Abstract
We examined a novel linkage of national U.S. donor registry data with records from a pharmacy claims warehouse (2007–2016) to examine associations (adjusted hazard ratio, LCL aHR UCL) of postdonation fills of antidiabetic medications (ADM, insulin or non-insulin agents) with body mass index (BMI) at donation and other demographic and clinical factors. In 28,515 living kidney donors (LKDs), incidence of ADM use at 9 years rose in a graded manner with higher baseline BMI: underweight, 0.9%; normal weight, 2.1%; overweight, 3.5%; obese, 8.5%. Obesity was associated with higher risk of ADM use compared to normal BMI (aHR, 3.364.596.27). Metformin was the most commonly used ADM and was filled more often by obese than by normal weight donors (9-year incidence, 6.87% vs. 1.85%, aHR, 3.555.007.04). Insulin use was uncommon and did not differ significantly by BMI. Among a subgroup with BMI data at the 1-year post-donation anniversary (n=19,528), compared with stable BMI, BMI increase >0.5 kg/m2 by year 1 was associated with increased risk of subsequent ADM use (aHR, 1.031.48 2.14, P=0.036). While this study did not assess the impact of donation on the development of obesity, these data support that among LKD, obesity is a strong correlate of ADM use.
Keywords: Antidiabetic medications, Diabetes mellitus, Health outcome, Living donation, Pharmacy claims, Registries
INTRODUCTION
Medical evaluation of living kidney donor candidates focuses on screening for known risk factors for adverse outcomes, and supporting donors with acceptable risks in informed decision making.1 As in the general population, diabetes mellitus has been considered a leading etiology of the uncommon end-stage renal disease (ESRD) events that occur in donors, particularly for ESRD late after donation. In a U.S. registry analysis of 125,427 living donors, ESRD beyond 10 years post-donation was predominantly reported as diabetic and hypertensive (later vs. early incidence rate ratios, 7.7 and 2.6, respectively).2 In a single-center study of 3956 predominantly white living donors, 25% of ESRD events with known causes (n = 6/25) were related to diabetes.3 While predonation diabetes is typically considered an exclusion for donation (mandated in current Organ Procurement and Transplantation Network [OPTN] policy),4 risk factors for postdonation diabetes mellitus (PDDM), and implications for donor counseling and selection, are not well defined.
Obesity is a well-established risk factor for diabetes and for chronic kidney disease and ESRD in the general population,5,6 but the outcome implications of obesity in living donors are controversial. A recent meta-analysis based on data from nearly 5 million healthy persons identified from seven general population cohorts, designed to inform donor candidate evaluation, found a modest association of baseline body mass index (BMI) >30 kg/m2 with increased risk of ESRD over median cohort follow-up of 4 to 16 years (adjusted hazard ratio (aHR), 1.04 1.16 1.29).7 A study of 119,769 donors from the U.S. found a stronger impact, such that each unit increase in BMI among overweight and obese donors increased the risk of ESRD by 7%.8 In contrast, a single-center study of 3752 predominantly white donors (1994–2016) found that obesity increased the risk of PDDM 3-fold but was not associated with ESRD (P = 0.46).9
Uncertainty about the long-term risks of obesity in living donors is reflected by variation in both clinical practice guidelines and clinical practice. Prior guidelines for the evaluation and care of living kidney donor candidates recommend BMI >35 kg/m2 as an absolute or relative contraindication to donation,10–16 while other guidelines recommend careful consideration of other comorbid conditions in donor candidates with BMI >30 kg/m2.11,13,17 In contrast, based on limited evidence in the donor population, the 2017 Kidney Disease: Improving Global Outcomes “Guideline for the Evaluation and Care of Living Donors” recommends that the decision to approve donor candidates with BMI >30 kg/m2 should be individualized based on demographic and health profile in relation to the transplant program’s acceptable risk threshold.1 In the context of the national obesity epidemic, overweight and obese donors now account for two-thirds of all accepted living donors nationally, although there is substantial center-level variation in acceptance practices by BMI, particularly for very obese donors.18
At the crossroads of controversy in the implications of obesity in living donor selection, and in recognition of diabetes mellitus as an important cause of ESRD when it occurs in donors, pursuit of better understanding of the implications of predonation obesity for comorbidity such as diabetes is warranted. Pharmacy claims offer a non-obtrusive measure of prescribed health care that do not rely on patient self-report and are increasingly used in observational investigations of large populations including transplant and donation-related epidemiologic studies.19–25 To this end, we examined a novel linkage of national U.S. transplant registry data with records from a pharmacy claims warehouse that identifies antidiabetic medication (ADM). Our goals were to identify the incidence of ADM use after donation, and to assess variation in risk according to baseline BMI and other demographic and clinical factors.
MATERIALS AND METHODS
Data Sources
We conducted a retrospective cohort study using linked health care databases in the United States.26 This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donors, waitlisted candidates, and transplant recipients in the United States, submitted by the members of OPTN. The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services, provides oversight of the activities of the OPTN and SRTR contractors. The study was approved by the Saint Louis University Institutional Review Board.
Pharmacy fill data were assembled by linking SRTR records for living kidney donors with billing claims from a large U.S. pharmaceutical claims data (PCD) warehouse that maintains prescription drug fill records including self-paid fills and those reimbursed by private and public payers. The PCD comprises National Council for Prescription Drug Program 5.1-format prescription claims aggregated from multiple sources including data clearinghouses, retail pharmacies, and prescription benefit managers for approximately 60% of U.S. retail pharmacy transactions. Individual claim records include the date of a given pharmacy fill with the National Drug Code identifying agent and dosage. After institutional review board and HRSA approvals, PCD records were linked with SRTR records for living donors.
We applied a deterministic deidentification strategy wherein patient identifiers (last name, first name, sex, date of birth, and ZIP code of residence) were transformed before delivery to the Saint Louis University researchers with Health Information Portability and Accountability Act and HITECH-certified encryption technology. The patient deidentification software uses multiple encryption algorithms in succession to guarantee that the resulting “token” containing encrypted patient identifiers can never be decrypted. However, the algorithm yields the same results for a given set of data elements, such that linkages by unique anonymous tokens are possible.
Population and Covariates
We included living kidney donors in the SRTR registry who donated between 2007 and 2016, and had linked pharmacy fill records covering the donation event and post-donation follow-up. Baseline donor demographic and clinical information ascertained from SRTR at the time of donation included: age, sex, race, donor-recipient relationship, health insurance status, BMI, physical limitations, education level, employment status, history of smoking, pre-donation hypertension, pre-donation estimated glomerular filtration rate (eGFR), and type of nephrectomy as reported by the transplant center. BMI was categorized according to World Health Organization criteria as: underweight, <18.5; normal weight, 18.5 to <25; overweight, 25 to <30; obese, >30 kg/m2.
Outcomes
The primary outcome was time to ADM use as defined by a pharmacy fill dates. Use of insulin and use of non-insulin agents were also examined separately (Appendix Table 1). Categories of non-insulin agents were examined in secondary analyses.
Statistical Analyses
Data management and analyses were performed with Statistical Analysis Software (SAS) for Windows, version 9.3 (SAS Institute Inc., Cary, NC). In all outcome analyses, we interpreted 2-tailed P-values less than 0.05 as statistically significant. Distributions of baseline traits among living kidney donors with and without ADM use were compared by the Chi square test. The cumulative incidence of ADM use was estimated by the Kaplan-Meier method, with a time scale of years since donation, and stratified by BMI categories for comparison. We also estimated the cumulative incidence of insulin use and use of each category of antidiabetic agent by 9 years. Participants were censored at the end of pharmacy data eligibility, death, or end of the study (December 31, 2016). We examined associations (LCL aHR UCL) of BMI at donation and other baseline donor demographic and clinical factors with ADM fills using multivariable Cox regression.
RESULTS
There were 58,601 living donors recorded in the SRTR in the study period. Of these, 28,530 had linked PCD data covering the donation event and some follow-up; 321 were excluded for missing BMI data, and 15 were exclude based on indication of predonation diabetes in the registry. The mean duration from donation to end of follow-up was 3.8 years (maximum 10.2 years). Among the final study sample of 28,515 living donors, the mean age was 42.9 years, 73.7% white, nearly 50% were biologically related to their recipient, 11% had hypertension, and 96% of the donor nephrectomies were laparoscopic (Table S1). The baseline characteristics of the study sample were similar to those of all donors recorded in the SRTR database during the same time period (Appendix, Table S2). The BMI distribution of the study sample included: <18.5 kg/m2, 0.9%; 18.5 to <25 kg/m2, 33.4%; 25 to <30 kg/m2, 41.5%; >30 kg/m2, 23.3%. Obese donors were more likely to be older, men, African American or Hispanic, past or current smokers, have lower than college education, have no or unknown insurance status at donation, donate to a related recipient with diabetes, hypertensive, and have slightly lower predonation eGFR (Table 1).
Table 1.
Distribution of clinical factors in the study sample of living kidney donors according to BMI at donation.
| BMI at Donation (kg/m2) | ||||
|---|---|---|---|---|
| <18.5 (n=268) |
18.5 to <25 (n=9,533) |
25 to <30 (n=11,836) |
≥30 (n=6,557) |
|
| (%) | (%) | (%) | (%) | |
| Donor characteristics | ||||
| Age (years) | † | ‡ | ‡ | |
| 18 to 30 | 31.8 | 20.7 | 15.4 | 16.3 |
| 31 to 44 | 29.1 | 34.6 | 35.5 | 39.5 |
| 45 to 59 | 31.3 | 36.4 | 39.9 | 37.8 |
| ≥60 | 7.8 | 8.3 | 9.1 | 6.5 |
| Female | 82.5* | 77.2 | 60.7ǂ | 63.1ǂ |
| Race | * | ‡ | ‡ | |
| White | 79.5 | 77.4 | 73.4 | 68.4 |
| Black | 7.5 | 7.8 | 10.5 | 15.2 |
| Hispanic | 5.2 | 9.5 | 12.7 | 14.2 |
| Other | 7.8 | 5.2 | 3.4 | 2.2 |
| Donor/recipient relationship | * | ‡ | ||
| Related Recipient with diabetes | 12.3 | 11.1 | 12.3 | 14.4 |
| Related Recipient without diabetes | 33.2 | 34.3 | 33.2 | 32.6 |
| Unrelated | 54.5 | 54.7 | 54.4 | 53.0 |
| Highest level of education | ǂ | ‡ | ||
| College & Higher | 69.4 | 70.4 | 68.0 | 62.7 |
| Grade/High School | 20.5 | 22.3 | 24.5 | 29.2 |
| Unknown | 76.5 | 80.2 | 7.5 | 8.1 |
| Employment status | ‡ | ‡ | ||
| Working | 76.5 | 80.2 | 83.0 | 83.3 |
| Not Working | 20.5 | 17.3 | 15.0 | 14.4 |
| Unknown | 3.0 | 2.5 | 2.0 | 2.3 |
| Insurance status | * | ǂ | ||
| Insured | 71.3 | 80.1 | 80.3 | 76.8 |
| Uninsured | 14.9 | 9.5 | 9.1 | 10.6 |
| Unknown | 13.8 | 10.4 | 10.6 | 12.6 |
| Hypertension history | 5.6 | 7.4 | 12.2ǂ | 14.5ǂ |
| Smoking history | 30.2 | 26.6 | 27.7* | 28.6* |
| eGFR at donation (mL/min per 1.73 m2) | * | ǂ | ||
| ≥90 | 74.0 | 68.2 | 65.7 | 66.7 |
| 60 to <90 | 24.2 | 29.5 | 32.2 | 31.1 |
| <60 | 1.1 | 1.5 | 1.6 | 1.7 |
| Unknown | 0.7 | 0.7 | 0.5 | 0.6 |
| Nephrectomy Type | ||||
| Open | 2.6 | 4.1 | 4.0 | 4.0 |
| Laparoscopic/ Unknown | 97.4 | 95.9 | 96.0 | 96.0 |
| Year of donation | * | * | ||
| 2007–2010 | 36.9 | 33.0 | 32.0 | 32.3 |
| 2011–2013 | 37.7 | 37.3 | 38.0 | 38.1 |
| 2014–2016 | 25.4 | 29.7 | 30.0 | 29.6 |
Data presented as percentages (%) of donors in each BMI category with the indicated baseline trait (column percentages).
P-values vs reference:
P <0.05–0.002
P=0.001–0.0002
P <0.0001.
Association of Obesity with ADM Use
The incidence of ADM use at 9 years rose in a graded manner with higher BMI: underweight, 0.9%; normal weight, 2.1%; overweight, 3.5%; obese, 8.5% (Figure 1). Compared to donors with normal BMI, after adjustment for age, sex, and race, overweight donors were 2-times more likely to fill ADM (aHR, 1.632.243.07), and obese donors were 4-times more likely (aHR, 3.394.626.30). These relationships persisted after adjustment for other demographic and clinical factors recorded in the registry (Table 2), such that overweight and obese baseline BMI was associated with 2- and 4-times the likelihood of ADM use, respectively (aHR, 1.622.233.06 and 3.364.596.27). When comparing ADM treatments, these differences were primarily due to non-insulin-based therapies. After covariate adjustment, other significant correlates of ADM included female sex (aHR, 1.662.172.83) and more recent years of donation (Table 2). Compared with donations in 2007–2010, donations in 2014–2016 were associated with more than twice the likelihood of ADM use (aHR, 1.602.363.48) (Table 2, Figure 2). Donation to a related recipient with diabetes was associated with trend toward higher risk of ADM use, but this relationship was not statistically significant. Compared with white race, black race and Hispanic ethnicity were not significantly associated with ADM use overall, but were associated with 3.5- and 2.9-times the likelihood of insulin use after donation.
Figure 1.
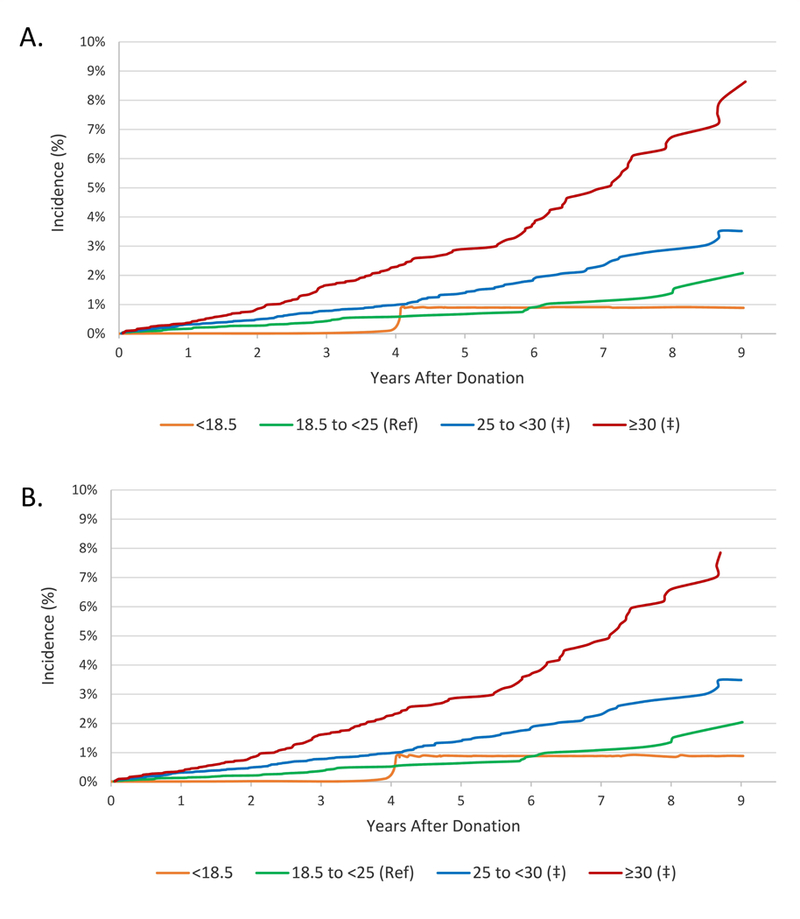
Incidence of ADM use according to BMI at donation. (A) Any antidiabetic medication use. (B) Non-insulin antidiabetic medication use. ‡P <0.0001 compared with reference
Table 2.
Adjusted associations of BMI and other baseline factors with ADM use in living kidney donors.
| Characteristics at Donation | Any ADM use aHR (95% CL) |
Non-insulin ADM use aHR (95% CL) |
Insulin use aHR (95% CL) |
|---|---|---|---|
| BMI (kg/m2) | |||
| <18.5 | 0.63 (0.09–4.52) | 0.67 (0.09–4.85) | N/A |
| 18.5 to <25 | Reference | Reference | Reference |
| 25 to <30 | 2.23 (1.62–3.06) ‡ | 2.40 (1.74–3.33) ‡ | 0.79 (0.28–2.22) |
| ≥30 | 4.59 (3.36–6.27) ‡ | 4.91 (3.56–6.77) ‡ | 1.44 (0.53–3.92) |
| Age (years) | |||
| 18 to 30 | 1.18 (0.86–1.60) | 1.16 (0.85–1.58) | 1.68 (0.55–5.10) |
| 31 to 44 | Reference | Reference | Reference |
| 45 to 59 | 0.80 (0.61–1.04) | 0.79 (0.61–1.04) | 1.03 (0.38–2.81) |
| ≥60 | 0.92 (0.59–1.45) | 0.96 (0.61–1.51) | N/A |
| Female | 2.17 (1.66–2.83) ‡ | 2.23 (1.70–2.93) ‡ | 0.59 (0.26–1.34) |
| Race | |||
| White | Reference | Reference | Reference |
| Black | 1.26 (0.92–1.73) | 1.26 (0.91–1.73) | 3.48 (1.26–9.63) * |
| Hispanic | 1.29 (0.95–1.77) | 1.27 (0.92–1.75) | 2.91 (1.05–8.12) * |
| Other | 1.73 (1.04–2.89) * | 1.79 (1.07–2.99) * | N/A |
| Donor/recipient relationship | |||
| Related recipient with diabetes | 1.13 (0.83–1.54) | 1.10 (0.80–1.51) | 1.46 (0.49–4.38) |
| Related recipient without diabetes | Reference | Reference | Reference |
| Unrelated | 0.89 (0.70–1.13) | 0.88 (0.69–1.12) | 1.03 (0.42–2.52) |
| Highest level of education | |||
| College & Higher | 0.99 (0.77–1.27) | 1.04 (0.81–1.33) | 0.44 (0.19–1.04) |
| Grade/High School | Reference | Reference | Reference |
| Unknown | 1.01 (0.67–1.52) | 1.06 (0.70–1.60) | 0.97 (0.26–3.61) |
| Employment status | |||
| Working | 1.02 (0.78–1.36) | 1.02 (0.77–1.36) | 0.75 (0.27–2.05) |
| Not Working | Reference | Reference | Reference |
| Unknown | 1.01 (0.67–1.52) | 1.06 (0.70–1.60) | 0.97 (0.26–3.61) |
| Insurance status | |||
| Insured | Reference | Reference | Reference |
| Uninsured | 0.93 (0.64–1.33) | 0.96 (0.67–1.38) | 0.22 (0.03–1.68) |
| Unknown | 0.74 (0.53–1.04) | 0.76 (0.55–1.07) | 0.28 (0.06–1.27) |
| Hypertension history | 1.26 (0.74,2.14) | 1.41 (0.86,2.33) | 5.36 (1.49,19.26)* |
| Smoking history | 1.03 (0.81–1.31) | 1.03 (0.81–1.32) | 1.10 (0.45–2.69) |
| eGFR at donation (mL/min per 1.73 m2) |
|||
| <60 | 1.64 (0.86–3.13) | 1.75 (0.94–3.25) | 1.77 (0.22–13.94) |
| 60 to <90 | 1.04 (0.81–1.34) | 1.02 (0.80–1.31) | 0.80 (0.30–2.12) |
| ≥90 | Reference | Reference | Reference |
| Nephrectomy Type | |||
| Open | 0.87 (0.52–1.47) | 0.89 (0.53–1.50) | N/A |
| Laparoscopic/unknown | Reference | Reference | Reference |
| Year of donation | |||
| 2007–2010 | Reference | Reference | Reference |
| 2011–2013 | 1.41 (1.08–1.86) * | 1.45 (1.10–1.91) * | 0.49 (0.17–1.46) |
| 2014–2016 | 2.36 (1.60–3.48) ‡ | 2.43 (1.63–3.62) ‡ | 1.30 (0.30–5.67) |
P-value vs reference:
P <0.05–0.002
P=0.001–0.0002
P <0.0001.
Figure 2.
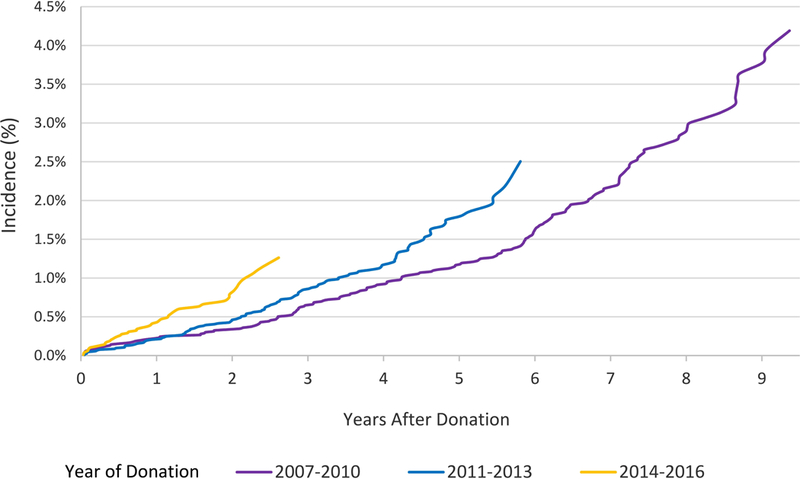
Incidence of ADM use according to year of donation.
Patterns of Types of ADM Use
Metformin was the most commonly used ADM after living donation. By 9 years post-donation, use of metformin rose with higher pre-donation BMI: underweight, 0.9%; normal weight, 1.8%; overweight, 3.3%; obese, 6.9% (Figure 3). Use of most classes of diabetes agents was higher in obese donors. Compared with normal BMI, obesity at donation was associated with 5-times the likelihood of metformin use (aHR,3.555.007.04), 3-times the likelihood of sulfonylurea use (aHR, 1.363.167.34), and 8-times the likelihood of other agent use (aHR, 2.428.3228.61) (Figure 4). Obesity at donation was not significantly associated with use of insulin or glitazones, which were both filled infrequently (<0.6% and no fill at 9 years).
Figure 3.
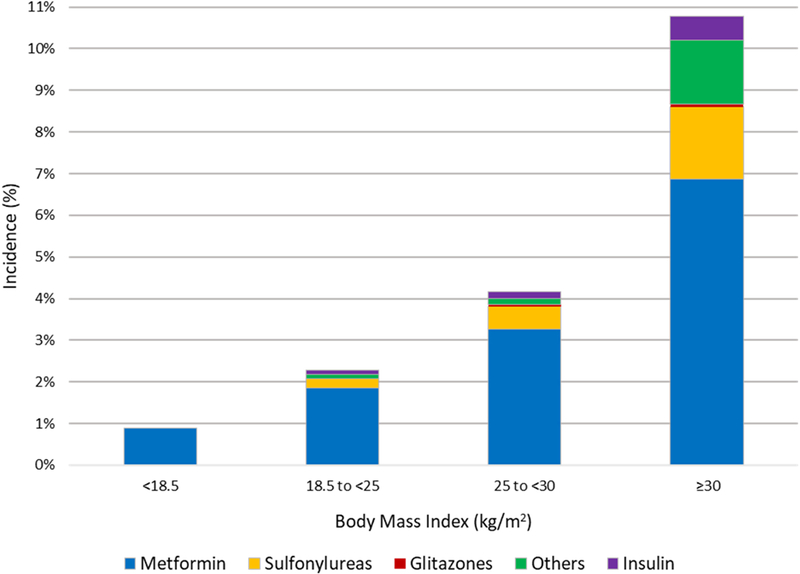
Distribution of ADM categories filled by 9 years post-donation, according to BMI at donation. Medication categories are not mutually exclusive.
Figure 4.
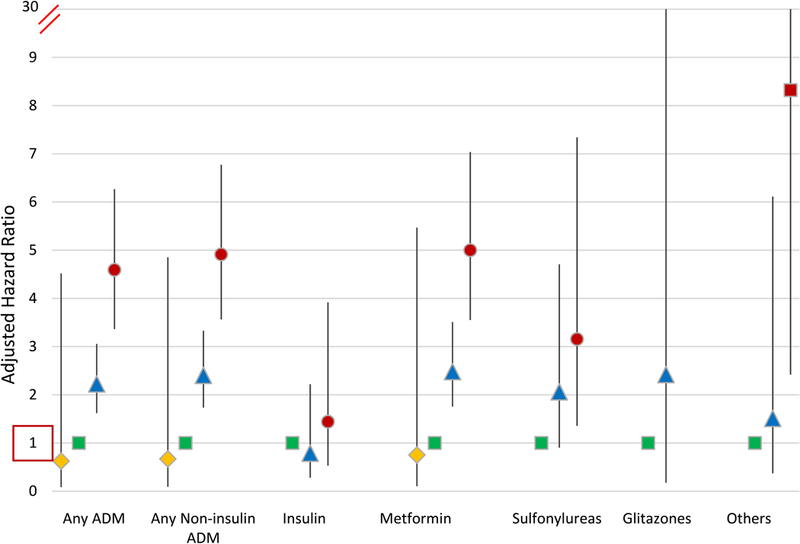
Adjusted associations of BMI at donation with ADM use categories after donation. Adjusted for other baseline factors in Table 1.
Secondary Analysis of Weight Change After Donation
Among a subgroup of 19,528 donors who also had BMI data at the 1-year post-donation anniversary and were free of ADM use in the first year, compared with stable BMI (defined as +0.5 kg/m2 change), BMI increase >0.5 kg/m2 by year 1 was associated with increased risk of subsequent ADM use (aHR, 1.031.48 2.14, P=0.036) after adjustment for all baseline factors including BMI at donation.
DISCUSSION
Pharmacy claims can provide efficient, non-obtrusive surrogate measures of treated clinical conditions that can help advance understanding of health outcomes in living donors, an important population that is not followed long-term in the U.S. national transplant registry. To improve the understanding of the frequency and correlates of ADM use in living kidney donors, and specifically examine associations of ADM use with BMI, we integrated the national U.S. donor registry with a pharmacy claims database that captures medication fills after donation. This analysis of a large, national donor sample yielded several notable observations: 1) The incidence of ADM use rose in a graded manner with higher BMI at donation, from 0.9% in underweight donors to 8.5% in obese donors by 9 years. 2) Associations of higher baseline BMI with ADM treatments persisted after covariate adjustment, such that obesity was associated with more than 4-times the likelihood of ADM use. 3) Metformin was the most commonly used ADM after donation, and use was 5-times more common among obese than among normal-weight donors. 4) Overweight and obese BMI were not associated with postdonation insulin use. 5) Use of ADM was more common in recent years. 6) BMI increase over the first year postdonation was also associated with increased risk of subsequent ADM use, independent of BMI at donation.
Our observation of graded increase in ADM use with higher BMI is consistent with prior studies reporting associations of obesity with measures of diabetes mellitus, both in the general population and in living donors. In one study that reported findings from two general population cohorts of 77,690 women and 46,060 men, higher BMI was associated with increased risk of diabetes at 10 years of follow-up: compared with normal BMI, BMIs of 25 to <30, 30 to <35, and ≥35 kg/m2 were associated with approximately 4, 10, and 20-times higher adjusted risk of diabetes.5 A single-center U.S. study that included 3752 predominantly white donors (1975–2014, 17% obese) found PDDM rates by the end of follow-up in 12% vs. 6% of donors with baseline BMI >30 vs. <30 kg/m2, and adjusted risk related to obesity was 3-times that of normal weight.9 Another smaller single-center study of 388 donors from Egypt (1976–2014) also found increased PDDM risk with higher BMI (4.1% in normal weight, 6% in overweight, and 25% in obese donors over follow-up to 2014).27 The higher incidence of PDDM in these two single-center cohorts likely reflects maximal follow-up over decades. Our study expands upon these single-center reports by examining a national U.S. cohort and using ADM as a non-obtrusive measure of a clinical condition that does not require return to the center for ascertainment.
The racial disparity in obesity observed in our study sample is present in the general population and was previously reported among both the general population28 and living donors.18 We previously identified racial and ethnic disparity in PDDM ascertained by diagnostic codes on medical claims; black and Hispanic donors had 1.52 and 1.65- times the likelihood of PDDM compared with white donors, respectively, based on clinical diagnoses after an average 7.7 years of follow-up.29 That sample was limited to 4650 privately insured donors, and importantly, baseline BMI was not available. In the current study, black race and Hispanic ethnicity were not significantly associated with ADM treatments overall, which may reflect the impact of adjustment for BMI (given the higher prevalence of obesity in these racial/ethnic groups), difference in the study samples, or the impact of using treatment compared with billing claims as the outcome measure. However, black and Hispanic donors had 3.5 and 2.9-times the likelihood of insulin use, respectively, compared with white donors in the current study, perhaps reflecting a greater severity of PDDM. Our finding of increased risk of ADM use for women in the multivariate model contrasts with the increased risk for men reported in the large single-center study of white donors, which may reflect differences in the outcome, and possibly care seeking for treatment.30
The temporal increase in ADM use observed in the current study warrants attention. While the 2017 diabetes mellitus report card by the Centers for Disease Control and Prevention noted that the annual incidence of diabetes in the U.S. peaked in 2008 at 0.85% and then declined to 0.65% in 2015,31 use of oral antidiabetic medications appears to be rising worldwide.32 Although reasons for the temporal trend in ADM use in living donors are not known, liberalization in accepting donors with metabolic abnormalities, including prediabetes, in more recent years could be contributing.18,33 One study of 8951 donors from three U.S. centers (1963 and 2007) reported that median values of fasting glucose at donation steadily increased over time.33 Notably, wide acceptance of hemoglobin A1c in place of oral glucose tolerance testing as one of the diagnostic criteria for diabetes around 2011 may also have contributed to this observed trend.34 Future work should monitor ongoing trends in ADM use and other measures of PDDM, and associations with baseline factors.
Metformin is the first-line pharmaceutical treatment for diabetes mellitus because of its efficacy in glucose control, lower risk of adverse effect profile, such as weight gain and hypoglycemia, and lower costs.35 Beginning diabetes treatment with metformin has also been associated with reduced subsequent need for treatment intensification.36 However, metformin has been linked to rare but fatal lactic acidosis complications, especially in patients with eGFR <30 ml/min per 1.73 m2, which has led the U.S. Food and Drug Administration (FDA) to label it as contraindicated for such patients. Since most donors are expected to have eGFR levels >30 ml/min per 1.73 m2, use is likely safe and appropriate, but notably, concern for reduced kidney function was not a deterrent to use, in contrast with infrequent use in the transplant population at higher eGFR levels.19 In the context of obesity, even independent of diabetes, metformin may have benefits for weight loss. In a randomized placebo controlled study of adults (BMI ≥24 kg/m2 and prediabetes) metformin was associated with 3.5% weight loss over 9 years of follow-up.37 However, the current FDA labeled indication for metformin is type 2 diabetes alone, and weight loss and prediabetes are not approved indications. Thus, while it is possible our study measure of ADM use captured indications other than type 2 diabetes, such uses are off-label, or uncommon use for treatment of polycystic ovarian syndrome, type 2 diabetes is the primary prescribing indication for metformin.
While ongoing work is needed, our study has implications for living donor care. Given the recently identified associations of obesity with post-onation ESRD,2 obesity should be considered a renal risk factor in donors, and of particular importance because of potential modifiability. Lifestyle factors that contribute to obesity include low physical activity levels, high caloric intake, and poor sleep quality.38 Interventional data among donors are lacking, but previous studies have shown that weight loss among obese adults is associated with reduced risk of diabetes which, in turn, would translate into reduced risk of ESRD. In a general population study of 114,281 women (1976–1990), the more weight gain during follow-up, the higher the risk of diabetes; in contrast, women who lost >5 kg had reduced risk of diabetes.39 However, achieving and sustaining weight loss is not trivial. Issa et al. reported that only 16% (14/90) of high BMI (>30 kg/m2) donors at one center were able to achieve weight loss to a BMI of <30 kg/m2 prior to donation.40 At 15 years of follow up, nearly all the high BMI donors (12/14) who had lost weight predonation gained weight, with a mean increase in BMI of 4.8.40 In our subgroup analysis of donors who also had BMI data at the 1-year post-donation anniversary, compared with stable BMI, BMI increase >0.5 kg/m2 by year 1 was associated with increased risk of subsequent ADM use. This is consistent with recent data that post-donation weight gain is associated with higher risk of diabetes.41 Although prospective evaluations of dietary interventions and monitored exercise programs in donors are needed to advance effective care, donor candidates should be counseled on lifestyle interventions to support achievement and maintenance of healthy body weight, and regular exercise according to guidelines for the general population.1 Importantly, these interventions should be initiated before donation and maintained lifelong, including as part of annual postdonation follow-up.1
There are several strengths to our study. We used pharmacy claims as a measure of diabetes, a non-obtrusive surrogate that avoids recall bias associated with survey studies, and complements information acquired though other study designs. Using this large, diverse national dataset, we confirmed associations of obesity at the time of donation with increased incidence of PDDM, previously reported among a single-center sample of predominantly white donors.9 We also found that black and Hispanic donors, compared to white donors, had higher risk of insulin use after donation. We also observed that donation to a related recipient with diabetes was associated with a trend towards higher ADM use, but the association was not statistically significant. Future work is needed to define the impacts of family history of diabetes, race and genetics in the complex relationships of obesity, diabetes and postdonation ESRD.
Our study has limitations. Linked pharmacy data were available for 49% of U.S. donors in the study period; thus, while our national study is larger than prior single-center cohorts, results may not generalize beyond the study sample. However, baseline characteristics of the sample were similar to those of all U.S. donors. We also lacked data for comparisons to healthy nondonors, and focused on comparisons of donor subgroups.42 Our measure was based on pharmacy fills, and we lacked laboratory tests for confirmation, such as hemoglobin A1c, fasting glucose, or oral glucose tolerance testing. However, our inability to capture diabetes controlled by life style measures should under-estimate the risk associated with obesity in donors. As discussed above, off-label prescribing of metformin may be captured as part of ADM use. We lacked data on fat distribution, which may be a stronger predictor for risk of ADM use than BMI. We were able to identify donation to related recipients with diabetes mellitus, but the registry does not include information on diabetes status in other relatives, or family history information for unrelated donors, or lifestyle risk factors. We lacked information on BMI changes for the entire follow-up period. However, in a subset with BMI data available at one-year anniversary, we found that BMI increase is associated with subsequent ADM use, independent of BMI at donation.
In conclusion, linkage of national U.S. donor registry data with pharmacy fill records demonstrates that being overweight or obese at the time of living kidney donation is strongly associated with ADM use after donation. In addition, the risk of ADM use appeared to increase over the period of our study. While this study did not assess the impact of donation on the development of obesity, these data advance understanding of the relationship obesity, when present among donors, with subsequent ADM use. Future work should monitor ongoing trends in ADM use and other measures of diabetes, associations with baseline factors including BMI and metabolic profile, and impact on renal and cardiovascular outcomes among living donors. All stakeholders—including patients, primary physicians, transplant programs, policy makers, insurers, and researchers—should recognize the importance of obesity as a contributor to postdonation health and work together to develop resources and interventions to support optimal donor outcomes.
Supplementary Material
ACKNOWLEDGEMENTS:
The authors thank SRTR colleague Nan Booth, MSW, MPH, ELS, for manuscript editing. An abstract describing portions of this work was an “Abstract of Distinction” at the American Transplant Congress 2017, Chicago, IL, and highlighted by Renal & Urology News, https://bit.ly/2pmCvFj.
FUNDING: This work was conducted under the auspices of the Minneapolis Medical Research Foundation (MMRF), contractor for the Scientific Registry of Transplant Recipients (SRTR), as a deliverable under contract no. HHSH250201500009C (U.S. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation). As a U.S. Government-sponsored work, there are no restrictions on its use. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government. Supported in part by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases, R01-DK096008. NNL was supported by a KRESCENT New Investigator Award.
ABBREVIATIONS:
- ADM
antidiabetic medication
- aHR
adjusted hazard ratio
- BMI
body mass index
- eGFR
estimated glomerular filtration rate
- ESRD
end-stage renal disease
- FDA
Food and Drug Administration
- HRSA
Health Resources and Services Administration
- OPTN
Organ Procurement and Transplantation Network
- PCD
pharmaceutical claims data
- PDDM
post-donation diabetes mellitus
- SAS
Statistical Analysis Software
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
DISCLOSURES: The authors declare no conflicts of interest.
Institution at which work was performed: Saint Louis University, St. Louis, MO, USA
REFERENCES
- 1.Lentine KL, Kasiske BL, Levey AS, et al. KDIGO Clinical Practice Guideline on the Evaluation and Care of Living Kidney Donors. Transplantation. 2017;101(8S Suppl 1):S1–S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anjum S, Muzaale AD, Massie AB, et al. Patterns of End-Stage Renal Disease Caused by Diabetes, Hypertension, and Glomerulonephritis in Live Kidney Donors. Am J Transplant. 2016;16(12):3540–3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ibrahim HN, Foley RN, Reule SA, et al. Renal function profile in white kidney donors: The first 4 decades. J Am Soc Nephrol. 2016;27(9):2885–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.OPTN (Organ Procurement and Transplantation Network)/UNOS (United Network for Organ Sharing). OPTN Policies, Policy 14: Living Donation. http://optn.transplant.hrsa.gov/governance/policies/ Accessed: July 7, 2019.
- 5.Field AE, Coakley EH, Must A, et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Archives of internal medicine. 2001;161(13):1581–1586. [DOI] [PubMed] [Google Scholar]
- 6.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Annals of internal medicine. 2006;144(1):21–28. [DOI] [PubMed] [Google Scholar]
- 7.Grams ME, Sang Y, Levey AS, et al. Kidney-failure risk projection for the living kidney-donor candidate. New Engl J Med. 2016;374(5):411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Locke JE, Reed RD, Massie A, et al. Obesity increases the risk of end-stage renal disease among living kidney donors. Kidney Int. 2017;91(3):699–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serrano OK, Sengupta B, Bangdiwala A, et al. Implications of excess weight on kidney donation: Long-term consequences of donor nephrectomy in obese donors. Surgery. 2018;164(5):1071–1076. [DOI] [PubMed] [Google Scholar]
- 10.Delmonico F A Report of the Amsterdam Forum On the Care of the Live Kidney Donor: Data and Medical Guidelines. Transplantation. 2005;79(6 Suppl):S53–66. [PubMed] [Google Scholar]
- 11.The British Transplant Society and The Renal Association. The United Kingdom Guidelines for Living Donor Kidney Transplantation. Fourth Edition, 2018.. [Google Scholar]
- 12.Abramowicz D, Cochat P, Claas FH, et al. European Renal Best Practice Guideline on kidney donor and recipient evaluation and perioperative care. Nephrol Dial Transplant. 2015;30(11):1790–1797. [DOI] [PubMed] [Google Scholar]
- 13.Spanish Society of Nephrology (S.E.N.) and Spanish Transplant Organisation (ONT). Recommendations for living-donor kidney transplantation. Nefrologia. 2010;30(Suppl 2):1–105. [Google Scholar]
- 14.AST Live Donor Community of Practice. Live Kidney Donor Medical Toolkit for Medical Providers. The Obese Kidney Donor. Available at: https://www.myast.org/patient-information/live-donor-toolkit. Accessed: July 7, 2019.
- 15.Pascual J, Abramowicz D, Cochat P, et al. European renal best practice guideline on the management and evaluation of the kidney donor and recipient. Nefrologia. 2014;34(3):293–301. [DOI] [PubMed] [Google Scholar]
- 16.Isbel N The CARI guidelines. Donors at risk: obesity. Nephrology (Carlton). 2010;15 Suppl 1:S121–132. [DOI] [PubMed] [Google Scholar]
- 17.Ethics Committee of the Transplantation Society. The consensus statement of the Amsterdam Forum on the Care of the Live Kidney Donor. Transplantation. 2004;78(4):491–492. [DOI] [PubMed] [Google Scholar]
- 18.Naik AS, Cibrik DM, Sakhuja A, et al. Temporal trends, center-level variation, and the impact of prevalent state obesity rates on acceptance of obese living kidney donors. Am J Transplant. 2018;18(3):642–649. [DOI] [PubMed] [Google Scholar]
- 19.Vest LS, Koraishy FM, Zhang Z, et al. Metformin use in the first year after kidney transplant, correlates, and associated outcomes in diabetic transplant recipients: A retrospective analysis of integrated registry and pharmacy claims data. Clinical transplantation. 2018;32(8):e13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lentine KL, Schnitzler MA, Garg AX, et al. Understanding antihypertensive medication use after living kidney donation through linked national registry and pharmacy claims data. American journal of nephrology. 2014;40(2):174–183. [DOI] [PubMed] [Google Scholar]
- 21.Lentine KL, Lam NN, Schnitzler MA, et al. Gender differences in use of prescription narcotic medications among living kidney donors. Clinical transplantation. 2015;29(10):927–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lentine KL, Lam NN, Schnitzler MA, et al. Predonation Prescription Opioid Use: A Novel Risk Factor for Readmission After Living Kidney Donation. Am J Transplant. 2017;17(3):744–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lam NN, Garg AX, Segev DL, et al. Gout after living kidney donation: correlations with demographic traits and renal complications. American journal of nephrology. 2015;41(3):231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lam NN, McArthur E, Kim SJ, et al. Gout after living kidney donation: a matched cohort study. Am J Kidney Dis. 2015;65(6):925–932. [DOI] [PubMed] [Google Scholar]
- 25.Lentine KL, Segev DL. Better understanding live donor risk through big data. Clin J Am Soc Nephrol. 2013;8(10):1645–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lentine KL, Yuan H, Tuttle-Newhall JE, et al. Quantifying prognostic impact of prescription opioid use before kidney transplantation through linked registry and pharmaceutical claims data. Transplantation. 2015;99(1):187–196. [DOI] [PubMed] [Google Scholar]
- 27.Abuelmagd MM, Nagib AM, Abuelmagd MM, et al. Study of the risk factors and complications of diabetes mellitus after live kidney donation. Transplant Proc. 2015;47(4):1152–1157. [DOI] [PubMed] [Google Scholar]
- 28.Center For Disease Control. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. Available at: https://www.cdc.gov/nchs/data/databriefs/db288.pdf. Accessed: July 7, 2019 2017;2018(December 18).
- 29.Lentine KL, Schnitzler MA, Xiao H, et al. Racial variation in medical outcomes among living kidney donors. The New England journal of medicine. 2010;363(8):724–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibrahim HN, Kukla A, Cordner G, Bailey R, Gillingham K, Matas AJ. Diabetes after kidney donation. Am J Transplant. 2010;10(2):331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. Diabetes Report Card 2017. Available at: https://www.cdc.gov/diabetes/pdfs/library/diabetesreportcard2017-508.pdf. Accessed: July 7, 2019. (December 19).
- 32.Fazeli Farsani S, Souverein PC, Overbeek JA, et al. Long term trends in oral antidiabetic drug use among children and adolescents in the Netherlands. British journal of clinical pharmacology. 2015;80(2):294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taler SJ, Messersmith E, Leichtman A, et al. Demographic, metabolic, and blood pressure characteristics of living kidney donors spanning five decades. American Journal of Transplantation. 2013;13(2):390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes care. 2012;35 Suppl 1:S64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes care. 2015;38(1):140–149. [DOI] [PubMed] [Google Scholar]
- 36.Berkowitz SA, Krumme AA, Avorn J, et al. Initial choice of oral glucose-lowering medication for diabetes mellitus: a patient-centered comparative effectiveness study. JAMA Intern Med. 2014;174(12):1955–1962. [DOI] [PubMed] [Google Scholar]
- 37.Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes care. 2012;35(4):731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Padwal RS. Obesity, diabetes, and the metabolic syndrome: the global scourge. Can J Cardiol. 2014;30(5):467–472. [DOI] [PubMed] [Google Scholar]
- 39.Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Annals of internal medicine. 1995;122(7):481–486. [DOI] [PubMed] [Google Scholar]
- 40.Issa N, Askandarani S, Gillingham K, et al. Fate of Living Kidney Donors Who Lost Weight to Become Donors. (Abstract # A331). Transplantation. 2014;98 (Supplement 1)(Supplement 1):494. [Google Scholar]
- 41.Issa N, Sanchez OA, Kukla A, et al. Weight gain after kidney donation: Association with increased risks of type 2 diabetes and hypertension. Clinical transplantation. 2018;32(9):e13360. [DOI] [PubMed] [Google Scholar]
- 42.Lentine KL, Segev DL. Understanding and Communicating Medical Risks for Living Kidney Donors: A Matter of Perspective. J Am Soc Nephrol. 2017;28(1):12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


