ABSTRACT
As an important second messenger in adipocytes, calcium ions (Ca2+) are essential in regulating various intracellular signalling pathways that control critical cellular functions. Calcium channels show selective permeability to Ca2+ and facilitate Ca2+ entry into the cytoplasm, which are normally located in the plasmatic and intracellular membranes. The increase of cytosolic Ca2+ modulates a variety of signalling pathways and results in the transcription of target genes that contribute to adipogenesis, a key cellular event includes proliferation and differentiation of adipocyte. In the past decades, the involvement of some Ca2+-permeable ion channels, such as Ca2+ release-activated Ca2+ channels, transient receptor potential channels, voltage-gated calcium channels and others, in adipogenesis has been extensively explored. In the present review, we provided a summary of the expression and contributions of these Ca2+-permeable channels in mediating Ca2+ influxes that drive adipogenesis. Moreover, we discussed their potentials as future therapeutic targets.
KEYWORDS: Calcium channels, calcium signalling, trp channels, adipogenesis, adipocyte differentiation, proliferation, obesity, body weight
Adipogenesis & obesity
Obesity is believed to be the result from an imbalance between energy intake and energy expenditure [1], and it is characterized by increased adipose tissue mass (fat deposition) that results from increased fat cell size (hypertrophy) and number (hyperplasia), suggesting that the main contributor to obesity is adipose tissue (Figure 1) [2]. Adipose tissue has been reported plays a fundamental role in the maintenance of energy homoeostasis and diverse biological processes, such as haematopoiesis, insulin sensitivity, vascular remodelling, immune response and metabolic derangements associated with obesity [3]. Two types of adipose tissue have been reported existing in humans and mammals, which are white adipose tissue (WAT) and brown adipose tissue (BAT), and function in the opposite way to maintain energy homoeostasis. WAT stores excess energy as triglyceride in lipid droplets, while BAT provides basal and inducible energy consumption by utilizes stored lipid droplets to generate heat in a process that is known as thermogenesis [4,5].
Figure 1.
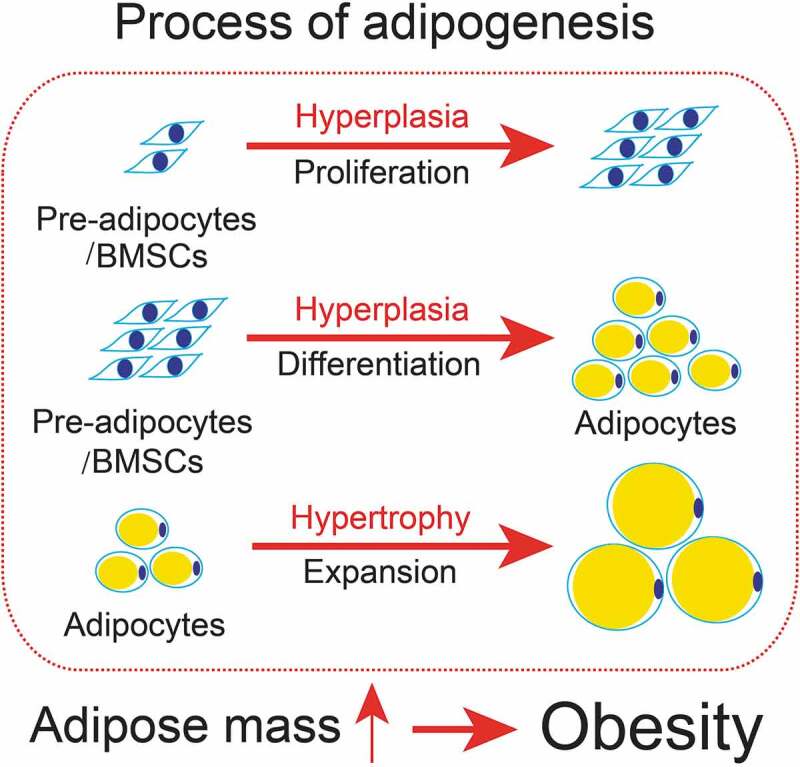
The schematic diagram of the process of adipogenesis
Adipocytes are derived from pre-adipocytes/bone marrow mesenchymal stem cells (BMSCs). The processes of adipogenesis, including two steps, hyperplasia (proliferation and differentiation, increased fat cell number) and hypertrophy (expansion, increased fat cell size). Upon adipogenesis, the increased adipose tissue mass (fat deposition) causes obesity.
Adipocytes are derived from mesenchymal precursor cells [6]. The processes of adipogenesis involve a variety of cellular processes (Figure 1), including division, proliferation, expansion, and differentiation from pre-adipocytes to mature adipocytes [7]. It has been reported that remarkable changes occur during adipocyte differentiation in both morphology and molecular levels. For instance, the fibroblast-like preadipocytes is morphologically rounded up and the elevated expression of mRNAs including lipoprotein lipase and several transcriptional factors, such as CCAAT-enhancer-binding protein β (C/EBPβ) and C/EBPδ, followed by the increased expression of C/EBPα and peroxisome proliferator-activated receptor γ (PPARγ), which upregulate the expression of genes that characterize the adipocyte phenotype [8]. During the process of adipogenesis, lipid-droplets start to be generated and appear in the cytoplasm, and over time, these droplets fuse into one or several large droplets. Adipogenesis has also been reported to be involved in various physiological functions. White adipose tissue (WAT) has been previously reported that plays important roles in insulation, mechanical support and as a major tissue type for energy storage [9]. Mature adipocytes have also been demonstrated to show hormonal and sympathetic functions [10]. BAT has been reported in adult humans to function as the main type of thermogenic tissue in response to generate heat to provide basal and inducible energy consumption [11,12]. Under positive energy balance, the excessive energy from food intake leads to the accumulation of triglycerides in WAT by lipogenic enzymes. By contrast, when under negative energy balance, lipases hydrolyse the excessive lipid drops into free fatty acids and/or glycerol which reserve as free energy circulating in the blood to muscle, liver, BAT and other tissues [13]. Excessive accumulation of triglycerides will induce obesity which affects normal adipocyte function and elevates the risk in the development of other metabolic disorders [14]. Therefore, investigating the underlying mechanisms in regulating adipogenesis is essential to understand and prevent obesity and related metabolic disorders.
Calcium signalling in adipogenesis
Calcium signalling plays a vital role in maintaining normal cellular functions, including proliferation, differentiation, homoeostasis, and apoptosis. The level of total body calcium is normally regulated. About 98% of total body calcium is stored in the skeleton as calcium phosphate. The calcium concentration of the extracellular fluid (ECF) is about 2.2–2.6 mmol/L in the form of total calcium, and about 1.3–1.5 mmol/L in the form of free calcium ions (Ca2+) [15]. The calcium concentration of intracellular fluid (ICF) is about 50–200 nmol/L, depending on the cell types, which is 20,000 to 100,000 times less than that in the ECF [16]. Therefore, the intracellular Ca2+ functions as an excellent messenger molecule that is widely involved in various cellular events, including generating action potentials, regulating enzyme activity, and bridging the extracellular and intracellular signal transduction [17].
Calcium channels play fundamental roles in regulating the concentration of cytosolic Ca2+, which could trigger a sudden increase of the cytosolic Ca2+ level up to 500–1000 nm/L. The cytosolic Ca2+ rises either by entering the cell through Ca2+-permeable ion channels on the plasma membrane, such as voltage-gated Ca2+ channels and transient receptor potential (TRP) channels and/or by release from the intracellular Ca2+ stores, such as the ER and mitochondria [18]. Once the action potentials generated or the signal transduction is completed, the cytosolic Ca2+ is then quickly removed from cytoplasm by various types of Ca2+ pumps, including the Ca2+-ATPase and Na+/Ca2+ exchanger on the plasma membrane pumping Ca2+ out of cells, and Sarco/endoplasmic reticulum Ca2+ pump (SERCA) on the ER membrane and the mitochondria calcium uniporter (MCU) on the mitochondria membrane, which pump Ca2+ back into the ER and mitochondria, respectively [19,20]. The timing and increasing level of cytosolic Ca2+ is precisely regulated by these proteins to balance the intracellular Ca2+ homoeostasis, which is fundamental for maintaining normal cell functions [17].
Calcium signalling is also a vital event in the process of adipogenesis, as it regulates various fundamental processes including proliferation, differentiation, energy metabolism and obesity. Cytosolic Ca2+ has been implicated in regulating adipocyte differentiation [21], which plays a key role in metabolic derangements associated with obesity in humans. Evidence have revealed that cytosolic Ca2+ concentrations are increased in 3T3-L1 pre-adipocytes after Ca2+-ATPase inhibitor treatment, such as thapsigargin, which in turn efficiently inhibits adipocyte differentiation, and impairs specific gene expression of adipocytes and reduces the accumulation of lipid drops [22,23]. These inhibitory effects could be also induced by either enhancing the activity of calcineurin, a Ca2+-dependent phosphatase [24] or by activation of calcineurin effectors, such as NFAT [25]. By contrast, cyclosporine A (CsA) treatment has been proved that inhibits calcineurin activity and increases adipocyte differentiation and lipid accumulation in 3T3-L1 pre-adipocytes [24]. These results are consistent with data that the treatment of CsA promotes obesity development in humans [26]. These results demonstrated that the elevation of cytosolic Ca2+ levels has negative effects on 3T3-L1 adipocyte differentiation similar to that seen in other cell types [27].
Extracellular Ca2+ is also involved in the modulation of adipogenesis. It has been reported that low extracellular Ca2+ promotes adipogenesis and high extracellular Ca2+ attenuates adipogenesis [28] in 3T3-L1 pre-adipocytes, which is consistent with the data observed in rats when feeding with dietary calcium [29,30]. Extracellular calcium modulates brown adipocyte differentiation as well. Low extracellular Ca2+ accelerates differentiation and high extracellular Ca2+ suppresses differentiation in mouse brown adipocytes [31]. Moreover, ionomycin-induced the increase of cytosolic Ca2+ enhances the proliferation of primary mouse bone marrow mesenchymal stem cells (BMSCs) but not differentiation [32]. And high extracellular Ca2+ promotes the proliferation of BMSCs via a calcium-sensing receptor and ERK signalling pathway [33]. However, several papers have reported that high extracellular Ca2+ enhances adipogenesis probably through L-type Cav channel [34] and calcium-sensing receptor [35] in porcine BMSCs. These contradictory data indicate the complicated mechanisms of calcium signalling in the regulation of adipogenesis. The mechanisms that govern the levels of intracellular Ca2+ involve membrane receptors, signalling molecules, and a diverse array of Ca2+ channels. In the next session, we summarized the calcium channels involved in regulating the concentration of cytosolic Ca2+ and adipogenesis.
Ca2+ release-activated calcium channels in adipogenesis
Ca2+ influx via store-operated Ca2+ channels (SOCs) in the plasma membrane provides increased cytosolic Ca2+ level and sustains the activity of several intracellular enzymes including calcineurin, which is critical for adipogenesis [36]. SOCs channels are activated by a mechanism critically dependent on the depletion of endoplasmic reticulum (ER) Ca2+ stores [37], which is widespread in adipocyte tissue [38]. Selective calcium release-activated Ca2+ (CRAC) channels are one type of SOCs. A well-described mechanism of extracellular Ca2+ entering adipocytes is through the CRAC channel, which is found by stromal interaction molecule 1 (STIM1) [37] and Orai1 proteins [39,40]. It is known that the CRAC channel contains two components: Orai1, the pore-forming subunit, which is situated in the plasma membrane and regulates Ca2+ entry, and STIM1, the regulatory subunit located in the ER membrane. STIM1 function as a Ca2+ sensing protein monitoring the fluctuation of Ca2+ levels in ER, which normally spread out in the ER membrane and oligomerize during the low level of Ca2+. Orai1 and STIM1 function together to induce Ca2+ influx from the extracellular space into intracellular fluid, which is termed store-operated Ca2+ entry (SOCE) [41].
The functional presence of STIM1 and ORAI1 has also been confirmed in many non-excitable cell types [38]. It has been reported that the up-regulation of STIM1 increases Ca2+ influx via CRAC channels and inhibits the 3T3-L1 adipocyte differentiation [42]. These results are consistent with the data by over-expression of STIM1 in 3T3-L1 pre-adipocytes, which increases Ca2+ influx and inhibits 3T3-L1 adipocyte differentiation, without affecting their proliferation and growth arrest. The over-expression of STIM1 also induces the down-regulation of adiponectin and C/EBPα. Besides, the down-regulation of endogenous STIM1 promotes 3T3-L1 adipocyte differentiation, resulting in the up-regulation of C/EBPα and adiponectin [42]. Another study has demonstrated that STIM and SOCE also play an important role in the adiposity of Drosophila [43]. Impairment of STIM1, the core component of SOCE, causes adiposity in Drosophila. Acute dysfunction of STIM1 in the fat storage tissue triggers hyperphagia in flies [43]. These studies suggested that CRAC channels are critically contributed to the Ca2+ influx in adipogenesis and obesity.
Transient receptor potential (TRP) channels in adipogenesis
Another major class of calcium-permeable channels is TRP family. TRP channels are normally non-selective to Ca2+ and sodium (Na+) permeation [44]. The structure of TRP channels is composed of six transmembrane (TM) domains, with both N-termini and C-termini located in the cytosol and a loop between TM5 and TM6 formed a pore for ion entry [45–49]. The TRP superfamily is further classified into TRPV (Vanilloid), TRPA (Ankyrin), TRPC (Canonical), TRPP (Polycystic), TRPM (Melastatin), TRPN (NomPC), and TRPML (Mucolipin), according to their primary amino acid sequences [50,51]. TRP channels are widely expressed and have a variety of physiological functions, such as detection of various mechanical and chemical stimuli in sensory transduction such as vision, hearing, olfaction, taste, touch, pain and thermosensation [52]. To date, accumulative evidence have shown that several TRP channels are involved in adipogenesis and function, suggesting that these TRP channels could be potential targets for human obesity treatment and prevention [53]. In the next session of the current review, we provided a brief introduction to the recent progress of TRP channels in adipogenesis and function.
TRPV family in adipogenesis
In terms of TRPV family, several TRPV channels have been reported to be expressed in adipocytes and play important roles in proliferation, differentiation, thermogenesis of adipocytes and obesity.
In the past years, TRPV1 has been studied for its involvement in adipocyte differentiation and energy metabolism, which further related to obesity management. As the first identified TRP channels, TRPV1 is activated by capsaicin, a highly selective agonist of TRPV1, or when the surrounding temperature is higher than 43°C. TRPV1 is expressed in 3T3-L1 preadipocytes and adipose tissue both in animals and humans. TRPV1 has also been reported to play a key role in the regulation of food intake and glucose homoeostasis in WAT during the development of obesity [54]. Activation of TRPV1 by dietary capsaicin treatment induces a significant increase of Ca2+ influx and impaired differentiation in 3T3-L1 pre-adipocytes [55], which is probably through the calcineurin pathway [56]. In mature adipocytes, downregulation of TRPV1 significantly reduces the calcium increase which is activated by capsaicin during adipogenesis [55]. Lacking TRPV1 exacerbates obesity and promotes insulin resistance, which is associated with diabetes and ageing [54]. However, dietary capsaicin treatment has also been reported to prevent high fat diet (HFD)-induced obesity in wild-type (WT) mice in vivo, but not in TRPV1 knockout mice [55,57]. Recently, TRPV1 has been shown to play a key role in regulating the browning of WAT, which could be a novel strategy to counteract obesity [58]. It has been reported that capsaicin increases intracellular Ca2+ level of adipocytes and promotes the browning of WAT. Moreover, activation of TRPV1 increases the expression level of thermogenic genes, such as UCP1, and induces the browning process in 3T3-L1 pre-adipocytes [59]. In BAT, TRPV1 activation is involved in the stimulation of metabolism and energy expenditure to protect against obesity [60]. Similarly, monoacylglycerol, another TRPV1 agonist, increases UCP1 expression in BAT and significantly reduces the mass of visceral fat in HFD-treatment mice [61]. However, knockout of TRPV1 prevents HFD-treatment-induced obesity [62] and obesity-induced hypertension [63]. Furthermore, the lack of TRPV1 promotes obesity and induces leptin and insulin resistance, which in turn, resulted in increased food intake and decreased physical activity [54]. These contradictory data indicate the complicated effects of TRPV1 in regulating obesity. Therefore, TRPV1 could be a potential target for obesity management and drug application.
TRPV3 often form functional heteromeric channels with TRPV1 [64], which also shows similar effects in regulating adipogenesis and obesity with TRPV1 [65]. TRPV3 could be primarily activated by a high noxious threshold which is over 50°C and then becomes responsive to warm temperatures [66]. It has been reported that the expression level of TRPV3 was decreased in visceral adipose tissue in HFD‐treatment mice, ob/ob and db/db mice [67], and also reduced in subcutaneous WAT and interscapular BAT in HFD-treated and db/db mice, which is similar to TRPV1 [68]. HFD feeding increases TRPV3 expression in the medial nucleus tractus solitarius (mNTS) and hypoglossal nucleus (HN) in rats, which is accompanied by a reduced expression of proopiomelanocortin (POMC) and resulted in increased food intake and a gain of body‐weight [69]. It has been reported that activation of TRPV3 prevented adipogenesis in 3T3-L1 preadipocytes and played an anti-adipogenic role in vivo [65]. Activation of TRPV3 by its agonist, such as diphenylborinic anhydride and (‐)‐epicatechin, prevents adipogenesis in 3T3‐L1 pre-adipocytes. Besides, chronic activation of TRPV3 prevented adipogenesis and weight gain in mice. However, the detailed role of the TRPV3-mediated Ca2+ influx in adipogenesis has not been fully understood.
TRPV2 and TRPV4 function as an osmo- and/or mechano-sensor, which could be activated by hypotonic solution or mechanical stimulation [70–73]. TRPV2 has also been reported to be expressed in both WAT and BAT [68,74], which can be activated by noxious heat with the threshold above 52°C [75]. The expression level of TRPV2 is higher in mature adipocytes than in pre-adipocytes. Additionally, TRPV2 has been reported to play a role in adipocyte differentiation. It is reported that knockdown of TRPV2 reduces the differentiation of human white adipocytes [76]. TRPV2 has been proved to participate in thermogenesis and brown adipocyte differentiation [77,78]. The knockout of TRPV2 significantly decreases the mRNA expression levels of thermogenic genes, including PGC1α and UCP1. TRPV2 knockout mice have increased body weight, which is more fat upon HFD-treatment, accompanied by accumulated lipid droplets and enlarged sizes of brown adipocyte [77]. Moreover, activation of TRPV2 has been reported to prevent the brown adipocyte differentiation in mouse brown pre-adipocytes, which is probably via a calcineurin pathway [78]. These findings suggested that the TRPV2-mediated Ca2+ influx plays an important role in BAT differentiation and thermogenesis. And TRPV2 could be a target for preventing human obesity and other metabolic-related diseases [77–79]. However, the detailed mechanisms of TRPV2 in adipocyte differentiation are still unknown, which needs further studies in the future.
TRPV4 has been reported to be highly expressed in adipose tissue [80], such as WAT and BAT in mouse, as well as in human adipocytes, and the expression level of TRVP4 is higher in WAT than in BAT [81]. In adipocytes, TRPV4 functions as both hypotonic and major Ca2+ permeable channels. The amount of Ca2+ influx through one single TRPV4 channel is assessed to be around 100 times more than that of the L-type Ca2+ channel, which in turn to simulate various Ca2+-dependent signalling cascades [82]. Downregulation of TRPV4 did not affect adipogenesis in 3T3-F442A adipocytes. However, the administration of GSK205, an inhibitor of TRPV4, up-regulates the expression level of thermogenic genes such as Ppargc1a and Ucp1, which further promotes the browning process in 3T3-F442A adipocytes. Besides, pharmacological inhibition or deletion of TRPV4 also activates energy expenditure and protects mice from HFD-induced obesity in vivo [81]. These results suggested that inhibition of TRPV4 promotes browning of WAT by reducing the intracellular Ca2+ level [81]. However, knockout of TRPV4 has been proved to increase weight gain and promotes obesity during HFD-treatment in mice [83]. These results suggested a contradictory role of TRPV4 in adipogenesis and obesity. Therefore, further investigation is necessary to understand the role of TRPV4 in regulating Ca2+ influx, adipogenesis and obesity.
Other TRP members in adipogenesis
Several TRPC (TRPC1, 4, 5) channels have been reported function as SOCs, by interacting with the key players of SOCE, such as ORAI1 and STIM1, which we have discussed previously. TRPC1 usually forms a tetrameric complex with TRPC4 or TRPC5 and interacts with each other to stimulate the intracellular Ca2+ signalling pathway. The homomeric TRPC1 alone does not functionally work on the plasma membrane [84]. It has been recently reported that increasing the level of extracellular adenosine triphosphate (ATP) induces Ca2+ influx in adipocytes via CRAC channels, such as ORAI1 and STIM1 [85]. The activation of ORAI1 not only induces the SOCE, but also stimulates the translocation of TRPC1 onto the plasma membrane by which mediates an additional Ca2+ influx [86]. TRPC1, TRPC4, TRPC5, and TRPC6 have been reported to be expressed in both preadipocytes and adipocytes, suggesting that these TRPCs may participate in adipogenesis [87]. Indeed, it has been proved that TRPC1 negatively regulates HFD-treatment induced obesity [88]. Besides, TRPC4 and TRPC6 were differentially expressed in pre‐adipocytes and mature adipocytes. These results suggested that TRPCs may play critical roles in adipogenesis [88]. Since TRPCs allow both the entry of Ca2+ and Na+ ions, the exact role of TRPCs mediate signalling in adipogenesis and obesity needs further studies.
Other TRP channels, such as TRPM8 and TRPPs, have also been reported to be involved in adipogenesis and obesity. TRPM8 is known as a cold sensing channel with a temperature threshold lower than 26-28°C [89], which can be activated by menthol or icilin [89,90]. TRPM8 has been reported to be functionally presented in BAT of the mouse, and the expression levels of TRPM8 are significantly increased during adipocyte differentiation [91]. Activation of TRPM8 in adipocytes by menthol up-regulates UCP1 expression and requires protein kinase A activation, which in turn enhances the BAT thermogenesis and browning of WAT in mice [92,93]. Besides, TRPM8 also expresses in a cell line of human white adipocyte. Activation of TRPM8 induces UCP1 expression, WAT browning, mitochondrial activation, and heat production [91]. TRPM8 has also been proved to be involved in the regulation of clock and clock-controlled genes in BAT [94].
TRPP is a type of non-selective ion channel, which has been proved to be associated with autosomal dominant polycystic kidney [95]. TRPP has three family members, TRPP2, TRPP3, and TRPP5. TRPP2, also known as PKD2 or polycystin‐2, has been reported to be expressed in adipose tissue, and the expression level of TRPP2 in mature adipocytes is higher than in pre-adipocytes [87,95]. However, the role of TRPP2 in adipogenesis has not been investigated yet. TRPP3 plays a role in BAT differentiation and thermogenesis [96]. Down-regulation of TRPP3 suppresses the expression of thermogenic genes, such as UCP1 and PGC1α, and attenuates the mitochondrial respiration but with no effect on adipogenesis. These results suggested that TRPP3 may facilitate BAT differentiation by enhancing mitochondrial function [96]. Taken together, it has been proved that some TRP channels are involved in adipogenesis and adiposity. However, the direct role of these channels in adipogenesis and obesity needs to be further investigated.
Cav channels in adipogenesis
Cav channels, namely voltage-gated calcium channels, are one of the major calcium-permeable channels, which are widely expressed in numerous cell types such as neurons and adipocytes, and play important roles in regulating cellular processes, including release of neurotransmitters in neurons, and activation, differentiation and proliferation in adipocytes, respectively [97,98]. Cav channels are grouped into three subtypes: L-type high-voltage-activated (HVA) Cav channel is encoded by Cav1; other HVA Cav channels, such as P/Q-type, R-type, and N-type, are encoded by Cav2; and low-voltage-activated (LVA) T-type Cav channel is encoded by Cav3. Some of these Cav channels have been proved to be involved in the regulation of intracellular calcium homoeostasis and adipocyte differentiation. The presence of L-type Cav has been reported to be expressed in adipocytes by experimental and clinical studies. L-type Cav is also involved in high extracellular Ca2+-stimulated adipogenesis in porcine BMSCs [34], because the effects of extracellular calcium on adipogenesis can be inhibited by an L-type Cav blocker, Nifedipine. Besides, the distribution of L-type Cav and the basal levels of intracellular calcium have been proved to be regulated by the growth hormone in rat adipocytes [99,100]. The T-type Cav has been firstly reported to be expressed in 3T3-F442A pre-adipocytes [101]. Then Cav3.1 has been proved to be expressed in cultured pre-adipocytes and 3T3-L1 pre-adipocytes, and the protein-expression level of Cav3.1 is significantly decreased in the differentiated adipocytes [102]. It has been confirmed that the selective block of T-type Cav by NNC55-0396, a T-type Cav inhibitor, inhibits the proliferation in pre-adipocytes [102]. It has been further confirmed that mice lacking Cav3.1 or treated with TTA-A2, another selective blocker of T-type Cav, are resistant to HFD induced increase of fat mass and body weight gain [102]. These results suggested that Cav channels, such as Cav3.1, appear to be potential targets for the prevention and treatment of obesity.
P2 receptors in adipogenesis
P2 receptors are one type of purinergic receptor, which is in response to the release of ATP. They have been classified into five subclasses, including ionotropic receptors such as P2X, P2Z, and metabotropic receptors such as P2Y, P2 U, and P2 T. P2 receptors have been reported to be involved in several cellular functions, such as vascular reactivity, cytokine secretion, and cell proliferation and migration [103]. However, the effects of purinergic stimulation seem to vary between cell types. A series of evidence have reported that extracellular ATP induces various cellular functions in both adipocytes and pre-adipocytes. In WATs, external ATP has been reported to be involved in glucose transport [104], glycogen synthase [105], lipolysis [106] and cytosolic Ca2+ homoeostasis [107]. In BATs, it has been reported to modulate membrane trafficking [108], cytosolic Ca2+ homoeostasis [109], and cell proliferation [110]. In 3T3-L1 pre-adipocytes, the extracellular ATP has been demonstrated to regulate hormone-induced adipocyte differentiation, which is mediated by P2Y receptors without affects pre-adipocyte proliferation [111]. It has been recently reported that knockdown of CD36, a membrane protein that has been demonstrated to participate in the progression of adipogenesis, resulted in a reduction of adipocyte differentiation and downregulation of purinergic receptor P2X7 expression, suggesting that the suppression of adipogenesis mediated by CD36 is probably via P2X7 pathway in 3T3-L1 cells [112]. Although P2 receptors have been proved to play a role in adipogenesis, the underlying mechanisms are still needed for further investigation.
Concluding Remarks
In the present review, we summarized the recent research progress of Ca2+-permeable channels, such as CRAC channels, TRPs, Cav and other channels in adipogenesis (Figure 2). Disturbance in Ca2+ homoeostasis through manipulating these calcium channels affects the downstream signalling pathways which in turn promotes or inhibits adipocyte differentiation and subsequently affects obesity. However, there are still some open questions that should be carefully considered in future studies. First, how do pre-adipocytes and mature adipocytes balance the Ca2+ fluxes among different components to maintain Ca2+ homoeostasis? Second, is there any other Ca2+ permeable channels involved in adipogenesis and obesity, such as high-voltage-activated Cav channels and NMDA receptors. Third, is the effect of cytosolic Ca2+ on adipocyte differentiation phase-dependent? Further in-depth studies are required to determine the best therapeutic targets of calcium channels for clinical treatment of obesity and related metabolic disorders. Such studies could pave the way for new clinical approaches to treating human obesity and related metabolic diseases.
Figure 2.
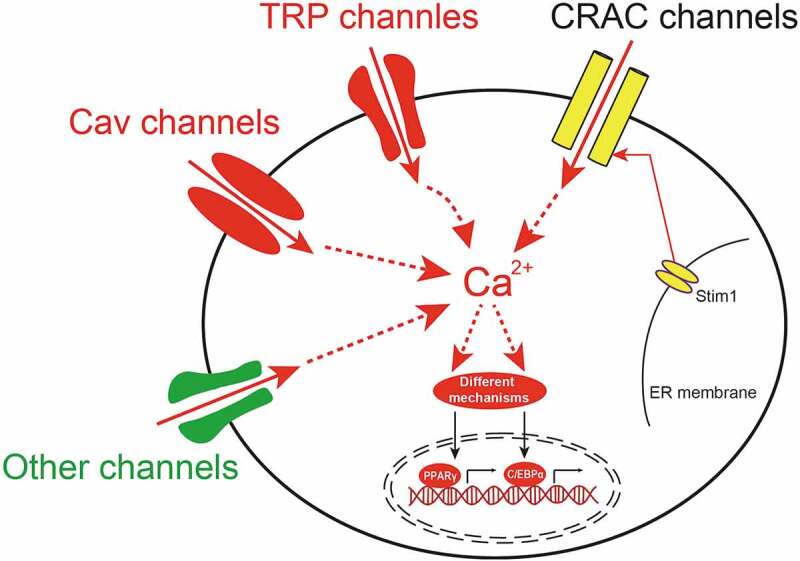
The schematic diagram of the involvement of Ca2+-permeable ion channels in adipogenesis
In pre-adipocytes or bone marrow mesenchymal stem cells (BMSCs), several different types of Ca2+-permeable ion channels, such as Ca2+ release-activated Ca2+ (CRAC) channels, transient receptor potential (TRP) channels, voltage-gated calcium (Cav) channels and other channels, are expressed and contributed in adipogenesis. Upon these channels activation by respective stimuli, extracellular Ca2+ entry into the cytoplasm. And the increase of cytosolic Ca2+ modulates adipogenesis by promoting transcription via different mechanisms. PPARγ: peroxisome proliferator-activated receptor γ; C/EBPα: CCAAT-enhancer-binding protein α.
Acknowledgments
We are grateful to Dr. Makoto Tominaga (National Institute for Physiological Sciences) and Dr. Kunitoshi Uchida (Fukuoka Dental College of Japan) for their kind suggestions and comments.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China [No. 81700741], Shenzhen Municipal Science, Technology and Innovation Commission [No. JCYJ20180302144710880] and Shenzhen Nanshan District Scientific Research Program of China [No. 2017003].
Author Contributions
The authors’ contributions were as follows: M Zhai, D Yang, W Yi and W Sun were involved in literature collection and summarization; M Zhai, D Yang, W Yi and W Sun written the review manuscript. W Yi and W Sun obtained the funding.
Disclosure Statement
The authors declare that they have no conflict of interest.
References
- [1].Ahn J, Lee H, Kim S, et al. The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem Biophys Res Commun. 2008;373:545–549. [DOI] [PubMed] [Google Scholar]
- [2].Hajer GR, van Haeften TW, Visseren FL.. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29:2959–2971. [DOI] [PubMed] [Google Scholar]
- [3].Rull A, Camps J, Alonso-Villaverde C, et al. Insulin resistance, inflammation, and obesity: role of monocyte chemoattractant protein-1 (or CCL2) in the regulation of metabolism. Mediators Inflamm. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Argyropoulos G, Harper ME. Uncoupling proteins and thermoregulation. J Appl Physiol. 2002;92:2187–2198. [DOI] [PubMed] [Google Scholar]
- [5].Mozo J, Emre Y, Bouillaud F, et al. Thermoregulation: what role for UCPs in mammals and birds? Biosci Rep. 2005;25:227–249. [DOI] [PubMed] [Google Scholar]
- [6].Rosen ED, Walkey CJ, Puigserver P, et al. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14:1293–1307. [PubMed] [Google Scholar]
- [7].Poulos SP, Dodson MV, Hausman GJ. Cell line models for differentiation: preadipocytes and adipocytes. Exp Biol Med (Maywood). 2010;235:1185–1193. [DOI] [PubMed] [Google Scholar]
- [8].Niemelä S, Miettinen S, Sarkanen J, et al. Adipose tissue and adipocyte differentiation: molecular and cellular aspects and tissue engineering applications. Top Tissue Eng. 2008;4:26. [Google Scholar]
- [9].Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. [DOI] [PubMed] [Google Scholar]
- [10].Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316:129–139. [DOI] [PubMed] [Google Scholar]
- [11].Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. [DOI] [PubMed] [Google Scholar]
- [13].Sethi JK, Vidal-Puig AJ. Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007;48:1253–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pi-Sunyer FX. Medical hazards of obesity. Ann Intern Med. 1993;119:655–660. [DOI] [PubMed] [Google Scholar]
- [15].Boron WF, Boulpaep EL. Intracellular pH regulation in the renal proximal tubule of the salamander. Na-H exchange. J Gen Physiol. 1983;81:29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Takahashi A, Camacho P, Lechleiter JD, et al. Measurement of intracellular calcium. Physiol Rev. 1999;79:1089–1125. [DOI] [PubMed] [Google Scholar]
- [17].Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. [DOI] [PubMed] [Google Scholar]
- [18].Koch GL. The endoplasmic reticulum and calcium storage. Bioessays. 1990;12:527–531. [DOI] [PubMed] [Google Scholar]
- [19].Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. [DOI] [PubMed] [Google Scholar]
- [20].Misquitta CM, Mack DP, Grover AK. Sarco/endoplasmic reticulum Ca2+ (SERCA)-pumps: link to heart beats and calcium waves. Cell Calcium. 1999;25:277–290. [DOI] [PubMed] [Google Scholar]
- [21].Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. [DOI] [PubMed] [Google Scholar]
- [22].Ntambi JM, Takova T. Role of Ca2+ in the early stages of murine adipocyte differentiation as evidenced by calcium mobilizing agents. Different Res Biol Divers. 1996;60:151–158. [DOI] [PubMed] [Google Scholar]
- [23].Shi H, Halvorsen YD, Ellis PN, et al. Role of intracellular calcium in human adipocyte differentiation. Physiol Genomics. 2000;3:75–82. [DOI] [PubMed] [Google Scholar]
- [24].Neal JW, Clipstone NA. Calcineurin mediates the calcium-dependent inhibition of adipocyte differentiation in 3T3-L1 cells. J Biol Chem. 2002;277:49776–49781. [DOI] [PubMed] [Google Scholar]
- [25].Neal JW, Clipstone NA. A constitutively active NFATc1 mutant induces a transformed phenotype in 3T3-L1 fibroblasts. J Biol Chem. 2003;278:17246–17254. [DOI] [PubMed] [Google Scholar]
- [26].Mathieu RL, Casez JP, Jaeger P, et al. Altered body composition and fuel metabolism in stable kidney transplant patients on immuno-suppressive monotherapy with cyclosporine A. Eur J Clin Invest. 1994;24:195–200. [DOI] [PubMed] [Google Scholar]
- [27].Berridge MJ. Calcium microdomains: organization and function. Cell Calcium. 2006;40:405–412. [DOI] [PubMed] [Google Scholar]
- [28].Jensen B, Farach-Carson MC, Kenaley E, et al. High extracellular calcium attenuates adipogenesis in 3T3-L1 preadipocytes. Exp Cell Res. 2004;301:280–292. [DOI] [PubMed] [Google Scholar]
- [29].Park S, Kang S, Kim DS. Severe calcium deficiency increased visceral fat accumulation, down-regulating genes associated with fat oxidation, and increased insulin resistance while elevating serum parathyroid hormone in estrogen-deficient rats. Nutr Res. 2019;73:48–57. [DOI] [PubMed] [Google Scholar]
- [30].Zhang F, Ye J, Zhu X, et al. Anti-Obesity effects of dietary calcium: the evidence and possible mechanisms. Int J Mol Sci. 2019;20(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pramme-Steinwachs I, Jastroch M, Ussar S. Extracellular calcium modulates brown adipocyte differentiation and identity. Sci Rep. 2017;7:8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hashimoto R, Katoh Y, Miyamoto Y, et al. Increased extracellular and intracellular Ca(2)(+) lead to adipocyte accumulation in bone marrow stromal cells by different mechanisms. Biochem Biophys Res Commun. 2015;457:647–652. [DOI] [PubMed] [Google Scholar]
- [33].Ye J, Ai W, Zhang F, et al. Enhanced proliferation of porcine bone marrow mesenchymal stem cells induced by extracellular calcium is associated with the activation of the calcium-sensing receptor and erk signaling pathway. Stem Cells Int. 2016;(2016):6570671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhang F, Ye J, Meng Y, et al. Calcium supplementation enhanced adipogenesis and improved glucose homeostasis through activation of camkii and PI3K/Akt Signaling pathway in porcine bone marrow mesenchymal stem cells (pBMSCs) and mice fed high fat diet (HFD). Cell Physiol Biochem. 2018;51:154–172. [DOI] [PubMed] [Google Scholar]
- [35].Hashimoto R, Katoh Y, Miyamoto Y, et al. High extracellular Ca(2+) enhances the adipocyte accumulation of bone marrow stromal cells through a decrease in cAMP. Cell Calcium. 2017;67:74–80. [DOI] [PubMed] [Google Scholar]
- [36].Cooper DM, Karpen JW, Fagan KA, et al. Ca(2+)-sensitive adenylyl cyclases. Adv Second Messenger Phosphoprotein Res. 1998;32:23–51. [PubMed] [Google Scholar]
- [37].Venkatachalam K, van Rossum DB, Patterson RL, et al. The cellular and molecular basis of store-operated calcium entry. Nat Cell Biol. 2002;4:E263–272. [DOI] [PubMed] [Google Scholar]
- [38].Tojyo Y, Morita T, Nezu A, et al. Key components of store-operated Ca2+ entry in non-excitable cells. J Pharmacol Sci. 2014;125:340–346. [DOI] [PubMed] [Google Scholar]
- [39].Wu MM, Buchanan J, Luik RM, et al. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Xu P, Lu J, Li Z, et al. Aggregation of STIM1 underneath the plasma membrane induces clustering of Orai1. Biochem Biophys Res Commun. 2006;350:969–976. [DOI] [PubMed] [Google Scholar]
- [41].Soboloff J, Spassova MA, Tang XD, et al. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281:20661–20665. [DOI] [PubMed] [Google Scholar]
- [42].Graham SJ, Black MJ, Soboloff J, et al. Stim1, an endoplasmic reticulum Ca2+ sensor, negatively regulates 3T3-L1 pre-adipocyte differentiation. Differentiation. 2009;77:239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Baumbach J, Hummel P, Bickmeyer I, et al. A Drosophila in vivo screen identifies store-operated calcium entry as a key regulator of adiposity. Cell Metab. 2014;19:331–343. [DOI] [PubMed] [Google Scholar]
- [44].Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. [DOI] [PubMed] [Google Scholar]
- [45].Liao M, Cao E, Julius D, et al. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 2013;504:107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Cao E, Liao M, Cheng Y, et al. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature. 2013;504:113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Paulsen CE, Armache JP, Gao Y, et al. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature. 2015;520:511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zubcevic L, Herzik MA Jr., Chung BC, et al. Cryo-electron microscopy structure of the TRPV2 ion channel. Nat Struct Mol Biol. 2016;23:180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Huynh KW, Cohen MR, Jiang J, et al. Structure of the full-length TRPV2 channel by cryo-EM. Nat Commun. 2016;7:11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–647. [DOI] [PubMed] [Google Scholar]
- [51].Wu LJ, Sweet TB, Clapham DE. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev. 2010;62:381–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Uchida K, Sun W, Yamazaki J, et al. Role of thermo-sensitive transient receptor potential channels in brown adipose tissue. Biol Pharm Bull. 2018;41:1135–1144. [DOI] [PubMed] [Google Scholar]
- [54].Lee E, Jung DY, Kim JH, et al. Transient receptor potential vanilloid type-1 channel regulates diet-induced obesity, insulin resistance, and leptin resistance. Faseb J. 2015;29:3182–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zhang LL, Yan Liu D, Ma LQ, et al. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ Res. 2007;100:1063–1070. [DOI] [PubMed] [Google Scholar]
- [56].Cioffi DL. The skinny on TRPV1. Circ Res. 2007;100:934–936. [DOI] [PubMed] [Google Scholar]
- [57].Chen J, Li L, Li Y, et al. Activation of TRPV1 channel by dietary capsaicin improves visceral fat remodeling through connexin43-mediated Ca2+ influx. Cardiovasc Diabetol. 2015;14:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014;10:24–36. [DOI] [PubMed] [Google Scholar]
- [59].Baboota RK, Singh DP, Sarma SM, et al. Capsaicin induces “brite” phenotype in differentiating 3T3-L1 preadipocytes. PloS One. 2014;9:e103093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Baskaran P, Krishnan V, Fettel K, et al. TRPV1 activation counters diet-induced obesity through sirtuin-1 activation and PRDM-16 deacetylation in brown adipose tissue. Int J Obes (Lond). 2017;41:739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Iwasaki Y, Tamura Y, Inayoshi K, et al. TRPV1 agonist monoacylglycerol increases UCP1 content in brown adipose tissue and suppresses accumulation of visceral fat in mice fed a high-fat and high-sucrose diet. Biosci Biotechnol Biochem. 2011;75:904–909. [DOI] [PubMed] [Google Scholar]
- [62].Motter AL, Ahern GP. TRPV1-null mice are protected from diet-induced obesity. FEBS Lett. 2008;582:2257–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Marshall NJ, Liang L, Bodkin J, et al. A role for TRPV1 in influencing the onset of cardiovascular disease in obesity. Hypertension. 2013;61:246–252. [DOI] [PubMed] [Google Scholar]
- [64].Cheng W, Yang F, Liu S, et al. Heteromeric heat-sensitive transient receptor potential channels exhibit distinct temperature and chemical response. J Biol Chem. 2012;287:7279–7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Cheung SY, Huang Y, Kwan HY, et al. Activation of transient receptor potential vanilloid 3 channel (TRPV3) suppresses adipogenesis. Endocrinology. 2015;156(6):2074–2086. [DOI] [PubMed] [Google Scholar]
- [66].Liu B, Qin F. Single-residue molecular switch for high-temperature dependence of vanilloid receptor TRPV3. Proc Natl Acad Sci U S A. 2017;114:1589–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Cheung SY, Huang Y, Kwan HY, et al. Activation of transient receptor potential vanilloid 3 channel suppresses adipogenesis. Endocrinology. 2015;156:2074–2086. [DOI] [PubMed] [Google Scholar]
- [68].Sun W, Li C, Zhang Y, et al. Gene expression changes of thermo-sensitive transient receptor potential channels in obese mice. Cell Biol Int. 2017;41:908–913. [DOI] [PubMed] [Google Scholar]
- [69].Hu J, Choo HJ, Ma SX. Infrared heat treatment reduces food intake and modifies expressions of TRPV3-POMC in the dorsal medulla of obesity prone rats. Int J Hyperthermia. 2011;27:708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Muraki K, Iwata Y, Katanosaka Y, et al. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res. 2003;93:829–838. [DOI] [PubMed] [Google Scholar]
- [71].Iwata Y, Katanosaka Y, Arai Y, et al. Dominant-negative inhibition of Ca2+ influx via TRPV2 ameliorates muscular dystrophy in animal models. Hum Mol Genet. 2009;18:824–834. [DOI] [PubMed] [Google Scholar]
- [72].Phan MN, Leddy HA, Votta BJ, et al. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheumatism. 2009;60:3028–3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Liedtke W, Tobin DM, Bargmann CI, et al. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2003;100(Suppl 2):14531–14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Bishnoi M, Kondepudi KK, Gupta A, et al. Expression of multiple transient receptor potential channel genes in murine 3T3-L1 cell lines and adipose tissue. Pharmacol Rep. 2013;65:751–755. [DOI] [PubMed] [Google Scholar]
- [75].Caterina MJ, Rosen TA, Tominaga M, et al. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. [DOI] [PubMed] [Google Scholar]
- [76].Che H, Yue J, Tse HF, et al. TRPM channels in human preadipocytes. Pflugers Arch. 2014;466:947–959. [DOI] [PubMed] [Google Scholar]
- [77].Sun W, Uchida K, Suzuki Y, et al. Lack of TRPV2 impairs thermogenesis in mouse brown adipose tissue. EMBO Rep. 2016;17:383–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Sun W, Uchida K, Takahashi N, et al. Activation of TRPV2 negatively regulates the differentiation of mouse brown adipocytes. Pflugers Arch. 2016;468:1527–1540. [DOI] [PubMed] [Google Scholar]
- [79].Sun W, Uchida K, Tominaga M. TRPV2 regulates BAT thermogenesis and differentiation. Channels (Austin). 2017;11:94–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Liedtke W, Choe Y, Marti-Renom MA, et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ye L, Kleiner S, Wu J, et al. TRPV4 is a regulator of adipose oxidative metabolism, inflammation, and energy homeostasis. Cell. 2012;151:96–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Mercado J, Baylie R, Navedo MF, et al. Local control of TRPV4 channels by AKAP150-targeted PKC in arterial smooth muscle. J Gen Physiol. 2014;143:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].O’Conor CJ, Griffin TM, Liedtke W, et al. Increased susceptibility of Trpv4-deficient mice to obesity and obesity-induced osteoarthritis with very high-fat diet. Ann Rheum Dis. 2013;72:300–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Myeong J, Ko J, Hong C, et al. The interaction domains of transient receptor potential canonical (TRPC)1/4 and TRPC1/5 heteromultimeric channels. Biochem Biophys Res Commun. 2016;474:476–481. [DOI] [PubMed] [Google Scholar]
- [85].El Hachmane MF, Ermund A, Brannmark C, et al. Extracellular ATP activates store-operated Ca(2+) entry in white adipocytes: functional evidence for STIM1 and ORAI1. Biochem J. 2018;475:691–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Cheng KT, Liu X, Ong HL, et al. Local Ca(2)+ entry via Orai1 regulates plasma membrane recruitment of TRPC1 and controls cytosolic Ca(2)+ signals required for specific cell functions. PLoS Biol. 2011;9:e1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Sukumar P, Sedo A, Li J, et al. Constitutively active TRPC channels of adipocytes confer a mechanism for sensing dietary fatty acids and regulating adiponectin. Circ Res. 2012;111:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Krout D, Schaar A, Sun Y, et al. The TRPC1 Ca(2+)-permeable channel inhibits exercise-induced protection against high-fat diet-induced obesity and type II diabetes. J Biol Chem. 2017;292:20799–20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].de la Pena E, Malkia A, Cabedo H, et al. The contribution of TRPM8 channels to cold sensing in mammalian neurones. J Physiol. 2005;567:415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Peier AM, Moqrich A, Hergarden AC, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. [DOI] [PubMed] [Google Scholar]
- [91].Rossato M, Granzotto M, Macchi V, et al. Human white adipocytes express the cold receptor TRPM8 which activation induces UCP1 expression, mitochondrial activation and heat production. Mol Cell Endocrinol. 2014;383:137–146. [DOI] [PubMed] [Google Scholar]
- [92].Ma S, Yu H, Zhao Z, et al. Activation of the cold-sensing TRPM8 channel triggers UCP1-dependent thermogenesis and prevents obesity. J Mol Cell Biol. 2012;4:88–96. [DOI] [PubMed] [Google Scholar]
- [93].Jiang C, Zhai M, Yan D, et al. Dietary menthol-induced TRPM8 activation enhances WAT “browning” and ameliorates diet-induced obesity. Oncotarget. 2017;8:75114–75126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Moraes MN, de Assis LVM, Henriques FDS, et al. Cold-sensing TRPM8 channel participates in circadian control of the brown adipose tissue. Biochim Biophys Acta Mol Cell Res. 2017;1864:2415–2427. [DOI] [PubMed] [Google Scholar]
- [95].Moran MM, Xu H, Clapham DE. TRP ion channels in the nervous system. Curr Opin Neurobiol. 2004;14:362–369. [DOI] [PubMed] [Google Scholar]
- [96].Goralczyk A, van Vijven M, Koch M, et al. TRP channels in brown and white adipogenesis from human progenitors: new therapeutic targets and the caveats associated with the common antibiotic, streptomycin. Faseb J. 2017;31:3251–3266. [DOI] [PubMed] [Google Scholar]
- [97].Lory P, Bidaud I, Chemin J. T-type calcium channels in differentiation and proliferation. Cell Calcium. 2006;40:135–146. [DOI] [PubMed] [Google Scholar]
- [98].Panner A, Wurster RD. T-type calcium channels and tumor proliferation. Cell Calcium. 2006;40:253–259. [DOI] [PubMed] [Google Scholar]
- [99].Gaur S, Morton ME, Frick GP, et al. Growth hormone regulates the distribution of L-type calcium channels in rat adipocyte membranes. Am J Physiol. 1998;275:C505–514. [DOI] [PubMed] [Google Scholar]
- [100].Gaur S, Yamaguchi H, Goodman HM. Growth hormone regulates cytosolic free calcium in rat fat cells by maintaining L-type calcium channels. Am J Physiol. 1996;270:C1478–1484. [DOI] [PubMed] [Google Scholar]
- [101].Green H, Kehinde O. Formation of normally differentiated subcutaneous fat pads by an established preadipose cell line. J Cell Physiol. 1979;101:169–171. [DOI] [PubMed] [Google Scholar]
- [102].Uebele VN, Gotter AL, Nuss CE, et al. Antagonism of T-type calcium channels inhibits high-fat diet-induced weight gain in mice. J Clin Invest. 2009;119(6):1659–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Burnstock G. Introduction to purinergic signalling in the brain. Adv Exp Med Biol. 2013;986:1–12. [DOI] [PubMed] [Google Scholar]
- [104].Halperin ML, Mak ML, Taylor WM. Control of glucose transport in adipose tissue of the rat: role of insulin, ATP, and intracellular metabolites. Can J Biochem. 1978;56:708–712. [DOI] [PubMed] [Google Scholar]
- [105].Tamura S, Dubler RE, Larner J. Stimulation of maximal intracellular insulin action on glycogen synthase by preincubation of adipocytes with adenosine 5ʹ-triphosphate. J Biol Chem. 1983;258:719–724. [PubMed] [Google Scholar]
- [106].Schmidt M, Loffler G. Induction of aromatase activity in human adipose tissue stromal cells by extracellular nucleotides–evidence for P2-purinoceptors in adipose tissue. Eur J Biochem. 1998;252:147–154. [DOI] [PubMed] [Google Scholar]
- [107].Kelly KL, Deeney JT, Corkey BE. Cytosolic free calcium in adipocytes. Distinct mechanisms of regulation and effects on insulin action. J Biol Chem. 1989;264:12754–12757. [PubMed] [Google Scholar]
- [108].Pappone PA, Lee SC. Purinergic receptor stimulation increases membrane trafficking in brown adipocytes. J Gen Physiol. 1996;108:393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Omatsu-Kanbe M, Isono T, Matsuura H. Multiple P2 receptors contribute to a transient increase in intracellular Ca2+ concentration in ATP-stimulated rat brown adipocytes. Exp Physiol. 2002;87:643–652. [DOI] [PubMed] [Google Scholar]
- [110].Wilson SM, Barsoum MJ, Wilson BW, et al. Purine nucleotides modulate proliferation of brown fat preadipocytes. Cell Prolif. 1999;32:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Omatsu-Kanbe M, Inoue K, Fujii Y, et al. Effect of ATP on preadipocyte migration and adipocyte differentiation by activating P2Y receptors in 3T3-L1 cells. Biochem J. 2006;393:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Gao H, Li D, Yang P, et al. Suppression of CD36 attenuates adipogenesis with a reduction of P2X7 expression in 3T3-L1 cells. Biochem Biophys Res Commun. 2017;491:204–208. [DOI] [PubMed] [Google Scholar]


