Abstract
Microbial communities are complex and dynamic, composed of hundreds of taxa interacting across multiple spatial scales. Understanding the composition of these complex communities became tractable with the advent of high-throughput DNA sequencing. Understanding their biogeography across spatial scales was made possible by new imaging technologies that enable analysis of community spatial organization with micron-scale resolution. In the human mouth sequencing results indicate that distinct sites host microbial communities that are not only compositionally distinguishable but to a meaningful degree are composed of entirely different microbes. Imaging suggests that the spatial organization of these communities is also distinct. Together, the literature supports the idea that most oral microbes are site specialists. A clear understanding of microbiota structure at different sites in the mouth enables mechanistic studies and informed hypothesis testing and strengthens the position of oral microbiology as a model system for microbial ecology in general.
Keywords: biofilm, fluorescence microscopy, human microbiome, imaging, metagenomics, microbial ecology
The Landscape of the Mouth
To a colonizing bacterium, the human mouth presents a varied landscape. The teeth are a permanent, smooth hard surface whose juncture with the gums creates a nutrient-rich crevice. By contrast, a flexible mucosal epithelium coats the cheeks, the soft palate, the ventral side of the tongue, and the floor of the mouth. A keratinized epithelium covers the gums and the hard palate. Densely packed papillae give the dorsum of the tongue a rough topography, and deep crypts occur in the lingual and palatine tonsils.
Overlaid on this topography are surface features of varying levels of complexity. An enamel pellicle coats the teeth and a mucosal pellicle of different composition coats the mucosa (39). Small ridges termed microplicae are present on epithelial surfaces (5). Membrane-bound mucins project from epithelial surfaces and may serve as anchoring points for interaction with secreted mucins (5, 49). Among the papillae of the tongue, circumvallate and fungiform papillae have broad smooth keratinized surfaces while filiform papillae are capped by hairs that project into the saliva (50).
Spatial gradients exist in the mouth because of localized sources and sinks of nutrients, heat, oxygen, saliva and crevicular fluid (78, 83). Saliva is produced by hundreds of minor salivary glands as well as several major glands, each of which produces characteristic secretions (25, 84, 98). The velocity of salivary flow and thus the steepness of gradients influenced by saliva depend on the local topography and proximity to salivary glands. The gingival crevice is a localized source of crevicular fluid derived from serum (6). Temperature in the gingival sulcus varies with position in the mouth and with health and disease (52). Microbes themselves also constitute point sources of nutrients and strongly influence pH, oxygen levels, and the nutritional environment over short spatial scales and in the interior of microbial biofilms (90, 100, 101).
As well as being varied in space, the environment in the mouth is subject to regular disturbances in time. Oral hygiene practices, movements of the lips, cheeks, and tongue, and the periodic transit and mastication of ingested food create more or less regular disturbance within the mouth. Salivary flow and electrolyte content vary not only with stimuli such as eating but also in a circadian rhythm (15, 106). Shedding of epithelial cells, a process called desquamation, also varies over the course of a day (40).
Thus, the mouth is not a unitary environment. Sites within the mouth, although connected by salivary flow, constitute distinct habitats. As pointed out in a recent review by Proctor and Relman (78), landscape ecology provides a conceptual framework for analyzing how the spatial organization of host-determined habitat influences spatial patterning in the resident microbial community. An understanding of how microbial communities function in specific habitats within the mouth will require a firm foundation of information on how microbial communities are structured. The word “structure” has been used in the microbiome literature with both a compositional and a spatial meaning: in the compositional sense, it describes the relative proportions of the microbes that make up a community. In the spatial sense, it means how these microbes are physically arranged with respect to each other and to host tissues. Bacteria need nutrients, moisture, and a habitat that meets their physiological requirements for temperature, pH, and oxygen concentration. Spatial gradients of these factors seem likely to be key determinants of microbial distribution, and an important question is the scale on which these gradients operate. Bacteria also need to adhere to habitat sites (33, 46) or be washed out of the mouth by salivary flow and swallowed into the gut. Thus, the surface features of the landscape that are available for adhesion are also likely to be key determinants of microbial distribution. The interplay between nutrient gradients and adhesion, with contributions from both host and microbe, plays out across multiple spatial scales in the oral environment.
For understanding microbial community organization, resolution and scale are important considerations. Composition can be assessed over a range of taxonomic resolution, including phylum, family, genus, species or strain levels. Taxonomic resolution is limited by the type of sequence data available (ribosomal RNA gene or whole-genome), the bioinformatic analysis method, and the comprehensiveness of available databases. Similarly, spatial scale can be assessed over a range of resolutions including centimeter, millimeter or micron scales. Spatial scale is determined by sampling protocol and analysis technology. A key question in sampling is the resolution and scale that are most relevant to the underlying biology that we wish to understand. For some questions, broad overviews are sufficient. However, coarse resolution and coarse scales of analysis run the risk of averaging over heterogeneous populations and obscuring meaningful biological distinctions.
In this review, we will describe the advancing understanding of how microbial communities are structured, not only spatially but also compositionally, at distinct sites in the mouth. Based on our analysis of the literature, we advocate taking a new look at what we call the site-specialist hypothesis of the oral microbiome. In this view, separate sites in the mouth such as the teeth, tongue dorsum, and buccal mucosa, while connected by a single environment and bathed in the same saliva, are profoundly different in the composition, organization, and biology of the microbiotas they support. This hypothesis is not new; in slightly different terms it was first presented almost five decades ago. However, the wealth of information that has been acquired since then allows for a deeper, more comprehensive reformulation of the hypothesis. Paradoxically, the magnitude of the new information made available by the advent of high-throughput sequencing has also had the effect of obscuring the simple conclusion. A motivation of this review is to organize the new information in such a way that the merits of the site-specialist hypothesis can be readily evaluated.
Early Work on Site-specific Oral Microbial Communities
From decades of work in oral microbiology we know that the microbial communities of the mouth vary from site to site. The earliest comprehensive characterization of oral microbial communities across the mouth was by Gibbons, Gordon, Socransky, and their colleagues. In a series of studies during the 1960s using cultivation on rich medium under anaerobic conditions, they investigated the overall composition of the microbiota of saliva, dental plaque, the gingival crevice, the cheeks, and the tongue dorsum (30, 31, 32, 35, 36, 85). They found organisms such as Corynebacterium, Actinomyces, and Streptococcus sanguinis colonizing teeth, treponemes in the gingival crevice, and S. salivarius on the tongue. By 1971, Socransky and Manganiello (87) could write that “[e]vidence is mounting that different microorganisms colonize different sites in the oral cavity. Although all indigenous organisms can be found in each of the sites at one time or another it is becoming clear that many organisms have a ‘primary ecologic niche.’”
Cultivation and testing of individual strains are painstaking methods for assessing the composition of the oral microbiota. Consequently, investigators sought new, faster ways of evaluating microbial community complexity. Checkerboard DNA-DNA hybridization was developed as a higher-throughput way to take a census of oral microbial communities (88). This method involved isolation of DNA from a set of about 40 key oral reference strains and arraying this DNA on a nylon membrane. DNA was then extracted from oral samples and hybridized with the membrane, allowing quantification of the abundance of each reference strain in each oral sample. Using this method, Mager et al. (59) and Socransky and Haffajee (86) discovered that Actinomyces naeslundii and several other Actinomyces species were abundant in dental plaque, that Veillonella parvula and Prevotella melaninogenica reached their highest abundance in saliva and on the dorsal and lateral surfaces of the tongue, and that Streptococcus mitis, S. oralis, and Gemella morbillorum were most abundant on soft tissue surfaces such as the ventral side of the tongue, the floor of the mouth, the cheeks, hard palate, and gums. Thus, the outlines of site-specific oral microbial communities were in place before the age of high-throughput DNA sequencing began.
High Throughput Tag Sequencing Provides a Systems-Level Overview
Sequencing of the 16S ribosomal RNA gene from natural samples permitted a more comprehensive, systems-level view of microbial community composition. By virtue of the 16S rRNA gene variable and conserved regions, it is possible to identify a 16S rRNA sequence that can serve as an identification tag for a microbial species. As was becoming clear from extensive study over decades, oral microbial communities are complex, with several hundred species detectable in any given sample from a human subject and close to a thousand in the human population overall (17). Characterization of this level of complexity became feasible with the advent of high-throughput sequencing. In addition to the good match between the quantity of data that sequencing could produce and the complexity of the community represented by the data, an additional benefit of 16S rRNA gene sequencing was that it allowed detection of previously unknown, unexpected, or uncultivable bacteria. Balancing these benefits, a limitation of the sequencing approach is that the sequence tag provides no direct information about the biochemistry of the organism, which must be inferred by comparison of the tag to gene sequences of known, cultivated strains.
Early sequencing studies in the oral cavity (1, 7) used nearly full-length 16S rRNA gene sequences for maximum taxonomic resolution and revealed complex communities with commonalities across individuals and differences across oral sites. Aas et al. (1) sampled 9 oral sites from 5 volunteers and showed that the different oral sites harbored communities of distinctive composition, consistent with the earlier cultivation-based work described above. By sequencing a total of 2,589 clones – an average of about 80 clones per sample – Aas et al. found that sites such as the cheek, palate, and anterior vestibule were dominated by S. mitis and Gemella haemolysans in all volunteers. The tongue dorsum community included S. mitis, S. salivarius, S. parasanguinis, Granulicatella adiacens, and Veillonella. The most abundant bacteria in dental plaque included S. mitis, S. sanguinis, S. gordonii, Rothia dentocariosa, and Actinomyces. Bik et al. (7) sequenced more deeply, approximately 1000 clones per sample for each of 10 volunteers, from samples pooled across oral sites within each volunteer to gain an overall view of the oral community and its consistency across individuals. Like Aas et al., they found complex communities with some individual variability on a background of similarity across individuals. The core set of 11 species that Bik et al. found in every volunteer included many of the same species reported by Aas et al. Although Bik et al. identified a provisional group of core species in the oral microbiome, they did not differentiate composition across oral sites.
In a quest for yet greater sequencing depth at lower cost, work shifted to short-read, high-throughput sequencing, using technologies that could generate hundreds of thousands to millions of sequencing reads at a time, where each individual read was only a few hundred nucleotides in length (12, 19, 44, 48, 58, 81, 105). The pioneering studies of Keijser et al. (44) and Zaura et al. (105) produced an in-depth overview of cultivable and uncultivable members of the oral microbiome and were among the first to demonstrate that many low-abundance microbes can be detected in an oral sample by deep sequencing. The initial study’s estimate of multiple thousands of taxa (44) was, however, an overestimate that later studies avoided as algorithms were developed for noise reduction (8, 27, 105). Costello et al. (12) pioneered the use of high-throughput sequencing for a comprehensive analysis of sites across the human body while Diaz et al. applied the method to two sites in depth. With this greater sequencing capacity, the Human Microbiome Project (41) analyzed the microbiome of several hundred volunteers at 18 body sites, including 9 sites in the mouth. Segata et al. presented a comprehensive analysis of the HMP data that was consistent with earlier findings while providing a deeper and richer view of the distinctiveness of oral sites. Analyzing primarily at phylum, family, and genus levels, Segata et al. showed that oral sites could be clustered according to the composition of their microbiota. Three mucosal sites (buccal mucosa, keratinized gingiva, and hard palate) were characterized by a very high relative abundance of Streptococcus (47% +/− 18%) and a high abundance of Gemella (5.2 +/− 5.1%), in agreement with the results of Mager, Socransky and Haffajee, Aas, and Diaz. Other oral sites had a more even distribution of taxon abundance, but Segata et al. were able to identify biomarkers, i.e., genera whose high abundance was characteristic for a given group of sites. Examples included Veillonella and Oribacterium as biomarkers for tongue dorsum and saliva, and Corynebacteriaceae as a biomarker for plaque. Zhou et al. (107) highlight the consistent distribution pattern in human microbiome samples, in which a modest number of genera dominate any given sample but hundreds of low-abundance taxa create a “long tail” on rank abundance curves. Xu et al. (104) sampled children, young adults and the elderly to characterize variation across the life cycle. Collectively, these results are in broad agreement with earlier findings that plaque is distinctive from other oral sites and that the composition of saliva is similar to that of the tongue. The comprehensive nature of the HMP study lent generality to its results, and the discovery of biomarker genera began to build a bridge between high-level metrics of community composition, on the one hand, and detailed studies of oral microbial biology, on the other.
High Resolution Bioinformatics Sharpens Understanding of Site Distinctiveness
The distinctive communities at different oral sites came into sharper focus with the realization that high-throughput data could be mined at single-nucleotide resolution and that this data could then provide a detailed, species-level look at oral communities. Several high-resolution bioinformatics procedures were developed—for example, one based on Shannon entropy to distinguish between noise and useful information at a nucleotide position (27) and another based on modeling patterns of noise in order to employ a de-noising algorithm to differentiate signal from noise (8). With both procedures, it was possible to go beyond the 97% OTU or genus level achieved with previous bioinformatic approaches.
Using the Shannon entropy pipeline, a re-analysis of the HMP data (26) identified exact sequence variants called oligotypes and mapped the oligotypes to species in the Human Oral Microbiome Database (17). This work demonstrated that closely related bacteria, differing in some cases by only a single nucleotide in the sequenced region of the 16S rRNA gene, showed dramatically different distribution patterns across oral sites, suggesting a higher level of habitat specificity than had previously been recognized.
A salient feature of the oral microbiome that emerged from the work of Eren et al. is that many of the genera had sub-types specialized for two or more of the three distinct habitat zones represented by dental plaque, tongue dorsum, and keratinized gingiva. A graphical representation of this habitat specificity is shown in Figure 1 in which the position of a species indicates its relative abundance in tongue dorsum, supragingival plaque, and keratinized gingiva samples. The figure illustrates, for example, that the genus Actinomyces has species strongly specialized to either tongue or teeth: A. naeslundii in dental plaque and A. graevenitzii and A. odontolyticus (and its close relatives) on the tongue. A. graevenitzii is notable for its extreme specialization, with a mean relative abundance on the tongue that is 3900-fold higher than on teeth. The genus Fusobacterium has subtypes in all three of the habitats: F. nucleatum on teeth, F. periodonticum on tongue, and an unnamed species, F. sp. HMT (Human Microbial Taxon) 248, on the keratinized gingiva. Within the genus Streptococcus, S. salivarius and S. parasanguinis seem specialized for tongue and S. sanguinis and S. gordonii for dental plaque. Some species, by contrast, were apparent generalists. S. mitis and its close relatives were abundant in all habitats, although in keratinized gingiva they reached an exceptionally high mean relative abundance of approximately 50%. Other apparent generalists were Haemophilus parainfluenzae, which was approximately equally abundant in all habitats, and Porphyromonas pasteri. A detailed list of the major taxa in 4 distinct oral sites is presented in Table 1.
Figure 1. Site specificity in the oral microbiome.
The position of each species represents its relative abundance in three distinct oral sites: supragingival plaque, tongue dorsum, and keratinized gingiva. The y-axis shows the ratio of the taxon’s normalized abundance in supragingival plaque to its normalized abundance in tongue dorsum; the x-axis shows the ratio of normalized abundance in keratinized gingiva to the mean of the supragingival plaque and tongue dorsum normalized abundance. The area of points is proportional to the mean abundance of the taxon in its site of greatest normalized abundance. Color indicates the genus; species are as follows: An, Actinomyces naeslundii group; Ao, Actinomyces odontolyticus group; Ag, Actinomyces graevenitzii; Cm, Corynebacterium matruchotii; Fn, Fusobacterium nucleatum; Fp, Fusobacterium periodonticum; F. sp. 248, Fusobacterium sp. HMT 248; Gh, Gemella haemolysans; Gs, Gemella sanguinis; Ga, Granulicatella adiacens; Ge, Granulicatella elegans; Hh, Haemophilus haemolyticus group; Hp, Haemophilus parainfluenzae; Lm, Lautropia mirabilis; Lb, Leptotrichia buccalis group; L. sp. 221, Leptotrichia sp. HMT 221; Nf, Neisseria flavescens group; Nm, Neisseria mucosa group; Pp, Porphyromonas pasteri; P. sp. 930, Porphyromonas sp. HMT 930; Pm, Prevotella melaninogenica; A. sp. 473, Alloprevotella sp. HMT 473; Rd, Rothia dentocariosa; Rm, Rothia mucilaginosa; Sg, Streptococcus gordonii; Ssan, Streptococcus sanguinis; Sm, Streptococcus mitis; Ssal; Streptococcus salivarius; Sp, Streptococcus parasanguinis group; Va, Veillonella atypica; Vp1, Veillonella parvula/dispar group 1; Vp2, Veillonella parvula/dispar group 2; Vr, Veillonella rogosae; V. sp. 780 Veillonella sp. HMT 780. Data is from the Human Microbiome Project analyzed with oligotyping (26) and mapped to eHOMD version15.1 (www.homd.org).
Table 1.
The most abundant bacteria in samples from tongue dorsum, supragingival plaque, keratinized gingiva, and buccal mucosa. The first three rows show species abundant at all sites; the remaining rows show other species with >0.5% mean normalized abundance in alphabetical order. Mean relative abundance was calculated from Human Microbiome Project data from the V1-V3 region of 16S ribosomal RNA as analyzed by oligotyping (26) and mapped to eHOMD version15.1 (www.homd.org). Site distinctiveness is shown as ratios of mean normalized abundance in tongue dorsum vs. supragingival plaque (column 3), supragingival plaque vs. tongue dorsum (column 6), keratinized gingiva vs. the mean of supragingival plaque and tongue dorsum (column 9), and buccal mucosa vs. the mean of supragingival plaque and tongue dorsum (column 12). Bold font indicates the taxon has a site distinctiveness ratio of at least 40; blue font indicates the taxon has a mean normalized abundance of at least 4%.
| Supragingival Plaque | % SUPP | SUPP/TD | Tongue Dorsum | % TD | TD/SUPP | Keratinized Gingiva | % KG | KG/(mean SUPP,TD) |
Buccal Mucosa | % BM | BM/(mean SUPP, TD) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Streptococcus mitis group | 13.1% | 1.2 | Streptococcus mitis group | 11.2% | 0.9 | Streptococcus mitis group | 54.6% | 4.5 | Streptococcus mitis group | 55.4% | 4.6 |
| Porphyromonas pasteri | 1.2% | 0.3 | Porphyromonas pasteri | 3.9% | 3.3 | Porphyromonas pasteri | 1.3% | 0.5 | Porphyromonas pasteri | 2.2% | 0.9 |
| Haemophilus parainfluenzae | 2.1% | 0.8 | Haemophilus parainfluenzae | 2.6% | 1.2 | Haemophilus parainfluenzae | 3.5% | 1.5 | Haemophilus parainfluenzae | 2.5% | 1.0 |
| Actinomyces massiliensis | 0.5% | 2060.5 | Actinomyces graevenitzii | 2.5% | 3946.0 | Actinomyces naeslundii group | 0.6% | 0.1 | Actinomyces naeslundii group | 1.8% | 0.4 |
| Actinomyces naeslundii group | 10.1% | 323.6 | Actinomyces odontolyticus group | 7.7% | 43.0 | Alloprevotella sp. HMT 473 | 3.7% | 50.8 | Actinomyces odontolyticus group | 0.7% | 0.2 |
| Bergeyella sp. HMT 322 | 1.6% | 6.1 | Atopobium parvulum | 1.0% | 36.8 | Bergeyella sp. HMT 322 | 0.7% | 0.8 | Fusobacterium nucleatum | 0.5% | 0.4 |
| Campylobacter concisus | 0.5% | 0.2 | Campylobacter concisus | 2.9% | 5.5 | Campylobacter concisus | 1.2% | 0.7 | Fusobacterium periodonticum | 0.5% | 0.4 |
| Campylobacter showae | 0.7% | 13.2 | Fusobacterium periodonticum | 2.8% | 22.8 | Fusobacterium nucleatum | 0.5% | 0.5 | Gemella haemolysans | 7.8% | 28.3 |
| Capnocytophaga gingivalis, C. granulosa | 3.5% | 16.2 | Gemella sanguinis | 1.1% | 99.6 | Fusobacterium sp. HMT 248 | 1.0% | 222.2 | Granulicatella adiacens | 0.6% | 0.4 |
| Capnocytophaga leadbetteri | 2.4% | 28.5 | Granulicatella adiacens | 2.8% | 5.1 | Gemella haemolysans | 5.8% | 20.9 | Granulicatella elegans | 1.1% | 21.5 |
| Capnocytophaga sp. HMT 336 | 0.5% | 86.9 | Lachnoanaerobaculum spp. | 0.8% | 2.6 | Granulicatella elegans | 3.2% | 61.5 | Haemophilus haemolyticus group | 0.7% | 37.7 |
| Capnocytophaga sputigena | 2.4% | 9.2 | Lachnospiraceae [G-2] HMT 096 | 0.5% | 86.6 | Haem. haemolyticus group | 2.8% | 144.2 | Lautropia mirabilis | 0.8% | 0.3 |
| Corynebacterium durum | 2.8% | 338.2 | Leptotrichia sp. HMT 215 | 0.5% | 9.2 | Neisseria flavescens group | 1.6% | 0.3 | Neisseria mucosa group | 1.2% | 0.7 |
| Corynebacterium matruchotii | 5.7% | 603.6 | Leptotrichia sp. HMT 221 | 1.0% | 70.8 | Porphyromonas sp. HMT 930 | 2.5% | 114.3 | Neisseria flavescens group | 1.4% | 0.3 |
| Fusobacterium nucleatum | 2.2% | 28.1 | Leptotrichia wadei, L. sp. HMT 417 | 0.6% | 2.1 | Streptococcus sanguinis group | 0.8% | 0.4 | Porphyromonas sp. HMT 930 | 0.6% | 26.9 |
| Granulicatella adiacens | 0.5% | 0.2 | Neisseria flavescens group | 8.8% | 31.5 | Streptococcus salivarius group | 4.3% | 1.0 | Prevotella melaninogenica | 0.5% | 0.2 |
| Kingella oralis | 1.3% | 231.0 | Neisseria subflava | 1.0% | 0.8 | Veillonella parvula/dispar 2 | 1.4% | 0.9 | Rothia aeria, R. dentocariosa | 1.6% | 0.3 |
| Lautropia mirabilis | 4.9% | 127.7 | Oribacterium spp. | 1.2% | 22.4 | Veillonella sp. HMT 780 | 2.8% | 249.7 | Rothia mucilaginosa | 2.3% | 0.9 |
| Leptotrichia buccalis group | 2.7% | 95.6 | Prevotella histicola, P. veroralis, P. sp. HMT 313 | 1.9% | 14.4 | total | 92.3% | Streptococcus gordonii | 0.6% | 1.4 | |
| Neisseria elongata | 1.7% | 88.1 | Prevotella melaninogenica | 4.4% | 53.6 | Streptococcus sanguinis group | 0.9% | 0.4 | |||
| Neisseria mucosa group | 3.2% | 78.9 | Prevotella nanceiensis | 0.8% | 57.5 | Streptococcus parasanguinis group | 0.8% | 0.3 | |||
| Neisseria subflava | 1.3% | 1.2 | Prevotella pallens | 0.8% | 78.9 | Streptococcus salivarius group | 2.9% | 0.6 | |||
| Rothia aeria, R. dentocariosa | 9.8% | 265.9 | Prevotella salivae | 0.6% | 82.3 | Veillonella parvula/dispar 1 | 0.9% | 0.3 | |||
| Saccharibacteria (TM7) [G-1] HMT 347 | 0.7% | 171.1 | Rothia mucilaginosa | 4.9% | 66.8 | Veillonella parvula/dispar 2 | 1.3% | 0.9 | |||
| Saccharibacteria (TM7) [G-1] HMT 348 | 0.5% | 286.6 | Saccharibacteria (TM7) [G-1] HMT 352, HMT 952 | 1.8% | 5.6 | Veillonella rogosae | 0.7% | 0.5 | |||
| Selenomonas noxia | 0.7% | 301.9 | Streptococcus parasanguinis group | 5.1% | 48.3 | total | 90.4% | ||||
| Streptococcus gordonii | 0.9% | 85.1 | Streptococcus salivarius group | 9.0% | 98.8 | ||||||
| Streptococcus sanguinis group | 4.0% | 14.9 | Veillonella atypica | 1.8% | 313.0 | ||||||
| Veillonella parvula/dispar 2 | 2.9% | 42.2 | Veillonella parvula/dispar 1 | 6.1% | 14.5 | ||||||
| total | 84.7% | Veillonella rogosae | 3.0% | 159.1 | |||||||
| total | 93.2% | ||||||||||
| bold = site specialists (ratio at least 40) | |||||||||||
| blue = abundant taxa (at least 4%) |
Streptococcus mitis group includes S. mitis, S. oralis (all subspecies), S. infantis (both clades), S. australis, S. cristatus clade 578, S. pneumoniae, and S. spp. HMT 061, 064, 066, 074, and 423.
Actinomyces naeslundii group includes A. naeslundii, A. oris, A. johnsonii, A. viscosus, and A. spp. HMT 169, 170, 171 and 175.
Actinomyces odontolyticus group includes A. odontolyticus, A. lingnae, A. meyeri, and A. spp. HMT 172 and 180.
Fusobacterium nucleatum includes F. nucleatum ss animalis, F. nucleatum ss polymorphum, F. nucleatum ss vincentii, and F. naviforme.
Fusobacterium periodonticum includes F. periodonticum and F. sp. HMT 370.
Haemophilus haemolyticus group includes H. haemolyticus, H. parahaemolyticus, H. paraphrohaemolyticus, and H. sp. HMT 036.
Leptotrichia buccalis group includes L. buccalis, L. hongkongensis, and L. spp. HMT 212 and 225.
Neisseria mucosa group includes N. mucosa, N. flava, N. macacae, and N. sicca.
Neisseria flavescens group includes N. flavescens, N. perflava, N. cinerea, N. meningitidis, and N. polysaccharea.
Streptococcus parasanguinis group includes S. parasanguinis clade 411, S. parasanguinis clade 721, S. peroris, and S. spp. HMT 056, 057, and 066.
Streptococcus salivarius group includes S. salivarius and S. vestibularis.
Streptococcus sanguinis group includes S. sanguinis and S. lactarius.
Veillonella parvula/dispar 1 is made up of oligotypes at least 99% identical to V. parvula clone BU083 and V. dispar strain DSM 20735. Veillonella parvula/dispar 2 is made up of oligotypes identical to V. parvula strain DSM 2008, clone _X002, clone AA050, or clone _X042 and V. dispar clone _X031.
The overall picture that emerged from oligotyping analysis of HMP data in combination with mapping to HOMD is that each site in the mouth is colonized by a community in which a subset of major taxa collectively make up the majority of the cells. Strikingly, these taxa are disparate, belonging to diverse families and even different phyla, consistent with Darwin’s original observation on ‘divergence of character’ that organisms inhabiting the same habitat tend to be taxonomically diverse (13). Our analysis buttressed and extended the basic conclusion of Socransky and Manganiello from more than 40 years earlier: that microorganisms in the mouth have a primary ecological niche, a site where they thrive and are found in greatest relative abundance. The earlier studies were limited to those organisms that could be cultured. The significance of the high-throughput sequencing studies is that they are culture-independent, comprehensive, and of high taxonomic resolution and thus able to generalize the conclusions of Socransky and Manganiello.
Whole Genome Sequences Distinguish Closely Related Organisms
As the outlines of a comprehensive description of the microbiota came into focus using 16S ribosomal RNA gene tags, interpreting the meaning of the 16S rRNA gene tag became more pressing. Bacterial strains with very different biological functions can have near-identical 16S rRNA gene sequences (109). Recognizing the need for more detailed genomic information, the HMP incorporated not only tag sequencing, but also whole-genome “shotgun” sequencing which has further reinforced and refined our knowledge of the habitat-selectiveness of oral microbes. This method, in which millions of randomly selected small fragments of DNA from the sample are sequenced, makes it possible to distinguish strains or closely related species that may have identical 16S ribosomal RNA genes but very different gene complements and functional capabilities. As a method for identifying prevalent strains within the human microbiome, Kraal et al. (48) compared individual reads from whole-genome shotgun sequence data to 1,751 sequenced reference genomes. Their analysis focused largely on the stool microbiome but included data from the supragingival plaque, tongue dorsum, and buccal mucosa communities. The abundant strains they identified were largely consistent with the oligotyping analysis. Using the more extensive dataset produced during subsequent years of the HMP, Lloyd-Price et al. (58) examined the strain composition of the oral microbiota in more detail. They showed that a number of species have distinct sub-types or strains specialized to different individuals or different body sites. For example, subtypes of H. parainfluenzae were detected that specialized for plaque, tongue dorsum, and buccal mucosa. Interestingly, many of the species that appeared from earlier studies to be generalist taxa abundant throughout the mouth are among the species identified by Lloyd-Price et al. as having site-specific sub-types, including not only H. parainfluenzae but also Porphyromonas pasteri (a.k.a. Porphyromonas sp. oral taxon 279) and Streptococcus infantis. A similar result was found by Costea et al. (11) who detected plaque-specific and tongue-specific subtypes of Granulicatella adiacens as well as subtypes of taxa such as H. parainfluenzae, Capnocytophaga gingivalis and C. sputigena that were differentially abundant in plaque and tongue. These findings suggest that many taxa that appear to be generalists are, instead, conglomerations of closely related species or strains with distinct biology that have not yet been teased apart by taxonomists. In summary, studies over several decades, using a variety of methods of steadily increasing taxonomic resolving power, have led to a major conclusion: that most oral microbes show strong preferences for individual habitats within the mouth.
Site-Specialist Hypothesis for the Oral Microbiome
As a way of thinking about the differences between the microbiomes of different oral sites, we advocate for an idea we will call the site-specialist hypothesis: that oral sites differ not just in the compositional proportions of their microbiota but in the specific identities of their microbes. It is now widely recognized that the microbiotas of the various oral sites are distinguishable (1, 19, 51, 59, 78, 81, 82, 83, 86, 103, 105). However, the distinction is often blurred between having the same set of microbes in different proportions, and having a substantially different set of microbes. In the analysis of Segata et al. (81), for example, the oral sites could be clearly differentiated, in a statistically robust way, based purely on the relative proportions of common genera. Analysis of the same data with single-nucleotide resolution showed that the common genera were composed of different underlying species in different oral sites (26). Analysis at the level of genus (or even phylum) was sufficient to detect an overall difference between sites but hid the extent of the disparity between the communities. We hypothesize that most microbes in the mouth are site specialists; they grow primarily in only one of the microhabitats of the mouth. The less-abundant taxa that occur at each site, but that are highly abundant elsewhere in the mouth, we interpret as accidental tourists that have been dislodged from their preferred environment. They may be metabolically inert, or they may be active but they do not become established and they influence the site biology only transiently.
From an operational point of view, it is unlikely that a microbe will be found to be exclusively confined to a single oral site, because all oral sites shed microbes into saliva, and saliva, in turn, coats all of the oral sites. Microbes transported by saliva could become lodged in cracks or crevices of the biofilm distant from their site of origin, or could adhere at sites other than their primary habitat due to the stochasticity of binding. During the process of sampling itself, microbes from the superficial coating of saliva are likely to be sampled along with the underlying biofilm. The degree of admixture of saliva with samples has not been investigated to our knowledge, but is likely to depend on the method of sampling (swab, buccal brush, paper point, or scaler) and the biomass being sampled. For example, a clump of dental plaque from between the teeth or at the gingival margin is likely to have more bacterial cells, and proportionally less salivary contamination, than a thin layer of plaque scraped from a relatively clean tooth surface. To make a back-of-the-envelope calculation about the degree to which saliva could contribute to the apparent mixing of communities, we postulate for the sake of argument (1) that the bacteria that grow in dental plaque are entirely different from the bacteria that grow on the tongue; (2) that 95% of the microbes in a sample from the mouth will be from the intended sample site while 5% will be from saliva coating the site; and (3) that 50% of the microbes in saliva come from the tongue dorsum and 20% come from plaque – which is clearly an oversimplification but we make it here to illustrate the point. A microbe that makes up 10% of the tongue community would then comprise about 5% of the salivary microbiota, and would contribute 0.25% of the microbes in a sample from dental plaque. In other words, a microbe that lived exclusively on the tongue would also be found in a plaque sample, where its relative abundance would be ~40-fold less than its relative abundance on the tongue. Conversely, a microbe that lived exclusively in plaque would have a relative abundance ~100-fold higher in a plaque sample than its apparent abundance on the tongue. These ratios, which would be expected purely from cross-contamination with saliva during the sampling process, are the same order of magnitude as many of the ratios for oral species in the mouth (Figure 1, Table 1). Thus, the data on site composition of the oral microbiome are broadly consistent with the idea that many microbes are primarily resident in only one of the oral habitats, and are detected in the other habitats as contaminants via saliva.
What does it mean, then, to be “resident” in a habitat? We suggest that in the dynamic environment of the mouth, a resident microbe is one that actively localizes itself in its habitat and grows and divides there. The mechanism of site localization represents an important but understudied problem. Adhesion to a site is clearly essential as was recognized many years ago by early investigators (33). Because of extensive and rapid salivary flow (~1 liter/day), if a microbe does not adhere to an oral surface, it will be swallowed and pass into the gut. Thus, a key element of survival in the mouth is the ability to remain in a preferred habitat through binding to available surfaces. Gibbons and Van Houte (33) argued that the local distribution of nutrients likely has minimal influence on the differential localization of organisms because closely related organisms with apparently similar nutrient requirements localize to different parts of the mouth, and conversely, some organisms with wildly different nutrient requirements co-localize to the same part of the mouth. For most oral microbes, adhesion either directly to a site or indirectly via attachment to other microbes that directly adhere to the site is likely to be the major mechanism of site localization. For motile organisms such as treponemes or campylobacters, chemotaxis is also likely to be important. A site-specialist hypothesis then makes testable predictions for site localization. An oral microbe should adhere selectively to substrates from its preferred oral site or demonstrate chemotaxis toward attractants from its preferred site compared to other oral sites.
Consistent with the site-specialist hypothesis, we note that a well-defined and consistent set of bacterial species is routinely observed to make up the bulk of the oral microbiome, and that this set of species differs from site to site within the mouth, but not from person to person. Specifically, the preponderance of cells (~80-90%) in a healthy oral microbiome come from a set of about two dozen species or groups of closely related species for each oral site (Table 1). These abundant and characteristic taxa are the structural and functional core of the community and are key to understanding its microbial biology.
An objection might be raised that the picture just described is an over-simplification of the situation in the mouth. Admittedly, it is. Hundreds of taxa can be found in the mouth at low abundance including some that can bloom sporadically and others that are present consistently but in low abundance. Sporadic blooms are likely important to understand for both health and disease. Distinguishing whether consistently low abundance organisms are residents or transients is a challenging issue. Low-abundance taxa could conceivably act as keystone taxa shaping the rest of the community with an influence out of proportion to their numbers. They could fill key metabolic niches, without which the community would be in disequilibrium. However, we suggest that hypotheses about the role or function of rare or sporadic taxa can be more incisively formulated by addressing them as outliers within the framework of abundant core taxa.
Apparent Exceptions to the Framework of Site-Specific Taxa
Several of the taxa that we consider site specialists for tongue or keratinized gingiva are sometimes reported in the literature as members of the plaque microbiota, or as part of the oral core microbiome found in both tongue dorsum and plaque; conversely, plaque specialists sometimes are reported as part of the core tongue microbiota (e.g., 24, 29, 38, 63, 76, 77, 79, 104). These findings are not in themselves contrary to the site-specialist hypothesis because abundant site specialists would be expected to be dispersed by saliva and detectable throughout the mouth. The hypothesis does make predictions, however, about the relative proportions of taxa at different sites, and about the functional role and likely organization of these taxa within the community. Namely, we predict that when microbes are outside their preferred site, their growth would be reduced and their gene expression would be reduced or significantly altered. The gene expression profile of a microbe when in its native site would generally include expression of pathways for DNA replication and cell division, whereas that microbe when found outside its native site should show significantly less expression of these pathways. If imaged with micron-scale resolution, microbes outside of their preferred site would be expected to be at the exterior of the local biofilm, as a salivary coating, or would be present as isolated cells or clumps rather than as structurally integrated members of the community.
For the most part, samples from the oral microbiota from global populations have the same major genera in approximately the same proportions as those described from the United States in the Human Microbiome Project (81). Samples from Canada, Saudi Arabia, China, Korea, and Japan follow this pattern (4, 38, 80, 82, 91, 104). Some apparent differences that have been reported may be artifacts of differing analysis protocols. In dental plaque in volunteers from China, for example, Jiang et al. (43) reported that 8% of the sequences consisted of Parascardovia, a genus in the Bifidobacteriaceae that is not abundant in HMP data. Reanalysis of the data of Jiang et al. using an oligotyping pipeline (94) showed no Parascardovia and instead showed abundant Actinomyces, suggesting that the apparent presence of the unusual taxon was due to a difference in analysis pipelines. Similarly, the identification of genus Derxia in high abundance (e.g., 44, 104, 105) may be an analysis pipeline variant on Lautropia mirabilis. Reproducible workflows that are comparable across studies can be achieved using HOMD as a reference database and a protocol that generates species-level identifications with a standardized bioinformatic pipeline (28).
In other cases, presence of high-abundance taxa not reflected in the HMP data may reflect global differences in lifestyle and cultural practices. Nasidze et al. (66) surveyed the salivary microbiota in diverse populations from around the globe and found the genera Enterobacter and Serratia in substantial blooms, 45-95% of the salivary microbiota, in 10% of healthy individuals. Li et al. (55) similarly found high proportions of enterobacteriaceae in African and Alaskan native donors compared to Europeans. A study of toddlers in China detected enterobacteriaceae making up 50% of the microbiota of 18- and 24-month-old, but not 12-month-old, children (54). As well as being a component of the gut microbiota, enterobacteriaceae are commonly present in raw milk cheeses and other fermented foods and beverages (9, 92) that could provide an inoculum into the oral microbiota. It would be interesting to conduct follow-up studies to establish whether sporadic or regular colonization with enterobacteriaceae is a consistent feature of human oral microbiomes and what cultural practices or environmental characteristics may influence their abundance.
Several studies have reported shifts in proportions of both common and rare members of the normal microbiota among subjects of different nationalities or ethnicities. Mason et al. (64) compared people of four different ethnicities living in the United States. Takeshita et al. (91) compared healthy adults in South Korea to those in Japan; Clemente et al. (10) compared the oral mucosa of Yanomami to US populations. All of these studies found statistically significant differences in the relative abundance of both high-abundance and low-abundance genera across groups. These studies may well point to the effect of cultural, environmental, or genetic factors that influence the microbiota. However, it would be important to validate these findings of ethnic differences by analyzing the data using newer statistical methods. Microbiome sequence data are effectively normalized; they report relative abundances of taxa and therefore belong to a category of data termed “compositional”. As such, they must be analyzed using methods that not only account for sampling variation and the simultaneous testing of multiple hypotheses, but also account for the compositional nature of the data – methods that are not currently in common use in the microbiome literature but that, when used, identify fewer taxa as significantly different between groups (34). We do not yet understand why individuals differ from one another in the proportions of major or minor microbial taxa, nor do we understand to what degree those differences may be caused by diet, environment, cultural factors, genetics, or historical accident. This subject needs further research and the results of Gloor et al. indicate that we, as a field, need to adapt our statistical methods to account for the compositional properties of microbiome data.
Person-to-Person Differences in the Oral Microbiome
Our hypothesis that site-specific microbiomes are predominantly composed of a few dozen well-defined species may seem to contradict the often-reported finding that each individual person has a distinctive and characteristic oral microbiome. Numerous studies have shown that individuals carry distinctive oral microbiomes (38, 58, 94, 105). However, studies carried out with species-level resolution have also demonstrated that the most abundant species are widely shared across individuals, and that individuals are differentiated by distinctive sets of strains within these species as well as by distinctive proportions of the major taxa (1, 4, 7, 38, 58, 61, 80, 94, 105). Thus, the distinctiveness of individual oral microbiomes appears to arise from strain-level divergence and the long-term stability of strain profiles within individuals. The individuality of oral microbiomes is an important finding that raises questions about the process of site colonization and evolution but is not inconsistent with the presence of a consistent set of species at each oral site across individuals.
Spatial Differentiation Within a Site
Even as major differences in the microbial composition at different oral sites are becoming clear, the degree of variation within an oral site remains under-studied. At millimeter scales, on the different aspects of teeth and across the plaque biofilm, physical conditions such as the rate of salivary flow, the chemistry of saliva, and the biology of the microbes interact with one another to produce complex and time-variable effects, with the result that associating the microbiota with the local conditions may not be straightforward. The seminal work of Kleinberg and Jenkins (45), for example, demonstrated gradients in the pH of dental plaque across the mouth associated with differences in the rate of salivary flow. These experiments were carried out on a thick plaque biofilm produced by asking volunteers to refrain from oral hygiene for three days, and the plaque biofilm itself had complex effects on pH; the plaque generated acid when exposed to dietary carbohydrates, but plaque microbes also metabolized salivary urea so that in the fasting state the plaque became substantially more alkaline than the saliva that washed over it. These experiments showed that position in the mouth influences the pH fluctuations generated by a thick plaque biofilm but did not necessarily indicate that we should expect a pH gradient on exposed tooth surfaces subjected to regular oral hygiene. This study laid the foundations for study of how local differences in topography and salivary flow affect the biochemistry and, likely, the composition of plaque.
Applying high-throughput methods to this finer spatial scale, several investigators have reported differences in the composition of dental plaque from tooth to tooth within the mouth and at different tooth surfaces. Haffajee et al. (37) used checkerboard DNA hybridization to investigate abundance of 40 strains in plaque from 187 subjects and found shifts in the proportions of taxa based on tooth position within the mouth; for example, Veillonella parvula was in the highest abundance on the lower molars and Streptococcus sanguinis was highest on the lower incisors and canines. Zaura et al. (105) sequenced deeply from 3 individuals and likewise found shifts in proportions of taxa between incisors and molars and between lingual, buccal, and interproximal sites, but no clearly consistent trends. Simon-Soro et al. (83) sequencing from 2 people found Veillonella in high abundance on canine teeth and Streptococcus in higher abundance on vestibular (buccal) surfaces, whereas Fusobacterium was more abundant on lingual surfaces. Proctor et al. (77) compared buccal and lingual surfaces by high-throughput sequencing from 31 individuals and detected a gradient in sample composition from incisors to molars particularly on the lingual surfaces of teeth. These differences may be shaped by the abundance of saliva, as well as by other features of the local landscape.
Micron-Scale Biogeography
Analysis of the biogeography of oral microbial communities at an even finer scale – that of microns to hundreds of microns – sheds light on the role of both adhesion and gradients of nutrients and oxygen in structuring the distribution of bacteria.
Early studies of dental plaque development in situ, allowing plaque to accumulate on an epoxy resin crown and then imaging it using electron microscopy, showed densely packed, species-rich communities. Intimate microbe-microbe interactions within these communities were visualized, including a structure called a “corn-cob” in which a central filament was surrounded by cocci attached directly to the central filament (56, 57). Corncobs were found in three-day-old plaque but were also found in three-week-old plaque as a surface layer atop a thick bed of filaments. This corncob structure was a direct demonstration of the highly specific adhesion of microbes to one another that represents a mechanism by which microbes may localize to a specific habitat within the mouth. These studies also established that plaque communities are initially rich in cocci and over the course of several days to a week become more filament-rich. This change suggests an ecological succession, the modification of the environment by the initial colonizers in a way that enables later colonizers to enter. The earliest stages of the succession were imaged by electron microscopy of pieces of human dental enamel and root surfaces, fixed in an acrylic carrier and worn inside the mouth for up to 48 hours (71, 72). These images show coccus-rich plaque and then, at 24 hours, a single filament projecting perpendicular to the plaque biofilm, followed by a complex mat of filaments at 48 hours. Wood and colleagues (102) used similar devices and allowed plaque to develop for 4 days, then used confocal light microscopy to investigate the overall structure of the plaque biofilm. They found an open architecture with lobes of the biofilm separated by fluid-filled voids or channels, in contrast to the apparently more compact structure seen in electron micrographs.
Identifying the partners involved in taxon-taxon interactions became possible with the advent of fluorescence in situ hybridization and immunofluorescence applied to oral microbial biofilms. The initial stages of plaque formation, from 4 to 8 hours after exposure of an enamel chip to the oral environment, were investigated by Palmer, Diaz, Kolenbrander, and colleagues using probes for streptococci, Actinomyces, Prevotella, and Veillonella. They found mixed communities, taxon-taxon interactions, and interpersonal diversity beginning from the earliest stages of plaque formation (18, 73, 74). Dige, Kilian, Nyvad, and colleagues (21, 22) allowed plaque biofilms to form on glass slabs worn inside the mouth for up to 48 hours. Using probes for Streptococcus and Actinomyces, they determined that Actinomyces, while slower-growing, localized on the inner side of the biofilm, suggesting that it bound directly to the acquired pellicle.
More complex imaging of dental plaque biofilms was initiated by Thurnheer and colleagues (93) who carried out fluorescence in situ hybridization with probes targeting 6 taxa (in two sets of three) and found that hybridization could be carried out simultaneously on gram-positive and gram-negative organisms. Extending this finding by hybridizing simultaneously with probes for 5 taxa, Al-Ahmad et al. (2, 3) found the initial colonizers Streptococcus and Actinomyces in high relative abundance in the first few days of plaque development, with their abundance rising over the first 12 hours and then decreasing over 7 days while the abundance of Fusobacterium increased. Palmer and colleagues (75) employed both sequence analysis and FISH to investigate the complexity of adhesion and nearest-neighbor interactions in 4- to 8-hour biofilms.
Studies of mature biofilms investigated the spatial arrangement of microbes in periodontal pockets using electron microscopy, immunohistochemistry, and fluorescence in situ hybridization. Carriers could be placed into periodontal pockets for variable periods of time to permit imaging of various stages of biofilm growth at different depths in the periodontal pocket (99). These studies found differences in colonization based on spatial position, with spirochaetes colonizing a carrier placed deep into a periodontal pocket and streptococci colonizing a shallowly-placed carrier. The biofilms had a mosaic architecture, with initial colonizers at the surface of the carrier and later colonizers in a patchy distribution (23, 62). In a series of studies of microbial localization within intact periodontal pockets that were extracted, embedded, and sectioned, Noiri and colleagues also found organisms distributed into different regions of the pocket, with for example, Campylobacter rectus deep in the pocket, treponemes and Fusobacterium in unattached plaque, and Actinomyces viscosus and Eikenella corrodens in plaque attached to the tooth (68, 70). Direct attachment of the periodontal pathogen Porphyromonas gingivalis to the biofilm via fimbrae could also be seen (69).
Analysis of oral microbial biofilms on extracted teeth that were embedded and sectioned was carried out by Zijnge and colleagues (108) who used probes targeting most groups of oral bacteria and found complex biofilms with distinctive taxon-taxon interactions. They detected a multi-layered biofilm with distinctive microbes in the basal, the intermediate, and the surface layer. A similar method was employed by Dige and colleagues (20) to study the spatial organization of bacteria in caries. They produced beautiful images of patchy biofilms occupying cavities and crevices in teeth.
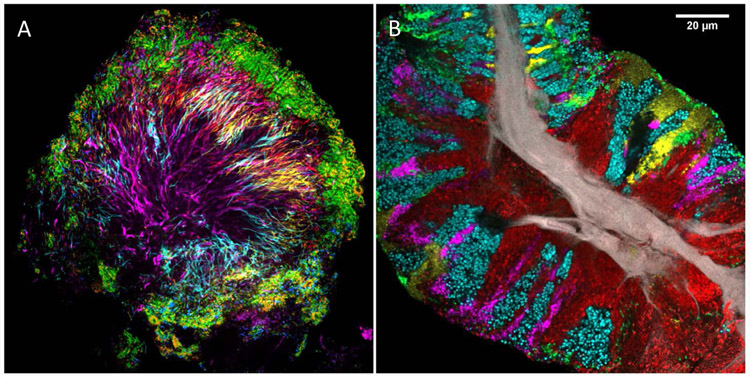
Comprehensive analysis of the spatial organization of complex biofilms was advanced by the development of CLASI-FISH, Combinatorial Labeling and Spectral Imaging – Fluorescence in situ Hybridization, which permitted the simultaneous imaging and analysis of potentially dozens of microbial taxa (95, 96, 97). By imaging all the major constituents of the supragingival plaque biofilm simultaneously, it was possible to visualize the spatial organization of a complex microbial consortium called a “hedgehog” (60) (Figure 2A). The organization of this structure, with known aero-tolerant taxa on the exterior and anaerobes and micro-aerophiles in the interior, suggested the existence of oxygen and nutrient gradients over scales of tens of microns. Further, the images revealed that the “corncob” structures previously identified by electron microscopy were more complex than previously appreciated, consisting of at least 3 different coccoid taxa, two of which (Streptococcus and Porphyromonas) bound directly to the central filament and the third of which (Haemophilus/Aggregatibacter) bound to the Streptococcus cells. The central filament itself was identified as Corynebacterium. Thus, the hedgehog structure demonstrated at micron scales the significance of both adhesion and gradients of nutrients and oxygen for the colonization and spatial organization of the microbial community.
Figure 2. Imaging reveals dense and highly organized communities in the human oral microbiome.
(A): a “hedgehog” structure from dental plaque. Corynebacterium (magenta filaments) radiate outward from a central point forming a spatially structured habitat inhabited by other taxa at characteristic positions. Cocci (Streptococcus, green; Porphyromonas, blue, Haemophilus/Aggregatibacter, orange) occupy an outer shell; microaerophilic taxa (Fusobacterium, yellow; Leptotrichia, cyan) occupy a presumably low-oxygen zone inside the outer shell. See Mark Welch et al 2016. (B): a biofilm scraped from the surface of the tongue and imaged using CLASI-FISH. Human epithelial tissue forms a central core (gray). Actinomyces (red) occupy a region close to the core; Streptococcus (green) is localized in an exterior crust and in stripes in the interior. Other taxa (Rothia, cyan; Neisseria, yellow; Veillonella, magenta) are present in clusters and stripes that suggest growth of the community outward from the central core. S. Wilbert et al., manuscript in preparation.
Application of CLASI-FISH to other sites in the mouth shows promise for illuminating the role of micron-scale structure and microbe-microbe interactions in establishing biogeography of microbes. Imaging reveals a community on the tongue dorsum (Fig. 2B) whose structure is completely different from that of the hedgehog, but whose degree of organization is similar. Both communities are characterized by intimate and direct interactions among disparate microbes, with different taxa localized to different regions of the structure. Microbes themselves clearly make up a major portion of the environment for adjacent microbes, both directly by presenting surfaces for binding and indirectly by modulating the local environment. The differential distribution of microbes throughout the mouth may be driven by coevolution of microbe-microbe interactions and simultaneous adaptation of the microbes to one another and to the features of the host.
Integrating biogeography across spatial scales
The highly organized structures visualized in both dental plaque and tongue, composed of microbes with widely differing habitat requirements, suggest that for the biogeography of oral microbes some critical gradients occur at micron scales. Dense concentrations of microbes in biofilms are capable of absorbing and secreting nutrients rapidly enough to create strong micron-scale gradients (89, 90, 100). Microbes can, for example, generate an anaerobic environment within 20 to 30 microns from the oxygenated surface of a biofilm (101). The relatively sharp segregation of corncob structures carrying oxygen-tolerant microbes at the surface of plaque from anaerobes buried more deeply (57, 60, 108) demonstrates the shaping of microbial distribution by steep gradients at micron scales.
The magnitude and range of these gradients, however, is modulated by a number of factors working across spatial scales. One important determinant is likely to be the thickness of the biofilm itself, which ranges from a few microns to a millimeter or more, because gradients may be generated and shaped by the metabolism of the bacteria that make up the biofilm. Characteristics of the bacteria, such as the efficiency with which they take up substrates and the rate at which they secrete waste products, are likely drivers in shaping the gradients. Thickness of the biofilm, however, will depend not only on the growth habit and reproduction of the microbes but also on the degree to which the site is exposed to abrasion and the rate at which the underlying surface is shed. Finally, an additional factor likely influencing both the magnitude and the range of gradients is salivary flow. At first glance, salivary flow would seem to attenuate gradients, as material from a point source can be distributed throughout the mouth within a few minutes (42). However, when saliva flows over a biofilm, differences in the velocity of flow influence the rate at which small molecules are transported to and from the biofilm (16, 45, 53, 77), potentially modulating microbial metabolism and shifting the composition of the microbiota.
Topography and spatial features of the landscape at scales of hundreds of microns to a few millimeters can also influence the characteristics of the niche and the composition of the local microbiota. For example, the tooth surface supports a characteristic dental plaque microbiota that is similar at both supragingival and subgingival sites. The bottom of the periodontal pocket not only has a tooth surface but also has gingival tissue forming one side of the pocket, and in deeper pockets a low-oxygen micro-habitat forms (65) that supports a distinctive microbiota richer in treponemes and other anaerobes compared to supragingival plaque. Other topographically distinctive locations include the tonsillar crypts, the circumvallate and foliate papillae of the tongue, and the hairs that project from the filiform papillae; these hairs are more heavily colonized by microbes than the base of the same papillae (50). Spatially resolved sampling of the microbiota will be necessary to determine the degree to which such small-scale topographical features influence the local composition of the microbiota.
If small-scale biogeography is primarily driven by micron-scale gradients and modulated by local topography, biogeography at the larger scale of variation between teeth, tongue, cheeks, and gums appears to be driven by differences in the substrate. Descriptions of the landscape of the mouth, including the one at the beginning of this review, generally open by mentioning the primary distinctions among the major oral surfaces: the teeth as a fixed, non-shedding surface vs. the mucosa which sheds, and then different types of mucosa: keratinized, non-keratinized, and specialized, such as the tongue dorsum with its dense population of papillae. These major types of surfaces correspond rather well to the major types of microbiota; the microbiota of the teeth are clearly distinguishable from the microbiota of the tongue and of the keratinized gingiva, while the non-keratinized floor of the mouth and the soft palate bear microbiota similar to that of the cheeks, and the keratinized hard palate microbiota resembles that of the tongue surface (1, 26, 59, 81).
Horizons for Oral Microbial Ecology
The likely importance of the substrate in shaping the microbiota suggests that adhesion of initial colonizers to the substrate and of later colonizers to the initial colonizers will be key to understanding how distinctive microbiotas assemble and interact at the different oral sites. Extensive studies of adhesion and coaggregation have been carried out on a variety of oral species and strains, primarily from dental plaque (47, 67). Of the species and strains that we now know to be major constituents of the healthy oral microbiome, however, many have barely been studied (48). How do these microbes localize to their preferred environment? To what surface structures, of the host or of other microbes, might they adhere? How much of the differential distribution of organisms across the mouth can be explained solely on the basis of this adhesion? The time may be right for a renaissance of studies on the adhesion of oral microbes, to illuminate the mechanisms by which prevalent and abundant but little-studied microbes build communities in the mouth.
New methods may open new frontiers for research in initial colonization and in micron-scale analysis of the biochemical environment. Studies of the stages of colonization and growth of dental plaque in different micro-environments were greatly advanced by the development of devices that permitted an enamel chip or other hard substrate to be worn in the mouth for a period of time, then retrieved and analyzed. To understand colonization of mucosal surfaces we need to develop an equivalent system or an in vitro strategy, perhaps with organoids, to characterize the stages of colonization of a new mucosal surface following desquamation of the overlying cells. We also need to move to a higher level of spatial resolution. Analyzing microbial distribution with micron-scale resolution can reveal the micro-environment that the organism needs to thrive. Biosensors, micro-electrodes, and stains may be used to define these habitats more precisely by determining levels of substrates, metabolites, or oxygen with micron-scale precision. The goal is to understand at a mechanistic level how oral microbes are interacting with one another and with host tissue, and how and why microbial distribution patterns are established.
At a systems level, oral microbiology is poised to take full advantage of a higher level of genomic resolution. As we enter an era in which sequencing entire microbial genomes is straightforward and inexpensive, genomics will offer an ever-clearer view of how well any given cultivated strain might represent the more complex population found in the mouth. Can we identify a well-defined set of genes that characterizes each bacterial species in the mouth? If there is genomic variability, can we determine how that variability affects the organism’s niche, its spatial localization and function? Are even the apparently generalist species composed of distinct strains that specialize to different habitats within the mouth? How does the genome reveal the niche an organism occupies, and the potential and realized breadth of that niche?
Lastly, we need to understand dynamics and variation. Even highly abundant organisms vary widely in relative abundance from day to day in samples from the mouth (14, 94). What drives the volatility in community composition over time? Why is there not only strain-level variation, but also variation in the overall composition of the microbiota from person to person? Why do some people have unusually high levels of one or another taxon, consistently over time? With the increasing affordability of 16S rRNA gene sequencing, it may be possible to find patterns or associations between community composition and geography, diet, genetics, or other features of host biology or behavior. Longitudinal studies of individuals over time, with experimental perturbations of diet or behavior, may provide insight into the sources of both variation and volatility.
The varied landscape of the mouth presents an opportunity to understand microbial community structure across spatial scales and in relation to substrates, gradients, and other key environmental features that differentiate one site in the mouth from another. Building on foundations laid more than 50 years ago, investigations of the oral microbiota with ever-increasing taxonomic resolution have revealed highly specialized communities at the different sites in the mouth, and strong specialization of individual microbes to one or another habitat within the mouth. Future work to investigate microbial interactions at micron scale and at molecular scale will enable us to understand the intimate co-habitation on which these communities are founded, and how site specificity is achieved.
Acknowledgements.
We thank the Micro-Eco Discussion Group at the Marine Biological Laboratory for comments and suggestions. This work was supported by NIH National Institute of Dental and Craniofacial Research Grants DE022586 (to G.G.B.) and R37DE016937 (to F.E.D.).
Contributor Information
Jessica L. Mark Welch, Marine Biological Laboratory, Woods Hole MA 02543.
Floyd E. Dewhirst, The Forsyth Institute, Cambridge MA 02142 and Harvard School of Dental Medicine, Boston MA 02115.
Gary G. Borisy, The Forsyth Institute, Cambridge MA 02142.
Literature Cited
- 1.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. 2005. Defining the normal bacterial flora of the oral cavity. Journal of Clinical Microbiology 43: 5721–5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Ahmad A, Follo M, Selzer A-C, Hellwig E, Hannig M, Hannig C. 2009. Bacterial colonization of enamel in situ investigated using fluorescence in situ hybridization. Journal of Medical Microbiology 58: 1359–1366. [DOI] [PubMed] [Google Scholar]
- 3.Al-Ahmad A, Wunder A, Auschill TM, Follo M, Braun G, Hellwig E, Arweiler NB. 2007. The in vivo dynamics of Streptococcus spp., Acinomyces naeslundii, Fusobacterium nucleatum and Veillonella spp. in dental plaque biofilm as analysed by five-colour multiplex fluorescence in situ hybridization. Journal of Medical Microbiology 56: 681–687. [DOI] [PubMed] [Google Scholar]
- 4.Al-hebshi NN, Abdulhaq A, Albarrag A, Basode VK, Chen T. 2016. Species-level core oral bacteriome identified by 16S rRNA pyrosequencing in a healthy young Arab population. Journal of Oral Microbiology 8: 31444. doi: 10.3402/jom.v8.31444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asikainen P, Sirviö E, Mikkonen JJW, Singh SP, Schulten EAJM, ten Bruggenkate CM, Koistinen AP, Kullaa AM. 2015. Microplicae – Specialized Surface Structure of Epithelial Cells of Wet-Surfaced Oral Mucosa. Ultrastructural Pathology 39(5): 299–305. [DOI] [PubMed] [Google Scholar]
- 6.Barros SP, Williams R, Offenbacher S, Morelli T. 2016. Gingival crevicular fluid as a source of biomarkers for periodontitis. Periodontology 2000 70: 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bik EM, Long CD, Armitage GC, Loomer P, Emerson J, Mongodin EF, Nelson KE, Gill SR, Fraser-Liggett CM, Relman DA. 2010. Bacterial diversity in the oral cavity of 10 healthy individuals. The ISME Journal 4(8): 962–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callahan B, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. 2016. DADA2: High-resolution sample inference from Illumina amplicon data. Nature Methods 13(7): 581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chavez-López C, De Angelis M, Martuscelli M, Serio A, Paparella A, Suzzi G. 2006. Characterization of the Enterobacteriaceae isolated from an artisanal Italian ewe’s cheese (Pecorino Abruzzese). Journal of Applied Microbiology 101: 353–360. doi: 10.1111/j.1365-2672.2006.02941.x. [DOI] [PubMed] [Google Scholar]
- 10.Clemente JC, Pehrsson EC, Blaser MJ, Sandhu K, Gao Z, Wang B, et al. 2015. The microbiome of uncontacted Amerindians. Sci. Adv 1:e1500183. doi: 10.1126/sciadv.1500183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costea PI, Coelho LP, Sunagawa S, Munch R, Huerta-Cepas J, Forslund K, Hildebrand F, Kushugulova A, Zeller G, Bork P. 2017. Subspecies in the global human gut microbiome. Molecular Systems Biology 13: 960. doi: 10.15252/msb.20177589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. 2009. Bacterial community variation in human body habitats across space and time. Science 326: 1694–1697. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darwin C 2009. The Origin of Species by Means of Natural Selection Or, the Preservation of Favoured Races in the Struggle for Life, 6th edition. www.gutenberg.org/ebooks/2009. [PMC free article] [PubMed] [Google Scholar]
- 14.David LA, Materna AC, Friedman J, Campos-Baptista MI, Blackburn MC, Perrotta A, et al. 2014. Host lifestyle affects human microbiota on daily timescales. Genome Biology 15, 1. doi: 10.1186/gb-2014-15-7-r89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawes C 1972. Circadian rhythms in human salivary flow rate and composition. Journal of Physiology 220: 529–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dawes C 1989. An analysis of factors influencing diffusion from dental plaque into a moving film of saliva and the implications for caries. Journal of Dental Research 68: 1483–1488. [DOI] [PubMed] [Google Scholar]
- 17.Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner ACR, Yu W-H, et al. 2010. The human oral microbiome. Journal of Bacteriology 192: 5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diaz PI, Chalmers NI, Rickard AH, Kong C, Milburn CL, Palmer RJ Jr., Kolenbrander PE. 2006. Molecular Characterization of Subject-Specific Oral Microflora during Initial Colonization of Enamel. Applied and Environmental Microbiology 72(4): 2837–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz PI, Dupuy AK, Abusleme L, Reese B, Obergfell C, Choquette L, Dongari-Bagtzoglou A, Peterson DE, Terzi E, Strausbaugh LD. 2012. Using high throughput sequencing to explore the biodiversity in oral bacterial communities. Molecular Oral Microbiology 27: 182–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dige I, Grønkjær L, Nyvad B. 2014. Molecular Studies of the Structural Ecology of Natural Occlusal Caries. Caries Research 48: 451–460. [DOI] [PubMed] [Google Scholar]
- 21.Dige I, Nilsson H, Kilian M, Nyvad B. 2007. In situ identification of streptococci and other bacteria in initial dental biofilm by confocal laser scanning microscopy and fluorescence in situ hybridization. European Journal of Oral Sciences 115: 459–467. [DOI] [PubMed] [Google Scholar]
- 22.Dige I, Raarup MK, Nyengaard JR, Kilian M, Nyvad B. 2009. Actinomyces naeslundii in initial dental biofilm formation. Microbiology 155: 2116–2126. [DOI] [PubMed] [Google Scholar]
- 23.Drescher J, Schlafer S, Schaudinn C, Riep B, Neumann K, Friedmann A, Petrich A, Göbel UB, Moter A. 2010. Molecular epidemiology and spatial distribution of Selenomonas spp. in subgingival biofilms. European Journal of Oral Sciences 118: 466–474. [DOI] [PubMed] [Google Scholar]
- 24.Edlund A, Yang Y, Yooseph S, He X, Shi W, McLean JS. 2018. Uncovering complex microbiome activities via metatranscriptomics during 24 hours of oral biofilm assembly and maturation. Microbiome 6: 217. doi: 10.1186/s40168-018-0591-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eliasson L, Carlén A. 2010. An update on minor salivary gland secretions. European Journal of Oral Sciences 118: 435–442. doi: 10.1111/j.1600-0722.2010.00766.x [DOI] [PubMed] [Google Scholar]
- 26.Eren AM, Borisy GG, Huse SM, Mark Welch JL. 2014. Oligotyping analysis of the human oral microbiome. Proceedings of the National Academy of Sciences U.S.A 111, E2875–E2884. doi: 10.1073/pnas.1409644111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eren AM, Maignien L, Sul WJ, Murphy LG, Grim SL, Morrison HG, et al. 2013. Oligotyping: differentiating between closely related microbial taxa using 16S rRNA gene data. Methods in Ecology and Evolution 4: 1111–1119. doi: 10.1111/2041-201X.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Escapa IF, Chen T, Huang Y, Gajare P, Dewhirst FE, Lemon K. 2018. New Insights into Human Nostril Microbiome from the Expanded Human Oral Microbiome Database (eHOMD): a Resource for the Microbiome of the Human Aerodigestive Tract. mSystems 3(6): e00187–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Espinoza JL, Harkins DM, Torralba M, Gomez A, Highlander SK, Jones MB, et al. 2018. Supragingival Plaque Microbiome Ecology and Functional Potential in the Context of Health and Disease. mBio 9(6): e01631–18. doi: 10.1128/mBio.01631-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gibbons RJ, Kapsimalis B, Socransky SS. 1964. The Source of Salivary Bacteria. Arch. oral Biol 9: 101–103. [DOI] [PubMed] [Google Scholar]
- 31.Gibbons RJ, Socransky SS, de Araujo WC, van Houte J. 1964. Studies of the Predominant Cultivable Microbiota of Dental Plaque. Arch. oral Biol 9: 365–370. [DOI] [PubMed] [Google Scholar]
- 32.Gibbons RJ, Socransky SS, Sawyer S, Kapsimalis B, and MacDonald JB. 1963. The Microbiota of the Gingival Crevice Area of Man – II. The Predominant Cultivable Organisms. Arch. oral Biol 8: 281–289. [DOI] [PubMed] [Google Scholar]
- 33.Gibbons RJ, van Houte J. 1975. Bacterial adherence in oral microbial ecology. Annual Review of Microbiology 29: 19–42. [DOI] [PubMed] [Google Scholar]
- 34.Gloor GB, Wu JR, Pawlowsky-Glahn V, Egozcue JJ. 2016. It’s all relative: analyzing microbiome data as compositions. Annals of Epidemiology 26: 322–329. doi: 10.1016/j.annapidem.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 35.Gordon DF Jr., Gibbons RJ. 1966. Studies of the Predominant Cultivable Micro-Organisms from the Human Tongue. Arch. oral Biol 11: 627–632. [DOI] [PubMed] [Google Scholar]
- 36.Gordon DF Jr., Jong BB. 1968. Indigenous Flora from Human Saliva. Applied Microbiology 16(2): 428–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haffajee AD, Teles RP, Patel MR, Song X, Yaskell T, Socransky SS. 2009. Factors affecting human supragingival biofilm composition. II. Tooth position. Journal of Periodontal Research 44: 520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hall MW, Singh N, Ng KF, Lam DK, Goldberg MB, Tenenbaum HC, Neufeld JD, Beiko RG, Senadheera DB. 2017. Inter-personal diversity and temporal dynamics of dental, tongue, and salivary microbiota in the healthy oral cavity. npj Biofilms and Microbiomes 3(2); doi: 10.1038/s41522-016-0011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hannig C, Hannig M, Kensche A, Carpenter G. 2017. The mucosal pellicle – An underestimated factor in oral physiology. Arch. oral Biol 80: 144–152. [DOI] [PubMed] [Google Scholar]
- 40.Helmerhorst EJ, Dawes C, Oppenheim FG. 2018. The complexity of oral physiology and its impact on salivary diagnostics. Oral Diseases 24: 363–371. doi: 10.1111/odi.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Human Microbiome Project Consortium. 2012. Structure, function and diversity of the healthy human microbiome. Nature 486: 207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jenkins GN, Krebsbach PH. 1985. Experimental study of the migration of charcoal particles in the human mouth. Arch. oral Biol 30(9): 697–699. [DOI] [PubMed] [Google Scholar]
- 43.Jiang W-X, Hu Y-J, Gao L, He Z-Y, Zhu C-L, Ma R, et al. 2015. The impact of various time intervals on the supragingival plaque dynamic core microbiome. PLoS ONE 10:e0124631. doi: 10.1371/journal.pone.0124631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keijser BJF, Zaura E, Huse SM, van der Vossen JMBM, Schuren FHJ, Montijn RC, ten Cate JM, Crielaard W. 2008. Pyrosequencing analysis of the Oral Microflora of healthy adults. Journal of Dental Research 87: 1016. doi: 10.1177/154405910808701104. [DOI] [PubMed] [Google Scholar]
- 45.Kleinberg I, Jenkins GN. 1964. The pH of dental plaques in the different areas of the mouth before and after meals and their relationship to the pH and rate of flow of resting saliva. Arch. oral Biol 9: 493–516. [DOI] [PubMed] [Google Scholar]
- 46.Kolenbrander PE, Palmer RJ Jr., Periasamy S, Jakubovics NS. 2010. Oral multispecies biofilm development and the key role of cell-cell distance. Nature Reviews Microbiology 8: 471–480. [DOI] [PubMed] [Google Scholar]
- 47.Kolenbrander PE, Palmer RJ Jr., Rickard AH, Jakubovics NS, Chalmers NI, Diaz PI. 2006. Bacterial interactions and successions during plaque development. Periodontology 2000 42: 47–79. [DOI] [PubMed] [Google Scholar]
- 48.Kraal L, Abubucker S, Kota K, Fischbach MA, Mitreva M. 2014. The Prevalence of Species and Strains in the Human Microbiome: A Resource for Experimental Efforts. PLoS ONE 9(5): e97279. doi: 10.1371/journal.pone.0097279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kullaa AM, Asikainen P, Herrala M, Ukkonen H, Mikkonen JJW. 2014. Microstructure of Oral Epithelial Cells as an Underlying Basis for Salivary Mucosal Pellicle. Ultrastructural Pathology 38(6): 382–386. doi: 10.3109/01913123.2014.944732. [DOI] [PubMed] [Google Scholar]
- 50.Kullaa-Mikkonen A, Hynynen M, and Hyvönen P. 1987. Filiform Papillae of Human, Rat and Swine Tongue. Acta Anatomica 130: 280–284. [DOI] [PubMed] [Google Scholar]
- 51.Kumar P, Mason MR. 2015. Mouthguards: does the indigenous microbiome play a role in maintaining oral health? Frontiers in Cellular and Infection Microbiology 5: 35. doi: 10.3389/fcimb.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kung RTV, Ohs B, Goodson JM. 1990. Temperature as a periodontal diagnostic. Journal of Clinical Periodontology 17: 557–563. [PubMed] [Google Scholar]
- 53.Lecomte P, Dawes C. 1987. The influence of salivary flow rate on diffusion of potassium chloride from artificial plaque at different sites in the mouth. Journal of Dental Research 66: 1614–1618. [DOI] [PubMed] [Google Scholar]
- 54.Li F, Tao D, Feng X, Wong MCM, Lu H. 2018. Establishment and Development of Oral Microflora in 12–24 Month-Old Toddlers Monitored by High-Throughput Sequencing. Frontiers in Cellular and Infection Microbiology 8:422. doi: 10.3389/fcimb.2018.00422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li J, Quinque D, Horz H-P, Li M, Rzhetskaya M, Raff JA, et al. 2014. Comparative analysis of the human saliva microbiome from different climate zones: Alaska, Germany, and Africa. BMC Microbiol. 14: 316. doi: 10.1186/s12866-014-0316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Listgarten MA, Mayo H, Amsterdam M. 1973. Ultrastructure of the Attachment Device between Coccal and Filamentous Micro-organisms in “Corn Cob” Formations of Dental Plaque. Arch. oral Biol 18: 651–656. [DOI] [PubMed] [Google Scholar]
- 57.Listgarten MA, Mayo HE, Tremblay R. 1975. Development of Dental Plaque On Epoxy Resin Crowns In Man. A Light and Electron Microscopic Study. Journal of Periodontology 46: 10–26. [DOI] [PubMed] [Google Scholar]
- 58.Lloyd-Price J, Mahurkar A, Rahnavard G, Crabtree J, Orvis J, Hall AB, et al. 2017. Strains, functions and dynamics in the expanded Human Microbiome Project. Nature 550: 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mager DL, Ximinez-Fyvie LA, Haffajee AD, Socransky SS. 2003. Distribution of selected bacterial species on intraoral surfaces. Journal of Clinical Periodontology 30: 644–654. [DOI] [PubMed] [Google Scholar]
- 60.Mark Welch JL, Rossetti BJ, Rieken CW, Dewhirst FE, Borisy GG. 2016. Biogeography of a human oral microbiome at the micron scale. Proceedings of the National Academy of Sciences U.S.A 113, E791–E800. doi: 10.1073/pnas.1522149113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mark Welch JL, Utter DR, Rossetti BJ, Mark Welch DB, Eren AM, Borisy GG. 2014. Dynamics of tongue microbial communities with single-nucleotide resolution using oligotyping. Front. Microbiol 5:568. doi: 10.3389/fmicb.2014.00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marsh PD, Moter A, Devine DA 2011. Dental plaque biofilms: communities, conflict and control. Periodontology 2000 55: 16–35. [DOI] [PubMed] [Google Scholar]
- 63.Mason MR, Chambers S, Dabdoub SM, Thikkurissy S, Kumar PS. 2018. Characterizing oral microbial communities across dentition states and colonization niches. Microbiome 6(1): 67. doi: 10.1186/s40168-018-0443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mason MR, Nagaraja HN, Camerlengo T, Joshi V, Kumar PS. 2013. Deep sequencing identifies ethnicity-specific bacterial signatures in the oral microbiome. PLoS ONE 8:e77287. doi: 10.1371/journal.pone.0077287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mettraux GR, Gusberti FA, Graf H. 1984. Oxygen tension (pO2) in untreated human periodontal pockets. Journal of Periodontology 55(9): 516–521. [DOI] [PubMed] [Google Scholar]
- 66.Nasidze I, Li J, Quinque D, Tang K, Stoneking M. 2009. Global diversity in the human salivary microbiome. Genome Research 19, 636–643. doi: 10.1101/gr.084616.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nobbs AH, Lamont RJ, Jenkinson HF. 2009. Streptococcus Adherence and Colonization. Microbiology and Molecular Biology Reviews 73(3): 407–450. doi: 10.1128/MMBR.00014-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Noiri Y, Li L, Ebisu S. 2001. The Localization of Periodontal-Disease-Associated Bacteria in Human Periodontal Pockets. Journal of Dental Research 80(10): 1930–1934. [DOI] [PubMed] [Google Scholar]
- 69.Noiri Y, Li L, Yoshimura F, Ebisu S. 2004. Localization of Porphyromonas gingivalis-carrying Fimbriae in situ in Human Periodontal Pockets. Journal of Dental Research 83(12): 941–945. [DOI] [PubMed] [Google Scholar]
- 70.Noiri Y, Ozaki K, Nakae H, Matsuo T, Ebisu S. 1997. An Immunohistochemical Study on the Localization of Porphyromonas gingivalis, Campylobacter rectus and Actinomyces viscosus in Human Periodontal Pockets. J. Periodontal Research 32: 598–607. [DOI] [PubMed] [Google Scholar]
- 71.Nyvad B, Fejerskov O. 1987. Transmission electron microscopy of early microbial colonization of human enamel and root surfaces in vivo. Scandinavian Journal of Dental Research 85: 297–307. [DOI] [PubMed] [Google Scholar]
- 72.Nyvad B, Fejerskov O. 1987. Scanning electron microscopy of early microbial colonization of human enamel and root surfaces in vivo. Scandinavian Journal of Dental Research 95: 287–296. [DOI] [PubMed] [Google Scholar]
- 73.Palmer RJ Jr., Diaz PI, Kolenbrander PE. 2006. Rapid Succession within the Viellonella Population of a Developing Human Oral Biofilm In Situ. Journal of Bacteriology 188(11): 4117–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Palmer RJ Jr., Gordon SM, Cisar JO, Kolenbrander PE. 2003. Coaggregation-Mediated Interactions of Streptococci and Actinomyces Detected in Initial Human Dental Plaque. Journal of Bacteriology 185(11): 3400–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Palmer RJ Jr., Shah N, Valm A, Paster B, Dewhirst F, Inui T, Cisar JO. 2017. Interbacterial Adhesion Networks within Early Oral Biofilms of Single Human Hosts. Applied and Environmental Microbiology 83: e00407–17. doi: 10.1128/AEM.00407-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peterson SN, Meissner T, Su AI, Snesrud E, Ong AC, Schork NJ, Bretz WA. 2014. Functional expression of dental plaque microbiota. Frontiers in Cell and Infection Microbiology 14 August 2014. doi: 10.3389/fcmib.2014.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Proctor DM, Fukuyama JA, Loomer PM, Armitage GC, Lee SA, Davis NM, Ryder MI, Holmes SP, Relman DA. 2018. A spatial gradient of bacterial diversity in the human oral cavity shaped by salivary flow. Nature Communications 9: 681. doi: 10.1038/s41467-018-02900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Proctor DM, Relman DA. 2017. The Landscape Ecology and Microbiota of the Human Nose, Mouth, and Throat. Cell Host & Microbe 21: 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sands KM, Twigg JA, Lewis MAO, Wise MP, Marchesi JR, Smith A, Wilson MJ, Williams DW. 2016. Microbial profiling of dental plaque from mechanically ventilated patients. Journal of Medical Microbiology 65(2): 147–159. doi: 10.1099/jmm.0.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sato Y, Yamagishi J, Yamashita R, Shinozaki N, Ye B, Yamada T, Yamamoto M, Nagasaki M, Tsuboi A. 2015. Inter-Individual Differences in the Oral Bacteriome Are Greater than Intra-Day Fluctuations in Individuals. PLoS One 10(6): e0131607. doi: 10.1371/journal.pone.0131607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Segata N, Kinder Haake S, Mannon P, Lemon KP, Waldron L, Gevers D, Huttenhower C, Izard J. 2012. Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biology 13: R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shi W, Tian J, Xu H, Zhou Q, Qin M. 2018. Distinctions and associations between the microbiota of saliva and supragingival plaque of permanent and deciduous teeth. PLoS One 13(7): e0200337. doi: 10.1371/journal.pone.0200337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Simón-Soro Á, Tomás I, Cabrera-Rubio R, Catalan MD, Nyvad B, Mira A. 2013. Microbial Geography of the Oral Cavity. Journal of Dental Research 92(7): 616–621. [DOI] [PubMed] [Google Scholar]
- 84.Siqueira WL, Salih E, Wan DL, Helmerhorst EJ, Oppenheim FG. 2008. Proteome of Human Minor Salivary Gland Secretion. Journal of Dental Research 87(5): 445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Socransky SS, Gibbons RJ, Dale AC, Bortnick L, Rosenthal E, MacDonald JB. 1963. The Microbiota of the Gingival Crevice Area of Man – I. Total Microscopic and Viable Counts and Counts of Specific Organisms. Arch. oral Biol 8: 275–280. [DOI] [PubMed] [Google Scholar]
- 86.Socransky SS, Haffajee AD. 2005. Periodontal Microbial Ecology. Periodontology 2000 38: 135–187. [DOI] [PubMed] [Google Scholar]
- 87.Socransky SS, Manganiello SD. 1971. The Oral Microbiota of Man From Birth to Senility. Journal of Periodontology 42(8): 485–496. [DOI] [PubMed] [Google Scholar]
- 88.Socransky SS, Smith C, Martin L, Paster BJ, Dewhirst FE, Levin AE. 1994. “Checkerboard” DNA-DNA hybridization. Biotechniques 17: 788–782. [PubMed] [Google Scholar]
- 89.Stewart PS. 2003. Diffusion in Biofilms. Journal of Bacteriology 185(5): 1485–1491. doi: 10.1128/JB.185.5.1485-1491.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stewart PS, Franklin MJ. 2008. Physiological heterogeneity in biofilms. Nature Reviews Microbiology 6: 199–210. doi: 10.1038/nrmicro1838. [DOI] [PubMed] [Google Scholar]
- 91.Takeshita T, Matsuo K, Furuta M, Shibata Y, Fukami K, Shimazaki Y, et al. 2014. Distinct composition of the oral indigenous microbiota in South Korean and Japanese adults. Sci. Rep 4:6990. doi: 10.1038/srep06990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tamang JP, Watanabe K, Holzapfel WH. 2016. Review: Diversity of Microorganisms in Global Fermented Foods and Beverages. Front. Microbiol 7:377. doi: 10.3389/fmicb.2016.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thurnheer T, Gmür R, Guggenheim B. 2004. Multiplex FISH analysis of a six-species bacterial biofilm. Journal of Microbiological Methods 56: 37–47. [DOI] [PubMed] [Google Scholar]
- 94.Utter DR, Mark Welch JL, Borisy GG. 2016. Individuality, Stability, and Variability of the Plaque Microbiome. Front. Microbiol 7:564. doi: 10.3389/fmicb.2016.00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Valm AM, Mark Welch JL, Borisy GG. 2012. CLASI-FISH: principles of combinatorial labeling and spectral imaging. Systematic and Applied Microbiology 35(8): 496–502. doi: 10.1016/j.syapm.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Valm AM, Mark Welch JL, Rieken CW, Hasegawa Y, Sogin ML, Oldenbourg R, Dewhirst FE, Borisy GG. 2011. Systems-level analysis of microbial community organization through combinatorial labeling and spectral imaging. PNAS 108(10): 4152–4157. doi: 10.1073/pnas/1101134108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Valm AM, Oldenbourg R, Borisy GG. 2016. Multiplexed Spectral Imaging of 120 Different Fluorescent Labels. PLoS ONE 11(7): e0158495. doi: 10.1371/journal.pone.0158495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Veerman ECI, Van den Keybus PAM, Vissink A, Nieuw Amerongen AV. 1996. Human glandular salivas: their separate collection and analysis. European Journal of Oral Sciences 104: 346–352. [DOI] [PubMed] [Google Scholar]
- 99.Wecke J, Kersten T, Madela K, Moter A, Göbel UB, Friedmann A, Mernimoulin J-P. 2000. A novel technique for monitoring the development of bacterial biofilms in periodontal pockets. FEMS Microbiology Letters 191: 95–101. [DOI] [PubMed] [Google Scholar]
- 100.Wessel AK, Arshad TA, Fitzpatrick M, Connell JL, Bonnecaze RT, Shear JB, Whiteley M. 2014. Oxygen limitation within a bacterial aggregate. mBio 5(2): e00992–14. doi: 10.1128/mBio.00992-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wimpenny JWT, Coombs JP. 1983. Penetration of Oxygen into Bacterial Colonies. Journal of General Microbiology 129: 1239–1242. [DOI] [PubMed] [Google Scholar]
- 102.Wood SR, Kirkham J, Marsh PD, Shore RC, Nattress B, Robinson C. 2000. Architecture of Intact Natural Human Plaque Biofilms Studied by Confocal Laser Scanning Microscopy. Journal of Dental Research 79(1): 21–27. [DOI] [PubMed] [Google Scholar]
- 103.Ximénez-Fyvie LA, Haffajee AD, Socransky SS. 2000. Comparison of the microbiota of supra- and subgingival plaque in health and periodontitis. Journal of Clinical Periodontology 27: 648–657. [DOI] [PubMed] [Google Scholar]
- 104.Xu X, He J, Xue J, Wang Y, Li K, Zhang K, et al. 2015. Oral cavity contains distinct niches with dynamic microbial communities. Environmental Microbiology 17: 699–710. doi: 10.1111/1462-2920.12502. [DOI] [PubMed] [Google Scholar]
- 105.Zaura E, Keijser BJF, Huse SM, Crielaard W. 2009. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiology 9: 259. doi: 10.1186/1471-2180-9-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zheng L, Seon YJ, McHugh J, Papagerakis S, Papagerakis P. 2012. Clock Genes Show Circadian Rhythms in Salivary Glands. Journal of Dental Research 91(8): 783–788. doi: 10.1177/0022034512451450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou Y, Gao H, Mihindukulasuriya KA, La Rosa PS, Wylie KM, Vishnivetskaya T, et al. 2013. Biogeography of the ecosystems of the healthy human body. Genome Biology 14: R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zijnge VM, van Leeuwen BM, Degener JE, Abbas F, Thurnheer T, Gmür R, Harmsen HJM. 2010. Oral Biofilm Architecture on Natural Teeth. PLoS One 5(2): e9321. doi: 10.1371/journal.pone/0009321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zuo G, Xu Z, Hao B. 2013. Shigella Strains Are Not Clones of Escherichia coli but Sister Species in the Genus Escherichia. Genomics, Proteomics & Bioinformatics 11(1): 61–65. doi: 10.1016/j.gpb.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]