Introduction
Motor deficit is the most common physical complication after stroke and improving motor outcomes remains a challenging issue in the field of stroke recovery. Dynamic changes in motor cortical excitability across the lesional hemisphere (decreased cortical excitability) and the contra-lesional hemisphere (over activated cortical exctiablity) after stroke has been observed.1 This inter-hemispheric imbalance or inhibition has been the model for several proposed experimental brain modulation tools. Transcranial direct current stimulation (tDCS) can modulate cortical excitability (a typical configuration involves an anodal electrode on the lesional hemisphere and a cathodal electrode on the contra-lesional hemisphere) with a lasting after-effect in a somewhat dose-dependent fashion.2 tDCS may improve motor skill learning through augmentation of synaptic plasticity that requires BDNF secretion and TrkB activation.3 Several tDCS studies in post-stroke motor recovery, either single session or multiple sessions, have examined potential benefits as well as safety profiles. 4–7 The relatively low cost and the ease of administering tDCS has boosted this flurry of studies. However, data on tDCS efficacy in stroke motor recovery have been mixed and inconsistent, leaving several issues to be resolved before tDCS is ready for widespread clinical application in post-stroke motor recovery in the future.
In this review, we will systematically examine and discuss the hurdles and challenges in using tDCS as a brain modulation tool to enhance and facilitate recovery after stroke, and propose potential solutions pertinent to using tDCS with the goal of harnessing these opportunities.
Interhemispheric Inhibition Model and Montage
An influential theoretical model upon which much of non-invasive brain stimulation for stroke patients is based includes the following: (1) an interhemispheric inhibition of human motor cortex (i.e., each motor cortex inhibits the other one); and (2) the imbalance of such interhemispheric motor interactions after a stroke with the unaffected, and over-excited motor cortex exerting an unmatched transcallosal inhibitory effect onto the affected motor cortex, which in turn interferes with the recovery process.8, 9 Therefore, the approach for applying tDCS generally has been to either up-regulate the lesional hemisphere with excitatory anodal stimulation, down-regulate the contralesional hemisphere with inhibitory cathodal stimulation, or use bihemispheric stimulation applying anodal stimulation on the lesional side and cathodal stimulation on the contralesional side simultaneously.4 There have been several challenges to this conventional wisdom. For example, a recent Transcranial Magnetic Stimulation (TMS) study revealed corticomotor excitability did not change for the contralesional hemisphere during a period of motor recovery, thus challenging the notion that cathodal stimulation applied to the contralesional side is necessary.10 A subsequent meta-analysis of 112 published studies using TMS showed that the neurophysiological effects of stroke are mainly localized to the lesioned hemisphere, and there was no clear evidence for hyper-excitability of the contra-lesional hemisphere or interhemispheric imbalance after stroke. 11 One tDCS study demonstrated that cathodal stimulation on the ipsilesional hemisphere during the sub-acute phase of stroke could improve motor function as well as reduce spasticity.12 Recently, Waters et al13 also challenged this inter-hemispheric inhibition model, in which they proposed that two hemispheres interact cooperatively rather than competitively. Regardless, their experiment supported the notion that bi-hemispheric stimulation (using either montage) yielded substantial performance gains relative to uni-hemispheric (anodal or cathodal) or sham stimulation. Therefore, it is not clear whether the interhemispheric inhibition model still holds for stroke patients with unihemispheric infarcts and possibly altered interhemispheric interactions.
Different montages may generate different electric fields and may have differential brain modulatory effects. 14, 15 For example, extracephalic montages may lead to the significantly higher amount of currents passing through the brainstem when compared with other montages where both electrodes are positioned on the scalp. Extracephalic montages are no longer used in stroke patients due to this safety concern, but it may deserve further investigation. Three common electrode montages used in post-stroke motor recovery are: (1) Anodal montage (anode on ipsilesional C3/4, cathode over the supraorbital region on the contralesional hemisphere (e.g., FP2/1 in 10/20 EEG system); (2) cathodal montage (anode on ipsilesional FP1/2, cathode on contralesional C4/3); and (3) bihemispheric montage (C3/C4 montage with anode on the ipsilesional motor cortex and cathode on contralesional motor cortex). Theoretically, the bihemispheric montage may offer an advantage of simultaneous excitation of the hypoactive ipsilesional motor cortex, and suppression of contralesional motor cortex.8, 9, 16 Two studies in healthy subjects showed stronger motor learning effects after bi-hemispheric stimulation than after uni-hemispheric stimulation.16, 17 A recent meta-analysis of tDCS post-stroke motor studies demonstrated that bihemispheric montage might have better success odds than unihemispheric montages either with cathodal on the contralesional or anodal on the lesional side montage regarding reducing motor impairment as measured by the Fugl-Meyer Motor Scale.18
Optimal Dose and Safety Concerns
Hundreds of tDCS studies have been done in healthy control subjects as well as subjects with various disease conditions, including stroke. However, the optimal tDCS dose, with maximal efficacy and safety, has not been well established in humans, especially in stroke patients. Early data suggested that there is a dose-response relationship from 0.1 mA to 1 mA using an amplitude of motor evoked potentials (MEP) as a surrogate measure of cortical excitability in healthy controls.19 More recently, a positive dose-dependent relationship between the upper extremity motor impairment reduction and current density in the 0.03 – 0.09 mA/cm2 range (not current level) was demonstrated in a meta-regression using data from stroke patients.18 It is not clear whether this trend will extend beyond this dose range. Two proof-of-concept studies showed signs of promise. Higher current strengths led to higher cortical excitability in otherwise identical tDCS stimulation setup.20 Use of smaller pad sizes while controlling for tDCS current amplitudes and other stimulation parameters (leading to higher current density) also led to higher cortical excitability.21
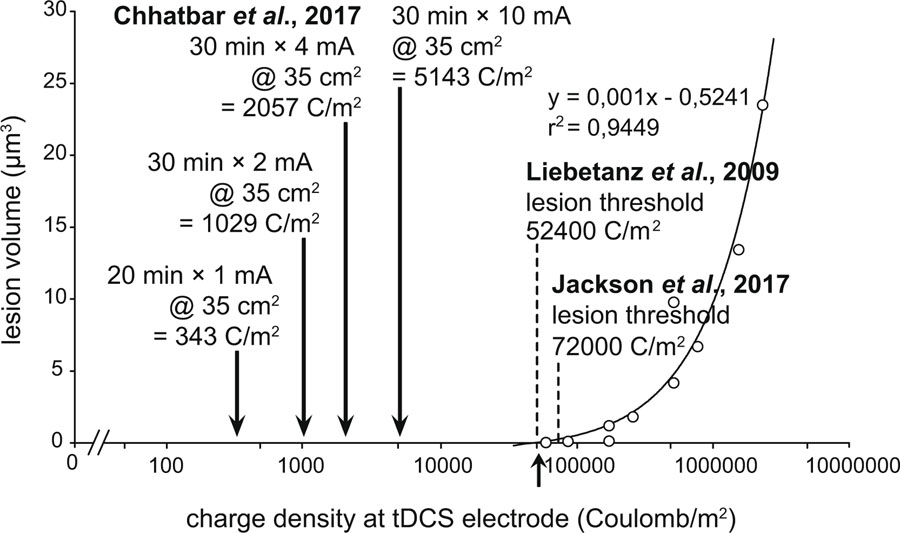
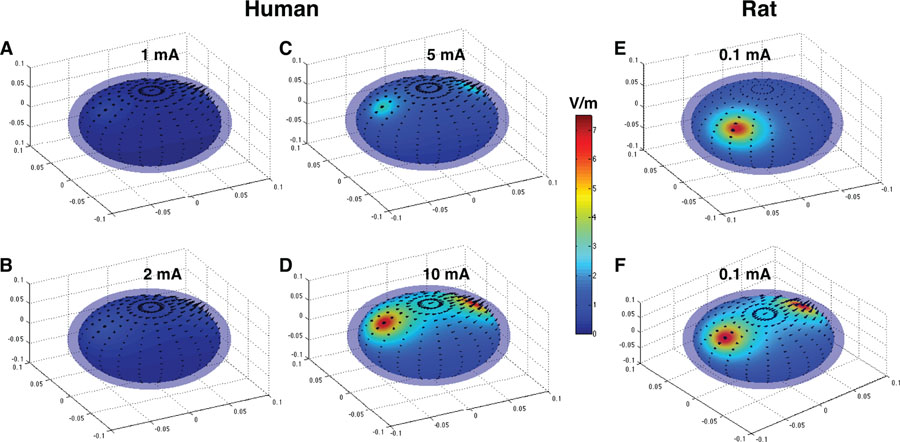
The primary concern of higher doses of tDCS is centered on potential injury to the brain, but an animal study suggests that up to two orders of magnitude higher doses, i.e., 14.29 mA/cm2, than the ones used in human protocols are required before any structural brain injury occurs.22 For example, tDCS at 10 mA with 35 cm2 pad size for 30 minutes translates to a current density of 0.286 mA/cm2 at the skin-electrode interface with the charge density of 5143 C/m2, which is an order of magnitude lower than the doses (charge density between 52400 to 72000 C/m2) that caused brain injury in rodents (Fig. 1). Charge density is a more comprehensive safety measure than current density for tDCS because the parameter of charge density takes into consideration of stimulation duration.23, 24 Simulations on spherical head models14 (with skull and scalp layers intactness assumption) suggest that current in the order of 10 mA likely leads to the generation of electric fields that are comparable to those generated in rodent brain without any apparent brain damage (Fig. 2). In cases of skull defect and/or electrically conductive implant, applied tDCS currents can “short” through such interfaces. This can result in much higher electric fields at the adjacent or nearby part of the brain. There might also be an overall increase in the electric field distribution throughout the brain because of relatively lower extracranial shunting of currents. As mentioned before, existing tDCS protocols use much smaller amounts of currents than have been thought to damage the brain.
Figure 1. Comparison of Safety Between Animal and Human Studies based on tDCS Dose Levels.
Typical human studies involve charge density (current amplitude × duration of stimulation ÷ pad size) of ~1 kC/m2 or less. A recent tDCS dose escalation study demonstrated the safety of ~2 kC/m2 in stroke subjects. Stimulation using 10 mA tDCS for 30 minutes on a standard 35 cm2 pad size offers ~5 kC/m2 charge density, which is still an order of magnitude lower than >50 kC/m2 as required in animal studies to cause brain injury.
Figure 2. Spheric Head Model Simulations Suggest that tDCS current in the order of 10 mA in humans generate electric fields that are experimentally shown to cause no damage in rodent brain.
A-D, A human spherical head model of radius 8 cm with bihemispheric C3/C4 montage is used to simulate electric fields using 1, 2, 5 and 10 mA currents. E-F, Rat spherical head model of radius 0.8 cm is used to simulate electric fields using 0.1 mA current with F3 montage (E) and C3/C4 montage (F). 0.1 mA current was shown to be safe in rodents for tDCS duration of 4.5 hours (270 minutes).22 Note close similarity of generated electric field intensity between 10 mA human model (D) and 0.1 mA rat model (F) and compare it with conventional 1 mA (A) and 2 mA (B) currents in human tDCS applications. Figure panels are generated with SPHERES14 and adapted.
In addition to safety concerns for brain tissue, there are also safety concerns for the skin. Skin is the most electrically resistive component of the human body. Higher resistance at the skin leads to highest electrical energy conversion and dissipation at skin level (P = I x R; where P is power, I is current, and R is resistance). This makes skin more prone to damage when compared with other less resistive body organs. The possibility of resulting skin damage is demonstrated by multiple reports of skin damage potentially from tDCS,25–27 but the exact etiology of skin damage remains to be elucidated. One possible explanation is the “edge effect” (i.e. higher electric fields at the edge of electrode pad) that is more pronounced at corners of rectangular electrode pads than with circular pads and can be lowered by use of the less conductive medium.28 Thin or worn out pads tend to make a patient more uncomfortable and irritated with the increased sensation of scalp tingling possibly because of heterogeneous distribution of current at electrode-skin interface. Thermal injury at electrode sites can be a possibility, but the 4 mA tDCS dose escalation study in the patients with ischemic stroke did not find a rise in skin temperature as a result of tDCS application at any dose level from 1 mA to 4 mA.29 In this aforementioned phase I study, all 18 patients completed the stimulation without meeting any pre-specified safety rules: including any skin injury or brain injury. It marks the first evidence about the safety profile and tolerability of tDCS intensity relatively higher than that conventionally used in most clinical trials in any disease conditions. It is a logical step to test the efficacy of tDCS at higher amplitudes in phase II multi-center study.
Inter-individual Variability and Modeling
Variability in response to tDCS has been observed in both studies as well as individual levels. The one-size-fits-all approach (same “applied dose” in all subjects) maybe one of contributing factors to this variability. The difference in individual head geometry and anatomy can result in variability in generation of electric fields in the brain region of interest (“targeted dose”) across subjects.30–32 Simulations of electric fields during the application of tDCS is potentially a good way to estimate the “targeted dose” in the brain area of interest based on the “applied dose” through a combination of electrode-related parameters, electrode montage and anatomical and physiochemical characteristics of the human body. Modeling has been a requirement in recent requests for application from National Institute of Health (NIH). While simulations seem realistic as many groups use head MRI to generate finite element models of the brain, skull, and scalp, such simulations are not free of limitations. Anisotropy created by white matter tracts, inhomogenous stroke lesion or defects in the skull itself, small vessel disease which can be bihemispheric and the cystic nature of a chronic stroke where the cyst is filled with protein-rich CSF and surrounded by a membrane can lead to drastic change in distribution of electric fields inside the brain.32–34 Many groups do not include anisotropy, introduced by fiber tracts in the white matter for example, which may lead to the suboptimal modeling of generated electric fields inside the brain.35, 36 Calculation of tissue anisotropy heavily depends on the quality of MRI sequences, especially the number of directions the diffusion is tested. This generates a trade-off between the richness of diffusion-weighted images (DWI) and the possibility of induced movement artifacts due to the duration of the sequence, and last but not least the amount of time and money that is required to obtain these kinds of sequences. More importantly, no predictive modeling has been attempted to date that can use regressors from existing DWI datasets and offer correction for the tDCS simulation models using voxel-by-voxel anisotropy measures. The accuracy of simulation models depends on the resolution of images and the amount of detail in the creation of finite element montage. Finally, no existing tDCS simulation model has been validated by an experimental sampling of tDCS-generated electric fields in the human brain. Recently there have been attempts to measure electric field in the human brain in vivo while applying alternative current 37, 38 or direct current39 through the scalp. Such measurements may provide critical data for validation and/or refinement of existing tDCS simulation models.
Subject Selection
Patient selection is always a critical factor in the success of any clinical trial, including tDCS trials. It may contribute to the inter-individual variability discussed above. For example, a large effect size was observed in Lindenberg’s study. 40 In this study, the subjects were stroke patients at the chronic stage with the mild and moderate deficit with an FM-UE score of ~39 (out of 66) at the baseline. By contrast, in another study with negative results, enrolled subjects were in the acute stroke phase, had severe deficits (e.g., an FM-UE < 20), or had large lesions that completely damaged the primary motor cortex and/or its descending corticospinal tract.18 Upon further analysis, authors discovered several patients with relatively milder deficit, as reflected by positive motor evoked potentials (MEPs), appear to respond to active tDCS much better than sham stimulation. Similarly, in an epidural cortical electric stimulation study, only those stroke patients with stimulation-induced upper limb movement during motor threshold assessment (suggesting partial corticospinal tract integrity) benefited from the stimulation protocol.41 This concept was also supported by an imaging biomarker study showing that regardless of rehabilitation therapy, a stroke patient is unlikely to recover even if achieving an FM-UE score of 25 if the corticospinal tract was severely damaged to a certain threshold in the acute phase.42 Careful subject selection based on behavioral assessment (i.e., FM-UE scale), electrophysiological measures (TMS-induced MEP assessing the functional integrity of CST), imaging approach (assessing the structural integrity of CST) or combination of these measures may enhance the likelihood of success of future tDCS trials.
Selecting patient from the acute-subacute vs. the chronic phase or determining the best timing for a tDCS intervention also deserves some discussion. There are many confounders and uncertainties for conducting stroke recovery trials, not just tDCS study, in the acute or subacute phase, such as, ongoing challenging medical issues; robust spontaneous recovery; lack of validated patient selection tool, etc. A prior meta-analysis also revealed that tDCS trial is likely to be successful in the chronic phase than in the subacute phase18. On other side, arguing for earlier intervention is that the natural biological recovery process early after a stroke can be robust and has not been well harnessed by previous stroke rehabilitation trials.43–45
Choice of Outcome Measures
Stroke recovery studies require a highly standardized, reliable, and clinically meaningful measure of outcome that is sensitive and responsive to changes related to the mechanisms of functional recovery induced and that shows low random variation and low. As stroke represents a leading global cause of adult disability, important considerations for any study of stroke rehabilitation are impairment reduction, recovery of functional skills, and quality of life improvement. These three aspects represent a hierarchy with the quality of life improvements predicated on functional improvements, and similarly, functional improvements first require a reduction in motor impairment. Outcome variable should have a stable psychometric property with excellent inter-rater and intra-rater reliability. Commonly used scales, also recommended by a panel of experts, in stroke motor recovery trial are FM-UE scale (motor impairment), the Wolf-Motor Function Test (functional outcomes) and the Stroke Impact Scale-Hand (quality of life).46 In general, the FM-UE scale is well correlated with the Wolf-Motor Function Test suggesting a reduction in motor impairment is associated with functional improvement. A majority of prior tDCS studies did not incorporate multiple outcome measures reflecting changes in impairment levels, functional outcomes, and the subject’s perspective into the study design. (Table 1).
Table 1. Outcomes Measures Used in tDCS Post-Stroke Upper Extremity Clinical Trials.
| Study | Motor Impairment | Functional Outcomes | Quality of Life | Comments |
|---|---|---|---|---|
| Lindenberg36 | FM-UE scale | WMFT | ||
| Viana41 | FM-UE scale | WMFT | SSQOL | |
| Fusco42 | FM-UE scale | 9HPT | BI | |
| Kim43 | FM-UE scale | mBI | ||
| Boggio44 | JHFT | |||
| Bolognini45 | FM-UE scale | JHFT | MAL | Electrophysiology data was collected |
| Hesse46 | FM-UE scale | BI | ||
| Di Lazzaro47 | NIHSS | ARAT | MAL | Electrophysiology data was collected |
| Rossi48 | FM-UE scale | BI, mRS | ||
| Nair49 | FM-UE scale | Imaging data was collected | ||
| Ang50 | FM-UE scale | EEG data was collected | ||
| Sattler51 | FM-UE scale | JHFT | Electrophysiology data was collected | |
| Andreda52 | FM-UE scale | BBT | ||
| Figlewski53 | WMFT |
FM-UE scale: Fugl-Meyer Upper Extremity scale; WMFT: Wolf Motor Function Test; SSQOL: stroke specific quality of life scale; JHFT: Jebsen and Taylor Hand Function Test; BI: Barthel Index; 9HPT: 9 hotel peg test; mBI: modified Barth Index; MAL: Motor Activity Log Rating Scale; BBT: Box and Block Test; ARAT: action research arm test.
Another important consideration of outcome measure in a study design is the minimal clinically important difference (MCID). Particularly for outcome measures which are highly sensitive to change, it is often possible to detect a statistically significant difference between groups with very few subjects. However, the trial should be powered to identify an effect size which is clinically relevant, defined to account for the cost-benefit tradeoff of treatment and large enough not to be explained by natural recovery.
Other Factors
Several factors related to clinical trials study design can lead to the confounding results, for example, blinding and concomitant medications. While blinding used to be a major issue, most of the new devices now have the capability to keep the investigators, the therapists, outcome assessors and the patients blind to the particular intervention. Nevertheless, several potential blinding issues remain. Examples include redness of the skin after tDCS (or lack thereof in case of sham), comparatively higher battery drain after multiple tDCS sessions (versus sham), and perception of the sensory irritation by the study participant. One often used approach to mediating these unblinding risks is the use of a separate, blinded outcome assessor who does not have contact with the treating team or patient.
Stroke survivors likely take one or more neuropsychiatric drugs, and those medications may interfere with the tDCS effect. Data suggest that tDCS may be associated with modulation of glutamatergic, GABAergic, dopaminergic, serotonergic, and cholinergic transmitter-receptor activities.47 It is neither ethical nor safe to discontinue any or all of these medications, but it is important to take these medications into consideration of trial design and/or to include them as a covariate in the data analysis. The role of peripheral sensorimotor activity along with tDCS should not be underestimated. Such activity can be, but not limited to, physical/occupational therapy, robotic therapy, virtual reality, etc. It is important to standardize and quantify such therapies to ensure both active stimulation and sham groups received an equal amount of therapy during the studies.
Summary
tDCS holds promise to be a therapeutical tool for post-stroke motor recovery or other domain specific post-stroke deficits. As dozens of tDCS motor recovery studies, mainly proof-of-concept single-center studies, in either single session or multiple-sessions, were conducted and had a sign of positivity, our understanding of this technology and its application is improving every day. Researchers are actively investigating and solving these issues highlighted above (Table 2), and new directions based on the generated knowledge will appear in the near future. The field is ready for a multi-center, well-designed, sham-controlled double blinded tDCS study to systematically investigate its efficacy in improving outcomes in stroke populations.
Table 2: Summary of Status, Issues, and Solution for tDCS Studies.
| Current Status | Key Issues | Potential Solutions | |
|---|---|---|---|
| Interhemispheric imbalance model | - Lesioned hemisphere shows lower excitability while the contralesional hemisphere shows normal or increased excitability. | - Model is simplified and does not generalize to all strokes - Stimulation location (lesional vs contralesional cortex) and timeline to stimulate (acute vs. chronic stage) will need to be determined |
- Dedicated clinical trials to evaluate the effects of anodal/cathodal stimulation at lesional/contralesional cortices at sub-acute or chronic stages of stroke. - further validate the model or develop a better model |
| Stimulation montages | - Anodal stimulation on lesioned hemisphere; cathodal stimulation on contralesional hemisphere; or simultaneous bihemispheric stimulation were all tested; - Meta-analysis shows bihemispheric stimulation is likely advantageous |
- Studies comparing efficacy among all three montages are lacking, and the effect size is unknown. | - Proof of concept study to examine electric field distribution across different montages; - Four-arm clinical trial comparing anodal, cathodal, bihemispheric and sham tDCS intervention are needed. |
| tDCS response variability and modeling | - High inter-subject variability noted possibly due to factors like scalp fat, head size, anisotropy, montage, lesion size, time since stroke (chronic vs. acute), small vessel lesion load, membrane around a cystic lesion, etc. | - Lack of experimental validation of simulation models; - No imaging or behavioral marker for patient selection. |
- Experimental measurement of intracranial electric fields in human subjects and use of this information to validate/refine existing simulation models; - Investigate whether behavioral, imaging or neurophysiology tool or combination can be used to predict therapeutic response. - Consider personalized/biomarker approach in tDCS research |
| Optimal stimulation dose and safety concerns | - Dose-response relationship is observed in current density, charge density, but not conventional current level in meta-analysis; - At least 2 orders of magnitude higher dose were safely administered in animal models. |
- Only up to 2 mA current with different pad sizes are tested, i.e., dose-response relationship was only observed in the range of 0.03–0.09 mA/cm²; - Safety, especially long-term safety, beyond 2 mA is unknown in humans. |
- Dedicated phase I dosage escalation and safety study; - Phase II equivalent dose-response study to find the most efficacious dosage; - Proof-of-concept study towards “individualized” dosage towards achieving comparable “targeted” dose. |
| Subject selection | - Effect size is not well defined in tDCS trial yet. - Patient selection was an issue in the past studies. |
- Lack of appropriate patient selection can likely lead to failure or small effect size of the study. | - Use of behavioral assessment, imaging marker and/or TMS as tools for patient selection. |
| Choice of Peripheral Therapy | - Various peripheral therapy options were used, including robotic, virtual reality, regular occupational therapy. | - Lack of standardization, quantification of these therapy protocol - Therapy dose may not be equal between active and sham group |
- Control therapy dose between groups - Choose peripheral therapy that can be standardized, such as. CIMT therapy |
| Outcome measures | - Majority of proof-concept of trials did not incorporate comprehensive hierarchy outcome measures; - the group difference was assessed for statistical significance only; - various outcomes were used. |
- Statistical significance may not be clinically meaningful; - Not all outcome measure used in the literature have been well validated |
- Only include outcomes with well-defined psychometric property and MCID. - Addressed outcomes in three aspects: motor impairment, functional improvement, and quality of life |
Acknowledgment
Drs. Feng, Chhatbar, George, and Kautz acknowledge grant support from National Institute of Health (P20GM109040, P2CHD086844). Drs. Meinzer, Schlaug, and Feng acknowledge grant support from National Institute of Health(1U01NS102353). Dr. Chhatbar acknowledges grant support from SC-CoAST/NIH StrokeNet (U10NS086490). Dr. Schlaug acknowledges grant support from the Brain Initiative (R01MH111874-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
None.
References:
- 1.Kreisel SH, Bazner H, Hennerici MG. Pathophysiology of stroke rehabilitation: Temporal aspects of neuro-functional recovery. Cerebrovascular diseases. 2006;21:6–17 [DOI] [PubMed] [Google Scholar]
- 2.Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial dc motor cortex stimulation in humans. Neurology. 2001;57:1899–1901 [DOI] [PubMed] [Google Scholar]
- 3.Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, et al. Direct current stimulation promotes bdnf-dependent synaptic plasticity: Potential implications for motor learning. Neuron. 2010;66:198–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlaug G, Renga V, Nair D. Transcranial direct current stimulation in stroke recovery. Archives of neurology. 2008;65:1571–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, et al. Clinical research with transcranial direct current stimulation (tdcs): Challenges and future directions. Brain Stimulation. 2012;5:175–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fregni F, Nitsche M, Loo C, Brunoni A, Marangolo P, Leite J, et al. Regulatory considerations for the clinical and research use of transcranial direct current stimulation (tdcs): Review and recommendations from an expert panel. Clinical Research and Regulatory Affairs. 2014:1–14 [DOI] [PMC free article] [PubMed]
- 7.Feng WW, Bowden MG, Kautz S. Review of transcranial direct current stimulation in poststroke recovery. Topics in stroke rehabilitation. 2013;20:68–77 [DOI] [PubMed] [Google Scholar]
- 8.Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol 1992;453:525–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Lazzaro V, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, et al. Direct demonstration of interhemispheric inhibition of the human motor cortex produced by transcranial magnetic stimulation. Experimental brain research. 1999;124:520–524 [DOI] [PubMed] [Google Scholar]
- 10.Stinear CM, Petoe MA, Byblow WD. Primary motor cortex excitability during recovery after stroke: Implications for neuromodulation. Brain Stimulation. 2015 [DOI] [PubMed]
- 11.McDonnell MN, Stinear CM. Tms measures of motor cortex function after stroke: A meta-analysis. Brain Stimul 2017;10:721–734 [DOI] [PubMed] [Google Scholar]
- 12.Wu D, Qian L, Zorowitz RD, Zhang L, Qu Y, Yuan Y. Effects on decreasing upper-limb poststroke muscle tone using transcranial direct current stimulation: A randomized sham-controlled study. Archives of physical medicine and rehabilitation. 2013;94:1–8 [DOI] [PubMed] [Google Scholar]
- 13.Waters S, Wiestler T, Diedrichsen J. Cooperation not competition: Bihemispheric tdcs and fmri show role for ipsilateral hemisphere in motor learning. J Neurosci 2017;37:7500–7512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Truong DQ, Huber M, Xie X, Datta A, Rahman A, Parra LC, et al. Clinician accessible tools for gui computational models of transcranial electrical stimulation: Bonsai and spheres. Brain Stimul 2014;7:521–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saturnino GB, Antunes A, Thielscher A. On the importance of electrode parameters for shaping electric field patterns generated by tdcs. NeuroImage. 2015 [DOI] [PubMed]
- 16.Vines BW, Cerruti C, Schlaug G. Dual-hemisphere tdcs facilitates greater improvements for healthy subjects’ non-dominant hand compared to uni-hemisphere stimulation. BMC neuroscience. 2008;9:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chi RP, Fregni F, Snyder AW. Visual memory improved by non-invasive brain stimulation. Brain Res 2010;1353:168–175 [DOI] [PubMed] [Google Scholar]
- 18.Chhatbar PY, Ramakrishnan V, Kautz S, George MS, Adams RJ, Feng W. Transcranial direct current stimulation post-stroke upper extremity motor recovery studies exhibit a dose-response relationship. Brain Stimulation. 2015 [DOI] [PMC free article] [PubMed]
- 19.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 2000;527 Pt 3:633–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bastani A, Jaberzadeh S. Differential modulation of corticospinal excitability by different current densities of anodal transcranial direct current stimulation. PloS one. 2013;8:e72254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bastani A, Jaberzadeh S. A-tdcs differential modulation of corticospinal excitability: The effects of electrode size. Brain Stimul 2013;6:932–937 [DOI] [PubMed] [Google Scholar]
- 22.Liebetanz D, Koch R, Mayenfels S, Konig F, Paulus W, Nitsche MA. Safety limits of cathodal transcranial direct current stimulation in rats. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2009;120:1161–1167 [DOI] [PubMed] [Google Scholar]
- 23.Chhatbar PY, George MS, Kautz SA, Feng W. Quantitative reassessment of safety limits of tdcs for two animal studies. Brain Stimul 2017;10:1011–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chhatbar PY, George MS, Kautz SA, Feng W. Charge density, not current density, is a more comprehensive safety measure of transcranial direct current stimulation. Brain Behav Immun 2017;66:414–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palm U, Keeser D, Schiller C, Fintescu Z, Nitsche M, Reisinger E, et al. Skin lesions after treatment with transcranial direct current stimulation (tdcs). Brain Stimul 2008;1:386–387 [DOI] [PubMed] [Google Scholar]
- 26.Frank E, Wilfurth S, Landgrebe M, Eichhammer P, Hajak G, Langguth B. Anodal skin lesions after treatment with transcranial direct current stimulation. Brain Stimul 2010;3:58–59 [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Wei Y, Wen J, Li X. Skin burn after single session of transcranial direct current stimulation (tdcs). Brain Stimul 2015;8:165–166 [DOI] [PubMed] [Google Scholar]
- 28.Minhas P, Datta A, Bikson M. Cutaneous perception during tdcs: Role of electrode shape and sponge salinity. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2011;122:637–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chhatbar PY, Chen R, Deardorff R, Dellenbach B, Kautz SA, George MS, et al. Safety and tolerability of transcranial direct current stimulation to stroke patients -a phase i current escalation study. Brain Stimul 2017;10:553–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kessler SK, Minhas P, Woods AJ, Rosen A, Gorman C, Bikson M. Dosage considerations for transcranial direct current stimulation in children: A computational modeling study. PloS one. 2013;8:e76112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Truong DQ, Magerowski G, Blackburn GL, Bikson M, Alonso-Alonso M. Computational modeling of transcranial direct current stimulation (tdcs) in obesity: Impact of head fat and dose guidelines. NeuroImage. Clinical. 2013;2:759–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Datta A, Bikson M, Fregni F. Transcranial direct current stimulation in patients with skull defects and skull plates: High-resolution computational fem study of factors altering cortical current flow. NeuroImage. 2010;52:1268–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Datta A, Baker JM, Bikson M, Fridriksson J. Individualized model predicts brain current flow during transcranial direct-current stimulation treatment in responsive stroke patient. Brain Stimul 2011;4:169–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suh HS, Lee WH, Kim T-S. Influence of anisotropic conductivity in the skull and white matter on transcranial direct current stimulation via an anatomically realistic finite element head model. Physics in medicine and biology. 2012;57:6961. [DOI] [PubMed] [Google Scholar]
- 35.Lee W, Seo H, Kim S, Cho M, Lee S, Kim T-S. Influence of white matter anisotropy on the effects of transcranial direct current stimulation: A finite element study. 13th International Conference on Biomedical Engineering. 2009:460–464 [Google Scholar]
- 36.Metwally MK, Han SM, Kim TS. The effect of tissue anisotropy on the radial and tangential components of the electric field in transcranial direct current stimulation. Medical & biological engineering & computing. 2015 [DOI] [PubMed]
- 37.Huang Y, Liu AA, Lafon B, Friedman D, Dayan M, Wang X, et al. Measurements and models of electric fields in the in vivo human brain during transcranial electric stimulation. Elife. 2017;6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Opitz A, Falchier A, Yan CG, Yeagle EM, Linn GS, Megevand P, et al. Spatiotemporal structure of intracranial electric fields induced by transcranial electric stimulation in humans and nonhuman primates. Sci Rep 2016;6:31236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chhatbar PY, Kautz SA, Takacs I, Rowland NC, Revuelta GJ, George MS, et al. Evidence of transcranial direct current stimulation-generated electric fields at subthalamic level in human brain in vivo. Brain Stimul 2018 [DOI] [PMC free article] [PubMed]
- 40.Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 2010;75:2176–2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levy RM, Harvey RL, Kissela BM, Winstein CJ, Lutsep HL, Parrish TB, et al. Epidural electrical stimulation for stroke rehabilitation: Results of the prospective, multicenter, randomized, single-blinded everest trial. Neurorehabil Neural Repair. 2015 [DOI] [PubMed]
- 42.Feng W, Wang J, Chhatbar PY, Doughty C, Landsittel D, Lioutas VA, et al. Corticospinal tract lesion load -a potential imaging biomarker for stroke motor outcomes. Annals of neurology. 2015 [DOI] [PMC free article] [PubMed]
- 43.Dromerick AW, Edwardson MA, Edwards DF, Giannetti ML, Barth J, Brady KP, et al. Critical periods after stroke study: Translating animal stroke recovery experiments into a clinical trial. Front Hum Neurosci 2015;9:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jorgensen HS, Nakayama H, Raaschou HO, Vive-Larsen J, Stoier M, Olsen TS. Outcome and time course of recovery in stroke. Part ii: Time course of recovery. The copenhagen stroke study. Archives of physical medicine and rehabilitation. 1995;76:406–412 [DOI] [PubMed] [Google Scholar]
- 45.Cortes JC, Goldsmith J, Harran MD, Xu J, Kim N, Schambra HM, et al. A short and distinct time window for recovery of arm motor control early after stroke revealed with a global measure of trajectory kinematics. Neurorehabil Neural Repair. 2017;31:552–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bushnell C, Bettger JP, Cockroft KM, Cramer SC, Edelen MO, Hanley D, et al. Chronic stroke outcome measures for motor function intervention trials: Expert panel recommendations. Circ Cardiovasc Qual Outcomes. 2015;8:S163–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Medeiros LF, de Souza ICC, Vidor LP, de Souza A, Deitos A, Volz MS, et al. Neurobiological effects of transcranial direct current stimulation: A review. Frontiers in Psychiatry. 2012;3:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Viana RT, Laurentino GE, Souza RJ, Fonseca JB, Silva Filho EM, Dias SN, et al. Effects of the addition of transcranial direct current stimulation to virtual reality therapy after stroke: A pilot randomized controlled trial. NeuroRehabilitation. 2014;34:437–446 [DOI] [PubMed] [Google Scholar]
- 49.Fusco A, Assenza F, Iosa M, Izzo S, Altavilla R, Paolucci S, et al. The ineffective role of cathodal tdcs in enhancing the functional motor outcomes in early phase of stroke rehabilitation: An experimental trial. Biomed Res Int 2014;2014:547290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim DY, Lim JY, Kang EK, You DS, Oh MK, Oh BM, et al. Effect of transcranial direct current stimulation on motor recovery in patients with subacute stroke. Am J Phys Med Rehabil 2010;89:879–886 [DOI] [PubMed] [Google Scholar]
- 51.Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain dc stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci 2007;25:123–129 [PubMed] [Google Scholar]
- 52.Bolognini N, Vallar G, Casati C, Latif LA, El-Nazer R, Williams J, et al. Neurophysiological and behavioral effects of tdcs combined with constraint-induced movement therapy in poststroke patients. Neurorehabilitation and neural repair. 2011;25:819–829 [DOI] [PubMed] [Google Scholar]
- 53.Hesse S, Waldner A, Mehrholz J, Tomelleri C, Pohl M, Werner C. Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: An exploratory, randomized multicenter trial. Neurorehabilitation and neural repair. 2011;25:838–846 [DOI] [PubMed] [Google Scholar]
- 54.Di Lazzaro V, Dileone M, Capone F, Pellegrino G, Ranieri F, Musumeci G, et al. Immediate and late modulation of interhemipheric imbalance with bilateral transcranial direct current stimulation in acute stroke. Brain Stimul 2014;7:841–848 [DOI] [PubMed] [Google Scholar]
- 55.Rossi C, Sallustio F, Di Legge S, Stanzione P, Koch G. Transcranial direct current stimulation of the affected hemisphere does not accelerate recovery of acute stroke patients. Eur J Neurol 2013;20:202–204 [DOI] [PubMed] [Google Scholar]
- 56.Nair DG, Renga V, Lindenberg R, Zhu L, Schlaug G. Optimizing recovery potential through simultaneous occupational therapy and non-invasive brain-stimulation using tdcs. Restor Neurol Neurosci 2011;29:411–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ang KK, Guan C, Phua KS, Wang C, Zhao L, Teo WP, et al. Facilitating effects of transcranial direct current stimulation on motor imagery brain-computer interface with robotic feedback for stroke rehabilitation. Archives of physical medicine and rehabilitation. 2015;96:S79–87 [DOI] [PubMed] [Google Scholar]
- 58.Sattler V, Acket B, Raposo N, Albucher JF, Thalamas C, Loubinoux I, et al. Anodal tdcs combined with radial nerve stimulation promotes hand motor recovery in the acute phase after ischemic stroke. Neurorehabilitation and neural repair. 2015;29:743–754 [DOI] [PubMed] [Google Scholar]
- 59.Andrade SM, Batista LM, Nogueira LL, de Oliveira EA, de Carvalho AG, Lima SS, et al. Constraint-induced movement therapy combined with transcranial direct current stimulation over premotor cortex improves motor function in severe stroke: A pilot randomized controlled trial. Rehabil Res Pract 2017;2017:6842549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Figlewski K, Blicher JU, Mortensen J, Severinsen KE, Nielsen JF, Andersen H. Transcranial direct current stimulation potentiates improvements in functional ability in patients with chronic stroke receiving constraint-induced movement therapy. Stroke. 2017;48:229–232 [DOI] [PubMed] [Google Scholar]