Abstract
Purpose of Review Tissue engineering has expanded into a highly versatile manufacturing landscape that holds great promise for advancing cardiovascular regenerative medicine. In this review, we provide a summary of the current state-of-the-art bioengineering technologies used to create functional cardiac tissues for a variety of applications in vitro and in vivo.
Recent Findings Studies over the past few years have made a strong case that tissue engineering is one of the major driving forces behind the accelerating fields of patient-specific regenerative medicine, precision medicine, compound screening, and disease modeling. To date, a variety of approaches have been used to bioengineer functional cardiac constructs, including biomaterial-based, cell-based, and hybrid (using cells and biomaterials) approaches. While some major progress has been made using cellular approaches, with multiple ongoing clinical trials, cell-free cardiac tissue engineering approaches have also accomplished multiple breakthroughs, although drawbacks remain.
Summary This review summarizes the most promising methods that have been employed to generate cardiovascular tissue constructs for basic science or clinical applications. Further, we outline the strengths and challenges that are inherent to this field as a whole and for each highlighted technology.
Keywords: Cardiac tissue engineering, Bioprinting, 3D modeling, Vascular network, Cardiovascular regenerative medicine, Patient-specific precision medicine
Introduction
Cardiovascular tissue engineering straddles the crossroads between biomaterials engineering, 3D design and modeling, heart biology, and medicine. It is a complex area of research, but in that complexity lie its strengths—the potential to create regenerative implants, improve drug screening platforms for more effective and controlled delivery of therapeutics, and model various diseases in vitro [1–6]. Borrowing expertise from these various areas enables a multidisciplinary approach towards biomanufacturing of functional, living tissues and organs, be it for basic science or translational research for cardiovascular applications.
With applications ranging from patient-specific medical implants, drug screening platforms, and in vitro disease models, the field of biological additive manufacturing has greatly expanded in recent years. Improved control over spatial resolution, biomaterial/hydrogel properties, and biological pathway understanding has enabled tissue engineering to begin its transition from a strictly research tool into the clinical and biotech areas. This review summarizes the current tissue engineering approaches that have shown most promise as a translational tool, focused predominantly on cardiac and cardiovascular engineered tissues.
Importantly, to maintain cell viability and functionality, the scaffolds that are employed in tissue engineering must satisfy several key biophysical and biochemical requirements, both during and post manufacturing, such as cell viability, proper niche recapitulation, spatial fidelity, and biodegradability [7–9]. These parameters, together with a reliable method to generate functional vascular networks within the engineered constructs, are critical for generating high-fidelity tissue analogs [10]. A balance must be maintained between the mechanical properties of scaffolds and their biocompatibility to allow for incorporated cells to remodel their microenvironment, which is a critical step towards intercellular connections and adequate tissue function [11, 12]. Here, we will explore several tissue engineering methods that have been used to generate viable and functional tissue analogs, for use in in vitro research or translational applications, outlining the specific methods are most capable of producing vascularized tissue/organ mimics for the heart and other target organs.
Scaffold-Free Cellular Approaches
A common, substantial issue in most cardiovascular diseases (CVDs) is the irreversible loss of cardiomyocytes (CMs) and scar tissue formation, which could eventually lead into arrhythmia and heart failure. While conventional therapies primarily focus on minimizing scar formation and adverse remodeling, they rarely address the massive loss of muscle tissue. Therefore, new therapies are currently being explored. In particular, cell-based therapies have become a new focal point for the treatment of various CVDs, aiming to restore function and structure of damaged heart by implantation of cells into the pathologic tissue using a variety of techniques (e.g., intracoronary, intravenous, intramyocardial, and trans-endocardial injection methods) [13].
To date, a variety of cell sources have been used in scaffold-free cardiovascular repair processes including bone marrow-derived (BMSCs), mesenchymal (MSCs), and embryonic stem cells (ESCs) [14–16], induced pluripotent stem cells (iPSCs) [17, 18], cardiac stem and progenitor cells [19, 20], skeletal myoblasts [21], cardiac fibroblast [22] and endothelial cells [23], and a variety of CMs [24–26]. While a large number(> 200) of clinical trials have established the effectiveness and safety of most cell types in treating CVDs, the efficacy of scaffold-free, cell-based therapies remains challenging due to factors including inadequate consistency of cell sources, poor cell retention and survival after transplantation, and risk of tumorigenicity [10, 27, 28].
Difficulties in replicating native tissue functionality and mechanical stability have limited the progress of scaffold-free approaches towards fabrication of viable cardiovascular tissues [29]. Considering the significance of spatial distribution of cells in recapitulating organ/tissue structure and function, cell 3D bioprinting has recently been explored as a robust tool to engineer tissue constructs [30, 31]. 3D bioprinting can also be helpful in creating highly complex vascular tissue constructs at dimensions greater than 500 μm, given that diffusion limit of oxygen in living tissues is ~ 100–200 μm [32]).
Direct cell printing has already been used successfully to create a variety of cardiovascular tissues. For example, smooth muscle cells (SMCs) and FBs were laid down to mimic layered tissue constructs. Printed tissues underwent perfusion mediated maturation [33]. Scaffold-free, near-solid tissue strands have been printed without any need for a liquid medium. This approach better matched the mechanical and biochemical characteristics of the host tissue [34]. In another study, ten-thousand spheroids, composed of CMs, FBs, and ECs, were fused together to form contractile cardiac patches [35]. The patches were grafted into rat hearts to assess their potential for translational applications. Multicellular patches showed remarkable electrophysiological and mechanical contractility coupling, and more mature tissue and host anastomosis [35]. While scaffold-free cell self-assembly has shown success for specific cell types, not all cells are conducive to forming aggregates and need anchoring (e.g., osteoblasts) [29].
Cell-Free Biomaterial Approaches
Cardiac tissue engineering aims to generate high-fidelity analogs of human heart tissue for in vitro disease modeling or in vivo repair of damaged tissue. Successful application of such efforts requires selecting proper cell sources, and importantly, developing functional biomaterials that can support tissue viability, maturation, and function. The field of cardiovascular tissue engineering has spent considerable resources to develop functional biomaterials for applications that can be addressed without the need for an exogenous cellular component. Such approaches are especially relevant to large-scale tissue/organ reconstruction [36, 37]. Acellular cardiac scaffolds can provide the unique signaling environment that will allow for endogenous cardiac tissue repair and improved restorative therapies [38].
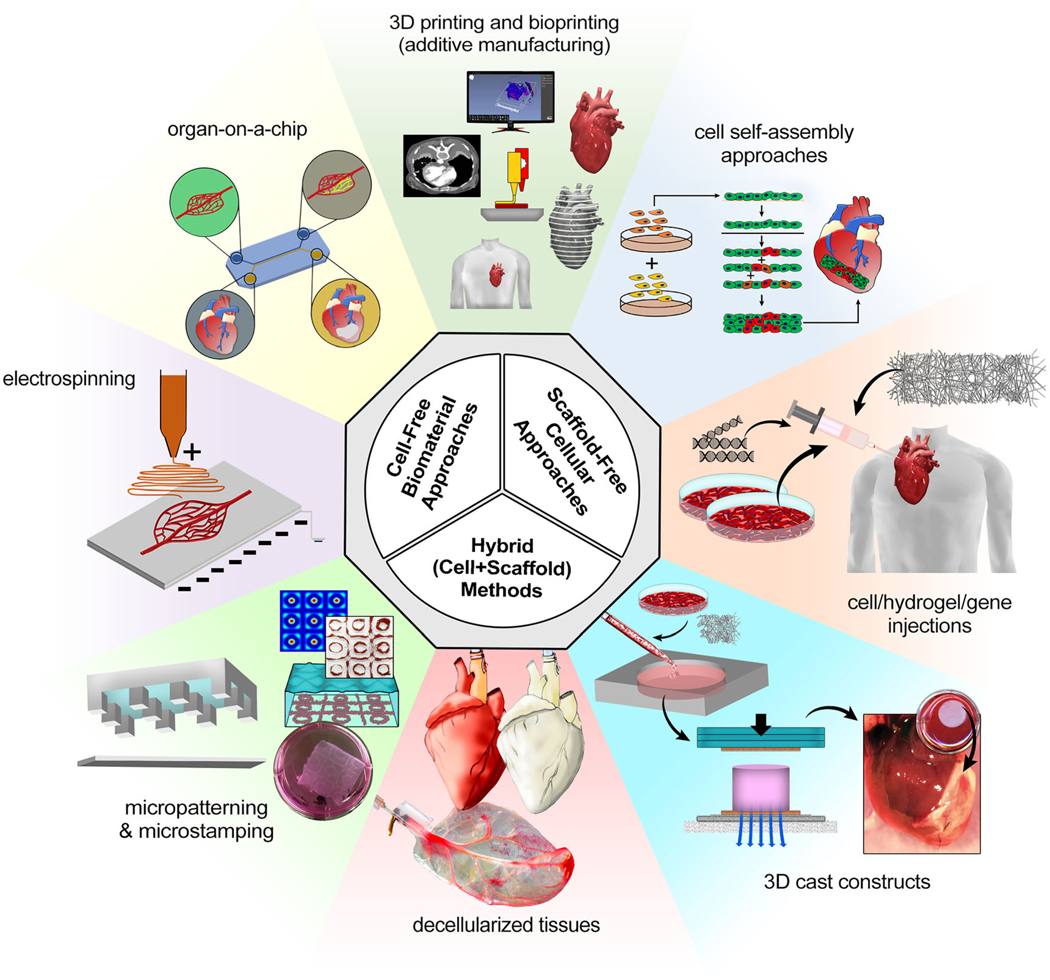
Biomaterials that can recapitulate the native heart extracellular matrix (ECM), while maintaining adequate mechanical integrity, can be difficult to create, due to the complexity and tight regulations inherent to these tissues. Such materials should exhibit relatively low, myocardial-mimetic stiffness (~ 1–10 KPa [11, 39]), biodegradable at proper rate to allow ECM remodeling and intercellular connections [11, 40], while supporting the cardiac cell viability and function. To address these requirements, a variety of bioengineering methods have used synthetic and natural biomaterials, including decellularization techniques, additive manufacturing approaches, electrospinning, 3D casting, and micropatterning (Fig. 1) [45].
Fig. 1.
Schematic summary of cardiovascular tissue engineering paradigms (inner circle) and commonly used bioengineering approaches (outer circle), including 3D printing and bioprinting, cell self-assembly approaches, cell/hydrogel/gene injection therapies, 3D cast tissue constructs (reconstructed from [41]), decellularized tissue scaffolds (reconstructed from [42, 43]), micropatterning and stamping (reconstructed from [44]), electrospinning, and organ-on-a-chip methods
Hybrid (Cellular Scaffold) Approaches
A majority of cardiovascular tissue engineering efforts utilize a combination of cells and biomaterials to create biomimetic, functional tissue constructs for a variety of in vitro and in vivo applications. In these strategies, engineered biomaterials will have to fulfill a wide spectrum of physiomechanical, biological, and biochemical requirements to recapitulate the highly complex microenvironment of the native heart tissue [46–48]. Employed biomaterials—in the form of injectable hydrogel or ex vivo fabricated patch/scaffolds—will help significantly to retain transplanted cells at the site of injury, provide 3D organization to the cells, protect, stimulate and guide their growth and function, and increase the thickness of cardiac tissue, resulting in diminished wall stress and adverse cardiac remodeling [13, 49].
Engineered scaffolds, when paired with patient-specific stem cells, can be used to deliver novel therapies and advance regenerative medicine [46, 50]. In the field of cardiac tissue engineering, polymeric biomaterials can be derived from natural or synthetic sources. Most common biologically derived polymers include collagen, gelatin, Matrigel, chitosan, alginate, and decellularized ECM [51, 52]. Synthetic cardiac biomaterials, such as polyesters (e.g., FDA-approved polycaprolactone, poly-L-lactic acid, and poly(lactic-coglycolic acid) [13]), could be attractive alternatives to natural polymers, offering facilitated chemical-physical modifications and enhanced reproducibility [13, 52]. Additionally, other optimized materials such as silk and conductive materials like graphene and carbon nanotubes have been recently utilized [53]. The range of validated scaffolds is further enhanced by the different crosslinking methods that are available to solidify the material, from ionic based, to enzymatic and UVor visible light initiated [46, 54, 55].
To accurately simulate their target environment, scaffolds require directed assembly of the biomaterial components using various approaches including casting, bioprinting, or electrospinning, which are covered in more detail later in this review. In the case of synthetic scaffolds, polymer functionalization using a variety of small molecules, peptides, and proteins is often required to facilitate cellular integration and maturation within the engineered tissue construct [48, 50]. For any of these scaffolds to be a viable long-term translational option, they must support vascularization, either directly, or through their porous structure. Below, we outline briefly some of the most common bioengineering approaches used to create functional cardiovascular constructs for in vitro and in vivo applications.
3D Printing and Bioprinting (Additive Manufacturing)
Current approaches of 3D bioprinting in CVDs primarily utilize a microextrusion method where the bioink is expelled from the nozzle at a controlled rate [9, 56]. This provides a significant control over the spatial and cellular composition of the manufactured tissues. The ability to incorporate patient-derived cardiac cells (e.g., iPSC-derived CMs, FBs, ECs, and SMCs) into supportive bioink allows fabrication of functional, personalized tissue constructs that can aim to repair the damaged heart, or other target organs [57, 58••]. While major progress has been made in bioengineering functional scaffolds for in vitro modeling and cardiac tissue repair and regeneration, these applications remain limited due to multiple complex features of cardiovascular tissues [57, 59••].
One of the most important components of 3D bioprinting is preparation of the bioinks that are used as a medium to support the cells, and maintain the tissue geometry and design at the end of the process [60]. Bioinks can be a biomaterial which acts as scaffolding support, one that can be incorporated with specific cells, or a cell-derived material that can imitate a biological tissue [60, 61]. Different types of bioinks offer a range of advantages, but also bring unique challenges to tissue manufacturing, depending on the instrument setup as well as the chemistry of the material [61]. In the case of cardiovascular tissue printing, bioinks often must contain different cell types including cardiac CMs, ECs, and SMCs [62], which makes formulating and optimization of cell-specific bioinks challenging. Materials used for this purpose must be biocompatible, exhibit adequate rheological-biomechanical properties to achieve printability and post-print stability, and biodegradable to allow cell remodeling and intercellular connection [9]. They should also allow for flexible design of complicated structures, resembling the target vascular structures. Examples of such hydrogels include gelatin [63, 64], fibrin-based composites [57, 65], and sodium alginate [66].
Current studies in bio-additive manufacturing have focused on biocompatibility, cell viability, and support for heterogeneous bioprinting, which imposes more strict constraints on viable bioinks. Thus, it is important to focus on developing improved methods for characterization and screening of novel bioinks (Table 1).
Table 1.
Most common cardiovascular bioprinting approaches, biolink solutions, and their strengths and weaknesses
| 3D printing method | Bioink | Cell source | Application | Advantages | Disadvantages | Refs |
|---|---|---|---|---|---|---|
| Extrusion | PCL carbon nanotube (PCL-CNT) composite | H9c2 myoblast cells | Cardiac tissue engineering | Crystallinity; mechanical strength; scaffold conductivity | Cell toxicity based on CNT concentration | [67] |
| Extrusion | Decellularized extracellular matrix | Stem cells (hESCs & hiPSCs) | Cardiac patch to regenerate ischemic tissue | Vascularization, cell survival, and therapeutic efficacy | Inclusion of stem cells in the bioink requires safety validation pre-clinic | [68, 69] |
| Digital Light Processing (DLP/SLA) | Polymer Resin | hiPSC-derived cardiomyocytes | Drug screening | Fast printing method, flexible device design and simple device fabrication | Requires prolonged exposure to UV light | [70] |
| Extrusion | Gelatin hydrogel | Human mesenchymal stem cell (hMSC) | In vitro cardiac models | Biocompatible; low toxicity; flexible design | Low printing resolution | [63] |
| Extrusion | Fibrin-based composite hydrogel | Ventricular cardiomyocytes | In vitro tissue modeling | Large-scale fabrication of cardiac tissues | Complicated fabrication process | [57] |
| Extrusion | Sodium alginate hydrogel | Human umbilical vein smooth muscle cells (HUVSMCs) | In vitro tissue modeling | Improves mechanical properties and bioink stability | Cell viability is concentration dependent | [33, 66, 71] |
| Inkjet-based Bioprinting | Gelatin; silk; alginate; PEGs; etc. | Cardiomyocytes and muscle cells | In vitro tissue modeling | Availability; cost; throughput | Poor Z-resolution; high cell stress; limited materials | [6, 72, 73] |
| F.R.E.S.H. | Alginate; fibrin; collagen | Myoblasts and fibroblasts, etc. | In vitro tissue engineering | Spatial resolution; multi-material; support-free | Specialized equipment; high cell numbers needed | [74] |
| Optical Tweezers | Any cell-supporting liquid | hESCs and cardiomyocytes | In vitro tissue modeling and engineering | Cell- and Subcellular-level of manipulation | Very low throughput; specialized equipment | [75, 76] |
| Laser-induced forward Transfer (L.I.F.T.) | Any laser, or light-based Cross-linkable material | Human mesenchymal stem cell (hMSC) | High-resolution tissue building | High resolution; Materials deposition as liquid or solid | High cost; cell damage | [77, 78] |
| Laser-guided direct writing (L.G.D.W.) | Any laser, or light-based crosslinkable material | Human umbilical vein endothelial cells (HUVECs) | Controlled multicellular tissue constructs | High resolution; Materials deposition as liquid or solid | High cost; cell damage | [79] |
Cell Self-Assembly Approaches
Cell self-assembly utilizes the inherent ability of the cells to organize in tissue-specific manner to recapitulate the desired tissue/organ structure. Such bottom-up approaches that exploit autonomous cell aggregation can significantly facilitate engineering of functional cardiovascular tissues [80]. As cardiac patches for treating myocardial infarct (MI), self-assembled cell sheet constructs have demonstrated engraftment associated with CM proliferation, neovascularization, and intercellular junctions [81–85]. Further, cardiac functional enhancement has been reported, with reduced left ventricular remodeling, less fibrosis, and preserved wall thickness and ejection fraction [81, 83, 86, 87]. Electromechanical coupling with synchronized pacing and limited arrhythmias has been also validated in cell sheet-treated MI hearts [82, 85]. Currently, various methods are being tested to move beyond the current diffusion barrier of three-sheet thickness. Perfusable vascular beds cultured via bioreactor systems have produced viable twelve-layer tissues [88, 89]. Future efforts must focus on scaling tissues to billions of cells, optimizing vascularization, and providing better nutritional media that also incorporate biological feedback assays [89].
Cell and Hydrogel Injection Therapies
Injection of a variety of cells and/or hydrogels has been evaluated as an alternative therapy to treat CVDs, including MI and severe end-stage heart failure (> 200 trials over past two decades) [90–92]. Direct cell/hydrogel injections are particularly promising methods as they offer less invasive therapy in comparison to other tissue engineering approaches [91, 93, 94]. For this purpose, to date, a variety of cardiac cell types (e.g., cardiac stem/ progenitor cells, FBs, ECs and SMCs, and CMs [16, 18, 22, 23]) and injectable hydrogels (e.g., collagen, dECM, alginate, chitosan, fibrin, poly(ethylene glycol) diacrylate (PEG-DA), PNIPAm, and gelatin methacrylate [91, 92, 95]) have been used to repair damaged or diseased heart. Some of the main requirements for these biomaterials include injectability (or printability), biocompatibility and bioactivity to support exogenous/endogenous cell function, enhanced mass transfer properties, and biodegradability [91]. Cells and/or biomaterials have been delivered via administration routes including intracoronary, intravenous, intramyocardial, and trans-endocardial [13].
While evidence-based standard of care therapies such as angiotensin-converting enzyme inhibitors and β-blockers have shown success in reducing heart failure (HF) mortality rates and healthcare costs, drug therapy is shown to be efficacious only in a subset of HF patients [90, 96]. Thus, cell transplantation has been proposed as an adjunct therapy to treat CVDs. Grafted cells may contribute to cardiac tissue repair in multiple ways [96]. Most commonly, paracrine signaling has been identified as the primary mechanism of action, through transplanted cell secretion of growth hormones, cytokines, exosomes, and metalloproteinases [97–99]. Such paracrine factors, in turn, can stimulate and modulate endogenous repair mechanisms. Considering that most cell therapies have shown poor long-term retention and engraftment of transplanted cells [100, 101], indirect paracrine mechanisms are more likely to be responsible for the regenerative effects and improved cardiac function. Although, stem cell differentiation into CMs (or other cardiac cell types), damaged tissue remuscularization [102, 103], and neoangiogenesis [104, 105] have been reported as other potential mechanisms.
Hydrogel injections for cardiovascular regeneration provide an opportunity to concurrently introduce patient-derived organ specific cells and bioactive agents, which can act locally to the injured area to regenerate cardiac tissue [106]. Biologically derived, synthetic, or hybrid materials have been designed for this purpose [92, 95]. In vivo studies have demonstrated significant functional benefits associated with injection of hydrogels with varying compositions. Injectable hydrogels can provide a functional delivery strategy for novel pharmacological agents and cellular therapies that can supplement existing strategies to improve cardiovascular recovery post-MI [91]. The field of tissue engineering has seen numerous injectable hydrogels that have been developed and tried for application in cardiac repair after MI, and there is hope that such therapies will make it to the clinic in the near future [92, 93, 107].
3D Cast Tissue Constructs
Casting 2D hydrogels into molds with shape/geometry of interest has been the most common bioengineering approach to create cardiac tissue constructs for both in vitro and in vivo applications [11, 41•, 108–111]. Ease of use, flexibility in employing a large variety of biomaterials, simple crosslinking/curing methods, and relatively rapid manufacturing processes are some of the main advantages of 3D casting [111]. For example, acellular type I collagen gels have been cast, encapsulating cardiogenic peptides (e.g., follistatin like-1 protein (FSTL1)), iron oxide nanoparticles, and other biological reagents, providing a 3D cardiac tissue analog with physiomechanical properties resembling those of the native heart tissue [1, 41•, 108, 110, 112]. Application of FSTL1laden cardiac patch in mouse and pig models of MI demonstrated significant effect of the cast patch in regenerating damaged tissue [41•].
The lack of functional vascular network in cast cellular scaffolds may lead to formation of necrotic/apoptotic cores within thick tissues [113]. Yet, there is a lack of standardized approaches to construct such vascular networks, which is a major challenge for bioengineering 3D tissue grafts at the clinical scale [114]. A promising approach utilizes simple vasculature casting that allows for consistent control of cardiovascular network geometry and endothelialization. It is compatible with a range of cell types, synthetic and natural ECMs, and crosslinking strategies. Such perfused vascular channels can also sustain metabolic function in engineered tissue constructs [115, 116]. For this purpose, sacrificial materials (e.g., carbohydrate glass or pluronic) are used for rapid casting of patterned vasculature within engineered tissues [115].
Notably, casting and 3D bioprinting methods have been recently combined together, to allow integration of important advantages of each technique to create complex, heterogeneous tissue constructs laden with a variety of cells and biological reagents [62, 64, 115]. These hybrid tissue engineering techniques are usually used to create 3D vascular scaffolds, where a sacrificial biomaterial is often used first to form the vasculature, followed by casting hydrogel (with or without cells) to fill the interstitial space in a mold [64]. Cast matrices contain either thrombin, transglutaminase (TG), or analogs that can diffuse into adjacent printed filaments, facilitating a continuous, interpenetrating polymer network. Such approaches can generate arbitrarily thick tissues, as the hydrogel matrix does not require UV curing, which can limit crosslinking due to lower penetration range [117].
Decellularized Tissues
Patients suffering from end-stage organ failure often require organ transplantation, which is complicated by organ shortage. For those patients, the need for chronic, lifelong immunosuppression and procedures to assess for rejection are not trivial. A promising approach in the field is to decellularize existing (donor) organs, recover their intact ECM, and reseed them with patient’s own cells to rebuild the target tissue/organ for implantation without the need for immune-suppressive drugs [118]. By taking advantage of cells self-assembly, decellularized ECM scaffolds can significantly facilitate engineering of whole functional organs at scales that are attractive for diverse clinical applications.
Decellularized tissues rely on isolation of the ECM from any given tissue with minimal loss, damage or disruption, while maximizing native cell removal. This is usually achieved through physical, chemical, and enzymatic methods. Some example methods include agitation in solution, thermal shock, convective flow, and manual disruption [119]. One of the more consistent methods is perfusion decellularization [120], which can be applied to cadaver hearts or any other target organ [121]. Importantly, this technique uses the organ’s existing vasculature for perfusion, while also preserving the vessels’ ECM structure at both macro- and micro-scale. Native vasculature perfusion-based techniques are well-suited for translating decellularization processes for whole human organs, as they would provide a more even distribution of decellularizing agents, hence avoiding overexposure and potential toxic effects [118, 121].
Decellularized heart scaffolds have been repopulated with human-derived ESCs and MSCs, delivered through coronary perfusion [122]. Differentiated cells expressed canonical cardiac markers (cardiac troponin T and Nkx2.5) and had differential expression of myosin heavy and light chains. Importantly, in case where the native vasculature was preserved during decellularization, there were CD31+ cells, pointing to some stem cells having differentiated into vascular ECs. Combining the properties of native acellular scaffolds with recellularization techniques will provide a functional platform for cardiovascular organ engineering and regeneration with significant potential for clinical applications. The use of decellularized scaffolds to bioengineer functional organs may overcome the most significant challenges in organ transplantation: donor shortage and immunosuppression [123].
Micropatterning and Microstamping
Micro patterning and stamping are well-suited for large-scale engineered tissue studies, drug discovery, and pharmacological testing, since they can generate reproducible and consistent patterns that can be leveraged for cell-cell interactions [124–126]. As these technologies are inspired by the semiconductor industry, they are capable of creating patterns with resolutions from sub-micron to multiple centimeters, while maintaining high fidelity. Micropatterning and microstamping can be used to produce vascular tissue scaffolds at scales that other techniques would struggle [126–128]. Microstamping techniques often rely on surface modifications to adhere proteins, such as peptides and antibodies, or surface functionalization, such as plasma treatment, to guide cell attachment and migration along predefined patterns [124, 128–131].
Micropatterning has been extensively used in the development of functional cardiovascular constructs, where cellular arrangement, ECM dimensions, and precise localization of factors are critical for functional recapitulation of the tissues [55, 125, 126, 132]. The ability to integrate multiple cell types on the same surface has also been shown to benefit maturation and functional development of the tissue mimics [124, 130–132].
In the field of cardiac and skeletal muscle tissue engineering, scaffolds that are capable of directing cellular alignment are of great significance, as they would provide a highly biomimetic microenvironment to support mechanical function of cultured cells [133, 134]. For example, micro-molding and micro-ablation methods have been used together in order to fabricate elastomeric scaffolds with well-defined anisotropic surface patterns. The micropatterned substrates directed formation of highly aligned, engineered muscle tissues consisting C2C12 cells [133]. In another study, micropatterning was used to align neonatal rat ventricular CMs, at both micro- and macroscopic scales, to follow the realistic murine ventricular microstructure [134].
A novel micropatterning approach to create cardiac tissues was recently developed by using bio-acoustic wave patterning (BAWP) to generate 3D cellular assemblies [135]. The process uses the Faraday waves to pattern cells into dense aggregates in a predefined 3D structure. To sustain these patterns, the hydrogel will be subsequently crosslinked using light-based or chemical crosslinking. This approach could be potentially used for alignment or separation of a variety of cells [135, 136]. In comparison to other tissue engineering techniques, BAWP is relatively quick and straightforward. Further, BAWP’s inherent ability to generate reproducible and repeating patterns over large surface areas [137] makes this approach an attractive candidate for large-scale, high-throughput drug screening and tissue manufacturing applications. For example, BAWP was recently used to pattern hiPSC-CMs into 3D tissue constructs at a cell density similar to that of native myocardium (108 cells/mL), much greater than the packing densities achievable by alternative methods [136].
Electrospinning
Electrospinning is a simple and cost-effective biofabrication approach that is increasingly used to create tissue constructs. Common electrospinning setups consist of a syringe pump loaded with a polymer solution with voltage applied to the syringe tip [138]. The system is then grounded at the collecting metal surface where fibers are collected. The pump drives the polymer solution out of the syringe needle at a controlled dispensation rate and the DC voltage applied to the metal needle tip induces a charge in the solution, which repulses similar charges in the solution. This forms a Taylor cone when the electrical forces are balanced by the polymer surface tension [139]. When the forces exceed this balance, a fiber jet is ejected from the Taylor cone and accelerates to the grounded surface. Varying the different parts of the setup and polymer composition can create discrete fiber morphology at diameters ranging from 100 nm to 5 μm [138].
Considering that electrospun fibers can replicate the native ECM in terms of morphology and scale, and can be modified for enhanced cellular adhesion, proliferation, and infiltration, this technique has been used to produce scaffolds for a range of tissue engineering applications including cardiac, vascular, nerve, bone, and tendon/ligament tissues [140–142]. Electrospinning methods can be readily scaled up to produce tissue scaffolds for clinical and industrial applications [140]. Further, a large variety of polymers can be used. These characteristics have made electrospinning a great candidate for the biofabrication of cardiac tissue engineering scaffolds. For instance, modulating the rotating drum and mandrel collectors in electrospinning systems can help to form aligned nanofibers, which can in turn generate aligned/guided cardiac muscle assemblies [143].
A variety of both natural and synthetic polymers have been used to electrospun cardiovascular tissue constructs. These include fibrinogen, silk fibroin, chitosan, gelatin, and collagen (natural polymers) [140, 144], and polycaprolactone (PCL), poly-L-lactic acid (PLLA), poly(lactic-co-glycolic acid) (PLGA), and polyethylene glycol (PEG) (synthetic polymers) [145]. Electrospun cardiac scaffolds have been cultured with a variety of cells including rat neonatal cardiac FBs and CMs, human aortic ECs, iPSC-CMs, for both in vitro and in vivo testing [140]. To date, the electrospun nanofiber cardiac scaffolds have found many applications as drug delivery and disease modeling systems, cardiac patches, heart valve, and prosthesis [146].
Organ-on-a-Chip Methods
Over the past decades, the emergence of micro- and nano-biofabrication technologies has made major contributions to the advancement of patient-specific, cardiac tissue engineering [147]. In particular, high-throughput applications such as compound screening for novel drug candidates for CVDs require organ-on-a-chip devices that can incorporate whole-organ functionality in a reproducible production pipeline [148]. As new market pharmaceuticals, on average, take 12 years in research and development (R&D), and clinical trials before making it to patients [149], organ-on-a-chip platforms can serve to reduce time in R&D by mimicking human physiological environments in vitro to assay the effects of target drugs [148].
To increase functional mimicry of the engineered device, important parameters such as vascularization, cell density and organization, and biomechanical properties must be considered during the device development. For the heart, for example, an organ-on-a-chip device would require a functional myocardium, blood vessels that deliver nutrients to the myocardium, and various smooth muscle and fibroblast support cells that are involved in the proper function of the heart [150, 151]. Appropriate organization and density of these cell types must be accounted for in the device to successfully imitate the heart tissue. For example, CMs can be seeded on top of an established vascular bed and perfused [152]. The density of such vasculature allows for transport of nutrients and subsequently provides energy for CMs.
Soft polymer materials can be used to generate microvascular networks with microfluidic channels capable of leakage free perfusion. Such networks can support vasculogenesis, angiogenesis, and anastomosis in addition to co-culture of different cells inside the same tissue chamber across multiple microfluidic devices [153]. Taken together, a successful heart-on-a-hip device will have to incorporate appropriate valve function, pressure points, and flow rates. Cardiac microsystems that could accomplish these include biomimetic contractile myocardium-on-a-chip, tissue-engineered bio-hybrid actuator, and cardiovascular systems incorporating synthetic engineered cardiac pumps and valves [151]. Ability to record electrophysiological responses and contractile motion of CMs under various biochemical factors and fluidic conditions would also be critical. Further, patterned CMs can be stimulated using electrodes and their electrical signals can be monitored to assess contractility in the presence of changing environmental factors [154]. On-chip sensors can be also integrated into a microfluidic circulatory system to verify the physiological systemic circulation measured in vitro [155].
Summary and Future Directions
This review provides a summary of tissue engineering approaches as it pertains to cardiovascular tissues, specifically, and other organs in general. The field of tissue/organ biomanufacturing has been progressively expanding and is in the transitional phase, moving beyond a tool predominantly used for basic science research, into the more clinical and translational applications. This is aided in large part by the wide range of specialized methods that can be used to generate rationally engineered tissue mimics, the major ones of which we have summarized here.
The one method that tends to stand out when it comes to bioengineering functional tissues is 3D bioprinting, which has shown great promise to translate tissue scaffolds towards clinical-industrial applications. Bioprinted tissues are also being used as improved platforms for drug testing and disease modeling in vitro. It is envisioned that the field of cardiac tissue engineering will move towards techniques that would allow for higher spatial control over the cellular arrangement in tissue construct. Bioprinting excels in producing such predefined, high-resolution architectures that can better recapitulate the complex structure and function of cardiovascular tissues. There has been increasing reliance on bioprinting as a one-of-a-kind technique capable of creating biomimetic vascular tissues.
Recent advances in bioprinting technology allow for fabrication of complex, patient-specific, 3D architectures in a spatial resolution where rational design of organ/tissue is possible, while supporting the viability and function of the incorporated cells. There remain several challenges for the clinical application of bioprinted cardiac and other tissue constructs. Most importantly, there is a lack of functional tissue-specific FDA-approved bioinks. Development of cardiac-specific bioinks using tailored biomaterials and a selection of macromolecules, implicated in cardiovascular development, would be an important milestone towards clinical bioprinting of the cardiac tissues.
The ability to design and generate vascular networks that enable perfusing thick tissue constructs and integrating them into the target tissue post-engraftment is another unique capability of bioprinting technologies. Being able to generate large perfused tissue mimics brings us one step closer to being able to treat severe cardiac injuries such as MI, and congenital heart defects. As tissue engineering advances in its biofabrication capabilities, there will be increasingly more faithful tissue constructs, ushering breakthroughs that would allow for truly regenerative, rather than palliative, medicine, which will improve long-term outcomes, decrease direct and indirect medical costs, and improve drug discovery and disease modeling effectiveness.
Footnotes
Compliance with Ethical Standards
Conflict of Interest Martin L. Tomov, Carmen J. Gil, Alexander Cetnar, Andrea S. Theus, Bryanna J. Lima, Joy E. Nish, Holly D. Bauser-Heaton, and Vahid Serpooshan declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Serpooshan V, Zhao M, Metzler SA, Wei K, Shah PB, Wang A, et al. Use of bio-mimetic three-dimensional technology in therapeutics for heart disease. Bioengineered. 2014;5(3):193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doppler SA, Deutsch MA, Serpooshan V, Li G, Dzilic E, Lange R, et al. Mammalian heart regeneration: the race to the finish line. Circ Res. 2017;120(4):630–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duan B State-of-the-art review of 3D bioprinting for cardiovascular tissue engineering. Ann Biomed Eng. 2017;45(1):195–209. [DOI] [PubMed] [Google Scholar]
- 4.Ahadian S, Khademhosseini A. Smart scaffolds in tissue regeneration. Regen Biomater. 2018;5(3):125–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashammakhi N, et al. Advances and future perspectives in 4D bioprinting. Biotechnol J. 2018;13(12):e1800148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derakhshanfar S, Mbeleck R, Xu K, Zhang X, Zhong W, Xing M. 3D bioprinting for biomedical devices and tissue engineering: a review of recent trends and advances. Bioact Mater. 2018;3(2): 144–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomov ML, Olmsted ZT, Paluh JL. The human embryoid body cystic core exhibits architectural complexity revealed by use of high throughput polymer microarrays. Macromol Biosci. 2015;15(7):892–900. [DOI] [PubMed] [Google Scholar]
- 8.Tomov ML, Tsompana M, Olmsted ZT, Buck M, Paluh JL. Human embryoid body transcriptomes reveal maturation differences influenced by size and formation in custom microarrays. J Nanosci Nanotechnol. 2016;16(9):8978–88. [Google Scholar]
- 9.Hu JB, Tomov ML, Buikema JW, Chen C, Mahmoudi M, Wu SM, et al. Cardiovascular tissue bioprinting: physical and chemical processes. Appl Phys Rev. 2018;5(4):041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang NF, Serpooshan V, Morris VB, Sayed N, Pardon G, Abilez OJ, et al. Big bottlenecks in cardiovascular tissue engineering. Commun Biol. 2018;1(1):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee S, Serpooshan V, Tong X, Venkatraman S, Lee M, Lee J, et al. Contractile force generation by 3D hiPSC-derived cardiac tissues is enhanced by rapid establishment of cellular interconnection in matrix with muscle-mimicking stiffness. Biomaterials. 2017;131: 111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bitar KN, Zakhem E. Design strategies of biodegradable scaffolds for tissue regeneration. Biomed Eng Comput Biol. 2014;6:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnal-Pastor M, et al. Biomaterials for cardiac tissue engineering. Regen Med Tissue Eng. 2013:275–323. [Google Scholar]
- 14.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701–5. [DOI] [PubMed] [Google Scholar]
- 15.Kulandavelu S, Balkan W, Hare JM. Next-generation stem cell therapy: genetically modified mesenchymal stem cells for cardiac repair: editorial to: “Mesenchymal Stem Cells with eNOS Over Expression Enhance Cardiac Repair in Rats with Myocardial Infarction” by Leilei Chen et al. Cardiovasc Drugs Ther. 2017;31(1):5–7. [DOI] [PubMed] [Google Scholar]
- 16.Thakker R, Yang P. Mesenchymal stem cell therapy for cardiac repair. Curr Treat Options Cardiovasc Med. 2014;16(7):323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zwi-Dantsis L, Gepstein L. Induced pluripotent stem cells for cardiac repair. Cell Mol Life Sci. 2012;69(19):3285–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lalit PA, Hei DJ, Raval AN, Kamp TJ. Induced pluripotent stem cells for post-myocardial infarction repair: remarkable opportunities and challenges. Circ Res. 2014;114(8):1328–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leong YY, Ng WH, Ellison-Hughes GM, Tan JJ. Cardiac stem cells for myocardial regeneration: they are not alone. Front Cardiovasc Med. 2017;4(4):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serpooshan V, Liu YH, Buikema JW, Galdos FX, Chirikian O, Paige S, et al. Nkx2.5+ cardiomyoblasts contribute to cardiomyogenesis in the neonatal heart. Sci Rep. 2017;7(1): 12590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, Hutcheson KA, et al. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med. 1998;4(8):929–33. [DOI] [PubMed] [Google Scholar]
- 22.Chistiakov DA, Orekhov AN, Bobryshev YV. The role of cardiac fibroblasts in post-myocardial heart tissue repair. Exp Mol Pathol. 2016;101(2):231–40. [DOI] [PubMed] [Google Scholar]
- 23.Yuan P, Ma X. Endothelial cells facilitate cell-based cardiac repair: progress and challenge. Curr Stem Cell Res Ther. 2014;9(5):415–23. [DOI] [PubMed] [Google Scholar]
- 24.Dunn KK, Palecek SP. Engineering scalable manufacturing of high-quality stem cell-derived cardiomyocytes for cardiac tissue repair. Front Med. 2018;5:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masoudpour H, Laflamme MA. Cardiac repair with pluripotent stem cell-derived cardiomyocytes: proof of concept but new challenges. J Thorac Cardiovasc Surg. 2017;154(3):945–8. [DOI] [PubMed] [Google Scholar]
- 26.Lundy SD, Gantz JA, Pagan CM, Filice D, Laflamme MA. Pluripotent stem cell derived cardiomyocytes for cardiac repair. Curr Treat Options Cardiovasc Med. 2014;16(7):319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H, Chen H, Wang W, Wei Y, Hu S. Cell survival and redistribution after transplantation into damaged myocardium. J Cell Mol Med. 2010;14(5):1078–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baldari S, di Rocco G, Piccoli M, Pozzobon M, Muraca M, Toietta G. Challenges and strategies for improving there generative effects of mesenchymal stromal cell-based therapies. Int J Mol Sci. 2017;18(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JK, Link JM, Hu JCY, Athanasiou KA. The self-assembling process and applications in tissue engineering. Cold Spring Harb Perspect Med. 2017;7(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moldovan NI. Progress in scaffold-free bioprinting for cardiovascular medicine. J Cell Mol Med. 2018;22(6):2964–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aguilar IN, Smith LJ, Olivos DJ III, Chu TMG, Kacena MA, Wagner DR. Scaffold-free bioprinting of mesenchymal stem cells with the regenova printer: optimization of printing parameters. Bioprinting. 2019;15:e00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rouwkema J, et al. Supply of nutrients to cells in engineered tissues. Biotechnol Genet Eng Rev. 2010;26:163–78. [DOI] [PubMed] [Google Scholar]
- 33.Norotte C, Marga FS, Niklason LE, Forgacs G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials. 2009;30(30):5910–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu Y, Moncal KK, Li J, Peng W, Rivero I, Martin JA, et al. Three dimensional bioprinting using self-assembling scalable scaffold free “tissue strands” as a new bioink. Sci Rep. 2016;6:28714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noguchi R, Nakayama K, Itoh M, Kamohara K, Furukawa K, Oyama JI, et al. Development of a three-dimensional pre-vascularized scaffold-free contractile cardiac patch for treating heart disease. J Heart Lung Transplant. 2016;35(1):137–45. [DOI] [PubMed] [Google Scholar]
- 36.Esmaeili Pourfarhangi K, Mashayekhan S, Asl SG, Hajebrahimi Z. Construction of scaffolds composed of acellular cardiac extracellular matrix for myocardial tissue engineering. Biologicals. 2018;53:10–8. [DOI] [PubMed] [Google Scholar]
- 37.Courtman DW, Pereira CA, Kashef V, McComb D, Lee JM, Wilson GJ. Development of a pericardial acellular matrix biomaterial: biochemical and mechanical effects of cell extraction. J Biomed Mater Res. 1994;28(6):655–66. [DOI] [PubMed] [Google Scholar]
- 38.Burdick JA, et al. Acellular biomaterials: an evolving alternative to cell-based therapies. Sci Transl Med. 2013;5(176):176ps4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.da Silveira JS, Scansen BA, Wassenaar PA, Raterman B, Eleswarpu C, Jin N, et al. Quantification of myocardial stiffness using magnetic resonance elastography in right ventricular hypertrophy: initial feasibility in dogs. Magn Reson Imaging. 2016;34(1):26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee S, et al. Hydrogel degradation modulates human iPSC-derived cardiomyocyte fates in 3D. Front Bioeng Biotechnol. [Google Scholar]
- 41.Wei K, et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature. 2015;525(7570):479–85• Reporting a novel effect of an embryonic epicardial patch, loaded with a paracrine cardiokine (FSTL1), on stimulating adult cardiomyocytes to re-enter cell cylce and proliferate.
- 42.Galvez-Monton C, et al. Cardiac Tissue Engineering and the Bioartificial Heart. Rev Esp Cardiol. 2013;66(5):391–9. [DOI] [PubMed] [Google Scholar]
- 43.Gershlak JR, Hernandez S, Fontana G, Perreault LR, Hansen KJ, Larson SA, et al. Crossing kingdoms: using decellularized plants as perfusable tissue engineering scaffolds. Biomaterials. 2017;125:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serpooshan V, Chen P, Wu H, Lee S, Sharma A, Hu DA, et al. Bioacoustic-enabled patterning of human iPSC-derived cardiomyocytes into 3D cardiac tissue. Biomaterials. 2017;131: 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Momtahan N, Poornejad N, Struk JA, Castleton AA, Herrod BJ, Vance BR, et al. Automation of pressure control improves whole porcine heart decellularization. Tissue Eng Part C Methods. 2015;21(11):1148–61. [DOI] [PubMed] [Google Scholar]
- 46.Dar A, Shachar M, Leor J, Cohen S. Optimization of cardiac cell seeding and distribution in 3D porous alginate scaffolds. Biotechnol Bioeng. 2002;80(3):305–12. [DOI] [PubMed] [Google Scholar]
- 47.Sapir Y, Kryukov O, Cohen S. Integration of multiple cell-matrix interactions into alginate scaffolds for promoting cardiac tissue regeneration. Biomaterials. 2011;32(7):1838–47. [DOI] [PubMed] [Google Scholar]
- 48.Khan K, Gasbarrino K, Mahmoud I, Makhoul G, Yu B, Dufresne L, et al. Bioactive scaffolds in stem-cell-based therapies for cardiac repair: protocol for a meta-analysis of randomized controlled preclinical trials in animal myocardial infarction models. Syst Rev. 2018;7(1):225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen QZ, Harding SE, Ali NN, Lyon AR, Boccaccini AR. Biomaterials in cardiac tissue engineering: ten years of research survey. Mater Sci Eng Rep. 2008;59(1–6):1–37. [Google Scholar]
- 50.Sun Y, Han X, Wang X, Zhu B, Li B, Chen Z, et al. Sustained release of IGF-1 by 3D mesoporous scaffolds promoting cardiac stem cell migration and proliferation. Cell Physiol Biochem. 2018;49(6):2358–70. [DOI] [PubMed] [Google Scholar]
- 51.Leor J, Amsalem Y, Cohen S. Cells, scaffolds, and molecules for myocardial tissue engineering. Pharmacol Ther. 2005;105(2): 151–63. [DOI] [PubMed] [Google Scholar]
- 52.Huyer LD, Montgomery M, Zhao Y, Xiao Y, Conant G, Korolj A, et al. Biomaterial based cardiac tissue engineering and its applications. Biomed Mater. 2015;10(3):034004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keane TJ, Badylak SF. Biomaterials for tissue engineering applications. Semin Pediatr Surg. 2014;23(3):112–8. [DOI] [PubMed] [Google Scholar]
- 54.Di Felice V, et al. Silk fibroin scaffolds enhance cell commitment of adult rat cardiac progenitor cells. J Tissue Eng Regen Med. 2015;9(11):E51–64. [DOI] [PubMed] [Google Scholar]
- 55.Izadifar M,Chapman D, Babyn P, Chen X, Kelly ME. UV-assisted 3D bioprinting of nanoreinforced hybrid cardiac patch for myocardial tissue engineering. Tissue Eng Part C Methods. 2018;24(2):74–88. [DOI] [PubMed] [Google Scholar]
- 56.Serpooshan V, Mahmoudi M, Hu DA, Hu JB, Wu SM. Bioengineering cardiac constructs using 3D printing. J 3D Printing Med. 2017;1(2):123–39. [Google Scholar]
- 57.Wang Z, Lee SJ, Cheng HJ, Yoo JJ, Atala A. 3D bioprinted functional and contractile cardiac tissue constructs. Acta Biomater. 2018;70:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kang HW, et al. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol. 2016;34(3):312–9•• Demonstrated capabilities of integrated tissue–organ printers by fabricating mandible and calvarial bone, cartilage and skeletal muscle.
- 59.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32(8):773–85•• A seminal work reviewing the application of 3D bioprinting to tissue and organ engineering.
- 60.Gungor-Ozkerim PS, Inci I, Zhang YS, Khademhosseini A, Dokmeci MR. Bioinks for 3D bioprinting: an overview. Biomater Sci. 2018;6(5):915–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gopinathan J, Noh I. Recent trends in bioinks for 3D printing. Biomater Res. 2018;22:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li X, Liu L, Zhang X, Xu T. Research and development of 3D printed vasculature constructs. Biofabrication. 2018;10(3): 032002. [DOI] [PubMed] [Google Scholar]
- 63.Tijore A, Irvine SA, Sarig U, Mhaisalkar P, Baisane V, Venkatraman S. Contact guidance for cardiac tissue engineering using 3D bioprinted gelatin patterned hydrogel. Biofabrication. 2018;10(2). [DOI] [PubMed] [Google Scholar]
- 64.Hu JB, et al. Bioengineering of vascular myocardial tissue; a 3D bioprinting approach. Tissue Eng A. 2017;23:S158–9. [Google Scholar]
- 65.Lee S, et al. 3D bioprinted functional and contractile cardiac tissue constructs. Tissue Eng A. 2017;23:S96–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y, Yu Y, Akkouch A, Dababneh A, Dolati F, Ozbolat IT. In vitro study of directly bioprinted perfusable vasculature conduits. Biomater Sci. 2015;3(1):134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ho CM, et al. 3D printed polycaprolactone carbon nanotube composite scaffolds for cardiac tissue engineering. Macromol Biosci. 2017;17(4). [DOI] [PubMed] [Google Scholar]
- 68.Jang J, Park HJ, Kim SW, Kim H, Park JY, Na SJ, et al. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials. 2017;112:264–74. [DOI] [PubMed] [Google Scholar]
- 69.Pati F, Jang J, Ha DH, Won Kim S, Rhie JW, Shim JH, et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun. 2014;5:3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma X, Dewan S, Liu J, Tang M, Miller KL, Yu C, et al. 3D printed micro-scale force gauge arrays to improve human cardiac tissue maturation and enable high throughput drug testing. Acta Biomater. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pattanaik S, Arbra C, Bainbridge H, Dennis SG, Fann SA, Yost MJ. Vascular tissue engineering using scaffold-free prevascular endothelial-fibroblast constructs. Biores Open Access. 2019;8(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jang J 3D bioprinting and in vitro cardiovascular tissue modeling. Bioengineering (Basel). 2017;4(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim YK, Park JA, Yoon WH, Kim J, Jung S. Drop-on-demand inkjet-based cell printing with 30-mum nozzle diameter for cell level accuracy. Biomicrofluidics. 2016;10(6):064110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hinton TJ, Jallerat Q, Palchesko RN, Park JH, Grodzicki MS, Shue HJ, et al. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci Adv. 2015;1(9):e1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tan Y, Kong CW, Chen S, Cheng SH, Li RA, Sun D. Probing the mechanobiological properties of human embryonic stem cells in cardiac differentiation by optical tweezers. J Biomech. 2012;45(1):123–8. [DOI] [PubMed] [Google Scholar]
- 76.JingP LiuY, Keeler EG CruzNM, FreedmanBS Lin LY. Optical tweezers system for live stem cell organization at the single-cell level. Biomed Opt Express. 2018;9(2):771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gruene M, Deiwick A, Koch L, Schlie S, Unger C, Hofmann N, et al. Laser printing of stem cells for biofabrication of scaffold-free autologous grafts. Tissue Eng Part C Methods. 2011;17(1):79–87. [DOI] [PubMed] [Google Scholar]
- 78.Gaebel R, Ma N, Liu J, Guan J, Koch L, Klopsch C, et al. Patterning human stem cells and endothelial cells with laser printing for cardiac regeneration. Biomaterials. 2011;32(35):9218–30. [DOI] [PubMed] [Google Scholar]
- 79.Nahmias Y, Schwartz RE, Verfaillie CM, Odde DJ. Laser-guided direct writing for three-dimensional tissue engineering. Biotechnol Bioeng. 2005;92(2):129–36. [DOI] [PubMed] [Google Scholar]
- 80.Tomov ML, Olmsted ZT, Dogan H, Gongorurler E, Tsompana M, Otu HH, et al. Distinct and shared determinants of cardiomyocyte contractility in multi-lineage competent ethnically diverse human iPSCs. Sci Rep. 2016;6:37637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dergilev K, et al. C-kit cardiac progenitor cell based cell sheet improves vascularization and attenuates cardiac remodeling following myocardial infarction in rats. Biomed Res Int. 2018;2018: 3536854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Furuta A, Miyoshi S, Itabashi Y, Shimizu T, Kira S, Hayakawa K, et al. Pulsatile cardiac tissue grafts using a novel three-dimensional cell sheet manipulation technique functionally integrates with the host heart, in vivo. Circ Res. 2006;98(5):705–12. [DOI] [PubMed] [Google Scholar]
- 83.Kamata S, Miyagawa S, Fukushima S, Nakatani S, Kawamoto A, Saito A, et al. Improvement of cardiac stem cell sheet therapy for chronic ischemic injury by adding endothelial progenitor cell transplantation: analysis of layer-specific regional cardiac function. Cell Transplant. 2014;23(10):1305–19. [DOI] [PubMed] [Google Scholar]
- 84.Sekiya S, Shimizu T, Yamato M, Kikuchi A, Okano T. Bioengineered cardiac cell sheet grafts have intrinsic angiogenic potential. Biochem Biophys Res Commun. 2006;341(2):573–82. [DOI] [PubMed] [Google Scholar]
- 85.Shimizu T, Yamato M, Isoi Y, Akutsu T, Setomaru T, Abe K, et al. Fabrication of pulsatile cardiac tissue grafts using a novel 3dimensional cell sheet manipulation technique and temperature responsive cell culture surfaces. Circ Res. 2002;90(3):e40. [DOI] [PubMed] [Google Scholar]
- 86.Harada S, Nakamura Y, Shiraya S, Fujiwara Y, Kishimoto Y, Onohara T, et al. Smooth muscle cell sheet transplantation preserve cardiac function and minimize cardiac remodeling in a rat myocardial infarction model. J Cardiothorac Surg. 2016;11(1): 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zakharova L, Mastroeni D, Mutlu N, Molina M, Goldman S, Diethrich E, et al. Transplantation of cardiac progenitor cell sheet onto infarcted heart promotes cardiogenesis and improves function. Cardiovasc Res. 2010;87(1):40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Masuda S, Shimizu T. Three-dimensional cardiac tissue fabrication based on cell sheet technology. Adv Drug Deliv Rev. 2016;96:103–9. [DOI] [PubMed] [Google Scholar]
- 89.Sakaguchi K, Shimizu T, Okano T. Construction of three dimensional vascularized cardiac tissue with cell sheet engineering. J Control Release. 2015;205:83–8. [DOI] [PubMed] [Google Scholar]
- 90.Banerjee MN, Bolli R, Hare JM. Clinical studies of cell therapy in cardiovascular medicine: recent developments and future directions. Circ Res. 2018;123(2):266–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pena B, et al. Injectable hydrogels for cardiac tissue engineering. Macromol Biosci. 2018;18(6):e1800079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hasan A, Khattab A, Islam MA, Hweij KA, Zeitouny J, Waters R, et al. Injectable hydrogels for cardiac tissue repair after myocardial infarction. Adv Sci (Weinh). 2015;2(11):1500122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101(7):1869–79. [DOI] [PubMed] [Google Scholar]
- 94.Park H, Kang SW, Kim BS, Mooney DJ, Lee KY. Shear-reversibly crosslinked alginate hydrogels for tissue engineering. Macromol Biosci. 2009;9(9):895–901. [DOI] [PubMed] [Google Scholar]
- 95.Reis LA, Chiu LLY, Feric N, Fu L, Radisic M. Biomaterials in myocardial tissue engineering. J Tissue Eng Regen Med. 2016;10(1):11–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Muller P, Lemcke H, David R. Stem cell therapy in heart diseases—cell types, mechanisms and improvement strategies. Cell Physiol Biochem. 2018;48(6):2607–55. [DOI] [PubMed] [Google Scholar]
- 97.Golpanian S, Wolf A, Hatzistergos KE, Hare JM. Rebuilding the damaged heart: mesenchymal stem cells, cell-based therapy, and engineered heart tissue. Physiol Rev. 2016;96(3):1127–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11(4): 367–8. [DOI] [PubMed] [Google Scholar]
- 99.Hodgkinson CP, Bareja A, Gomez JA, Dzau VJ. Emerging concepts in paracrine mechanisms in regenerative cardiovascular medicine and biology. Circ Res. 2016;118(1):95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kanda P, Davis DR. Cellular mechanisms underlying cardiac engraftment of stem cells. Expert Opin Biol Ther. 2017;17(9):1127–43. [DOI] [PubMed] [Google Scholar]
- 101.Li X, et al. Improving cell engraftment in cardiac stem cell therapy. Stem Cells Int. 2016;2016:7168797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107(7):913–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang S, Wang D, Estrov Z, Raj S, Willerson JT, Yeh ETH. Both cell fusion and trans differentiation account for the transformation of human peripheral blood CD34-positive cells into cardiomyocytes in vivo. Circulation. 2004;110(25):3803–7. [DOI] [PubMed] [Google Scholar]
- 104.Mathison M, Rosengart TK. Heart regeneration: the endothelial cell comes first. J Thorac Cardiovasc Surg. 2018;155(3):1128–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Domenech M, Polo-Corrales L, Ramirez-Vick JE, Freytes DO. Tissue engineering strategies for myocardial regeneration: acellular versus cellular scaffolds? Tissue Eng Part B Rev. 2016;22(6): 438–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pal A, Vernon BL, Nikkhah M. Therapeutic neovascularization promoted by injectable hydrogels. Bioact Mater. 2018;3(4):389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Radhakrishnan J, Krishnan UM, Sethuraman S. Hydrogel based injectable scaffolds for cardiac tissue regeneration. Biotechnol Adv. 2014;32(2):449–61. [DOI] [PubMed] [Google Scholar]
- 108.Mahmoudi M, Zhao M, Matsuura Y, Laurent S, Yang PC, Bernstein D, et al. Infection-resistant MRI-visible scaffolds for tissue engineering applications. Bioimpacts. 2016;6(2):111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Serpooshan V, Mahmoudi M, Zhao M, Wei K, Sivanesan S, Motamedchaboki K, et al. Protein corona influences cell biomaterial interactions in nanostructured tissue engineering scaffolds. Adv Funct Mater. 2015;25(28):4379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Serpooshan V, Zhao M, Metzler SA, Wei K, Shah PB, Wang A, et al. The effect of bioengineered acellular collagen patch on cardiac remodeling and ventricular function post myocardial infarction. Biomaterials. 2013;34(36):9048–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vunjak Novakovic G, Eschenhagen T, Mummery C. Myocardial tissue engineering: in vitro models. Cold Spring Harb Perspect Med. 2014;4(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Serpooshan V, Ruiz-Lozano P. Ultra-rapid manufacturing of engineered epicardial substitute to regenerate cardiac tissue following acute ischemic injury. Methods Mol Biol. 2014;1210:239–48. [DOI] [PubMed] [Google Scholar]
- 113.Nelson CM, VanDuijn MM, Inman JL, Fletcher DA, Bissell MJ. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science. 2006;314(5797):298–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface. 2011;8(55):153–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen DHT, Cohen DM, et al. Rapid casting of patterned vascular networks for perfusable engineered 3D tissues. Nat Mater. 2012;11(9):768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen DHT, Cohen DM, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. 2012;11(9):768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jayakumar MK, Idris NM, Zhang Y. Remote activation of biomolecules in deep tissues using near-infrared-to-UV upconversion nanotransducers. Proc Natl Acad Sci U S A. 2012;109(22): 8483–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guyette JP, Gilpin SE, Charest JM, Tapias LF, Ren X, Ott HC. Perfusion decellularization of whole organs. Nat Protoc. 2014;9(6):1451–68. [DOI] [PubMed] [Google Scholar]
- 119.Gilbert TW. Strategies for tissue and organ decellularization. J Cell Biochem. 2012;113(7):2217–22. [DOI] [PubMed] [Google Scholar]
- 120.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14(2):213–21. [DOI] [PubMed] [Google Scholar]
- 121.Gerli MFM, Guyette JP, Evangelista-Leite D, Ghoshhajra BB, Ott HC. Perfusion decellularization of a human limb: a novel platform for composite tissue engineering and reconstructive surgery. PLoS One. 2018;13(1):e0191497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ng SL, et al. Lineage restricted progenitors for the repopulation of decellularized heart. Biomaterials. 2011;32(30):7571–80. [DOI] [PubMed] [Google Scholar]
- 123.Seetapun D, Ross JJ. Eliminating the organ transplant waiting list: the future with perfusion decellularized organs. Surgery. 2017;161(6):1474–8. [DOI] [PubMed] [Google Scholar]
- 124.Cimetta E, Pizzato S, Bollini S, Serena E, deCoppi P, Elvassore N. Production of arrays of cardiac and skeletal muscle myofibers by micropatterning techniques on a soft substrate. Biomed Microdevices. 2009;11(2):389–400. [DOI] [PubMed] [Google Scholar]
- 125.Kim JJ, Hou L, Huang NF. Vascularization of three-dimensional engineered tissues for regenerative medicine applications. Acta Biomater. 2016;41:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee BW, Liu B, Pluchinsky A, Kim N, Eng G, Vunjak-Novakovic G. Modular assembly approach to engineer geometrically precise cardiovascular tissue. Adv Healthc Mater. 2016;5(8):900–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Salick MR, Napiwocki BN, Sha J, Knight GT, Chindhy SA, Kamp TJ, et al. Micropattern width dependent sarcomere development in human ESC-derived cardiomyocytes. Biomaterials. 2014;35(15): 4454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pacharra S, et al. Surface patterning of a novel PEG-functionalized poly-l-lactide polymer to improve its biocompatibility: applications to bioresorbable vascular stents. J Biomed Mater Res B Appl Biomater. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yamaguchi M, Ikeda K, Suzuki M, Kiyohara A, Kudoh SN, Shimizu K, et al. Cell patterning using a template of microstructured organosilane layer fabricated by vacuum ultraviolet light lithography. Langmuir. 2011;27(20):12521–32. [DOI] [PubMed] [Google Scholar]
- 130.Atmanli A, Hu D, Domian IJ. Molecular etching: a novel methodology for the generation of complex micropatterned growth surfaces for human cellular assays. Adv Healthc Mater. 2014;3(11):1759–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sugiura S, Cha JM, Yanagawa F, Zorlutuna P, Bae H, Khademhosseini A. Dynamic three-dimensional micropatterned cell co-cultures within photocurable and chemically degradable hydrogels. J Tissue Eng Regen Med. 2016;10(8):690–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yu H, Tay CY, Pal M, Leong WS, Li H, Li H, et al. A bio-inspired platform to modulate myogenic differentiation of human mesenchymal stem cells through focal adhesion regulation. Adv Healthc Mater. 2013;2(3):442–9. [DOI] [PubMed] [Google Scholar]
- 133.Guillemette MD, Park H, Hsiao JC, Jain SR, Larson BL, Langer R, et al. Combined technologies for microfabricating elastomeric cardiac tissue engineering scaffolds. Macromol Biosci. 2010;10(11):1330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Badie N, Bursac N. Novel micropatterned cardiac cell cultures with realistic ventricular microstructure. Biophys J. 2009;96(9): 3873–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Entcheva E, Bien H. Acoustic micromachining of three dimensional surfaces for biological applications. Lab Chip. 2005;5(2):179–83. [DOI] [PubMed] [Google Scholar]
- 136.Zhu Y, et al. Tissue engineering of 3Dorganotypic microtissues by acoustic assembly. Methods Mol Biol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tian L, Martin N, Bassindale PG, Patil AJ, Li M, Barnes A, et al. Spontaneous assembly of chemically encoded two-dimensional coacervate droplet arrays by acoustic wave patterning. Nat Commun. 2016;7:13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Senthamizhan A, Balusamy B, Uyar T. Electrospinning: a versatile processing technology for producing nano fibrous materials for biomedical and tissue-engineering applications. In: Electrospun materials for tissue engineering and biomedical applications: research, design and commercialization; 2017. p. 3–41. [Google Scholar]
- 139.Xie J, Wang CH. Encapsulation of proteins in biodegradable polymeric microparticles using electrospray in the Taylor cone-jet mode. Biotechnol Bioeng. 2007;97(5):1278–90. [DOI] [PubMed] [Google Scholar]
- 140.Kitsara M, Agbulut O, Kontziampasis D, Chen Y, Menasché P. Fibers for hearts: a critical review on electrospinning for cardiac tissue engineering. Acta Biomater. 2017;48:20–40. [DOI] [PubMed] [Google Scholar]
- 141.Senel Ayaz HG, et al. Textile-templated electrospun anisotropic scaffolds for regenerative cardiac tissue engineering. Biomaterials. 2014;35(30):8540–52. [DOI] [PubMed] [Google Scholar]
- 142.Prabhakaran MP, Nair AS, Kai D, Ramakrishna S. Electrospun composite scaffolds containing poly(octanediol-co-citrate) for cardiac tissue engineering. Biopolymers. 2012;97(7):529–38. [DOI] [PubMed] [Google Scholar]
- 143.Kai D, Prabhakaran MP, Jin G, Ramakrishna S. Guided orientation of cardiomyocytes on electrospun aligned nanofibers for cardiac tissue engineering. J Biomed Mater Res B Appl Biomater. 2011;98b(2):379–86. [DOI] [PubMed] [Google Scholar]
- 144.Joanne P, Kitsara M, Boitard SE, Naemetalla H, Vanneaux V, Pernot M, et al. Nanofibrous clinical-grade collagen scaffolds seeded with human cardiomyocytes induces cardiac remodeling in dilated cardiomyopathy. Biomaterials. 2016;80:157–68. [DOI] [PubMed] [Google Scholar]
- 145.Ishii O, Shin M, Sueda T, Vacanti JP. In vitro tissue engineering of a cardiac graft using a degradable scaffold with an extracellular matrix-like topography. J Thorac Cardiovasc Surg. 2005;130(5): 1358–63. [DOI] [PubMed] [Google Scholar]
- 146.Hekmati AH, Norouzi M. 12—electrospun scaffolds for cardiac tissue engineering In: Uyar T, Kny E, editors. Electrospun materials for tissue engineering and biomedical applications: Woodhead Publishing; 2017. p. 289–97. [Google Scholar]
- 147.Kitsara M, et al. Heart on a chip: micro-nanofabrication and microfluidics steering the future of cardiac tissue engineering. Microelectron Eng. 2019;203:44–62. [Google Scholar]
- 148.Ronaldson-Bouchard K, Vunjak-Novakovic G. Organs-on-a-chip: a fast track for engineered human tissues in drug development. Cell Stem Cell. 2018;22(3):310–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Grabowski H Are the economics of pharmaceutical research and development changing?: productivity, patents and political pressures. Pharmacoeconomics. 2004;22(2 Suppl 2):15–24. [DOI] [PubMed] [Google Scholar]
- 150.Zhang YS, Arneri A, Bersini S, Shin SR, Zhu K, Goli-Malekabadi Z, et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials. 2016;110:45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang YS, Aleman J, Arneri A, Bersini S, Piraino F, Shin SR, et al. From cardiac tissue engineering to heart-on-a-chip: beating challenges. Biomed Mater. 2015;10(3):034006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lin DSY, Guo F, Zhang B. Modeling organ-specific vasculature with organ-on-a-chip devices. Nanotechnology. 2019;30(2):024002. [DOI] [PubMed] [Google Scholar]
- 153.Wang X, Phan DTT, Sobrino A, George SC, Hughes CCW, Lee AP. Engineering anastomosis between living capillary networks and endothelial cell-lined microfluidic channels. Lab Chip. 2016;16(2):282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ribas J, Sadeghi H, Manbachi A, Leijten J, Brinegar K, Zhang YS, et al. Cardiovascular organ-on-a-chip platforms for drug discovery and development. Appl In Vitro Toxicol. 2016;2(2):82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen Y, Chan HN, Michael SA, Shen Y, Chen Y, Tian Q, et al. A microfluidic circulatory system integrated with capillary-assisted pressure sensors. Lab Chip. 2017;17(4):653–62. [DOI] [PubMed] [Google Scholar]