Abstract
Contingency management (CM) interventions are among the most effective behavioral interventions for smoking. This study assessed the effects of CM and electronic cigarettes (ECs) on smoking reductions and abstinence for durations of 30-36 days. Twelve participants were exposed to Baseline, EC alone, and EC + CM conditions. An internet-based platform was used to monitor smoking via breath carbon monoxide (CO) and deliver CM for smoking abstinence (CO ≤4 ppm). A Bluetooth-enabled EC monitored daily EC puffs. Abstinence rates were equivalent between EC (34.4%) and EC + CM (30.4%) conditions. Both conditions promoted smoking reductions. We observed an inverse correlation between smoking and EC puffs (r = −.62, p < .05). Results suggest the use of electronic cigarettes can promote smoking reductions and abstinence, and CM did not improve these outcomes. Larger magnitude consequences or tailoring EC characteristics (e.g., flavor) may have improved outcomes. Technology-based methods to collect intensive, longitudinal measures of smoking and electronic cigarette use may be useful to characterize their environmental determinants.
Keywords: contingency management, cigarette smoking, e-cigarette, smoking cessation, technology
Contingency management (CM) interventions are among the most effective behavioral interventions for problematic substance use, including cigarette smoking (Lussier, Heil, Mongeon, Badger, & Higgins, 2006; Prendergast, Podus, Finney, Greenwell, & Roll, 2006). CM intervention to reduce cigarette smoking might include consequences with monetary value provided to individuals based on objective evidence of smoking abstinence. Objective evidence of smoking can be obtained through breath carbon monoxide (CO) or cotinine in urine or saliva (cotinine is a metabolite of nicotine; Benowitz & Jacob, 1994). CM has resulted in smoking abstinence in a variety of populations including heavy, rural, pregnant, adolescent, and difficult-to-treat smokers (for reviews see Dallery, Kurti, & Martner, 2015; Ledgerwood, 2008; Sigmon & Patrick, 2012). Recently, remote, internet-based technology has been used to monitor CO and deliver CM (Dallery & Glenn, 2005; Dallery, Glenn, & Raiff, 2007; Dallery et al., 2017; Meredith, Grabinski, & Dallery, 2011; Stoops et al., 2009), which could promote widespread access to the intervention. The present study focused on the effects of internet-based CM and electronic cigarettes (ECs) on cigarette smoking reductions (i.e., reductions in breath CO) and abstinence.
ECs are battery-operated devices that contain a liquid consisting mainly of water, propylene glycol, vegetable glycerin, nicotine, and flavorings. “Drawing” on the device or pressing a button (depending on the model) ignites an atomizer to produce an aerosol, a process commonly referred to as “vaping.” Since they were first introduced in 2004, ECs have gained popularity at an exponential rate (U.S. Department of Health and Human Services, 2016). A large part of this growth is fueled by the belief that ECs help people quit smoking and reduce cravings to smoke (Adkison et a1., 2013; Glasser et al., 2017). Several features of ECs could make them useful smoking cessation aids. ECs can deliver nicotine at doses and rates that approach combustible cigarettes, and although there are some differences in puff topography between ECs and combustible cigarettes, they both involve several obvious similarities (i.e., inhaling, exhaling, hand-to-mouth gestures; Farsalinos, Romagna, Tsiapras, Kyrzopoulos, & Voudris, 2013). These similarities could facilitate substitution of smoking with vaping. Indeed, several studies suggest that ECs may function as a substitute for cigarettes in laboratory settings (Grace, Kivell, & Laugesen, 2015; Johnson, Johnson, Rass, & Pacek, 2017; Pope et al., 2019; Quisenberry, Koffarnus, Epstein, & Bickel, 2017; Quisenberry, Koffarnus, Hatz, Epstein, & Bickel, 2015; Snider, Cummings, & Bickel, 2017; Stein, Koffarnus, Stepanov, Hatsukami, & Bickel, 2018), and a small number of randomized controlled studies suggest that vaping may promote smoking cessation (Adriaens, Van Gucht, Declerck, & Baeyens, 2014; Bullen et al., 2013; Caponnetto et al., 2013). Another reason to favor a transition to ECs for smokers is that ECs contain fewer toxicants and carcinogens than cigarettes (Goniewicz et al., 2014; Shahab et al., 2017). As such, to the extent ECs promote smoking abstinence, and possibly smoking reductions, they may represent a useful harm reduction strategy (Farsalinos, 2018).
EC devices have evolved rapidly, and many of the first-generation ECs used in previous studies are outdated (Lopez & Eissenberg, 2015). First-generation ECs have low-capacity batteries and use prefilled cartridges, whereas newer-generation ECs have larger batteries and a reservoir that is refillable with e-liquid. These devices may differ in their nicotine delivery profiles and ability to reduce withdrawal symptoms (Farsalinos et al., 2015; Lechner et al., 2015; Wagener et al., 2017). The majority of people who use ECs have newer-generation ECs (Dawkins, Turner, Roberts, & Soar, 2013; Farsalinos et al., 2013a), yet only one randomized controlled trial evaluated newer-generation ECs (Adriaens et al., 2014). Adriaens et al. (2014) observed abstinence rates of 34% and 0% for the EC groups and control groups after week 8. After week 8, the control group received an EC. An overall verified abstinence (CO < 5 ppm) rate of 21% was obtained at 8-month followup. Additional research is needed on the potential for newer ECs to promote smoking cessation.
Previous studies examining changes in smoking and vaping have generally relied on self-reported smoking and vaping (e.g., Adkison et al., 2013; Etter & Bullen, 2014; Prochaska & Grana, 2014; Vickerman, Carpenter, Altman, Nash, & Zbikowski, 2013; Vickerman et al., 2016). Even when an objective measure of smoking (e.g., breath CO) was obtained, it was generally at fixed, weekly time points or during follow-up visits (e.g., Adriaens et al., 2014; Caponnetto et al., 2013; Caponnetto, Polosa, Russo, Leotta, & Campagna, 2011; Halpern et al., 2018; Jorenby, Smith, Fiore, & Baker, 2017; Manzoli et al., 2015; Tseng et al., 2016; Wagener et al., 2014). An isolated, single CO sample can only indicate recent smoking abstinence; it cannot capture dynamic changes in smoking over time. Similarly, only a few studies attempted to verify EC use by collecting used e-liquid cartridges (Bullen et al., 2013; Tseng et al., 2016). A more fine-grained temporal resolution of changes in smoking and vaping may reveal important interactions between these behaviors, and enable single-case experimental approaches to evaluate interventions (Dallery & Raiff, 2014; McDonald et al., 2017).
The primary goals of this study were to compare the effects of ECs to a combination of ECs and CM on smoking during the initial weeks of a quit attempt, and to assess interactions between vaping and smoking. We also assessed the social validity of the intervention components via questionnaire at study completion. We measured vaping using a Bluetooth-enabled EC that recorded each puff via ignition button clicks (Dautzenberg & Bricard, 2015; Pearson et al., 2017) and used an internet-based method to obtain measures of CO twice per day and deliver CM (Dallery, Raiff, & Grabinski, 2013). Breath CO is not affected by vaping (Vansickel, Cobb, Weaver, & Eissenberg, 2010). We hypothesized that combining ECs and CM would result in higher rates of abstinence or reduction than ECs alone, and that EC puffs per day would be inversely related to CO levels. To our knowledge, this study is the first to use frequent, objective measures of vaping and smoking in users’ natural environments.
METHOD
Participants
Participants (N = 12) were recruited locally using print (flyers), online media (e.g., Craigslist and Facebook advertisements), and community outreach methods through HealthStreet-Gainesville. Eligible participants met the following criteria: (1) 18-65 years old, (2) smoked ≥2 years, (3) smoked ≥8 cigarettes per day on average, (4) smoked in the past 24 hours, (5) expressed a desire to quit smoking (yes/no), (6) had reliable access to the internet and a computer or smartphone, and (7) breath CO ≥10 ppm at set-up. Exclusion criteria included (1) current or previous medical condition that would pose an increased risk to participation, (2) use of benzodiazepines, cocaine, or opiates in the previous 6 months, (3) smoke marijuana more than two times per month, (4) exposed to elevated CO levels (e.g., spouse smokes in house), and (5) pregnant or expected to become pregnant in the next 6 months. A total of 22 individuals were screened for eligibility. Three were not eligible because they did not have access to a computer or the internet, two were exposed to elevated CO levels, and one had a preexisting medical condition. Four participants withdrew from the study prior to completing baseline (two before any data were collected); we do not report their results.
Participants were not excluded for previous EC use. Three participants (P1, P7, P9) never tried an EC prior to the study. Two participants owned an EC but quit using it more than a month prior to t he study (P8 used a first-generation EC, and P12 used a second-generation EC). The remaining seven participants had tried an EC more than a year prior to the study but never owned one.
Materials and Set-Up
Set-up meetings occurred at the University of Florida Behavioral Health and Technology Research Clinic. Researchers obtained informed consent and assigned participants a randomly generated ID. Participants completed a psychosocial questionnaire on demographics, smoking and other tobacco use, psychological and medical conditions, and nicotine dependence using the Fagerstrom Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991). Researchers provided participants with a copy of the National Cancer Institute’s brochure, Clearing the Air (smokefree.gov). Then, researchers and participants read through a manual that described the study procedures, how to use Mōtiv8 (described below), and a manual for their EC model. Participants completed a short verbal quiz and had time to ask questions about the study procedures. Participants practiced submitting video samples and using Mōtiv8. Participants were also given a CO meter (Bedfont piCO+ Smokerlyzer, calibrated before each intake), an EC (Smokio or Joyetech eMode), and e-liquid (V2). Research staff demonstrated how to assemble and use the EC (i.e., how to power on/off the device and press the button to power the atomizer). Participants read and signed a property contract that included all loaned items.
Mōtiv8 platform and CO sample collection.
An internet-based web application, Mōtiv8, tracked participants’ smoking. Participants logged into the secure website using a username and password and completed the video sample collection following simple on-screen instructions. Mōtiv8 enabled the collection of video-based CO samples and allowed users to track their progress (CO levels) on a graph. CO levels were automatically updated in their progress graph after each submission. Mōtiv8 also displayed users’ earnings during CM phases. Researchers verified Breath CO samples and earnings were updated at least once daily. Participants were instructed to submit breath CO samples twice daily with at least 8 hr between samples. The first sample of each day was submitted before 4:00 PM. The second sample of each day was submitted before 11:59 PM. After each sample was submitted, Mōtiv8 prohibited the user from submitting another sample for 8 hr. The Mōtiv8 website was inoperable during baseline for P11 and P12, so participants were instructed to send their videos (containing a timestamp and password sent prior to each sample recording) via email to the researcher.
Electronic cigarette.
Smokio electronic cigarettes are second-generation ECs that consist of a 650 mAh or 900 mAh lithium-ion battery, a refillable reservoir holding 1 ml of e-liquid, a replaceable 2.2-Ohm atomizer head, and a mouthpiece. The EC connects to a mobile phone application via Bluetooth and records each button press to supply electricity to the atomizer. Button presses longer than 0.5 s are recorded as a puff by the device to account for the time required to raise the temperature to vaporize the e-liquid. After 10 s, the EC shuts off power to the atomizer to prevent over-heating. Participants did not have access to the application during the study. However, as of May 2016, Smokio is no longer supported in the United States. Thus, two participants (P9, P12) used Joyetech eMode electronic cigarettes. The eMode is similar to Smokio and is also a second-generation EC. The eMode syncs to computer software, myVapors, and has a 2100 mAh lithium-ion battery and replaceable 2.4-Ohm atomizer head.
E-liquid.
We provided participants with V2 e-liquid with a concentration of 24 mg/ml (2.4%) of nicotine. This concentration was chosen based on previous research that suggested concentrations higher than 18 mg/ml may be necessary to delivery nicotine comparable to a conventional cigarette (Farsalinos et al., 2013b, 2014). We used V2 e-liquid in this study because this manufacturer has actual concentrations within 10% of labeled concentrations (Davis, Dang, Kim, & Talbot, 2015). This accuracy is considered acceptable for nicotine patches and is the nicotine tolerance level set by the American E-Liquid Manufacturing Standards Association. Participants were provided with “tobacco” flavored e-liquid if they smoked traditional cigarettes or “menthol” flavored e-liquid if they smoked menthol cigarettes.
Behavior change inventory.
Approximately once every 4 days, participants completed an online survey (Qualtrics, Provo, UT) that asked about their withdrawal, adverse events, new medications, use of other tobacco products, and if they had tried new methods to initiate or maintain abstinence. Participants indicated whether anyone else used their EC and if they had used another EC or e-liquid.
Social Validity
Participants completed a 23-item acceptability questionnaire about their experience with the EC and other aspects of the program. The questionnaire included nine statements about the EC: (1) Overall, it was easy to use, (2) Overall, it was helpful in my quit attempt, (3) Overall, the electronic cigarette decreased craving for the conventional cigarette, (4) Overall, it was convenient, (5) Overall, I did not feel embarrassed to use it in public, (6) I would recommend trying an electronic cigarette to other smokers who want to quit, (7) Overall, it tasted like smoking a conventional cigarette, (8) Overall, it feels like smoking a conventional cigarette, and (9) Overall, it was pleasant. Seven statements were answered about the program in general (e.g., Overall, the Mōtiv8 website was easy to use). Participants rated their agreement to each statement on a visual analog scale (0-100) with anchors for Very Strongly Disagree (0) and Very Strongly Agree (100). Open-ended questions asked what participants liked least and most about the EC and Mōtiv8 website, what aspect of the treatment they found most helpful, and suggestions for changes.
Measures
The primary dependent variables were CO levels and EC puffs per day. Secondary measures were the percentage of negative CO samples, mean EC puffs per day across phases, and acceptability ratings.
Experimental Procedures
We used a nonconcurrent multiple baseline across participants design (Watson & Workman, 1981). Three phases were included: Baseline, EC, and EC + CM. Half the participants received the EC phase following baseline; the other half received EC + CM following baseline.
Baseline.
Each participant was randomly assigned to experience baseline phases between 2-8 days. Baseline sessions served three purposes: to familiarize participants with the study procedures, to collect data on natural cigarette smoking levels, and to assess the potential effects of monitoring CO on smoking (Beard & West, 2012). Participants were instructed to follow the breath sample collection procedure and review cessation resources from the Clearing the Air guide to quitting smoking. There were no contingencies for CO samples in this phase. Participants were then given a quit date with instructions to begin using the EC in the next phase (one participant, P9, did not follow these instructions and took nine puffs on his EC during the final day of baseline).
EC.
Participants were instructed to continue breath sample collection, quit smoking, and use the EC as needed. No contingencies were programmed for CO samples or vaping. Participants completed the online behavior change inventory approximately once every 4 days during this phase and earned $4 for each survey. This phase lasted at least 14 days. A 14-day period was selected because the first 2 weeks are a critical period for a quit attempt and are a predictor of long-term abstinence (e.g., Kenford et al., 1994). If participants failed to reduce CO levels during the EC phase, they received CM in addition to the EC. Participants were told their behavior in the EC phase might qualify them for a third phase of the study. Participants did not know the criteria or what the third phase would entail because the potential for additional compensation in the third phase might influence their behavior in the second phase. Participants qualified for EC + CM if they submitted fewer than 11 of 14 CO samples during the last 7 days of the EC condition, with less than 50% of samples negative for smoking with a stable or upward trend observed by visual analysis. The rationale for this inclusion criteria was that effects of EC + CM would be difficult to observe if participants had already reduced their CO levels in the EC phase. If participants did not qualify for EC + CM, they continued in the EC phase for an additional 14 days to equate time with those in the EC + CM phase.
EC + CM.
This phase lasted 14 days. Participants continued to follow breath sample collection procedures, used the EC as needed, and completed the behavior change inventory about every 4 days. Instead of earning money for completing the behavior change inventory, participants received incentives for negative samples (CO ≤4 ppm). Participants earned $1.00 for the first negative sample, and the amount increased by $0.05 for each consecutive negative sample. In addition, every third consecutive negative CO sample resulted in a $0.25 bonus. If a participant missed a sample or submitted a sample that was positive for smoking, they earned $0.00 and the schedule reset to $1.00 for the next negative sample. This escalating schedule of reinforcement is similar to schedules used in previous research, albeit with lower magnitudes (Dallery et al., 2007; Roll, Higgins, & Badger, 1996). Participants who experienced EC + CM after baseline proceeded to the EC phase for 14 days. Participants who received EC + CM after the EC phase proceeded to study completion.
Study completion.
At the end of the study, participants returned equipment and completed the acceptability questionnaire. To encourage safe return of the equipment, we offered a $10 voucher upon receipt of all equipment.
RESULTS
Demographic characteristics are displayed in Table 1. Participants were 20-64 years old (M = 37.5). Most participants reported smoking 12-20 cigarettes per day (M = 16.3, SD = 6.0) and a mean FTND score of 5 (SD = 1.5), reflecting moderate nicotine dependence. Eight participants smoked traditional cigarettes and four participants smoked menthol cigarettes. Menthol and traditional cigarette smokers showed similar outcomes.
Table 1.
Participant Demographics
| ID | Sex | Age | Race | Education | Weekly Income ($) | CPD | Years Smoked | FTND |
|---|---|---|---|---|---|---|---|---|
| P1 | F | 35 | White, Cuban | Some College | 100-200 | 20 | 20 | 7 |
| P2 | M | 35 | White | Some College | 401-500 | 30 | 22 | 7 |
| P3 | F | 25 | White | Graduate School | 401-500 | 20 | 10 | 6 |
| P4 | F | 43 | White | College Graduate | 401-500 | 10 | 11 | 3 |
| P5 | M | 26 | White | Some College | 100-200 | 20 | 10 | 5 |
| P6 | M | 22 | White | High School | < 100 | 20 | 10 | 5 |
| P7 | F | 39 | White | College Graduate | 501-600 | 15 | 3 | 4 |
| P8 | M | 47 | White | College Graduate | < 100 | 15 | 30 | 6 |
| P9 | M | 64 | Black | College Graduate | 601-700 | 12 | 19 | 6 |
| P10 | F | 38 | Black | Some College | 201-300 | 12 | 14 | 5 |
| P11 | F | 20 | American Indian | High School | < 100 | 9 | 2 | 3 |
| P12 | F | 56 | Black | Some College | 100-200 | 12 | 40 | 3 |
Note. CPD = Cigarettes Per Day. FTND = Fagerstrom Test for Nicotine Dependence scores.
Figure 1 shows individual CO levels and EC puffs per day across phases for the participants who received the EC phase first. Figure 2 shows individual CO levels and EC puffs per day across phases for participants who received EC + CM first. All participants had high levels of CO in baseline. Following baseline, five participants (P1, P2, P3, P7, P8) showed immediate reductions in CO and high levels of EC use. Three (P4, P9, P10) showed more gradual reductions in CO and gradual increases in EC use. Two participants (P6, P11) showed initial, but brief, reductions in CO and high levels of EC use, and two (P5, P12) failed to appreciably reduce CO levels and showed relatively low EC use. While P2 substantially reduced CO, several samples were missed during the EC phase, which qualified him to receive EC + CM.
Figure 1.
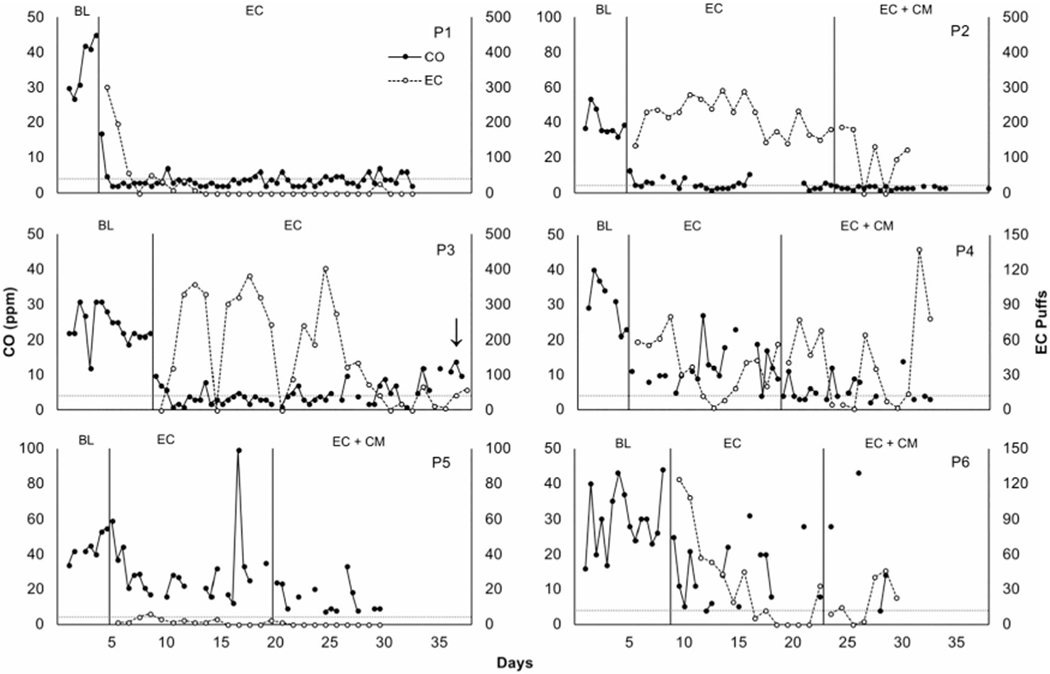
Carbon monoxide (CO) levels in parts per million (filled circles, primary axis) and number of EC puffs per day (open circles, secondary axis) across phases. BL = baseline. Horizontal dashed lines represent abstaining goals (CO ≤4 ppm). Arrows indicate that a participant reported using e-liquid not provided by researchers. The first day in EC phase corresponded with the quit date. Note that two CO samples are plotted consecutively along the x-axis per day.
Figure 2.
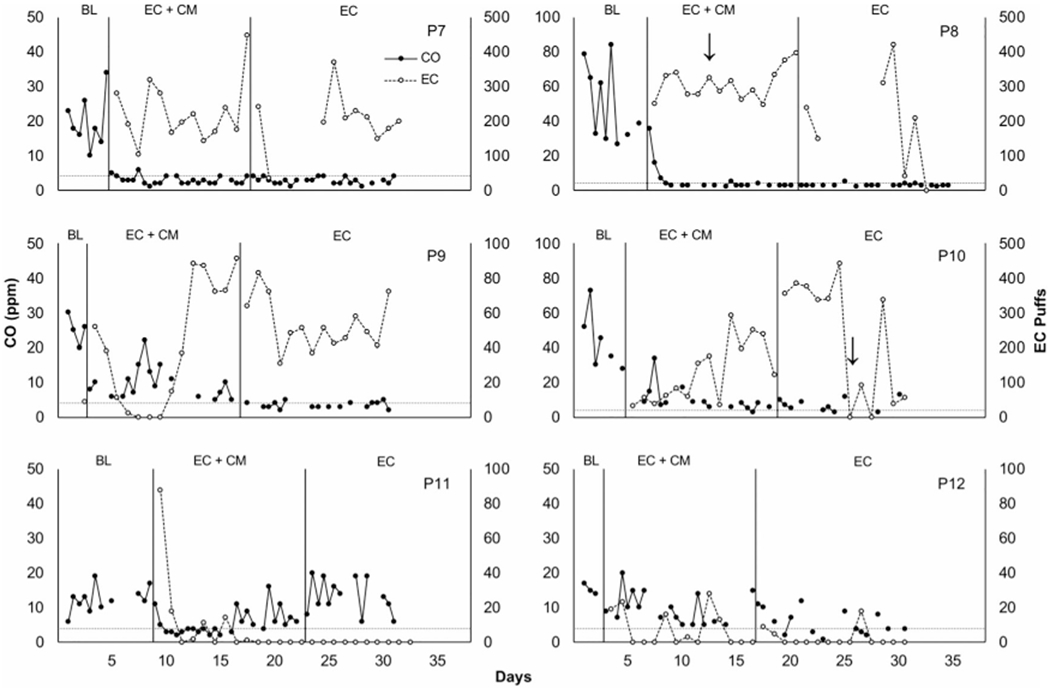
Individual carbon monoxide (CO) levels in parts per million (filled circles, primary axis) and number of EC puffs per day (open circles, secondary axis) across phases. BL = baseline. Horizontal dashed lines represent abstaining goals (CO ≤4 ppm). Arrows indicate that a participant reported using e-liquid not provided by researchers. The first day in EC + CM phase corresponded with the quit date. Note that two CO samples are plotted consecutively along the x-axis per day.
EC data were lost for P2 (days 31-38), P7, and P8 due to failures with the Smokio. P5 and P6 withdrew from the study early. Participants earned a mean of $32.04 (range = $18-52.95, SD = $11.30).
Figure 3 shows the percentage of CO samples that met the abstinence criterion (≤ 4 ppm) across each phase. No samples met the abstinence criterion during baseline, and 34.4% and 30.4% of all EC and EC + CM samples, respectively, met the criterion. Missing samples were counted as positive. There were few missing samples during baseline (9.4%). In the EC phase, 33.3% of all samples were missing and 34.5% of all samples were missing in the EC + CM phase.
Figure 3.
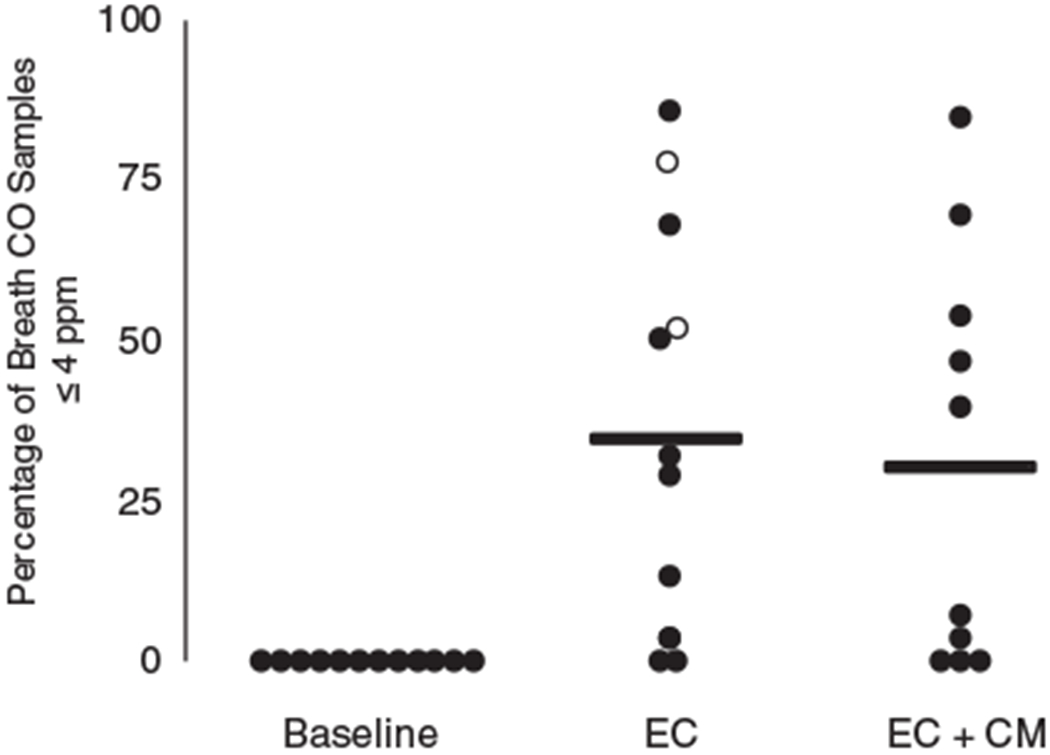
Percentage of breath carbon monoxide (CO) samples that were less than or equal to abstinence criteria of 4 parts per million (ppm) across each phase. Dots represent data for each participant. Open circles represent data for participants who met abstinence criteria in EC phase and did not receive EC + CM. Horizontal lines represent means.
To assess the relation between EC puff frequency and smoking reductions, we calculated the mean EC puffs per day across each phase for each participant and the mean percentage change in breath CO for that phase relative to his or her mean baseline CO level. Figure 4 shows participants’ mean percentage change in CO from baseline plotted as a function of mean EC puffs per day during each phase. Changes in breath CO from baseline in EC and EC + CM phases were inversely related to the mean number of EC puffs taken daily during those phases (rs = −.72, p < .01).
Figure 4.
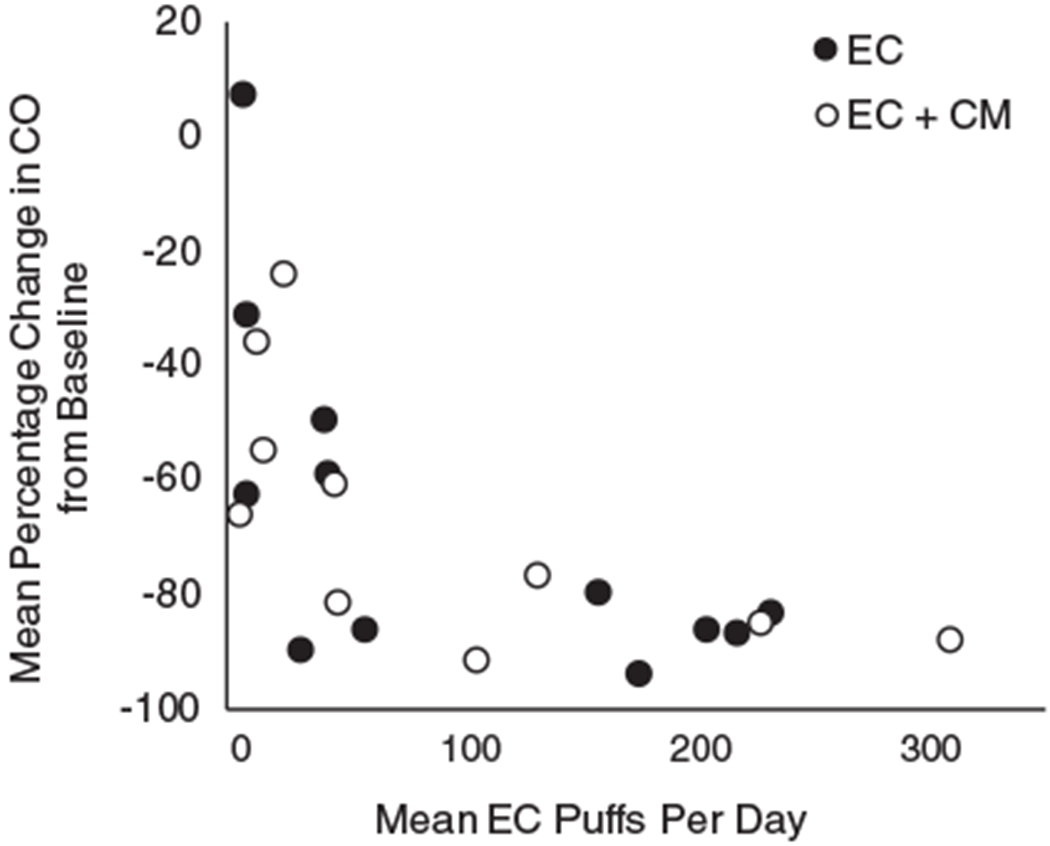
Mean percentage change in breath carbon monoxide (CO) samples from baseline plotted as a function of mean EC puffs per day for each phase. Each dot represents data for a participant. Filled circles represent participants in the EC phase and open circles represent participants in the EC + CM phase.
Figure 5 displays acceptability ratings for the EC. One participant (P6) did not complete the acceptability questionnaire. Generally, the participants agreed the EC was helpful in their quit attempt (M = 68, SD = 39.4) and decreased their craving for cigarettes (M = 66.4, SD = 41.2). Three participants, P5, P11, and P12, consistently provided the lowest ratings for the EC. All but one participant (P12) agreed they would recommend EC to others who are trying to quit (M = 83.7, SD = 30.8). Responses to the open-ended questions about the EC are provided in the Supporting Information.
Figure 5.
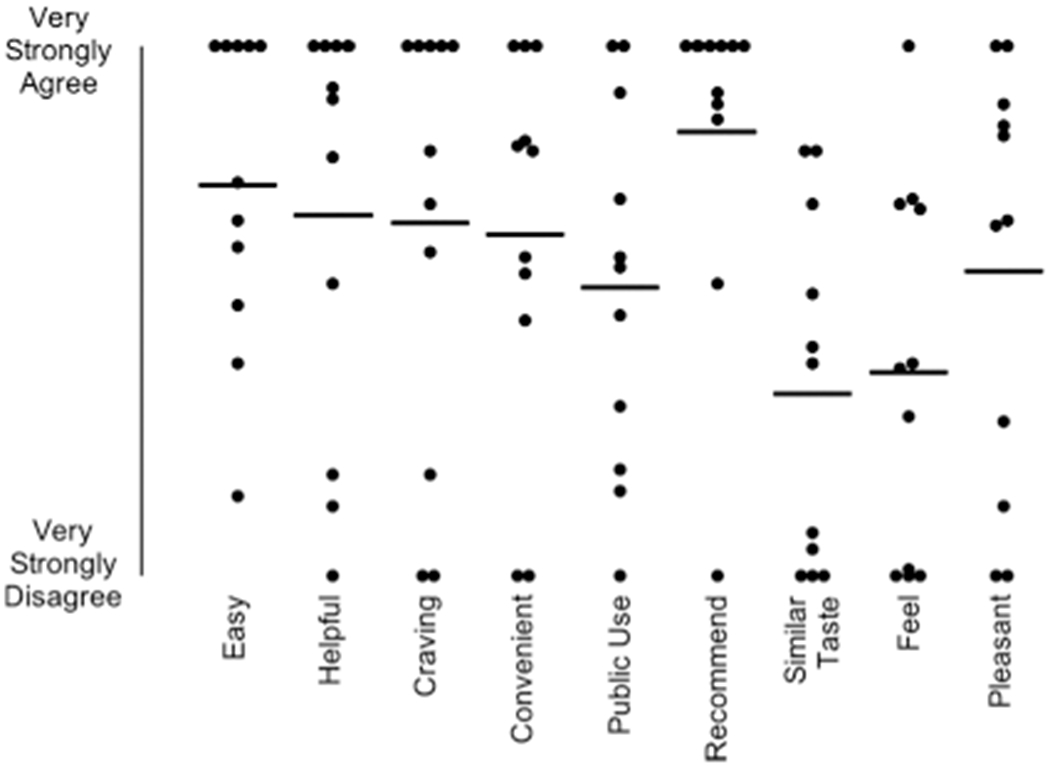
Acceptability ratings for the electronic cigarette. Each dot represents a score for a participant. Horizontal lines represent means. Responses were provided on a visual analog scale from 0-100 (0 = Very Strongly Disagree, 100 = Very Strongly Agree).
Figure 6 shows acceptability ratings for the CM procedures and the Clearing the Air guide. Participants generally agreed that the program was helpful in their quit attempt (M = 68.2, SD = 41.7), with the exception of P5, P11, and P12. Mōtiv8 was considered easy to use (M = 87.3, SD = 16.2) and participants liked seeing their progress on the Mōtiv8 graphs (M = 88.3, SD = 15.9). (Data from participants P11 and P12 were removed for these two items because they did not use Mōtiv8.) Lower agreement was seen for the Clearing the Air guide to quitting smoking: half of the participants read the manual and liked it (M = 55.4, SD = 41.7), and three agreed it was helpful (M = 39.4, SD = 41.2). Responses to the open-ended questions about CM and Clearing the Air are provided in the Supporting Information.
Figure 6.
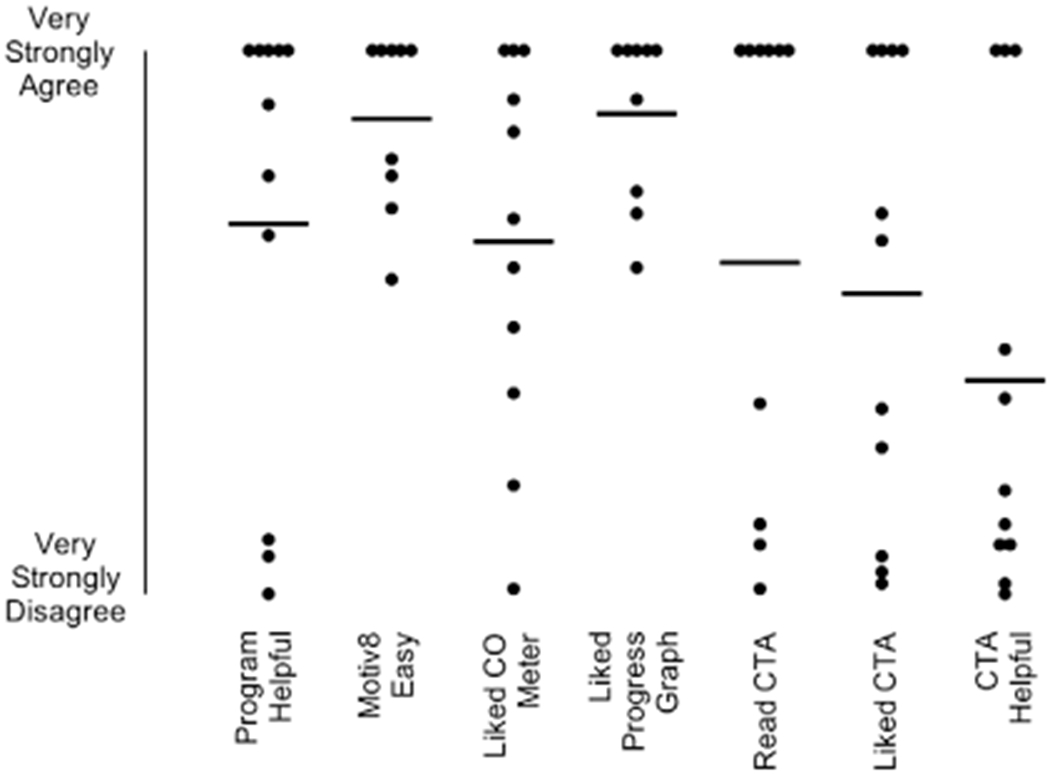
Acceptability ratings for intervention procedures. Each dot represents a score for a participant. Horizontal lines represent means. Responses were provided on a visual analog scale from 0-100 (0 = Very Strongly Disagree, 100 = Very Strongly Agree). CTA = Clearing the Air.
Responses to the behavior change inventory revealed that two participants (P2, P3) purchased 24 mg/ml tobacco flavored e-liquid from another retailer, P8 used 24 mg/ml clove and vanilla flavored e-liquid, and P10 used 16 mg/ml fruit flavored e-liquid. Marijuana use was reported once by P10. No participants reported that anyone else had used their ECs.
DISCUSSION
The present study assessed the effects of ECs alone and ECs combined with a CM intervention on cigarette smoking. We used technology-based tools to intensively monitor breath CO levels and EC use in the natural environment during the initial 4 weeks of a quit attempt. Twelve adult1 smokers with interest in quitting participated. Baseline CO levels remained elevated and stable for all participants, suggesting that monitoring alone did not influence CO. Contrary to our hypothesis, we did not observe differences in smoking abstinence or reductions between the EC alone and EC + CM conditions. Relative to baseline conditions, however, both conditions promoted smoking abstinence and reductions. We also observed an inverse relation between vaping and smoking: the more EC puffs, the greater the decrease in smoking relative to baseline (Figure 4). Overall, the results suggest that ECs may promote smoking abstinence and reductions, and the addition of a CM intervention did not improve these outcomes.
The inverse correlation between vaping and smoking is consistent with other findings: Frequent EC use is associated with lower self-reported cigarette smoking (Biener & Hargraves, 2015; Brose, Hitchman, Brown, West, & McNeill, 2015; Giovenco & Delnevo, 2018; Hitchman, Brose, Brown, Robson, & McNeill, 2015; Levy, Yuan, Luo, & Abrams, 2017; Subialka Nowariak, Lien, Boyle, Amato, & Beebe, 2018). Given the inverse correlation between EC use and CO levels, future research should determine if directly reinforcing EC use produces reductions in CO. The present results are also consistent with two RCTs that indicated higher rates of cessation with nicotine-containing versus placebo ECs (Bullen et al., 2013; Caponnetto et al., 2013). Bullen et al. (2013) also compared ECs to a nicotine patch and found no significant differences in 6-month outcomes. These results suggest that ECs may decrease smoking via nicotine replacement. In the present study, participants received e-liquid with 24 mg/ml of nicotine. Research suggests that ECs with equivalent nicotine concentrations can yield the same plasma nicotine boost and pharmacokinetic profile as conventional cigarettes (Farsalinos et al., 2013b; Wagener et al., 2017) even in naive smokers (Lopez et al., 2016), over the duration of smoking one conventional cigarette (i.e., about 5 min). Several factors affect absorption (EC device characteristics, puff topography, experience level of the user); thus, conclusions about nicotine replacement in the present study are tentative. In addition to the role of nicotine replacement, laboratory evidence also suggests that even when ECs do not deliver nicotine they can reduce self-reports of cigarette craving and urge to smoke (Vansickel et al., 2010). These findings parallel reductions in craving and smoking in human laboratory settings following smoking of denicotinized conventional cigarettes (Dallery, Houtsmuller, Pickworth, & Stitzer, 2003; Rose, Salley, Behm, Bates, & Westman, 2010). Smoking placebo ECs or cigarettes may reduce craving and smoking by mimicking some of the sensory and behavioral effects associated with regular smoking (Rose, 2006). However, it is unclear to what extent these acute effects seen in the laboratory extend to tobacco abstinence relief over longer periods, such as those observed during a quit attempt (Perkins, Karelitz, & Michael, 2015). Thus, ECs may decrease smoking by providing an alternative source of nicotine, and by substituting behaviors and sensations associated with smoking conventional cigarettes. Individual differences in the relative contributions of these effects are unknown.
Two variables may have limited the impact of CM on smoking. First, we used a relatively low magnitude monetary consequence compared to previous research (e.g., Dallery et al., 2013; Halpern et al., 2018). Although some research suggests that low-magnitude CM may be efficacious (Corby, Roll, Ledgerwood, & Schuster, 2000; Correia & Benson, 2006; Petry & Martin, 2002), abstinence rates increase as an orderly function of reinforcer magnitude (Dallery et al., 2001; Lussier et al., 2006; Silverman, Chutuape, Bigelow, & Stitzer, 1999). Thus, employing higher magnitude CM during the initial weeks of the quit attempt would have probably promoted greater abstinence, and it may have also promoted a complete transition to EC use for a greater number of participants. It is possible that following such a transition, EC use could be sustained without relapse to smoking following removal of CM. Second, we did not employ a shaping phase during which gradual reductions in smoking were reinforced. In our previous research, we arranged a 3-4-day phase in which reductions from baseline CO were reinforced, and by the end of the phase CO levels needed to indicate abstinence for reinforcement (Dallery et al., 2013, 2017; Jarvis & Dallery, 2017). This procedure was included to increase the likelihood that behavior would contact the contingency. Indeed, in the present study, several participants (P5, P9, P12) never contacted the abstinence contingency. Although it is unknown if a shaping phase (as arranged in previous studies) would generate greater abstinence than an abrupt transition to abstinence, some research suggests that alternative methods to shape abstinence hold promise (Lamb et al., 2010; cf. Romanowich & Lamb, 2014).
There were considerable individual differences in ratings on the social validity questionnaire. Not surprisingly, participants who were the least successful also provided the lowest ratings. For example, P5, P11, and P12 used the EC device less than the other participants and showed lower reductions in CO. Their ratings concerning the EC device were consistently the lowest (i.e., they “very strongly disagreed” with several aspects of the EC, Figure 5). In particular, several items that were rated negatively related to the gustatory and sensory characteristics of ECs, such as “Overall, it tasted like smoking a conventional cigarette,” “Overall, it was pleasant,” and “Overall, it feels like smoking a conventional cigarette.” Responses to what they liked least about the EC revealed a similar pattern: P5 stated, “Did not really simulate/feel like a real cigarette,” P11 stated, “Bad taste,” and P12 stated, “Adjusting the flow so it wuldnt [sic] taste as gross.” We used tobacco or menthol flavors (depending on conventional cigarette type reported to be smoked by each participant) in the present study to keep the flavor profile as consistent as possible across participants. Two participants, however, reported using different flavors (P8, P10). Future research should consider assessing flavor preferences, in addition to other EC characteristics (e.g., nicotine concentration), on EC use and subsequent changes in cigarette smoking (Audrain-McGovern, Strasser, & Wileyto, 2016; Robinson, Hensel, Al-Olayan, Nonnemaker, & Lee, 2018; Shiffman, Sembower, Pillitteri, Gerlach, & Gitchell, 2015). Despite the negative ratings by a few participants, most endorsed positive aspects of the EC such as its ability to reduce cravings, its convenience, and its overall helpfulness in quitting smoking. Sample responses to what they liked most about the EC indicated, “It was easy to use,” “It was a pain-free way to quit smoking,” “I quit,” and “Calm urges.” These results parallel a descriptive study of a Bluetooth-enabled EC (the Smokio) in five adults (Pearson et al., 2017). Participants reported that the device was convenient and acceptable, and they liked “the ability to use the Smokio in places where they could not smoke a cigarette” (p. 6). Participants also rated several aspects of the CM intervention favorably. These results are consistent with several other studies that have examined the social validity of technology-based CM (e.g., Dallery et al., 2013, 2017; Raiff, Jarvis, Turturici, & Dallery, 2013). Participants largely agreed or very strongly agreed that Mōtiv8 was easy, they liked the CO meter, they liked the CO progress graph, and that the program was helpful. Most open-ended responses also reflected ease of use, and the ability to monitor and track progress using Mōtiv8.
The study had several limitations. First, we assumed that recordings of EC puffs reflected actual puffs. We did not compare EC-recorded puffs to direct observation or biochemically verified nicotine levels (Thompson & Borrero, 2011). Second, we did not isolate the effects of goal setting in the form of setting a quit date from the effects of EC or EC + CM. However, because a goal of the study was to assess the effects of EC versus EC + CM during a quit attempt, we determined that arranging the quit attempt via goal setting was appropriate. In addition, because all participants were assigned a quit date, it is not a confound for comparisons between groups. Third, EC devices are changing rapidly, and the characteristics of the ECs we used may be different relative to future generation devices. Indeed, the Smokio is no longer available for purchase in U.S. markets, but at least one Joyetech EC is Bluetooth enabled. Fourth, the results only pertain to the initial weeks of a quit attempt, and thus longer-term patterns of EC and/or cigarette intake cannot be inferred. Indeed, research suggests that many EC users become “dual users” of ECs and cigarettes (King, Patel, Nguyen, & Dube, 2015; McMillen, Gottlieb, Shaefer, Winickoff, & Klein, 2014). From a harm reduction perspective, dual use may be preferable to exclusive cigarette smoking if it translates to a reduction in smoking. Smoking reductions can improve health outcomes (Hatsukami et al., 2005) and may lead to smoking abstinence (Hughes & Carpenter, 2006; Lindson-Hawley, Aveyard, & Hughes, 2012). However, a common concern is that dual use could perpetuate the addiction to nicotine and decrease the likelihood that an individual quits smoking (Grana, Benowitz, & Glantz, 2014; Kalkhoran & Glantz, 2016). Future research should investigate EC use over longer durations to determine the longer-term utility of ECs to promote smoking cessation.
The present study represents an important first step in employing technology-based tools to obtain objective measures of vaping and smoking in the natural environment. Although we observed no meaningful differences between EC and EC + CM, several manipulations could improve the impact of CM (reinforcer magnitude, shaping procedures) and possibly ECs (flavor, device, nicotine concentration, and directly reinforcing EC use) on smoking. In some respects, the current methods represent an unprecedented strategy to evaluate these manipulations using idiographic, single-case experimental designs. Indeed, we obtained fine grained profiles of how an EC device may influence smoking. Several individual profiles emerged, from frequent vaping and smoking abstinence, to various levels of vaping and smoking reductions, to low rates of vaping and no meaningful changes in smoking. Although a goal of the present study was merely to assess these interactions, a next step is to understand their environmental and biological determinants. Such understanding will have important public health implications in the context of the continued escalation of EC use.
Supplementary Material
Acknowledgments
The study was supported in part by crowdsourced funding enabled by Experiment.com. Preparation of this paper was supported in part by Grant P30DA029926. The authors declare no conflicts of interest.
Footnotes
The current study only pertains to daily, adult smokers. EC use in nonsmoking adolescents is a legitimate concern, but it is not addressed by the current study.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article at the publisher’s website.
REFERENCES
- Adkison SE, O’Connor RJ, Bansal-Travers M, Hyland A, Borland R, Yong HH, … Fong GT (2013). Electronic nicotine delivery systems: International tobacco control four-country survey. American Journal of Preventive Medicine, 44, 207–215. 10.1016/j.amepre.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriaens K, Van Gucht D, Declerck P, & Baeyens F (2014). Effectiveness of the electronic cigarette: An eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. International Journal of Environmental Research and Public Health, 11, 11220–11248. 10.3390/ijerphmn1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Strasser AA, & Wileyto EP (2016). The impact of flavoring on the rewarding and reinforcing value of e-cigarettes with nicotine among young adult smokers. Drug and Alcohol Dependence, 166, 263–267. 10.1016/j.drugalcdep.2016.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard E, & West R (2012). Pilot study of the use of personal carbon monoxide monitoring to achieve radical smoking reduction. Journal of Smoking Cessation, 7(1), 12–17. 10.1017/jsc.2012.1. [DOI] [Google Scholar]
- Benowitz NL, & Jacob P (1994). Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clinical Pharmacology and Therapeutics, 56, 483–493. 10.1038/clpt.1994.169. [DOI] [PubMed] [Google Scholar]
- Biener L, & Hargraves JL (2015). A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: Association with smoking cessation and motivation to quit. Nicotine & Tobacco Research, 17, 127–133. 10.1093/ntr/ntu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose LS, Hitchman SC, Brown J, West R, & McNeill A (2015). Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction, 110, 1160–1168. 10.1111/add.12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen C, Howe C, Laugesen M, McRobbie H, Parag V, Williman J, & Walker N (2013). Electronic cigarettes for smoking cessation: A randomised controlled trial. Lancet, 382, 1629–1637. 10.1016/S0140-6736(13)61842-5 [DOI] [PubMed] [Google Scholar]
- Caponnetto P, Campagna D, Cibella F, Morjaria JB, Caruso M, Russo C, & Polosa R (2013). Efficiency and safety of an electronic cigarette (ECLAT) as tobacco cigarettes substitute: A prospective 12-month randomized control design study. PLoS ONE, 8, e66317 10.1371/journal.pone.0066317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caponnetto P, Polosa R, Russo C, Leotta C, & Campagna D (2011). Successful smoking cessation with electronic cigarettes in smokers with a documented history of recurring relapses: A case series. Journal of Medical Case Reports, 5, 585 10.1186/1752-1947-5-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corby EA, Roll JM, Ledgerwood DM, & Schuster CR (2000). Contingency management interventions for treating the substance abuse of adolescents: A feasibility study. Experimental and Clinical Psychopharmacology, 8, 371–376. 10.1037//1064-1297.8.3.371. [DOI] [PubMed] [Google Scholar]
- Correia CJ, & Benson TA (2006). The use of contingency management to reduce cigarette smoking among college students. Experimental and Clinical Psychopharmacology, 14, 171–179. 10.1037/1064-1297.14.2.171. [DOI] [PubMed] [Google Scholar]
- Dallery J, & Glenn IM (2005). Effects of an Internet-based voucher reinforcement program for smoking abstinence: a feasibility study. Journal of Applied Behavior Analysis, 38, 349–357. 10.1901/jaba.2005.150-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallery J, Glenn IM, & Raiff BR (2007). An Internet-based abstinence reinforcement treatment for cigarette smoking. Drug and Alcohol Dependence, 86, 230–238. 10.1016/j.drugalcdep.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Dallery J Houtsmuller EJ Pickworth WB, & Stitzer ML (2003). Effects of cigarette nicotine content and smoking pace on subsequent craving and smoking. Psychopharmacology, 165, 172–180. 10.1007/s00213-002-1242-8. [DOI] [PubMed] [Google Scholar]
- Dallery J, Kurti AN, & Martner SG (2015). Technological approaches to assess and treat cigarette smoking In Marsch LA, Lord SE, & Dallery J (Eds.), Behavioral healthcare and technology (pp. 95–112). New York, NY: Oxford. [Google Scholar]
- Dallery J, & Raiff BR (2014). Optimizing behavioral health interventions with single-case designs: From development to dissemination. Translational Behavioral Medicine, 4, 290–303. 10.1007/s13142-014-0258-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallery J, Raiff BR, & Grabinski MJ (2013). Internet-based contingency management to promote smoking cessation: A randomized controlled study. Journal of Applied Behavior Analysis, 46, 750–764. 10.1002/jaba.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallery J, Raiff BR, Kim SJ, Marsch LA, Stitzer M, & Grabinski MJ (2017). Nationwide access to an Internet-based contingency management intervention to promote smoking cessation: A randomized controlled trial. Addiction, 112, 875–883. 10.1111/add.13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallery J, Silverman K, Chutuape MA, Bigelow GE, & Stitzer ML (2001). Voucher-based reinforcement of opiate plus cocaine abstinence in treatment-resistant methadone patients: Effects of reinforcer magnitude. Experimental and Clinical Psychopharmacology, 9, 317–325. 10.1037/1064-1297.9.3.317. [DOI] [PubMed] [Google Scholar]
- Dautzenberg B, & Bricard D (2015). Real-time characterization of e-cigarettes use: The 1 million puffs study. Journal of Addiction Research & Therapy, 6, 229 10.4172/2155-6105.1000229. [DOI] [Google Scholar]
- Davis B, Dang M, Kim J, & Talbot P (2015). Nicotine concentrations in electronic cigarette refill and do-it-yourself fluids. Nicotine & Tobacco Research, 17, 134–141. 10.1093/ntr/ntu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins L, Turner J, Roberts A, & Soar K (2013). “Vaping” profiles and preferences: An online survey of electronic cigarette users. Addiction, 108, 1115–1125. 10.1111/add.12150. [DOI] [PubMed] [Google Scholar]
- Etter JF, & Bullen C (2014). A longitudinal study of electronic cigarette users. Addictive Behaviors, 39, 491–494. 10.1016/j.addbeh.2013.10.028. [DOI] [PubMed] [Google Scholar]
- Farsalinos K (2018). Electronic cigarettes: An aid in smoking cessation, or a new health hazard? Therapeutic Advances in Respiratory Disease, 12, 1–20. 10.1177/1753465817744960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K, Romagna G, Tsiapras D, Kyrzopoulos S, Spyrou A, & Voudris V (2013a). Impact of flavour variability on electronic cigarette use experience: an Internet survey. International Journal of Environmental Research and Public Health, 10, 7272–7282. 10.3390/ijerph10127272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, & Voudris V (2013b). Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: Implications for research protocol standards definition and for public health authorities’ regulation. International Journal of Environmental Research and Public Health, 10, 2500–2514. 10.3390/ijerph10062500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Spyrou A, Stefopoulos C, Tsimopoulou K, Kourkoveli P, Tsiapras D, … Voudris V (2015). Nicotine absorption from electronic cigarette use: Comparison between experienced consumers (vapers) and naïve users (smokers). Scientific Reports, 5, 11269 10.1038/srep11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Spyrou A, Tsimopoulou K, Stefopoulos C, Romagna G, & Voudris V (2014). Nicotine absorption from electronic cigarette use: Comparison between first and new-generation devices. Scientific Reports, 4, 4133 10.1038/srep04133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovenco DP, & Delnevo CD (2018). Prevalence of population smoking cessation by electronic cigarette use status in a national sample of recent smokers. Addictive Behaviors, 76, 129–134. 10.1016/j.addbeh.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser AM, Collins L, Pearson JL, Abudayyeh H, Niaura RS, Abrams DB, & Villanti AC (2017). Overview of electronic nicotine delivery systems: A systematic review. American Journal of Preventive Medicine, 52, e33–e66. 10.1016/j.amepre.2016.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, … Benowitz N (2014). Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tobacco Control, 23, 133–139. 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace RC, Kivell B, & Laugesen M (2015). Estimating cross-price elasticity of e-cigarettes using a simulated demand procedure. Nicotine & Tobacco Research, 17, 592–598. 10.1093/ntr/ntu268. [DOI] [PubMed] [Google Scholar]
- Grana R, Benowitz N, & Glantz SA (2014). E-cigarettes: A scientific review. Circulation, 129, 1972–1986. 10.1161//CIRCULATIONAHA.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern SD, Harhay MO, Saulsgiver K, Brophy C, Troxel AB, & Volpp KG (2018). A pragmatic trial of e-cigarettes, incentives, and drugs for smoking cessation. New England Journal of Medicine, 378, 2302–2310. 10.1056/NEJMsa1715757. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Kotlyar M, Allen S, Jensen J, Li S, Le C, & Murphy S (2005). Effects of cigarette reduction on cardiovascular risk factors and subjective measures. Chest, 128, 2528–2537. 10.1378/chest.128.4.2528. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, & Fagerström KO (1991). The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction, 86(9), 1119–1127. [DOI] [PubMed] [Google Scholar]
- Hitchman SC, Brose LS, Brown J, Robson D, & McNeill A (2015). Associations between e-cigarette type, frequency of use, and quitting smoking: Findings from a longitudinal online panel survey in Great Britain. Nicotine & Tobacco Research, 17, 1187–1194. 10.1093/ntr/ntv078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, & Carpenter MJ (2006). Does smoking reduction increase future cessation and decrease disease risk? A qualitative review. Nicotine & Tobacco Research, 8, 739–749. 10.1080/14622200600789726. [DOI] [PubMed] [Google Scholar]
- Jarvis BP, & Dallery J (2017). Internet-based self-tailored deposit contracts to promote smoking reduction and abstinence. Journal of Applied Behavior Analysis, 50, 189–205. 10.1002/jaba.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Johnson PS, Rass O, & Pacek LR (2017). Behavioral economic substitutability of e-cigarettes, tobacco cigarettes, and nicotine gum. Journal of Psychopharmacology, 31, 851–860. 10.1177/0269881117711921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorenby DE, Smith SS, Fiore MC, & Baker TB (2017). Nicotine levels, withdrawal symptoms, and smoking reduction success in real world use: A comparison of cigarette smokers and dual users of both cigarettes and E-cigarettes. Drug and Alcohol Dependence, 170, 93–101. 10.1016/j.drugalcdep.2016.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkhoran S, & Glantz SA (2016). E-cigarettes and smoking cessation in real-world and clinical settings: A systematic review and meta-analysis. The Lancet Respiratory Medicine, 4, 116–128. 10.1016/S2213-2600(15)00521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, & Baker TB (1994). Who will quit with and without the nicotine patch. Journal of the American Medical Association, 271, 589–594. [DOI] [PubMed] [Google Scholar]
- King BA, Patel R, Nguyen KH, & Dube SR (2015). Trends in awareness and use of electronic cigarettes among US adults, 2010-2013. Nicotine & Tobacco Research, 17, 219–227. 10.1093/ntr/ntu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RJ, Kirby KC, Morral AR, Arlington R, Galbicka G, & Iguchi MY (2010). Shaping smoking cessation in hard-to-treat smokers. Journal of Consulting and Clinical Psychology, 78, 62–71. 10.1037/a0018323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner WV, Meier E, Wiener JL, Grant DM, Gilmore J, Judah MR, … Wagener TL (2015). The comparative efficacy of first- versus second-generation electronic cigarettes in reducing symptoms of nicotine withdrawal. Addiction, 110, 862–867. 10.1111/add.12870. [DOI] [PubMed] [Google Scholar]
- Ledgerwood DM (2008). Contingency management for smoking cessation: Where do we go from here? Current Drug Abuse Reviews, 1, 340–349. [DOI] [PubMed] [Google Scholar]
- Levy DT, Yuan Z, Luo Y, & Abrams DB (2017). The relationship of e-cigarette use to cigarette quit attempts and cessation: Insights from a large, nationally representative U.S. survey. Nicotine & Tobacco Research, 20, 931–939. 10.1093/ntr/ntx166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindson-Hawley N, Aveyard P, & Hughes JR (2012). Reduction versus abrupt cessation in smokers who want to quit. Cochrane Database of Systematic Reviews, 2012(11), 1–42. 10.1002/14651858.CD008033.pub3. [DOI] [PubMed] [Google Scholar]
- Lopez AA, & Eissenberg T (2015). Science and the evolving electronic cigarette. Preventive Medicine, 80, 101–106. 10.1016/j.ypmed.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez AA, Hiler MM, Soule EK, Ramôa CP, Karaoghlanian NV, Lipato T, … Eissenberg T (2016). Effects of electronic cigarette liquid nicotine concentration on plasma nicotine and puff topography in tobacco cigarette smokers: A preliminary report. Nicotine & Tobacco Research, 18, 720–723. 10.1093/ntr/ntv182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, & Higgins ST (2006). A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction, 101, 192–203. 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- Manzoli L, Flacco ME, Fiore M, La Vecchia C, Marzuillo C, Gualano MR, … Villari P (2015). Electronic cigarettes efficacy and safety at 12 months: Cohort study. PloS ONE, 10, e0129443 10.1371/journal.pone.0129443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald S, Quinn F, Vieira R, O’Brien N, White M, Johnston DW, & Sniehotta FF (2017). The state of the art and future opportunities for using longitudinal n-of-1 methods in health behaviour research: A systematic literature overview. Health Psychology Review, 11, 307–323. 10.1080/17437199.2017.1316672. [DOI] [PubMed] [Google Scholar]
- McMillen RC, Gottlieb MA, Shaefer RM, Winickoff JP, & Klein JD (2014). Trends in electronic cigarette use among U.S. adults: Use is increasing in both smokers and nonsmokers. Nicotine & Tobacco Research, 17, 1195–1202. 10.1093/ntr/ntu213. [DOI] [PubMed] [Google Scholar]
- Meredith SE, Grabinski MJ, & Dallery J (2011). Internet-based group contingency management to promote abstinence from cigarette smoking: A feasibility study. Drug and Alcohol Dependence, 118, 23–30. 10.1016/j.drugalcdep.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JL, Elmasry H, Das B, Smiley SL, Rubin LF, DeAtley T, … Abrams DB (2017). Comparison of ecological momentary assessment versus direct measurement of e-cigarette use with a Bluetooth-enabled e-cigarette: A pilot study. JMIR Research Protocols, 6, e84 10.2196/resprot.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, & Michael VC (2015). Reinforcement enhancing effects of acute nicotine via electronic cigarettes. Drug and Alcohol Dependence, 153, 104–108. 10.1016/j.drugalcdep.2015.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, & Martin B (2002). Low-cost contingency management for treating cocaine- and opioid-abusing methadone patients. Journal of Consulting and Clinical Psychology, 70, 398–405. 10.1037//0022-006X.70.2.398. [DOI] [PubMed] [Google Scholar]
- Pope DA, Poe L, Stein JS, Kaplan BA, Heckman BW, Epstein LH, & Bickel WK (2019). Experimental tobacco marketplace: Substitutability of e-cigarette liquid for cigarettes as a function of nicotine strength. Tobacco Control, 28, 206–211. 10.1136/tobaccocontrol-2017-054024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, & Roll J (2006). Contingency management for treatment of substance use disorders: a meta-analysis. Addiction, 101, 1546–1560. 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Prochaska JJ, & Grana RA (2014). E-cigarette use among smokers with serious mental illness. PloS ONE, 9, e113013 10.1371/journal.pone.0113013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quisenberry AJ, Koffarnus MN, Epstein LH, & Bickel WK (2017). The experimental tobacco marketplace II: Substitutability and sex effects in dual electronic cigarette and conventional cigarette users. Drug and Alcohol Dependence, 178, 551–555. 10.1016/j.drugalcdep.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quisenberry AJ, Koffarnus MN, Hatz LE, Epstein LH, & Bickel WK (2015). The experimental tobacco marketplace I: Substitutability as a function of the price of conventional cigarettes. Nicotine & Tobacco Research, 18, 1642–1648. 10.1093/ntr/ntv230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiff BR, Jarvis BP, Turturici M, & Dallery J (2013). Acceptability of an Internet-based contingency management intervention for smoking cessation: Views of smokers, nonsmokers, and healthcare professionals. Experimental and Clinical Psychopharmacology, 21, 204–213. 10.1037/a0032451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RJ, Hensel EC, Al-Olayan AA, Nonnemaker JM, & Lee YO (2018). Effect of e-liquid flavor on electronic cigarette topography and consumption behavior in a 2-week natural environment switching study. PLoS ONE, 13, e0196640 10.1371/journal.pone.0196640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, & Badger GJ (1996). An experimental comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Journal of Applied Behavior Analysis, 29, 495–505. 10.1901/jaba.1996.29-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanowich P, & Lamb RJ (2014). The effects of percentile versus fixed criterion schedules on smoking with equal incentive magnitude for initial abstinence. Experimental and Clinical Psychopharmacology, 22, 348–355. 10.1037/a0036935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE (2006). Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology, 184, 274–285. 10.1007/s00213-005-0250-x. [DOI] [PubMed] [Google Scholar]
- Rose JE, Salley A, Behm FM, Bates JE, & Westman EC (2010). Reinforcing effects of nicotine and non-nicotine components of cigarette smoke. Psychopharmacology, 210, 1–12. 10.1007/s00213-010-1810-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahab L, Goniewicz ML, Blount BC, Brown J, McNeill A, Alwis KU, … West R (2017). Nicotine, carcinogen, and toxin exposure in long-term e-cigarette and nicotine replacement therapy users. Annals of Internal Medicine, 166, 390–400. 10.7326/M16-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Sembower MA, Pillitteri JL, Gerlach KK, & Gitchell JG (2015). The impact of flavor descriptors on nonsmoking teens’ and adult smokers’ interest in electronic cigarettes. Nicotine & Tobacco Research, 17, 1255–1262. 10.1093/ntr/ntu333. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, & Patrick ME (2012). The use of financial incentives in promoting smoking cessation. Preventive Medicine, 55(Suppl), S24–S32. 10.1016/j.ypmed.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman K, Chutuape MA, Bigelow GE, & Stitzer ML (1999). Voucher-based reinforcement of cocaine abstinence in treatment-resistant methadone patients: Effects of reinforcer magnitude. Experimental and Clinical Psychopharmacology, 146, 128–138. [DOI] [PubMed] [Google Scholar]
- Snider SE, Cummings KM, & Bickel WK (2017). Behavioral economic substitution between conventional cigarettes and e-cigarettes differs as a function of the frequency of e-cigarette use. Drug and Alcohol Dependence, 177, 14–22. 10.1016/j.drugalcdep.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JS, Koffarnus MN, Stepanov I, Hatsukami DK, & Bickel WK (2018). Cigarette and e-liquid demand and substitution in e-cigarette-naïve smokers. Experimental and Clinical Psychopharmacology, 26, 233–243. 10.1037/pha0000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Dallery J, Fields NM, Nuzzo PA, Schoenberg NE, Martin CA, … Wong CJ (2009). An Internet-based abstinence reinforcement smoking cessation intervention in rural smokers. Drug and Alcohol Dependence, 105, 56–62. 10.1016/j.drugalcdep.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subialka Nowariak EN, Lien RK, Boyle RG, Amato MS, & Beebe LA (2018). E-cigarette use among treatment-seeking smokers: Moderation of abstinence by use frequency. Addictive Behaviors, 77, 137–142. 10.1016/j.addbeh.2017.09.023. [DOI] [PubMed] [Google Scholar]
- Thompson RH, & Borrero JC (2011). Direct observation In Fisher WW, Piazza CC, & Roane HS (Eds.), Handbook of applied behavior analysis (pp. 191–205). New York, NY: Guilford. [Google Scholar]
- Tseng T-Y, Ostroff JS, Campo A, Gerard M, Kirchner T, Rotrosen J, & Shelley D (2016). A randomized trial comparing the effect of nicotine versus placebo electronic cigarettes on smoking reduction among young adult smokers. Nicotine & Tobacco Research, 18, 1937–1943. 10.1093/ntr/ntw017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. (2016). E-cigarette use among youth and young adults: A report of the Surgeon General. MD: Retrieved from: Rockville: https://e-cigarettes.surgeongeneral.gov/documents/2016_sgr_full_report_non-508.pdf. [Google Scholar]
- Vansickel AR, Cobb CO, Weaver MF, & Eissenberg TE (2010). A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: Nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiology, Biomarkers & Prevention, 19, 1945–1953. 10.1158/1055-9965.EPI-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickerman KA, Carpenter KM, Altman T, Nash CM, & Zbikowski SM (2013). Use of electronic cigarettes among state tobacco cessation quitline callers. Nicotine & Tobacco Research, 15, 1787–1791. 10.1093/ntr/ntt061. [DOI] [PubMed] [Google Scholar]
- Vickerman KA, Schauer GL, Malarcher AM, Zhang L, Mowery P, & Nash CM (2016). Reasons for electronic nicotine delivery system use and smoking abstinence at 6 months: A descriptive study of callers to employer and health plan-sponsored quitlines. Tobacco Control, 26, 126–134. 10.1136/tobaccocontrol-2015-052734. [DOI] [PubMed] [Google Scholar]
- Wagener TL, Floyd EL, Stepanov I, Driskill LM, Frank SG, Meier E, … Queimado L (2017). Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tobacco Control, 26, e23–e28. 10.1136/tobaccocontrol-2016-053041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagener TL, Meier E, Hale JJ, Oliver ER, Warner ML, Driskill LM, … Foster S (2014). Pilot investigation of changes in readiness and confidence to quit smoking after E-cigarette experimentation and 1 week of use. Nicotine & Tobacco Research, 16, 108–114. 10.1093/ntr/ntt138. [DOI] [PubMed] [Google Scholar]
- Watson PJ, & Workman EA (1981). The nonconcurrent multiple baseline across-individuals design: An extension of the traditional multiple baseline design. Journal of Behavior Therapy and Experimental Psychiatry, 12, 257–259. 10.1016/0005-7916(81)90055-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


