Abstract
Background:
Biofilms are involved in many Staphylococcus aureus infections. Relation of biofilm forming S. aureus strains and the infection types or the clinical outcomes remain unclear.
Methods:
We measured biofilm formation, with a microtiter plate assay, of a collection of methicillin-sensitive clinical isolates from 159 invasive S. aureus infections, encompassing all cases occurring within a hospital catchment area during two years, and of additional 49 non-invasive skin infection isolates from the same region. These results were related to available clinical and microbiological documentation.
Results:
Isolates from medical device infections (intravenous line-associated and prosthetic joint infections), as well as isolates from superficial skin infections, were particularly proficient in forming biofilms. No increased biofilm-forming capacity was seen in isolates from endocarditis, osteomyelitis, or from other infections. There was also a correlation of biofilm formation with the agr type of the isolates. Thicker biofilms appeared to be more resistant to antibiotic treatment in vitro. No correlation between biofilm formation and clinical outcomes was noted.
Conclusions:
S. aureus isolates from ‘classical’ biofilm-related infections, but also from superficial skin infections, are especially proficient in forming biofilms. There is, however, no obvious relation of biofilm-forming capacity of isolates and the clinical outcome of the infection, and more studies on this issue are needed.
Keywords: biofilm, Staphylococcus aureus, clinical outcome, skin, antibiotic sensitivity, agr
Introduction
Many Staphylococcus aureus infections involve formation of biofilms, that is, sessile communities of bacteria attached to surfaces and encased in extracellular matrix [1]. Staphylococcal biofilms appear on implantable medical devices (catheters, prosthetic joints, implants, etc.), but also on host tissues in biofilm-like infections of chronic wounds, endocarditis, or osteomyelitis [1]. As biofilms are resistant to host immune system and antibiotics, they contribute to the persistent and hard-to-treat character of staphylococcal diseases [1].
Despite the importance of biofilms, systematic research on the biofilm-forming capacity of S. aureus clinical isolates from human infections is limited [2]. Several studies investigated correlations of disease types and biofilm formation [3, 4, 5, 6, 7, 8, 9, 10, 11], but these usually compared isolates from only two different infection types, and frequently involved only methicillin-resistant S. aureus (MRSA). Moreover, almost nothing is known about the correlation of the clinical outcomes with the biofilm forming capacity of the infecting isolates.
In this study, we examined in vitro biofilm formation by methicillin-sensitive S. aureus (MSSA) clinical isolates from a wide range of invasive infections, and demonstrated that it correlates with the infection type, but not with the clinical outcome.
Materials and methods
A previously described collection of 159 S. aureus isolates from invasive infections (that is, from blood or other normally sterile body sites) treated in Skaraborg Hospital, Sweden, in 2003–2005, encompassing all cases occurring within the hospital catchment area, was used [12, 13, 14, 15]. Additional 49 isolates from non-invasive (superficial) skin infections were collected in the same region in 2011 [14]. All collected isolates were MSSA.
Biofilm formation by isolates in tryptic soy broth (TSA) supplemented with 0.25 % w/v glucose after 24 h at 37°C in wells of 96-well cell culture plate (Sarstedt, Germany) was determined in triplicates using classic crystal violet staining biofilm assay [16], and expressed as the mean absorbance at 570 nm for each isolate (A570). Before the experiment, plates were coated overnight at 4°C with 20 % v/v human plasma in PBS. In case of invasive isolates, biofilm formation values were correlated with the previously collected and published data on their agr type, occurrence of the tst gene, and the clinical data of the infected patients [12, 13]. Results were analyzed using the Mann-Whitney U test in SPSS (v.22, IBM Corporation, USA). The study was approved by the Ethical Board of Gothenburg.
To measure sensitivity of biofilms to antibiotics, the 24 h established biofilms were washed with PBS, and the wells were filled with TSA with 512 mg/ml of rifampicin, and incubated for additional 24 h at 37°C. Afterwards, viability of the biofilms was measured with the XTT (2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) reduction assay, and expressed as % viability of the control untreated biofilms [17]. Viability differences were analyzed using the unpaired t test in Prism (v.7, GraphPad, USA).
Results and discussion
Infection type
Most S. aureus isolates can form biofilms in vitro [2, 5], yet it remains unclear how this ability correlates with clinical infection. Our study, encompassing isolates from all cases of invasive S. aureus infections in the hospital catchment area (all of them MSSA, as MRSA have a very low prevalence in Sweden [18]), found a clear correlation of in vitro biofilm formation with certain infection types (Table 1). Nearly all isolates showed some capacity to form biofilms, but isolates from ‘classical’ biofilm-related infections (line-associated infections and infected joint prostheses) formed biofilms significantly better than the other invasive isolates. Analyzed separately, isolates from line-associated infections still formed significantly better biofilms (p=0.004), and while the number of isolates from infected joint prostheses was too small to reach a significant difference (n=5, p=0.3), their mean biofilm formation was notably high.
Table 1.
Correlation of infection type and biofilm formation in collection of S. aureus clinical isolates. Biofilm formation was measured as A570. Mann-Whitney U test was used for statistical comparisons. p>0.05 was considered non-significant (n.s.).
| infection type | n | Biofilm formation [mean ± SEM] | p compared to other invasive isolates |
|---|---|---|---|
| all invasive | 159 | 1.9 ± 0.1 | - |
| bacteraemia without focus | 31 | 1.7±0.2 | n.s. |
| invasive skin and soft tissue | 37 | 1.9 ± 0.2 | n.s. |
| biofilm – related: | 27 | 2.5 ± 0.2 | 0.002 |
| - line-associated | 22 | 2.6 ± 0.2 | 0.004 |
| - prosthetic joint | 2 | 2.4 ± 0.5 | n.s. |
| native joint arthritis | 17 | 2.0±0.3 | n.s. |
| endovascular | 11 | 1.7±0.4 | n.s. |
| osteomyelitis (all): | 13 | 1.9±0.3 | n.s. |
| - vertebral osteomyelitis | 6 | 2.3 ± 0.6 | n.s. |
| respiratory tract infection | 7 | 1.2 ±0.4 | n.s. |
| urinary tract infection | 8 | 1.7±0.5 | n.s. |
| intraabdominal | 7 | 1.1±0.2 | n.s. |
| other type | 1 | 2.1 | n.s. |
| non-invasive skin infections | 49 | 2.3 ± 0.2 | 0.031 |
Previous studies, restricted to MRSA only, showed better biofilm formation by isolates from urinary catheters compared to other urinary tract infections [3], and by isolates from device-related orthopedic infections compared to non-device orthopedic infections [4]. Other studies, however, did not find correlation of biofilm formation with isolates coming from implant-related or unrelated orthopedic infection [6], from catheter-related bacteraemia or nasal colonization [8], or from bloodstream infections with different infection foci [10]. Unlike these studies, comparing only two or three selected groups of isolates, our study included all invasive S. aureus isolates from a single hospital. This unbiased approach confirmed previous assumption that isolates from ‘classical’ biofilm-related infections have increased propensity to form biofilms. It remains to be explained if good biofilm forming strains preferably cause biofilm-related infections, or if their ability to form biofilm increases while bacteria adapt to the new environment during infection. Indeed, longitudinal observations (though limited to only 3 patients) suggested that adaptation of S. aureus to biofilm lifestyle might sometimes occur in the course of a chronic infection [19].
We observed no statistically significant differences in biofilm formation for isolates from bacteraemia without focus, invasive soft tissue infections, native joint septic arthritis, endovascular infections, or osteomyelitis (Table 1). A marked trend for increased biofilm formation was observed in isolates from vertebral osteomyelitis, probably not reaching significance due to the small number of cases (n=6, p=0.1).
Our finding of no differences for osteomyelitis and endovascular isolates might be surprising, as endovascular infections and osteomyelitis are thought to involve biofilms as part of their pathogenesis [1], and as osteomyelitis isolates were previously noted to form more biofilm than sepsis or colonizing isolates [9]. This could be due to several factors. First, the lack of significant differences might be due to the small sample size, especially in the case of vertebral osteomyelitis. Second, biofilm formation is only a part of these diseases’ pathology, and it might be overshadowed by other properties, such as the ability to persist intracellularly in osteomyelitis [9] or to clump during endovascular infections [20]. Finally, the conditions used for biofilm formation assay can affect the outcome, and it remains unclear how in vitro conditions relate to various conditions in vivo [5]. Measurements of biofilm formation on plasma-coated plastic surfaces – as used in this study – is probably representative for biofilm formation on artificial surface of catheters and implants, but not for biofilms forming inside the bone matrix or in the clot on a damaged vascular wall, as it happens in osteomyelitis and endovascular infections.
Previous studies noted good biofilm formation by isolates from skin infections [5, 7, 21]. Also in our study, isolates from superficial, non-invasive skin infections formed significantly better biofilms than the invasive isolates (p=0.031; Table 1). This suggests that biofilm formation might be important for S. aureus skin colonization and for superficial skin infections, but not for promotion of deeper invasion or systemic dissemination. One could even speculate that biofilm formation on skin surface and invasion into deeper tissues are two distinct behaviors controlled by opposite factors – as was shown for example for staphylokinase, which promotes penetration into the skin, but decreases biofilm formation [14, 15].
S. aureus genotype
While some studies showed correlation of biofilm formation with the agr types [4, 6, 22], others did not [10, 23]. In our sample, isolates with the agr type I had smaller (p=0.003) and isolates from the type III had larger (p<0.001) capacity to form biofilms than the isolates from other agr types (Table 2). The possibility that different agr types regulate biofilm formation in different ways warrants further attention. More likely, however, the observed differences barely represent different distribution of agr types amongst local clonal lineages, as S. aureus clonal lineages are known to differ in biofilm formation [11, 23, 24, 25, 26].
Table 2.
Correlation of agr type and biofilm formation in collection of S. aureus clinical isolates from invasive infections. Biofilm formation was measured as A570. Mann-Whitney U test was used for statistical comparisons. p>0.05 was considered non-significant (n.s.).
| agr type | n | Biofilm formation [mean ± SEM] | p compared to other isolates |
|---|---|---|---|
| all typed | 159 | 1.9 ± 0.1 | - |
| agr I | 58 | 1.5 ± 0.1 | 0.003 |
| agr II | 39 | 1.6 ± 0.2 | n.s. |
| agr III | 53 | 2.6 ± 0.2 | <0.001 |
| agr IV | 9 | 1.6 ±0.4 | n.s. |
The same reasoning as for agr types applies also to isolates with or without the tst gene. When biofilm formation was compared between isolates harbouring the tst gene (n=29; OD570=2.6±0.2) or not (n=130; OD570=1.2±0.1), the tst-positive isolates formed better biofilms (p=0.001). The tst might therefore be a marker of genetic lineages with increased biofilm formation.
Biofilm sensitivity to antibiotics
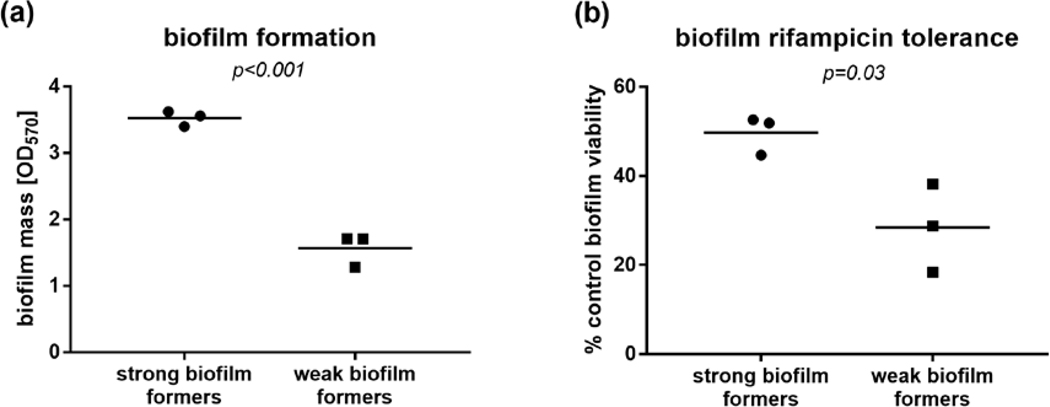
Biofilms are considered resistant to antibiotics [1]. When we measured viability of biofilms formed by three representative strains of the ‘strong’ and ‘weak’ biofilms formers (Figure 1a) after exposure to high concentration of rifampicin (frequently used in combination therapy of S. aureus biofilms [1]), we indeed observed that rifampicin failed to completely eradicate the biofilms. The decline in viability was, however, significantly more pronounced in weak biofilm formers (p=0.03, Figure 1b), suggesting that differences in biofilm formation observed between our clinical isolates might translate to altered tolerance to antimicrobial treatment. This prompted us to investigate if biofilm formation in clinical isolates correspond to clinical outcomes.
Figure 1.
Biofilm formation in the in 96-well plate crystal violet assay (a) and viability after 24 h exposure to rifampicin, measured with XTT assay (b) of representative ‘strong’ and ‘weak’ biofilm-forming S. aureus strains from the invasive isolate collection. P values calculated with the unpaired t test.
Clinical outcomes
In some studies, MRSA isolates from persistent bacteraemia formed better biofilms than from resolving bacteraemia [27], and patients infected with strong biofilm producing MRSA had higher re-admission rate, but lower mortality rate [24]. Other large size studies, however, failed to find any correlation between biofilm formation and disease outcome in S. aureus bloodstream infections [10, 28]. In our comprehensive MSSA sample, no clear correlation of patient characteristics or disease outcomes and biofilm forming capacity was observed. There were no significant differences in biofilm formation depending on sex, nosocomial or community acquired infection, nor presence or absence of any of the comorbidities (diabetes mellitus, rheumatoid arthritis, atopic dermatitis, psoriasis, renal disease, heart disease). The only difference was that isolates from patients with history of previous invasive S. aureus infection showed decreased biofilm formation compared to other isolates (previous infection n=12, OD570=1.2±0.3; no previous infection n=130, OD570=2.0±0.1; p=0.038). Biofilm formation also had no statistical impact on any of the disease outcomes (development of complicated bacteraemia or severe sepsis, 28-day mortality, disease recurrence, presence of residual symptoms).
The lack of correlation of disease outcome with in vitro biofilm formation is probably not surprising, considering that differences in biofilm formation observed between the isolates were small, and that the invasive infections differ from each other in their severity and prognosis. Microplate biofilm assays, as used in this study, have a good correlation with in vivo mouse catheter biofilm models [29], but differences observed between the groups in our study might be too small to translate to meaningful differences in the clinical outcome. Notably, studies reporting significant correlations between biofilm formation and clinical outcome also reported more pronounced differences in biofilm formation between isolates [24, 27]. Moreover, biofilm-related infections differ greatly in respect to prognosis and ease of treatment. This variation might overshadow the clinical impact of differences in biofilm formation. It was also recently suggested that the importance of biofilm formation for the clinical outcome depends on the genotype of the infecting strain [30], what introduces yet another confounding factor. Therefore, future studies aiming at investigating the impact of biofilm formation on disease outcome should preferably use large number of isolates, stratified based on their clonal lineages and infection types.
Summary
This study observed that good biofilm forming S. aureus isolates are predominantly associated with ‘classical’ biofilm-related infections (intravenous line-associated infections and prosthetic joints infections), and with superficial skin infections. Correlations with genotype and history of previous invasive S. aureus infection were also observed, but not with the disease outcome. These findings indicate a need for future studies to decipher the clinical impact of the capacity to form biofilms.
Acknowledgments
Funding information
This work was supported by the by the Swedish Society for Medical Research under Fellowship for Postdoctoral Scholars; Swedish Medical Research Council under Grant 523-2013-2750; Gothenburg Medical Society under Grant 778031; Scandinavian Society for Antimicrobial Chemotherapy Foundation under Grant 781191; Rune och Ulla Amlövs Stiftelse för Neurologisk och Reumatologisk Forskning; Adlerbertska Forskningsstiftelsen; Institute for Medicine, Gothenburg University; NIH public health service grant AI083211 (Project 3); and Department of Veteran Affairs Merit Award (I01 BX002711).
Footnotes
Disclosure of interest
The authors declare no conflicts of interest.
References
- 1.Archer NK, Mazaitis MJ, Costerton JW, et al. Staphylococcus aureus biofilms: properties, regulation, and roles in human disease. Virulence. 2011. Sep-Oct;2(5):445–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akers KS, Cardile AP, Wenke JC, et al. Biofilm formation by clinical isolates and its relevance to clinical infections. Adv Exp Med Biol. 2015;830:1–28. [DOI] [PubMed] [Google Scholar]
- 3.Ando E, Monden K, Mitsuhata R, et al. Biofilm formation among methicillin-resistant Staphylococcus aureus isolates from patients with urinary tract infection. Acta Med Okayama. 2004. August;58(4):207–14. [DOI] [PubMed] [Google Scholar]
- 4.Kawamura H, Nishi J, Imuta N, et al. Quantitative analysis of biofilm formation of methicillin-resistant Staphylococcus aureus (MRSA) strains from patients with orthopaedic device-related infections. FEMS Immunol Med Microbiol. 2011. October;63(1):10–5. [DOI] [PubMed] [Google Scholar]
- 5.Kwiecinski J, Kahlmeter G, Jin T. Biofilm Formation by Staphylococcus aureus Isolates from Skin and Soft Tissue Infections. Curr Microbiol. 2015. May;70(5):698–703. [DOI] [PubMed] [Google Scholar]
- 6.Post V, Wahl P, Uckay I, et al. Phenotypic and genotypic characterisation of Staphylococcus aureus causing musculoskeletal infections. Int J Med Microbiol. 2014. July;304(5–6):565–76. [DOI] [PubMed] [Google Scholar]
- 7.Smith K, Perez A, Ramage G, et al. Biofilm formation by Scottish clinical isolates of Staphylococcus aureus. J Med Microbiol. 2008. August;57(Pt 8):1018–23. [DOI] [PubMed] [Google Scholar]
- 8.Asai K, Yamada K, Yagi T, et al. Effect of incubation atmosphere on the production and composition of staphylococcal biofilms. J Infect Chemother. 2015. January;21(1):55–61. [DOI] [PubMed] [Google Scholar]
- 9.Kalinka J, Hachmeister M, Geraci J, et al. Staphylococcus aureus isolates from chronic osteomyelitis are characterized by high host cell invasion and intracellular adaptation, but still induce inflammation. Int J Med Microbiol. 2014. November;304(8):1038–49. [DOI] [PubMed] [Google Scholar]
- 10.Cha JO, Yoo JI, Yoo JS, et al. Investigation of Biofilm Formation and its Association with the Molecular and Clinical Characteristics of Methicillin-resistant Staphylococcus aureus. Osong Public Health Res Perspect. 2013. October;4(5):225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naicker PR, Karayem K, Hoek KG, et al. Biofilm formation in invasive Staphylococcus aureus isolates is associated with the clonal lineage. Microb Pathog. 2016. January;90:41–9. [DOI] [PubMed] [Google Scholar]
- 12.Jacobsson G, Dashti S, Wahlberg T, et al. The epidemiology of and risk factors for invasive Staphylococcus aureus infections in western Sweden. Scand J Infect Dis. 2007;39(1):6–13. [DOI] [PubMed] [Google Scholar]
- 13.Jacobsson G, Gustafsson E, Andersson R. Outcome for invasive Staphylococcus aureus infections. Eur J Clin Microbiol Infect Dis. 2008. September;27(9):839–48. [DOI] [PubMed] [Google Scholar]
- 14.Kwiecinski J, Jacobsson G, Karlsson M, et al. Staphylokinase promotes the establishment of Staphylococcus aureus skin infections while decreasing disease severity. J Infect Dis. 2013. September;208(6):990–9. [DOI] [PubMed] [Google Scholar]
- 15.Kwiecinski J, Peetermans M, Liesenborghs L, et al. Staphylokinase Control of Staphylococcus aureus Biofilm Formation and Detachment Through Host Plasminogen Activation. J Infect Dis. 2016. January 1;213(1):139–48. [DOI] [PubMed] [Google Scholar]
- 16.Christensen GD, Simpson WA, Younger JJ, et al. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985. December;22(6):996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alonso B, Cruces R, Perez A, et al. Comparison of the XTT and resazurin assays for quantification of the metabolic activity of Staphylococcus aureus biofilm. J Microbiol Methods. 2017. August;139:135–137. [DOI] [PubMed] [Google Scholar]
- 18.Johnson AP. Methicillin-resistant Staphylococcus aureus: the European landscape. J Antimicrob Chemother. 2011. May;66 Suppl 4:iv43-iv48. [DOI] [PubMed] [Google Scholar]
- 19.Trouillet-Assant S, Lelievre L, Martins-Simoes P, et al. Adaptive processes of Staphylococcus aureus isolates during the progression from acute to chronic bone and joint infections in patients. Cell Microbiol. 2016. October;18(10):1405–14. [DOI] [PubMed] [Google Scholar]
- 20.Crosby HA, Kwiecinski J, Horswill AR. Staphylococcus aureus Aggregation and Coagulation Mechanisms, and Their Function in Host-Pathogen Interactions. Adv Appl Microbiol. 2016;96:1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Land AD, Hogan P, Fritz S, et al. Phenotypic Variation Is Almost Entirely Independent of the Host-Pathogen Relationship in Clinical Isolates of S. aureus. PLoS One. 2015;10(6):e0129670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cafiso V, Bertuccio T, Santagati M, et al. agr-Genotyping and transcriptional analysis of biofilm-producing Staphylococcus aureus. FEMS Immunol Med Microbiol. 2007. October;51(1):220–7. [DOI] [PubMed] [Google Scholar]
- 23.Croes S, Deurenberg RH, Boumans ML, et al. Staphylococcus aureus biofilm formation at the physiologic glucose concentration depends on the S. aureus lineage. BMC Microbiol. 2009;9:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luther MK, Parente DM, Caffrey AR, et al. Clinical and Genetic Risk Factors for Biofilm-Forming Staphylococcus aureus. Antimicrob Agents Chemother. 2018. May;62(5):e02252–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez CJ Jr., Mende K, Beckius ML, et al. Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infect Dis. 2013;13:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King JM, Kulhankova K, Stach CS, et al. Phenotypes and Virulence among Staphylococcus aureus USA100, USA200, USA300, USA400, and USA600 Clonal Lineages. mSphere. 2016. May-Jun;1(3): e00071–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seidl K, Bayer AS, Fowler VG Jr., et al. Combinatorial phenotypic signatures distinguish persistent from resolving methicillin-resistant Staphylococcus aureus bacteremia isolates. Antimicrob Agents Chemother. 2011. February;55(2):575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guembe M, Alonso B, Lucio J, et al. Biofilm production is not associated with poor clinical outcome in 485 patients with Staphylococcus aureus bacteraemia. Clin Microbiol Infect. 2018. June;24(6):659.e1–659.e3. [DOI] [PubMed] [Google Scholar]
- 29.Ferreira FA, Souza RR, Bonelli RR, et al. Comparison of in vitro and in vivo systems to study ica-independent Staphylococcus aureus biofilms. J Microbiol Methods. 2012. March;88(3):393–8. [DOI] [PubMed] [Google Scholar]
- 30.Recker M, Laabei M, Toleman MS, et al. Clonal differences in Staphylococcus aureus bacteraemia-associated mortality. Nat Microbiol. 2017. October;2(10):1381–1388. [DOI] [PubMed] [Google Scholar]