Abstract
Objective: We sought to ascertain whether baseline anxiety/depression and oppositional defiant disorder (ODD) symptoms impacted the experience of short-term methylphenidate (MPH) adverse effects (AEs) in 7- to 11-year-old children with attention-deficit/hyperactivity disorder (ADHD) (n = 171) undergoing a double-blind MPH crossover trial.
Method: The Vanderbilt ADHD Diagnostic Parent Rating Scale measured baseline child anxiety/depression and ODD symptomology. The parent-completed Pittsburgh Side Effect Rating Scale assessed the AEs of anxiety, sadness, and irritability at baseline, on placebo, and on three MPH dosages. For each AE, we evaluated comorbidity main effects, dose main effects, and comorbidity × dose interactions.
Results: Baseline anxiety/depression × dose and ODD × dose interactions were significant for the AEs of anxiety, sadness, and irritability. Compared with premedication baseline, these AEs attenuated on MPH for children with initially higher comorbidity symptoms, whereas those with initially lower comorbidity symptoms tended toward no change or increasing AE levels.
Conclusion: Premedication anxiety/depressive and ODD symptoms may be important predictors of short-term MPH emotional AEs.
Keywords: ADHD, methylphenidate, adverse effects, side effects, stimulants
Introduction
Attention-deficit/hyperactivity disorder (ADHD) affects >5.4 million children in the United States (Danielson et al. 2018). Stimulants are first-line medications for ADHD treatment (Wolraich et al. 2011), and methylphenidate (MPH) is the most commonly prescribed ADHD treatment worldwide (Raman et al. 2018). MPH effectively decreases ADHD symptoms but can cause adverse effects (AEs)—including anxiety, sadness, irritability, headaches, stomachaches, loss of appetite, trouble sleeping, and social withdrawal (MTA 1999)—which can impact adherence (Brinkman et al. 2018). Identifying adverse event predictors could help caregivers and providers to identify the most tolerable ADHD medication, facilitate the medication titration process, and eliminate patient discomfort.
Past research has investigated whether a variety of physical and clinical factors predict MPH AEs. Most studies of school age children have found that physical factors such as age, gender, height, and weight do not predict stimulant AEs (Sonuga-Barke et al. 2009; Ogrim et al. 2013). ADHD subtype also does not appear to predict MPH AEs (Ogrim et al. 2013).
Presence of coexisting psychological disorders is another factor that has been examined as an MPH AE predictor with mixed results. Two of the most common ADHD mental health comorbidities are anxiety (estimated to be present in 25%–35% of pediatric ADHD patients) (Barkley 2006) and oppositional defiant disorder (ODD, estimated to be present in >40% of pediatric ADHD patients) (Barkley 2006). Numerous studies have failed to find a relationship between comorbid anxiety or ODD and stimulant AEs (Diamond et al. 1999; Greenhill et al. 2001; Sonuga-Barke et al. 2009; Gadow and Nolan 2011; Ogrim et al. 2013). Karabekiroglu et al. (2008), however, compared children who experienced an increase in stimulant AEs with those who did not and found that children experiencing AEs had higher rates of a range of comorbid mental health diagnoses. In contrast, two studies by Golubchik et al. (2014a, 2014b) suggested an association between MPH treatment and reduced anxiety in children with comorbid ADHD and anxiety disorders. Similarly, two additional studies that accounted for baseline symptomatology found reduced irritability and/or anxiety with MPH treatment (Efron et al. 1997; Gurkan et al. 2010).
Methodological complexities make examining the relationship between ADHD comorbidities and stimulant AEs challenging, and may explain the inconsistent results across studies. For example, many stimulant “AEs” (e.g., sleep problems) are actually present in children with ADHD before medication initiation. This is especially true among children with comorbid anxiety and ODD, where the putative “side effects” of anxiety and irritability, respectively, are elevated at premedication baseline. However, many studies have not accounted for baseline manifestations of AE behaviors (Diamond et al. 1999). Among studies that did assess change in anxiety between premedication baseline and MPH treatment conditions, methodological limitations include lack of placebo control (Efron et al. 1997; Gurkan et al. 2010; Golubchik et al. 2014a, 2014b), leading to concerns that parent AE ratings may be confounded by expectancy effects. Furthermore, since many studies have not enrolled stimulant-naive children, measuring baseline AE levels may not be possible or might not accurately reflect symptom presence when unmedicated (Greenhill et al. 2001; Sonuga-Barke et al. 2009).
Our study objective was to ascertain whether having varying degrees of baseline comorbid mental health symptoms impact the experience of specific stimulant AEs among stimulant-naive children. We do so in a double blind placebo-controlled randomized trial by examining both the presence of comorbidities and experience of AEs continuously rather than dichotomously, which allows examination of the effects of subthreshold symptom levels and maximizes statistical power. Our primary hypotheses concern the AEs most related to each comorbidity. We hypothesized that higher premedication levels of comorbid anxiety and depression will be linked to higher on-medication anxiety and sadness ratings, whereas higher premedication ODD levels will be associated with higher on-medication irritability ratings. However, we predicted that after accounting for premedication AE levels, these AEs would no longer appear medication related.
Methods
Participants
We recruited stimulant-naive children aged 7–11 years from the community and local schools for an ambulatory clinic study examining stimulant medication response between 9/2006 and 6/2013, when the study fulfilled its recruitment goals. As per the Institutional Review Board-approved protocol, all parents/caregivers and participants gave written and informed consent/assent.
Participants were required to meet ADHD Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (American Psychiatric Association 1994) criteria for onset age, pervasiveness, and impairment based on the Diagnostic Interview Schedule for Children-Parent version (DISC-P) (Shaffer et al. 2000) and the Vanderbilt ADHD diagnostic teacher rating scale (Wolraich et al. 1998). Similar to algorithms used in the Multimodal Treatment Study of Children with ADHD (MTA 1999), parent and teacher must have reported six nonoverlapping DSM-IV symptoms in a symptom domain and both parent and teacher each reported ≥4 symptoms in that domain to meet ADHD criteria. A clinician (pediatrician or psychologist) also interviewed families and rendered a Clinical Global Impression (Guy 1976) functional severity rating of at least “moderately ill.”
We used the DISC-P to evaluate for psychiatric comorbidities and excluded participants with mania/hypomania. Comorbid depression, anxiety, oppositional defiant, and conduct disorders were allowed unless judged to be the principal cause of ADHD symptoms or requiring different treatment. Children whose medical history indicated significant brain injury were excluded, as were children with Wechsler Abbreviated Scale of Intelligence (Wechsler 1999) IQ <80 and Wechsler Individual Achievement Test—Second Edition (Wechsler 2001) word reading and numerical operations subtest scores <80.
Among the 194 children who met all study inclusion criteria, 171 participated in the medication trial and had data included in the analyses (see CONSORT Flow Diagram in Supplementary Fig. S1). Nonparticipants differed from participants on race/ethnicity (i.e., smaller proportion of non-Hispanic whites; p = 0.007) and IQ score (i.e., mean IQ = 99 vs. 107; p = 0.04), but these groups did not vary based on gender, comorbid mental health conditions, parent or teacher ADHD symptom scores, and academic achievement scores (all ps > 0.20). Among participants, mean age was 8.4 years (standard deviation [SD] 1.3). Participants were primarily boys (71%) and non-Hispanic white (81% vs. 16% African American, Hispanic, or other race).
Study data were drawn from a medication trial that oversampled ADHD-predominantly inattentive (ADHD-I) presentation (n = 126 vs. n = 45 for combined presentation) to examine medication response in children with ADHD-I specifically as this is the most common subtype in population-based studies (Froehlich et al. 2007). On the DISC-P 26% of participants met criteria for a disruptive behavior disorder, whereas 32% met criteria for any anxiety disorder (i.e., social phobia, simple phobia, separation anxiety, panic agoraphobia, generalized anxiety disorder, obsessive compulsive disorder, and/or posttraumatic stress disorder) and 1% met criteria for a major depressive episode or dysthymia. See Supplementary Table S1 for sample demographic and clinical characteristics.
Medication trial
Subjects participated in a 4-week double-blind crossover trial of long-acting osmotic-release oral system MPH. A computer-generated list was used to randomize participants equally to one of six dosing schedules consisting of three active dosage weeks [18, 27, or 36 mg for children ≤25 kg; 18, 36, or 54 mg for children >25 kg; sample mean maximum dose = 1.57 mg/(kg·day)] and 1 week of placebo [dosing schedules listed in prior publication (Froehlich et al. 2011)]. Study pills were identical capsules filled with either an inert white powder (placebo) or the prescribed dose of MPH overencapsulated for blinding. An investigational pharmacist (who was kept independent of participants and study staff) conducted the randomization and intervention allocation to ensure that participant families and study staff were blind to each child's intervention allocation.
Measures
Vanderbilt ADHD Diagnostic Parent Rating Scale
At premedication baseline, parents completed the Vanderbilt ADHD Diagnostic Parent Rating Scale (VADPRS) to measure our primary predictors (e.g., baseline anxiety/depression and oppositional-defiant symptomatology) using the anxiety/depression and ODD comorbidity screening scales, both of which have adequate reliability, factor structure, and concurrent validity (Wolraich et al. 2003). On these comorbidity scales, each item is rated on a 4-point scale to indicate how frequently it occurs (0 = never, 1 = occasionally, 2 = often, and 3 = very often). We summed responses from the eight ODD screening scale items to create an ODD total score (mean = 7.95 [SD 6.7], range 0–24) and the seven anxiety/depression items to create an anxiety/depression score (mean = 5.25 [SD 4.7], range 0–21). Our use of continuous comorbidity scores, as opposed to dichotomous diagnostic status, allowed for a more nuanced examination of the relationship between these common comorbidities and AEs.
Pittsburgh Side Effects Rating Scale
Our primary outcome measure was the Pittsburgh Side Effects Rating Scale (PSERS), a frequently used measure of pre-existing problems and medication-related AEs (Pelham 1993). On the PSERS, parents rated each AE on a 4-point scale indicating severity (0 = none, 1 = mild, 2 = moderate, and 3 = severe) at premedication baseline as well as at the end of each medication trial week. We utilized parent AE ratings since parents are best positioned to observe AEs (Lee et al. 2011).
Statistical analyses
Missing data ranged between 0.6% and 5.3% across predictors and dependent variables and were handled through multiple imputation in Mplus (Version 8) (Muthen and Muthen 2017). Mplus allows all available information (i.e., means, variances, and covariances) from dependent variables, and from both item-level (Mazza et al. 2015) and construct-level auxiliary correlates of missing data (Graham 2003), to be included in alternative hypothesis multiple imputation models (Muthen and Muthen 2017). Specifically, ODD, anxiety, and depression items and total scores from the VADPRS and DISC-P were included in the imputation model to help make the implicit MAR assumption more plausible. Mplus can also account for the preponderance of zero responses in the AE item Likert-scale ratings (i.e., “censored from below” or a “floor effect”) by accounting for censoring in the imputation. A total of 100 imputed data sets were created.
Because Mplus has no analytic equivalent to a fixed-level within-subjects (i.e., baseline [BL], placebo [PL], and high dose MPH [HD] conditions) design, the imputed data sets were imported into SAS (version 9.4). Owing to evidence of a significant dose–response relationship for MPH side effects, with effects accentuated at the highest dose in prior studies (Greenhill et al. 2001) as well in the present sample, we chose to focus our analytic models and figures on the high dose condition.
Generalized estimating equations (GEE; SAS “Proc Genmod” with a “/repeated” statement to address the correlations between within-subjects factors) were used to model our primary analyses (i.e., effect of baseline anxiety/depression on anxiety and sadness AEs; effect of baseline ODD on the AE irritability), as well as secondary analyses. Research shows that log-transformations of Likert-scale response data, combined with maximum likelihood estimation (in GEE), provide accurate parameter estimates (Duan 1983; Olsen and Schafer 2001; Muthen and Muthen 2017). Two GEE model sets were conducted: one for comorbid anxiety/depression and another for comorbid ODD. In each model, comorbidity, dose (BL, PL, and HD), and their interaction were analyzed as predictors. To facilitate interaction interpretation, the “dose” within-subjects factor (BL, PL, and HD) was centered such that zero represented baseline, and both anxiety/depression and ODD scores were grand-mean centered (Enders and Tofighi 2007) such that zero represented sample mean levels of both disorders. Each AE was analyzed separately. All GEE results were pooled across imputed data sets using Proc MIAnalyze in SAS (Gantz 2006).
With three dose levels (BL, PL, and HD), a quadratic effect of dose was possible and examined, along with a quadratic “dose × comorbidity” (dose2 × comorbidity) interaction. The GEE model main effects can be interpreted as follows: a positive main effect of comorbidity signifies that increasing comorbidity symptoms are associated with higher AEs scores, a positive main effect of dose signifies that there is a linear increase in AEs across baseline, placebo, and high dose conditions, and a positive dose2 (quadratic dose) main effect signifies that there is a nonlinear increase in side effects across baseline, placebo, and high dose conditions. Of note, however, when significant interaction effects are present, main effects cannot be interpreted in isolation: rather, the beta weights for the individual main effects (i.e., both comorbidity main effect and dose main effect) must be considered in conjunction with the beta weights for the higher-order (comorbidity × dose) interaction effect to determine the summary effect.
Given that both linear and quadratic dose main effects and interaction effects were evaluated, only the most parsimonious GEE model for each AE was retained: (1) if the quadratic “dose × comorbidity” interaction was significant, that model was retained; (2) if the quadratic “dose × comorbidity” interaction was not significant, but the quadratic dose main effect was significant, only the quadratic dose main effect remained in the model, (3) if neither the quadratic “dose × comorbidity” interaction effect nor the quadratic dose main effect were significant, neither was retained in the GEE and a linear “dose × comorbidity” interaction model was interpreted.
To localize and interpret significant linear dose × comorbidity interactions, we ran post hoc models of comorbidity effects at each dose that are detailed in the “Interaction Deconstruction” columns in Tables 2 and 3. To assess dose × comorbidity interactions when there was a main dose effect and no higher-order interaction effects, we also ran post hoc analyses comparing pairwise conditions (i.e., BL vs. PL, BL vs. HD, and PL vs. HD), which are detailed in the “Dose Comparisons of Adverse Effect Scores” columns in Tables 2 and 3. To understand MPH AEs in children with different initial comorbidity severity profiles, we created figures using the general linear model estimates to demonstrate the hypothetical AE patterns in children with baseline comorbidity symptoms at very high (+2 SD), high (+1 SD), average (mean), and low (−1 SD) levels.
Table 2.
Effects of Baseline Anxiety/Depression Symptom Score, Dose, Dose2, and Their Interaction on Methylphenidate Adverse Effect Ratings (n = 171)
| |
Interaction deconstruction |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AE | Intercept | ANX/DEP main effect (β) | Dose main effect (β) | Dose2 main effecta(β) | Dose comparisons of AE scores | ANX/DEP × dose interaction (β) | ANX/DEP × dose2 interactiona(β) | Effect of ANX/DEP at baseline (β) | Effect of ANX/DEP on placebo (β) | Effect of ANX/DEP on high dose MPH (β) |
| Anxiety | 0.41b | 0.06b | −0.46b | 0.17b | NI | −0.07b | 0.03c | 0.06b | 0.02d | 0.01e |
| Sadness | 0.27b | 0.06b | −0.28b | 0.14b | NI | −0.08b | 0.02b | 0.06b | NS | NS |
| Social Withdrawal | 0.11b | 0.02b | −0.15c | 0.09b | NI | −0.01e | 0.02b | NS | NS | |
| Headaches | 0.26b | 0.03b | −0.29b | 0.14b | NI | −0.02b | 0.03b | NS | NS | |
| Stomachaches | 0.26b | 0.03c | −0.21c | 0.14b | NI | −0.01e | 0.03b | NS | NS | |
| Tiredness | 0.25b | 0.02d | −0.23c | 0.09d | NI | −0.01e | 0.02e | NS | NS | |
| Irritability | 0.38b | 0.04b | 0.01 | NI | −0.02d | 0.04b | 0.02e | NS | ||
| Trouble Sleeping | 0.11b | 0.01e | −0.07 | 0.13b | BL < PL < HD | −0.01 | NA | NA | NA | |
| Appetite | 0.04d | 0.01e | 0.09 | 0.09d | BL < PL < HD | −0.00 | NA | NA | NA | |
| Picking | 0.35b | 0.02e | −0.34b | 0.13b | BL > PL, PL = HD, BL > HD | −0.00 | NA | NA | NA | |
If the dose2 main effect and/or and comorbidity × dose2 interaction effect were not significant, they were not retained in the models.
p < 0.0001.
p < 0.001.
p < 0.01.
p < 0.05.
Findings from primary analyses are presented in boldface.
β, standardized beta coefficient; AE, adverse effect; ANX/DEP, anxiety/depression symptom score; BL, baseline condition; HD, high dose methylphenidate; MPH, methylphenidate; NA, not applicable due to the absence of significant interaction effects; NI, not interpretable due to higher-order significant interaction effect; NS, not significant; PL, placebo.
Table 3.
Effects of Baseline Oppositional Defiant Disorder Symptom Score, Dose, Dose2, and Their Interaction on Methylphenidate Adverse Effect Ratings (n = 171)
| |
Interaction deconstruction |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AE | Intercept | ODD main effect (β) | Dose main effect (β) | Dose2 main effecta(β) | Dose comparisons of AE scores | ODD × dose interaction (β) | ODD × dose2 interactiona(β) | Effect of ODD at baseline (β) | Effect of ODD on placebo (β) | Effect of ODD on high dose MPH (β) |
| Irritability | 0.38b | 0.04b | 0.01 | NI | −0.02b | 0.04b | 0.02c | NS | ||
| Anxiety | 0.41b | 0.03b | −0.46b | 0.17b | NI | −0.03d | 0.01e | 0.03b | NS | NS |
| Sadness | 0.27b | 0.04b | −0.28b | 0.14b | NI | −0.05b | 0.02b | 0.04b | NS | NS |
| Headaches | 0.26b | 0.02b | −0.29b | 0.14b | NI | −0.01b | 0.02b | NS | NS | |
| Stomachaches | 0.26d | 0.03b | −0.21c | 0.14b | NI | −0.01c | 0.02b | 0.01d | NS | |
| Tiredness | 0.25b | 0.01e | −0.23c | 0.09d | NI | −0.01e | 0.01d | NS | NS | |
| Social withdrawal | 0.11b | 0.01d | −0.15c | 0.09b | BL > PL, PL < HD, BL = HD | −0.00 | NA | NA | NA | |
| Trouble sleeping | 0.11b | 0.01 | −0.07 | 0.14b | BL < PL < HD | −0.01 | NA | NA | NA | |
| Appetite | 0.04d | 0.01e | 0.09 | 0.09d | BL < PL < HD | −0.00 | NA | NA | NA | |
| Picking | 0.35b | 0.01 | −0.34b | 0.13b | BL > PL, PL = HD, BL > HD | −0.00 | NA | NA | NA | |
If the dose2 main effect and/or and comorbidity × dose2 interaction effect were not significant, they were not retained in the models.
p < 0.0001.
p < 0.001.
p < 0.01.
p < 0.05.
Findings from primary analyses are presented in boldface.
β, standardized beta coefficient; AE, adverse effect; BL, baseline condition; HD, high dose methylphenidate; MPH, methylphenidate; NA, not applicable due to absence of significant interaction effects; NI, not interpretable due to higher-order significant interaction effect; NS, not significant; ODD, oppositional defiant disorder; PL, placebo.
Results
Table 1 lists AE frequencies during the baseline, placebo, and high dose weeks. Owing to their low frequencies, statistical models for tics and hallucinations failed to converge and they were dropped from all further analyses.
Table 1.
Frequencies of Each Parent-Rated Adverse Effect During Baseline, Placebo, and Methylphenidate High Dose Conditions (n = 171)
| |
Baseline |
Placebo |
High dose |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| None | Mild | Moderate | Severe | None | Mild | Moderate | Severe | None | Mild | Moderate | Severe | |
| Appetite | 159 (94%) | 7 (4%) | 1 (1%) | 1 (1%) | 125 (74%) | 31 (18%) | 10 (6%) | 4 (2%) | 61 (36%) | 57 (34%) | 35 (21%) | 14 (8%) |
| Trouble sleeping | 117 (69%) | 33 (19%) | 16 (9%) | 1 (1%) | 132 (78%) | 26 (15%) | 10 (6%) | 1 (1%) | 71 (42%) | 53 (31%) | 27 (16%) | 14 (8%) |
| Irritability | 91 (54%) | 46 (27%) | 29 (17%) | 2 (1%) | 103 (61%) | 41 (24%) | 21 (12%) | 5 (3%) | 87 (51%) | 51 (30%) | 17 (10%) | 9 (5%) |
| Stomachaches | 113 (66%) | 39 (23%) | 12 (7%) | 2 (1%) | 130 (76%) | 33 (19%) | 6 (4%) | 1 (1%) | 94 (55%) | 45 (26%) | 23 (14%) | 5 (3%) |
| Sadness | 116 (68%) | 33 (19%) | 16 (9%) | 3 (2%) | 146 (86%) | 15 (9%) | 9 (5%) | 0 (0%) | 119 (70%) | 30 (18%) | 12 (7%) | 4 (2%) |
| Picking | 101 (59%) | 42 (25%) | 20 (12%) | 5 (3%) | 142 (84%) | 21 (12%) | 6 (4%) | 1 (1%) | 131 (77%) | 22 (13%) | 11 (6%) | 2 (1%) |
| Social withdrawal | 143 (84%) | 22 (13%) | 3 (2%) | 0 (0%) | 157 (92%) | 11 (6%) | 1 (1%) | 0 (0%) | 134 (79%) | 21 (12%) | 11 (6%) | 1 (1%) |
| Anxiety | 84 (49%) | 57 (34%) | 23 (14%) | 3 (2%) | 114 (85%) | 21 (12%) | 5 (3%) | 0 (0%) | 134 (79%) | 20 (12%) | 11 (6%) | 1 (1%) |
| Tiredness | 143 (84%) | 18 (11%) | 5 (3%) | 0 (0%) | 146 (86%) | 18 (11%) | 6 (4%) | 0 (0%) | 134 (79%) | 25 (15%) | 6 (4%) | 2 (1%) |
| Headaches | 114 (67%) | 39 (23%) | 14 (8%) | 1 (1%) | 143 (84%) | 26 (15%) | 0 (0%) | 0 (0%) | 122 (72%) | 28 (16%) | 11 (6%) | 4 (2%) |
| Tics | 150 (88%) | 9 (5%) | 4 (2%) | 1 (1%) | 164 (96%) | 4 (2%) | 2 (1%) | 0 (0%) | 155 (91%) | 4 (2%) | 5 (3%) | 3 (2%) |
| Hallucinations | 165 (96%) | 2 (1%) | 0 (0%) | 0 (0%) | 168 (98%) | 1 (1%) | 1 (1%) | 0 (0%) | 160 (93%) | 3 (2%) | 1 (1%) | 2 (1%) |
Missing data ranged between 0% and 4%.
Primary analyses
Joint effects of baseline anxiety/depression symptoms and MPH dose on anxiety and sadness AEs
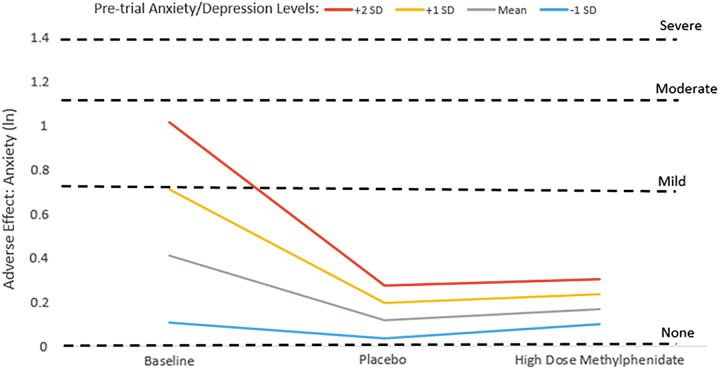
For the AE anxiety, there were significant baseline anxiety/depression × linear dose (ANX/DEP × dose) as well as anxiety/depression × nonlinear dose (ANX/DEP × dose2) interaction effects (Table 2) such that children with higher premedication anxiety/depression ratings continued to have higher anxiety ratings during baseline, placebo, and high dose conditions compared with children with lower premedication anxiety/depression levels (Table 2, Interaction Deconstruction). However, a pattern emerged whereby children with higher baseline anxiety/depression ratings appeared to have diminishing anxiety AE scores during placebo and high dose weeks compared with their own premedication ratings (Fig. 1). In contrast, children with lower baseline anxiety/depression symptoms seemed to experience little change in their anxiety levels across baseline, placebo, and high dose weeks (Fig. 1).
FIG. 1.
Anxiety adverse effect ratings during baseline, placebo, and high dose methylphenidate conditions by level of premedication anxiety/depression symptoms. ln, natural log of adverse effect rating, with dashed lines indicating the ln score that corresponds to adverse effect parent ratings of mild, moderate, and severe. SD, standard deviation.
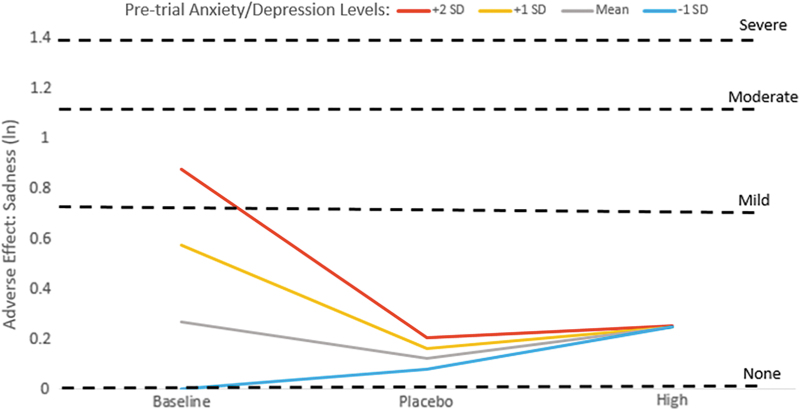
There were also significant baseline anxiety/depression × linear dose (ANX/DEP × dose) as well as anxiety/depression × nonlinear dose (ANX/DEP × dose2) interaction effects for the AE sadness (Table 2) such that children who had higher premedication levels of anxiety/depression appeared to have decreasing levels of sadness on placebo and high dose MPH compared with their own baselines (Fig. 2). However, sadness ratings did not seem to change markedly across baseline, placebo, and high dose conditions for children who had mean premedication anxiety/depression levels, whereas those with lower premedication anxiety/depression levels appeared to experience increased sadness on high dose MPH compared with baseline or placebo conditions (Fig. 2). The differential effects across groups was so striking that higher baseline anxiety/depression symptomatology did not predict higher sadness ratings on placebo or high dose MPH (Table 2, Interaction Deconstruction; Fig. 2, trajectories for all four groups converge on placebo and high dose MPH).
FIG. 2.
Sadness adverse effect ratings during baseline, placebo, and high dose methylphenidate conditions by level of premedication anxiety/depression symptoms. ln, natural log of adverse effect rating, with dashed lines indicating the ln score that corresponds to adverse effect parent ratings of mild, moderate, and severe. SD, standard deviation.
Joint effects of baseline ODD symptoms and MPH dose on the AE irritability
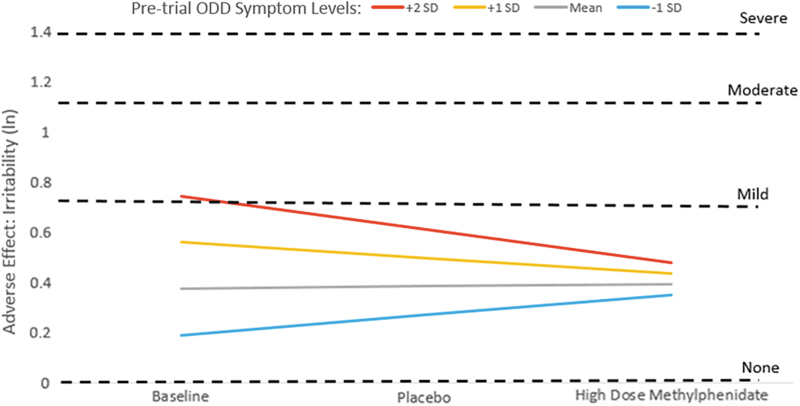
For the AE irritability, we observed a significant joint effect of baseline ODD symptoms and MPH dose for the linear dose (ODD × dose) but not the nonlinear dose (ODD × dose2) interaction models (Table 3) such that having higher premedication ODD symptoms predicted higher irritability levels at baseline and on placebo but not high dose MPH (Table 3, Interaction Deconstruction). This pattern appears to be due to an amelioration in irritability on high dose MPH (compared with premedication state) for children with higher baseline ODD symptoms (+1 or +2 SD above mean), whereas children with initially lower ODD symptom levels seemed to experience somewhat higher irritability ratings on high dose MPH compared with their baseline ratings (Fig. 3).
FIG. 3.
Irritability adverse effect ratings during baseline, placebo, and high dose methylphenidate conditions by level of premedication ODD symptoms. ln, natural log of adverse effect rating, with dashed lines indicating the ln score that corresponds to adverse effect parent ratings of mild, moderate, and severe. ODD, oppositional defiant disorder; SD, standard deviation.
Secondary analyses
Joint effects of baseline anxiety/depression symptoms and MPH dose on the AE irritability
We also observed a significant baseline anxiety/depression × linear dose (ANX/DEP × dose) interaction for the AE irritability: having higher premedication anxiety/depression symptoms predicted having higher irritability ratings at baseline and on placebo but not on high dose MPH (Table 2). In fact, children who had higher baseline anxiety/depression symptoms appeared to have less irritability on high dose MPH compared with their premedication state (Supplementary Fig. S2a), whereas children with lower baseline anxiety/depression seemed to increase somewhat in irritability from baseline to the high dose MPH condition (Supplementary Fig. S2a).
Joint effects of baseline ODD symptoms and MPH dose on anxiety and sadness AEs
Significant baseline ODD symptom × dose interactions were seen for both the linear and nonlinear dose (ODD × dose and ODD × dose2) models for the AEs anxiety and sadness (Table 3): having higher premedication oppositional-defiant symptom levels predicted having more anxiety and sadness at baseline but not on placebo or high dose MPH (Table 3, Interaction Deconstruction). In fact, children with more pretrial ODD symptoms seemed to diminish in anxiety and sadness on high dose MPH compared with their baseline (Supplementary Fig. S3a, b). However, children with initially lower oppositional symptom levels appeared little changed in their anxiety from baseline to high dose MPH (Supplementary Fig. S3a) and seemed to worsen in their sadness from baseline to high dose MPH (Supplementary Fig. S3b).
Joint effects of baseline comorbidity symptoms and MPH dose on other AEs
Tables 2 and 3 show the models predicting other AEs, with Supplementary Figures S2b–h and 3c–i showing AE trajectories for different initial comorbidity symptom levels.
One pattern—involving significant higher-order comorbidity rating by MPH dose interactions—emerged for these AEs: headaches, stomachaches, social withdrawal, and tiredness. Specifically, having higher baseline comorbidity ratings (i.e., anxiety/depression or ODD symptom ratings) predicted higher baseline ratings of headaches, stomachaches, social withdrawal, and tiredness. However, children with higher versus lower baseline comorbidity ratings did not differ in ratings for these AEs on high dose MPH. This effect appeared to be generally due to attenuating headache, stomachache, social withdrawal, and tiredness ratings on high dose MPH (compared with baseline ratings) for children with higher pretrial comorbidity symptoms, whereas those with initially lower comorbidity symptomatology tended to show increased ratings for these AEs on high dose MPH (compared with baseline ratings).
For the other AEs (appetite suppression, trouble sleeping, and picking), there were no significant interactions between baseline comorbidity ratings and MPH dose. However, for these outcomes, we did observe significant dose main effects that were similar across anxiety/depression and ODD models. For appetite suppression and trouble sleeping—the two most prevalent AEs—there were main dose effects such that appetite and sleep problem ratings were highest on high dose MPH, intermediate for placebo, and lowest at pretrial baseline. For the AE picking, children were rated as having fewer problems during the placebo and high dose conditions compared with their premedication baseline, with picking ratings being equivalent for the placebo and high dose conditions.
Main effects of baseline anxiety/depression were seen for the AEs appetite suppression, trouble sleeping, and picking, such that more premedication anxiety/depression symptoms predicted more appetite suppression, trouble sleeping, and picking behaviors across dose conditions. Main effects of baseline ODD symptoms were seen for the AE appetite problems (more baseline oppositionality predicted more appetite problems across dose conditions). However, we did not observe a main effect of premedication oppositionality on sleep problem or picking AE ratings.
Discussion
Despite both public perception and published studies (Storebo et al. 2018) finding that children with ADHD who take MPH are at risk for worsening anxiety, mood issues such as sadness, and irritability, we found that risk for these AEs may not be universal but appears to be moderated by premedication levels of anxiety, depression, and oppositional-defiant symptoms. In fact, we found that children with the higher levels of ODD symptoms at baseline demonstrated decreased irritability on high dose MPH and those with higher anxiety/depression symptoms at baseline showed attenuated anxiety and sadness on both placebo and high dose MPH. Conversely, children with initially lower comorbid symptom levels appeared to be at greater risk for these MPH AEs. Results such as these underscore the possibility of a personalized and predictive (rather than a “one size fits all”) approach to ADHD treatment planning.
By showing differential risk for MPH AEs by baseline anxiety/depression and oppositionality symptom levels, our findings help to bridge the disconnect between studies indicating worsening of emotionality with MPH, and those that do not. For example, a recent Cochrane Systematic Review of nonrandomized studies found that rates of anxiety, sadness, and irritability with MPH treatment were 18.4%, 16.8%, and 17.2%, respectively (Storebo et al. 2018), whereas a Cochrane Systematic Review of randomized controlled trials (RCTs) found no significant effect of MPH on the AEs of “worried or anxious” (risk ratio, RR [95% confidence interval, CI]: 1.37 [0.84–2.25]), “nervousness” (RR [95% CI]: 2.52 [0.82–7.76]), “sad, tearful, depressed” (RR [95% CI]: 1.41 [0.86–2.29]), or “irritability (RR [95% CI]: 1.11 [0.77–1.60]) (Storebo et al. 2016). Additional recent meta-analyses and individual studies found that MPH reduced risk of both anxiety (Gurkan et al. 2010; Golubchik et al. 2014a, 2014b; Coughlin et al. 2015; Snircova et al. 2016; Pozzi et al. 2018) and irritability (Efron et al. 1997; Sonuga-Barke et al. 2009; Stuckelman et al. 2017; Pozzi et al. 2018; Winters et al. 2018). Our findings illustrate that both scenarios can in fact be at play—MPH may worsen or improve these emotional symptoms, with the key to predicting direction of effect hinging upon baseline anxiety/depression and oppositionality levels.
Intriguingly, a similar pattern was documented in a placebo-controlled MPH crossover study of adults: participants with baseline anxiety in the higher range of “normal” experienced a reduction in state anxiety when treated with MPH, whereas those in the lower range of “normal” anxiety at baseline experienced the opposite effect (Segev et al. 2016). In addition, this explanation appears plausible after examining the relative levels of baseline anxiety and the pattern of findings observed in the two studies included in Coughlin et al.'s (2015) meta-analysis that detailed participants' premedication anxiety levels. In a sample of participants who had low baseline anxiety-related ratings (mean = 0.39 for “shy” and mean = 0.59 for “fearful,” with 0 = none and 3 = very much), Gittelman-Klein et al. (1976) found an elevated RR (95% CI) of 22.1 (1.3–364.0) for experiencing anxiety with MPH treatment. In contrast, in a sample with higher mean baseline anxiety ratings (Child Behavior Checklist internalizing disorder mean t score = 60.7), Stein et al. (1996) found a decrease in anxiety with MPH treatment (RR [95% CI]: 0.7 [0.5–1.0]).
It may be that MPH exerts these salutary effects on anxiety and irritability in individuals with higher baseline levels of these comorbidities due to its beneficial effects on emotional dysregulation (Kutlu et al. 2017; Moukhtarian et al. 2017; Winters et al. 2018). Other investigators have surmised that MPH's agonist activity at the serotonin type 1A receptor could explain its amelioration of anxiety when comorbid with ADHD (Faraone 2018).
In our secondary analyses, we found that the AEs of headaches, stomachaches, social withdrawal, and tiredness evinced baseline comorbidity × dose interactions that were similar to those demonstrated for our main outcomes (the AEs anxiety, sadness, and irritability). Specifically, headache, stomachache, social withdrawal, and tiredness ratings generally attenuated on high dose MPH (vs. pretrial) for those with higher baseline comorbidity symptoms levels, whereas those with initially lower comorbidity symptom ratings tended to show an increase in these AEs on high dose MPH (vs. pretrial). It is plausible that these effects are seen because these particular AEs may represent corollaries of mental health symptomatology (i.e., headaches and stomachaches may represent somatization symptoms; social withdrawal may be related to social anxiety; tiredness may be linked to depressed mood (2013)), which, similar to anxiety, sadness, and irritability, may be ameliorated by MPH treatment in those with high baseline mental health comorbidity symptoms.
Our study had many strengths, including the enrollment of stimulant-naive children that allowed for increased accuracy in measuring baseline levels of putative AEs. Furthermore, using a stimulant-naive sample may confer less risk for sampling bias, since studies allowing participants with prior stimulant experience are likely biased toward enrolling those for whom stimulants have been effective and well tolerated. Our use of a community-based rather than a specialty referral sample enhances the generalizability of study findings to primary care pediatric practices, although likely reduces applicability to psychiatric subspecialty practices.
Our RCT design is also a significant strength. In prior studies assessing effects of comorbidities on MPH AEs that lacked blinding and placebo control (Karabekiroglu et al. 2008; Ogrim et al. 2013), it is difficult to separate cases in which children actually experienced MPH AEs versus cases in which families' fears about the intervention being poorly tolerated increased sensitivity to reporting potential AEs. Our finding that parents rated children as having elevated difficulties with sleep and appetite suppression on placebo compared with their premedication baseline highlights the substantial biases that families bring to AE ratings and highlights the need for placebo control in medication tolerability studies.
In addition, we examined both our comorbidity predictors (anxiety/depression and ODD symptoms) and AE outcomes as continuous measures due to concerns about low numbers of children meeting full diagnostic criteria for depressive disorders, to allow examination of subthreshold symptoms, and to confer maximal analytic power. Many prior studies treated the comorbidity and/or AE constructs as dichotomous (present/absent) variables (Diamond et al. 1999; Greenhill et al. 2001; Ogrim et al. 2013) even though they are present on a continuum, limiting power, and perhaps helping to explain why we found a significant influence of comorbidity on MPH AEs, whereas these previous studies did not. In addition, studies that grouped the full range of AE together into a single outcome variable may have obscured the moderating effects of anxiety/depression and ODD (Karabekiroglu et al. 2008; Ogrim et al. 2013), since we observed different patterns of comorbidity × dose interactions for different side effects.
Our study also had several limitations. Specifically, our use of parent ratings to assess baseline anxiety/depression and ODD symptoms, rather than child self-report ratings, may have led to some underidentification of anxiety/depression if parents were unaware of their child's internalizing symptoms (Manassis et al. 2009). We also used Vanderbilt ADHD rating scale comorbidity items to assess anxiety/depression and ODD-related behaviors, rather than diagnostic measures for these disorders or measures with more extensive psychometric support. However, use of the Vanderbilt comorbidity items ensures that our findings are clinically meaningful in real-world practices where the Vanderbilt scale is used extensively. Furthermore, it should be noted that the Vanderbilt oppositional defiant, anxiety, and depression summary scores have been shown to be highly related to these comorbid diagnoses (Becker et al. 2012).
In addition, one of our ADHD diagnostic measures, the DISC-P, is limited in that it was developed primarily for epidemiological purposes. However, the DISC-P does have evidence of validity and reliability (Shaffer et al. 2000), and our ADHD diagnostic process additionally incorporated Vanderbilt ADHD diagnostic teacher rating scale results as well as a clinical interview with a PhD-level psychologist and/or pediatrician trained in ADHD care (who completed CGI ratings). A further limitation is that, as is common in clinical practice, we used single items to measure AEs. In addition, outcomes were measured through parent rather than teacher AE ratings, as there is some evidence that parents, who have a limited number of children to observe, may be more sensitive reporters of child AEs than teachers who oversee whole classrooms (Lee et al. 2011).
Because our participants tried each MPH dose for 1 week, it should also be noted that our findings only apply to short-term side effects. Although some AEs may have a delayed onset (Schachar et al. 1997), two recent studies comparing parallel and crossover ADHD medication RCTs provide some evidence that side effects in short- and longer-term MPH studies may not vary. Krogh et al.'s (2019) meta-analysis found no difference in RR for either serious or nonserious AEs between the parallel trials (the vast majority of which administered MPH for >4 weeks) and the crossover MPH RCTs (which, by and large, provided 1 week on each MPH dose as we did in our study). Similarly, Coughlin et al.'s (2015) meta-analysis reported no significant difference in the association of anxiety with psychostimulants in crossover studies compared with parallel-group studies, with all 11 included parallel group studies involving ≥3 weeks of active treatment and 10 of 12 crossover studies providing 1 week of treatment on each active stimulant dose. Moreover, documenting the experience of side effects soon after starting ADHD medication may be highly clinically salient, as these short-term side effects appear to exert an important influence on treatment adherence: Toomey et al. (2012) found that among families stopping ADHD medication treatment, the discontinuation most commonly occurred within the first month of treatment initiation, and AEs were the dominant reason given for discontinuation.
Conclusion
This study demonstrates that premedication levels of anxious/depressive or oppositional-defiant symptoms may be important predictors of short-term MPH AEs in school-age children with ADHD. Specifically, we found improvements in anxiety, sadness, and irritability with MPH initiation for children with higher levels of internalizing and oppositional symptoms at baseline. In contrast, children with lower premedication comorbidity symptom levels appeared more prone to emotional AEs.
Clinical Significance
Our findings underscore the importance of assessing for emotional comorbidity symptoms before starting MPH treatment, as baseline symptomatology may have important implications for ADHD medication management counseling. Although caregivers of children who have higher baseline anxiety, mood, and oppositional symptoms are often the most concerned about MPH emotional AEs, our results suggest that these families can be advised of possible improvement in emotional symptoms with MPH treatment.
Disclaimer
This article is solely the authors' responsibility and does not represent the official views of the NIMH (which had no role in study design, data collection, or data analysis).
Supplementary Material
Disclosures
Dr. Epstein has received research support from Akili Interactive Labs, received royalties from Multi-Health Systems, Inc., received consulting fees from the American Academy of Pediatrics and American Board of Pediatrics, and received licensing fees from Optimal Medicine, Inc. and IXICO. Dr. Froehlich, Dr. Brinkman, Dr. Peugh, Ms. Piedra, and Mr. Vitucci have no conflicts of interest or financial disclosures.
Supplementary Material
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV). Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- Barkley RA: Attention-Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment (3rd ed). New York (NY), The Guilford Press, 2006 [Google Scholar]
- Becker SP, Langberg JM, Vaughn AJ, Epstein JN: Clinical utility of the Vanderbilt ADHD diagnostic parent rating scale comorbidity screening scales. J Dev Behav Pediatr 33:221–228, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman WB, Simon JO, Epstein JN: Reasons why children and adolescents with attention-deficit/hyperactivity disorder stop and restart taking medicine. Acad Pediatr 18:273–280, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin CG, Cohen SC, Mulqueen JM, Ferracioli-Oda E, Stuckelman ZD, Bloch MH: Meta-analysis: reduced risk of anxiety with psychostimulant treatment in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 25:611–617, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson ML, Bitsko RH, Ghandour RM, Holbrook JR, Kogan MD, Blumberg SJ: Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. J Clin Child Adolesc Psychol 47:199–212, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond IR, Tannock R, Schachar RJ: Response to methylphenidate in children with ADHD and comorbid anxiety. J Am Acad Child Adolesc Psychiatry 38:402–409, 1999 [DOI] [PubMed] [Google Scholar]
- Duan N: Smearing estimate: A nonparametric retransformation method. J Am Stat Assoc 78:605–610, 1983 [Google Scholar]
- Efron D, Jarman F, Barker M: Side effects of methylphenidate and dexamphetamine in children with attention deficit hyperactivity disorder: A double-blind, crossover trial. Pediatrics 100:662–666, 1997 [DOI] [PubMed] [Google Scholar]
- Enders CK, Tofighi D: Centering predictor variables in cross-sectional multilevel models: A new look at an old issue. Psychol Methods 12:121–138, 2007 [DOI] [PubMed] [Google Scholar]
- Faraone SV: The pharmacology of amphetamine and methylphenidate: Relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev 87:255–270, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich TE, Epstein JN, Nick TG, Melguizo Castro MS, Stein MA, Brinkman WB, Graham AJ, Langberg JM, Kahn RS: Pharmacogenetic predictors of methylphenidate dose-response in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 50:1129–1139.e1122, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich TE, Lanphear BP, Epstein JN, Barbaresi WJ, Katusic SK, Kahn RS: Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of U.S. children. Arch Pediatr Adolesc Med 161:857–864, 2007 [DOI] [PubMed] [Google Scholar]
- Gadow KD, Nolan EE. Methylphenidate and comorbid anxiety disorder in children with both chronic multiple tic disorder and ADHD. J Atten Disord 15:246–256, 2011 [DOI] [PubMed] [Google Scholar]
- Gantz M: Combining PROC GENMOD Models with Multinomial Outcomes Using PROC MIANALYZE (Paper ST-12). SAS Global Forum, 2006
- Gittelman-Klein R, Klein DF, Katz S, Saraf K, Pollack E: Comparative effects of methylphenidate and thioridazine in hyperkinetic children. I. Clinical results. Arch Gen Psychiatry 33:1217–1231, 1976 [DOI] [PubMed] [Google Scholar]
- Golubchik P, Golubchik L, Sever JM, Weizman A: The beneficial effect of methylphenidate in ADHD with comorbid separation anxiety. Int Clin Psychopharmacol 29:274–278, 2014a [DOI] [PubMed] [Google Scholar]
- Golubchik P, Sever J, Weizman A: Methylphenidate treatment in children with attention deficit hyperactivity disorder and comorbid social phobia. Int Clin Psychopharmacol 29:212–215, 2014b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JW: Adding missing-data-relevant variables to FIML-based structural equation models. Struct Equ Modeling 10:80–100, 2003 [Google Scholar]
- Greenhill LL, Swanson JM, Vitiello B, Davies M, Clevenger W, Wu M, Arnold LE, Abikoff HB, Bukstein OG, Conners CK, Elliott GR, Hechtman L, Hinshaw SP, Hoza B, Jensen PS, Kraemer HC, March JS, Newcorn JH, Severe JB, Wells K, Wigal T: Impairment and deportment responses to different methylphenidate doses in children with ADHD: The MTA titration trial. J Am Acad Child Adolesc Psychiatry 40:180–187, 2001 [DOI] [PubMed] [Google Scholar]
- Gurkan K, Bilgic A, Turkoglu S, Kilic BG, Aysev A, Uslu R: Depression, anxiety and obsessive-compulsive symptoms and quality of life in children with attention-deficit hyperactivity disorder (ADHD) during three-month methylphenidate treatment. J Psychopharmacol 24:1810–1818, 2010 [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU Assessment Manual for Psychopharmacology, Revised. Bethesda, US Department of Health, Education, and Welfare, 1976 [Google Scholar]
- Karabekiroglu K, Yazgan YM, Dedeoglu C: Can we predict short-term side effects of methylphenidate immediate-release? Int J Psychiatry Clin Pract 12:48–54, 2008 [DOI] [PubMed] [Google Scholar]
- Krogh HB, Storebo OJ, Faltinsen E, Todorovac A, Ydedahl-Jensen E, Magnusson FL, Holmskov M, Gerner T, Gluud C, Simonsen E: Methodological advantages and disadvantages of parallel and crossover randomised clinical trials on methylphenidate for attention deficit hyperactivity disorder: A systematic review and meta-analyses. BMJ Open 9:e026478, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu A, Akyol Ardic U, Ercan ES: Effect of methylphenidate on emotional dysregulation in children with attention-deficit/hyperactivity disorder + oppositional defiant disorder/conduct disorder. J Clin Psychopharmacol 37:220–225, 2017 [DOI] [PubMed] [Google Scholar]
- Lee J, Grizenko N, Bhat V, Sengupta S, Polotskaia A, Joober R: Relation between therapeutic response and side effects induced by methylphenidate as observed by parents and teachers of children with ADHD. BMC Psychiatry 11:70, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manassis K, Tannock R, Monga S: Anxious by maternal—Versus self-report: Are they the same children? J Can Acad Child Adolesc Psychiatry 18:103–109, 2009 [PMC free article] [PubMed] [Google Scholar]
- Mazza GL, Enders CK, Ruehlman LS: Addressing item-level missing data: A comparison of proration and full information maximum likelihood estimation. Multivariate Behav Res 50:504–519, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moukhtarian TR, Cooper RE, Vassos E, Moran P, Asherson P: Effects of stimulants and atomoxetine on emotional lability in adults: A systematic review and meta-analysis. Eur Psychiatry 44:198–207, 2017 [DOI] [PubMed] [Google Scholar]
- MTA. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry 56:1073–1086, 1999 [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO: Mplus User's Guide (1998–2017), 8th ed. Los Angeles (CA), Muthen & Muthen, 2017 [Google Scholar]
- Ogrim G, Hestad KA, Brunner JF, Kropotov J: Predicting acute side effects of stimulant medication in pediatric attention deficit/hyperactivity disorder: Data from quantitative electroencephalography, event-related potentials, and a continuous-performance test. Neuropsychiatr Dis Treat 9:1301–1309, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen MK, Schafer JL: A two-part random-effects model for semicontinuous longitudinal data. J Am Stat Assoc 96:730–745, 2001 [Google Scholar]
- Pelham WE: Pharmacotherapy for children with attention deficit hyperactivity disorder. School Psychol Rev 22:199–227, 1993 [Google Scholar]
- Pozzi M, Carnovale C, Peeters G, Gentili M, Antoniazzi S, Radice S, Clementi E, Nobile M: Adverse drug events related to mood and emotion in paediatric patients treated for ADHD: A meta-analysis. J Affect Disord 238:161–178, 2018 [DOI] [PubMed] [Google Scholar]
- Raman SR, Man KKC, Bahmanyar S, Berard A, Bilder S, Boukhris T, Bushnell G, Crystal S, Furu K, KaoYang YH, Karlstad O, Kieler H, Kubota K, Lai EC, Martikainen JE, Maura G, Moore N, Montero D, Nakamura H, Neumann A, Pate V, Pottegard A, Pratt NL, Roughead EE, Macias Saint-Gerons D, Sturmer T, Su CC, Zoega H, Sturkenbroom M, Chan EW, Coghill D, Ip P, Wong ICK: Trends in attention-deficit hyperactivity disorder medication use: A retrospective observational study using population-based databases. Lancet Psychiatry 5:824–835, 2018 [DOI] [PubMed] [Google Scholar]
- Schachar RJ, Tannock R, Cunningham C, Corkum PV: Behavioral, situational, and temporal effects of treatment of ADHD with methylphenidate. J Am Acad Child Adolesc Psychiatry 36:754–763, 1997 [DOI] [PubMed] [Google Scholar]
- Segev A, Gvirts HZ, Strouse K, Mayseless N, Gelbard H, Lewis YD, Barnea Y, Feffer K, Shamay-Tsoory SG, Bloch Y: A possible effect of methylphenidate on state anxiety: A single dose, placebo controlled, crossover study in a control group. Psychiatry Res 241:232–235, 2016 [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME: NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry 39:28–38, 2000 [DOI] [PubMed] [Google Scholar]
- Snircova E, Marcincakova-Husarova V, Hrtanek I, Kulhan T, Ondrejka I, Nosalova G: Anxiety reduction on atomoxetine and methylphenidate medication in children with ADHD. Pediatr Int 58:476–481, 2016 [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Coghill D, Wigal T, DeBacker M, Swanson J: Adverse reactions to methylphenidate treatment for attention-deficit/hyperactivity disorder: Structure and associations with clinical characteristics and symptom control. J Child Adolesc Psychopharmacol 19:683–690, 2009 [DOI] [PubMed] [Google Scholar]
- Stein MA, Blondis TA, Schnitzler ER, O'Brien T, Fishkin J, Blackwell B, Szumowski E, Roizen NJ: Methylphenidate dosing: Twice daily versus three times daily. Pediatrics 98:748–756, 1996 [PubMed] [Google Scholar]
- Storebo OJ, Pedersen N, Ramstad E, Kielsholm ML, Nielsen SS, Krogh HB, Moreira-Maia CR, Magnusson FL, Holmskov M, Gerner T, Skoog M, Rosendal S, Groth C, Gillies D, Buch Rasmussen K, Gauci D, Zwi M, Kirubakaran R, Hakonsen SJ, Aagaard L, Simonsen E, Gluud C: Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents—Assessment of adverse events in non-randomised studies. Cochrane Database Syst Rev 5:CD012069, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storebo OJ, Simonsen E, Gluud C: Methylphenidate for attention-deficit/hyperactivity disorder in children and adolescents. JAMA 315:2009–2010, 2016 [DOI] [PubMed] [Google Scholar]
- Stuckelman ZD, Mulqueen JM, Ferracioli-Oda E, Cohen SC, Coughlin CG, Leckman JF, Bloch MH: Risk of irritability with psychostimulant treatment in children with ADHD: A meta-analysis. J Clin Psychiatry 78:e648–e655, 2017 [DOI] [PubMed] [Google Scholar]
- Toomey SL, Sox CM, Rusinak D, Finkelstein JA: Why do children with ADHD discontinue their medication? Clin Pediatr (Phila) 51:763–769, 2012 [DOI] [PubMed] [Google Scholar]
- Wechsler D: Wechsler Abbreviated Scale of Intelligence. San Antonio (TX), Psychological Corporation, 1999 [Google Scholar]
- Wechsler D: Wechsler Individual Achievement Test—Second Edition (WIAT-II). San Antonio (TX), Psychological Corporation, 2001 [Google Scholar]
- Winters DE, Fukui S, Leibenluft E, Hulvershorn LA: Improvements in irritability with open-label methylphenidate treatment in youth with comorbid attention deficit/hyperactivity disorder and disruptive mood dysregulation disorder. J Child Adolesc Psychopharmacol 28:298–305, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolraich M, Brown L, Brown RT, DuPaul G, Earls M, Feldman HM, Ganiats TG, Kaplanek B, Meyer B, Perrin J, Pierce K, Reiff M, Stein MT, Visser S: ADHD: Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 128:1007–1022, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolraich ML, Feurer ID, Hannah JN, Baumgaertel A, Pinnock TY: Obtaining systematic teacher reports of disruptive behavior disorders utilizing DSM-IV. J Abnorm Child Psychol 26:141–152, 1998 [DOI] [PubMed] [Google Scholar]
- Wolraich ML, Lambert W, Doffing MA, Bickman L, Simmons T, Worley K: Psychometric properties of the Vanderbilt ADHD diagnostic parent rating scale in a referred population. J Pediatr Psychol 28:559–567, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.