Short abstract
Temporomandibular joint disorder is a common chronic craniofacial pain condition, often involving persistent, widespread craniofacial muscle pain. Although the etiology of chronic muscle pain is not well known, sufficient clinical and preclinical information supports a contribution of trigeminal nociceptors to craniofacial muscle pain processing under various experimental and pathological conditions. Here, we review cellular and molecular mechanisms underlying sensitization of muscle nociceptive afferents. In particular, we summarize findings on pronociceptive roles of peripheral glutamate in humans, and we discuss mechanistic contributions of glutamate receptors, including N-methyl-D-aspartate receptors and metabotropic glutamate receptors, which have considerably increased our understanding of peripheral mechanisms of craniofacial muscle pain. Several members of the transient receptor potential (TRP) family, such as transient receptor potential vanilloid 1 (TRPV1) and transient receptor potential ankyrin 1, also play essential roles in the development of spontaneous pain and mechanical hypersensitivity in craniofacial muscles. Furthermore, glutamate receptors and TRP channels functionally and bi-directionally interact to modulate trigeminal nociceptors. Activation of glutamate receptors invokes protein kinase C, which leads to the phosphorylation of TRPV1. Sensitization of TRPV1 by inflammatory mediators and glutamate receptors in combination with endogenous ligands contributes to masseter hyperalgesia. The distinct intracellular signaling pathways through which both receptor systems engage and specific molecular regions of TRPV1 are offered as novel targets for the development of mechanism-based treatment strategies for myogenous craniofacial pain conditions.
Keywords: muscle pain, craniofacial pain, TRP channels, glutamate receptors, primary afferents
Introduction
Craniofacial musculoskeletal pain conditions, including those related to temporomandibular disorders (TMD), are the most common persistent pain conditions arising from oral and craniofacial structures. The presentation of signs and symptoms of TMD vary and often involve the temporomandibular joint (TMJ), nerves, tendons, ligaments, disks, bones, connective tissue, and muscles of mastication.1 The pathophysiology of TMD-related pain conditions is not well understood; it is multifactorial and characterized by multiple symptoms involving structural and inflammatory components.2–4 Management of the debilitating pain associated with craniofacial deep tissues is still largely inadequate due to its unclear etiology and pathology, and the development of novel and effective therapeutic interventions has been an ongoing effort in both research and clinical communities.
Chronic muscle pain conditions, such as TMD, are characterized by localized myalgia, tenderness upon manual palpation, reduced force output, and limited range of motion,5–7 all of which could result from sensitization of nociceptors.8 Thus, the prominent features of persistent muscle pain conditions are spontaneous pain and primary mechanical hyperalgesia. In addition, pain referral is also a common characteristic of chronic muscle pain conditions, including TMD.7,8 To this end, there has been a steady increase in studies over the past few decades exploring the roles of trigeminal nociceptors, either by exogenously administering pronociceptive/inflammatory mediators or by inducing tissue inflammation with various inflammatory agents. Among many algogens and inflammatory mediators implicated in muscle pain,9 peripheral glutamate and its receptors are the most extensively studied in the context of craniofacial muscle pain and hyperalgesia in humans and rodents. Furthermore, there is intriguing evidence that glutamate signaling pathways functionally interact with transient receptor potential (TRP) ion channels, which are another set of important players in trigeminal nociceptor sensitization.10 Recent studies have significantly advanced our knowledge of the neural mechanisms of pathological craniofacial musculoskeletal pain, and they offer important new insights for the development of mechanism-based pharmacological therapies in treating myogenous TMD. This review will examine key findings from human and animal experiments on peripheral glutamate, its receptors, and TRP channels under acute and persistent pain conditions, with a focus on the muscles of mastication.
Peripheral glutamate induces craniofacial muscle pain and hyperalgesia in humans and experimental animals
Glutamate is a pain modulator in the human central nervous system. In the periphery, glutamate acts as an algogen and modulates nociceptor activities. Local application of glutamate into the periphery also induces excitation and sensitization of nociceptors.11,12 Consistent with these electrophysiological findings, glutamate injection into the hindpaw produces rapid pain responses and edema, indicating that peripheral glutamate and its receptors play an important role in nociception as well as in inflammation.13 In the orofacial muscles, locally administered glutamate activates and sensitizes muscle nociceptors.14,15 In addition, glutamate injected into the masseter muscle produces significantly greater edema volume and extracellular water content than is produced by isotonic saline control injection.15 However, glutamate administered in the TMJ and the knee joint does not lead to gross inflammatory signs,12,16 indicating the need for further examination of the role of exogenous glutamate on muscle inflammation. The activity of small diameter afferents evoked by masseter injection of glutamate in rats closely correlates with pain reports from human subjects who received local injections of comparable dose of glutamate in the masseter muscle.14 Interestingly, glutamate injection in different craniofacial muscles produces different subjective pain levels. A greater subjective pain level is experienced upon injection into the temporalis muscle than upon injection into the masseter muscle.17 In the same experiment, less pain was induced by injection in neck muscle than in masseter muscle.18 Pain responses evoked by masseter injection of glutamate in healthy subjects are similar to pain experienced by persistent myofascial TMD pain patients, with respect to sensory-discriminative and affective-unpleasantness components.19 Glutamate injection into latent myofascial trigger points in humans produces greater pain intensity, greater area of referred pain, and increased mechanical hyperalgesia than injection into nontrigger point control sites.20 These results suggest that exogenous glutamate can be noxious in experimental animals and humans.
Endogenous glutamate also produces and modulates pain under pathophysiological conditions. The source of interstitial glutamate is not entirely clear, and neuronal and nonneuronal sources could contribute.21–23 It is presumed, however, that peripheral terminals of primary afferents could be a dominant source. Approximately 80% of trigeminal primary afferents express vesicular glutamate transporter,24 suggesting that a majority of trigeminal ganglia (TG) afferents are glutamatergic. Electrical stimulation of sensory afferents or chemical activation of nociceptors by capsaicin or formalin increases glutamate concentration in tissue,23,25,26 suggesting neural release of glutamate. Injection of botulinum toxin into the temporalis muscle produces a rapid decrease in interstitial levels of muscular glutamate,27 suggesting constitutive release of glutamate as well. Inflammation increases glutamate release in preclinical models of osteoarthritis.28,29 Interestingly, masseter inflammation induced by complete Freund’s adjuvant (CFA) in rats upregulates the vesicular glutamate transporter 2 gene in rat TG,30 indicating that glutamate release is upregulated following tissue inflammation. Acid injection into rat masseter muscle produces ectopic discharge of muscle spindle afferents, which can cause the release of glutamate from spindle afferent terminals to affect adjacent nociceptors.31
In humans, tissue injury and inflammation also produces sustained increases of glutamate, as shown in patients with chronic knee joint or tendon pain.32,33 Patients with trapezius myalgia show greater serotonin and glutamate levels in trapezius muscle.34–36 Subjects with chronic widespread pain, including fibromyalgia, show increased glutamate concentrations in trapezius muscle.37 Myofascial TMD patients show increased interstitial glutamate in masseter muscle, but not plasma glutamate, compared to healthy subjects.38 Interstitial glutamate concentration in masseter muscle of TMD patients appears to be affected by injury or insult. Acute pain due to the insertion of microdialysis needle to masseter muscle results in higher levels of interstitial glutamate in myofascial TMD patients than in healthy controls.39 Masseter injection of hypertonic saline increases the release of glutamate.40 Monosodium glutamate ingestion induces a greater increase of interstitial glutamate and spontaneous pain in masseter muscle of myofascial TMD patients than in healthy subjects.41 However, not all conditions are associated with increases in the interstitial glutamate level in orofacial muscles. Tooth clenching does not induce the release of glutamate in masseter muscles of either myofascial TMD patients or healthy subjects.42 Additionally, acidic saline injection into masseter muscle does not increase glutamate release in healthy subjects.43
Glutamate receptors in primary afferents mediate masseter pain and hyperalgesia
Glutamate acts via N-methyl-D-aspartate (NMDA) receptors, 2-amino-3–(3-hydroxy-5-methyl-isoxazol-4-yl) propanoic acid (AMPA) receptors, kainate receptors, and metabotropic glutamate receptors (mGluRs). Since all of these receptors are expressed in the peripheral terminals of small diameter cutaneous afferents,44,45 exogenous or endogenous glutamate could evoke or modulate nociception through these receptors. The role of glutamate receptors and endogenously released glutamate is suggested in a series of studies using antagonists of glutamate receptors. Masseter injection of MK801, an NMDA receptor antagonist, attenuates nocifensive behaviors in lightly anesthetized rats, reduces masseter muscle swelling, and decreases c-fos expression in the subnucleus caudalis (Vc) induced by the masseteric injection of mustard oil.46,47 Masseter injection of MK801 also alleviates nocifensive responses during masseter infusion of hypertonic saline, which is accompanied by a reduction in c-fos upregulation in Vc.48 Pretreatment of masseter with AP5, another antagonist of the NMDA receptor, attenuates development of CFA-induced reduction in bite force, but posttreatment does not reverse the hyperalgesia.49 The blockade of NMDA receptors in the masseter muscle also decreases glutamate-evoked masseter afferent activities.50 Consistently, in humans, pain and mechanical hyperalgesia induced by masseter injection of glutamate is attenuated by treatment of masseter with ketamine, which blocks NMDA receptors.51 These results indicate that peripheral NMDA receptors contribute to inflammation, nociception, and hyperalgesia in masseter muscles.
Peripheral mGluRs are also implicated in masseter hyperalgesia. R,S-3,5-dihydroxyphenylglycol (DHPG), a group I mGluR agonist, produces mechanical hyperalgesia in masseter muscle, which is prevented by pretreatment of the masseter with 2-methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP), a selective mGluR 5 antagonist, but not by 7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxylate ethyl ester, a selective mGluR 1 antagonist.52,53
Transient receptor potential vanilloid 1 and transient receptor potential ankyrin 1 contribute to pain and hyperalgesia from craniofacial muscles
Microneurographic recordings in human peroneal nerve from muscles show that intramuscular injection of capsaicin produces cramping pain and activates mechanosensitive group III (thinly myelinated) and group IV (unmyelinated) fibers.54 Capsaicin injection into masseter muscle also produces intense deep pain sensations and inhibits motor unit firing.55 Transient receptor potential vanilloid 1 (TRPV1), a receptor for capsaicin, is expressed in a small subpopulation of small to medium-diameter masseter afferents in rat TG.56,57 TRPV1 and transient receptor potential ankyrin 1 (TRPA1) are expressed in approximately 25% and 10% of masseter afferents.56 Direct injection of capsaicin activates mechanosensitive group IV nociceptors in gastrocnemius muscle in rats.58 Capsaicin or mustard oil injection into rat masseter muscle produces characteristic nocifensive responses followed by the development of mechanical hyperalgesia of masseter muscle in lightly anesthetized rats.56,59,60 These capsaicin- and mustard oil-evoked nociception and hyperalgesia are inhibited by TRPV1 and TRPA1 antagonists, respectively, supporting a role of TRPV1 and TRPA1 in acute and pathological pain responses in the masseter muscle.56
Preclinical studies using various muscle pain models further support the contribution of TRPV1 and TRPA1 in muscle pain. Hydrogen peroxide injected into gastrocnemius muscle in mice produces ongoing pain, assessed by guarding behaviors and conditioned place aversion, which is attenuated by pharmacological and genetic knockout of TRPA1.61 TRPV1 antagonist reduces exercise-induced muscle hyperalgesia in rats.62 In rat masseter muscle, CFA-induced mechanical hyperalgesia is inhibited by the injection of TRPV1 or TRPA1 antagonists.30,63 In mice, genetic and pharmacological inhibition of TRPV1 or TRPA1 antagonist attenuates CFA-induced spontaneous pain from masseter muscle, assessed by mouse grimace scale and conditioned place preference.64,65 Simultaneous inhibition of TRPV1 and TRPA1 produces greater conditioned place preference than the respective inhibition, suggesting additive inhibitory effects on ongoing pain.64 Since masseter inflammation increases the expression of TRPV1 and TRPA1 in TG,30,63 upregulation of TRPV1 and TRPA1 likely contributes to the hyperalgesia. These results suggest that activation of TRPV1 and TRPA1 mediates spontaneous pain and mechanical hyperalgesia in a rodent model of masseter inflammation. It is likely that inflamed masseter muscle increases levels of endogenous ligands of TRPV1 and TRPA1. For example, 9(S)-hydroxyoctadecadienoic acid (HODE) or 13(S)-HODE are oxidized linoleic acid metabolites that have been suggested to be endogenous ligands of TRPV1.66 TRPA1 is activated by multiple endogenous electrophiles such as hydrogen peroxide.61,67 CFA-induced spontaneous pain from masseter muscle is reduced in mice by scavenging the oxidized linoleic acid metabolites or hydrogen peroxide.65
These findings indicate that the TRPV1-expressing population of nociceptors are likely primary mediators of masseter inflammation. Supporting this notion, chemical ablation of TRPV1-expressing primary afferents using resiniferatoxin injected into Vc or chemogenetic inhibition of TRPV1-lineage masseter afferent terminals attenuates both development of spontaneous pain and reduction in bite force following masseter inflammation.65 Furthermore, ablation of neurokinin1 receptor-expressing projection neurons in Vc also produces the same effects. In contrast to the inhibitory effects of ablating TRPV1-expressing afferents, the inhibition of TRPV1 molecules only modestly reversed decreased bite force following CFA injection into masseter muscle and the inhibition of TRPA1 did not affect bite force at all.64,65 Although the source of this discrepancy is unknown, it is hypothesized that unknown molecules expressed in TRPV1-positive masseter afferents mediate bite-evoked nociception under masseter inflammation. Masseter inflammation influences expression of multiple genes from TG implicated in pain processing,68 and further investigation of their contribution to muscle hyperalgesia, especially in bite-evoked pain, is necessary.
Activation of TRP channels leads to glutamate receptor signaling
The signaling through glutamate receptors and TRP channels are important mediators of craniofacial muscle pain. Interestingly, these two pathways intersect in the trigeminal nociceptive mechanisms. It has been suggested that interactions of glutamate receptors and TRPV1 occur in trigeminal deep tissue afferents including TMJ and masseter muscle nociceptors.69,70 Deep tissue injection of glutamate sensitizes trigeminal nociceptors to enhance the activation of deep tissue afferents and Vc neurons by subsequent capsaicin injection.69,70 In contrast, deep tissue injection of capsaicin reduces the activation of Vc neurons and prevents glutamate-induced activation of deep tissue afferents.69,70 Results in humans are consistent with this mode of interaction between glutamate receptors and TRPV1 signaling: Preceding glutamate administration into masseter causes sensitization to subsequent administration of capsaicin resulting in increasing pain levels and electromyographic activities of the masseter muscle,71,72 whereas preceding capsaicin administration is associated with a desensitization of nociceptors to subsequent injection of glutamate.72 Injection of hot or acidic glutamate solution produces greater pain,73,74 which also suggests heat- and acid-mediated regulation of TRPV1.10
Activation of TRP channels in nociceptors can induce the release of neuropeptides and glutamate from peripheral terminals at the site of injection to produce neurogenic inflammation. TRPV1 is a Ca2+-permeable ion channel, and its activation in primary afferents induces Ca2+ influx to produce Ca2+-dependent vesicular release. This process is independent of voltage-dependent sodium channel activation or action potential firing75 and is consistent with the lack of effect of local anesthetics on capsaicin-induced inflammation of TMJ.76 Capsaicin injection into masseter muscle increases baseline discharge of Vc neurons and masseter nerve sensitivity.77 Masseter injection of MPEP, a mGluR5 antagonist, prevents capsaicin-induced hypersensitivity of masseter nerve.77 In skin, capsaicin injection increases glutamate release in subcutaneous tissue, which can be attenuated by treatment with a mixture of antagonists against NMDA, AMPA, and mGluR1/5.25 The same mixture of antagonists attenuates capsaicin-induced thermal hyperalgesia.25 Similarly, an NMDA receptor antagonist inhibits capsaicin-induced release of neuropeptides in spinal cord,78 and NMDA-mediated release of substance P in central afferent terminals is eliminated by capsaicin-induced ablation of nociceptive afferents.79 These reports suggest that release of neuropeptides from TRPV1-expressing afferent terminals is enhanced by positive feedback from peripheral glutamate receptors. Likewise, nocifensive behavioral and neuronal activation in Vc by mustard oil, an agonist of TRPA1, is also reduced by NMDA receptor antagonist.46,47 Overall, these results indicate that the activation of nociceptive terminals by TRP channel agonists can induce the release of glutamate from peripheral terminals and the activation of peripheral glutamate receptors, which subsequently produces further release of glutamate and peripheral sensitization. This interpretation appears to be contradictory to the desensitizing effects of capsaicin injection on glutamate-induced responses.69,70,72 Although the source of discrepancy is not clear, it is possibly due to a lack of understanding of the different functional consequences of TRPV1 activation by endogenous ligands versus capsaicin. Strong activation of TRPV1 by capsaicin produces Ca2+-dependent desensitization of TRPV1 and afferent terminals, whereas persistent low-level TRPV1 activation may contribute to long-lasting pain.80,81 The mechanisms of TRP channel activation leading to glutamate receptor regulation need to be further determined.
Activation of glutamate receptors regulates TRP channel signaling
NMDA and AMPA receptors, as well as mGluR5 are excitatory receptors, and their activation in nociceptive afferents likely leads to the activation of nociceptors. Interestingly, however, mechanical hyperalgesia of masseter muscle induced by the injection of NMDA is prevented by pretreatment of the masseter with TRPV1 antagonist.82 NMDA receptor subunits and TRPV1 are colocalized in masseter afferents, and NMDA treatment sensitizes capsaicin responses in dissociated TG neurons.82 Masseter injection of NMDA increases serine phosphorylation of TRPV1, which is dependent on calmodulin-dependent kinase II and protein kinase C (PKC), but not protein kinase A (PKA).82 In particular, phosphorylation at TRPV1 S800 is increased by NMDA in dissociated TG neurons.83 This effect is dependent on PKC and A-kinase anchoring protein 150 (AKAP150),83 which is a scaffolding protein for harboring PKC and TRPV1.84 In addition to NMDA, mechanical hyperalgesia of masseter muscle induced by masseter injection of DHPG, an agonist of mGluR1/5, is also prevented by the treatment of masseter muscle with TRPV1 antagonist.85 mGluR1/5 can cause TRPV1 sensitization in primary afferents through multiple pathways. For example, DHPG directly activates TRPV1 by inducing de novo synthesis of diacylglycerol.86 DHPG has also been shown to decrease capsaicin-induced desensitization of TRPV1 through PKA by inducing de novo synthesis of prostaglandin E2.87 These mechanisms do not require the involvement of PKC, whereas DHPG-induced mechanical hyperalgesia of masseter muscle is attenuated by the pharmacological inhibition of PKC or by disrupting the interaction of TRPV1 with AKAP150.85 DHPG-induced sensitization of capsaicin-evoked currents depends on PKC but not PKA. Interestingly, TRPV1 antagonist prevents mechanical hyperalgesia of masseter muscle produced by the injection of PKC activator but not by PKA activator.85 Masseter injection of DHPG induces PKC-dependent phosphorylation of TRPV1 S800, suggesting that TRPV1 S800 phosphorylation is a common site of convergence in pathways of glutamate-induced regulation of TRPV1. TRPV1 S800 is located within the carboxy-terminal domain, which includes multiple regulatory domains for TRPV1 function.81 TRPV1 S800 is a PKC-specific phosphorylation site that produces functional sensitization upon activation, which can be mediated by multiple modalities of agonistic stimuli such as capsaicin, heat, and proton.88 Therefore, glutamate receptor-mediated regulation of TRPV1 through PKC-induced phosphorylation of S800 could be implicated in hyperalgesia. Indeed, a recent study determined a causal role of TRPV1 phosphorylation to masseter hyperalgesia using a knock-in mouse line in which mouse TRPV1 S801, an orthologue residue of rat TRPV1 S800, is mutated to alanine preventing PKC-induced phosphorylation of the residue.89 Spontaneous pain following CFA injection into masseter muscle is reduced in the knock-in mice. Masseter injection of TRPV1 antagonist further decreases spontaneous pain in both knock-in and wild-type (WT) genotypes, and the extent of inhibition is greater in WT than knock-in, suggesting that CFA-induced spontaneous pain is mediated by TRPV1 S801 phosphorylation-dependent and independent mechanisms.89
Masseter hyperalgesia induced by CFA or the injection of NMDA is also attenuated by TRPA1 inhibitor, suggesting interaction of NMDA receptor and TRPA1 in masseter afferents.63 Although mechanisms underlying glutamate receptor and TRPA1 are not known, and need to be determined, it is noteworthy that TRPA1 phosphorylation also contributes to nociception.90
Conclusions and future studies
Glutamate receptor and TRPV1 channel mechanisms in craniofacial muscle pain are summarized in Figure 1. Based on the current literature discussed herein, we hypothesize that intricate interactions of glutamate receptors and TRP channels contribute to the development and maintenance of craniofacial muscle nociception and hyperalgesia. In our model, glutamate receptors and TRP channels interact bi-directionally to modulate trigeminal nociceptors. Glutamate receptor activation leads to PKC-dependent phosphorylation of TRPV1, which contributes to hyperalgesia. Despite the advances in understanding of these mechanisms, questions remain. Activation of glutamate receptors does not directly activate TRP channels; therefore, endogenous ligands for TRP channels must participate. It will be critical to determine if putative endogenous ligands for TRP channels are increased in craniofacial muscles under chronic muscle pain conditions, including TMD. It will be also interesting to determine if glutamate receptor-TRP channel interactions contribute to hyperalgesia in other craniofacial muscle pain models such as prolonged mouth opening.91 Importantly, glutamate-evoked masseter pain was greater in women than in men.14,17 Masseteric injection of ketamine attenuates pain and mechanical hyperalgesia evoked by masseter injection of glutamate in men, but this effect was not observed in women.51,92 Estrogen upregulates NMDA receptor subunits, which may lead to sex differences in NMDA-evoked masseter afferent discharge.93 It will be of interest to determine sex differences in glutamate-TRP interaction in trigeminal muscle nociceptors. Better understanding of peripheral mechanisms of craniofacial muscle pain conditions should help us to develop better strategies for the management of chronic craniofacial muscle pain. For example, manipulation of TRP channel phosphorylation or disruption of glutamate receptor-TRP channel interactions can attenuate masseter hyperalgesia. Functional silencing of masseter nociceptors may also provide effective treatment for widespread craniofacial muscle pain.
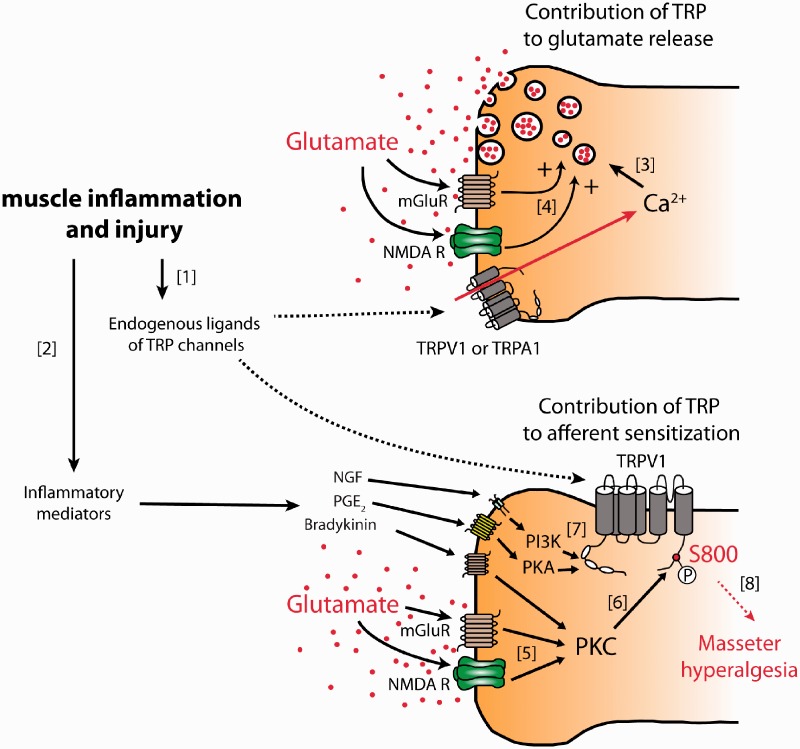
Figure 1.
Model of glutamate receptor-TRP channel mechanisms in craniofacial muscle hyperalgesia.
Two TRPV1-expressing afferent terminals within masseter muscle are diagrammed. TRP channels contribute to both glutamate release from masseter afferents and their sensitization. Although glutamate release (top) and sensitization (bottom) are diagrammed in separate afferent terminals for clarity, these two processes should occur in the same afferent terminals. [1] Injury or inflammation of craniofacial muscle should produce various chemical substances that either directly activate nociceptive TRP channels (e.g., oxidized linoleic acid metabolites or hydrogen peroxide) or [2] sensitize TRPV1 through the activation of protein kinases (e.g., nerve growth factor (NGF), prostaglandin E2 (PGE2), or bradykinin). [3] Activation of TRP channels mediates calcium influx to induce glutamate release from afferent terminals. [4] Released glutamate activates metabotropic or ionotropic glutamate receptor, which further enhances glutamate release providing positive feedback. [5] Released glutamate should act in an autocrine or paracrine manner to activate glutamate receptors. Glutamate receptor activation leads to activation of PKC, [6] which in turn phosphorylates TRPV1, especially S800 in rats. [7] Receptor activation by inflammatory mediators leads to signaling through PKC, PKA, and PI3K to further sensitize TRPV1. [8] TRPV1 integrates the effects of endogenous ligands, signaling from inflammatory mediators and glutamate receptors to eventually produce masseter hyperalgesia.
TRP: transient receptor potential; mGluR: metabotropic glutamate receptor; NMDAR: N-methyl-D-aspartate receptor; TRPV1: transient receptor potential vanilloid 1; TRPA1: transient receptor potential ankyrin 1; NGF: nerve growth factor; PGE2: prostaglandin E2; PI3K: phosphoinositide 3-kinase; PKA: protein kinase A; PKC: protein kinase C.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by DE023846 (to M-KC), DE027731 (to M-KC), and DE016062 (to JYR).
ORCID iD
Man-Kyo Chung https://orcid.org/0000-0001-7637-1148
References
- 1.Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, List T, Svensson P, Gonzalez Y, Lobbezoo F, Michelotti A, Brooks SL, Ceusters W, Drangsholt M, Ettlin D, Gaul C, Goldberg LJ, Haythornthwaite JA, Hollender L, Jensen R, John MT, De Laat A, de Leeuw R, Maixner W, van der Meulen M, Murray GM, Nixdorf DR, Palla S, Petersson A, Pionchon P, Smith B, Visscher CM, Zakrzewska J, Dworkin SF, International RDC/TMD Consortium Network, International association for Dental Research, Orofacial Pain Special Interest Group, International Association for the Study of Pain. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the international RDC/TMD consortium network* and orofacial pain special interest group†. J Oral Facial Pain Headache 2014; 28: 6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kopp S. Neuroendocrine, immune, and local responses related to temporomandibular disorders. J Orofac Pain 2001; 15: 9–28. [PubMed] [Google Scholar]
- 3.Kaneyama K, Segami N, Yoshimura H, Honjo M, Demura N. Increased levels of soluble cytokine receptors in the synovial fluid of temporomandibular joint disorders in relation to joint effusion on magnetic resonance images. J Oral Maxillofac Surg 2010; 68: 1088–1093. [DOI] [PubMed] [Google Scholar]
- 4.Kaneyama K, Segami N, Nishimura M, Suzuki T, Sato J. Importance of proinflammatory cytokines in synovial fluid from 121 joints with temporomandibular disorders. Br J Oral Maxillofac Surg 2002; 40: 418–423. [PubMed] [Google Scholar]
- 5.Lundh H, Westesson PL. Clinical signs of temporomandibular joint internal derangement in adults. An epidemiologic study. Oral Surg Oral Med Oral Pathol 1991; 72: 637–641. [DOI] [PubMed] [Google Scholar]
- 6.Molin C. Vertical isometric muscle forces of the mandible. A comparative study of subjects with and without manifest mandibular pain dysfunction syndrome. Acta Odontol Scand 1972; 30: 485–499. [DOI] [PubMed] [Google Scholar]
- 7.Hedenberg-Magnusson B, Ernberg M, Kopp S. Symptoms and signs of temporomandibular disorders in patients with fibromyalgia and local myalgia of the temporomandibular system. A comparative study. Acta Odontol Scand 1997; 55: 344–349. [DOI] [PubMed] [Google Scholar]
- 8.Mense S. Nociception from skeletal muscle in relation to clinical muscle pain. Pain 1993; 54: 241–289. [DOI] [PubMed] [Google Scholar]
- 9.Capra NF, Ro JY. Human and animal experimental models of acute and chronic muscle pain: intramuscular algesic injection. Pain 2004; 110: 3–7. [DOI] [PubMed] [Google Scholar]
- 10.Chung MK, Jung SJ, Oh SB. Role of TRP channels in pain sensation. Adv Exp Med Biol 2011; 704: 615–636. [DOI] [PubMed] [Google Scholar]
- 11.Du J, Koltzenburg M, Carlton SM. Glutamate-induced excitation and sensitization of nociceptors in rat glabrous skin. Pain 2001; 89: 187–198. [DOI] [PubMed] [Google Scholar]
- 12.Lawand NB, Willis WD, Westlund KN. Excitatory amino acid receptor involvement in peripheral nociceptive transmission in rats. Eur J Pharmacol 1997; 324: 169–177. [DOI] [PubMed] [Google Scholar]
- 13.Beirith A, Santos AR, Calixto JB. Mechanisms underlying the nociception and paw oedema caused by injection of glutamate into the mouse paw. Brain Res 2002; 924: 219–228. [DOI] [PubMed] [Google Scholar]
- 14.Cairns BE, Hu JW, Arendt-Nielsen L, Sessle BJ, Svensson P. Sex-related differences in human pain and rat afferent discharge evoked by injection of glutamate into the masseter muscle. J Neurophysiol 2001; 86: 782–791. [DOI] [PubMed] [Google Scholar]
- 15.Cairns BE, Gambarota G, Svensson P, Arendt-Nielsen L, Berde CB. Glutamate-induced sensitization of rat masseter muscle fibers. Neuroscience 2002; 109: 389–399. [DOI] [PubMed] [Google Scholar]
- 16.Fiorentino PM, Cairns BE, Hu JW. Development of inflammation after application of mustard oil or glutamate to the rat temporomandibular joint. Arch Oral Biol 1999; 44: 27–32. [DOI] [PubMed] [Google Scholar]
- 17.Castrillon EE, Cairns BE, Wang K, Arendt-Nielsen L, Svensson P. Comparison of glutamate-evoked pain between the temporalis and masseter muscles in men and women. Pain 2012; 153: 823–829. [DOI] [PubMed] [Google Scholar]
- 18.Svensson P, Wang K, Arendt-Nielsen L, Cairns BE, Sessle BJ. Pain effects of glutamate injections into human jaw or neck muscles. J Orofac Pain 2005; 19: 109–118. [PubMed] [Google Scholar]
- 19.Castrillon EE, Cairns BE, Ernberg M, Wang K, Sessle B, Arendt-Nielsen L, Svensson P. Glutamate-evoked jaw muscle pain as a model of persistent myofascial TMD pain? Arch Oral Biol 2008; 53: 666–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C, Ge HY, Ibarra JM, Yue SW, Madeleine P, Arendt-Nielsen L. Spatial pain propagation over time following painful glutamate activation of latent myofascial trigger points in humans. J Pain 2012; 13: 537–545. [DOI] [PubMed] [Google Scholar]
- 21.Klegeris A, McGeer PL. Beta-amyloid protein enhances macrophage production of oxygen free radicals and glutamate. J Neurosci Res 1997; 49: 229–235. [DOI] [PubMed] [Google Scholar]
- 22.Parpura V, Liu F, Jeftinija KV, Haydon PG, Jeftinija SD. Neuroligand-evoked calcium-dependent release of excitatory amino acids from Schwann cells. J Neurosci 1995; 15: 5831–5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.deGroot J, Zhou S, Carlton SM. Peripheral glutamate release in the hindpaw following low and high intensity sciatic stimulation. Neuroreport 2000; 11: 497–502. [DOI] [PubMed] [Google Scholar]
- 24.Li JL, Xiong KH, Dong YL, Fujiyama F, Kaneko T, Mizuno N. Vesicular glutamate transporters, VGluT1 and VGluT2, in the trigeminal ganglion neurons of the rat, with special reference to coexpression. J Comp Neurol 2003; 463: 212–220. [DOI] [PubMed] [Google Scholar]
- 25.Jin YH, Yamaki F, Takemura M, Koike Y, Furuyama A, Yonehara N. Capsaicin-induced glutamate release is implicated in nociceptive processing through activation of ionotropic glutamate receptors and group I metabotropic glutamate receptor in primary afferent fibers. J Pharmacol Sci 2009; 109: 233–241. [DOI] [PubMed] [Google Scholar]
- 26.Omote K, Kawamata T, Kawamata M, Namiki A. Formalin-induced release of excitatory amino acids in the skin of the rat hindpaw. Brain Res 1998; 787: 161–164. [DOI] [PubMed] [Google Scholar]
- 27.Gazerani P, Au S, Dong X, Kumar U, Arendt-Nielsen L, Cairns BE. Botulinum neurotoxin type A (BoNTA) decreases the mechanical sensitivity of nociceptors and inhibits neurogenic vasodilation in a craniofacial muscle targeted for migraine prophylaxis. Pain 2010; 151: 606–616. [DOI] [PubMed] [Google Scholar]
- 28.Lawand NB, McNearney T, Westlund KN. Amino acid release into the knee joint: key role in nociception and inflammation. Pain 2000; 86: 69–74. [DOI] [PubMed] [Google Scholar]
- 29.Jean YH, Wen ZH, Chang YC, Hsieh SP, Lin JD, Tang CC, Chen WF, Chou AK, Wong CS. Increase in excitatory amino acid concentration and transporters expression in osteoarthritic knees of anterior cruciate ligament transected rabbits. Osteoarthr Cartil 2008; 16: 1442–1449. [DOI] [PubMed] [Google Scholar]
- 30.Chung MK, Park J, Asgar J, Ro JY. Transcriptome analysis of trigeminal ganglia following masseter muscle inflammation in rats. Mol Pain 2016; 12: pii: 1744806916668526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lund JP, Sadeghi S, Athanassiadis T, Caram Salas N, Auclair F, Thivierge B, Arsenault I, Rompre P, Westberg KG, Kolta A. Assessment of the potential role of muscle spindle mechanoreceptor afferents in chronic muscle pain in the rat masseter muscle. PLoS One 2010; 5: e11131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alfredson H, Forsgren S, Thorsen K, Lorentzon R. In vivo microdialysis and immunohistochemical analyses of tendon tissue demonstrated high amounts of free glutamate and glutamate NMDAR1 receptors, but no signs of inflammation, in Jumper’s knee. J Orthop Res 2001; 19: 881–886. [DOI] [PubMed] [Google Scholar]
- 33.Alfredson H, Thorsen K, Lorentzon R. In situ microdialysis in tendon tissue: high levels of glutamate, but not prostaglandin E2 in chronic Achilles tendon pain. Knee Surg Sports Traumatol Arthrosc 1999; 7: 378–381. [DOI] [PubMed] [Google Scholar]
- 34.Rosendal L, Larsson B, Kristiansen J, Peolsson M, Sogaard K, Kjaer M, Sorensen J, Gerdle B. Increase in muscle nociceptive substances and anaerobic metabolism in patients with trapezius myalgia: microdialysis in rest and during exercise. Pain 2004; 112: 324–334. [DOI] [PubMed] [Google Scholar]
- 35.Larsson B, Rosendal L, Kristiansen J, Sjogaard G, Sogaard K, Ghafouri B, Abdiu A, Kjaer M, Gerdle B. Responses of algesic and metabolic substances to 8 h of repetitive manual work in myalgic human trapezius muscle. Pain 2008; 140: 479–490. [DOI] [PubMed] [Google Scholar]
- 36.Kreiner F, Galbo H. Elevated muscle interstitial levels of pain-inducing substances in symptomatic muscles in patients with polymyalgia rheumatica. Pain 2011; 152: 1127–1132. [DOI] [PubMed] [Google Scholar]
- 37.Gerdle B, Larsson B, Forsberg F, Ghafouri N, Karlsson L, Stensson N, Ghafouri B. Chronic widespread pain: increased glutamate and lactate concentrations in the trapezius muscle and plasma. Clin J Pain 2014; 30: 409–420. [DOI] [PubMed] [Google Scholar]
- 38.Castrillon EE, Ernberg M, Cairns BE, Wang K, Sessle BJ, Arendt-Nielsen L, Svensson P. Interstitial glutamate concentration is elevated in the masseter muscle of myofascial temporomandibular disorder patients. J Orofac Pain 2010; 24: 350–360. [PubMed] [Google Scholar]
- 39.Bajramaj E, Haggman-Henrikson B, Dawson A, Gerdle B, Ghafouri B. The effect of microdialysis catheter insertion on glutamate and serotonin levels in masseter muscle in patients with myofascial temporomandibular disorders and healthy controls. Diagnostics (Basel) 2019; 9: pii: E14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Louca S, Christidis N, Ghafouri B, Gerdle B, Svensson P, List T, Ernberg M. Serotonin, glutamate and glycerol are released after the injection of hypertonic saline into human masseter muscles - a microdialysis study. J Headache Pain 2014; 15: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimada A, Castrillon EE, Baad-Hansen L, Ghafouri B, Gerdle B, Wahlen K, Ernberg M, Cairns BE, Svensson P. Increased pain and muscle glutamate concentration after single ingestion of monosodium glutamate by myofascial temporomandibular disorders patients. Eur J Pain 2016; 20: 1502–1512. [DOI] [PubMed] [Google Scholar]
- 42.Dawson A, Ghafouri B, Gerdle B, List T, Svensson P, Ernberg M. Effects of experimental tooth clenching on pain and intramuscular release of 5-HT and glutamate in patients with myofascial TMD. Clin J Pain 2015; 31: 740–749. [DOI] [PubMed] [Google Scholar]
- 43.Ernberg M, Castrillon EE, Ghafouri B, Larsson B, Gerdle B, List T, Svensson P. Experimental myalgia induced by repeated infusion of acidic saline into the human masseter muscle does not cause the release of algesic substances. Eur J Pain 2013; 17: 539–550. [DOI] [PubMed] [Google Scholar]
- 44.Carlton SM, Hargett GL, Coggeshall RE. Localization and activation of glutamate receptors in unmyelinated axons of rat glabrous skin. Neurosci Lett 1995; 197: 25–28. [DOI] [PubMed] [Google Scholar]
- 45.Coggeshall RE, Carlton SM. Ultrastructural analysis of NMDA, AMPA, and kainate receptors on unmyelinated and myelinated axons in the periphery. J Comp Neurol 1998; 391: 78–86. [DOI] [PubMed] [Google Scholar]
- 46.Ro JY. Contribution of peripheral NMDA receptors in craniofacial muscle nociception and edema formation. Brain Res 2003; 979: 78–84. [DOI] [PubMed] [Google Scholar]
- 47.Ro JY, Capra NF, Masri R. Contribution of peripheral N-methyl-D-aspartate receptors to c-fos expression in the trigeminal spinal nucleus following acute masseteric inflammation. Neuroscience 2004; 123: 213–219. [DOI] [PubMed] [Google Scholar]
- 48.Ro JY, Capra NF, Lee JS, Masri R, Chun YH. Hypertonic saline-induced muscle nociception and c-fos activation are partially mediated by peripheral NMDA receptors. Eur J Pain 2007; 11: 398–405. [DOI] [PubMed] [Google Scholar]
- 49.Ro JY, Nies M, Zhang Y. The role of peripheral N-methyl-D-aspartate receptors in muscle hyperalgesia. Neuroreport 2005; 16: 485–489. [DOI] [PubMed] [Google Scholar]
- 50.Cairns BE, Svensson P, Wang K, Hupfeld S, Graven-Nielsen T, Sessle BJ, Berde CB, Arendt-Nielsen L. Activation of peripheral NMDA receptors contributes to human pain and rat afferent discharges evoked by injection of glutamate into the masseter muscle. J Neurophysiol 2003; 90: 2098–2105. [DOI] [PubMed] [Google Scholar]
- 51.Cairns BE, Svensson P, Wang K, Castrillon E, Hupfeld S, Sessle BJ, Arendt-Nielsen L. Ketamine attenuates glutamate-induced mechanical sensitization of the masseter muscle in human males. Exp Brain Res 2006; 169: 467–472. [DOI] [PubMed] [Google Scholar]
- 52.Lee JS, Ro JY. Peripheral metabotropic glutamate receptor 5 mediates mechanical hypersensitivity in craniofacial muscle via protein kinase C dependent mechanisms. Neuroscience 2007; 146: 375–383. [DOI] [PubMed] [Google Scholar]
- 53.Lee HJ, Choi HS, Ju JS, Bae YC, Kim SK, Yoon YW, Ahn DK. Peripheral mGluR5 antagonist attenuated craniofacial muscle pain and inflammation but not mGluR1 antagonist in lightly anesthetized rats. Brain Res Bull 2006; 70: 378–385. [DOI] [PubMed] [Google Scholar]
- 54.Marchettini P, Simone DA, Caputi G, Ochoa JL. Pain from excitation of identified muscle nociceptors in humans. Brain Res 1996; 740: 109–116. [DOI] [PubMed] [Google Scholar]
- 55.Sohn MK, Graven-Nielsen T, Arendt-Nielsen L, Svensson P. Inhibition of motor unit firing during experimental muscle pain in humans. Muscle Nerve 2000; 23: 1219–1226. [DOI] [PubMed] [Google Scholar]
- 56.Ro JY, Lee JS, Zhang Y. Activation of TRPV1 and TRPA1 leads to muscle nociception and mechanical hyperalgesia. Pain 2009; 144: 270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takeda M, Tanimoto T, Ito M, Nasu M, Matsumoto S. Role of capsaicin-sensitive primary afferent inputs from the masseter muscle in the C1 spinal neurons responding to tooth-pulp stimulation in rats. Exp Brain Res 2005; 160: 107–117. [DOI] [PubMed] [Google Scholar]
- 58.Hoheisel U, Reinohl J, Unger T, Mense S. Acidic pH and capsaicin activate mechanosensitive group IV muscle receptors in the rat. Pain 2004; 110: 149–157. [DOI] [PubMed] [Google Scholar]
- 59.Ro JY, Lee J, Capra NF, Zhang Y. Role of soluble guanylate cyclase in the trigeminal subnucleus caudalis in capsaicin-induced muscle hypersensitivity. Brain Res 2007; 1184: 141–148. [DOI] [PubMed] [Google Scholar]
- 60.Ro JY, Capra NF. Assessing mechanical sensitivity of masseter muscle in lightly anesthetized rats: a model for craniofacial muscle hyperalgesia. Neurosci Res 2006; 56: 119–123. [DOI] [PubMed] [Google Scholar]
- 61.Sugiyama D, Kang S, Arpey N, Arunakul P, Usachev YM, Brennan TJ. Hydrogen peroxide induces muscle nociception via transient receptor potential ankyrin 1 receptors. Anesthesiology 2017; 127: 695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fujii Y, Ozaki N, Taguchi T, Mizumura K, Furukawa K, Sugiura Y. TRP channels and ASICs mediate mechanical hyperalgesia in models of inflammatory muscle pain and delayed onset muscle soreness. Pain 2008; 140: 292–304. [DOI] [PubMed] [Google Scholar]
- 63.Asgar J, Zhang Y, Saloman JL, Wang S, Chung MK, Ro JY. The role of TRPA1 in muscle pain and mechanical hypersensitivity under inflammatory conditions in rats. Neuroscience 2015; 310: 206–215. [26393428] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang S, Brigoli B, Lim J, Karley A, Chung MK. Roles of TRPV1 and TRPA1 in spontaneous pain from inflamed masseter muscle. Neuroscience 2018; 384: 290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang S, Lim J, Joseph J, Wang S, Wei F, Ro JY, Chung M-K. Spontaneous and bite-evoked muscle pain are mediated by a common nociceptive pathway with differential contribution by TRPV1. J Pain 2017; 18: 1333–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ruparel S, Green D, Chen P, Hargreaves KM. The cytochrome P450 inhibitor, ketoconazole, inhibits oxidized linoleic acid metabolite-mediated peripheral inflammatory pain. Mol Pain 2012; 8: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trevisan G, Hoffmeister C, Rossato MF, Oliveira SM, Silva MA, Silva CR, Fusi C, Tonello R, Minocci D, Guerra GP, Materazzi S, Nassini R, Geppetti P, Ferreira J. TRPA1 receptor stimulation by hydrogen peroxide is critical to trigger hyperalgesia and inflammation in a model of acute gout. Free Radic Biol Med 2014; 72: 200–209. [DOI] [PubMed] [Google Scholar]
- 68.Chung MK, Asgar J, Jennifer P, Ro JY. Transcriptome analysis of trigeminal ganglia following masseter muscle inflammation in rats. Mol Pain 2016; 12: 174480691666852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lam DK, Sessle BJ, Hu JW. Glutamate and capsaicin effects on trigeminal nociception II: activation and central sensitization in brainstem neurons with deep craniofacial afferent input. Brain Res 2009; 1253: 48–59. [DOI] [PubMed] [Google Scholar]
- 70.Lam DK, Sessle BJ, Hu JW. Glutamate and capsaicin effects on trigeminal nociception I: activation and peripheral sensitization of deep craniofacial nociceptive afferents. Brain Res 2009; 1251: 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang K, Svensson P, Sessle BJ, Cairns BE, Arendt-Nielsen L. Interactions of glutamate and capsaicin-evoked muscle pain on jaw motor functions of men. Clin Neurophysiol 2010; 121: 950–956. [DOI] [PubMed] [Google Scholar]
- 72.Arendt-Nielsen L, Svensson P, Sessle BJ, Cairns BE, Wang K. Interactions between glutamate and capsaicin in inducing muscle pain and sensitization in humans. Eur J Pain 2008; 12: 661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sato H, Castrillon EE, Cairns BE, Bendixen KH, Wang K, Nakagawa T, Wajima K, Svensson P. Intramuscular temperature modulates glutamate-evoked masseter muscle pain intensity in humans. J Oral Facial Pain Headache 2015; 29: 158–167. [DOI] [PubMed] [Google Scholar]
- 74.Sato H, Castrillon EE, Cairns BE, Bendixen KH, Wang K, Nakagawa T, Wajima K, Svensson P. Intramuscular pH modulates glutamate-evoked masseter muscle pain magnitude in humans. Eur J Pain 2016; 20: 106–115. [DOI] [PubMed] [Google Scholar]
- 75.Spitzer MJ, Reeh PW, Sauer SK. Mechanisms of potassium- and capsaicin-induced axonal calcitonin gene-related peptide release: involvement of L- and T-type calcium channels and TRPV1 but not sodium channels. Neuroscience 2008; 151: 836–842. [DOI] [PubMed] [Google Scholar]
- 76.Tang ML, Haas DA, Hu JW. Capsaicin-induced joint inflammation is not blocked by local anesthesia. Anesth Prog 2004; 51: 2–9. [PMC free article] [PubMed] [Google Scholar]
- 77.Chun YH, Ro JY. Electrophysiological characterization of the rat trigeminal caudalis (Vc) neurons following intramuscular injection of capsaicin. Neurosci Lett 2010; 469: 289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Malcangio M, Fernandes K, Tomlinson DR. NMDA receptor activation modulates evoked release of substance P from rat spinal cord. Br J Pharmacol 1998; 125: 1625–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu H, Mantyh PW, Basbaum AI. NMDA-receptor regulation of substance P release from primary afferent nociceptors. Nature 1997; 386: 721–724. [DOI] [PubMed] [Google Scholar]
- 80.Chung MK, Campbell JN. Use of capsaicin to treat pain: mechanistic and therapeutic considerations. Pharmaceuticals (Basel) 2016; 9: E66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Joseph J, Wang S, Lee J, Ro JY, Chung MK. Carboxyl-terminal domain of transient receptor potential vanilloid 1 contains distinct segments differentially involved in capsaicin- and heat-induced desensitization. J Biol Chem 2013; 288: 35690–35702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee J, Saloman JL, Weiland G, Auh QS, Chung MK, Ro JY. Functional interactions between NMDA receptors and TRPV1 in trigeminal sensory neurons mediate mechanical hyperalgesia in the rat masseter muscle. Pain 2012; 153: 1514–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee J, Chung MK, Ro JY. Activation of NMDA receptors leads to phosphorylation of TRPV1 S800 by protein kinase C and A-Kinase anchoring protein 150 in rat trigeminal ganglia. Biochem Biophys Res Commun 2012; 424: 358–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jeske NA, Diogenes A, Ruparel NB, Fehrenbacher JC, Henry M, Akopian AN, Hargreaves KM. A-kinase anchoring protein mediates TRPV1 thermal hyperalgesia through PKA phosphorylation of TRPV1. Pain 2008; 138: 604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chung MK, Lee J, Joseph J, Saloman J, Ro JY. Peripheral group I metabotropic glutamate receptor activation leads to muscle mechanical hyperalgesia through TRPV1 phosphorylation in the rat. J Pain 2015; 16: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim YH, Park CK, Back SK, Lee CJ, Hwang SJ, Bae YC, Na HS, Kim JS, Jung SJ, Oh SB. Membrane-delimited coupling of TRPV1 and mGluR5 on presynaptic terminals of nociceptive neurons. J Neurosci 2009; 29: 10000–10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu HJ, Bhave G, Gereau RWt. Prostaglandin and protein kinase A-dependent modulation of vanilloid receptor function by metabotropic glutamate receptor 5: potential mechanism for thermal hyperalgesia. J Neurosci 2002; 22: 7444–7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang S, Joseph J, Ro JY, Chung MK. Modality-specific mechanisms of protein kinase C-induced hypersensitivity of TRPV1: S800 is a polymodal sensitization site. Pain 2015; 156: 931–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Joseph J, Qu L, Wang S, Kim M, Bennett D, Ro J, Caterina M, Chung MK. Phosphorylation of TRPV1 S801 contributes to modality-specific hyperalgesia in mice. J Neurosci 2019; 39: 9954–9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hall BE, Prochazkova M, Sapio MR, Minetos P, Kurochkina N, Binukumar BK, Amin ND, Terse A, Joseph J, Raithel SJ, Mannes AJ, Pant HC, Chung MK, Iadarola MJ, Kulkarni AB. Phosphorylation of the transient receptor potential ankyrin 1 by cyclin-dependent kinase 5 affects chemo-nociception. Sci Rep 2018; 8: 1177–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hawkins JL, Durham PL. Prolonged jaw opening promotes nociception and enhanced cytokine expression. J Oral Facial Pain Headache 2016; 30: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Castrillon EE, Cairns BE, Ernberg M, Wang K, Sessle BJ, Arendt-Nielsen L, Svensson P. Effect of a peripheral NMDA receptor antagonist on glutamate-evoked masseter muscle pain and mechanical sensitization in women. J Orofac Pain 2007; 21: 216–224. [PubMed] [Google Scholar]
- 93.Dong XD, Mann MK, Kumar U, Svensson P, Arendt-Nielsen L, Hu JW, Sessle BJ, Cairns BE. Sex-related differences in NMDA-evoked rat masseter muscle afferent discharge result from estrogen-mediated modulation of peripheral NMDA receptor activity. Neuroscience 2007; 146: 822–832. [DOI] [PMC free article] [PubMed] [Google Scholar]