type cells rescue genotypically Math1-null hair cells in the inner ears of chimeric mice. Dev. Biol 305, 430–8. 10.1016/j.ydbio.2007.02.028 [DOI] [PubMed] [Google Scholar]
- Eatock RA, 2018. Specializations for Fast Signaling in the Amniote Vestibular Inner Ear. Integr. Comp. Biol 58, 341–350. 10.1093/icb/icy069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatock RA, Songer JE, 2011. Vestibular Hair Cells and Afferents: Two Channels for Head Motion Signals. Annu. Rev. Neurosci 34, 501–534. 10.1146/annurev-neuro-061010-113710 [DOI] [PubMed] [Google Scholar]
- Forge A, Li L, Corwin J, Nevill G, 1993. Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science 259, 1616–1619. 10.1126/science.8456284 [DOI] [PubMed] [Google Scholar]
- Forge A, Li L, Nevill G, 1998. Hair cell recovery in the vestibular sensory epithelia of mature guinea pigs. J. Comp. Neurol 397, 69–88. [DOI] [PubMed] [Google Scholar]
- Gao Z, Kelly MC, Yu D, Wu H, Lin X, Chi F, Chen P, 2016. Spatial and Age-Dependent Hair Cell Generation in the Postnatal Mammalian Utricle. Mol. Neurobiol 53, 1601–1612. 10.1007/s12035-015-9119-0 [DOI] [PubMed] [Google Scholar]
- Gleeson M, Felix H, 1987. A comparative study of the effect of age on the human cochlear and vestibular neuroepithelia. Acta Otolaryngol. Suppl 436, 103–9. [DOI] [PubMed] [Google Scholar]
- Golub J, Tong L, Ngyuen T, Hume C, Palmiter RD, Rubel EW, Stone JS, 2012. Hair cell replacement in adult mouse utricles after targeted ablation of hair cells with diphtheria toxin. J. Neurosci 32, 15093–15105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbels SP, Woessner DW, Mitchell JC, Ricci AJ, Brigande JV, 2008. Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature 455, 537–541. 10.1038/nature07265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinand N, Boselie F, Guyot J-P, Kingma H, 2012. Quality of Life of Patients with Bilateral Vestibulopathy. Ann. Otol. Rhinol. Laryngol 121, 471–477. 10.1177/000348941212100708 [DOI] [PubMed] [Google Scholar]
- Herdman SJ, Blatt P, Schubert MC, Tusa RJ, 2000. Falls in patients with vestibular deficits. Am. J. Otol 21, 847–51. [PubMed] [Google Scholar]
- Hilber P, Cendelin J, Le Gall A, Machado M-L, Tuma J, Besnard S, 2019. Cooperation of the vestibular and cerebellar networks in anxiety disorders and depression. Prog. Neuro-Psychopharmacology Biol. Psychiatry 89, 310–321. 10.1016/j.pnpbp.2018.10.004 [DOI] [PubMed] [Google Scholar]
- Itoh M, Chitnis AB, 2001. Expression of proneural and neurogenic genes in the zebrafish lateral line primordium correlates with selection of hair cell fate in neuromasts. Mech. Dev 102, 263–6. [DOI] [PubMed] [Google Scholar]
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y, 2005. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat. Med 11, 271–276. 10.1038/nm1193 [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Ishimoto S, Minoda R, 2003. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J. Neurosci 23, 4395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto K, Izumikawa M, Beyer LA, Atkin GM, Raphael Y, 2009. Spontaneous hair cell regeneration in the mouse utricle following gentamicin ototoxicity. Hear. Res 247, 17–26. 10.1016/j.heares.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M, Chang Q, Pan A, Lin X, 2012. Atoh1 directs the formation of sensory mosaics and induces cell proliferation in the postnatal mammalian cochlea in vivo. J. Neurosci 32, 6699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp JL, Dubois CL, Schaffer AE, Hao E, Shih HP, Seymour PA, Ma J, Sander M, 2011. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development 138, 653–665. 10.1242/dev.056499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo BR, Baldwin EM, Layman WS, Taketo MM, Zuo J, 2015. In Vivo Cochlear Hair Cell Generation and Survival by Coactivation of Beta-Catenin and Atoh1. J Neurosci 35, 10786–98. 10.1523/JNEUROSCI.0967-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanford PJ, Shailam R, Norton CR, Gridley T, Kelley MW, 2000. Expression of Math1 and HES5 in the cochleae of wildtype and Jag2 mutant mice. J. Assoc. Res. Otolaryngol 1, 161–171. 10.1007/s101620010023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RM, Hume CR, Stone JS, 2012. Atoh1 expression and function during auditory hair cell regeneration in post-hatch chickens. Hear. Res 289, 74–85. 10.1016/j.heares.2012.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin V, Golub JS, Nguyen TB, Hume CR, Oesterle EC, Stone JS, 2011. Inhibition Of Notch Activity Promotes Nonmitotic Regeneration of Hair Cells in the Adult Mouse Utricles. J. Neurosci 31, 15329–15339. 10.1523/JNEUROSCI.2057-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Dearman JA, Cox BC, Walters BJ, Zhang L, Ayrault O, Zindy F, Gan L, Roussel MF, Zuo J, 2012. Age-Dependent In Vivo Conversion of Mouse Cochlear Pillar and Deiters’ Cells to Immature Hair Cells by Atoh1 Ectopic Expression. J. Neurosci 32, 6600–6610. 10.1523/JNEUROSCI.0818-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma EY, Rubel EW, Raible DW, 2008. Notch Signaling Regulates the Extent of Hair Cell Regeneration in the Zebrafish Lateral Line. J. Neurosci 28, 2261–2273. 10.1523/JNEUROSCI.4372-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass JC, Berndt FA, Cánovas J, Kukuljan M, 2013. P27Kip1 knockdown induces proliferation in the organ of Corti in culture after efficient shRNA lentiviral transduction. J. Assoc. Res. Otolaryngol 14, 495–508. 10.1007/s10162-013-0383-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H, 2010. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci 13, 133–40. 10.1038/nn.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matei V, Pauley S, Kaing S, Rowitch D, Beisel KW, Morris K, Feng F, Jones K, Lee J, Fritzsch B, 2005. Smaller inner ear sensory epithelia in Neurog1 null mice are related to earlier hair cell cycle exit. Dev. Dyn 234, 633–650. 10.1002/dvdy.20551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall AA, Miller DM, Yates BJ, 2017. Descending Influences on Vestibulospinal and Vestibulosympathetic Reflexes. Front. Neurol 8, 112 10.3389/fneur.2017.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInturff S, Burns JC, Kelley MW, 2018. Characterization of spatial and temporal development of Type I and Type II hair cells in the mouse utricle using new cell-type-specific markers. Biol. Open 7, bio038083 10.1242/bio.038083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millimaki BB, Sweet EM, Dhason MS, Riley BB, 2007. Zebrafish atoh1 genes: classic proneural activity in the inner ear and regulation by Fgf and Notch. Development 134, 295–305. 10.1242/dev.02734 [DOI] [PubMed] [Google Scholar]
- Oesterle EC, Campbell S, Taylor RR, Forge A, Hume CR, 2008. Sox2 and Jagged1 expression in normal and drug-damaged adult mouse inner ear. J. Assoc. Res. Otolaryngol 9, 65–89. 10.1007/s10162-007-0106-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan N, Jahan I, Kersigo J, Duncan JS, Kopecky B, Fritzsch B, 2012. A novel Atoh1 “self-terminating” mouse model reveals the necessity of proper Atoh1 level and duration for hair cell differentiation and viability. PLoS One 7, e30358 10.1371/journal.pone.0030358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol R, Pickett SB, Nguyen TB, Stone JS, 2014. Large basolateral processes on type II hair cells are novel processing units in mammalian vestibular organs. J. Comp. Neurol 522, 3141–3159. 10.1002/cne.23625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SD, Velazquez-Villasenor L, Dimitri PS, Merchant SN, 2006. Decreasing Hair Cell Counts in Aging Humans. Ann. N. Y. Acad. Sci 942, 220–227. 10.1111/j.1749-6632.2001.tb03748.x [DOI] [PubMed] [Google Scholar]
- Roberson DW, Alosi JA, Cotanche DA, 2004. Direct transdifferentiation gives rise to the earliest new hair cells in regenerating avian auditory epithelium. J. Neurosci. Res 78, 461–471. 10.1002/jnr.20271 [DOI] [PubMed] [Google Scholar]
- Rose MF, Ren J, Ahmad KA, Chao H-T, Klisch TJ, Flora A, Greer JJ, Zoghbi HY, 2009. Math1 Is Essential for the Development of Hindbrain Neurons Critical for Perinatal Breathing. Neuron 64, 341–354. 10.1016/j.neuron.2009.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenhall U, Rubin W, 1975. Degenerative Changes in the Human Vestibular Sensory Epithelia. Acta Otolaryngol. 79, 67–80. 10.3109/00016487509124657 [DOI] [PubMed] [Google Scholar]
- Sedó-Cabezón L, Boadas-Vaello P, Soler-Martín C, Llorens J, 2014. Vestibular damage in chronic ototoxicity: A mini-review. Neurotoxicology 43, 21–27. 10.1016/j.neuro.2013.11.009 [DOI] [PubMed] [Google Scholar]
- Shailam R, Lanford PJ, Dolinsky CM, Norton CR, Gridley T, Kelley MW, 1999. Expression of proneural and neurogenic genes in the embryonic mammalian vestibular system. J. Neurocytol 28, 809–19. [DOI] [PubMed] [Google Scholar]
- Shang J, Cafaro J, Nehmer R, Stone J, 2010. Supporting cell division is not required for regeneration of auditory hair cells after ototoxic injury in vitro. J. Assoc. Res. Otolaryngol 11, 203–222. 10.1007/s10162-009-0206-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou J, Zheng JL, Gao WQ, 2003. Robust generation of new hair cells in the mature mammalian inner ear by adenoviral expression of Hath1. Mol. Cell. Neurosci 23, 169–79. [DOI] [PubMed] [Google Scholar]
- Shroyer NF, Helmrath MA, Wang VY-C, Antalffy B, Henning SJ, Zoghbi HY, 2007. Intestine-Specific Ablation of Mouse atonal homolog 1 (Math1) Reveals a Role in Cellular Homeostasis. Gastroenterology 132, 2478–2488. 10.1053/j.gastro.2007.03.047 [DOI] [PubMed] [Google Scholar]
- Slowik AD, Bermingham-Mcdonogh O, 2013. Hair cell generation by notch inhibition in the adult mammalian cristae. J. Assoc. Res. Otolaryngol 14, 813–828. 10.1007/s10162-013-0414-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PF, 2017. The vestibular system and cognition. Curr. Opin. Neurol 30, 84–89. 10.1097/WCO.0000000000000403 [DOI] [PubMed] [Google Scholar]
- Stone JS, Phillips J, Nguyen TB, Gantz J, Gamlin C, Trullngger-Dwyer C, Pujol R, Rubel EW, 2015. Morphological and functional analyses of vestibular hair cell loss and replacement in adult Pou4f3DTR mice after treatment with two different doses of diphtheria toxin. Assoc. Res. Otolaryngol. 38th Annu. Midwinter Meet February 21–25, Baltimore, MD. [Google Scholar]
- Stone JS, Wisner SR, Bucks SA, Mellado Lagarde MM, Cox BC, 2018. Characterization of Adult Vestibular Organs in 11 CreER Mouse Lines. J. Assoc. Res. Otolaryngol 19, 381–399. 10.1007/s10162-018-0676-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RR, Filia A, Paredes U, Asai Y, Holt JR, Lovett M, Forge A, 2018. Regenerating hair cells in vestibular sensory epithelia from humans. Elife 7, e34817 10.7554/eLife.34817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RR, Jagger DJ, Saeed SR, Axon P, Donnelly N, Tysome J, Moffatt D, Irving R, Monksfield P, Coulson C, Freeman SR, Lloyd SK, Forge A, 2015. Characterizing human vestibular sensory epithelia for experimental studies: New hair bundles on old tissue and implications for therapeutic interventions in ageing. Neurobiol. Aging 36, 2068–2084. 10.1016/j.neurobiolaging.2015.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L, Strong MK, Kaur T, Juiz JM, Oesterle EC, Hume C, Warchol ME, Palmiter RD, Rubel EW, 2015. Selective Deletion of Cochlear Hair Cells Causes Rapid Age-Dependent Changes in Spiral Ganglion and Cochlear Nucleus Neurons. J. Neurosci 35, 7878–7891. 10.1523/JNEUROSCI.2179-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters BJ, Coak E, Dearman J, Bailey G, Yamashita T, Kuo B, Zuo J, 2017. In Vivo Interplay between p27Kip1, GATA3, ATOH1, and POU4F3 Converts Non-sensory Cells to Hair Cells in Adult Mice. Cell Rep. 19, 307–320. 10.1016/j.celrep.2017.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GP, Chatterjee I, Batts SA, Wong HT, Gong TW, Gong SS, Raphael Y, 2010. Notch signaling and Atoh1 expression during hair cell regeneration in the mouse utricle. Hear. Res 267, 61–70. 10.1016/j.heares.2010.03.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Chai R, Kim GS, Pham N, Jansson L, Nguyen D-H, Kuo B, May L. a., Zuo J, Cunningham LL, Cheng AG, 2015. Lgr5+ cells regenerate hair cells via proliferation and direct transdifferentiation in damaged neonatal mouse utricle. Nat. Commun 6, 6613 10.1038/ncomms7613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warchol ME, Lambert PR, Goldstein BJ, Forge A, Corwin JT, 1993. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science 259, 1619–1622. 10.1126/science.8456285 [DOI] [PubMed] [Google Scholar]
- Warchol ME, Massoodnia R, Pujol R, Cox BC, Stone JS, 2019. Development of hair cell phenotype and calyx nerve terminals in the neonatal mouse utricle. J. Comp. Neurol 527, 1913–1928. 10.1002/cne.24658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods C, Montcouquiol M, Kelley MW, 2004. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat. Neurosci 7, 1310–8. 10.1038/nn1349 [DOI] [PubMed] [Google Scholar]
- Wu J, Li W, Lin C, Chen Y, Cheng C, Sun S, Tang M, Chai R, Li H, 2016. Co-regulation of the Notch and Wnt signaling pathways promotes supporting cell proliferation and hair cell regeneration in mouse utricles. Sci. Rep 6, 29418 10.1038/srep29418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Xie X, Deng M, Chen X, Gan L, 2010. Generation and characterization of Atoh1-Cre knock-in mouse line. Genesis 48, 407–414. 10.1002/dvg.20633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates BJ, Bolton PS, Macefield VG, 2014. Vestibulo-Sympathetic Responses, in: Comprehensive Physiology. John Wiley & Sons, Inc., Hoboken, NJ, USA, pp. 851–887. 10.1002/cphy.c130041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Gao W-Q, 2000. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat. Neurosci 3, 580–586. [DOI] [PubMed] [Google Scholar]


