Abstract
We have implemented a linear ion trap (LIT)-based SIM-stitching method for ultra-high-resolution Fourier transform mass spectrometry (FTMS) that increases the S/N over a wide m/z range compared to non-segmented wide full-scan (WFS) spectra. Here we described an improved segmented spectral scan stitching method that was based on quadrupole mass filter (QMF)-SIM, overcame previous limitations of ion signal loss in LIT. This allowed for accurate representation of isotopologue distributions, both at natural abundance and in stable isotope-resolved metabolomics (SIRM)-based experiments. We also introduced a new spectral binning method that provided more precise and resolution-independent bins for irreversibly noise-suppressed FTMS spectra. We demonstrated a substantial improvement in S/N and sensitivity (typically > 10-fold) for 13C labeled lipid extracts of human macrophages grown as three-dimensional (3D) cell culture, with detection of an increased number of 13C isotopologue ions. The method also enabled analysis of extracts from very limited biological samples.
Keywords: Spectral Stitching, Spectral Binning, Stable Isotope-Resolved Metabolomics (SIRM), Ultra-High-Resolution Fourier Transform Mass Spectrometry
Graphical Abstract

1. Introduction
Direct infusion nanoelectrospray ultra-high-resolution Fourier transform mass spectrometry (DI nESI UHR-FTMS) instruments enable analyses of crude metabolite extracts without prior separation while achieving the high mass accuracy and resolution needed for determining of molecular formula in complex mixtures. However, high-resolution ion trap instruments such as ion-cyclotron resonance (ICR) and Orbitrap™ MS can experience loss of spectral quality due to space charge effects. These effects decrease the dynamic range, which compromises wide scan spectral quality. This is particularly problematic for biochemical pathway-tracing approaches such as stable isotope-resolved metabolomics (SIRM) [1–3], which can result in 40 or more metabolite isotopologues within a range of 40 m/z. Some of these isotopologue ions are not detectable due to low S/N, and can heavily overlap with other constituents, thus requiring high S/N, accuracy, resolution wide scan MS1 acquisitions for proper analysis of isotopologue distribution (Figure S1) and metabolic interpretation [2, 4].
To address these issues, Southam et al. [5] developed the SIM-stitching method using ultrahigh resolution ICR-FTMS with a front end linear ion trap or LIT (Thermo LTQ-FT). Their approach took advantage of a well-recognized property of MS that higher S/N can be achieved using a narrower m/z range for a fixed number of ions. Their method consists of multiple narrow SIM segments, each with a set, optimum automatic gain control (AGC) target value, which controls the number of ions injected into detectors. These SIM segments are then stitched together post-acquisition to produce a wide range spectrum, which is necessary for multivariate statistical analysis of metabolomic data and useful for visual inspection [5]. Generating single, contiguous spectra is equally important when searching for interspersed isotopologue peaks from different metabolites (Figure S1c) [6]. The spacings for these isotopologue ions can span 40 m/z or more, with isotopologue clusters dispersed across the entire spectral range (Figure S1a). We have implemented Southam’s method for our DI nESI UHR-FTMS analyses of SIRM lipid data, where the spectra had very high peak density and dynamic range (Figure S1). Implementation of this method on our QMF-SIM-based instrument helps eliminate the edge effect [5, 7]. This is because ion isolation utilizing QMF-SIM does not suffer from the space charge effect seen in the LIT, which contributes to the edge effect. In addition to the edge effect, we encountered another limitation of the method, i.e. applicability of a conventional noise filtering method only for full-profile spectra (true raw spectra with intact baseline noise), which are unavailable in some commercial form of Orbitrap instruments [8].
Many QMF-FTMS instruments automatically truncate the baseline noise and provide only the reduced-profile spectra, which lack the critical information for the conventional noise reduction process [5, 8]. For the reduced-profile spectra, a recently reported intensity-independent noise filtering method [9] can be substituted for the conventional method since it does not require baseline noise information from full-profile spectra. While this method provides a reasonable noise threshold with minimal introduction and removal of false signals, the intensities are unfortunately distorted due to the introduction of noise signals when averaging spectra followed by a resolution-dependent spectral binning [9]. We employed a new spectral binning method that accounts for and leverages the way computers store decimal numbers to overcome the intensity distortion introduced by the resolution-dependent method.
Here we describe an improved segmented-scan spectral stitching method for QMF-FTMS instruments that provide only reduced-profile spectra and demonstrate it on an Orbitrap Fusion™ Tribrid™ (Thermo Scientific). In addition, the method can be optimized based on specific sample ion distribution profiles. We demonstrated this sample-specific silhouette segmented-scan spectral stitching (S7) on the extremely dense, highly demanding lipid analysis for SIRM studies, including human macrophages grown as 3D cultures.
2. Materials and Methods
2.1. Materials
2.1.1. Isolation of human monocytes
Human whole blood (WB) was obtained from healthy volunteers as part of a lung cancer metabolism study according to a University of Kentucky IRB approved protocol (IRB# 43807). Monocytes were isolated from WB using the RosetteSep™ human monocyte enrichment cocktail kit (StemCell Technologies Inc., Cambridge). Each mL of WB was mixed and incubated for 20 min with 2 μL of 0.5 mM EDTA, and 50 μL of the RosetteSep™ monocyte negative selection cocktail. The WB was diluted 1:1 with phosphate-buffered saline (PBS) (Ca2+ and Mg2+ free) + 1× antibiotic-antimycotic (Thermo fisher) + 2 % endotoxin-free fetal bovine serum (FBS) and then added to a Ficoll-Hypaque (GE) gradient in SepMate™-50 tubes (StemCell Technologies Inc.), followed by centrifugation at 1,200 ×g for 20 min. The top layers were pooled and then spun for 10 min at 400 ×g. The resulting cell pellets were resuspended in the same 2 % FBS-PBS dilution buffer and washed twice via centrifugation at 600 ×g and 120 ×g for 10 minutes each. Finally, the cells were counted and seeded into 6-well plates at a density of 3 – 5 × 106 cells per well in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 0.2 % glucose, 2 mM glutamine, 10 % FBS, 1× antibiotic-antimycotic, and 50 ng/mL of monocyte colony stimulating factor (M-CSF) (M0 medium).
2.1.2. Macrophage spheroid formation and 13C6-Glc SIRM experiment
Isolated human monocytes were differentiated into macrophages in 6-well plates for 5 days in M-CSF supplemented medium at 37 °C-5% CO2 before treatment with NanoShuttles™-PL (NS; n3D Biosciences) at 1 μL per 104 cells overnight according to the vendor’s protocol. After 16 h, NS-loaded cells were then detached using accutase, counted, and bioprinted for 1 h in cell-repellent flat-bottom 96-well plates (Greiner Bio-One) at a density of 200,000 cells per well using the magnetic spheroids drive (n3D, Biosciences) for spheroid formation [10]. Cells were either not activated (M0) or classically activated to the M1 subtype by treating the cells with 100 ng/mL lipopolysaccharide (LPS) plus 20 ng/mL interferon gamma (IFNγ) for 3 days [11, 12]. For the SIRM experiment, cells were incubated for another 24 h in fresh M0 medium with glucose replaced by 0.2 % 13C6-glucose.
2.1.3. Extraction of metabolites from macrophage spheroids
Macrophage spheroids were held with the 96-well magnetic holder and washed twice with cold PBS and once briefly (30 sec) with Nanopure water to remove salts. The spheroids were quenched in 200 μL of cold 70 % methanol per well, which also served to extract polar metabolites [10]. The remaining pellets were extracted for lipids by adding 600 μL of methyl tert- butyl ether (MTBE) and 180 μL of 100 % methanol to each pellet. The mixtures were vortexed at 3,000 rpm for 1 h at room temperature. After adding 210 μL of Nanopure water, the mixtures were vortexed for 30 sec and centrifuged at 14,800 ×g for 10 min at 4 °C. The top lipid layer was collected, and the residue was extracted again as described above. The two pooled lipid extracts were lyophilized and dissolved in 200 μL of chloroform : methanol : butylated hydroxytoluene (2:1:1 mM) solvent mixture before FTMS analyses.
2.2. Methods
2.2.1. AGC target optimization
An nESI spray solvent mixture of isopropanol : methanol : chloroform : ammonium formate (4 : 2 : 1 v/v : 20 mM) containing 2,800-fold diluted deuterated-lipid (D-lipid) standard mixture (SPLASH™ LipidoMix®, Avanti Polar Lipids, Alabaster, AL) was prepared. The mixture was injected into the FTMS (THERMO Scientific™ Orbitrap Fusion™ Tribrid™) via nESI (Advion TriVersa NanoMate®) in positive ion mode. The applied voltage was 1.5 kV with 0.4 psi head pressure and was controlled by ChipSoft 8.3.3 software (Advion). AGC targets of 1 × 103, 2 × 103, 5 × 103, 1 × 104, 2 × 104, 5 × 104, 1 × 105, 2 × 105, 5 × 105, and 1 × 106 were selected for three different 100 m/z segments of 480–580, 625–725, and 740–840, which is where the D-lipid standards for calibration ([18:1(d7) lysophosphatidylcholine (LPC) + H]+: m/z 529.39936, [18:1(d7) cholesteryl ester (CE) + NH4]+ : m/z 675.67794, and [15:0–18:1(d7)−15:0 triacylglycerol (TG) + NH4]+: m/z 829.79845) are centered. Ion injection times were 25, 50, 100, and 500 ms for AGC targets 1 × 103–5 × 103, 1 × 104–5 × 104, 1 × 105–2 × 105, and 5 × 105–1 × 106, respectively. All the spectra were acquired for 2 minutes with five microscans per scan at a measured R400 m/z ≈ 370,000. The scans were averaged using Xcalibur 3.0.63 (THERMO Scientific™). Each segment was calibrated against the three D-lipid standards. The mass errors were obtained using the other D-lipid standards within each segment in combination with known sample contamination, such as polydimethylsiloxane (PDMS), polyethylene glycol (PEG), etc. [13, 14] (Table S1) using the in-house software Precalculated Exact Mass Isotopologue Search Engine (PREMISE) [4, 15].
2.2.2. Evaluating edge effects
The Thermo Fusion mass spectrometers include QMF, LIT, and Orbitrap; this enabled selection of QMF or LIT to directly compare ion isolation for SIM-scans. The nESI spray solvent mixture described above (2.1.1) was injected into the Fusion via the nESI in positive ion mode. Maximum ion injection time was 100 ms with an AGC target of 1 × 105. All the spectra were acquired for 12 seconds with 5 microscans per scan, then the scans were averaged using Xcalibur. Intensity ratios of monoisotopic to m + 2 isotopologue ions of the D-lipids (18:1(d7) LPC (529.39936 / 531.40607), 18:1(d7) CE (675.67794 / 677.68465), and 18:1(d7)−15:0 TG (829.79845 / 831.80516)) at different locations in 100 m/z segments were measured in triplicate in a similar way to Weber et al. [7].
2.2.3. Mass spectrometry of human macrophage lipids from SIRM experiments
The non-polar phase extracted from the human macrophages was diluted 1 : 5 with the spray solvent mixture. The samples were injected into the FTMS via nESI in positive ion mode. The applied voltage was 1.5 kV with 0.4 psi head pressure and was controlled by the ChipSoft. Xcalibur was used to set up the S7 method. MS1 QMF-SIM was used to filter the segments to the desired m/z ranges. For the S7 method, each stored scan was composed of 1 microscan with a total acquisition time of 15 minutes including dummy scans. For WFS spectra, 5 microscans were accumulated per stored scan for a total acquisition time of 10.5 minutes including dummy scans. The AGC target was 1 × 105 with 1000 ms of maximum ion injection time for both S7 and WFS. Fluoranthene (m/z 202.07771) was injected into the detector by the instrument to serve as a lock mass (EASY-IC™) for WFS spectra only.
2.2.4. Processing FTMS spectra
Xcalibur 3.0.63 was used for visual inspection of acquired spectral raw files and to extract an exact m/z list from the WFS spectra. MSFileReader 3.1 (Thermo Scientific™) was used to export a series of m/z lists from individual scans and the maximum ion injection time from both WFS and S7 spectra to Microsoft Excel spreadsheets. MSFileReader is a library with a component object model (COM) standard that supports read access to Thermo raw files and can be used in any programming language that supports the COM interface. Two-step internal calibration has been conducted for WFS spectra. The spectra were first automatically calibrated using fluoranthene by the instrument during the acquisition, and the acquired spectra were subsequently calibrated using a D-lipid standard [15:0–18:1(d7) PC + H]+ (m/z 753.61337) either by PREMISE or the Python script described below. Unique internal calibrants (Table 1) were used to calibrate each segment for S7 spectra. The instrument has been calibrated on a two-week basis using a calibration solution (Pierce™ LTQ ESI Positive/Negative Ion Calibration Solution). A Python script was used for centroided m/z list (what Thermo calls “label data”) extraction from .raw files, poor scan elimination and inter-scan normalization [5] using maximum injection time, alignment of m/z in an ascending order across scans within a segment, 32-bit floating point format-based spectral binning, m/z median and intensity average, m/z correction using internal calibrants, intensity-independent noise filtering [9], inter-segment normalization using intensity ratios of peaks mutually present in the overlapping regions, and stitching for S7 and WFS spectra from the macrophage samples (the last two steps are only for S7). The Python script is available at https://zenodo.org/badge/latestdoi/150272490. PREMISE was used for both WFS and S7 to assign observed m/z values to an in-house lipid database [4, 15] and common contaminants lists (Table S1) [14] for the mass error measurement.
Table 1. Parameters of the optimized S7 for lipid extracts of M0 and M1 macrophage spheroids cultured in 13C6-glucose.
| Number of microscan | Minimun number of scan | Duration time (min) | Low m/z | High m/z | Calibrant | ||||
|---|---|---|---|---|---|---|---|---|---|
| mass | Molecular ID |
Molecular Formula |
Adduct | ||||||
| 1 | 4 | 0.09 | 150 | 350 | 223.06365 | PDMS* | C6H18O3Si3 | [+H]+ | |
| 1 | 4 | 0.09 | 330 | 530 | 445.12002 | PDMS | C12H36O6Si6 | [+H]+ | |
| 1 | 2 | 0.06 | 510 | 610 | 529.39936 | 18:1(d7) LPC | C26H52N1O7P1 | [+H]+ | |
| 1 | 2 | 0.06 | 600 | 700 | 675.67794 | 18:1(d7) CE 15:0- | C45H78O2 | [+NH4]+ | |
| 1 | 2 | 0.06 | 690 | 790 | 753.61337 | 18:1(d7) PC 15:0- | C41H80N1O8P1 | [+H]+ | |
| 1 | 1 | 0.03 | 780 | 830 | 829.79845 | 18:1(d7)-15:0 TG | C51H96O6 | [+NH4]+ | |
| 1 | 1 | 0.03 | 820 | 870 | 832.24053 | PDMS | C22H66O11Si11 | [+NH4]+ | |
| 1 | 1 | 0.03 | 860 | 910 | 906.25932 | PDMS | C24H72O12Si12 | [+NH4]+ | |
| 1 | 2 | 0.06 | 900 | 1000 | 980.27811 | PDMS | C26H78O13Si13 | [+NH4]+ | |
| 1 | 4 | 0.09 | 980 | 1180 | 1054.29690 | PDMS | C28H84O14Si14 | [+NH4]+ | |
| 1 | 4 | 0.09 | 1160 | 1360 | 1276.35327 | PDMS | C34H102O17Si17 | [+NH4]+ | |
| 1 | 5 | 0.12 | 1340 | 1600 | 1424.39085 | PDMS | C38H114O19Si19 | [+NH4]+ | |
PDMS: polydimethylsiloxane contaminant
2.2.5. Optimizing settings of sample-specific silhouette segmented-scans
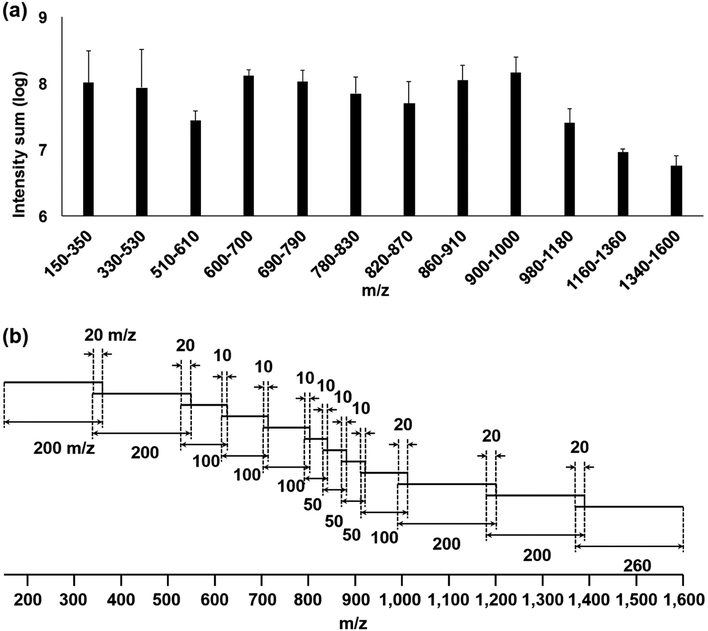
We found five major factors to be considered when designing the S7 method. The first step was determining the best sizes of the segments. While Southam et al. [16] recommended using an optimized and fixed SIM segment width for the spectral stitching method, Taylor et al. [17] segmented a wide mass range into multiple different widths of SIM segments based on the sample-specific m/z density distribution. Specifically, they used SIM widths of 100 m/z for 600– 900 m/z regions with denser m/z population, and any segments outside the range were widened to avoid under fill of LIT and hence the ICR detector. Their data were similar to our experiences with lipid extracts from various types of cancer, including those from patients, where we observed a denser m/z population between 600 and 1,000 m/z [2, 3, 15, 18, 19]. The intensity log sum from the WFS spectra of macrophage lipid extracts was used to design segments with evenly distributed total ion population density (Figure 1a), which was used throughout this study. We used 12 segments with different widths in the mass range of 150–1,600 m/z (Figure 1a, b). In order to avoid any computational backlog and errors due to large numbers of scans (more than 150 scans per segment) being processed when running the Python script, the m/z range of 1,160–1,600 was separated into two segments despite its lower intensity log sum. The shorter segments were set to acquire proportionally lower numbers of scans in order to expedite coverage of the wide mass range [5] (duration time, Table 1). When setting the width of segments for SIRM experiments, the size of the overlapping regions should be considered to ensure the transfer of accurate isotopologue silhouette (Figure S1b, c). This also has the advantage of enabling more robust normalization between segments by providing many mutually present peaks within the region. The overlapping region can be set to less than or close to 10 m/z in width when natural abundance isotopologue profiling is expected, since the peak spacings of observable isotopologues in general do not span more than 5 m/z. Second, each segment must have internal standards for m/z calibration. Any standard mixture that is composed of repeating units of oligomers, such as the PEG standard [5], is potentially useful. It has been reported that peaks of common chemical background such as plasticizers have served as lock masses [20], thus any known contaminants [14] for a given sample workflow can be used as internal calibrants. We selected eight PDMS ions as internal calibrants in combination with ions from four D-lipid standards as unique external calibrants for the 12 segments as shown in Table 1.
Figure 1. Schematic of S7 optimized for the ion density silhouette of mammalian lipids.
(a) Average log intensity sum of 12 m/z ranges from WFS (113 scans averaged) spectra of macrophage M0 subtype lipid extracts (triplicate). Error bar is RSD. (b) Distribution of 12 segments of a single S7 cycle with different m/z widths and different number of scans overlap by 10 or 20 m/z. The mass range is m/z 150–1,600 with an AGC target of 1 × 105. A total of 18 S7 cycles comprised a single analytical run.
Unstable spray may occur intermittently in DI nESI, across multiple scans. Although this unstable spray results in a loss of MS signal, we find the DI nESI is typically stable for about 20 minutes with 200–600 nL min−1 of flow rate with up to 15 μL of injection volume, as evidenced by consistent spray current at around 100 nA for the types of samples in this study. Any major spray instability can introduce errors in normalization of intensities based on injection time [5] when more than 1 microscan per scan is used. For the Thermo MS instruments, including the Fusion, a “microscan” comprises a single cycle of ion injection and storage or scan-out of ions followed by detection, whereas a “scan” is the disc-stored spectrum from the average of specified microscans. Since the instrument sums the microscans at the transient level followed by Fourier transformation, not only is it impossible to know which one of the multiple microscans lost their signal during the nESI instability but also the ion injection times for the individual microscans are lost. Therefore, we set the number of microscans to 1 for every stored scan, which allows rejection of individual poor scans to more accurately normalize inter-scan intensities. The maximum ion injection time was set to 1,000 ms to accommodate wide-ranging differences in segments among samples. We observed that the only instances when injection time reached 1,000 ms occurred when the signal was lost due to the nESI instability. The Python script described here assumes scans with an injection time equaling the maximum injection time are poor scans that experienced an unstable nESI profile and ignores them during analysis at an early processing stage.
To adjust the number of scans of WFS to be proportional to the size of its m/z range (1450 m/z width), approximately 870 or more scans are required. However, with the current Python script, more than 150 scans in combination with wide segments (> 200 m/z width) can cause computational backlog and errors due to the extremely large number of peaks detected. Thus, acquiring a reduced number of scans by increasing the number of microscans (5 in this study) per scan was necessary. This is a compromise between number of scans and quality control of microscans based on ESI spray stability.
We iteratively rotated the acquisition of the segments, to minimize the effects of any DI nESI instability or sample composition stratification. We found that a total of 18 cycles of 12-segments (ca. 49 seconds per cycle), for a total of 15 minutes of acquisition time with the first 30 seconds allocated to the dummy scans (Table 1, Figure 1b) provided an acceptable compromise between speed and reliability. The dummy scans minimize the effects of unstable nESI in the first several seconds of spraying [7].
It is critical to use resolving power that is sufficient to resolve natural abundance isotopic profiles for compound identification [21, 22], i.e. R400 m/z ≥ 200,000 for SIRM experiments [4, 23]. Although it has been reported that low mass accuracy combined with relative isotope abundance information produced better assignments than high mass accuracy alone [7, 24], this is true only if natural abundance isotopic distribution can be assumed; this is clearly not the case for SIRM (e.g. Figure S1). Thus, the full capacity of the instrument has been utilized throughout; R200 m/z = 500,000, corresponding to a measured R400 m/z ≈ 370,000 that takes approximately 1 second per microscan.
Considering all the above factors, the parameters of the method can be tailored according to the sample silhouette of ion density distributions, or to preselected analytical targets as discussed in the Methods (2.2.5) and in Figure 1.
3. Results and Discussion
3.1. Optimization of AGC target for mass accuracy in Orbitrap Fusion
For acquiring extremely high density, wide-scan MS1 data, such as those obtained from the 13C lipid profiles of SIRM experiments, a high S/N is required to distinguish low abundance isotopologue peaks from noise while minimizing the mass error. In such SIRM experiments, it is crucial to keep the mass error to around 0.1 ppm to reduce the number of possible candidate molecular formulae. For example, at 0.1 ppm mass accuracy, the number of candidates is typically 1 when calculated for an m/z of 150 with the search pool limited to the elements CHONPS, whereas it increases to 10 and 32 candidates for the m/z values of 700 and 900, respectively. The candidate list increases approximately 10-fold at 1 ppm of mass accuracy [24]. In practice, a small improvement in mass accuracy can be achieved by setting lower AGC target values, at the cost of reduced sensitivity.
In our case, the mass error started to increase with increasing AGC targets from 2 × 105 to 1 × 106 while the error remained consistent with AGC targets of 1 × 103 through 1 × 105 (Figure S2). Thus, we chose 1 × 105 as the AGC target value throughout this study to maximize the S/N while maintaining an acceptably low mass error (ca. 0.2 ppm). In practice, the AGC target can be tailored for the particular silhouette of ion density in each segment, to optimize the S/N for a given application.
3.2. Elimination of edge effects by using wide quadrupole mass filter (QMF) ion isolation width
Southam et al. reported the loss of ions at the ends of SIM segments using instruments with either LIT or QMF [5, 7, 16]. The mechanism behind the “edge effect” in LIT [25, 26] and QMF [27–29] has been well described.
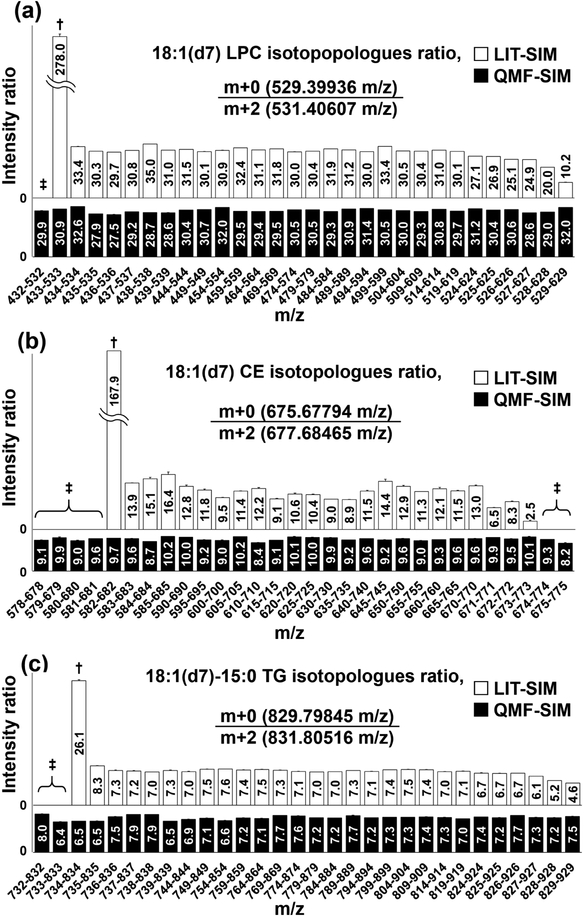
As previously reported [5, 7], LIT-SIM suffered from considerable ion loss at both ends of the segments, while QMF-SIM did not (Figure 2). As seen in Figure 2, the intensity ratios of monoisotopic (m+0) to m+2 ions remained consistent even though the accurate masses of m+2 ions of CE (m/z 677.68465) and TG (m/z 831.80516) and the m+0 ion of LPC (m/z 529.39936) fell in the +/− 0.5 m/z ends of the QMF-SIM segments. This is presumably due to a more rectangular ion isolation efficiency profile of the extended QMF-SIM segment compared with the narrower ones (Figure S3) [27, 28]. With our Orbitrap Fusion instruments, setting a segment window width of at least 50 m/z minimized the ion loss when using QMF-SIM (data not shown).
Figure 2. Differences in edge effect between LIT and QMS based acquisitions.
Values in the boxes are ion intensity ratios of m+0 : m+2 of D-lipids (a) 18:1(d7) LPC, (b) 18:1(d7) CE, and (c) 18:1(d7)−15:0 TG acquired by specifying 30 different 100 m/z segments. †: in these cases, the ratio was very large because the intensity of m+0 (numerator) remains consistent while that of m+2 (denominator) dropped due to the edge effect of the LIT. ‡: in these cases, the ratio was effectively zero or could not be defined because the intensity of either m+0 (numerator) or m+2 (denominator) was below detection limits.
3.3. Improved spectral binning method for intensity-independent noise filtering
Southam et al. [5] acquired the spectra as transient files and used MATLAB-based processing to obtain “full-profile” spectra with intact baseline and noise information. As indicated earlier, the noise from the “full-profile” spectra can be suppressed by conventional approaches [5, 8] but not the noise-truncated spectra such as those acquired from Thermo Orbitrap Fusion. The noise in these “reduced-profile” spectra can be removed using the recently reported intensity-independent method [9]. This method utilizes repetition rate filtering (RRF) which takes advantage of how often each peak appears within a small m/z interval (bin) across multiple scans. Although commonly used visual inspection-based noise thresholding can be applied to both types of spectra, it is impractical for S7 since each m/z segment has different noise levels. Considering these difficulties, we employed the RRF concept to determine the noise level of S7 spectra.
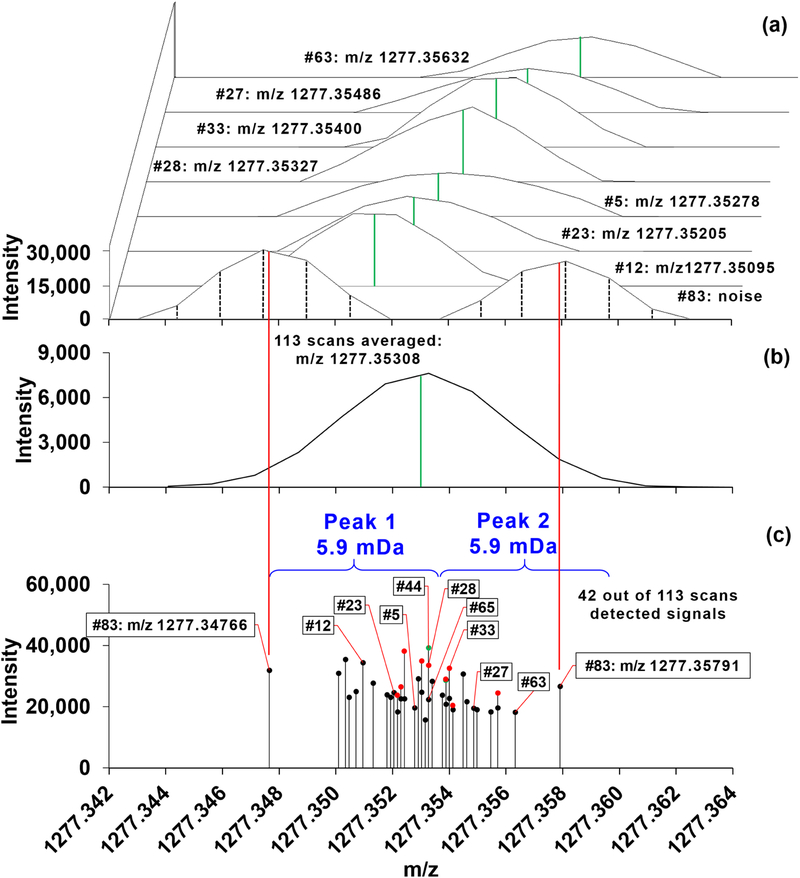
Spectral binning, necessitated by the RRF approach, requires the unique centroided m/z’s across scans to be put into a list in an ascending m/z order. We found that using the trend line of full width at half maximum (FWHM) as a criterion for the single-step bin width determination across the entire m/z range under ultra-high-resolution (R400 m/z ≈ 370,000) acquisition produced imprecise bin boundaries (Figure 3). Also, in SIRM experiments, no assumptions can be made about the number of detectable isotoplogues or their intensity ratios, unlike in unlabled experiments where 13C>5 ions would not be detectable for even very abundant metabolites of up to 50 carbons. Moreover, the detection of the 13C0 isotopologue ions of metabolites is not assured (Figure S1c, [6]). Therefore the binning method needed to be as accurate as possible under ultra-high-resolution acquisition to avoid both false positives and negatives of stable istotope incorporation [4].
Figure 3. FWHM-based bin width threshold and averaging peaks under WFS acquisition.
Solvent mixture from 2.2.1 was injected and a single raw file was acquired via WFS with 5 microscans per scan spanning 10 minutes of acquisition time, up to 113 scans. (a) Skewed-overlay spectra from 8 selected scans out of the total 42 scans that detected signals in the m/z range of m+1 of PDMS [C34H102O17 Si17 + NH4]+ across 113 scans. The scan numbers are annotated as #63, #27, #33, #28, #5, #23, #12, and #83 from top to bottom. The acquired raw files could be presented by either stick plots of profile data (black vertical dashed lines of 83rd scan) or spectra that were generated by drawing the lines from point to point of the profile data. Centroided m/z values along with the profile data were available using the default centroiding algorithm (Thermo calls “label data”). These centroided m/z values were used in this study. The centroided m/z values are put next to the scan numbers and noted in the spectra as solid green lines. (b) The 113 scans were averaged via Xcalibur with the solid green line marking the centroided m/z value. Note that Xcalibur reports a single peak with the noise signals from the 83rd scan merged into its base. (c) Distribution of the 43 peaks as the centroided m/z values from the 42 scans. Several scans had the exact same m/z values, which are indicated with colored dots (green and red); for instance, scans 28, 44, and 65 have m/z 1,277.35327. When the m/z range was binned based on FWHM, a putative single peak evident in (b) was separated into two (peaks 1 and 2) in (c) while merging the noise peaks into the analyte signals. The ~6 mDa bin width was calculated from the equation of a trend line of FWHM across m/z range of 150–1,600. The solid red lines from (a) to (c) show where the centroided m/z values of the noise peaks from the 83rd scan are located in relation to the other peaks in the region.
In an effort to resolve the problem with FWHM-based binning in Figure 3, the difference between sequential m/z’s in the m/z list was obtained. These differences were found to be either equal to the minimum neighboring difference (MiND) or its integral multiples. The MiNDs were also observed to increase by a factor of 2 as the m/z range increased from 128–256 through 256–512 and 512–1,024 to 1,024–2,048 (Table S2), which our program took into account. A detailed example of how to determine the MiND is discussed in Table S2. The origin of this phenomenon is that digital computers store and do arithmetic on decimal numbers in binary floating-point format.
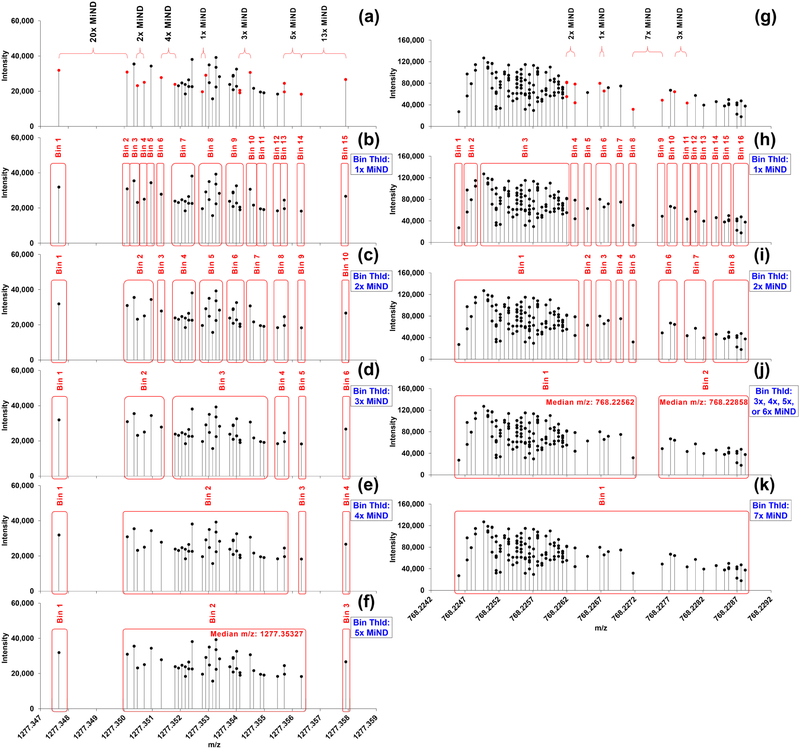
To determine the boundaries of bins, we inspected the maximum difference possible between adjacent m/z’s for given peaks of known contaminants (Figure 4), lipid standards (Figure S4), and prominent PC peaks from an unpolarized human macrophage (M0) extract identified based on the accurate mass (Figure S5, Table S3). Most of the known compound peaks were 1×, 2x, and/or 3× – 5×MiNDs apart from each other (Figures 4, S4). Also, most of the peaks identified as lipid isotopologue ions in the M0 extract showed narrow MiND distribution (1× – 5×MiNDs) as in Figure S5. The minimum distances we observed in this study were 6x or 7×MiNDs for two distinct, neighboring authentic peaks as in Figure 4j. This led to decision on creating a new bin each time the difference exceeds 5×MiND as the best practice for these samples acquired with ultra-high-resolution (R400 m/z ≈ 370,000) and a moderate m/z range (150 – 1,600 m/z). Using this MiND-cognizant correction, we could accurately bin the PDMS peak (Figure 4f) whereas the FWHM-based approach and Xcalibur could not (Figure 3). For general practice, the bin boundaries should be user-defined and sample-, MS parameter-dependent.
Figure 4. BFP-based bin width threshold determination.
Distribution of the peaks with m/z values of: (a-f) m+1 of PDMS [C34H102O17Si17 + NH4]+ and (g-k) m+2 of PDMS [C50H120O21Si17 + 2H]2+ across 113 scans in WFS range (150–1,600 m/z). (a, g) Differences in m/z values between the red dots are indicated above the distribution plot in terms of nxMiND (n is positive integer). Bins generated via (b, h) 1×MiND, (c, i) 2×MiND, (d, e, f, j) 3×, 4×, 5×, and 6×MiNDs, and (k) 7×MiND binning.
Once the size of each bin was determined, the number of peaks that fall into each bin across all scans was counted for RRF. In order to determine a repetition rate threshold that was less strict than that set by Schuhmann et al. [9], yet remained practical, we examined the repetition rate of m + n (n ≥ 2) isotopologue peaks of known contaminants (e.g. polyethylene glycol) in different m/z segments. The minimum repetition rate was in general above 5 % for WFS and 10 % throughout the segments of S7 spectra (data not shown).
However, there were some peaks with 4×, 5×, 6× and 7×MiNDs on the boundaries of the bins in both standard mixtures and M0 extracts (Figures 4, S4, S5). The influence of those peaks on the downstream RRF could vary depending on how strict the RRF threshold was set. For instance, at 5 % RRF threshold with either 3×, 4×, 5×, or 6×MiND binning, two peaks of m+2 PDMS ions (Figure 4j) were reported, which corresponds to the ions [C50H120 O2128 Si1630 Si + 2H]2+ and [C4913CH120 O2128Si1628Si + 2H]2+. The lighter and the heavier ions exhibited 97 and 15 % repetition rate, respectively. If the RRF threshold were as strict as [9] (70 %), only the lighter ion would be reported. The total number of detected signals in the bin (131) exceeded that of scans (113) with 7×MiND binning (Figure 4k), which is unrealistic, and the Python script would drop this bin from the final peak list. Most of the peaks separated by 6× or 7×MiND were found on the boundaries of the bins (Figure S4), and exclusion of these peaks did not affect the integrity of the bins at the given RRF threshold.
We used a 10 % repetition rate as the noise threshold for the S7 method, which removed more than ~ 85 % of signals detected in each segment (Figure S6). These settings can be used as default values but may need optimization for different sample types that have very different distribution of ions.
3.4. Application of S7 to SIRM lipid profiling of 3D human macrophage spheroids
3D cell cultures are increasingly preferred biological models that better reflect the microenvironment of cells in tissues than their traditional 2D counterparts [10, 30]. 3D cell cultures typically show lower proliferation rates than their 2D counterparts [10, 30], leading to less de novo lipid biosynthesis or 13C incorporation in SIRM experiments. This issue is exacerbated with human macrophage spheroids as they do not proliferate and the amount of materials that can be obtained from donors are very limited. As a result, many 13C isotopologue ions have relatively low intensities and fall below detection limits using WFS. By greatly enhancing the S/N, the S7 method can detect many more 13C isotopologue ions in SIRM studies, leading to improved biochemical interpretability of 3D cell cultures and human macrophage spheroids in particular.
The lipid extracts from M0 and M1 type human macrophage spheroids were prepared as described in 2.1.3 for comparing the performance of the WFS and S7 methods. The MiND-binning followed by 5 % and 10 % RRF was applied to WFS and S7, respectively, as determined in 3.3. Over the full range of 150–1600 m/z, we obtained ~0.38 ppm RMS error using the WFS method with AGC = 1 × 105, which was improved to ~0.25 ppm RMS error using the S7 method (Table 2).
Table 2. Mass errors for S7 and WFS with AGC targets of 1 × 105.
Nic and Nme indicate the number of internal calibrants and m/z values used for mass error calculation, respectively. Av: average, RMSE: RMS error, MAE: max absolute error, and SDE: SD error.
| Scan type | Macroph age subtype | Replicate | Nic | Nme | RMS error (ppm) | Max absolute error (ppm) | SDof error (ppm) | Av RMSE (ppm) | Av MAE (ppm) | Av SDE (ppm) |
|---|---|---|---|---|---|---|---|---|---|---|
| S7 | M0 | 1 | 12 | 29 | 0.250 | 0.718 | 0.166 | 0.263 | 0.767 | 0.176 |
| 2 | 12 | 29 | 0.243 | 0.791 | 0.164 | |||||
| 3 | 12 | 29 | 0.292 | 0.791 | 0.197 | |||||
| M1 | 1 | 12 | 29 | 0.244 | 0.718 | 0.165 | 0.235 | 0.658 | 0.150 | |
| 2 | 12 | 29 | 0.230 | 0.608 | 0.137 | |||||
| 3 | 12 | 29 | 0.230 | 0.644 | 0.148 | |||||
| WFS | M0 | 1 | 2 | 31 | 0.433 | 1.088 | 0.453 | 0.391 | 1.011 | 0.411 |
| 2 | 2 | 31 | 0.361 | 0.985 | 0.392 | |||||
| 3 | 2 | 31 | 0.374 | 0.955 | 0.390 | |||||
| M1 | 1 | 2 | 31 | 0.343 | 0.822 | 0.363 | 0.369 | 0.972 | 0.404 | |
| 2 | 2 | 31 | 0.399 | 1.066 | 0.433 | |||||
| 3 | 2 | 31 | 0.364 | 1.011 | 0.414 |
S/N was overall improved (Figure S7) and the number of detected 13C isotopologue ions for the example [PC + H]+ species (Table S4, Figure 5a, b), increased considerably using the S7 method. We calculated the % 13C isotopic enrichment of two of the PC lipids, which are pulmonary surfactants, dipalmitoylphosphatidylcholine (DPPC) and palmitoyloleoylphosphatidylcholine (POPC) in Figure 5c, d. The improved detection of isotopologue ions was evident for the heavier isotopologue ions (e.g. >m+17 for DPPC in M0 data, >m+8–11 for POPC in M1 data) where they were undetected using WFS method but detected with good S/N using S7 method.
Figure 5. S7 increases S/N and detects many more 13C isotopologue peaks than WFS method in lipid data of 13C labeled human macrophage spheroids, especially at lower abundance.
The same lipid extract as in Figure S7 were analyzed. Peaks detected in only one of three spectra were removed from the final m/z list for the assigned glycerophospholipids. Numbers of detected and assigned (a) 13C0 ions and (b) 13C>0 ions of [PC + H]+. **P-value < 0.01 by paired Student’s t test. Error bars represent the standard error of the sample mean. 13C fractional enrichment distribution of (c) DPPC and (d) POPC after natural abundance correction [34]. Inset figures show relative intensities (before natural abundance correction [34]) of selected isotopologue ions of each PC species demonstrating a substantial S/N improvement. m+3, m+even number, and m+odd number represent respectively 13C incorporation into the glycerol backbone, fatty acyl chains, and glycerol+fatty acyl chains.
DPPC is a major pulmonary surfactant and has been shown to modulate inflammatory response of human monocytes [31–33], thus it is important to understand its metabolism in macrophages. Regardless of the acquisition methods used, the natural abundance (NA) corrected [34] % 13C distribution of DPPC and POPC showed a decreased % 13C enrichment in the fatty acyl chains in M1 versus M0 subtype with little or no changes in the extent of 13C incorporation into the glycerol backbone (Figure 5c, d). In fact, the entire PC class exhibited the same trend (data not shown), which is consistent with decreased fatty acid biosynthesis or PC degradation in LPS+IFNγ-activated M1 macrophages. This is in contrast to the notion that fatty acid biosynthesis is enhanced in M1 macrophages based on the overexpression of mitochondrial citrate carrier and ATP-citrate lyase, which together could promote cytoplasmic acetyl CoA production from citrate, which is required for fatty acid synthesis [35]. However, citrate-derived acetyl CoA is also required for histone acetylation [36]. Our direct measurement of lipid biosynthesis using 13C6-glucose as tracer suggests that the synthesis of at least the PC class of lipids was not enhanced by M1 activation of human macrophage. Instead, reduced synthesis of DPPC can lead to DPPC depletion, which can promote inflammatory response in macrophages [32].
4. Conclusions
Recognizing the edge effect in both the LIT-[25, 26] and the QMF-based acquisition [27–29] is of great importance to properly perform SIM-spectral stitching. This issue is even more problematic for proper data analysis and interpretation of SIRM data [1–3, 15, 18], where preserving complex isotopologue patterns in stitched spectra is critical. We found that using QMF for ion isolation along with optimizing the SIM widths helped minimize the edge effect while greatly improving the S/N of stitched SIRM lipid data. Moreover, our MiND-cognizant binning approach solved the problem of inaccurate FWHM-based binning method. However, the threshold of RRF needs to be user-defined and experimental-design dependent. For example, for biomarker discovery purposes, one may want to remove low natural abundance isotopologue ions with low repetition rate. In contrast, in SIRM studies, one would need to optimize the detection of low-intensity, isotopically-enriched peaks that are important for biochemical interpretation. It is also impractical to use large numbers of standards to determine the lipid-species-specific repetition rate threshold, particularly for SIRM studies where the vast majority of isotopically-enriched standards are unavailable [37]. Thus, further efforts for determining the optimum RRF threshold by inspecting the repetition rate of m + n (n ≥ 2) isotopologue peaks of known contaminants is under way, with the goal of incorporating different threshold values for various sample settings.
When applying the S7 method to the challenging SIRM study of non-proliferating, low sample size human macrophages, we demonstrated the ability to detect very low abundant (< 0.5% 13C enriched), heavily labeled isotopologue ions (e.g. 13C>17), which were undetected with the WFS method. Such higher sensitivity affords a much greater information throughput [6] without compromising the sample throughput, and has the potential to alter the biochemical interpretation. Lastly, the method is readily tailored to other types of samples that require wide range MS1 with high ion population density, different ion abundance silhouettes, and/or low S/N.
Supplementary Material
Highlights.
Sample-specific segments were designed for the improved spectral stitching method
Use of quadrupole mass filter-selected ion monitoring eliminates edge effect
Binary floating point-based binning creates precise and resolution-independent bins
Method was used on carbon-13 incorporated lipid profiling of human macrophage samples
Method provides higher sensitivity and dynamic range compared with wide full-scan
Acknowledgements
We thank François Allain for providing the Thermo MSFileReader Python library.
Funding
This work was supported in part by NIH grants 1P01CA163223–01A1 (to ANL and TWMF), 1U24DK097215–01A1 (to RMH, TWMF, and ANL), 1R01CA118434–01A2 (to TWMF), 3R01CA118434–02S1 (to TWMF), and the Redox Metabolism Shared Resource(s) of the University of Kentucky Markey Cancer Center (P30CA177558).
Abbreviations:
- AGC
automatic gain control
- CE
cholesterol ester
- COM
component object model
- DG
diacylglycerol
- DI nESI FTMS
Direct infusion nanoelectrospray Fourier transform mass spectrometry
- DMEM
Dulbecco’s Modified Eagle Medium
- DPPC
Dipalmitoyl phosphatidylcholine
- FBS
fetal bovine serum
- FWHM
full width at half maximum
- LIT
linear ion trap
- LPC
lysophosphatidylcholine
- LPS
lipopolysaccharide
- MiND
minimum neighboring difference
- NS
NanoShuttles
- PBS
Phosphate-buffered saline
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- POPC
Palmitoyloleoylphosphatidylcholine
- PREMISE
Precalculated Exact Mass Isotopologue Search Engine
- QMF
quadrupole mass filter
- RRF
repetition rate filtering
- S7
sample-specific silhouette segmented-scan spectral stitching
- SIRM
stable isotope-resolved metabolomics
- SM
sphingomyelin
- TG
triacylglycerol
- WB
whole blood
- WFS
wide full-scan
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Lane AN, Fan TWM, Higashi RM, Tan J, Bousamra M, Miller DM, Prospects for clinical cancer metabolomics using stable isotope tracers, Experimental and molecular pathology, 86 (2009) 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fan TW, Lorkiewicz PK, Sellers K, Moseley HN, Higashi RM, Lane AN, Stable isotope-resolved metabolomics and applications for drug development, Pharmacology & therapeutics, 133 (2012) 366–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lane AN, Tan J, Wang Y, Yan J, Higashi RM, Fan TW, Probing the metabolic phenotype of breast cancer cells by multiple tracer stable isotope resolved metabolomics, Metabolic engineering, 43 (2017) 125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Higashi RM, Structural Mass Spectrometry for Metabolomics, The Handbook of Metabolomics 2012, pp. 61–97. [Google Scholar]
- [5].Southam AD, Payne TG, Cooper HJ, Arvanitis TN, Viant MR, Dynamic range and mass accuracy of wide-scan direct infusion nanoelectrospray fourier transform ion cyclotron resonance massspectrometry-based metabolomics increased by the spectral stitching method, Anal Chem, 79 (2007) 4595–4602. [DOI] [PubMed] [Google Scholar]
- [6].Lorkiewicz P, Higashi RM, Lane AN, Fan TWM, High information throughput analysis of nucleotides and their isotopically enriched isotopologues by direct-infusion FTICR-MS, Metabolomics, 8 (2012) 930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Weber RJ, Southam AD, Sommer U, Viant MR, Characterization of isotopic abundance measurements in high resolution FT-ICR and Orbitrap mass spectra for improved confidence of metabolite identification, Anal Chem, 83 (2011) 3737–3743. [DOI] [PubMed] [Google Scholar]
- [8].Zhurov KO, Kozhinov AN, Fornelli L, Tsybin YO, Distinguishing analyte from noise components in mass spectra of complex samples: where to cut the noise?, Anal Chem, 86 (2014) 3308–3316. [DOI] [PubMed] [Google Scholar]
- [9].Schuhmann K, Thomas H, Ackerman JM, Nagornov KO, Tsybin YO, Shevchenko A, Intensity-Independent Noise Filtering in FT MS and FT MS/MS Spectra for Shotgun Lipidomics, Anal Chem, 89 (2017) 7046–7052. [DOI] [PubMed] [Google Scholar]
- [10].Fan TW, El-Amouri SS, Macedo JKA, Wang QJ, Song H, Cassel T, Lane AN, Stable Isotope-Resolved Metabolomics Shows Metabolic Resistance to Anti-Cancer Selenite in 3D Spheroids versus 2D Cell Cultures, Metabolites, 8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mosser DM, Zhang X, Activation of murine macrophages, Current protocols in immunology, Chapter 14 (2008) Unit-14.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liu M, Luo F, Ding C, Albeituni S, Hu X, Ma Y, Cai Y, McNally L, Sanders MA, Jain D, Kloecker G, Bousamra, M 2nd, Zhang HG, Higashi RM, Lane AN, Fan TW, Yan J, Dectin-1 Activation by a Natural Product beta-Glucan Converts Immunosuppressive Macrophages into an M1-like Phenotype, Journal of immunology (Baltimore, Md. : 1950), 195 (2015) 5055–5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Makarov A, Denisov E, Kholomeev A, Balschun W, Lange O, Strupat K, Horning S, Performance evaluation of a hybrid linear ion trap/orbitrap mass spectrometer, Anal Chem, 78 (2006) 2113–2120. [DOI] [PubMed] [Google Scholar]
- [14].Keller BO, Sui J, Young AB, Whittal RM, Interferences and contaminants encountered in modern mass spectrometry, Analytica chimica acta, 627 (2008) 71–81. [DOI] [PubMed] [Google Scholar]
- [15].Lane AN, Fan TW, Xie Z, Moseley HN, Higashi RM, Isotopomer analysis of lipid biosynthesis by high resolution mass spectrometry and NMR, Analytica chimica acta, 651 (2009) 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Southam AD, Weber RJ, Engel J, Jones MR, Viant MR, A complete workflow for high-resolution spectral-stitching nanoelectrospray direct-infusion mass-spectrometry-based metabolomics and lipidomics, Nature protocols, 12 (2016) 310–328. [DOI] [PubMed] [Google Scholar]
- [17].Taylor NS, White TA, Viant MR, Defining the Baseline and Oxidant Perturbed Lipidomic Profiles of Daphnia magna, Metabolites, 2nd, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sellers K, Fox MP, Bousamra M 2nd, Slone SP, Higashi RM, Miller DM, Wang Y, Yan J, Yuneva MO, Deshpande R, Lane AN, Fan TW, Pyruvate carboxylase is critical for non-small-cell lung cancer proliferation, The Journal of clinical investigation, 125 (2015) 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fan TWM, Zhang X, Wang C, Yang Y, Kang WY, Arnold S, Higashi RM, Liu J, Lane AN, Exosomal lipids for classifying early and late stage non-small cell lung cancer, Analytica chimica acta, 1037 (2018) 256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Papan C, Penkov S, Herzog R, Thiele C, Kurzchalia T, Shevchenko A, Systematic screening for novel lipids by shotgun lipidomics, Anal Chem, 86 (2014) 2703–2710. [DOI] [PubMed] [Google Scholar]
- [21].Xian F, Hendrickson CL, Marshall AG, High Resolution Mass Spectrometry, Analytical Chemistry, 84 (2012) 708–719. [DOI] [PubMed] [Google Scholar]
- [22].Almeida R, Pauling JK, Sokol E, Hannibal-Bach HK, Ejsing CS, Comprehensive lipidome analysis by shotgun lipidomics on a hybrid quadrupole-orbitrap-linear ion trap mass spectrometer, Journal of the American Society for Mass Spectrometry, 26 (2015) 133–148. [DOI] [PubMed] [Google Scholar]
- [23].Yang Y, Fan TWM, Lane AN, Higashi RM, Chloroformate derivatization for tracing the fate of Amino acids in cells and tissues by multiple stable isotope resolved metabolomics (mSIRM), Analytica chimica acta, 976 (2017) 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kind T, Fiehn O, Metabolomic database annotations via query of elemental compositions: mass accuracy is insufficient even at less than 1 ppm, BMC bioinformatics, 7 (2006) 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Belov ME, Nikolaev EN, Harkewicz R, Masselon CD, Alving K, Smith RD, Ion discrimination during ion accumulation in a quadrupole interface external to a Fourier transform ion cyclotron resonance mass spectrometer, International Journal of Mass Spectrometry, 208 (2001) 205–225. [Google Scholar]
- [26].Tolmachev AV, Udseth HR, Smith RD, Radial stratification of ions as a function of mass to charge ratio in collisional cooling radio frequency multipoles used as ion guides or ion traps, Rapid communications in mass spectrometry: RCM, 14 (2000) 1907–1913. [DOI] [PubMed] [Google Scholar]
- [27].Gillet LC, Navarro P, Tate S, Rost H, Selevsek N, Reiter L, Bonner R, Aebersold R, Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: a new concept for consistent and accurate proteome analysis, Mol Cell Proteomics, 11 (2012) O111.016717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Scheltema RA, Hauschild JP, Lange O, Hornburg D, Denisov E, Damoc E, Kuehn A, Makarov A, Mann M, The Q Exactive HF, a Benchtop mass spectrometer with a pre-filter, high-performance quadrupole and an ultra-high-field Orbitrap analyzer, Mol Cell Proteomics, 13 (2014) 3698–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Meier F, Geyer PE, Virreira Winter S, Cox J, Mann M, BoxCar acquisition method enables single-shot proteomics at a depth of 10,000 proteins in 100 minutes, Nature methods, 15 (2018) 440–448. [DOI] [PubMed] [Google Scholar]
- [30].Edmondson R, Broglie JJ, Adcock AF, Yang L, Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors, Assay and drug development technologies, 12 (2014) 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Agassandian M, Mallampalli RK, Surfactant phospholipid metabolism, Biochimica et biophysica acta, 1831 (2013) 612–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tonks A, Morris RH, Price AJ, Thomas AW, Jones KP, Jackson SK, Dipalmitoylphosphatidylcholine modulates inflammatory functions of monocytic cells independently of mitogen activated protein kinases, Clinical and experimental immunology, 124 (2001) 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Han S, Mallampalli RK, The Role of Surfactant in Lung Disease and Host Defense against Pulmonary Infections, Annals of the American Thoracic Society, 12 (2015) 765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Moseley HN, Correcting for the effects of natural abundance in stable isotope resolved metabolomics experiments involving ultra-high resolution mass spectrometry, BMC bioinformatics, 11 (2010) 139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kelly B, O’Neill LA, Metabolic reprogramming in macrophages and dendritic cells in innate immunity, Cell research, 25 (2015) 771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB, ATP-citrate lyase links cellular metabolism to histone acetylation, Science (New York, N.Y.), 324 (2009) 1076–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Higashi RM, Fan TWM, Lorkiewicz PK, Moseley HNB, Lane AN, Stable Isotope Labeled Tracers for Metabolic Pathway Elucidation by GC-MS and FT-MS, Methods in molecular biology, 1198 (2014) 147–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.