Abstract
Although both are characterized by the presence of an IgM monoclonal gammopathy, IgM multiple myeloma and Waldenstrom’s macroglobulinemia are two distinct hematologic entities. Differentiation of each however, may be challenging, but obviously critical to ensuring appropriate therapeutic decision-making and patient prognostication. Herein we report a case of a patient with Waldenstrom’s macroglobulinemia presenting with bone marrow morphology mimicking plasma cell myeloma, highlighting the importance of clinical correlation and ancillary studies to reach an appropriate diagnosis.
Keywords: Multiple myeloma, Waldenstrom’s macroglobulinemia, Lymphoplasmacytic lymphoma, Paraproteinemia, Bone marrow
Introduction
Waldenstrom’s macroglobulinemia (WM) is a rare disorder of B lymphocytes, which is defined as a lymphoplasmacytic lymphoma (LPL) with bone marrow involvement and an IgM monoclonal gammopathy [1]. LPL is usually composed of small lymphocytes, with variable numbers of plasma cells, plasmacytoid lymphocytes, and increased mast cells [2, 3]. Distinct clusters of plasma cells may form separate from the lymphoid component [2, 3]. With marked plasmacytic differentiation it may be clinically challenging to differentiate LPL from plasma cell myeloma. However, this differentiation is critical to prognostication and therapeutic decision-making. Herein we describe a case of WM presenting with morphology mimicking plasma cell myeloma, outlining the importance of clinical correlation and ancillary studies to lead to an accurate diagnosis.
Case Report
A 63-year-old Caucasian female was referred to hematology for assessment of an IgM lambda monoclonal paraprotein detected in the evaluation for anemia. She reported fatigue and unintentional weight loss, but no other constitutional symptoms. There was no clinical evidence of bleeding or peripheral neuropathy; however, she was experiencing intermittent headaches and blurred vision, concerning for potential hyperviscosity syndrome. There was no peripheral lymphadenopathy or hepatosplenomegaly.
Laboratory testing demonstrated white blood cell count of 3.3 × 109/L (normal 4.5 – 11 × 109/L), hemoglobin of 104 g/L (normal 120 - 160 g/L), mean cell volume of 95 fL (normal 80 - 98 fL), platelet count of 144 × 109/L (normal 140 - 440 ×109/L), and LDH of 140 U/L (normal 120 - 230 U/L). There was mild hypercalcemia (corrected calcium 2.67 mmol/L; normal 2.1 - 2.6 mmol/L), but otherwise normal electrolytes, renal and liver function. Total serum protein was elevated at 116 g/L (normal 60 - 80 g/L). Serum protein electrophoresis demonstrated an IgM lambda monoclonal protein of 50 g/L, with an elevated serum viscosity ratio (at 37 degrees) of 7.8 (normal 1.1 - 1.8) and beta-2 microglobulin of 2.6 mg/L (normal 1.1 - 2.4g/L). There were no osseous lesions identified on skeletal survey.
Bone marrow aspirate and biopsy demonstrated a hypercellular marrow with extensive plasma cell infiltration in large sheets (91.4% of 500 cell differential; Fig. 1A). Lymphocytes were rare, accounting for 1% in the 500 cell differential (Fig. 1B). Tri-lineage hematopoiesis was markedly decreased with otherwise unremarkable morphology. No amyloid deposits were seen, and Congo red staining was negative. Immunohistochemistry demonstrated a marked increase in CD138+ (Fig. 1C), lambda restricted monotypic plasma cells (Fig. 1D), which were BCL1+, CD20- and CD56-. Flow cytometry revealed a population of clonal lambda-restricted plasma cells that were CD38+, CD138+, CD56-, CD19+, CD20-, and CD117-, and a separate a clonal B cell population which were CD19+, CD20+ (dim), surface lambda+ (dim), CD10+ (dim), and CD38+ (high). The flow cytometry findings suggested a B cell lymphoma with plasma cell differentiation.
Figure 1.
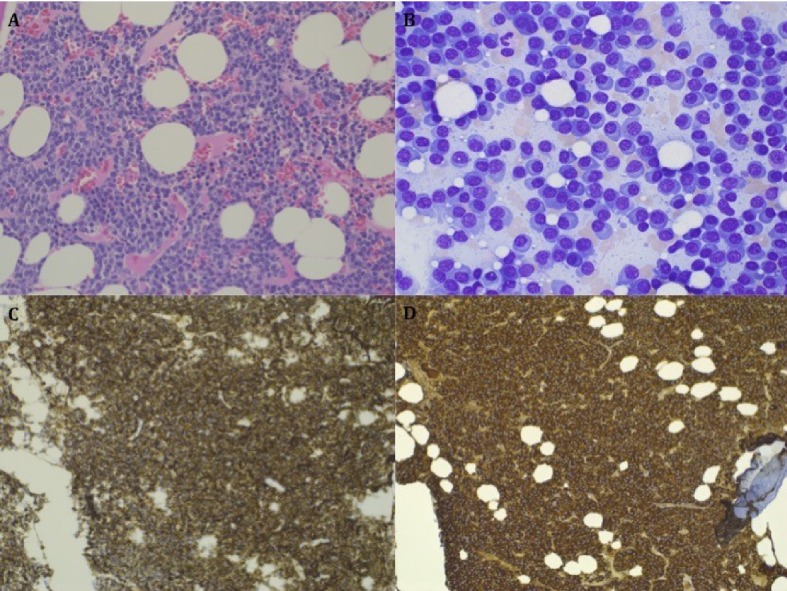
Bone marrow biopsy specimen. (A) Hypercellular marrow with extensive sheet forming plasma cell infiltration. (B) Higher magnification demonstrating extensive plasma cell infiltration with few lymphocytes. (C)Immunohistochemistry demonstrating presence of marked CD138 positive population. (D) Immunohistochemistry demonstrating lambda restricted population.
While the morphologic findings favored a diagnosis of plasma cell myeloma, the composite of the patient’s clinical presentation and immunophenotypic findings favored a diagnosis of WM. She was started on bendamustine with the later addition of rituximab, and completed six cycles with resolution of her symptoms and a notable decrease in monoclonal paraproteinemia, supporting our presumptive diagnosis.
Discussion
Herein we present a case of a patient with IgM lambda monoclonal paraprotein, possible hyperviscosity syndrome and laboratory findings of anemia and hypercalcemia. Morphologically, the bone marrow demonstrated extensive plasma cell infiltration with a CD19+, CD20- CD56+, CD138+ clonal population by flow cytometry.
When considered separately, the clinical features of myeloma (“CRAB” criteria: hypercalcemia, renal dysfunction, anemia and bone involvement), can be observed in LPL and other lymphoproliferative disorders. Furthermore, symptomatic hyperviscosity, although classically associated with WM, can occur in up to 6% of IgM myeloma cases [4]. Flow cytometry was particularly informative in our case. In contrast to normal plasma cells, the ubiquitous B cell marker CD19 is usually absent in myeloma cells [5]. Furthermore, myeloma cells often express CD56, which is typically absent in normal plasma cells [6]. While the evaluation of these two markers can aid in differentiating mature B-cell lymphomas from plasma cell myelomas [7], they have not yet been described in a case with extreme plasmacytic differentiation. The reported phenotypic heterogeneity of CD20 has challenged the prior assertion that IgM myeloma can be distinguished based on the presence of a CD20 negative plasma cell population [2].
To arrive at the appropriate diagnosis, it is prudent that the clinician be aware of the spectrum of pathology linking B-cell lymphomas and plasma cell disorders. This clinical case highlights the importance of integrating both clinical and laboratory studies to differentiate WM from IgM plasma cell myeloma.
References
- 1.Owen RG, Treon SP, Al-Katib A, Fonseca R, Greipp PR, McMaster ML, Morra E. et al. Clinicopathological definition of Waldenstrom's macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom's Macroglobulinemia. Semin Oncol. 2003;30(2):110–115. doi: 10.1053/sonc.2003.50082. [DOI] [PubMed] [Google Scholar]
- 2.Lin P, Molina TJ, Cook JR, Swerdlow SH. Lymphoplasmacytic lymphoma and other non-marginal zone lymphomas with plasmacytic differentiation. Am J Clin Pathol. 2011;136(2):195–210. doi: 10.1309/AJCP8FOIVTB6LBER. [DOI] [PubMed] [Google Scholar]
- 3.Morice WG, Chen D, Kurtin PJ, Hanson CA, McPhail Ed. Novel immunophenotypic features of marrow lymphoplasmacytic lymphoma and correlation with Waldenstrom's macroglobulinemia. Mod Pathol. 2009;22(6):807–816. doi: 10.1038/modpathol.2009.34. [DOI] [PubMed] [Google Scholar]
- 4.Mehta J, Singhal S. Hyperviscosity syndrome in plasma cell dyscrasias. Semin Thromb Hemost. 2003;29(5):467–471. doi: 10.1055/s-2003-44554. [DOI] [PubMed] [Google Scholar]
- 5.Harada H, Kawano MM, Huang N, Harada Y, Iwato K, Tanabe O, Tanaka H. et al. Phenotypic difference of normal plasma cells from mature myeloma cells. Blood. 1993;81(10):2658–2663. [PubMed] [Google Scholar]
- 6.Lima M, Teixeira Mdos A, Fonseca S, Goncalves C, Guerra M, Queiros ML, Santos AH. et al. Immunophenotypic aberrations, DNA content, and cell cycle analysis of plasma cells in patients with myeloma and monoclonal gammopathies. Blood Cells Mol Dis. 2000;26(6):634–645. doi: 10.1006/bcmd.2000.0342. [DOI] [PubMed] [Google Scholar]
- 7.Seegmiller AC, Xu Y, McKenna RW, Karandikar NJ. Immunophenotypic differentiation between neoplastic plasma cells in mature B-cell lymphoma vs plasma cell myeloma. Am J Clin Pathol. 2007;127(2):176–181. doi: 10.1309/5EL22BH45PHUPM8P. [DOI] [PubMed] [Google Scholar]


