Summary
Background:
Outcomes for children with relapsed or refractory acute myeloid leukemia (AML) remain poor. The BCL-2 inhibitor venetoclax has shown promising activity in combination with hypomethylating agents and low-dose cytarabine in older, unfit adults with newly diagnosed AML. The aims of the present study were to determine the safety and explore the activity of venetoclax with standard and high-dose chemotherapy in pediatric patients with relapsed AML.
Methods:
Patients with relapsed or refractory AML or acute leukemia of ambiguous lineage who were 2–22 years old and who had adequate organ function and performance status were eligible. We tested the safety and activity of venetoclax (240 or 360mg/m2/day orally for 28 days) in combination with cytarabine (100mg/m2/dose IV every 12 hours for 20 doses or 1000mg/m2/dose IV every 12 hours for 8 doses) with or without idarubicin (12mg/m2 IV). The primary endpoint was the recommended phase 2 dose (RP2D) of venetoclax plus chemotherapy and the secondary endpoint was the proportion of patients treated at the RP2D who achieved complete remission (CR) or complete remission with incomplete count recovery (CRi). Analyses were done per protocol. The study was at ClinicalTrials.gov (NCT03194932). The primary objective has been reached and the study is enrolling to address exploratory objectives.
Findings:
Thirty-eight patients were enrolled from July 1, 2017 to July 2, 2019, with a median follow-up of 7.1 months (IQR 5.1–11.2). The RP2D of venetoclax was 360mg/m2 (600mg max) combined with cytarabine (1000mg/m2/dose for 8 doses) with or without idarubicin (12mg/m2 as a single dose). Overall responses were observed in 24 (69%) of 35 evaluable patients, including 16 CR (11 MRD negative), 4 CRi (2 MRD negative), and 4 PR. Among the 20 patients treated at the RP2D 14 (70%) achieved CR or CRi and 16 (80%) CR/CRi/PR. The most common grade 3–4 adverse events were febrile neutropenia (25 patients), bloodstream infections (6 patients), and invasive fungal infections (6 patients). Treatment-related death occurred in only one patient.
Interpretation:
The safety and activity of venetoclax plus chemotherapy in patients with heavily pre-treated, relapsed and refractory AML suggests that this combination should be tested in newly diagnosed patients with high-risk AML.
Funding:
ALSAC, AbbVie, Gateway for Cancer Research, National Institutes of Health CA21765
Introduction
Although 50–70% of children newly diagnosed with acute myeloid leukemia (AML) can be cured with frontline therapy,(1–3) those with relapsed disease have a dismal outcome and require novel therapies. Overexpression of the anti-apoptotic BCL-2 family of proteins is a documented mechanism of resistance in AML and other malignancies.(4–6) Preclinical data demonstrate that inhibitors of BCL-2 have activity against AML cell lines and patient-derived samples.(4, 6, 7) Encouraging results observed in elderly unfit patients with newly diagnosed AML treated with the specific BCL-2 inhibitor venetoclax in combination with hypomethylating agents(8) or low-dose cytarabine(9) led to recent approval of these combinations. However, these combinations appear to be less active in relapsed or refractory AML.(10, 11)
In this first study of venetoclax in pediatric patients, we describe the safety and activity of its use in combination with high-dose cytarabine and idarubicin. We also describe the associations between BH3 profiling of AML blasts, response to window therapy with venetoclax, leukemic mutational pattern, and the clinical response to combination of venetoclax and conventional high-dose chemotherapy.
Methods
Study design and participants
Patients aged 2–22 years with relapsed or refractory AML or acute leukemia of ambiguous lineage were eligible. Patients were required to have greater than 5% bone marrow blasts, adequate organ function, a performance status (Lanksy for those ≤ 16 years, Karnofsky for those >16) of at least 50, and have recovered from prior therapy. Patients who underwent prior hematopoietic cell transplantation had to be at least 60 days from transplantation and free from active graft-versus-host disease. Patients who were pregnant and those with Down syndrome, acute promyelocytic leukemia, juvenile myelomonocytic leukemia, or a bone marrow failure syndrome were excluded. Patients could receive hydroxyurea or low-dose cytarabine (≤100mg/m2/day) up to 24 hours prior to the start of therapy but had to be 14 days from prior myelosuppressive therapy or gemtuzumab ozogamicin. Patients were eligible to receive idarubicin if they had previously received <300mg/m2 of doxorubicin equivalents (using conversion ratios of 0.5:1 for daunorubicin, 5:1 for idarubicin, and 4:1 for mitoxantrone). The study was approved by the institutional review boards of participating institutions and registered at ClinicalTrials.gov (NCT03194932). Each patient and/or family provided written informed consent and, as appropriate, assent.
Procedures
We explored the safety of two dose levels of venetoclax (Abbvie Inc, North Chicago, IL, USA) and two regimens of cytarabine with or without idarubicin using a rolling-6 accrual strategy.(12) Venetoclax was given orally once daily on days 1–28, initially at 240mg/m2 (max 400mg, dose levels 1 and 2b) and escalating to 360mg/m2 (max 600mg, dose levels 2a, 3, and 4). Conventional chemotherapy began on day 8, starting with intermediate-dose cytarabine (100mg/m2/dose IV every 12 hours for 20 doses) at dose levels 1 and 2a and escalating to high-dose cytarabine (1000mg/m2/dose IV every 12 hours for 8 doses) at dose levels 2b, 3, and 4. After dose level 1 was deemed tolerable, patients were enrolled in alternating fashion on dose levels 2a and 2b to evaluate the toxicity of increasing doses of either venetoclax (dose level 2a) or cytarabine (dose level 2b). When both dose levels were deemed tolerable, patients were enrolled on dose level 3. Dose level 4 (including idarubicin 12mg/m2 IV) was opened to patients with limited prior anthracycline exposure after dose level 3 was determined to be tolerated. At all dose levels, 50% of the planned venetoclax dose was given on day 1 to mitigate potential tumor lysis syndrome. For patients with disease progression during the venetoclax monotherapy window, conventional chemotherapy could be started earlier than day 8 at the treating physician’s discretion. Patients could receive up to four cycles or could proceed to alternative therapy including transplant after one cycle. Patients were admitted for monitoring during the first 2 days of therapy and then were monitored at least twice weekly with laboratory and clinical evaluations for toxicity until the completion of cycle 1.
Patients received triple intrathecal therapy with methotrexate, hydrocortisone, and cytarabine during diagnostic evaluation. Patients with evidence of CNS leukemia received weekly triple intrathecal therapy until clearance of leukemic blasts from the cerebrospinal fluid. Prophylactic antimicrobial therapy was given according to institutional guidelines and typically included levofloxacin and micafungin, with vancomycin added after administration of cytarabine. The use of azole antifungals and other potent inhibitors of CYP3A4 was prohibited during treatment with venetoclax.
Criteria for removing patients from therapy included the development of a dose-limiting toxicity, no response to therapy, relapse, second malignancy, and completion of protocol therapy and evaluation period.
Toxicities were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. Any grade 3 or higher non-hematologic event attributable to venetoclax was considered a DLT except for grade 3/4 infection; grade 3/4 nausea, vomiting, or diarrhea manageable with supportive care; or grade 3/4 electrolyte disturbance resolving to ≤ grade 2 within 7 days. Failure to recover an ANC >300/μl or platelet count of 30,000/μl by day 50 was also deemed a DLT unless attributable to underlying leukemia or infection.
Response was determined by bone marrow evaluation between days 35 and 49. In patients with hypocellular bone marrows at first evaluation, a repeat marrow was performed at the time of hematological recovery but not later than day 50. The last bone marrow evaluation performed was used to classify disease response. Complete response (CR) was defined as MRD <5% and hematological recovery (ANC ≥500/μl and platelets ≥75,000/μl), CRi as MRD <5% without hematological recovery, and partial response (PR) as MRD of 5–25% with a greater than 50% decrease in leukemic blast percentage. MRD negative status was defined by a level of <0.1%. In patients with extramedullary disease at study entry, complete resolution of extramedullary disease was required. For patients without an MRD evaluable immunophenotype, response was determined by bone marrow morphology. Overall response (ORR) was the sum of the CR, CRi, and PR. Minimal residual disease testing and response evaluations were performed in the Department of Pathology at St. Jude Children’s Research Hospital.
Response to single-agent venetoclax was assessed by comparing absolute peripheral blood leukemic blast counts on days 1 and 8 as determined by flow cytometric-based minimal residual disease (MRD) assay. In patients without evaluable peripheral blood MRD, single-agent response to venetoclax was evaluated using optional day 8 bone marrow evaluation (N=4) by comparing MRD in bone marrow before therapy and on day 8.
For BH3 profiling, cryopreserved blood or bone marrow cells were stained with leukemia-specific antibodies and permeabilized as described (Appendix page 1). BAD, HRK, and NOXA peptides were tested for induction of cytochrome c loss from the mitochondria of digitonin permeabilized leukemic blasts. Dependence on BCL-2, BCL-XL, and MCL-1 were determined by the relative release of cytochrome c due to mitochondrial outer membrane permeabilization in the presence of these agents as measured by multicolor flow-cytometry.
Blood samples were collected 2, 4, and 6 hours post-dose on days 1 and 2 and prior to dosing (0 hour) and 2, 4 and 6 hours after dosing on day 8 to characterize venetoclax pharmacokinetics. Venetoclax plasma concentrations were determined using liquid–liquid extraction and liquid chromatography with tandem mass spectrometric detection.(13) The lower limit of quantitation was 2.05 ng/mL. The pharmacokinetic parameters of venetoclax in plasma were estimated using noncompartmental methods in Phoenix® WinNonlin® version 6.4 (Certara USA, Inc., Princeton, NJ). Pharmacokinetic parameters estimated included the maximum plasma concentration (Cmax), the time to maximum concentration (Tmax), the area under the concentration-time curve (AUC) from time 0 to 6 hours post-dose (AUC6), and the AUC over the 24-hour dose interval (AUC24). The pre-dose (0 hour) concentration was used as the 24-hour post-dose concentration value at steady state on that same day in order to calculate AUC24. The apparent oral clearance (CL/F, where F is the bioavailability) of venetoclax was calculated by dividing the administered dose by AUC24. Dose-normalized exposures were calculated using the dose received.
Patient-reported quality of life and hope measures were administered at baseline prior to the start of treatment, during the first week of therapy, and after completion of one cycle of venetoclax therapy. Physical, emotional, and social health were assessed in patients aged 8–17 years directly and in those aged 5–7 years using parent proxies with the 37-item Patient Reported Outcomes Measurement Information System (PROMIS) Pediatric Profile.(14) Participants responded based on their experience during the past 7 days, and raw scores were converted into age-normalized T-scores in each domain. Hopefulness and optimism were assessed using the Herth Hope Index (HHI),(15) in adolescents and young adults (13 years and older) as well as their parents.(16)
Outcomes
The primary endpoint was the recommended phase 2 dose (RP2D) and was defined as the highest dose level at which six participants were treated with at most one experiencing a dose-limiting toxicity (DLT). The secondary endpoint of the study was overall response. Prespecified exploratory objectives included BH3 profiling, pharmacokinetics, and quality of life assessments.
Statistical analysis
Statistical analysis was performed using Stata 15.1 (StataCorp, College Station, USA) using data available on August 24, 2019. For the primary endpoint, the rolling-6 design resulted in a minimum possible sample size of 2 patients if the first two patients each experienced a DLT and a maximum sample size of 54 patients. Any participant who experienced a non-hematologic DLT during the first 35 days after taking the initial dose of venetoclax was considered evaluable for toxicity. Participants without DLTs who received at least 21 of the planned 28 doses of venetoclax and at least 80% of the planned chemotherapy were also considered evaluable for toxicity. Post-hoc analyses included estimation of overall survival, analysis of associations between response to single-agent venetoclax (including identification of an optimal threshold to define a “good” window response) and overall response to combination therapy, and description of severe infection. Overall survival was defined as the time from study enrollment to death, with living patients censored at last follow-up, and was estimated by the Kaplan-Meier method. The Fisher’s exact test was used to compare the proportion of patients who achieved CR/CRi among those who experienced good response to the window versus those with a poor response to the window therapy. For analysis of infection, sepsis requiring urgent intervention, severe sepsis, and septic shock were defined as previously described.(17, 18) Invasive fungal infection (IFI) was categorized according to EORTC consensus criteria with the addition of the category of suspected IFI, comprising diagnosed IFI with imaging findings consistent with IFI but not meeting EORTC criteria.(19)
Role of the funding source
The funding source had no role in the study design, collection, data analysis, data interpretation, or writing of the report. SEK, SBP, LW, and JER had access to the complete trial database. JER had the final responsibility to submit for publication.
Results
Between July 1, 2017 and July 2, 2019, 38 patients were enrolled, including 4 patients with primary refractory AML, 33 patients with relapsed AML, and 1 patient with relapsed mixed-phenotypic acute leukemia (Appendix page 2). The median follow-up time was 7.1 months (inter-quartile range 5.1–11.2). The 21 females and 17 males had a median age of 10 years (range, 3–22) at enrollment (Table 1). Patients with primary refractory AML therapy had received 2, 3, 4, or 5 prior lines of therapy (N=1 each). Previous transplant had been performed in 18 patients, of whom 8 had two prior transplants and 1 had three prior transplants. Twenty-one patients were treated for first relapse, 11 for second relapse, and 2 for third relapse. Prior attempts with salvage therapy occurred in 18 relapsed patients, 9 of whom had failed at least 2 prior salvage attempts. Median marrow MRD level at time of study entry was 32.7%.
Table 1:
Baseline patient characteristics
| Total Patients | N= 38 |
|---|---|
| Age: median (range) | 10 (3–22) |
| Female | 21 (55%) |
| Prior CR | |
| • 0 | 4 (10.5%) |
| • 1 | 21 (55.3%) |
| • 2+ | 13 (34.2%) |
| Prior therapies since last CR | |
| • 0 | 16 (42.1%) |
| • 1 | 9 (23.7%) |
| • 2 | 7 (18.4%) |
| • 3+ | 6 (15.8%) |
| Prior Transplant | |
| • 0 | 20 (52.6%) |
| • 1 | 9 (23.7%) |
| • 2+ | 9 (23.7%) |
| Genetics: Favorable | |
| • RUNX1-RUNX1T1 | 4 (10.5%) |
| • CBFβ/MYH11 | 2 (5.3%) |
| • CEBPA mutations | 2 (5.3%) |
| Genetics: Neutral | |
| • KMT2A rearrangements | 12 (31.6%) |
| Genetics: Adverse | |
| • FLT3 internal tandem duplication | 3 (7.9%) |
| • FLT3 point mutations | 2 (5.3%) |
| • TP53 | 4 (10.5%) |
| • WT1 | 5 (13.2%) |
| • 5q- | 2 (5.3%) |
| • Monosomy 7 | 1 (3.1%) |
| Bone marrow leukemic burden at study entry: median (IQR) | 33% (18–68%) |
Two patients did not receive combination therapy because of rapid disease progression (N=1) or infection precluding ongoing therapy (N=1) and were excluded from further analyses (Figure 1). Although the protocol allowed early initiation of therapy in patients with disease progression during the venetoclax window, disease-induced organ dysfunction precluded ongoing therapy after the day 1 (1/2 dose) venetoclax, precluding evaluation of treatment related toxicity or activity in the patient with disease progression. Patients received 1 cycle (N=30), 2 cycles (N=5), or 4 cycles (N=1) of treatment.
Figure 1:
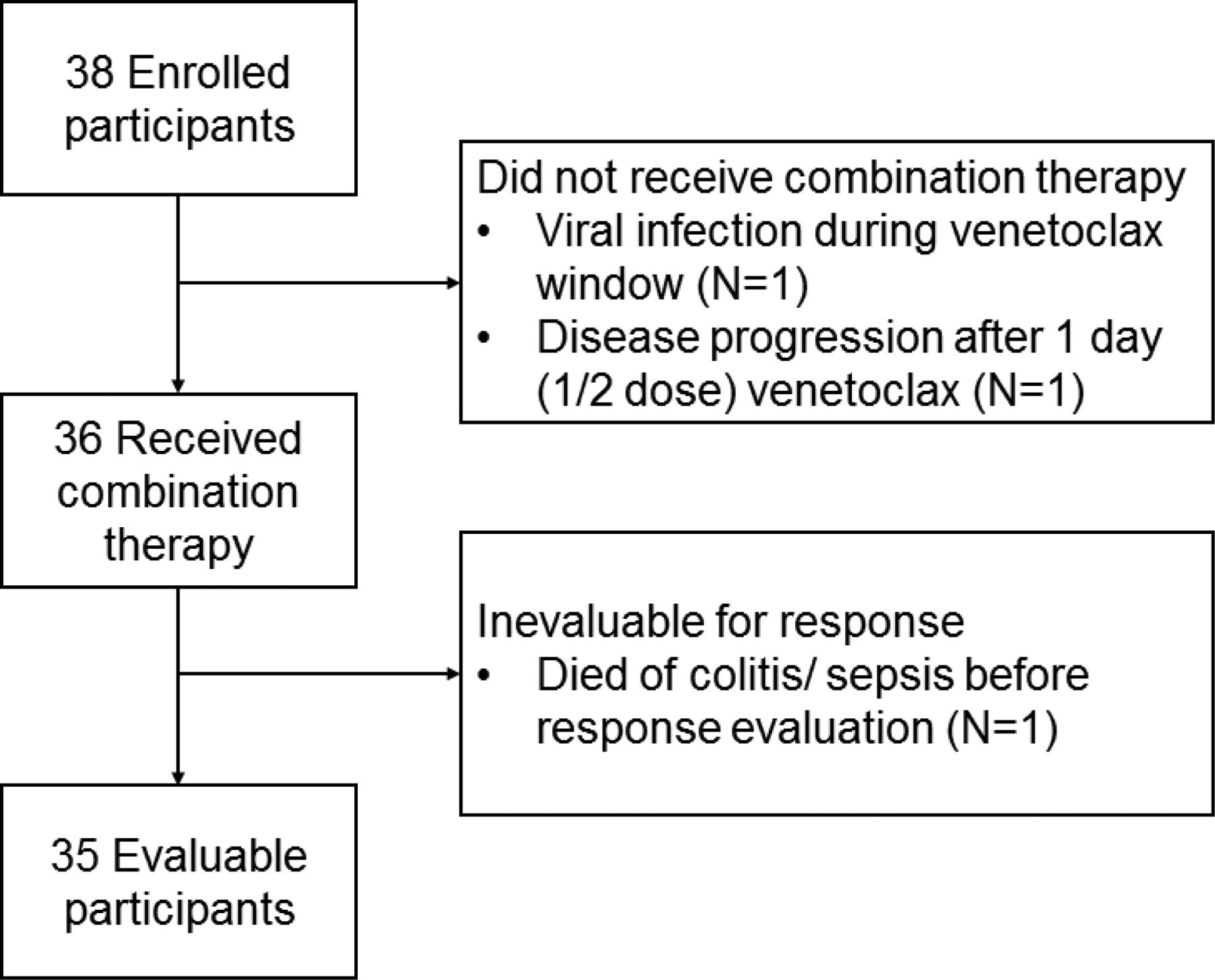
CONSORT diagram of enrolled and analyzed patients Of 38 enrolled participants, 3 were inevaluable for response. Two did not receive combination therapy either due to a viral infection during the venetoclax window (removed so as to avoid myelosuppressive therapy with an active infection) and a patient whose disease progression after the day 1 (1/2 dose venetoclax) precluded ongoing therapy (including the early introduction of chemotherapy as allowed in the protocol; N=1 each). Of 36 patients who received venetoclax and chemotherapy, 1 was inevaluable due to dying of toxicity prior to response evaluation and 35 were evaluable for response.
All dose levels were tolerated, with only one DLT observed (prolonged hematopoietic recovery in one patient at dose level 1). No patients required dose reduction or discontinuation due to venetoclax-related toxicity. There was one on-therapy death due to colitis/sepsis in a patient who had relapsed, was refractory to 3 prior courses of therapy, and had experienced colitis during his prior course of therapy. Venetoclax 360mg/m2 (max 600mg) combined with cytarabine 1000mg/m2/dose with or without idarubicin 12mg/m2 (dose levels 3 and 4) was the recommended phase 2 dose (RP2D; Table 2).
Table 2:
Dose escalation and response to cycle 1
| Dose level | Venetoclax | Cytarabine | Idarubicin | N | MRD neg | CR | CRi | PR | NR |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 240 mg/m2 (max 400 mg) | 100 mg/m2 q12h × 20 doses | 0 | 7 | 2 (29%) | 1 (14%) | 2 (29%) | 1 (14%) | 3 (43%) |
| 2a | 360 mg/m2 (max 600 mg) | 100 mg/m2 q12h × 20 doses | 0 | 5 | 0 | 0 | 1 (20%) | 1 (20%) | 3 (60%) |
| 2b | 240 mg/m2 (max 400 mg) | 1000 mg/m2 q12h × 8 doses | 0 | 3 | 1 (33%) | 2 (66%) | 0 | 0 | 1 (33%) |
| 3 | 360 mg/m2 (max 600 mg) | 1000 mg/m2 q12h × 8 doses | 0 | 11 | 7 (64%) | 7 (64%) | 1 (9%) | 1 (9%) | 2 (18%) |
| 4 | 360 mg/m2 (max 600 mg) | 1000 mg/m2 q12h × 8 doses | 12 mg/m2 | 9 | 3 (33%) | 6 (67%) | 0 | 1 (11%) | 2 (22%) |
N, evaluable patients; MRD neg, minimal residual disease negative (<0.1%) among patients obtaining a CR or CRi; CR, complete response; CRi, complete response with incomplete hematological recovery; PR, partial response; NR, no response
Note that MRD Negative patients are a subset of CR/CRi patients and not a discrete group.
Median time to hematological recovery (ANC> 300/μl, platelets >30/μl) in patients achieving a CR were 38 and 38.5 days, respectively. Patients experienced an average of 2.2 (range 0–8) grade 3 or higher toxicities during the first cycle of therapy, with infectious toxicities accounting for 65.7% of toxicities. Table 3 shows the toxicities that occurred during cycle 1. Serious adverse events occurred in 2 patients during cycle 1, including sepsis in 1 and the toxic death described above. Tumor lysis syndrome was observed in 1 patient with rapidly progressive disease during the venetoclax window and who was taken off-study after 1 dose of venetoclax due to disease-induced multisystem organ dysfunction prior to combination therapy. Febrile neutropenia was common, with 43 episodes in 25 (66%) study participants; 14 (33%) episodes were associated with clinically or microbiologically documented infection. There were 8 bloodstream infection episodes in 6 (16%) study participants during cycle 1 of therapy. Sepsis requiring urgent intervention occurred in 6/8 (75%), severe sepsis in 3/8 (38%), and septic shock in 2/8 episodes (25%). There were 6 episodes of IFI in 6 (16%) study participants, including 4 episodes of pulmonary infection and 2 of bloodstream or disseminated candidiasis; 3/6 episodes (50%) were probable or proven, with 2 episodes of possible and one suspected IFI.
Table 3:
Patients experiencing non-hematologic toxicities during cycle 1 of therapy
| Toxicity | Dose level | 1 | 2a | 2b | 3 | 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTCAE toxicity grade | 2 | 3 | 4 | 3 | 4 | 3 | 4 | 2 | 3 | 4 | 5 | 3 | 4 | |
| Gastrointestinal | ||||||||||||||
| Nausea/Vomiting | 1 (11%) | |||||||||||||
| Dental caries | 1 (11%) | |||||||||||||
| ALT increase | 1 (33%) | 1 (11%) | ||||||||||||
| AST increase | 1 (11%) | |||||||||||||
| Rectal hemorrhage | 1 (9%) | |||||||||||||
| Infections and infestations | ||||||||||||||
| Febrile Neutropenia | 4 (57%) | 2 (40%) | 3 (100%) | 6 (55%) | 7 (78%) | |||||||||
| Colitis or typhlitis | 1 (33%) | 2 (18%) | 2 (22%) | |||||||||||
| Lung or sinus infection | 1 (20%) | 1 (33%) | 2 (22%) | |||||||||||
| Mucosal | 1 (14%) | 3 (27%) | ||||||||||||
| Infections: other | 2 (29%) | 1 (33%) | 3 (27%) | 2 (22%) | ||||||||||
| Sepsis | 1 (9%) | 1 (11%) | ||||||||||||
| Multi-organ failure | 1 (9%) | |||||||||||||
| Metabolism and nutrition | ||||||||||||||
| Hyperglycemia | 1 (9%) | |||||||||||||
| Hyperkalemia | 1 (9%) | |||||||||||||
| Anorexia\ weight loss | 1 (14%) | 1 (11%) | ||||||||||||
| Hypokalemia | 1 (9%) | |||||||||||||
| Tumor lysis syndrome | 1 (9%) | |||||||||||||
| Respiratory | ||||||||||||||
| Respiratory failure | 1 (9%) | |||||||||||||
| Pulmonary edema | 1 (11%) | |||||||||||||
| Pulmonary hypertension | 1 (11%) | |||||||||||||
| Cardiac | ||||||||||||||
| Hypotension | 1 (11%) | |||||||||||||
| Hypertension | 1 (11%) | |||||||||||||
| Cardiac arrest | 1 (9%) | |||||||||||||
| Sinus tachycardia | 1 (9%) | |||||||||||||
| Other | ||||||||||||||
| Acute kidney injury | 1 (9%) | |||||||||||||
| Allergic reaction | 1 (14%) | |||||||||||||
| DIC | 1 (9%) | |||||||||||||
| Syncope | 1 (9%) | |||||||||||||
CTCAE: Common terminology criteria for adverse events version 4.03; ARDS: adult respiratory distress syndrome; DIC: disseminated intravascular coagulopathy. Listed toxicities include both those attributable to therapy and those deemed not attributable to therapy. The table includes all non-hematologic grade 3 or higher events and clinically significant grade 2 events occurring in at least 10% of the population. Grade 1 events were not recorded within the research database.
Response to cycle 1 was evaluable in 35 patients, with 16 (46%) achieving CR (11 MRD negative), 4 (11%) CRi (2 MRD negative), 4 (11%) PR, and 11 (31%) with no response (Table 2, Appendix page 5). One patient who received combination therapy but died from colitis/sepsis before response determination was inevaluable. Median MRD at the end of cycle 1 was 1.9%. Responses among the 12 evaluable patients who received intermediate-dose cytarabine (dose levels 1 and 2a) included 1 CR, 3 CRi, 2 PR, and 6 NR (CR/CRi 33%, ORR 50%). Responses in 23 patients who received high-dose cytarabine with or without idarubicin (dose levels 2b, 3, and 4) included 15 CR, 1 CRi, 2 PR, and 5 NR (CR/CRi 70%, ORR of 78%). Patients (N=20) treated at the RP2D had a CR/CRi of 70% (14 of 20; 95% confidence interval [95%CI] 46–88%) and an ORR of 80% (16 of 20; 95%CI 56–94%). Patients refractory to prior therapy (including 4 primary refractory and 18 relapsed/refractory patients) had an CR/CRi of 45% (6 CR, 4 CRi), with responses seen in patients refractory to 1 (N=6), 2 (N=3), and 4 (N=1) prior regimens. Durability of response was unable to be assessed, because 16 (80%) of the 20 patients who achieved a CR/CRi proceeded to allogeneic stem cell transplantation. All patients who were MRD negative after cycle 1 and proceeded to transplant remained MRD negative at the time of transplant. A post-hoc analysis of overall survival of study participants is shown in the Appendix (page 6). Death occurred off-therapy in 19 patients, all of which were due to progressive disease.
Response to the venetoclax window was evaluable in 33 patients who were also evaluable for end of cycle 1 response (Appendix page 5). Better response to the window was associated with achieving a CR/CRi at the end of cycle 1 (Kruskal-Wallis p=0.045). A receiver operating curve identified an optimal cutoff of at least a 50% reduction in blasts to identify patients likely to achieve a CR/CRi (73.5% correctly classified, sensitivity 84.2%, specificity 57%, Appendix page 7).
In post-hoc analyses, the proportion of patients who achieved CR/CRi among the 22 patients who experienced good response to the window (>50% blast reduction) was 73% (13 CR, 3 CRi; 95%CI 50–89%), in contrast to the 27% (3 CR; 95%CI 6–61%) in the 11 patients with a poor response to the window therapy (p=0.02 by Fisher’s exact test, Appendix pages 3 and 8). Patients with a good window response were MRD negative after cycle 1 in 10/22 vs. 2/11 in those with poor response (p=0.2). This trend was maintained when the analysis was restricted to patients treated at the RP2D: the CR/CRi was 86% (11 CR, 1 CRi, 9 MRD negative; 95%CI 57–98%) in the 14 patients with a good response and 40% (2 CR, 1 MRD negative; 95%CI 5–85%) in the 5 patients with a poor response (p=0.08 for CR/CRi, and p=0.1 for MRD negative).
Recurrent genetic lesions in this relapsed population included FLT3 alterations (N=3 internal tandem duplications, N=2 activating mutations), KMT2A rearrangements (N=12), TP53 mutations (N=4), WT1 mutations (N=5), CBFβ/MYH11 (N=2) and RUNX1-RUNX1T1 (N=4) fusions, NF1 mutations (N=3), and biallelic CEBPA, PTPN11, DNM2, and NRAS mutations (N=2 each; Appendix pages 2 and 5). Among the 8 patients with favorable genetic alterations (CBFβ/MYH11, RUNX1-RUNX1T1, or CEBPA mutations), 6 obtained CR. Among the 12 patients with KMT2A rearrangements, 4 achieved a CR, 1 CRi, 1 PR, and 5 patients had no response; 1 patient with KMT2A rearrangement did not receive combination therapy because of multiorgan system failure due to progressive disease within window therapy. Responses in 4 patients with TP53 mutations included 2 CR, 1 CRi, and 1 PR. None of the 5 patients with FLT3 alterations responded.
BH3 dependency profiling prior to protocol therapy was available for 19 patients. BCL-2 dependence was observed in 9 patients. BCL-XL was the primary observed dependence in 5 patients, while 5 patients showed minimal or no dependence on any tested BH3 family member. No patients’ leukemia was primarily dependent on MCL-1, although 1 patient who was more dependent on BCL-XL or BCL-2 also showed some dependence on MCL-1.
BH3 profiles were associated with both window response to therapy as well as end of cycle 1 marrow response. Patients with BCL-2 dependence included 5 patients with CR and 1 with CRi (CR/CRi 67%), 1 PR, and 2 NR. Patients with BCL-2 dependence had a >50% reduction in circulating blasts after window therapy in 7 of the 9 evaluable cases. Of the 2 patients not responding at the end of the cycle, 1 showed a poor response to window therapy and profiling following cycle 1 demonstrated a switch to BCL-XL dependence. Patients with BCL-XL dependence included 1 CR, 1 CRi (CR/CRi 40%), 1 PR, and 2 NR. Window response was good in 1 of the 5 patients with BCL-XL dependence, with the single patient showing a good window response having secondary BCL-2 dependence in vitro. In contrast, none of the patients lacking secondary BCL-2 dependence achieved a CR. Patients with minimal or no in vitro BH3 dependence included 1 CR and 1 PR (both showing minimal response to BCL-2), and 3 NR. (Appendix page 3)
Pharmacokinetic data was available for 10 patients, including 6 patients on dose levels 3 and 4 patients on dose level 4. The median time to maximal venetoclax concentration was 6 hours with maximal concentrations increasing across each day measured: 0.7±0.7 μg/mL on day 1, 1.4±0.9 μg/mL on day 2, and 2.2±1.4 μg/mL on day 8. Pharmacokinetic parameters were similar across age groups; day 8 AUC24 was 21.9–34.3 μg•h/mL in 2 children under 6 years, 53.5±40.7 μg•h/mL in 5 children 7–12 years, and 41.9±19.7 μg•h/mL in 3 children ≥13 years old. Pharmacokinetic parameters were also similar across weight groups and with and without idarubicin (Appendix page 9).
Quality of life scores were within the average range for nearly all domains at baseline (Appendix Table 4), although mobility concerns were higher at baseline per parent report. At the On-Treatment (1-week) and Post-Treatment time points, all PROMIS domains were within normal limits for both parent proxy and self-report. Descriptive statistics suggested parents and adolescent patients endorsed hopefulness at all time points (Appendix page 3).
Discussion
To our knowledge, this is the first study of venetoclax in children and young adults with relapsed AML. We found that the combination of venetoclax and high-dose cytarabine with or without idarubicin was well-tolerated and active, with 14 (70%) of the 20 evaluable patients who were treated at the RP2D achieving CR or CRi after 1 cycle of therapy. Notably, 10 (71%) of these 14 patients were MRD negative. The ORR of patients treated at the RP2D (80%) was similar to that observed in the AML 2001/01 study (using FLAG ± liposomal daunorubicin) of patients in first relapse (75%) despite the greater prior therapy burden in our population. Additionally, the proportion of patients who achieved CR after 1 cycle of therapy at the RP2D (65%) was similar to that obtained after two cycles of intensive therapy in AML 2001/01 (59%).(20) This CR estimate also compared favorably to that reported in AAML1421 (utilizing CPX-351) after 1 cycle of therapy (57%).(21) Limited availability of patients for randomized relapsed AML trials makes direct prospective comparisons between retrieval therapies impractical. The hematologic parameters used to define count recovery in our study have been used by our group historically.(22) Although differences between these parameters and those used by other groups may result in slight differences in the definitions of response, the ability of patients to proceed to transplantation following attainment of a CR/CRi on therapy suggests that the definitions used within the trial adequately identified patients whose response enabled more definitive therapy.
Despite our patient population being heavily pretreated, the side effect profile of the combination was consistent with toxicity seen with intensive chemotherapy in this population and included febrile neutropenia in 63% of patients, similar to prior studies at our institution in patients with relapsed AML.(22, 23) One (2.6%) of 38 patients died due to infectious complications, a proportion similar to that seen in studies of patient with first relapse of AML.(20) In addition to the objective tolerability of the regimen, survey results from patient and parent indicated that the therapy did not adversely affect quality of life. Patients and their parents also maintained normal Herth Hope Index scores throughout the first cycle, suggesting that the adverse effects did not diminish either hope or quality of life.
Patients in this study suffered grade 3/4 infections at similar proportions to those seen in prior relapsed AML trials (28.9% vs. 19.4%).(20) The overall number of IFI was similar to other pediatric patients with relapsed leukemia, with 6 participants (16%) experiencing IFI.(24) However, the impact of each infection was high; 3 of 6 participants with IFI discontinued venetoclax therapy because of the infection. Notably, the prohibition on azole use during the study precluded both azole prophylaxis and treatment of IFI; the use of azoles in future studies (combined with lower doses of venetoclax due to interactions via cytochrome P450 3A4)(25) may address this issue.
Our data suggest that responses may be more frequent when venetoclax is combined with high-dose chemotherapy compared to intermediate-dose cytarabine. This is consistent with data from studies of venetoclax in adults with relapsed AML.(10) In that retrospective cohort, objective responses were seen in only 21% of patients treated with combinations of venetoclax and either hypomethylating agents or low-dose cytarabine. Our data are also consistent with preclinical data showing synergy between venetoclax, cytarabine, and idarubicin, with greater synergy observed with higher doses of chemotherapy.(26)
Despite overall encouraging responses, discreet subsets of patients experienced poor responses. For example, none of the 5 patients with FLT3 activation responded. This is consistent with prior reports in which FLT3 activated samples were resistant to venetoclax.(27) Combining venetoclax with FLT3 inhibitors is an attractive therapeutic approach for these patients.(28) Additional previously proposed mechanisms of resistance to venetoclax were also observed in our patients, with exclusively BCL-XL dependent samples having poor responses to therapy (1/4 CR/CRi) and one patient converting from BCL-2 dependence to BCL-XL dependence after one cycle of therapy. This aligns with preclinical data, in which resistance to BCL-2 inhibition often occurred through a transition to reliance on other BH3 family members.(7, 29, 30)
The body-surface-area (BSA)-based dosing of venetoclax in this study resulted in similar venetoclax concentrations across different age and weight groups. In addition, the venetoclax concentrations in this study were similar to those observed in the adult AML subjects.(9) This finding indicates that BSA-based dosing adequately accounts for developmental pharmacokinetics of venetoclax. Idarubicin showed no effect on venetoclax pharmacokinetics in this study.
Limitations of this study include the heterogeneous patient population in terms of prior therapy, genetics, and BH3 dependency, as well as the small number of patients treated at each dose level. Nevertheless, the results strongly suggest that the combination of venetoclax and chemotherapy should be tested in newly diagnosed patients with high-risk AML. Future studies should evaluate not only the activity of such combinations but should seek to validate leukemic blast reduction and BH3 dependencies as biomarkers of response to combination therapy.
Supplementary Material
Research in Context.
Evidence before this study
We searched PubMed for clinical reports published in English from inception until October 17, 2019. Search terms included “acute myeloid leukemia”, “venetoclax”, “BCL-2”, “ABT-199”, “clinical”, “pediatric”, “children”, and “patient”. We excluded pre-clinical studies and reviews. Published literature indicated venetoclax in combination with low-dose cytarabine (i.e. 20mg/m2/day) or the hypomethylating agents decitabine and azacitidine has activity in adults who were treatment naïve or had relapsed acute myeloid leukemia (AML). Prior reports of venetoclax in relapsed AML have been in retrospective cohorts treated outside of clinical trials. There were no published reports of the use of venetoclax alone or in combination with conventional AML regimens in pediatric patients.
Added value of this study
Our results indicate that the specific BCL-2 inhibitor venetoclax is safe and active in combination with conventional intensive chemotherapy in pediatric patients with relapsed AML. The combination of venetoclax with high-dose cytarabine with or without idarubicin demonstrated excellent activity in this heavily pretreated population, which are higher than published data using venetoclax in alternative combinations. These results also compare favorably with results from previous relapsed AML trials. To our knowledge, this is the first report of combination therapy with BCL-2 inhibition and intensive chemotherapy in patients with AML.
Implications of all available evidence
Results of this study support further evaluation of venetoclax in combination with intensive chemotherapy in patients with AML.
Acknowledgments
This study was funded by the Cancer Center Support grant P30 CA021765 from the National Institutes of Health, the American Lebanese Syrian Associated Charities (ALSAC), AbbVie Inc, and Gateway for Cancer Research. We thank Lauren Wheeler and Christie Daniels for data collection and management.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
SYK and TP are employed by AbbVie. Inc. AHS is employed by AbbVie and own stocks. JTO and JER received research funding from AbbVie, Inc and from Gateway for Cancer Research during the conduct of the study. JTO received additional funding from AbbVie outside the submitted work. TBA received travel funds from AbbVie. SEK, AB, SBP, KC, LW, JW, JMK, PEM, SDG, NJL, and CHP declare no competing interests.
Data sharing
Trial data will be posted on https://clinicaltrials.gov/ as required. Individual participant de-identified datasets containing the variables analyzed in the published article will be made available. Supporting documents such as the protocol, statistical analyses plan, and informed consent are available through the CTG website for the specific study. Data used to generate the published article will be made available at the time of article publication. Investigators who seek access to individual level de-identified data will contact the computing team in the Department of Biostatistics (ClinTrialDataRequest@stjude.org) who will respond to the data request.
Contributor Information
Seth E. Karol, Department of Oncology, St. Jude Children’s Research Hospital, Memphis, TN, USA
Thomas B. Alexander, Department of Pediatrics, University of North Carolina, Chapel Hill, NC, USA
Amit Budhraja, Department of Cell and Molecular Biology, St. Jude Children’s Research Hospital, Memphis, TN, USA.
Stanley B. Pounds, Department of Biostatistics, St. Jude Children’s Research Hospital, Memphis, TN, USA
Kristin Canavera, Department of Psychology, St. Jude Children’s Research Hospital, Memphis, TN, USA.
Lei Wang, Department of Biostatistics, St. Jude Children’s Research Hospital, Memphis, TN, USA.
Joshua Wolf, Department of Infectious Diseases, St. Jude Children’s Research Hospital, Memphis, TN, USA.
Jeffery M. Klco, Department of Pathology, St. Jude Children’s Research Hospital, Memphis, TN, USA
Paul E. Mead, Department of Pathology, St. Jude Children’s Research Hospital, Memphis, TN, USA
Soumyasri Das Gupta, Department of Cell and Molecular Biology, St. Jude Children’s Research Hospital, Memphis, TN, USA.
Su Y. Kim, Abbvie Inc, North Chicago, IL, USA
Ahmed H. Salem, Abbvie Inc, North Chicago, IL, USA Department of Clinical Pharmacy, Faculty of Pharmacy, Ain Shams University, Cairo, Egypt.
Tammy Palenski, Abbvie Inc, North Chicago, IL, USA.
Norman J. Lacayo, Department of Pediatrics, Stanford University, Palo Alto, CA, USA
Ching-Hon Pui, Department of Oncology, St. Jude Children’s Research Hospital, Memphis, TN, USA.
Joseph T. Opferman, Department of Cell and Molecular Biology, St. Jude Children’s Research Hospital, Memphis, TN, USA
Jeffrey E. Rubnitz, Department of Oncology, St. Jude Children’s Research Hospital, Memphis, TN, USA
References
- 1.Rasche M, Zimmermann M, Borschel L, Bourquin JP, Dworzak M, Klingebiel T, et al. Successes and challenges in the treatment of pediatric acute myeloid leukemia: a retrospective analysis of the AML-BFM trials from 1987 to 2012. Leukemia. 2018;32(10):2167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubnitz JE, Inaba H, Dahl G, Ribeiro RC, Bowman WP, Taub J, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010;11(6):543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gamis AS, Alonzo TA, Meshinchi S, Sung L, Gerbing RB, Raimondi SC, et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children’s Oncology Group trial AAML0531. J Clin Oncol. 2014;32(27):3021–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan RQ, Hogdal LJ, Benito JM, Bucci D, Han L, Borthakur G, et al. Selective BCL-2 Inhibition by ABT-199 Causes On-Target Cell Death in Acute Myeloid Leukemia. Cancer Discov. 2014;4(3):362–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konopleva M, Zhao S, Hu W, Jiang S, Snell V, Weidner D, et al. The anti-apoptotic genes Bcl-X(L) and Bcl-2 are over-expressed and contribute to chemoresistance of non-proliferating leukaemic CD34+ cells. Br J Haematol. 2002;118(2):521–34. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed N, Sammons J, Hassan HT. Bcl-2 protein in human myeloid leukaemia cells and its down-regulation during chemotherapy-induced apoptosis. Oncol Rep. 1999;6(2):403–7. [DOI] [PubMed] [Google Scholar]
- 7.Niu XJ, Zhao JY, Ma J, Xie CZ, Edwards H, Wang G, et al. Binding of Released Bim to Mcl-1 is a Mechanism of Intrinsic Resistance to ABT-199 which can be Overcome by Combination with Daunorubicin or Cytarabine in AML Cells. Clinical Cancer Research. 2016;22(17):4440–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiNardo CD, Pratz KW, Letai A, Jonas BA, Wei AH, Thirman M, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19(2):216–28. [DOI] [PubMed] [Google Scholar]
- 9.Wei AH, Strickland SA Jr., Hou JZ, Fiedler W, Lin TL, Walter RB, et al. Venetoclax Combined With Low-Dose Cytarabine for Previously Untreated Patients With Acute Myeloid Leukemia: Results From a Phase Ib/II Study. J Clin Oncol. 2019;37(15):1277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiNardo CD, Rausch CR, Benton C, Kadia T, Jain N, Pemmaraju N, et al. Clinical experience with the BCL2-inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. Am J Hematol. 2018;93(3):401–7. [DOI] [PubMed] [Google Scholar]
- 11.Aldoss I, Yang D, Aribi A, Ali H, Sandhu K, Al Malki MM, et al. Efficacy of the combination of venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Haematologica. 2018;103(9):e404–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skolnik JM, Barrett JS, Jayaraman B, Patel D, Adamson PC. Shortening the timeline of pediatric phase I trials: the rolling six design. J Clin Oncol. 2008;26(2):190–5. [DOI] [PubMed] [Google Scholar]
- 13.Salem AH, Hu BB, Freise KJ, Agarwal SK, Sidhu DS, Wong SL. Evaluation of the Pharmacokinetic Interaction between Venetoclax, a Selective BCL-2 Inhibitor, and Warfarin in Healthy Volunteers. Clin Drug Invest. 2017;37(3):303–9. [DOI] [PubMed] [Google Scholar]
- 14.Hinds PS, Nuss SL, Ruccione KS, Withycombe JS, Jacobs S, DeLuca H, et al. PROMIS pediatric measures in pediatric oncology: valid and clinically feasible indicators of patient-reported outcomes. Pediatric blood & cancer. 2013;60(3):402–8. [DOI] [PubMed] [Google Scholar]
- 15.Herth K. Abbreviated instrument to measure hope: development and psychometric evaluation. Journal of advanced nursing. 1992;17(10):1251–9. [DOI] [PubMed] [Google Scholar]
- 16.Phillips-Salimi CR, Haase JE, Kintner EK, Monahan PO, Azzouz F. Psychometric properties of the Herth Hope Index in adolescents and young adults with cancer. Journal of nursing measurement. 2007;15(1):3–23. [DOI] [PubMed] [Google Scholar]
- 17.Wolf J, Tang L, Flynn PM, Pui CH, Gaur AH, Sun Y, et al. Levofloxacin Prophylaxis During Induction Therapy for Pediatric Acute Lymphoblastic Leukemia. Clin Infect Dis. 2017;65(11):1790–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein B, Giroir B, Randolph A, International Consensus Conference on Pediatric S. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. [DOI] [PubMed] [Google Scholar]
- 19.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008;46(12):1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaspers GJ, Zimmermann M, Reinhardt D, Gibson BE, Tamminga RY, Aleinikova O, et al. Improved outcome in pediatric relapsed acute myeloid leukemia: results of a randomized trial on liposomal daunorubicin by the International BFM Study Group. J Clin Oncol. 2013;31(5):599–607. [DOI] [PubMed] [Google Scholar]
- 21.Cooper TM, Absalon M, Alonzo TA, Gerbing RB, Leger KJ, Hirsch BA, et al. AAML 1421, a phase I/II study of CPX-351 followed by fludarabine, cytarabine, and G-CSF (FLAG) for children with relapsed acute myeloid leukemia (AML): A Report from the Children’s Oncology Group. J Clin Oncol. 2019;37(15_suppl):10003-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alexander TB, Lacayo NJ, Choi JK, Ribeiro RC, Pui CH, Rubnitz JE. Phase I Study of Selinexor, a Selective Inhibitor of Nuclear Export, in Combination With Fludarabine and Cytarabine, in Pediatric Relapsed or Refractory Acute Leukemia. J Clin Oncol. 2016;34(34):4094–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inaba H, Rubnitz JE, Coustan-Smith E, Li L, Furmanski BD, Mascara GP, et al. Phase I pharmacokinetic and pharmacodynamic study of the multikinase inhibitor sorafenib in combination with clofarabine and cytarabine in pediatric relapsed/refractory leukemia. J Clin Oncol. 2011;29(24):3293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hale KA, Shaw PJ, Dalla-Pozza L, MacIntyre CR, Isaacs D, Sorrell TC. Epidemiology of paediatric invasive fungal infections and a case-control study of risk factors in acute leukaemia or post stem cell transplant. Br J Haematol. 2010;149(2):263–72. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal SK, Salem AH, Danilov AV, Hu B, Puvvada S, Gutierrez M, et al. Effect of ketoconazole, a strong CYP3A inhibitor, on the pharmacokinetics of venetoclax, a BCL-2 inhibitor, in patients with non-Hodgkin lymphoma. Br J Clin Pharmacol. 2017;83(4):846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teh TC, Nguyen NY, Moujalled DM, Segal D, Pomilio G, Rijal S, et al. Enhancing venetoclax activity in acute myeloid leukemia by co-targeting MCL1. Leukemia. 2018;32(2):303–12. [DOI] [PubMed] [Google Scholar]
- 27.Chyla B, Daver N, Doyle K, McKeegan E, Huang X, Ruvolo V, et al. Genetic Biomarkers Of Sensitivity and Resistance to Venetoclax Monotherapy in Patients With Relapsed Acute Myeloid Leukemia. Am J Hematol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma J, Zhao S, Qiao X, Knight T, Edwards H, Polin L, et al. Inhibition of Bcl-2 Synergistically Enhances the Antileukemic Activity of Midostaurin and Gilteritinib in Preclinical Models of FLT3-Mutated Acute Myeloid Leukemia. Clin Cancer Res. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramsey HE, Fischer MA, Lee T, Gorska AE, Arrate MP, Fuller L, et al. A Novel MCL1 Inhibitor Combined with Venetoclax Rescues Venetoclax-Resistant Acute Myelogenous Leukemia. Cancer Discov. 2018;8(12):1566–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konopleva M, Pollyea DA, Potluri J, Chyla B, Hogdal L, Busman T, et al. Efficacy and Biological Correlates of Response in a Phase II Study of Venetoclax Monotherapy in Patients with Acute Myelogenous Leukemia. Cancer Discov. 2016;6(10):1106–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


